In his Clinical Note, “Amnestic MCI in ADNI: Maybe Not Enough Memory Impairment?” Duff1 raises important issues regarding the diagnosis of mild cognitive impairment (MCI). As he comments, MCI is often a transitional condition between the cognitive changes of aging and dementia. The criteria for MCI have been reasonably consistent and accepted, but the implementation of the criteria has posed significant issues.2,3
With respect to the specific operationalization of MCI in the Alzheimer's Disease Neuroimaging Initiative (ADNI), several points are relevant. ADNI was designed as a simulated randomized controlled trial. As such, the specific implementation of the criteria for MCI had to be as unambiguous as possible to allow maximal reliability across participating sites. As such, we chose to use the same instruments and cutoff scores for amnestic MCI in ADNI as we used in the vitamin E and donepezil MCI trial as that implementation strategy worked well in the study, yielding an annual progression rate from MCI to dementia of 16% per year.4
Duff1 takes issue with the cutoff scores used for inclusion in ADNI for amnestic MCI. We chose to use data from the Religious Orders Study employing delayed recall of 1 paragraph from the Logical Memory Test corrected for education.5 There is controversy in the field with respect to age and cognition as to whether one should adjust for age in cutoff scores vs adjusting for age in subsequent analytical models.6
Do They Work?
Considering the caveats above in selecting the implementation of the MCI criteria, has the endeavor been successful?
From a clinical perspective, we have followed participants for over 10 years in ADNI, and the annual rate of progression from MCI to dementia has been consistent with what has been predicted. As is shown in Figure 1, those individuals who were diagnosed originally with amnestic MCI (now labeled late MCI [LMCI]) have progressed at the anticipated rate.7 However, we have been responsive to persons with Duff's concerns on the lack of sensitivity of the criteria, and in ADNI2, we softened the Logical Memory cutoff scores to capture a less impaired cohort of patients with MCI, who were labeled as early MCI (EMCI). As is shown in Figure 1, the EMCI participants progressed to dementia at a rate that was somewhat less than those characterized with the LMCI criteria, as one would expect. Those progression rates are presented in Figure 1.
Figure 1. Mild Cognitive Impairment (MCI) Transitions.
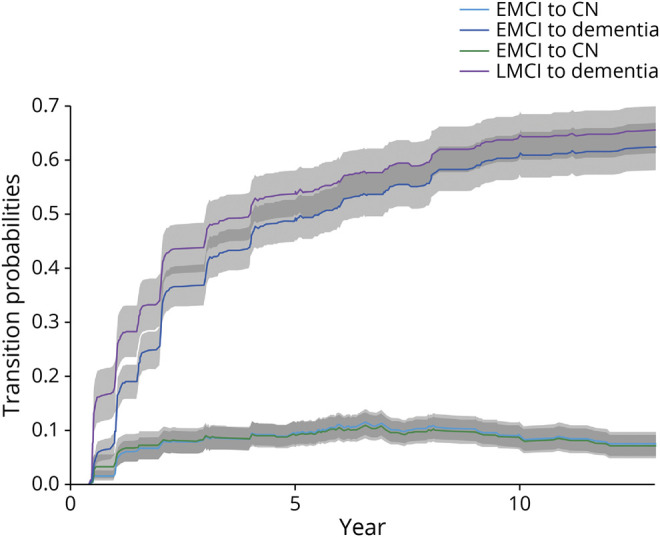
The progression of participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI) from early MCI (EMCI) to cognitively normal (CN) or dementia and progression of late MCI (LMCI) to cognitively normal or dementia.
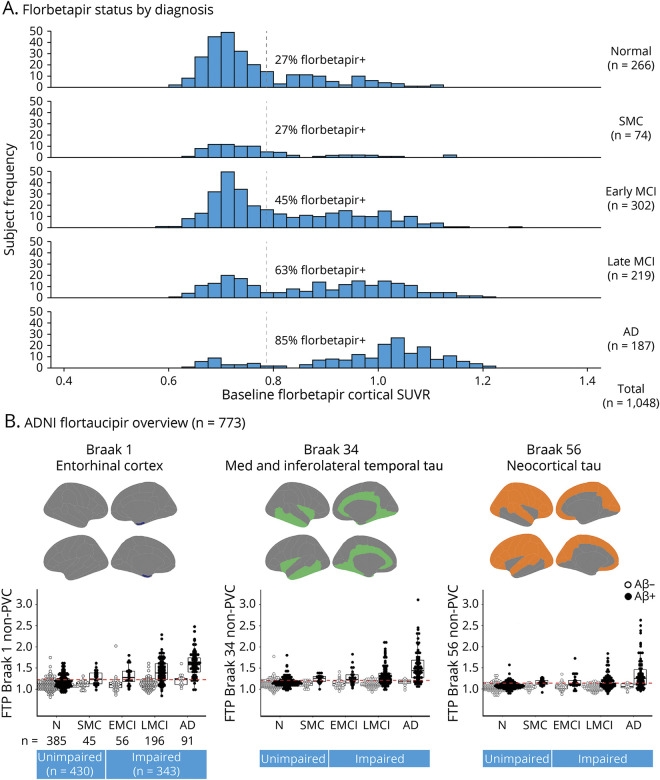
Because ADNI has been focused on Alzheimer disease (AD) biomarkers, we have been following amyloid and tau PET scans for many years. Figure 2 demonstrates the relative proportions of amyloid and PET positivity rates for cognitively unimpaired, subjective cognitive decline, EMCI, LMCI, and dementia. These rates are entirely comparable with the expectations and demonstrate that the positivity rates in EMCI and LMCI correspond to the criteria proposed, and the biomarkers suggest that the clinical criteria have performed well with respect to the underlying biological expectation of the cohorts. In particular, those with EMCI have an amyloid positivity rate of 45% and LMCI 63%, which is consistent with the literature. The flortaucipir-PET data also show expected rates among those who are amyloid positive.
Figure 2. Florbetapir Status by Diagnosis.
(A) Florbetapir distribution of participants in the Alzheimer's Disease Neuroimaging Initiative (ADNI) who are cognitively normal (N) or have subjective memory complaints (SMC), early mild cognitive impairment (EMCI), or late mild cognitive impairment (LMCI). (B) Flortaucipir (FTP) positivity according to amyloid status (β-amyloid [Aβ]+ or Aβ−) consistent with clinical diagnosis across Braak stage 1, Braak 3 and 4, and Braak 5 and 6. AD = Alzheimer disease; MCI = mild cognitive impairment; PVC = partial volume correction; SUVR = standardized uptake value ratio.
Finally, ADNI has attempted to obtain autopsy confirmation to validate the clinical and biomarker profiles of the participants in the various clinical groups. Unpublished data from the ADNI Neuropathology Core demonstrate that, of 39 persons diagnosed with LMCI at enrollment in the study who subsequently went on to have an autopsy, 31 had Thal Stages IV or V, and another 3 had Thal Stage III, suggesting that the ADNI MCI criteria do, in fact, predict progression and the presence of the biological substrate of AD on pathology.8
Duff1 has raised important questions regarding the implementation of the criteria for MCI. MCI is a clinical syndrome similar to dementia, and as such, there are no specific instruments or cutoff scores that define the condition. Rather, neuropsychological tests are useful for informing the clinician on the likelihood that the participant fulfills the criteria for MCI.9 Neuropsychological data can be useful in refining the diagnoses, but ultimately, MCI is a clinical diagnosis, and while the neuropsychological instruments and cutoff scores can influence the composition of the group, ultimately, the clinician must make the diagnosis. The data from ADNI appear to be working satisfactorily when verified with clinical progression, biomarker composition, and, ultimately, neuropathologic confirmation.
Acknowledgment
The author thanks Lea Dacy for help in preparation of the manuscript and Drs. William Jagust, Susan Landau, Michael Donohue, and Richard Perrin for providing unpublished data from ADNI.
Appendix. Authors

Footnotes
See page 595
Study Funding
Supported by the National Institute on Aging (P30 AG062677, U01 AG006786, U01 AG024904); the GHR Foundation; the Alzheimer's Association; and Mayo Medical Foundation for Education and Research.
Disclosure
Dr. Petersen receives research support from the National Institute on Aging; consulting fees from Roche, Inc., Merck, Inc., and Biogen, Inc.; serves on a DSMB for Genentech, Inc.; and receives royalties from Oxford University Press and UpToDate. Go to Neurology.org/N for full disclosures.
References
- 1.Duff K. Amnestic MCI in ADNI: maybe not enough memory impairment? Neurology. 2021;97(12):597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379-2388. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198-205. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169-176. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138(pt 5):1370-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machulda MM, Lundt ES, Albertson SM, et al. Neuropsychological subtypes of incident mild cognitive impairment in the Mayo Clinic Study of Aging. Alzheimers Dement. 2019;15(7):878-887. [DOI] [PMC free article] [PubMed] [Google Scholar]