Abstract
Dysregulated expression of the secretory protein renalase can promote pancreatic ductal adenocarcinoma (PDAC) growth in animal models. We characterized renalase expression in premalignant and malignant PDAC tissue and investigated whether plasma renalase levels corresponded to clinical PDAC characteristics. Renalase immunohistochemistry was used to determine the presence and distribution of renalase in normal pancreas, chronic pancreatitis, PDAC precursor lesions, and PDAC tissues. Associations between pretreatment plasma renalase and PDAC clinical status were assessed in patients with varied clinical stages of PDAC and included tumor characteristics, surgical resection in locally advanced/borderline resectable PDAC, and overall survival. Data were retrospectively obtained and correlated using non-parametric analysis. Little to no renalase was detected by histochemistry in the normal pancreatic head in the absence of abdominal trauma. In chronic pancreatitis, renalase immunoreactivity localized to peri-acinar spindle-shaped cells in some samples. It was also widely present in PDAC precursor lesions and PDAC tissue. Among 240 patients with PDAC, elevated plasma renalase levels were associated with worse tumor characteristics, including greater angiolymphatic invasion (80.0% vs. 58.1%, p = 0.012) and greater node positive disease (76.5% vs. 56.5%, p = 0.024). Overall survival was worse in patients with high plasma renalase levels with median follow-up of 27.70 months vs. 65.03 months (p < 0.001). Renalase levels also predicted whether patients with locally advanced/borderline resectable PDAC underwent resection (AUC 0.674; 95%CI 0.42–0.82, p = 0.04). Overall tissue renalase was increased in both premalignant and malignant PDAC tissues compared to normal pancreas. Elevated plasma renalase levels were associated with advanced tumor characteristics, decreased overall survival, and reduced resectability in patients with locally advanced/borderline resectable PDAC. These studies show that renalase levels are increased in premalignant pancreatic tissues and that its levels in plasma correspond to the clinical behavior of PDAC.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer death worldwide with an estimated 430,000 annual deaths and an overall survival rate of less than 10% [1, 2]. PDAC is often not detected until after it has disseminated systemically, is resistant to drug therapies, and is recognized as an urgent unmet medical need [3, 4]. A better understanding of the biology and requirements for PDAC growth is crucial to improving the diagnostic and therapeutic approaches to this disease.
Renalase (RNLS) is a novel secretory protein [5–7] that can be highly expressed in PDAC tissue [8]. Recombinant RNLS can promote the growth of human PDAC cell lines, and its inhibition leads to experimental tumor cell death in both in vitro and in vivo [8]. Overall PDAC patient survival correlates inversely with RNLS tissue levels in tumor samples [8]. These studies suggest that RNLS could be an important factor in maintaining PDAC growth and influence patient outcomes. Whether RNLS activity is associated with PDAC development, including in precursor lesions, has not been explored.
The risk of developing PDAC is increased in several premalignant lesions including chronic pancreatitis [13], pancreatic intraepithelial neoplasia (PanIN), and pancreatic cystic neoplasms. PanIN [9], the most common of these, progresses step-wise from non-invasive neoplasms (grades 1–3) to invasive PDAC with KRAS mutations being one of the earliest genetic events [10–12]. Pancreatic cystic neoplasms, comprising intraductal papillary mucinous neoplasms (IPMNs) and less frequently, mucinous cystic neoplasms (MCNs), can also progress to PDAC [13].
Since RNLS is a secretory protein, changes in its tissue levels could be reflected in its plasma levels. As seen with other tumors and biomarkers (e.g., CA19.9, CEA and PSA), it is possible that plasma RNLS levels could parallel tissue levels of RNLS in PDAC and serve as a new biomarker in PDAC.
Here we show that RNLS is present in premalignant (chronic pancreatitis, PanINs, and mucinous pancreatic cystic neoplasms) and malignant PDAC tissue suggesting a potential role for pancreatic RNLS in PDAC development. We also observed that elevations of a distinct form of plasma RNLS appear to correspond to PDAC prognosis in a subset of pancreas cancer patients with locally advanced disease at presentation.
Methods
Tissue specimens
Human pancreatic tissue samples from resection specimens of normal pancreas, chronic pancreatitis, PDAC precursor lesions (PanIN, IPMN, and MCN), and PDAC, were obtained from Yale Surgical Pathology (Yale HIC approval number: 2000021579). Additional chronic pancreatitis tissue samples were obtained from University of Minnesota Medical Center (IRB Approval Number: 0609M91887). Studies were given exceptions by the Veterans Administration HIC and Research committees under protocols FG0011-2020 and FSG0012-2020 (Oct 28, 2020)
Immunohistochemistry protocol
Immunohistochemistry was performed as described [8]. Sections (5-μm) from formalin-fixed paraffin-embedded tissues were mounted on slides and de-paraffinized and hydrated. After antigen retrieval in a pressure cooker containing citrate buffer (1g NaOH, 2.1 g citric acid in 1 L H2O pH 6), sections were blocked with DAKO Dual endogenous enzyme block (Agilent, Santa Clara, CA, USA) for 10 min and 2.5% normal horse serum for 1 hr followed by incubation with primary antibody and isotype control IgG overnight at 4°C. The m28-RNLS monoclonal antibody was diluted at 1μg/mL in buffer (TBS/1% Tween with 300 mM NaCl, pH = 7.4). ImmPRESS peroxidase-anti-rabbit IgG (Vector Laboratories, Burlingame, CA, USA) was used to detect primary antibodies. The color was developed using a Vector DAB substrate kit and tissue was counterstained with hematoxylin (Vector Laboratories). Hematoxylin and eosin stained and RNLS IHC stains were examined at the light microscope to confirm the histologic diagnosis and document the distribution of RNLS immunopositivity by a pancreaticobiliary pathologist (MER). RNLS IHC staining was categorized as present or absent in all components of the pancreas (benign exocrine, endocrine, pancreatic ducts and stroma; neoplastic tissues, either in-situ or invasive). The specificity of labeling on each tissue was confirmed by either labeling in tissue sections in the absence of the m28-RNLS primary antibody or2 using m28-RNLS antibody that had been pre-incubated with the peptide antigen (RP-220) [14].
Immunofluorescence protocol
The slides were deparaffinized and processed as above for immunofluorescence microscopy. After antigen retrieval, sections were blocked with TBS/0.3% TritonX-100/10% goat serum) for 1 hr and incubated with a cocktail of m28-RNLS (1:100) plus α-smooth-muscle actin (αSMA) mouse monoclonal (1:400 Sigma-Aldrich, St. Louis, MI, USA) at 4°C overnight. Alexa 488-conjugated goat anti-rabbit (1:2000, Invitrogen Corporation, Carlsbad, CA) and Alexa 594-conjugated goat anti-mouse (1:2000, Invitrogen Corporation, Carlsbad, CA) were used to detect the primary antibodies. Tissue autofluorescence was quenched using a commercial preparation (Vector TrueVIEW Autofluorescence Quenching Kit, Vector Laboratories, Burlingame, CA, USA). The specificity of labeling on each tissue was confirmed by labeling tissue sections in the absence of the m28-RNLS and αSMA primary antibodies.
Measurement of plasma RNLS and CA-19.9 levels
Plasma RNLS levels were determined by assaying the denaturation acid-sensitive pool and non-acid treated pool by ELISA as described [15]. Here we refer to the acid-treated value as RNLS and the untreated value as non-acid treated RNLS. The median value was used to separate low and high RNLS levels Serum CA19-9 levels were obtained from medical records before the first date of PDAC treatment.
Because of potentially spurious CA19-9 values in patients with biliary obstruction, patients with total bilirubin > 2.0 mg/dL were excluded from the CA19-9 analysis. CA19-9 levels of < 10 U/mL were also excluded because artifactually low CA19-9 levels can be seen in patients, especially those who have Lewis a-b-blood group antigen [16]. A threshold CA19-9 value of 300 U/mL was designated elevated based on studies showing that pre-operative CA19-9 values above 300 U/mL suggest advanced disease and unresectable cancer [17].
Clinical data collection
Prospectively collected plasma samples were obtained from consented patients with pathologically confirmed PDAC prior to initiation of treatment as part of the intake procedure for the Yale Gastrointestinal Tumor Biorepository from April 2012 to March 2019 (YGTB; Yale HIC #1203009817). Following definitive treatment, clinical and pathologic information were used to retrospectively annotate the plasma samples. Sociodemographic and clinicopathologic data were extracted from oncology visit notes, operation reports, and surgical pathology reports. Management was determined by the treating oncology team. Patients with locally advanced/borderline resectable (LA/BR) PDAC were determined at the time of diagnosis by cross-sectional imaging using a dedicated pancreatic contrast administration protocol and reviewed by a multidisciplinary PDAC management team that included an experienced pancreatic surgeon. Specifically, definitions of “resectable”, “borderline resectable”, and “locally advanced” were ascribed to patients by treating surgeons in accordance with the published National Comprehensive Cancer Network (NCCN) guidelines germane to the year of diagnosis [18] For the purposes of this study, patients who were identified as LA/BR at diagnosis who underwent resection with curative intent were considered “resectable” versus those who did not undergo surgery with curative intent. Any patients undergoing surgery for palliative purposes were not considered resectable.
Statistical analyses
Nonparametric statistical analyses were performed using SPSS Version 24 (IBM Statistics; Armonck, NY). Bivariate nonparametric analyses of sociodemographic and treatment-related variables were performed using the calculated cut-off value for RNLS. Multivariate analyses were performed for statistically significant variables within the bivariate analysis. Sensitivity and specificity values were calculated using the calculated cut-off value for RNLS and pre-determined cut-off value for CA19-9. Kaplan-Meier survival analyses were performed using log rank analysis to calculate statistical significance to calculate overall survival and resectability of patients. All quantitative data were reported as median (range) or mean when median was unavailable and a p-value < 0.05 was used to determine statistical significance.
Results
RNLS immunoreactivity in human pancreatic tissue
We examined the presence and distribution of RNLS in normal human pancreas (n = 11), chronic pancreatitis (n = 32), PDAC precursor lesions (PanIN: n = 5, IPMN: n = 6, MCN: n = 4), and PDAC (n = 9) by immunohistochemistry. Sociodemographic and labeling characteristics for the patients examined are summarized in Tables 1 and 2.
Table 1. Sociodemographic characteristics for RNLS immunohistochemistry patients.
| Histological variant | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Normal Pancreas (n = 11)a | Chronic Pancreatitis (n = 32)b | PanIN (n = 5) | IPMN (n = 6) | MCN (n = 4) | PDAC (n = 9) |
| Agec, years | 70 (23–94) | 56.8 (27–85) | 71 (62–82) | 70 (44–78) | 52 (26–74) | 67 (51–80) |
| Maled | 4 (36.4) | 13 (40.6) | 2 (40.0) | 4 (66.7) | 0 | 5 (55.6) |
| Whited | 7 (63.6) | 28 (87.6) | 5 (100) | 5 (83.3) | 4 (80) | 8 (88.9) |
| BMIc, kg/m2 | 28.0 (25.8–30.1) | 24.3 (13.3–37.1) | 26.0 (18.9–28.7) | 28.4 (14.9–39.6) | 22.6 (13.3–34.1) | 30.4 (23.3–34.9) |
| Current or past smokerd | 4 (36.4) | 17 (53.1) | 3 (60) | 4 (66.7) | 2 (40) | 5 (55.6) |
| Alcohol used | 0 | 5 (15.6) | 0 | 0 | 1 (20) | 1 (11.1) |
| Hypertensiond | 3 (27.3) | 13 (40.6) | 2 (40) | 2 (33.3) | 1 (20) | 3 (33.3) |
| Cardiovascular Diseased | 0 | 3 (8.4) | 1 (20) | 0 | 0 | 1 (11.1) |
| Chronic Kidney Diseased | 0 | 2 (6.3) | 0 | 0 | 1 (20) | 1 (11.1) |
| Diabetesd | 0 | 9 (28.1) | 3 (60) | 2 (33.3) | 2 (40) | 2 (22.2) |
a Seven normal pancreas cases were taken from patients who had undergone Whipple surgery for duodenal adenoma. Four normal pancreas cases were taken from patients who had undergone splenectomy associated with trauma.
b Five cases were taken from patients who had alcohol induced chronic pancreatitis. Four cases were taken from patients with idiopathic chronic pancreatitis. Six cases were taken from patients who had genetic chronic pancreatitis. One case came from a patient who had chronic pancreatitis due to pancreatic divisum. One case was taken from a patient who had chronic pancreatitis due to Sphincter of Oddi dysfunction. Two cases were taken from patients who had chronic pancreatitis associated with benign inflammatory pseudocyst. Thirteen cases were taken from patients with PDAC precursor lesions and PDAC.
c Data are given as median (range).
d Data are given as number (percentage).
Table 2. Labeling characteristics for RNLS immunohistochemistry patients.
| Pancreatic tissue type | n | Benign ductal epithelium | Acinar cell | Islets | Neoplastic cells | Stellate cells |
|---|---|---|---|---|---|---|
| Normal pancreas (trauma) | 4 | 4/4 (100) | 4/4 (100) | 4/4 (100) | N/A | 1/4 (25) |
| Normal pancreas (duodenal adenoma) | 7 | 1/7 (14) | 3/7 (43) | 1/7 (14) | N/A | 0/7 (0) |
| Chronic pancreatitis (Non-tumor associated) | 19 | 6/19 (32) | 8/19 (42) | 6/19 (32) | N/A | 6/19 (32) |
| Chronic pancreatitis (adjacent to pancreatic precursor lesion/PDAC) | 13 | 12/13 (92) | 13/13 (100) | 12/13 (92) | N/A | 2/13 (15) |
| Precursor Lesion (PanIN) | 5 | 5/5 (100) | 5/5 (100) | 5/5 (100) | 5/5 (100) | 0/5 (0) |
| Precursor Lesion (IPMN) | 6 | 6/6 (100) | 6/6 (100) | 5/6 (83.3) | 6/6 (100) | 0/6 (0) |
| Precursor Lesion (MCN) | 4 | 4/4 (100) | 4/4 (100) | 4/4 (100) | 4/4 (100) | 0/4 (0) |
| PDAC | 9 | 4/9 (44) | 4/9 (44) | 5/9 (55) | 9/9 (100) | 0/9 (11) |
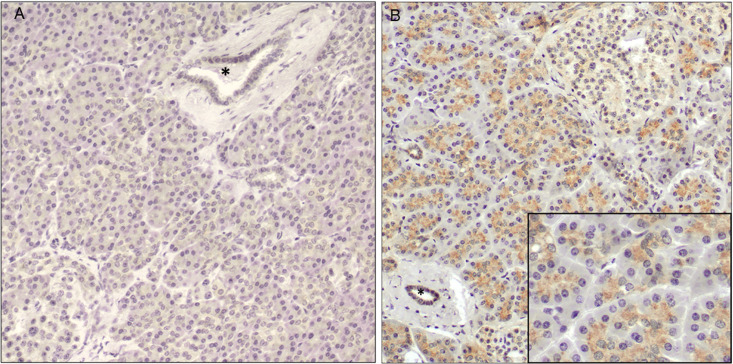
Among normal human pancreatic tissue from patients who had undergone Whipple procedure for duodenal adenoma (n = 7), there was little to no RNLS labeling (Fig 1A). In patients who had undergone distal pancreatectomy associated with trauma-related splenectomy (n = 4) there was granular RNLS labeling in the apical cytoplasm of pancreatic acinar cells as well as diffuse RNLS labeling in pancreatic islets and ducts (Fig 1B). One of these tissues showed RNLS labeling in spindle-shaped cells surrounding pancreatic acinar cells, a cellular distribution more often seen in chronic pancreatitis. Normal tissue from the pancreatic body or tail from non-abdominal trauma cases were not available for analysis. Representative images showing the spectrum of RNLS labeling in normal pancreas cases are presented in S1 Fig.
Fig 1. RNLS labeling in normal human pancreas.
Representative images of RNLS labeling detected by immunohistochemistry using the m28-RNLS antibody in the normal human pancreas from (A) a patient who had undergone Whipple procedure for duodenal adenoma with essentially no signal and (B) a patient who had undergone splenectomy for trauma, showing granular cytoplasmic staining in acinar cells. The asterisk indicates a normal pancreatic duct. Original magnification 200x; inset 400x.
Chronic pancreatitis tissues were obtained from patients with the following etiologies: alcoholic chronic pancreatitis (n = 5), genetic chronic pancreatitis (n = 6), idiopathic chronic pancreatitis (n = 4), chronic pancreatitis due to pancreatic divisum (n = 1), chronic pancreatitis due to Sphincter of Oddi dysfunction (n = 1), chronic pancreatitis adjacent to a benign inflammatory pseudocyst (n = 2), chronic pancreatitis associated with precursor lesions (n = 9), and chronic pancreatitis associated with PDAC (n = 4).
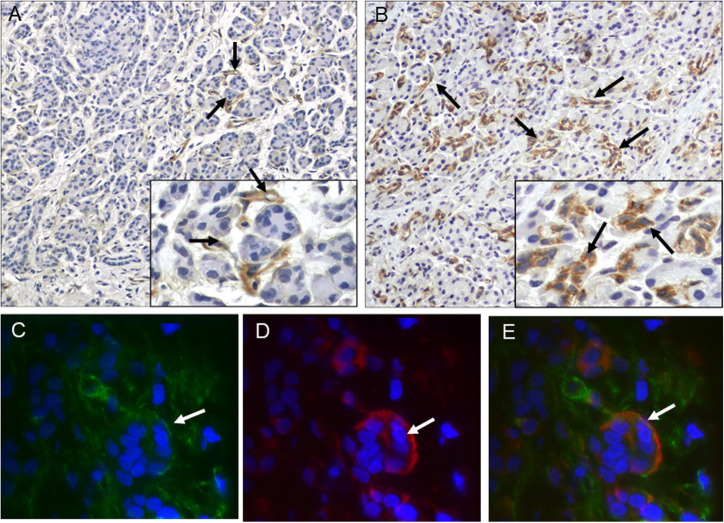
Overall, RNLS labeling in chronic pancreatitis was present, but variable, as noted below and in Table 2 and S2 Fig. RNLS immunoreactivity was found in spindle-shaped cells surrounding pancreatic acinar cells in six of the nineteen non-tumor associated chronic pancreatitis cases (Fig 2A) and two of the thirteen tumor-associated chronic pancreatitis cases (Fig 2B). These included one case each of the following etiologies: 1) Mutations in the cystic fibrosis transmembrane conductance regulator (CTFR), 2) Mutation in chymotrypsin C (CTRC), 3) Idiopathic chronic pancreatitis, 4) Alcoholic chronic pancreatitis, 5) Pancreatic divisum, 6) Associated with a pseudocyst, 7) Associated with MCN and 8) Chronic pancreatitis associated with PDAC. To determine the identity of the spindle-shaped containing RNLS immunoreactivity, we performed double-label (RNLS and αSMA- smooth muscle actin) immunofluorescence on six non-tumor associated and three tumor associated chronic pancreatitis cases. In a chronic pancreatitis case (associated CTRC mutation) we observed co-localization of RNLS and αSMA (Fig 2C–2E), suggesting that RNLS may be present in stellate cells. In the remainder of the cases the labeling was faint, and it was unclear whether the markers co-distributed. In non-tumor associated chronic pancreatitis samples, RNLS immunoreactivity was also observed in acinar cells (8/19 samples), pancreatic ducts (6/19) and islet cells (6/19). (Table 2). Granular cytoplasmic RNLS labeling of acinar cells, pancreatic ducts and islet cells was also present in regions of chronic pancreatitis associated with neoplastic conditions, including PanIN (n = 2), IPMN (n = 4), MCN (n = 3), and PDAC (n = 5). Finally, RNLS labeling was noted in stromal cells, including mononuclear inflammatory cells and fibroblasts, in regions of chronic pancreatitis associated with neoplastic precursor lesions in four samples.
Fig 2. RNLS is present in spindle-shaped cells in human chronic pancreatitis.
Representative images of RNLS immunoreactivity using the m28-RNLS antibody in (A) genetic chronic pancreatitis (CTRC mutation) and (B) PDAC-associated chronic pancreatitis. (C-E) Immunofluorescence labeling of RNLS with m28-RNLS antibody (green) and alpha-smooth muscle actin (red) in a patient with genetic chronic pancreatitis (CTRC mutation). The white arrows point to cells positive for both proteins (stellate cells). Original magnification A and B: 200x, insets 400x. Original magnification C-E: 10000x.
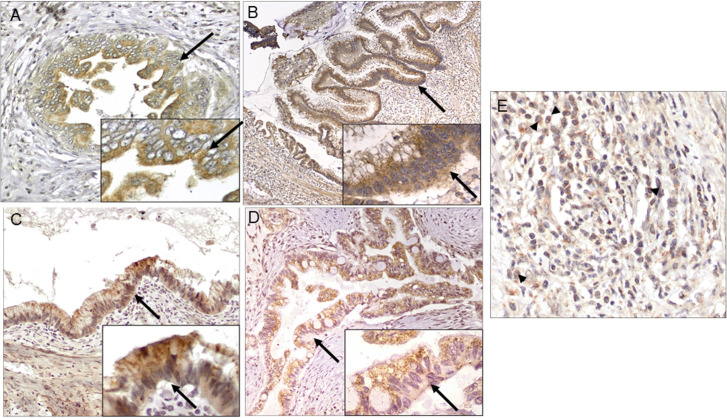
We labeled for RNLS immunoreactivity in PDAC precursor lesions (n = 15) including PanIN (n = 5), IPMN (n = 6), and MCN (n = 4), and in PDAC tumors (n = 9). In all premalignant and malignant PDAC tissues there was diffuse RNLS labeling in in-situ and invasive neoplastic epithelium, adjacent benign duct cells, acinar cells, and islets (Fig 3A–3D). In six of the fifteen precursor lesion cases and three of the nine PDAC cases, RNLS labeling was noted in stromal cells (comprising fibroblasts, endothelial cells and inflammatory cells) (Fig 3E). Additional representative images of RNLS labeling in PDAC precursor lesions and PDAC are presented in S3 Fig and S4 Fig. In-situ and invasive pancreatic ductal neoplasms and precursor lesions showed increased RNLS staining compared to benign samples (normal pancreas and most cases of pancreatitis). Specifically, in precursor and PDAC samples, RNLS was present in most cell types, including all benign elements. The intensity of labeling often appeared greater in neoplastic than in benign epithelium but was not quantified because of limitations of the labeling technique.
Fig 3. RNLS distribution in human precursor and PDAC tissue.
Representative images of RNLS labeling detected by immunohistochemistry using the m28-RNLS antibody in: (A) PanIN, (B) IPMN, (C) MCN, and (D-E) PDAC. Intense granular cytoplasmic staining is present in the majority neoplastic epithelial cells, and in a subset of mononuclear inflammation near tumor. The black arrows point to ductal cells in (A) PanIN, (B) IPMN, (C) MCN, and (D) PDAC. The black arrowheads point to mononuclear inflammatory cells in the PDAC stroma. Original magnification: A, E 400x, inset 600x; B 100x, inset 600x; C, D 200x, insets 400x.
Plasma RNLS in patients with PDAC
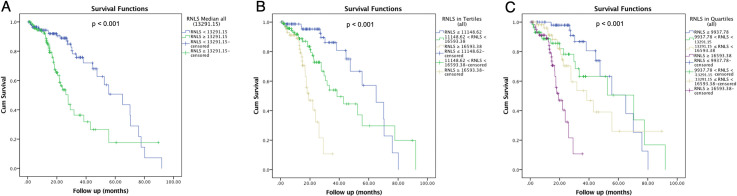
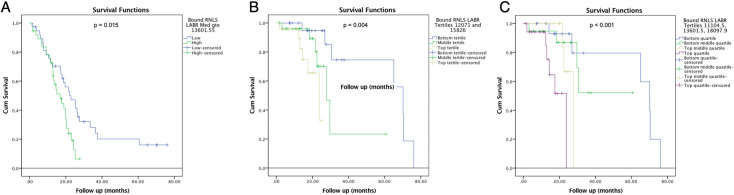
Three hundred and forty-seven patients with biopsy confirmed PDAC were identified. Of those, 240 patients had both plasma RNLS levels drawn prior to any PDAC treatment and medical records available for review. Median acid-treated RNLS level was 13291.15 ng/ml for all patients. Among patients with high or low acid-treated plasma RNLS, the median was 17337.52 vs 10135.56 ng/ml, respectively. The difference between these two groups was statistically different (p < 0.001). Median non-acid treated RNLS level was 1774.30 ng/ml. Median high non-acid treated RNLS level was 2824.05 ng/ml and median low non-acid treated RNLS level was 647.03 ng/ml (p < 0.001). High acid-treated plasma RNLS was associated with younger age at diagnosis but not associated with race/ethnicity, gender, or BMI. Patients with higher serum protein levels at diagnosis also had higher plasma RNLS levels at diagnosis (7.30 vs. 7.00, p = 0.004). Patients with high acid-treated RNLS levels also exhibited worse PDAC disease attributes (Table 3). This included higher angiolymphatic invasion (80.0% vs. 58.1%, p = 0.012), and greater node positive disease (76.5% vs. 56.5%, p = 0.024) particularly hepatic artery lymph node positivity (26.7% vs. 6.7%, p = 0.022). Patients with higher plasma acid-treated RNLS levels did not exhibit larger tumor size, or greater rates of margin positivity (Table 3). In concordance with worse disease features, patients with higher plasma acid-treated RNLS levels were less likely to be deemed clinically resectable at diagnosis (39.2% vs. 58.0%, p = 0.004) and were more likely to have disseminated disease (distant metastasis) at diagnosis (25.8% vs. 13.4%, p = 0.022). Higher plasma acid-treated RNLS levels at diagnosis were associated with decreased survival with a median of 27.70 months vs. 65.03 months (p < 0.001; Fig 4A). The survival difference persisted when acid-treated RNLS levels were divided into tertiles (n = 80 per group; Bottom tertile = 19.13 months, Middle tertile = 38.47 months, Top tertile = 65.03 months; p < 0.001; Fig 4B) and into quartiles (n = 60 per group; Bottom quartile = 19.13 months, Bottom middle quartile = 38.47 months, Top middle quartile = 70.43 months, Top quartile = 65.03 months, p < 0.001; Fig 4C). There was no correlation between non-acid treated RNLS levels and either tumor characteristics or survival (S1 Table, S5 Fig).
Table 3. Sociodemographic and clinicopathologic features among patients with PDAC with high vs. low plasma RNLS.
| Factor | Low RNLS | High RNLS | Total | p-value |
|---|---|---|---|---|
| Sociodemographic Characteristics | ||||
| Age at Diagnosis | 68.00 (9.83) | 66.00 (9.62) | 68.00 (9.80) | 0.018 |
| Race/ethnicity | 0.236 | |||
| White | 114/120 (95.0%) | 107/120 (89.2%) | 221/240 (92.1%) | |
| Black | 5/120 (4.2%) | 10/120 (8.3%) | 15/240 (6.3%) | |
| Other | 1/120 (0.8%) | 3/120 (2.5%) | 4/240 (1.7%) | |
| Male | 71/120 (59.2%) | 75/120 (62.5%) | 146/240 (60.8%) | 0.692 |
| BMI at Diagnosis | 26.37 (5.29) | 26.34 (6.92) | 26.29 (6.04) | 0.719 |
| Albumin | 4.00 (0.50) | 4.00 (0.50) | 4.00 (0.49) | 0.237 |
| Total Protein | 7.00 (0.71) | 7.30 (0.69) | 7.10 (0.73) | 0.004 |
| Clinicopathologic Characteristics | ||||
| Perineural Invasion | 61/75 (81.3%) | 46/50 (92.0%) | 107/125 (85.6%) | 0.122 |
| Angiolymphatic Invasion | 43/74 (58.1%) | 40/50 (80.0%) | 83/124 (66.9%) | 0.012 |
| Differentiation | 0.734 | |||
| Well | 3/75 (4.0%) | 1/50 (2.0%) | 4/125 (3.2%) | |
| Moderate | 37/75 (49.3%) | 23/50 (46.0%) | 50/125 (48.0%) | |
| Poor | 35/75 (46.7%) | 26/50 (52.0%) | 61/125 (48.8%) | |
| Node Positive | 43/76 (56.6%) | 39/51 (76.5%) | 82/127 (64.6%) | 0.024 |
| HALN Positive | 3/45 (6.7%) | 8/30 (26.7%) | 11/75 (14.7%) | 0.022 |
| Tumor Size ≥ 2cm | 58/73 (79.5%) | 44/49 (89.8%) | 102/122 (83.6%) | 0.144 |
| Any Margin | 15 (19.7) | 15 (29.4) | 30 (23.6) | 0.237 |
| Whipple Characteristics | Low (n = 53) | High (n = 36) | Total (n = 89) | |
| Neck Margin | 1 (1.9) | 2 (5.6) | 3 (3.4) | 0.563 |
| Bile Duct Margin | 0 | 0 | 0 | -- |
| Anterior Circumferential Margin | 0 | 0 | 0 | -- |
| Posterior Circumferential Margin | 11 (20.8) | 6 (16.7) | 17 (19.1) | 0.785 |
| Vascular Groove | 2 (3.8) | 0 (0.0) | 2 (2.2) | 0.513 |
| Uncinate Process | 10 (18.9) | 6 (16.7) | 16 (18.0) | 1.000 |
| Duodenal | 0 (0.0) | 1 (2.8) | 1 (1.1) | 0.404 |
| Invades Duodenum | 25 (472) | 19 (52.8) | 44 (49.4) | 0.669 |
| Gastric | 0 | 0 | 0 | -- |
| Pancreatic Duct | 13 (24.5) | 13 (36.1) | 26 (29.2) | 0.248 |
| CBD | 12 (22.6) | 9 (25.7) | 21 (23.9) | 0.801 |
| Peripancreatic soft tissue | 43 (81.1) | 32 (88.9) | 75 (84.3) | 0.386 |
| Any margin | 12 (22.6) | 9 (25.0) | 21 (23.6) | 0.805 |
| Whipple | 53 (68.8) | 36 (70.6) | 89 (69.5) | 1.000 |
| Distal Pancreatectomy Characteristics | Low (n = 24) | High (n = 15) | Total (n = 39) | |
| Peripancreatic soft tissue | 16 (69.6) | 13 (86.7) | 29 (76.3) | 0.273 |
| Pancreatic | 0 | 0 | 0 | -- |
| Anterior | 1 (4.3) | 1 (6.7) | 2 (5.3) | 1.000 |
| Posterior | 2 (8.7) | 4 (26.7) | 6 (15.8) | 0.188 |
| Spleen | 0 (0.0) | 1 (6.7) | 1 (2.6) | 0.395 |
| Any Margin | 3 (13.0) | 6 (40.0) | 9 (23.7) | 0.115 |
Low RNLS is defined as RNLS ≤ 13291.15ng/ml (median of data), high RNLS defined as > 13291.15ng/ml (median of data). Total is defined as data for all patients included in the dataset. P-value column includes p-value between low and high RNLS groups.
Fig 4. High plasma RNLS levels are associated with worse survival in patients with PDAC.
(A) When RNLS is cut by median, (B) by tertiles, and (C) by quartiles.
Plasma RNLS in patients with locally advanced/borderline resectable PDAC
Among patients with PDAC and available plasma RNLS levels, 76 patients presented with LA/BR PDAC according to the treating oncology team at diagnosis. Median acid-treated RNLS level among all LA/BR PDAC patients was 13601.55 ng/ml. Plasma acid-treated RNLS levels did not correlate with age, race/ethnicity, gender, BMI, serum albumin, or total blood protein at diagnosis (Table 4). High acid-treated RNLS level, defined as values above the median, did not correlate with high CA19-9 levels (Table 4). Of note, only 22 of the total 76 patients had CA19-9 values at diagnosis that met the exclusion criteria. Additionally, acid-treated RNLS levels were not associated with the reported tumor size, tumor location, or vessel encasement on imaging at initial presentation (Table 4). Among LA/BR patients, high acid-treated RNLS levels were associated with worse overall survival than were low RNLS levels (p = 0.015, Fig 5A). At a median follow-up of 19.60 months, median overall survival was 22.83 months among patients with low RNLS and 16.93 months among those with high RNLS levels. Survival correlations were especially discriminatory when RNLS levels were analyzed using tertiles (n = 25 per group; Bottom tertile = 70.13 months, Middle tertile = 27.70 months, Top tertile = 23.83 months; p = 0.004, Fig 5B) and quartiles (n = 19 per group; Bottom quartile = 60.63 months, Bottom middle quartile = 43.67 months, Top middle quartile = 25.81 months, Top quartile = 18.62 months; p < 0.001, Fig 5C). As a continuous variable, RNLS level was also associated with survival (p = 0.002). CA19-9 levels were not as predictive of survival. Median survival among those with low vs. high CA19-9 levels was 21.47 vs. 19.60 months (p = 0.548). There was no significant difference in survival when compared to values for non-acid treated RNLS (S6 Fig).
Table 4. Patient characteristics for low vs. high plasma RNLS levels in patients with locally advanced/borderline resectable (LA/BR) pancreatic adenocarcinoma (PDAC).
| Factor | Low RNLS (n = 38) | High RNLS (n = 38) | p-value |
|---|---|---|---|
| Age at Diagnosis | 67.0 (46–90) | 63.5 (37–86) | 0.651 |
| Race/ethnicity | 0.744 | ||
| White | 35 (92.1%) | 33 (86.8%) | |
| Black | 2 (5.3%) | 3 (7.9%) | |
| Other | 1 (2.6%) | 2 (5.3%) | |
| Gender | 0.818 | ||
| Male | 20 (52.6%) | 22 (57.9%) | |
| Female | 18 (47.4%) | 16 (42.1%) | |
| Chronic Kidney Disease | 1 (2.6%) | 2 (5.3%) | 1.000 |
| Hypertension | 12 (31.6%) | 14 (36.8%) | 0.809 |
| Heart Failure | 1 (2.6%) | 0 (0.0%) | 1.000 |
| Coronary Artery Disease | 4 (10.5%) | 2 (5.3%) | 0.674 |
| BMI at Diagnosis | 25.45 (17.81–40.04) | 26.34 (16.46–68.11) | 0.875 |
| Albumin at Diagnosis | 4.10 (2.50–4.70) | 4.00 (3.20–4.70) | 0.232 |
| Total Protein at Diagnosis | 7.10 (5.60–8.20) | 7.15 (5.70–8.30) | 0.555 |
| High CA19-9 at Diagnosis a | 6 (50.0%) | 2 (20.0%) | 0.204 |
| Large Vessel Encasement | 10 (26.3%) | 12 (31.6%) | 0.801 |
| PDAC Location | 0.377 | ||
| Head | 31 (81.6%) | 28 (73.7%) | |
| Body | 6 (15.8%) | 6 (15.8%) | |
| Tail | 1 (2.6%) | 4 (10.5%) | |
| PDAC Size by imaging | 30.50 (15.00–90.00) | 31.50 (18.00–92.00) | 0.705 |
a Only includes patients with total bilirubin < 2.0 and CA19-9 >10 (n = 22).
Fig 5. High plasma RNLS levels are associated with worse survival in patients with LA/BR PDAC.
(A) When RNLS is cut by median, (B) by tertiles, and (C) by quartiles.
Of the 76 patients with LA/BR PDAC, 18 (23.7%) underwent successful resection. Reasons for no eventual surgery in 58 patients were persistent local disease in 37 (48.7%), metastatic disease in 7 (9.2%), poor surgical candidacy in 4 (5.3%), and death in 3 (3.9%). The remaining 7 (9.2%) underwent exploratory laparoscopy and were subsequently found to have either unresectable local (5) or metastatic (2) disease upon surgical examination. Low RNLS levels were associated with greater adjusted odds ration (aOR) of undergoing resection (aOR = 0.29 (0.09–0.93, p = 0.036, Table 5). Of patients with low RNLS levels, 34.2% underwent resection compared to 13.2% patients with high RNLS levels, a > 2-fold difference. On the other hand, serum CA19-9 levels showed no relationship to resection status (aOR = 0.33, p = 0.272, Table 5).
Table 5. Treatments for high vs. low RNLS levels in patients with locally advanced/borderline resectable pancreatic adenocarcinoma as compared to CA19-9.
| RNLS | ||||
| Factor | Low (n = 38) | High (n = 38) | aOR (95%CI) | p-value |
| Resection | 13 (34.2%) | 5 (13.2%) | 0.29 (0.09–0.93) | 0.036 |
| Chemotherapy | 36 (94.7%) | 36 (97.3%) | 2.00 (0.17–23.06) | 0.578 |
| Radiotherapy | 18 (48.6%) | 21 (58.3%) | 1.48 (0.59–3.73) | 0.408 |
| CA19-9 | ||||
| Factor | Low (n = 14) | High (n = 8) | aOR (95%CI) | p-value |
| Resection | 3 (42.9%) | 3 (20.0%) | 0.33 (0.05–2.37) | 0.272 |
| Chemotherapy | 6 (85.7%) | 15 (100.0%) | -- | 1.000 |
| Radiotherapy | 5 (71.4%) | 7 (46.7%) | 0.35 (0.05–2.41) | 0.286 |
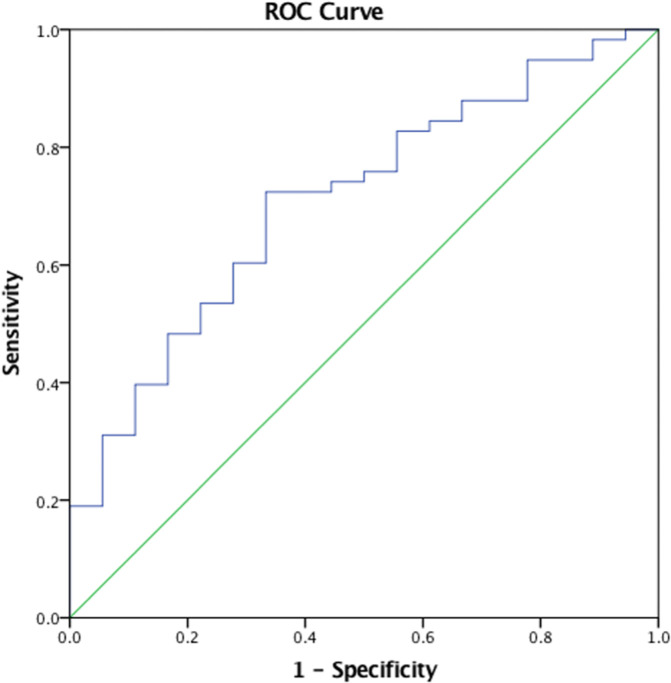
Further, receiver operating characteristic (ROC) analysis shows that lower plasma RNLS levels at diagnosis predicted resection among patients with LA/BR disease (AUC (area under curve) = 0.674; 95% CI 0.53–0.82; p = 0.037, Fig 6. The cut-off value with the highest sensitivity and specificity as determined by ROC analysis, 12548.46 ng/mL, was similar to the median of 13601.55 ng/mL and used as the cut-off for the designation of low vs high RNLS status. When using a cut-off value specified by AUC analysis, low RNLS was associated with resection with a sensitivity of 70.69% and a specificity of 66.67% (Table 6). Low CA19-9 levels showed lower sensitivity and lower specificity when compared to RNLS levels at AUC or median cut-off.
Fig 6. Plasma RNLS levels predict patients which will undergo resection in patients with locally advanced/borderline resectable pancreatic adenocarcinoma in ROC Curve.
AUC (area under curve) = 0.674.
Table 6. Sensitivity and specificity tests of CA19-9 and RNLS levels to predict resection in patients with locally advanced/borderline resectable pancreatic adenocarcinoma.
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| RNLS (Median) | 43.10% (20.16%-56.77%) | 27.78% (9.69%-53.48%) |
| RNLS (AUC) | 70.69% (57.27%-81.91%) | 66.67% (40.99%-86.66%) |
| CA19-9 | 0.00% (0.00%-45.93%) | 50.00% (24.65%-75.35%) |
Discussion
Our data show that little to no RNLS immunoreactive protein was present in normal pancreatic head tissues but that RNLS was detectable to varying degrees in premalignant PDAC and in PDAC tissues. The finding suggests a potential role for RNLS in the development of PDAC. Additionally, we found that high plasma RNLS levels correspond to worse PDAC tumor characteristics, worse overall survival, and less resectability in a subset of patients with LA/BR PDAC.
In a subset of samples primarily represented by chronic pancreatitis, RNLS was also present in stromal cells, including stellate cells with a myofibroblastic phenotype and other unidentified cell types. Stellate cells have important roles in the deposition of the extracellular matrix (stellate cells) and signaling to PDAC cells. The presence of RNLS in stellate cells shown in at least one chronic pancreatitis patient suggests that this protein could have a role in stellate cell function. Previous studies have shown that tumor microenvironment may exert selective pressures, including host immune response, proliferative or survival ability of cancer cells, or physiological restraints, which lead to the predominance of highly malignant PDAC cells [19, 20]. We have previously shown that RNLS is present in tumor-associated macrophages adjacent to melanoma, which can promote tumor growth through a STAT3-mediated mechanism [21]. Therefore, these findings suggest that RNLS could play a role in the tumor microenvironment during PDAC development. Future studies will determine the identity of the RNLS-labeled stromal cells that do not correspond to activated stellate cells and if RNLS levels can distinguish activated versus non-activated stellate cells.
Though essentially absent in other normal pancreatic head tissues taken from patients undergoing a Whipple procedure for duodenal adenomas, RNLS labeling was present in pancreatic tissue of patients who had undergone splenectomy with distal pancreatectomy for abdominal trauma. Normal pancreatic tissues from the pancreatic body or tail obtained in the absence of abdominal trauma were not available for analysis. Whether the RNLS immunoreactivity in the pancreatic tail reflects a local increase as an acute phase reactant [22, 23] in response to trauma or true differences in the RNLS content between distinct regions of the pancreas remains unclear.
A major finding of our study is that plasma levels of acid-treated RNLS correspond to PDAC prognosis. High plasma acid-treated RNLS levels were also associated with worse overall survival, angiolymphatic invasion, node positive disease, including sentinel hepatic artery lymph node positive disease, and metastasis. Plasma acid-treated RNLS levels were not associated with tumor size or surgical margin status among patients treated with Whipple or distal pancreatectomy surgery. It may be relevant that node positivity and metastasis reflect the clinically prognostic finding of tumor spread outside of the pancreas but that the effect of tumor size and margin status on prognosis in PDAC is controversial [24, 25]. Overall, these findings suggest that plasma acid-treated RNLS levels correlate with poor prognostic histopathologic features in PDAC. On the other hand, non-acid treated RNLS levels did not correspond with PDAC prognosis. Previous studies have found that non-acid treated plasma RNLS, considered its free form, is associated with mortality in patients with chronic kidney disease [15]. Our findings suggest that acid treated RNLS, hypothesized to represent a RNLS form in which its antigenic epitopes for RNLS antibody are hidden prior to acid treatment, however, may be associated with PDAC. The functional significance of this difference requires further evaluation.
Previous studies have suggested that tissue expression of RNLS is higher in select cancers than in benign tissues, including PDAC [26–30]. Tissue RNLS has been also described as a survival factor during ischemic or toxic injury and as a cytokine that activates several pathways, including PI3K/AKT, MAPK, p-ERK1/2, protein kinase B, and JAK/STAT pathways [28–30]. In PDAC tissue studies, high RNLS tumor expression was associated with worse overall survival [8]. Conditions that block RNLS can inhibit both in vitro and in vivo PDAC tumor growth [8]. Further studies would benefit from understanding the mechanism of elevated plasma RNLS levels in patients with PDAC, whether different forms of RNLS have distinct tumor effects, if tissue RNLS contributes the levels of plasma RNLS or whether the changes in plasma levels represents host responses to the tumor. Knowledge of whether plasma RNLS levels change with PDAC resection and can predict recurrence could also be clinically useful.
When considering patients who presented with LA/BC PDAC, our data show that reduced plasma RNLS, but not CA19-9, can predict both whether LA/BR PDAC patients will be subject to resection and their overall survival; CA-19 blood levels were not found to be predictive of these outcomes. Specifically, low plasma RNLS levels at diagnosis were associated with eventual eligibility for PDAC resection and with increased overall survival. There was a more than 2-fold increase in the rate of resection in patients with low plasma RNLS levels compared to high plasma RNLS levels. Previous studies have found survival improvements with resection in appropriately selected patients with locally advanced/borderline resectable PDAC [31]. Though our study includes a limited number of patients, we found a non-significant trend towards improved survival in patients undergoing resection. Future studies should prospectively assess the role of RNLS in resection clinical decision making and survival outcomes. Additionally, whether plasma RNLS levels can predict chemoresistance should be considered in future studies to determine the potential utility of plasma RNLS in tailoring chemotherapeutic strategies. Together with tumor characteristic findings in resected PDAC samples, these findings suggest that plasma RNLS levels may reflect a tumor biology that predict outcome independent of radiologic tumor size, location, or vessel encasement at presentation. Plasma RNLS levels could also reflect characteristics that were not analyzed such as genetic, epigenetic or stromal microenvironment characteristics that portend worse outcomes. Future studies should also consider measuring RNLS levels in patients throughout the development of PDAC from normal controls to chronic pancreatitis to develop a broader understanding of how RNLS may change throughout PDAC development.
Our study has a few limitations. First, the small sample size of each tissue type and immunohistochemical protocol limits quantitative comparisons of the levels of RNLS in pancreatic tissue between each group. Future studies using greater numbers of tissue samples and an automated immunohistochemistry platform are needed to quantitively compare tissue levels of RNLS across tissues. For plasma RNLS studies, the retrospective cohort study design limited the ability to accurately assess survival over long periods of time to predict resectability without bias from independent treatment team decisions. We were also limited in analyzing factors that may play a role in determining a patient’s candidacy for surgery such as comorbidities, fitness, and anatomical limitations that were not uniformly present in chart review. Without serial plasma RNLS measurements or concurrent tissue expression of RNLS on plasma RNLS samples, we cannot correlate plasma RNLS with underlying PDAC tissue expression. The small sample size of this study also limited power. A larger sample size may have yielded a potential relationship between CA19-9 and RNLS that was not seen here among LA/BR patients. Additionally, for example, we found the median survival among patients who underwent resection versus no resection was 29.67 months vs. 30.57 months (p = 0.060). This small sample size makes it difficult to evaluate this parameter in this cohort. Finally, there may have been selection bias associated with the type of patients who obtained care at our institution and subsequently were included in the biobank.
In conclusion, a key finding of our study is that RNLS is increased in both premalignant and malignant PDAC tissue compared to normal pancreas, suggesting a potential role for RNLS in the early development of PDAC. In addition, we found that the relationship between plasma RNLS levels and clinical outcomes of patients with PDAC complements published data that correlated tissue RNLS expression and PDAC survival. These findings suggest that plasma RNLS could be used as a predictive biomarker in patients with PDAC and guide therapies such as resectability in LA/BR PDAC. The RNLS levels in tissue and plasma suggest a potential pathophysiological mechanism of RNLS for the development of PDAC and severe PDAC disease. Further studies should explore the potential mechanism of action of RNLS in pre-malignant pancreatic tissue and its expression in stromal cells including stellate cells. Additionally, further studies should also explore disease progression patterns, plasma RNLS levels and tumor RNLS expression after resection, and the origin of plasma RNLS compared to tumor expression of RNLS. To further assess the ability of plasma RNLS to predict resectability in LA/BR pancreatic disease, larger prospective studies are needed that examine changes in plasma RNLS levels following neoadjuvant treatment. If plasma and tissue RNLS indeed reflects tumor biology and pathophysiology, it holds promise as a guide to surgical interventions and potential therapies that inhibit the pro-survival effects of RNLS in PDAC.
Supporting information
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Acknowledgments
We would like to acknowledge the staff members of the Yale Gastrointestinal Tumor Biorepository for providing access to patient samples for this study. The authors also wish to thank Christine Shugrue and Thomas Kolodecik for providing technical and constructive comments.
Data Availability
All original data files are available from the OSF database (DOI: 10.17605/OSF.IO/GT4V9).
Funding Statement
This study was financially supported by the U.S. Department of Defense (DOD) Cancer Program 2019 (CA180514) to FG and GD for salary support, laboratory analyses/equipment and materials necessary for this study, U.S. Veterans Administration Merit Award (BX00325) to FG for salary support and an NIH-NIDDK medical student fellowship award (DK007107) to MW for a stipend. Funding to FG from the Henry J. and Joan W Binder endowment also supported this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10(1):10–27. doi: 10.14740/wjon1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23Suppl 10:x135–8. doi: 10.1093/annonc/mds313 [DOI] [PubMed] [Google Scholar]
- 4.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9(4):e94928. doi: 10.1371/journal.pone.0094928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115(5):1275–80. doi: 10.1172/JCI24066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desir GV, Tang L, Wang P, Li G, Sampaio-Maia B, Quelhas-Santos J, et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. 2012;1(4):e002634. doi: 10.1161/JAHA.112.002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desir GV, Wang L, Peixoto AJ. Human renalase: a review of its biology, function, and implications for hypertension. J Am Soc Hypertens. 2012;6(6):417–26. doi: 10.1016/j.jash.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, et al. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Sci Rep. 2016;6:22996. doi: 10.1038/srep22996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1(4):306–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x [DOI] [PubMed] [Google Scholar]
- 11.Peters MLB, Eckel A, Mueller PP, Tramontano AC, Weaver DT, Lietz A, et al. Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: Results of a simulation model. Pancreatology. 2018;18(8):928–34. doi: 10.1016/j.pan.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–49. doi: 10.1101/gad.1415606 [DOI] [PubMed] [Google Scholar]
- 13.Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74(13):3381–9. doi: 10.1158/0008-5472.CAN-14-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Velazquez H, Moeckel G, Chang J, Ham A, Lee HT, et al. Renalase prevents AKI independent of amine oxidase activity. J Am Soc Nephrol. 2014;25(6):1226–35. doi: 10.1681/ASN.2013060665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang J, Guo X, Rao V, Gromisch E, Chung S, Kluger H, et al. Identification of Two Forms of Human Plasma Renalase, and Their Association With All-Cause Mortality. Kidney international reports. 2020;5(3):362–8. doi: 10.1016/j.ekir.2019.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19–9 and Lewis antigens in pancreatic cancer. Cancer research. 1987;47(20):5501–3. [PubMed] [Google Scholar]
- 17.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. Journal of surgical oncology. 2013;107(1):15–22. doi: 10.1002/jso.23192 [DOI] [PubMed] [Google Scholar]
- 18.NCCN.org. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2019.
- 19.Takahashi K, Ehata S, Koinuma D, Morishita Y, Soda M, Mano H, et al. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37(21):2757–72. doi: 10.1038/s41388-018-0144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball CR, Oppel F, Ehrenberg KR, Dubash TD, Dieter SM, Hoffmann CM, et al. Succession of transiently active tumor-initiating cell clones in human pancreatic cancer xenografts. EMBO Mol Med. 2017;9(7):918–32. doi: 10.15252/emmm.201607354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger H, et al. Renalase Expression by Melanoma and Tumor-Associated Macrophages Promotes Tumor Growth through a STAT3-Mediated Mechanism. Cancer Res. 2016;76(13):3884–94. doi: 10.1158/0008-5472.CAN-15-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haulik L, Toth B, Issekutz A, Gartner B. [Pancreatic injury in blunt abdominal trauma: early versus late diagnosis and surgical management]. Magy Seb. 2001;54(5):309–13. [PubMed] [Google Scholar]
- 23.Heuer M, Hussmann B, Lefering R, Taeger G, Kaiser GM, Paul A, et al. Pancreatic injury in 284 patients with severe abdominal trauma: outcome, course, and treatment algorithm. Langenbecks Arch Surg. 2011;396(7):1067–76. doi: 10.1007/s00423-011-0836-1 [DOI] [PubMed] [Google Scholar]
- 24.Li D, Hu B, Zhou Y, Wan T, Si X. Impact of tumor size on survival of patients with resected pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. BMC cancer. 2018;18(1):985. doi: 10.1186/s12885-018-4901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tummers W, Groen J, Sibinga Mulder B, Farina‐Sarasqueta A, Morreau J, Putter H, et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. British Journal of Surgery. 2019;106(8):1055–65. doi: 10.1002/bjs.11115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akkoc R, Aydin S, Goksu M, Ozcan Yildirim S, Eroksuz Y, Ogeturk M, et al. Can renalase be a novel candidate biomarker for distinguishing renal tumors? Biotechnic & Histochemistry. 2020:1–6. doi: 10.1080/10520295.2020.1825805 [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, et al. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Scientific reports. 2016;6(1):1–10. doi: 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger H, et al. Renalase expression by melanoma and tumor-associated macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer research. 2016;76(13):3884–94. doi: 10.1158/0008-5472.CAN-15-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Safirstein R, Velazquez H, Guo XJ, Hollander L, Chang J, et al. Extracellular renalase protects cells and organs by outside‐in signalling. Journal of cellular and molecular medicine. 2017;21(7):1260–5. doi: 10.1111/jcmm.13062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu X, Han P, Wang J, Sun H, Shao M. Renalase overexpression in ER-positive breast cancer. International Journal of Clinical and Experimental Pathology. 2018;11(3):1297. [PMC free article] [PubMed] [Google Scholar]
- 31.Del Chiaro M, Rangelova E, Halimi A, Ateeb Z, Scandavini C, Valente R, et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB. 2019;21(2):219–25. doi: 10.1016/j.hpb.2018.07.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Data Availability Statement
All original data files are available from the OSF database (DOI: 10.17605/OSF.IO/GT4V9).