Abstract
Recently developed molecular methods have made it possible to characterize mixed microflora in their entirety, including the substantial numbers of bacteria which do not grow on artificial culture media. In a previous study, molecular analysis of the microflora associated with acute oral infections resulted in the identification of three phylotypes, PUS3.42, PUS9.170, and PUS9.180, representing as-yet-uncultured organisms. The aim of this study was to design and validate specific PCR primers for these phylotypes and to determine their incidences in samples collected from healthy and diseased periodontal tissues. Two specific reverse primers were devised for each phylotype, and these were used in duplex PCRs with universal forward and reverse primers. All three phylotypes were detected in periodontal sites; PUS9.170, related to oral asaccharolytic Eubacterium spp., was significantly associated with disease. This study demonstrates the possibility of using unculturable, and therefore uncharacterized, organisms as markers of disease.
Periodontitis, the inflammatory disease leading to destruction of the supporting tissues of the teeth, affects around 10% of the population under 35 years old in its severe form (10). These individuals may lose teeth as a result of the disease and require life-long review. A number of bacteria, including Actinobacillus actinomycetemcomitans, Campylobacter rectus, Prevotella intermedia, Fusobacterium nucleatum, Porphyromonas gingivalis, and Bacteroides forsythus, have been associated with progressive disease (3). It is estimated that approximately 50% of the human oral flora has yet to be cultured (14). It is therefore likely, on numerical grounds alone, that currently unknown and uncharacterized bacterial species play roles in the etiology of periodontitis.
Recent advances in molecular biology have made it possible to study microbial communities, including unculturable species. Direct amplification by PCR of housekeeping genes from mixed culture biomass followed by purification and sequencing has allowed the analysis of complex communities (14). The gene encoding the small-subunit rRNA has been used particularly for this purpose, and large databases of 16S rRNA sequences, such as that made available by the Ribosomal Database Project (7), now exist. These techniques have been applied to the microflora associated with dentoalveolar abscesses (2, 13). A number of novel sequences which do not correspond to known, culturable organisms have been identified. Novel taxa identified by phylogenetic analysis in this way are designated “phylotypes.” Three of these, which each made up a substantial proportion of the flora in the abscesses in which they were detected, were selected for further study. On the basis of their phylogenetic positions, phylotype PUS3.42 represents a new genus related to the genera Bacteroides and Prevotella, PUS9.170 represents a new genus related to the oral asaccharolytic Eubacterium species, and PUS9.180 represents a new species in the genus Prevotella (2, 13).
The identification of novel organisms associated with infection, while of obvious academic interest, does not in itself advance our understanding of the disease process. However, sequence data from unculturable organisms can be used to design specific PCR primers and DNA probes for rapid detection of the organisms in clinical specimens. These can be used to determine the prevalence of the organisms in healthy and diseased tissues and, if specific associations are found, might be useful markers of disease activity.
The aim of this study was to design and validate phylotype-specific PCR primers for phylotypes PUS3.42, PUS9.170, and PUS9.180 and to use these primers to detect the target organisms in subgingival plaque samples from subjects with periodontitis and from healthy controls.
MATERIALS AND METHODS
Oligonucleotide primer design.
The sequences of three novel phylotypes, PUS3.42, PUS9.170, and PUS9.180 (2, 13), were aligned with their nearest neighbors in the phylogenetic tree with the ClustalW program (5) and inspected visually for regions specific to the target organisms. Oligonucleotides selected were screened for specificity by using the Ribosomal Database Project program Check_Probe (7) and for selfcomplementarity and melting temperature by using OLIGO (National Biosciences Inc.).
Bacterial strains.
The bacterial strains used in this study are listed in Tables 1, 2, and 3. Obligate anaerobes were maintained on fastidious anaerobe agar (LabM, Bury, United Kingdom) plus 5% horse blood (FAA) under anaerobic conditions. Facultative anaerobes were maintained aerobically on blood agar base 2 plus 5% horse blood (BA).
TABLE 1.
Specificities of the PUS3.42 and PUS9.180 reverse primers, used in conjunction with 27F, against the Cytophaga-Flavobacter-Bacteroides panel
| Strain | Specificitya
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUS3.42
|
PUS9.180
|
|||||||||
| 131R | 283R | 413R | 590R | 735R | 175R | 202R | 483R | 845R | 1287Rr | |
| Prevotella bivia NCTC 11156 | − | + | − | − | + | − | − | − | − | − |
| Prevotella buccalis ATCC 35310 | − | + | − | − | − | − | − | − | − | − |
| Prevotella corporis ATCC 33547 | − | + | − | − | + | − | − | − | − | − |
| Prevotella denticola ATCC 35308 | − | + | − | − | + | − | − | − | − | − |
| Prevotella intermedia ATCC 25611 | − | + | − | − | + | − | − | − | − | − |
| Prevotella loescheii NCTC 11321 | − | − | − | − | − | − | − | − | − | − |
| Prevotella melaninogenica ATCC 25845 | − | + | − | − | + | − | − | − | − | − |
| Prevotella nigrescens NCTC 9336 | − | + | − | − | + | − | − | − | − | − |
| Prevotella oralis NCTC 11459 | − | − | − | − | + | − | − | − | − | − |
| Prevotella oris ATCC 33573 | − | + | − | − | + | − | − | − | * | − |
| Prevotella oulorum ATCC 43324 | − | + | − | − | + | − | − | − | * | − |
| Prevotella ruminicola ATCC 19189 | − | + | − | − | − | − | − | − | * | − |
| Prevotella zoogleoformans ATCC 33285 | − | + | − | − | − | − | − | − | − | − |
| Bacteroides forsythus FDC 338 | − | ND | − | − | ND | − | − | − | − | − |
| Bacteroides fragilis ATCC 25285 | * | ND | + | − | ND | − | − | − | − | − |
| Bacteroides thetaiotaomicron NCTC 10582 | * | ND | * | − | ND | − | − | * | − | − |
| Bacteroides levii NCTC 11028 | − | ND | − | − | ND | − | − | − | − | − |
| Porphyromonas asaccharolytica ATCC 25260 | − | ND | − | − | ND | − | − | − | − | − |
| Porphyromonas endodontalis ATCC 35406 | − | ND | − | − | ND | − | − | − | − | − |
| Porphyromonas gingivalis ATCC 33277 | * | ND | − | − | ND | − | − | * | − | − |
| Flavobacterium meningosepticum NCTC 10016 | − | ND | − | − | ND | − | − | * | − | − |
| Rikenella microfusus NCTC 11190 | − | ND | − | − | ND | − | − | * | − | − |
+, PCR product of predicted size; −, no PCR product; *, PCR product of size other than that predicted; ND, not done.
TABLE 2.
Specificities of the PUS9.170 reverse primers, used in conjunction with 27F, against the Eubacterium panel
| Eubacterial strain | Specificitya
|
||||
|---|---|---|---|---|---|
| 189R | 417R | 589R | 835R | 1262R | |
| E. brachy ATCC 33089 | − | + | − | − | + |
| E. infirmum NCTC 12940 | − | + | − | − | − |
| E. nodatum ATCC 33099 | − | − | − | − | − |
| E. tardum NCTC 12941 | − | − | − | − | − |
| E. timidum ATCC 33093 | − | − | − | − | − |
| E. saphenum ATCC 49989 | − | − | + | − | − |
−, no PCR product; +, PCR product of predicted size.
TABLE 3.
Specificities of selected reverse primers, used in conjunction with 27F, against the wide-range panel
| Target strain | Specificitya
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PUS3.42
|
PUS9.180
|
PUS9.170
|
||||||
| 131R | 590R | 202R | 483R | 845R | 1287R | 189R | 835R | |
| Actinobacillus actinomycetemcomitans Y4 | * | − | − | − | − | − | − | − |
| Agrobacterium tumefaciens GMI G023 | * | − | − | − | * | − | − | − |
| Actinomyces viscosus NCTC 10951 | − | − | − | − | − | − | − | − |
| Clostridium perfringens W1678 | * | − | − | * | * | − | − | − |
| Campylobacter rectus ATCC 33238 | − | * | − | − | * | − | − | − |
| Eikenella corrodens P5-22 | − | − | − | − | * | − | − | − |
| Eubacterium brachy ATCC 33089 | * | * | − | * | * | * | − | − |
| Escherichia coli NCTC 10418 | − | − | − | * | − | − | − | − |
| Eubacterium infirmum W1471 | − | − | − | − | * | * | − | − |
| Eubacterium nodatum ATCC 33099 | − | − | − | − | * | − | − | − |
| Fusobacterium nucleatum NCTC 10562 | − | − | − | * | * | − | − | − |
| Lactobacillus casei W2005 | − | − | − | − | * | * | * | − |
| Peptostreptococcus anaerobius NCTC 11460 | − | − | − | * | * | − | − | − |
| Porphyromonas gingivalis ATCC 33277 | * | − | − | * | − | − | − | − |
| Peptostreptococcus micros ATCC 33270 | − | − | − | − | * | * | − | − |
| Prevotella nigrescens NCTC 9336 | − | − | − | − | − | − | − | − |
| Staphylococcus aureus NCTC 6715 | * | − | − | − | * | * | − | − |
| Streptococcus mutans NCTC 10832 | − | − | − | * | * | * | − | − |
| Veillonella parvula NCTC 11463 | − | − | − | − | * | − | − | − |
+, PCR product of predicted size; −, no PCR product; *, PCR product of size other than that predicted.
Patients and sampling.
Plaque samples were collected from 28 patients (12 men; mean age, 47.9 years) referred to Guy’s Dental Hospital for treatment of periodontitis and from 20 periodontally healthy subjects (11 men; mean age, 41.6 years). All patients had moderate-to-advanced chronic adult periodontitis, with at least four deep pockets with probing depths of 5 mm or more. None of the patients or controls had taken antibiotics or received scaling or root planing within the previous 6 months. All patients were clinically assessed and sampled by a single examiner. For each patient, two sites were selected for sampling: one with inflammation and a probing depth of at least 6 mm and one relatively healthy site with a probing depth of 3 mm or less. For the clinically healthy subjects, all samples were collected from sites probed 4 mm or less. The probing depth of each selected site was measured to the nearest millimeter, and the presence or absence of bleeding after probing was recorded. Supragingival plaque was removed if present, and subgingival plaque was then collected by using a sterile curette introduced to the depth of the pocket. Samples were placed in saline EDTA (0.15 M NaCl, 50 mM EDTA) and stored at −20°C until being processed.
DNA extraction procedures.
Bacteria were harvested by scraping the growth from 48-h FAA or BA plate cultures. DNA was extracted by methods optimized for gram-negative (1) and gram-positive (4) bacteria.
PCR.
Plaque samples were centrifuged for 5 min at 13,000 × g, the supernatant was discarded, and the pellet was resuspended in sterile distilled water. The suspensions were then incubated at 99.9°C for 5 min in a thermocycler (Biometra Uno II). PCR amplification was performed with PCR buffer (Bioline, London, United Kingdom) containing 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 1 mM (each) oligonucleotide primer, 1 U of Taq DNA polymerase (Bioline), and template DNA in a total volume of 100 μl. A touchdown protocol was used whereby in the first cycle, denaturation was performed at 94°C for 3 min, annealing was performed at 65°C for 1 min, and extension was performed at 72°C for 2 min. In subsequent cycles, the annealing temperature was decreased by 2°C each cycle for 8 cycles, after which 25 cycles were carried out under the same conditions. In the final cycle, extension was performed for 8 min. PCR products were separated by electrophoresis in 2% agarose gels and visualized under UV light following ethidium bromide staining.
Sequencing.
PCR products were sequenced directly with a dye terminator cycle sequencing kit with AmpliTaq FS (Perkin-Elmer), according to the manufacturer’s instructions, by using 3 μl of template at a concentration of 20 ng/μl. Sequencing was performed with an ABI 377 automated sequencer with the bacterial universal forward primer 27F (6) and the appropriate reverse primer for the PCR product being sequenced. Three sequencing runs were performed for each cloned gene fragment to ensure triple coverage at each base pair.
Statistical analysis.
The distributions of the three phylotypes in the paired samples from deep and shallow sites in the patients were examined by using the McNemar test, and the distribution in shallow pockets and controls was tested by Pearson chi-square analysis.
RESULTS
Multiple reverse primers specific for each phylotype were designed as shown in Table 4 and synthesized. The specificity of each primer was validated against both a wide panel of organisms representing the major branches of the bacterial phylogenetic tree and a narrow panel representing organisms closely related to the target sequence. Considerably more species were available for the validation of the Prevotella-like phylotypes than for the phylotype related to Eubacterium, because the oral asaccharolytic Eubacterium lineage of the phylogenetic tree is a deep branch with few members (12). All of the reverse primers, when used in conjunction with universal primer 27F, gave PCR products of the expected size when used in PCRs with the corresponding 16S rDNA sequences; products obtained with the primers specific for PUS3.42 are shown in Fig. 1. The validation results for the primers specific for PUS3.42 and PUS9.180 against a panel selected from the Cytophaga-Flavobacter-Bacteroides lineage are shown in Table 1. Primers 283R and 735R both gave PCR products of the predicted size with the majority of Prevotella species. 413R gave a product with Bacteroides fragilis, while 590R did not give a product with any of the species tested. 131R was also specific, although a product not of the predicted size was obtained with two Bacteroides and one Porphyromonas species. All of the PUS9.180 primers were specific, in that they did not give the predicted size product with any of the validation panels. 483R and 845R produced artefactual products of sizes other than those expected with template DNA from some strains. The validation results for PUS9.170 with the Eubacterium panel are shown in Table 2. Primers 417R, 589R, and 1262R gave products of the predicted sizes with one or more reference strains, while 189R and 835R were specific.
TABLE 4.
PCR reverse primer positions, sequences, and lengths synthesized for the novel 16S rRNA sequences PUS3.42, PUS9.180, and PUS9.170
| Phylotype | Primer | Sequence (5′→3′) |
|---|---|---|
| PUS3.42 | 131R | CCTGTGTGATGGGCAGGTTCGGT |
| 283R | CCCATCGAAGGATTGGTGGG | |
| 428R | TACGACCACAAGGGCYTTCAT | |
| 612R | TTTAACAGCTGACTTACACTCCCG | |
| 749R | GTCAGTTACGCTCCGGATGGCTG | |
| PUS9.170 | 189R | ACATCTTTAACATGTGTTATC |
| 417R | TAGGACAGAATTTTACGACCCTAAGGG | |
| 589R | TCTGCCTTTCACCATGCGCTTATATAC | |
| 835R | CGATATCTTACGATACCG | |
| 998R | TACTCGGGATTTCAGAAGGG | |
| 1262R | TAGTTTTGCTTACCTTCACAGGC | |
| PUS9.180 | 175R | AAGTGGAGGACATCGGGGATTAA |
| 202R | GCCTTTGCTCCACAAAAGATGT | |
| 483R | TACCTGCAGGGGGCCACGC | |
| 845R | CGACAAAAAGAGCCGGAGAACG | |
| 1287R | TGAGGATTGGACCGCACTTGCGCGC |
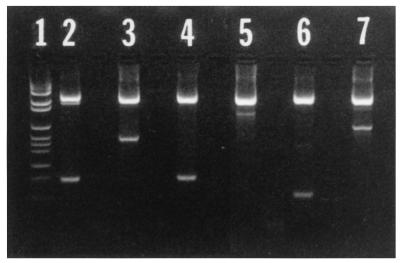
FIG. 1.
PUS3.42 plasmid DNA amplified by PCR with specific reverse primers and universal forward primer 27F. Lanes: 1, 131R (151 bp); 2, 283R (289 bp); 3, 413R (436 bp); 4, 590R (616 bp); 5, 735R (760 bp); 6, molecular size marker. Molecular marker bands are of the following sizes (top to bottom): 2,176, 1,766, 1,230, 1,033, 653, 517, 453, 394, 298, 234, 220, and 154 bp.
Primers showing specificity in the narrow-range panels were then tested against the wide-range panel, and the results are shown in Table 3. No PCR products of the predicted size were obtained with template DNAs from any of the strains, although other, artefactual, products were frequently seen, particularly with the PUS9.180 primers. As a result of these validation experiments, the following two reverse primers were chosen for each phylotype: 131R and 590R for PUS3.42, 202R and 483R for PUS.9180, and 189R and 835R for PUS9.170. These primers were then used separately with the universal primers 27F and 1492R in duplex PCRs (Fig. 2). 1492R was included as a positive control for PCR.
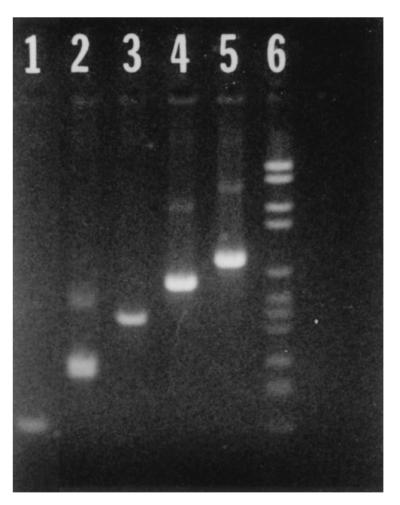
FIG. 2.
Duplex PCR analysis with PUS9.180, PUS9.170, and PUS3.42 plasmid DNA, specific reverse primers, and universal primers 27F and 1492R. Lanes: 1, molecular size marker; 2, 202R (PUS9.180 specific); 3, 483R (PUS9.180 specific); 4, 189R (PUS9.170 specific); 5, 835R (PUS9.170 specific); 6, 131R (PUS3.42 specific); 7, 590R (PUS3.42 specific). Molecular size markers are as described for Fig. 1.
The selected primers were then used in PCRs to detect the presence or absence of unculturable phylotypes in the clinical specimens. The results obtained with two primers for each phylotype are shown in Table 5 for all samples. For PUS3.42, with six samples a product was obtained with 131R only. Similarly, for PUS9.170, with seven samples a product was obtained with 189R only; for this phylotype, only four samples were positive for both primers. When seeking associations between phylotypes and the different disease states, the presence of the organism was recorded as positive only when both of the specific reverse primers gave PCR products.
TABLE 5.
Specificity of detection of target sequences in clinical samples
| Phylotype | No. of samples yielding PCR product with indicated primer(s) (n = 76) | ||
|---|---|---|---|
| PUS3.42 | 131R only | 590R only | 131R + 590R |
| 6 | 0 | 33 | |
| PUS9.180 | 202R only | 483R only | 202R + 483R |
| 1 | 1 | 12 | |
| PUS9.170 | 189R only | 835R only | 189R + 835R |
| 7 | 0 | 4 | |
The detection of phylotypes in shallow and deep pockets in periodontitis patients is shown in Table 6. Phylotype PUS9.170 were significantly associated with deep pockets (P < 0.05), while there was no significant difference in the detection of PUS3.42 or PUS9.180 at shallow or deep sites. There were no significant differences in the detection of target phylotypes in shallow pockets in periodontitis patients compared to the controls (Table 7). PUS9.170 was detected only in deep sites in periodontitis patients.
TABLE 6.
Sites in periodontitis patients with target phylotypes detectable by PCR
| Phylotype | No. of sites positive at pocket depth (mm) ofa:
|
Pb | |
|---|---|---|---|
| <4 | >5 | ||
| PUS3.42 | 9 | 15 | ns |
| PUS9.180 | 3 | 8 | ns |
| PUS9.170 | 0 | 4 | <0.05 |
Based on 28 pairs of shallow and deep pockets tested.
ns, not significant
TABLE 7.
Sites in shallow pockets in periodontitis patients and controls with target phylotypes detectable by PCR
| Phylotype | No. of sites positive
|
Pa | |
|---|---|---|---|
| Patients (n = 28) | Controls (n = 20) | ||
| PUS3.42 | 9 | 9 | ns |
| PUS9.180 | 3 | 1 | ns |
| PUS9.170 | 0 | 0 | ns |
ns, not significant.
To confirm the specificity of detection of the PCR primers, PCR products obtained from three patient samples that were positive for each of the phylotypes were sequenced and compared to the original cloned sequence. For PUS3.42, a product obtained with 590R was 97.8% similar to the original cloned sequence over 547 bases; similarly, a product from 483R was 98.8% similar to PUS9.180 over 403 bases, and a product obtained with 835R was 98.8% similar to PUS9.170 over 400 bases.
DISCUSSION
In this study, we designed multiple PCR primers specific for 16S rRNA phylotypes representing bacterial species that were not cultivable by conventional culture methods. The need for extensive validation of novel primers was demonstrated by the considerable cross-reactivity with related species that was seen with some of the primers. This was perhaps surprising, considering that a touchdown protocol, which should have ensured specificity, was used. It might be possible to devise alternative PCR protocols specifically for primers demonstrating cross-reactivity, if required. This was not attempted in this study, as two primers with adequate specificity were available for each phylotype.
Obviously, only culturable species can be included in validation studies. Given that 50% of the oral flora is unculturable, it is likely that other unculturable phylotypes may be related to those studied here. Therefore, multiple primers were used in an attempt to maintain high specificity of the detection system. The value of using two primers for each phylotype was demonstrated in this study, particularly for phylotypes PUS3.42 and PUS9.170. This presumably indicates that there are as-yet-uncharacterized taxa related to PUS3.42 and PUS9.170 that are perhaps also as yet unculturable but which have sequence homology in the 16S rRNA gene in the regions of the specific primers used. Future molecular analyses of the microflora in periodontitis tissues may identify these novel groups. In addition, the specificity of PCR detection was confirmed by sequencing for two of the phylotypes. The inclusion of the universal reverse primer 1492R in the reaction mixtures with the clinical samples was found to be useful, as it allowed the detection of any PCR-inhibitory substances from the sample itself. Without this, there would have been the possibility of recording false-negative reactions. We were fortunate that with the specific primers used there was no direct interaction with 1492R.
The results of investigations of the clinical samples showed that phylotype PUS9.170 was found only in deep pockets in the periodontitis patients. This phylotype is derived from an uncultured organism closely related to the oral asaccharolytic Eubacterium species, which are known to be strongly associated with periodontitis (8, 9, 11, 12). Although PUS9.170 was found in only 10% of disease sites, this finding nonetheless supports the association between Eubacterium species and advanced disease.
The development of rapid molecular testing methods as shown here means that, for the first time, mixed microflora associated with disease can be investigated in their entirety without the inherent biases of culture. The ability to identify novel organisms associated with disease will improve our knowledge of the etiology of periodontal disease and allow the discovery of additional marker organisms of value in disease diagnosis and treatment monitoring.
ACKNOWLEDGMENTS
R. Harper-Owen was in receipt of a University of Bristol Research Scholarship. D. Dymock was in receipt of an award to newly appointed science lecturers from the Nuffield Foundation.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1989. [Google Scholar]
- 2.Dymock D, Weightman A J, Scully C, Wade W G. Molecular analysis of microflora associated with dentoalveolar abscess. J Clin Microbiol. 1996;34:537–542. doi: 10.1128/jcm.34.3.537-542.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzink J L, Haffajee A D, Socransky S S. The predominant cultivable microbiota of active and inactive periodontal lesions. J Clin Periodontol. 1988;15:316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 4.Grimont F, Grimont P A D. DNA fingerprinting. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley and Sons Ltd.; 1991. pp. 249–276. [Google Scholar]
- 5.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 6.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley and Sons Ltd.; 1991. pp. 115–175. [Google Scholar]
- 7.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore W E C, Holdeman L V, Cato E P, Smibert R M, Burmeister J A, Ranney R R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983;52:510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore W E C, Holdeman L V, Smibert R M, Hash D E, Burmeister J A, Ranney R R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982;38:1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page R C, Altman L C, Ebersole J L, Vandesteen G E, Dahlberg W H, Williams B L, Osterberg S K. Rapidly progressive periodontitis. A distinct clinical condition. J Periodontol. 1983;54:197–209. doi: 10.1902/jop.1983.54.4.197. [DOI] [PubMed] [Google Scholar]
- 11.Uematsu H, Hoshino E. Predominant obligate anaerobes in human periodontal pockets. Periodontal Res. 1992;27:15–19. doi: 10.1111/j.1600-0765.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 12.Wade W G. The role of Eubacterium species in periodontal disease and other oral infections. Microb Ecol Health Dis. 1997;9:367–370. [Google Scholar]
- 13.Wade W G, Spratt D A, Dymock D, Weightman A J. Molecular detection of novel anaerobic species in dentoalveolar abscesses. Clin Infect Dis. 1997;25:S235–S236. doi: 10.1086/516215. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M J, Weightman A J, Wade W G. Applications of molecular ecology in the characterisation of uncultured microorganisms associated with human disease. Rev Med Microbiol. 1997;8:91–101. [Google Scholar]