Abstract
Background and Objectives
To test the hypothesis that a history of concussion (HOC) causes greater disturbances in cerebral blood flow (CBF) and white matter microstructure of midline brain structures after subsequent concussions, during the acute and chronic phases of recovery.
Methods
In this longitudinal MRI study, 61 athletes with uncomplicated concussion (36 with HOC) were imaged at the acute phase of injury (1–7 days after injury), the subacute phase (8–14 days), medical clearance to return to play (RTP), 1 month after RTP, and 1 year after RTP. A normative group of 167 controls (73 with HOC) were also imaged. Each session assessed CBF of the cingulate cortex, along with fractional anisotropy (FA) and mean diffusivity (MD) of the corpus callosum. Linear mixed models tested for interactions of HOC with time since injury. The Sport Concussion Assessment Tool (SCAT) was also used to evaluate effects of HOC on symptoms, cognition, and balance.
Results
Athletes with HOC had significantly greater declines in midcingulate CBF subacutely (z = −3.29, p = 0.002) and greater declines in posterior cingulate CBF at 1 year after RTP (z = −2.42, p = 0.007). No significant effects of HOC were seen for FA, whereas athletes with HOC had higher MD of the splenium at RTP (z = 2.54, p = 0.008). These effects were seen in the absence of significant differences in SCAT domains (|z| ≤ 1.14, p ≥ 0.256) or time to RTP (z = 0.23, p = 0.818).
Discussion
Results indicate subacute and chronic effects of HOC on cingulate CBF and callosal microstructure in the absence of differences in clinical indices. These findings provide new insights into physiologic brain recovery after concussion, with cumulative effects of repeated injury detected among young, healthy athletes.
Although concussion is a mild form of traumatic brain injury, there is evidence for long-term effects on brain physiology, with disturbances lasting beyond medical clearance to return to play (RTP).1,2 This raises concerns about whether multiple concussions have a cumulative effect on brain physiology. Retired athletes with a history of concussive and subconcussive impacts are at greater risk of cognitive deficits, mood dysregulation,3,4 and neurodegeneration.5,6 It is unclear, however, to what extent the effects of repeated concussion can be detected among young, otherwise healthy adults. MRI studies indicate that multiple concussions lead to greater acute physiologic disturbances,7-10 but there have been no investigations examining how history of concussion (HOC) influences recovery from acute injury (ACU) to RTP and beyond.
The present study addressed this gap by imaging concussed athletes with and without HOC from ACU to 1 year after RTP (1YR), with comparison to a large normative control group. Imaging assessed cerebral blood flow (CBF) and microstructural indices of fractional anisotropy (FA) and mean diffusivity (MD), which show both acute and chronic effects of concussion.1,11 This study focused on CBF of the cingulate cortex and microstructure of the corpus callosum because these deep midline structures experience substantial strain during concussive blows,12,13 with dense vascularization and tissue anisotropy (respectively) facilitating the sensitive measurement of concussion effects. There is also concern about the cumulative effects of injury in these regions, given their diverse functional roles and structural connections.14,15 It was hypothesized that concussed athletes with HOC would have greater declines in cingulate CBF and greater disturbances in callosal FA and MD, with effects lasting beyond RTP.
Methods
Study Participants
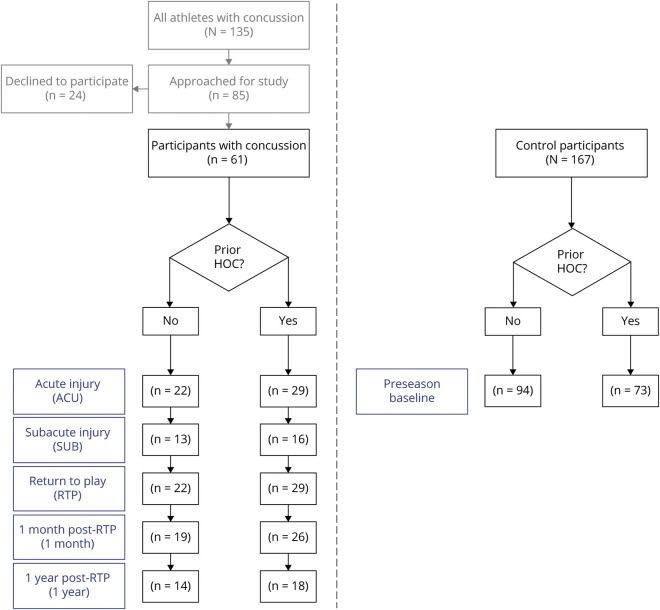
Two hundred forty-eight athletes were imaged for this study, conducted from October 2014 to March 2019. Sixty-one concussed athletes were recruited consecutively from university teams at a single institution through the sport medicine clinic (sport numbers in eAppendix 1, available from Dryad [doi.org/10.5061/dryad.z8w9ghxc4]). For all athletes, concussion diagnosis was made by a staff physician after head contact, direct or indirect, with signs or symptoms as per the Concussion in Sport Group guidelines.16 All athletes meeting criteria were approached at this time and, after screening for eligibility, were recruited for the study. For participants, imaging was performed at ACU (1–7 days after injury), subacute injury (SUB; 8–14 days after injury), medical clearance to RTP, 1 month after RTP (1MO), and 1YR. As a control group, a convenience sample of 167 athletes were also recruited and imaged at the start of their competitive season. These athletes were all approached during mandatory preseason baseline clinical assessments and, after screening for eligibility, were recruited for the study. Participants had no history of neurologic or psychiatric diseases or sensory/motor impairments and no MRI contraindications (i.e., claustrophobia or ferromagnetic implant). For concussed athletes, it was further required that athletes not exhibit any postconcussion neurologic impairments, determined by physician examination of cranial nerves, gait, balance, and gross motor function.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was carried out in accordance with Canadian Tri-Council Policy Statement 2 and with approval of the University of Toronto and St. Michael's Hospital research ethics boards, with all participants giving free and written informed consent.
Demographic and Clinical Data
For all athletes, demographic data were collected at intake (i.e., concussion diagnosis or baseline testing) and included self-reported HOC, along with number of concussions and months since last injury. All athletes were assessed with the Sport Concussion Assessment Tool (SCAT)17,18 before the beginning of their competitive seasons, and concussed athletes completed SCAT follow-up assessments at ACU and RTP. The SCAT evaluates multiple domains affected by concussion, including symptoms, cognition (using the Standardized Assessment of Concussion) and balance (using the Modified Balance Error Scoring System). For symptoms, athletes rated the severity of 22 items on a 7-point Likert scale; number of symptoms (counting symptoms with nonzero ratings) and symptom severity (summing symptom ratings) were obtained. Cognitive evaluation with Standardized Assessment of Concussion assessed orientation, concentration, immediate memory, and delayed recall, with scores summed to obtain a total cognitive score. Balance evaluation with the Modified Balance Error Scoring System assessed double-leg, single-leg, and tandem stances, with errors summed to obtain a balance errors total score.
Magnetic Resonance Imaging
Athlete imaging was conducted on a 3T MRI system (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) with 20-channel head coil. All acquisition and preprocessing details are provided in eAppendix 2, available from Dryad (doi.org/10.5061/dryad.z8w9ghxc4). Anatomic imaging for each session included T1-weighted magnetization-prepared rapid-acquisition gradient echo imaging, fluid-attenuated inversion recovery imaging, and susceptibility-weighted imaging. Structural images were inspected by an MRI technologist during imaging and later reviewed by a single neuroradiologist, with clinical reporting if abnormalities were identified. No abnormalities (white matter hyperintensities, contusions, microhemorrhage) were found for participants, indicating uncomplicated mild traumatic brain injury.
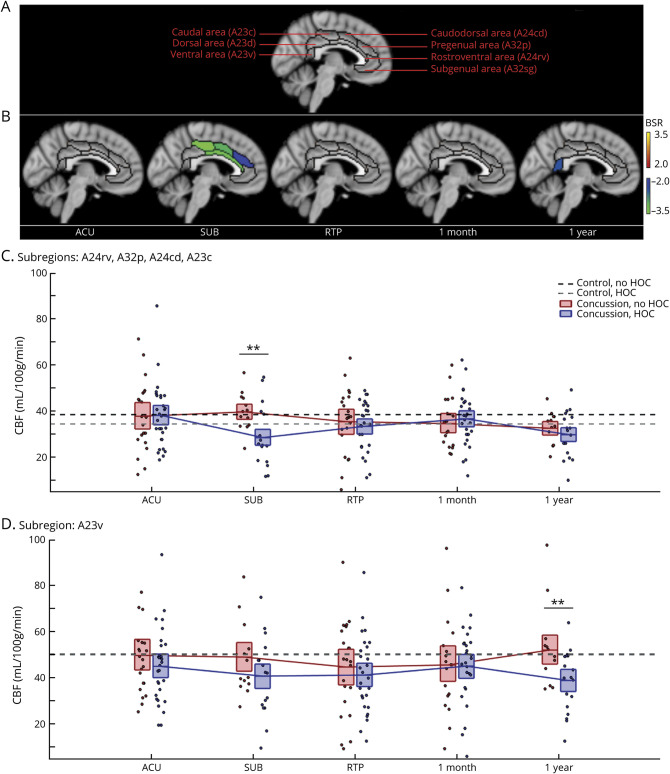
Voxel-wise CBF was estimated in milliliters per 100 g per minute (mL/100 g/min) using a 2-dimensional multislice pulsed arterial spin labeling sequence. A cingulate cortex mask was obtained with the Brainnetome Atlas, and mean CBF values were calculated for the 7 subdivisions (combining left and right hemispheric masks): dorsal area (A23d), rostroventral area (A24rv), pregenual area (A32p), ventral area (A23v), caudodorsal area (A24cd), caudal area (A23c), and subgenual area (A32sg). White matter was evaluated with a 30-direction diffusion tensor imaging sequence to obtain voxel-wise estimates of FA, MD, axial diffusivity (AD), and radial diffusivity (RD). A mask of the corpus callosum was obtained from the John Hopkins University atlas, and mean values of FA, MD, AD, and RD were calculated for the 3 subdivisions: genu of the corpus callosum (gCC), body of the corpus callosum, and splenium of the corpus callosum. The main analyses focused on FA and MD, with supplemental analyses of AD and RD used to further clarify the observed effects.
Statistical Analysis of Demographic and Clinical Data
The concussed and control groups were compared in terms of age, sex, and proportion of athletes participating in collision sports (i.e., having routine body-to-body contact).19 Statistical comparisons were performed with nonparametric 2-sample Wilcoxon tests. Concussed athletes also had SCAT domain scores (number of symptoms, symptom severity, cognitive score, balance errors) compared to both the control group and their own preinjury baseline using 2-sample and paired-measures Wilcoxon tests, respectively. Concussed and control groups were then divided into subgroups based on the presence or absence of HOC, with demographic and clinical data distributions reported. For the concussed subgroups, there was further statistical testing for differences in demographic and clinical variables, along with days to RTP, with 2-sample Wilcoxon tests. Significant effects of concussion and HOC were identified at a false discovery rate (FDR) threshold of 0.05.
Statistical Analysis of MRI Data
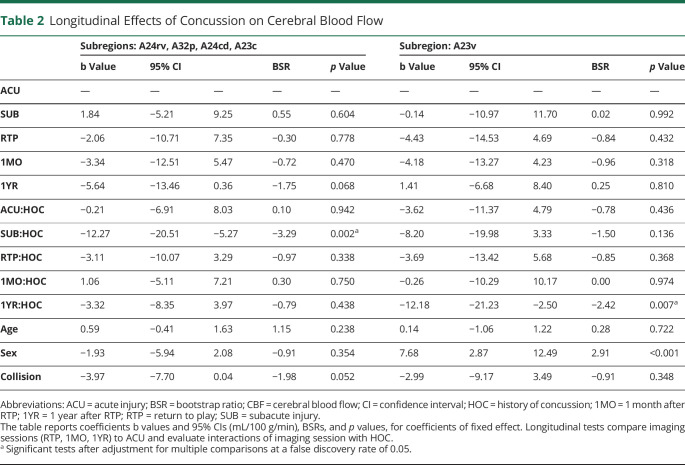
To model longitudinal changes in CBF, FA, and MD, the effects of imaging sessions on MRI parameters were estimated with a linear mixed-effects model (LMM), which handles missing data implicitly via maximum likelihood estimation. Fixed effects were obtained at postacute sessions (SUB, RTP, 1MO, 1YR) relative to ACU, with participant-specific random-effects intercepts. The influence of HOC on recovery was assessed by including this binary variable (yes = 1, no = 0) as an interaction term for each session, i.e., measuring the simple effects on concussed athletes. The model also included fixed-effect covariates controlling for age, sex, and collision sport participation. The last covariate adjusts for effects of subconcussive blows, distinguishing collision sports (with routine body-to-body contact) from limited-contact and noncontact sports.19 The models were fitted using the Matlab R2017b fitlme package (MathWorks, Natick, MA) with full covariance estimation via Cholesky parameterization. Analysis was done in a bootstrap resampling framework, in which a resampling unit consisted of all sessions for a participant (1,000 iterations). This produced fixed-effect coefficients b, their 95% confidence intervals (CIs), standardized effect sizes as bootstrap ratios (BSRs; a z score statistic calculated as the bootstrap mean/standard error), and p values. For each session, significant subregions were identified after thresholding at an FDR of 0.05.
For significant MRI parameters and brain regions, the group means of concussed athletes were also compared to those of athletic controls. A general linear model measured differences between concussed athletes and controls for each session (ACU, SUB, RTP, 1MO, 1YR), with covariates adjusting for age, sex, and collision sport participation. This was done in a bootstrap resampling framework (1,000 iterations) to obtain the coefficient b, 95% CI, BSR, and p value for each session, with significant sessions identified at an FDR of 0.05. To mitigate bias and efficiency loss from missing data, bootstrapped general linear models included multiple imputation using a Boot MI approach20: bootstrap samples were drawn from the complete dataset, and for each sample, repeated imputation generated 10 coefficient estimates, which were averaged to produce a point estimate. The 1,000 point estimates were treated as a conventional bootstrap distribution, and summary statistics were calculated. Imputations were done with the fitted LMMs to simulate missing values.
Exploratory whole-brain analyses also tested for effects of HOC beyond the hypothesized midline structures. For CBF, each of the 246 brain parcels in the Brainnetome Atlas were analyzed using the bootstrapped LMM approach described previously. For white matter parameters (FA, MD, AD, and RD), the 48 white matter parcels of the John Hopkins University atlas were reduced to 38 by excluding tracts overlapping with the brainstem (regions 1, 2, 7–14) and analyzed with the bootstrapped LMM procedure. For each imaging session, the BSRs were obtained for HOC interactions and plotted after thresholding at |BSR| > 2.0 (approximately p < 0.05, uncorrected). These analyses are presented as supplemental data (see Results).
Data Availability
The authors have documented all data, methods, and materials used to conduct this research study, and anonymized data will be shared by request from any qualified investigator.
Results
Demographic and Clinical Data
The study design is presented as a flowchart in Figure 1. Over the study period, 135 athletes in the varsity program were concussed, of whom 85 were approached and 61 agreed to participate. Reasons for nonparticipation included lack of interest, busy schedule, out of town for recovery, or medically cleared within 7 days of injury. Among participating concussed athletes, some had missing sessions. The number of participants imaged at each session was as follows: ACU 53 of 61, SUB 29 of 61, RTP 51 of 61, 1MO 45 of 61, and 1YR 32 of 61. Attrition was not significantly related to demographic or clinical variables (age, sex, HOC, collision sports, SCAT subtests, or days to RTP) for any of the sessions according to Spearman correlations at an FDR of 0.05. The first 44 of 61 concussed and 68 of 167 control athletes were evaluated with SCAT3; the remainder were evaluated with SCAT5 per evolving clinical guidelines. The cognitive scoring changed between versions (15–30 items for immediate memory, 5–10 items for delayed recall); hence, cognitive score distributions were reported separately for athletes assessed with each version in Table 1, and statistical testing was conducted separately for each. For all other SCAT domains, statistics were based on the complete dataset after verification that there were no significant differences in SCAT3 and SCAT5 scores (p ≥ 0.288, 2-sample Wilcoxon tests).
Figure 1. Study Flowchart Reporting Sample Sizes at Each Imaging Session.
HOC = history of concussion.
Table 1.
Demographic and Clinical Data for Control and Concussed Groups With and Without HOC
Demographic and clinical data are summarized in Table 1. Relative to controls, the concussed cohort was comparable in age (z = 0.39, p = 0.697) and sex (z = −0.17, p = 0.866) but had a higher proportion of collision sport athletes (z = 3.72, p < 0.001). Controls tended to have minimal but nonzero symptoms, consistent with in-clinic reporting trends.21 Concussed athletes (with and without HOC) had elevated number and severity of symptoms at ACU relative to controls and their own baseline (z ≥ 4.82, p < 0.001 for all tests) but were no longer elevated at RTP (z ≤ −3.24, p ≥ 0.999 for all tests), with acute effects remaining significant at an FDR of 0.05. The cognitive scores showed no significant effects of concussion (z ≥ −0.33, p ≥ 0.372 for all tests), while balance errors were elevated at ACU relative to controls (z = 2.38, p = 0.009) but not their baseline (z = 0.62, p = 0.267), and values were no longer elevated at RTP (z = −0.81, p = 0.792 and z = −2.11, p = 0.983, respectively). Supplemental testing of cognitive subscales (orientation, concentration, immediate memory, delayed recall) also showed no evidence of significantly reduced performance (z≥−1.61, p≥0.054 for all tests).
The concussed subgroups did not differ in age, sex, or participation in collision sports (|z| ≤ 1.45, p ≥ 0.147 for all tests). In terms of clinical outcome, they did not differ in days to RTP (z = 0.23, p = 0.818), nor did they differ in the number and severity of symptoms, cognitive scores, or balance errors reported at baseline, ACU, and RTP (|z| ≤ 1.14, p ≥ 0.256 for all tests). Hence, there was no evidence of significant difference in demographic or clinical variables between concussed athletes with and without HOC. None of the concussed athletes had acquired an additional concussion between ACU and 1YR, and all athletes had returned to normal school, work, and sport participation at this time.
MRI Data
The CBF analyses identified significant modifying effects of HOC on concussion response, summarized in Figure 2 and Table 2. eAppendix 3 provides complete LMM statistics, and eAppendix 4 supplies whole-brain analyses (available from Dryad [doi.org/10.5061/dryad.z8w9ghxc4]). Figure 1A depicts cingulate subdivisions, and Figure 1B plots regions with a significant effect of HOC on CBF at each imaging session. Two sets of regions were identified showing significant effects at different sessions at an FDR of 0.05. The first set includes midcingulate areas A24rv, A32p, A24cd, and A23c, with an anterior-to-posterior gradient of increasing effect size. In these regions, HOC had a negative effect at SUB, indicating greater postconcussion declines in CBF. Concussed athletes without HOC did not have significantly reduced CBF relative to controls without HOC (b = 1.41 [95% CI −2.84, 5.52] mL/100 g/min, BSR 0.65, p = 0.526). Concussed athletes with HOC, however, had reduced CBF relative to controls without HOC (−9.33 [95% CI −13.26, −4.97] mL/100 g/min, BSR −4.42, p < 0.001) and controls with HOC (−7.80 [95% CI −13.48, −2.50] mL/100 g/min, BSR −2.77, p = 0.006). At 1YR, values in these regions had declined below controls for both those without HOC (−5.50 [95% CI −9.67, −1.40] mL/100 g/min, BSR −2.63, p < 0.001) and those with HOC (−8.07 [95% CI−11.80, −4.23] mL/100 g/min, BSR −4.25, p < 0.001), but effects were not significantly larger for athletes with HOC.
Figure 2. Effects of HOC on Post-concussion Cerebral Blood Flow.
(A) Segmentation of the cingulate cortex based on the Brainnetome Atlas into 7 subregions. (B) For cingulate regions and imaging sessions showing significant history of concussion (HOC) effects on cerebral blood flow (CBF) during concussion recovery, standardized effects are shown as bootstrap ratios (BSRs). (C) Distribution of concussed athlete CBF values averaged over subregions showing significant effects at subacute injury (SUB), as denoted by double asterisks. (D) Distribution of concussed athlete CBF values for the subregion showing significant effects at 1 year after return to play (RTP), as denoted by double asterisks. For distribution plots, concussed athletes without and with HOC are plotted separately. Horizontal red/blue lines denote group means, and boxes indicate 95% confidence intervals of the mean. Distribution means are connected between sessions by solid red/blue lines, and the mean CBF values for controls without and with HOC are plotted as black and gray horizontal dashed lines, respectively. ACU = acute phase of injury.
Table 2.
Longitudinal Effects of Concussion on Cerebral Blood Flow
The second significant region is posterior cingulate area A23v, which shows a negative effect of HOC at 1YR, indicating greater postconcussion declines in CBF. Concussed athletes without HOC did not have significantly reduced CBF relative to controls without HOC (2.21 [95% CI −5.62, 10.36] mL/100 g/min, BSR 0.54, p = 0.602). Concussed athletes with HOC, however, had reduced CBF relative to both controls without HOC (−10.19 [95% CI −16.0, −3.86] mL/100 g/min, BSR −3.27, p < 0.001) and controls with HOC (−10.29 [95% CI −18.20, −2.51] mL/100 g/min, BSR −2.42, p = 0.019). For concussed athletes with HOC, CBF responses at SUB and 1YR were not related to the total number of prior concussions or months since last injury (|BSR| ≤ 0.94, p ≥ 0.306, all tests). Overall, these results suggest that the effects of concussion on CBF are amplified for athletes with HOC during recovery.
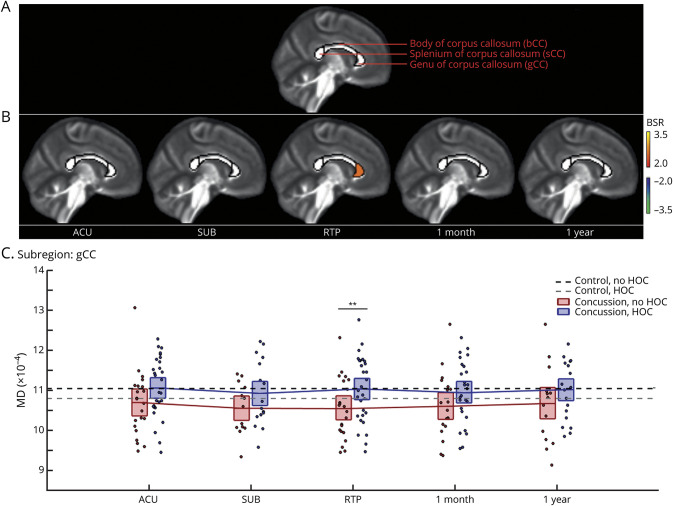
The analyses of diffusion tensor imaging parameters also found significant effects of HOC on concussion response. eAppendix 3 provides complete LMM statistics, and eAppendix 4 supplies whole-brain analyses (available from Dryad [doi.org/10.5061/dryad.z8w9ghxc4]). The FA analyses found no significant effects of HOC. Significant effects of HOC on MD were identified, however, with results summarized in Figure 3 and Table 3. Figure 2A depicts callosal subdivisions, and Figure 2B plots regions with a significant HOC effect on MD response at each imaging session. A significant effect was seen anteriorly within the gCC at an FDR of 0.05. This region shows a positive effect of HOC at RTP; thus, it is associated with increased MD. Compared to athletic controls, concussed athletes without HOC had significantly reduced MD values (−5.51 [95%CI −8.70, −1.92] × 10−5, BSR −3.04, p = 0.006). In contrast, concussed athletes with HOC showed no significant differences relative to controls without HOC (−0.27 [95%CI −3.36, 2.85] × 10−5, BSR −0.17, p = 0.857) or controls with HOC (b = 0.24 [95%CI −4.00, 4.96] × 10−5, BSR 0.11, p = 0.947). For concussed athletes with HOC, the MD response at RTP was not related to total number of prior concussions or months since last injury (|BSR| ≤ 0.98, p ≥ 0.316, all tests).
Figure 3. Effects of HOC on post-concussion mean diffusivity (MD).
(A) Segmentation of the corpus callosum based on the John Hopkins University atlas into 3 subregions. (B) For callosal regions and imaging sessions showing significant history of concussion (HOC) effects on mean diffusivity (MD) during concussion recovery, standardized effects are shown as bootstrap ratios (BSRs). (C) Distribution of concussed athlete MD values for the subregion showing effects at return to play (RTP), as denoted by double asterisks. For distribution plots, concussed athletes without and with HOC are plotted separately. Horizontal red/blue lines denote group means, and boxes indicate 95% confidence intervals. Distribution means are connected between sessions by solid red/blue lines, and the mean MD values for controls without and with HOC are plotted as black and gray horizontal dashed lines, respectively. ACU = acute phase of injury; SUB = subacute injury.
Table 3.
Longitudinal Effects of Concussion on MD
Analyses of AD and RD obtained a pattern of HOC effects identical to that seen for MD. For both parameters, a significant positive effect was seen in the gCC at RTP, indicating increased AD and RD values. Compared to athletic controls, concussed athletes without HOC had significantly reduced AD values (−5.67 [95%CI −9.02, −2.34] × 10−5, BSR −3.36, p < 0.001) and RD values (−5.61 [95%CI −9.91, −2.03] × 10−5, BSR −2.87, p < 0.001). Concussed athletes with HOC did not differ from controls without HOC for AD (−0.37 [95%CI −3.61, 2.75] × 10−5, BSR −0.22, p = 0.900) or RD (−0.53 [95%CI −3.89, 2.99] × 10−5, BSR −0.30, p = 0.768). Similarly, they did not differ from controls with HOC for AD (0.43 [95%CI −3.74, 4.84] × 10−5, BSR 0.20, p = 0.869) or RD (0.22 [95%CI −3.87, 4.54] × 10−5, BSR 0.10, p = 0.941). Overall, these results indicate differential microstructural responses for athletes with and without HOC during concussion recovery.
Associations between the perturbations in CBF and MD were also examined by correlating concussed athlete values for significant sessions and brain regions. Midcingulate CBF at SUB had minimal Spearman correlations with genual MD at RTP for athletes without HOC (ρ = −0.15 [95% CI −0.704, 0.380], p = 0.538 for n = 13 data points) and moderate but nonsignificant correlations for athletes with HOC (ρ = 0.409 [95% CI −0.371, 0.964], p = 0.226 for n = 12 data points). The opposite was seen for posterior cingulate CBF at 1YR, with moderate but nonsignificant correlations for athletes without HOC (ρ = 0.406 [95% CI −0.388, 0.849], p = 0.215 for n = 11 data points) and minimal correlations for athletes with HOC (ρ = 0.035 [95% CI −0.569, 0.658], p = 0.0933 for n = 16 data points).
Discussion
Concussion is a major health concern, and there is growing concern about the cumulative effects of repeated injury. This is driven by studies of former professional athletes in which neuropathology is associated with a history of repeated head injury.22,23 However, given the retrospective nature of these studies, it is unclear how the effects of multiple concussions emerge over time. The present study examined whether there are detectable effects of HOC on physiologic recovery for young, currently active athletes outside of the professional sport context. This study focused on CBF of the cingulate and microstructure of the corpus callosum because these deep midline structures experience substantial strain during impacts12,13 and the high levels of perfusion and structural anisotropy (respectively) facilitate more precise estimates of HOC effects. Postconcussion declines in CBF were significantly greater for athletes with multiple concussions, whereas microstructural indices were not significantly affected by prior concussion (FA) or showed greater perturbations for athletes without prior concussion (MD). Critically, neuroimaging effects were seen despite a lack of significant differences in clinical presentation or time to RTP, indicating that subclinical effects of HOC can be detected with MRI techniques. Moderate HOC-dependent correlations were also identified between CBF and MD, suggesting a complex relationship between these indices during concussion recovery. However, limited subsamples and correspondingly wide CIs indicate a need for further research investigating these relationships.
The analyses indicated that CBF tends to decrease within the cingulate after a concussion, with greater reductions for athletes with HOC relative to controls. Postconcussion declines in CBF are consistent with our understanding of concussive injury, which includes neurometabolic impairment, microvascular injury, and disrupted autoregulation.24,25 For both groups, CBF effects were nonsignificant at ACU, consistent with variable findings reported previously.26 At SUB, however, athletes with HOC declined significantly in posterior midcingulate CBF, while those without HOC showed no significant effects. Thus, in the symptomatic phase of injury, a single concussion has limited effects on midline CBF but appears to sensitize athletes to subsequent concussions. Studies have shown increasingly severe neurometabolic impairment and oxidative/nitrosative stress after multiple injuries,7,8,27 supporting this argument, although the effects on CBF have not been previously investigated. These data also support further studies of subacute clinical presentation to determine whether subacute CBF disturbances have corresponding behavioral effects. Of particular interest are measures of balance, coordination, and spatial orientation, given the key role of the posterior midcingulate in these domains.28,29
The analyses also identified declines in CBF that emerged at 1YR. These persistent reductions in CBF are supported by prior longitudinal and cross-sectional studies reporting post-RTP declines in cortical CBF.1,11 The mechanisms of delayed response are unclear but, combined with the whole-brain effects in eAppendix 4 (available from Dryad [doi.org/10.5061/dryad.z8w9ghxc4]) and our understanding of concussion pathophysiology, suggest a more taxed cerebrovascular system. One candidate mechanism is neuroinflammation,30 which causes microvascular injury with increased blood-brain barrier permeability.31 Effects may also be exacerbated by subconcussive blows32 and chronic exertion33 after an athlete has returned to training and competition. These changes may be enhanced by subtle declines in gray matter volume,11,34 further reducing local CBF demand. Significant CBF declines were observed at 1YR within midcingulate regions for concussed athletes with and without HOC. Although post-RTP effect sizes were comparable between subgroups, the effects were more spatially extensive for athletes with HOC, with significant effects extending into the posterior cingulate. The greater spatial extent may be a delayed consequence of the greater physiologic disturbances seen in athletes with HOC at SUB. Overall, results suggest more extensive chronic effects of concussion for athletes with HOC, although further studies are needed to characterize the evolution of CBF over the wide interval of 1MO to 1YR.
Compared to CBF, analyses of white matter microstructure revealed more complex effects of HOC in the corpus callosum. Changes in FA were nonsignificant, whereas callosal MD had decreased significantly, with greater effects among concussed athletes without HOC. Combined with analyses of AD and RD, which showed comparable postconcussion decreases, these findings suggest a decrease in overall diffusivity after injury without concurrent changes in directionality. The interpretation of postconcussion decreases in white matter MD is not straightforward and may be driven by multiple factors. The absence of FA changes, which are related to more severe impairments,35-37 suggests that diffuse axonal injury is not a predominant contributor. The decline in RD similarly argues against demyelination,38 and while a decline in AD may reflect axonal injury,39 the lack of directional specificity and absence of effect in the HOC cohort make this less likely. An alternative explanation is that axonal stretching after impact leads to osmotic imbalances, with increased intra-axonal and decreased extra-axonal water volume.40 This interpretation is supported by previous studies showing decreased diffusivity after concussion.41-43 This effect increases longitudinally and is greatest at RTP, suggesting that full recovery lasts beyond medical clearance, as has been previously reported.1,2
It is surprising to note, however, that significant diffusivity effects are limited to concussed athletes without HOC. Two mechanisms are proposed for the diminished response among athletes with HOC. The first is that postconcussion osmoregulatory response is altered by multiple injuries. This is aligned with a previous study of this cohort that found elevated cerebral myoinositol at RTP, but with diminished effects among athletes with HOC.44 This was interpreted as the brain accumulating osmolytes to counteract persistent ionic imbalances,45 with multiple concussions disrupting the response and leading to reduced MD. A second possibility is that measures of intra-axonal swelling are confounded by processes that increase diffusivity. Athletes with HOC may, for example, experience greater neuroinflammatory response46 or disrupted white matter integrity,38,39 leading to an apparent null effect of concussion on diffusivity. Although the present study demonstrates strong evidence of a differential microstructural response due to HOC, further work is needed to disentangle the underlying mechanisms. In particular, diffusion-weighted imaging protocols with more sophisticated modeling of tissue microstructure are needed,47 combined with neurometabolite and neuroinflammatory biomarkers.
In terms of clinical implications, previous literature has indicated that physiologic recovery persists beyond RTP.1,36,48 The present findings suggest that further caution is warranted when an athlete has HOC because these clinically silent disturbances may be exacerbated by repeated injury. Moreover, results indicate chronic perturbations, raising concerns about the cumulative effects of repeated injury later in life.3,4 As noted, the putative mechanisms of chronic declines in CBF also overlap with the effects of subconcussive blows and chronic exertion,32,33 making it important to examine whether athletes with HOC are more sensitive to the effects of collisions and overtraining. The HOC effects on brain physiology were seen in the absence of differences in clinical scores, supporting a need for more sensitive clinical tools that target these effects, for example, alternative neurocognitive and biomarker tests. However, it must be emphasized that the present findings were seen despite athletes being fully recovered clinically at RTP, indicating that they have effectively adapted to these perturbations. Findings should therefore be interpreted cautiously until cohort studies definitively establish (or refute) links between multiple concussions, chronic MRI effects, and long-term health outcomes.
The present study provides insights into the effects of multiple concussions on brain physiology; however, there are some limitations that should be considered. The determination of HOC was based on self-report with potential reporting errors. To address this limitation, future studies should follow up athletes prospectively over multiple injuries. The study was also based on a mixed cohort of male and female athletes from different sports, introducing heterogeneity in baseline physiology, injury mechanisms, and physiologic response. The presence of significant HOC effects suggests that findings generalize across cohorts, and the effects of age, sex, and collision sports were modeled as covariates of no interest. Nevertheless, there may be undetected interactions with HOC, requiring further investigation. Similarly, this study found no significant effects for number of prior concussions or time since last injury. Larger samples are needed to verify these results and to precisely estimate physiologic dose response for individual concussion events. Study recruitment was limited to a single institution, and imaging was conducted at a single site. This avoided confounding effects of heterogeneity in clinical management or magnetic resonance hardware, but multisite studies are needed to verify that the findings generalize beyond the studied population. There was also sample dropout, particularly at SUB and 1YR, when CBF effects were greatest. Dropout was without evident demographic or clinical biases according to correlation testing, and LMM analyses implicitly accounted for missing data. Nevertheless, unmodeled sources of attrition may exist, underscoring the importance of follow-up studies focusing on the early recovery phase and long-term outcome. Last, the study focused on HOC effects within the cingulate and corpus callosum, given their vulnerability to concussion and robust MRI signal. However, other nonmidline regions are also vulnerable to shear forces during impact,12,13,49 which is supported by the supplemental whole-brain analyses in eAppendix 4 (available from Dryad [doi.org/10.5061/dryad.z8w9ghxc4]), in which comparable HOC effects are seen outside of the midline regions. Further research is needed to discern which brain areas are most vulnerable to concussion in terms of both neurobiological change and long-term consequences for cognition, mood, and sensorimotor function.
This study provides one of the most comprehensive examinations to date of the cumulative effects of multiple concussions on longitudinal brain recovery. For a substantial cohort of concussed athletes and controls, persistent alterations in CBF and tissue microstructure were identified among young individuals in otherwise good health. These findings provide new evidence of the sensitivity of the brain to repeated concussion and help to better elucidate the mechanisms by which athletes with a history of repeated concussion may show long-term physiologic and mental health sequelae.
Glossary
- ACU
acute injury
- AD
axial diffusivity
- BSR
bootstrap ratio
- CBF
cerebral blood flow
- CI
confidence interval
- FA
fractional anisotropy
- FDR
false discovery rate
- gCC
genu of the corpus callosum
- HOC
history of concussion
- LMM
linear mixed-effects model
- MD
mean diffusivity
- 1MO
1 month after RTP
- 1YR
1 year after RTP
- RD
radial diffusivity
- RTP
return to play
- SCAT
Sport Concussion Assessment Tool
- SUB
subacute injury
Appendix. Authors
Footnotes
Editorial, page 567
Study Funding
This work was supported by the Canadian Institutes of Health Research (grant RN294001–367456), the Canadian Institute for Military and Veterans Health Research (grant W7714-145967), and Siemens Healthineers Canada.
Disclosure
N.W. Churchill, M.G. Hutchison, S.J. Graham, and T.A. Schweizer report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Churchill NW, Hutchison MG, Graham SJ, Schweizer TA. Mapping brain recovery after concussion: from acute injury to one year after medical clearance. Neurology. 2019;93(21):e1980-e1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, Schweizer TA. Neuroimaging of sport concussion: persistent alterations in brain structure and function at medical clearance. Sci Rep. 2017;7(1):8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57(4):719-726. [DOI] [PubMed] [Google Scholar]
- 4.Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903-909. [DOI] [PubMed] [Google Scholar]
- 5.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC. Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagnozzi R, Signoretti S, Tavazzi B, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes, part III. Neurosurgery. 2008;62(6):1286-1296. [DOI] [PubMed] [Google Scholar]
- 8.Vagnozzi R, Tavazzi B, Signoretti S, et al. Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment, part I. Neurosurgery. 2007;61(2):379-389. [DOI] [PubMed] [Google Scholar]
- 9.Johnson B, Gay M, Zhang K, et al. The use of magnetic resonance spectroscopy in the subacute evaluation of athletes recovering from single and multiple mild traumatic brain injury. J Neurotrauma. 2012;29(13):2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry LC, Tremblay S, Boulanger Y, Ellemberg D, Lassonde M. Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J Neurotrauma. 2010;27(1):65-76. [DOI] [PubMed] [Google Scholar]
- 11.Churchill N, Hutchison M, Richards D, Leung G, Graham S, Schweizer TA. Brain structure and function associated with a history of sport concussion: a multi-modal magnetic resonance imaging study. J Neurotrauma. 2017;34(4):765-771. [DOI] [PubMed] [Google Scholar]
- 12.Viano DC, Casson IR, Pellman EJ, Zhang L, King AI, Yang KH. Concussion in professional football: brain responses by finite element analysis, part 9. Neurosurgery. 2005;57(5):891-916. [DOI] [PubMed] [Google Scholar]
- 13.Viano DC, Casson IR, Pellman EJ, et al. . Concussion in professional football: comparison with boxing head impacts, part 10. Neurosurgery. 2005;57(6):1154-1172. [DOI] [PubMed] [Google Scholar]
- 14.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435-443. [DOI] [PubMed] [Google Scholar]
- 15.Jarbo K, Verstynen T, Schneider W. In vivo quantification of global connectivity in the human corpus callosum. Neuroimage. 2012;59(3):1988-1996. [DOI] [PubMed] [Google Scholar]
- 16.McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clin J Sport Med. 2013;23:89-117. [DOI] [PubMed] [Google Scholar]
- 17.Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: introducing the SCAT3. Br J Sports Med. 2013;47(5):289-293. [DOI] [PubMed] [Google Scholar]
- 18.Echemendia RJ, Broglio SP, Davis GA, et al. What tests and measures should be added to the SCAT3 and related tests to improve their reliability, sensitivity and/or specificity in sideline concussion diagnosis? A systematic review. Br J Sports Med. 2017;51(11):895-901. [DOI] [PubMed] [Google Scholar]
- 19.Meehan WP III, Taylor AM, Berkner P, et al. Division III collision sports are not associated with neurobehavioral quality of life. J Neurotrauma. 2016;33(2):254-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey RI, Hutchison MG, Comper P. Determining sensitivity and specificity of the Sport Concussion Assessment Tool 3 (SCAT3) components in university athletes. Brain Inj. 2018;32(11):1345-1352. [DOI] [PubMed] [Google Scholar]
- 22.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Len TK, Neary JP. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin Physiol Funct Imaging. 2011;31(2):85-93. [DOI] [PubMed] [Google Scholar]
- 25.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9. [DOI] [PubMed] [Google Scholar]
- 26.Churchill NW, Hutchison MG, Richards D, Leung G, Graham SJ, Schweizer TA. The first week after concussion: blood flow, brain function and white matter microstructure. Neuroimage Clin. 2017;14:480-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavazzi B, Vagnozzi R, Signoretti S, et al. Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses, part II. Neurosurgery. 2007;61(2):390-396. [DOI] [PubMed] [Google Scholar]
- 28.Hoffstaedter F, Grefkes C, Caspers S, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. 2014;35(6):2741-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt BA. Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat. 2016;74:28-46. [DOI] [PubMed] [Google Scholar]
- 30.Patterson ZR, Holahan MR. Understanding the neuroinflammatory response following concussion to develop treatment strategies. Front Cell Neurosci. 2012;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev. 2019;99(1):21-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8(3):e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey DM, Evans KA, McEneny J, et al. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp Physiol. 2011;96(11):1196-1207. [DOI] [PubMed] [Google Scholar]
- 34.Dean PJA, Sato JR, Vieira G, McNamara A, Sterr A. Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj. 2015;29(10):1211-1218. [DOI] [PubMed] [Google Scholar]
- 35.Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28(2):189-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103(2):298-303. [DOI] [PubMed] [Google Scholar]
- 37.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23(5):794-802. [PMC free article] [PubMed] [Google Scholar]
- 38.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132-140. [DOI] [PubMed] [Google Scholar]
- 39.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714-1722. [DOI] [PubMed] [Google Scholar]
- 40.Rosenblum WI. Cytotoxic edema: monitoring its magnitude and contribution to brain swelling. J Neuropathol Exp Neurol. 2007;66(9):771-778. [DOI] [PubMed] [Google Scholar]
- 41.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74(8):643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70(12):948-955. [DOI] [PubMed] [Google Scholar]
- 43.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007;24(9):1447-1459. [DOI] [PubMed] [Google Scholar]
- 44.Churchill NW, Hutchison MG, Graham SJ, Schweizer TA. Neurometabolites and sport-related concussion: from acute injury to one year after medical clearance. Neuroimage Clin. 2020;27:102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry LC, Tremblay S, Leclerc S, et al. Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 2011;11(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016. [DOI] [PubMed] [Google Scholar]
- 48.Dettwiler A, Murugavel M, Putukian M, Cubon V, Furtado J, Osherson D. Persistent differences in patterns of brain activation after sports-related concussion: a longitudinal functional magnetic resonance imaging study. J Neurotrauma. 2014;31(12):180-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor PA, Ludwigsen JS, Ford CC. Investigation of blast-induced traumatic brain injury. Brain Inj. 2014;28(7):879-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have documented all data, methods, and materials used to conduct this research study, and anonymized data will be shared by request from any qualified investigator.