Mild cognitive impairment (MCI), especially the amnestic subtype, has been proposed as a prodromal phase of Alzheimer disease (AD), where the patient has memory deficits but no/minimal functional impairment.1 Clinically, the demarcation point for memory deficits in MCI has typically been 1–1.5 SD units below normative data.1,2 Much of what we know about MCI has come from findings from the Alzheimer's Disease Neuroimaging Initiative (ADNI) studies.3
Whereas some of the inclusion criteria for MCI in ADNI are consistent with how this condition is diagnosed clinically (e.g., memory complaint, questionable/very mild functional changes [e.g., 0.5 rating on the Clinical Dementia Rating scale]), the level of objective memory seems very different. In this and subsequent versions of ADNI, the memory test that has been used to classify individuals as having MCI is Story A Logical Memory II from the Wechsler Memory Scale–Revised,4 which is delayed recall of a story presented 20–30 minutes earlier. Scores on this memory test range from 0 to 25, with higher scores indicating better memory functioning. ADNI had different raw score cutoffs on this memory test depending on the education level of the participant: ≤2 if 0–7 years of education, ≤4 if 8–15 years of education, and ≤8 if 16 or more years of education. However, no rationale is provided for these cutoffs or why education (and not age) is part of them. When compared to normative data, these cutoffs do not necessarily indicate impairment. For example, a 72-year-old patient with 6 years of education and a score of 2 on Story A Logical Memory II would fall at the 10th percentile according the normative data in the test manual.4 Although that score might fit the spirit of MCI, a 72-year-old patient with 12 years of education and a score of 4 on this story recall would fall at the 30th percentile, and a 72-year-old patient with 16 years of education and a score of 8 on this story recall would fall at the 60th percentile. Delayed memory performances of the 30th and 60th percentiles do not seem to reflect amnestic MCI. Over time, these cutoffs have increased. For example, in ADNI3, the Story A Logical Memory II cutoffs are ≤6, ≤9, and <11 for these same 3 educational groups, respectively. Using normative data from the test manual, this would equate to scores falling at the 46th, 72nd, and 81st percentiles, respectively. Again, these cutoffs would indicate average to high average memory, not an amnestic condition.
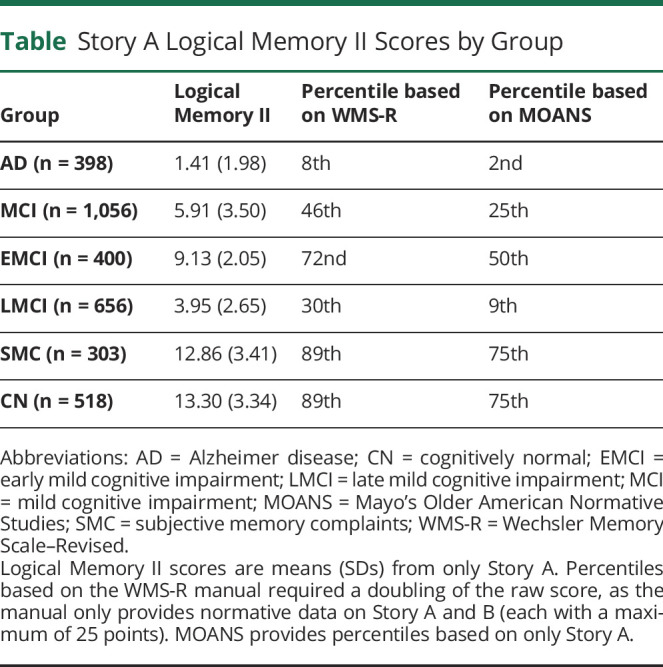
These generous cutoffs on Logical Memory II have led to ADNI samples that may not fully represent the target populations in the clinic. For example, using ADNI baseline data from all studies (i.e., the ADNIMERGE database, downloaded from adni.loni.usc.edu on December 3, 2020), the mean Story A Logical Memory II scores were calculated for participants with AD, MCI (combined across early and late), subjective memory complaints, and cognitively normal participants, and these are presented in the Table. These mean scores were compared to 2 normative sets: (1) 70- to 74-year-olds in the Wechsler Memory Scale–Revised manual4 (which included 50 nonimpaired adults who were screened for neurologic and psychiatric illness) and (2) midpoint 73-year-olds in Mayo's Older American Normative Studies5 (which included 139 adults who denied cognitive complaints and had no neurologic or psychiatric disease identified on a physical examination). Across these 2 normative sets, participants classified as having AD had moderately impaired (2nd percentile) to borderline (8th percentile) scores on this delayed recall test. Those with MCI had scores in the low average to average range (25th–46th percentiles). Those with subjective memory complaints or cognitively normal participants had high average to well above average scores (75th–89th percentiles). The test manual yielded higher percentile ranks for each group relative to the Mayo norms. It should be noted that the criteria for MCI1 were not yet published when the data were collected for these 2 normative samples, so some individuals with MCI may have been included in these samples.
Table.
Story A Logical Memory II Scores by Group
![]()
What are the ramifications of participants with amnestic MCI in ADNI who have much milder memory problems? Hundreds of studies have used ADNI data to examine biomarker and genetic profiles in MCI, describe the relationship between memory and neuroimaging findings, predict progression in this at-risk cohort, or develop new cognitive composites.6 Although these observations about the MCI cutoffs do not invalidate the rich results of ADNI, they do raise concerns about the generalizability of those findings to truly amnestic patients (e.g., patients with delayed recall scores ≤5th percentile). For example, clinicians who are using normative data from test manuals might be diagnosing MCI in patients who are more impaired/more advanced than those seen in ADNI. Does progression of MCI to AD in ADNI still apply in a clinic patient with MCI with more severe memory deficits? Do biomarkers in ADNI hold the same prognostic utility in patients with MCI whose Logical Memory II score is below the 5th percentile according to the test manual? Will treatments that are eventually effective in ADNI-defined MCI be as effective in clinic-defined MCI? These are empirical questions that can (and should) be examined with the various ADNI databases. In addition, it is possible that clinical practice needs to better calibrate with the research findings from ADNI and other studies. For example, instead of using 1.5 SD units below normative data as the demarcation point for MCI,1 Jak and colleagues2 observed that a lower cutoff (i.e., 1 SD units below normative data) for at least 2 scores within a cognitive domain yielded better stability of an MCI diagnosis. The clinician's use of practice and diagnostic guidelines may not be consistent with the research findings. These observations are meant to neither cast doubt on research findings from ADNI nor completely change current clinical diagnostic practices. However, it is important to understand when differences occur and to seek greater harmonization.
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
See page 597
Study Funding
The project described was supported by research grants from the National Institutes on Aging (R01AG055428). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Aging or the NIH.
Disclosure
The author reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. [DOI] [PubMed] [Google Scholar]
- 2.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner MW, Veitch DP, Aisen PS, et al. Recent publications from the Alzheimer's Disease Neuroimaging Initiative: reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13(4):e1-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechsler D. Wechsler Memory Scale–Revised. The Psychological Corporation; 1987. [Google Scholar]
- 5.Smith GE, Wong JS, Ivnik RJ, Malec JF. Mayo's Older American Normative Studies: separate norms for WMS-R Logical Memory Stories. Assessment. 1997;4(1):79-86. [Google Scholar]
- 6.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]


