IL-27 is a pleiotropic cytokine that plays important immunological roles in many disease settings. This study demonstrates that in addition to its known regulatory properties in preventing immune hyperactivity, gut epithelial IL-27 confers barrier immunity through promoting a distinct intraepithelial lymphocyte population.
Abstract
IL-27 controls a diverse range of immune responses in many disease settings. Here, we identify intestinal epithelial cells (IECs) as one of the major IL-27 cellular sources in the gut-associated tissue. Unlike IL-27 secreted by innate immune cells, gut epithelial IL-27 is dispensable for T-bet+ regulatory T cell (T reg cell) differentiation or IL-10 induction. Rather, IEC-derived IL-27 specifically promotes a distinct CD8αα+CD4+ intraepithelial lymphocyte (IEL) population that acquires their functional differentiation at the intestinal epithelium. Loss of IL-27 in IECs leads to a selective defect in CD8αα+CD4+ IELs over time. Consequently, mice with IEC-specific IL-27 ablation exhibited elevated pathogen burden during parasitic infection, and this could be rescued by transfer of exogenous CD8αα+CD4+ IELs. Collectively, our data reveal that in addition to its known regulatory properties in preventing immune hyperactivity, gut epithelial IL-27 confers barrier immunity by inducing a specific IEL subset and further suggest that IL-27 produced by different cell types plays distinct roles in maintaining intestinal homeostasis.
Introduction
In the gut mucosa, intestinal intraepithelial lymphocytes (IELs) represent the largest heterogeneous T cell population that plays a key role in serving as the first line of immune defense against enteric pathogens while avoiding deleterious immune-mediated inflammation and tissue damage resulting from continuous exposure to harmless foreign antigens derived from food and gut-colonized commensals. While some IELs, so-called natural IELs, develop unconventionally in the thymus in the presence of self-antigens, others, including CD8αα+CD4+ IELs and TCRαβ-bearing CD8αβ+ IELs, arise from the conventional CD4+ or CD8αβ+ T cells, respectively, in the periphery. Unlike natural IELs, the latter IEL populations, or so-called induced IELs, undergo further postthymic differentiation processes in the intestine in response to the exogenous antigens (Cheroutre et al., 2011; Mucida et al., 2013). Within the induced IEL populations, TCRαβ+CD8αβ+ IELs are generally shown to serve as long-lived epithelium-resident effector memory cells to confer protection against gut-associated pathogens (Hansen et al., 2009; Lepage et al., 1998; Müller et al., 2000). On the other hand, CD8αα+CD4+ IELs are predominantly considered to be immunoregulatory, as differentiation of conventional CD4+ T cells into CD8αα+CD4+ IELs not only allows them to reduce colitogenic potential in the presence of chronic antigen exposure but also empowers them with regulatory properties, including the expression of IL-10, to limit inflammatory responses that could jeopardize the integrity of the intestinal barrier (Das et al., 2003; Mucida et al., 2013). Recently, it has been further demonstrated that CD8αα+CD4+ IELs can also be converted from regulatory T cells (T reg cells) upon migration to the epithelium, and together, these two anti-inflammatory T cell subsets play complementary roles in establishing intestinal tolerance (Sujino et al., 2016).
In addition to TCR stimulation by luminal antigens, a specific cytokine milieu presented in the intestine provides essential cues for IEL maturation and terminal differentiation. In particular, induction of T-bet in response to IFNγ, IL-15, or IL-27 has been shown to play a role in differentiating IELs while suppressing the CD4+ T helper cell (Th cell) programs (Gangadharan et al., 2006; Reis et al., 2014). Interestingly, in addition to inducing T-bet expression in IELs, IL-27 was recently shown to promote the differentiation of T-bet–expressing T reg cells, a specific subset of T reg cells that are pivotal in controlling type I inflammation, particularly during enteric infections (Hall et al., 2012a; Lee et al., 2015; Macleod et al., 2020). Moreover, besides directly inhibiting various Th cell responses (Batten et al., 2006; Yoshimoto et al., 2007), IL-27 can also exert its immunosuppressive function through promoting IL-10 production by T cells (Awasthi et al., 2007; Stumhofer et al., 2007). As such, despite the reported proinflammatory role of IL-27 when this IL-6/IL-12 cytokine superfamily member and its receptor were first characterized (Chen et al., 2000; Pflanz et al., 2002; Yoshida et al., 2001), later studies have pointed to IL-27 as a major anti-inflammatory cytokine in limiting both autoimmune- and infection-driven inflammation in many different disease settings (Hunter and Kastelein, 2012). Nevertheless, even though the pleiotropic functions of IL-27 are now clearly recognized, considering the fact that multiple cell types are capable of expressing IL-27 (Hall et al., 2012b), the biological impact of IL-27 derived from different cellular sources remains poorly understood.
In this study, we identify intestinal epithelial cells (IECs) as one of the major IL-27–producing cell types in the gut-associated tissue. Our results demonstrate that IL-27 secreted by IECs is exclusively required for proper CD8αα+CD4+ IEL differentiation, while T-bet+ T reg cell development and IL-10 induction are driven by conventional dendritic cell (cDC)– or myeloid cell–derived IL-27, respectively. Interestingly, diminished CD8αα+CD4+ IELs in mice with IEC-specific IL-27 ablation did not lead to enhanced intestinal inflammation, which might be in part due to a corresponding increase of T reg cells within the intestinal epithelium. On the other hand, gut epithelial IL-27–driven CD8αα+CD4+ IEL responses appeared to be vital for providing optimal immune protection against enteric pathogens. To this end, adoptive transfer of exogenous CD8αα+CD4+ IELs was sufficient to eliminate the elevated pathogen burden detected in mice with IEC-specific IL-27 ablation during Toxoplasma gondii infection. Collectively, we show that in addition to its known regulatory properties in preventing immune hyperactivity, IL-27, secreted by gut epithelial cells, in particular, confers barrier immunity through inducing a specific CD8αα+CD4+ IEL subset whose role in immune defense has not been well appreciated (Mucida et al., 2013). Our data further suggest that IL-27 derived from different cellular sources plays distinct roles in maintaining intestinal tolerance while establishing efficient protective immunity.
Results and discussion
T cell–intrinsic IL-27 signaling is required for optimal CD8αα+CD4+ IEL differentiation
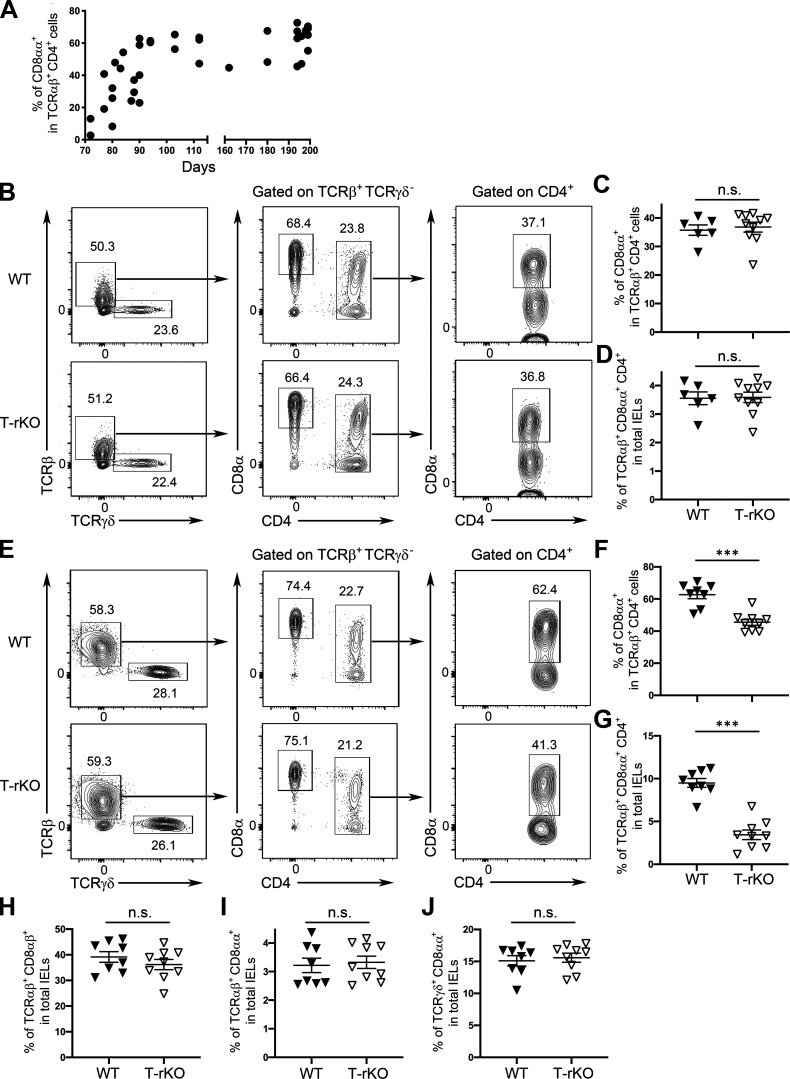
Previously, it was shown that while both IFNγ and IL-27 can drive T-bet–dependent CD8αα+CD4+ IEL differentiation in vitro, only mice devoid of IFNγ receptor 1 (Ifngr1−/−), but not IL-27 receptor subunit α (Il27ra−/−), harbored reduced CD8αα+CD4+ IELs in vivo despite diminished T-bet expression in IELs observed in both mouse lines (Reis et al., 2014). Nevertheless, it should be noted that the aforementioned analysis was conducted in Il27ra−/− mice at a relatively young age (∼8–10 wk), whereas Ifngr1−/− mice were examined at a later time point. Because CD8αα+CD4+ IELs, a subset of induced IELs, start as a small population early in life and accumulate over time in response to continuous exposure to exogenous antigens until they reach a plateau at ∼6 mo of age (Fig. 1 A; Cheroutre et al., 2011), it is thus possible that the impact of IL-27 on CD8αα+CD4+ IELs could only be observed in mice in old age. Indeed, consistent with what was reported in the previous study (Reis et al., 2014), we could not detect any clear difference in the size of CD8αα+CD4+ IELs between mice with T cell–specific deletion of IL-27Rα (CD4-cre Il27rafl/fl; T-rKO) and WT littermates at a similar age (∼10–12 wk; Fig. 1, B–D; Do et al., 2017). On the other hand, as shown in Fig. 1, E–G, a significant reduction of the CD8αα+CD4+ IEL population in T-rKO mice compared with that in WT controls was found when mice were aged (∼6 mo). It is noteworthy that T cell–intrinsic IL-27 signaling appeared to be required only for the CD8αα+CD4+ IEL subset, as deletion of IL-27Rα in T cells did not result in any alterations in other IEL populations, regardless of their age (Fig. 1, H–J). Hence, our results show that like IFNγ, IL-27 is also required for optimal CD8αα+CD4+ IEL development and that IL-27 signaling functions in a cell-autonomous manner to drive the differentiation of this specific IEL subset.
Figure 1.
Cell-intrinsic role of IL-27 in promoting CD8αα+CD4+ IEL differentiation. (A) Frequencies of small intestinal CD8αα+CD4+ IELs in naive WT mice over time. (B–D) Representative FACS profiles with gating strategy for different IEL subsets (B) and frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (C) and TCRβ+TCRγδ−CD8α+CD4+ cells (D) in total IELs from young (∼10–12 wk) T-rKO mice and WT littermates. (E–J) Representative FACS profiles with gating strategy for different IEL subsets (E) and frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (F) and TCRβ+TCRγδ−CD8α+CD4+ in total IELs (G), as well as frequencies of TCRαβ+CD8αβ+(H), TCRαβ+CD8αα+ (I), and TCRγδ+CD8αα+ (J) IEL subsets from old (>6 mo) T-rKO mice and WT littermates. Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: ***, P < 0.001.
IL-27 expressed by cDCs and myeloid cells respectively contributes to T-bet+ T reg cell and IL-10 induction but is dispensable for CD8αα+CD4+ IEL differentiation
In the intestine, cDCs and myeloid cells (i.e., monocytes and macrophages) have been shown to be the major IL-27–producing cells at both steady state and during parasitic infection (Hall et al., 2012a; Lee et al., 2015). By examining mice with DC–specific ablation of IL-27 (CD11c-cre Il27p28fl/fl; DC-KO), we have previously demonstrated that DC-derived IL-27 is critical for promoting T-bet+ T reg cell differentiation (Lee et al., 2015). Similar to what we have observed in CD8αα+CD4+ IELs, IL-27 also promotes T-bet+ T reg cell differentiation in a T reg cell–intrinsic manner, as T reg cell–specific IL-27Rα ablation (Foxp3creIl27rafl/fl; T reg-rKO) also led to a significant reduction in T-bet+ T reg cell frequencies (Fig. 2 A). However, despite its role in inducing T-bet+ T reg cells, DC-derived IL-27 appeared to play an expendable role in CD8αα+CD4+ IEL differentiation. As shown in Fig. 2, B and C, even in aged mice, comparable frequencies of CD8αα+CD4+ IELs were found in DC-KO mice and their WT littermates. Moreover, while IL-27 signaling has been previously implicated in the differentiation of TCRαβ+CD8αβ+CD8αα+ and TCRγδ+CD8αα+ IELs (Reis et al., 2014), we also could not observe any changes in these two as well as other IEL subsets in DC-KO mice (Fig. 2, D–F).
Figure 2.
IL-27 produced by either cDCs or other myeloid cells does not contribute to the differentiation of the CD8αα+CD4+ IEL subset but promotes T-bet+ T reg cells and IL-10 production, respectively. (A) FACS analysis and frequencies of T-bet+ T reg cells in LP of T reg-rKO mice and WT littermates (∼10–12 wk). (B–F) Frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (B) and TCRβ+TCRγδ−CD8α+CD4+ (C) in total IELs as well as frequencies of TCRαβ+CD8αβ+ (D), TCRαβ+CD8αα+ (E), and TCRγδ+CD8αα+ (F) IEL subsets from old (>6 mo) DC-KO mice and WT littermates. (G) FACS analysis and frequencies of T-bet+ T reg cells in LP of Mye-KO mice and WT littermates (∼10–12 wk). (H–N) FACS analysis and frequencies of IL-10+ conventional T cells in LP of Mye-KO mice (H), DC-KO mice (I), and their corresponding WT littermates (∼10–12 wk). Frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (J) and TCRβ+TCRγδ−CD8α+CD4+ in total IELs (K), as well as frequencies of TCRαβ+CD8αβ+ (L), TCRαβ+CD8αα+ (M), and TCRγδ+CD8αα+ (N) IEL subsets from old (>6 mo) Mye-KO mice and WT littermates. Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: *, P < 0.05; **, P < 0.01.
Next, we sought to determine whether myeloid cells could serve as the cellular source of IL-27 responsible for the generation of CD8αα+CD4+ IELs. Interestingly, unlike DC-KO mice, no alteration in T-bet+ T reg cell frequencies could be detected in mice with myeloid cell–specific deletion of IL-27 (LysozymecreIl27p28fl/fl; Mye-KO) compared with WT littermates (Fig. 2 G). On the other hand, myeloid cell–derived, but not DC-derived, IL-27 seemed to play a more important role in driving the production of IL-10 by T cells, a known function of IL-27 (Awasthi et al., 2007; Hall et al., 2012a; Stumhofer et al., 2007). To this end, as shown in Fig. 2, H and I, a decrease in IL-10 expression could only be found in T cells from Mye-KO mice, but not from DC-KO mice, compared with their corresponding littermate controls. Nevertheless, despite its role in IL-10 induction, like DC-derived IL-27, IL-27 produced by myeloid cells also seemed to be dispensable for promoting the differentiation of CD8αα+CD4+ IELs as well as other IEL subsets. As shown in Fig. 2, J–N, no differences in their frequencies between Mye-KO mice and littermate controls could be observed regardless of their age. Collectively, despite having distinct impacts on T-bet+ T reg cell differentiation and IL-10 induction, IL-27 produced by either cDCs or other myeloid cells could not account for the aforementioned IL-27–dependent CD8αα+CD4+ IEL differentiation.
Gut epithelial cells as one of the major IL-27 producers in the intestine
The development of CD8αα+CD4+ IELs from conventional CD4+ T cells and/or T reg cells occurs upon migration to the gut epithelium (Bilate et al., 2016; Sujino et al., 2016). Considering the pivotal role of gut microbiota in the establishment of normal IEL repertoire (Umesaki et al., 1993), these commensal bacteria could provide environmental cues essential for IEL maturation and terminal differentiation not only through serving as the major source of luminal antigens but also by shaping the cytokine milieu in the intestinal mucosa (Schirmer et al., 2016). Previously, it has been demonstrated that the production of IL-27 by lung epithelial cells can be induced in a TLR-dependent manner (Kim et al., 2011). While TLRs are instrumental in host defense against microbial infection, it has also long been appreciated that even under normal steady-state conditions, commensal bacteria recognized by TLRs are also crucial for the maintenance of intestinal homeostasis (Rakoff-Nahoum et al., 2004). Thus, it is plausible that like lung epithelial cells, IECs could secrete IL-27 upon TLR stimulation and that IEC-derived IL-27 is pivotal for CD8αα+CD4+ IEL differentiation in the gut epithelium. Supporting this notion, we have found significantly increased expression of transcripts of two IL-27 subunits, Il27p28 and Ebi3, in IECs upon in vitro culture with a TLR4 agonist, LPS (Fig. 3 A). Next, by taking an ELISA approach in which IL-27p28 and EBV-induced 3 (EBI3) were captured and detected, respectively, we have also detected a substantial amount of IL-27 protein produced by IECs stimulated with LPS as well as other TLR agonists (Fig. 3 B). Such IL-27 secretion was abolished in mice with IEC-specific deletion of IL-27 (Vil-cre Il27p28fl/fl; IEC-KO), while the production of IL-27 by the innate immune cells remained intact (Fig. 3 C). Moreover, as the expression of IL-27 could also be induced in an IFNγ-dependent manner (Lee et al., 2015; Liu et al., 2007), up-regulation of IL-27 in IECs in response to IFNγ stimulation was also detected (Fig. 3 D). Altogether, while the production of IL-27 by IECs might be relatively lower on a per-cell basis compared with other known IL-27 producers like cDCs or other myeloid cells (Fig. 3, C and D), given their high abundance in the intestinal epithelium, our data suggested that in response to environmental cues (e.g., microbiota and/or IFNγ), IECs function as one of the major cellular sources of IL-27 in the intestinal mucosa.
Figure 3.
IEC-derived IL-27 is required for optimal CD8αα+CD4+ IEL responses. (A) qPCR analyses for the expressions of Il27p28 and Ebi3 in CD45−EpCAM+ IECs from 8–12-wk-old WT and IEC-KO mice in the presence or absence of LPS stimulation. (B) ELISA analyses of the production of IL-27 by CD45−EpCAM+ IECs from 8–12-wk-old WT and IEC-KO mice in the presence or absence of Pam3CysSerLys4 (Pam3CSK4, a TLR2 agonist), LPS (TLR4 agonist), and CpG (TLR9 agonist) at different concentrations in vitro. (C and D) ELISA analyses of the production of IL-27 by CD45−EpCAM+ IECs, CD11chi cDCs, and CD11c−CD11b+ myeloid cells from 8–12-wk-old WT and IEC-KO mice in the presence or absence of LPS (1 µg/ml; C) or IFNγ (10 ng/ml; D) stimulation in vitro. (E–K) Frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (E) and TCRβ+TCRγδ−CD8α+CD4+ cells in total IELs (F), as well as frequencies of TCRαβ+CD8αβ+(G), TCRαβ+CD8αα+ (H), and TCRγδ+CD8αα+ (I) IEL subsets from old (>6 mo) IEC-KO mice and WT littermates. (J and K) FACS analysis and frequencies of Foxp3+ T reg cells in the intestinal epithelium of IEC-KO mice (J), T-rKO mice (K), and their corresponding WT littermates (>6 mo). Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Mice with IEC-specific IL-27 ablation exhibit a selective defect in CD8αα+CD4+ IELs
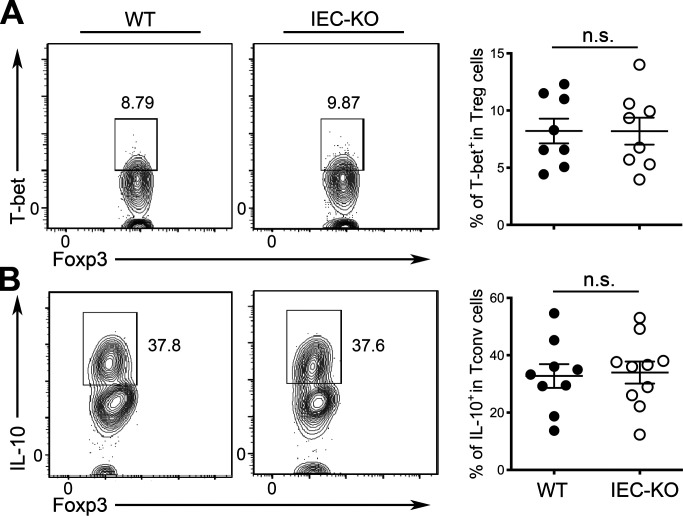
Next, we sought to determine whether IL-27 produced by IECs is required for the differentiation of CD8αα+CD4+ IELs. To this end, as shown in Fig. S1, analogous to the aforementioned findings in mice harboring T cells incapable of responding to IL-27, no clear difference in CD8αα+CD4+ IELs could be detected between IEC-KO mice and their WT littermates when they were at the young age. However, a significant reduction of the CD8αα+CD4+ IEL population in IEC-KO mice could be easily observed as they aged compared with the corresponding age-matched WT littermates (Fig. 3, E and F). The biological impact of IEC-derived IL-27 also seemed to be restricted to the CD8αα+CD4+ IEL subset. Like T-rKO mice, no other IEL populations were affected in IEC-KO mice irrespective of their age (Fig. 3, G–I). Intriguingly, in elder IEC-KO mice, accompanied by a decrease in CD8αα+CD4+ IELs, we could observe a corresponding increase in intraepithelial Foxp3+ T reg cells, a phenotype that could also be recapitulated in T-rKO mice (Fig. 3, J and K). Previously, it has been shown that upon migration to the epithelium, T reg cells lose Foxp3 and convert to CD8αα+CD4+ IELs in a microbiota-dependent manner and that CD8αα+CD4+ IELs and T reg cells coordinately maintain intestinal tolerance (Sujino et al., 2016). Considering the aforementioned findings of TLR stimulation–driven IL-27 expression by IECs and that IL-27 has been shown to suppress Foxp3 induction in the gut T reg cells (Cox et al., 2011), our data not only demonstrate an indispensable role of IEC-derived IL-27 in CD8αα+CD4+ IEL differentiation but further suggest that IL-27 produced by IECs could serve as a key molecular determinant in controlling the balance between CD8αα+CD4+ IELs and intraepithelial Foxp3+ T reg cells in the intestinal mucosa. Contrastingly, IL-27 produced by IECs did not appear to play a significant role in promoting T-bet+ T reg cells or IL-10 induction, demonstrating again that IL-27 from different cellular sources could exhibit distinct functions in maintaining intestinal homeostasis (Fig. S2).
Figure S1.
IEC-KO mice do not exhibit a CD8αα+CD4+ IEL phenotype at a young age. (A–C) Representative FACS profiles with gating strategy (A) for different IEL subsets and frequencies of CD8α+ in TCRβ+TCRγδ−CD4+ cells (B) and (C) TCRβ+TCRγδ−CD8α+CD4+ in total IELs (C) from young (∼10–12 wk) IEC-KO mice and WT littermates. Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: n.s., not significant.
Figure S2.
IL-27 produced by IECs is dispensable for both T-bet+ T reg cell differentiation and IL-10 induction in T cells. (A) FACS analysis and frequencies of T-bet+ T reg cells in LP of IEC-KO mice and WT littermates (∼10–12 wk). (B) FACS analysis and frequencies of IL-10+ T reg cells in LP of IEC-KO mice and WT littermates (∼10–12 wk). Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: n.s., not significant.
Loss of gut epithelial IL-27 results in impaired control of T. gondii infection
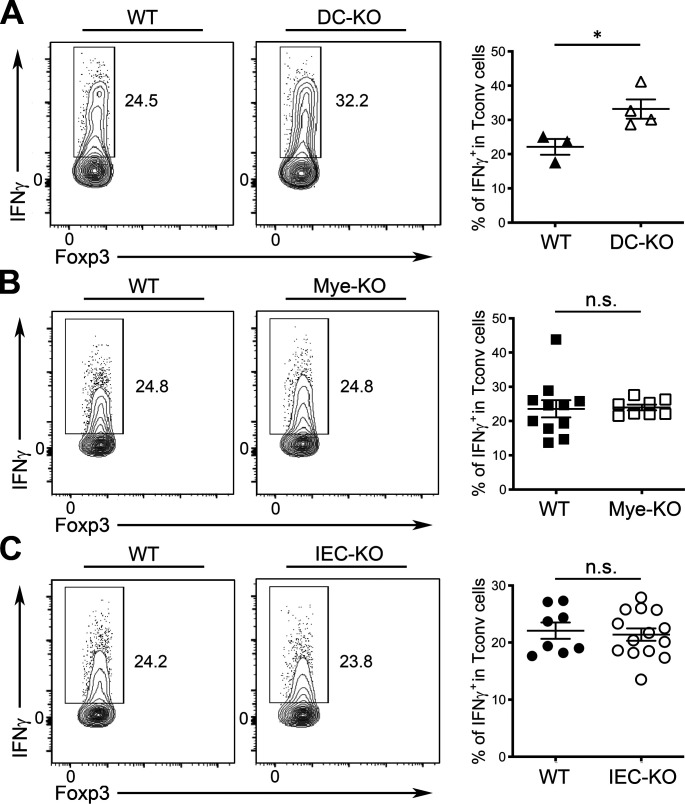
Although CD8αα+CD4+ IELs failed to accumulate properly in IEC-KO mice over time, these mice did not develop any detectable immune phenotypes in the intestinal tissue, which might be at least partially due to the aforementioned reciprocal increase of intraepithelial Foxp3+ T reg cells. Nevertheless, considering the well-recognized roles of IL-27 and CD8αα+CD4+ IELs in immune regulation, it is possible that deletion of IL-27 in IECs and the resultant defect in CD8αα+CD4+ IELs could still lead to dysregulated immune responses upon challenges. To this end, we have previously shown that mice with diminished T-bet+ T reg cells exhibited elevated IFNγ-mediated type I inflammation and exacerbated pathology during T. gondii infection (Lee et al., 2015). Consistently, as shown in Fig. S3, A and B, we could also observe a similar increase in IFNγ responses in T. gondii–infected DC-KO mice, but not in T. gondii–infected Mye-KO mice, as only the former mice harbored reduced T-bet+ T reg cells. On the other hand, despite having reduced CD8αα+CD4+ IELs, no alteration in IFNγ production by T cells could be found between aged IEC-KO mice and their WT littermates upon T. gondii infection (Fig. S3 C). Interestingly, while the loss of IL-27 in IEC does not seem to impact intestinal immune homeostasis at steady state or during T. gondii infection, IEC-KO mice, but not DC-KO or Mye-KO mice, were unable to control pathogens appropriately, as increased parasite burdens in IEC-KO mice were found not only in the intestine but also in the liver, a sign of unrestrained parasite expansion and dissemination (Fig. 4, A and B). Moreover, by using an avirulent Prugniaud (Pru) strain of T. gondii expressing tdTomato (Pru-tdTomato; Chtanova et al., 2008; Gregg et al., 2013), our analysis further revealed a markedly increased frequency of T. gondii–infected EpCAM+ IECs in IEC-KO mice compared with those in the littermate controls (Fig. 4 C). Together, despite that the immune regulatory function of IL-27 in retraining immune responses during T. gondii infection has been long established, we have identified a previously uncharacterized role of IL-27 produced by IECs but not by DCs or other myeloid cells in mounting a protective immune response against this particular parasite. Consistent with this notion, it has been shown that gut commensal bacteria confer host immunity during T. gondii infection through activating TLR2, TLR4, or TLR9 pathways (Benson et al., 2009). Considering that IL-27 production by IECs could be driven by stimulation of TLR2, TLR4, or TLR9 agonists (Fig. 3 B), our results further suggested that the previously reported gut commensal–mediated anti-parasitic immunity could act at least in part through an IEC-derived IL-27–dependent mechanism.
Figure S3.
IEC-derived IL-27 is not required to control IFNγ responses during T. gondii infection. (A) FACS analysis and frequencies of IFNγ+ T cells in LPof DC-KO mice and WT littermates (>6 mo). (B) FACS analysis and frequencies of IFNγ+ T cells in LP of Mye-KO mice and WT littermates (>6 mo). (C) FACS analysis and frequencies of IFNγ+ T cells in LP of IEC-KO mice and WT littermates (>6 mo). Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: *, P < 0.05.
Figure 4.
Loss of IL-27 production by IECs results in uncontrolled parasite burdens during T. gondii infection. (A and B) qPCR analysis of parasite burden in small intestine (A) and liver (B) of IEC-KO, DC-KO, or Mye-KO mice (>6 mo) along with their corresponding WT littermates at day 8 after T. gondii infection. (C) FACS analysis and frequencies of Pru-tdTomato+ in CD45−EpCAM+ IECs from IEC-KO mice and WT littermates (>6 mo) at day 8 after T. gondii infection. Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of Student’s t test: *, P < 0.05; **, P < 0.01.
Defective CD8αα+CD4+ IEL responses are responsible for uncontrolled parasite burdens in IEC-KO mice during T. gondii infection
Considering that IEC-derived IL-27 is selectively required for the differentiation of CD8αα+CD4+ IELs and that loss of IL-27 in IECs led to increased pathogen burdens, our findings implied a pivotal role of CD8αα+CD4+ IELs in conferring host immunity against enteric pathogens. To establish a causal relationship between a diminished CD8αα+CD4+ IEL population and elevated pathogen burdens observed in IEC-KO mice during T. gondii infection, we sought to determine whether IEC-KO mice could better control toxoplasmosis when exogenous CD8αα+CD4+ IELs were supplemented. Previously, it has been shown that transferred IELs were capable of trafficking back to the intestine to enhance resistance to T. gondii infection (Buzoni-Gatel et al., 1999). Nevertheless, early work has been focusing on the protective effect of CD8αβ+ IELs (Buzoni-Gatel et al., 1997), whereas the role of CD8αα+CD4+ IELs in establishing host immunity against this parasite has not been directly tested. To this end, as shown in Fig. 5 A, while the transfer of exogenous CD8αα+CD4+ IELs did not lead to further increases in WT littermates likely due to the filled niches, the frequencies of CD8αα+CD4+ IELs in the intestinal epithelium in IEC-KO mice were able to reach the same level as found in control mice upon adoptive transfer. Moreover, not only did the frequencies of total CD8αα+CD4+ IELs in the CD8αα+CD4+ IEL-repleted IEC-KO mice remained stable over time, but also the frequencies of exogenously transferred CD8αα+CD4+ IELs (CD45.1+) stayed unaltered (Fig. 5, B and C). While we could not completely exclude the possibility that IL-27 might still be needed in the long-term maintenance of this particular IEL population, our data provided firm evidence suggesting that IEC-derived IL-27 plays a predominant role in driving the differentiation of CD8αα+CD4+ IELs in the intestinal epithelium. Finally, when the difference in the frequencies of CD8αα+CD4+ IELs between IEC-KO mice and WT littermates was eliminated, these mice no longer exhibited increased parasite burdens (Fig. 5 D). These results strongly supported the aforementioned notion that IL-27 secreted by IECs protects the host from T. gondii infection through promoting the differentiation of CD8αα+CD4+ IELs.
Figure 5.
Defective CD8αα+CD4+ IEL responses in IEC-KO are responsible for impaired parasite control during T. gondii infection. (A) FACS analysis and frequencies of small intestinal CD8αα+CD4+ IELs in IEC-KO mice and WT littermates (>6 mo) with or without adoptive transfer of exogenous CD8αα+CD4+ IELs. (B) FACS analysis and frequencies of total CD8αα+CD4+ IELs in IEC-KO mice (>6 mo) at different time points after adoptive transfer of exogenous CD8αα+CD4+ IELs. (C) FACS analysis and frequencies of CD45.1+CD8αα+CD4+ IELs in IEC-KO mice (>6 mo) at different time points after adoptive transfer of exogenous CD8αα+CD4+ IELs. (D) qPCR analysis of parasite burden in small intestine (SI) and liver of IEC-KO and WT littermates with or without adoptive transfer of exogenous CD8αα+CD4+ IELs at day 8 after T. gondii infection. (E–I) qPCR analyses for the expressions of Il27ra (E), Ido1 (F), and Dmbt1 (G) in small intestinal CD45−EpCAM+ IECs and qPCR analysis of parasite burden in small intestine (H) and liver (I) of 8–12-wk-old IEC-rKO and WT littermates at day 8 after T. gondii infection. Each symbol represents an individual mouse, and the bar represents the mean. Data are pooled from at least three independent experiments. Results of one-way ANOVA in A–D and Student’s t test in E–I: ***, P < 0.001.
It should be noted, however, that an early study reported that IL-27 was capable of conferring intestinal epithelial barrier protection through the induction of antibacterial molecules in IECs (Diegelmann et al., 2012). It is thus probable that IEC-derived IL-27 could also exert its immune protective role against T. gondii by acting on IECs in an autocrine manner. To test this possibility, we generated a mouse line in which IL-27Rα is specifically deleted in IECs (ViI-cre Il27rafl/fl; IEC-rKO). As shown in Fig. 5, E–G, consistent with the previous study (Diegelmann et al., 2012), reduced expression of genes with antibacterial properties, indoleamine 2,3-sioxygenase 1 (Ido1) and deleted in malignant brain tumors 1 (Dmbt1), was found in IECs devoid of IL-27Rα. Nevertheless, loss of IL-27 signaling in IECs did not lead to any defect in controlling toxoplasmosis, as IEC-rKO mice and WT littermates exhibited similar parasite burdens (Fig. 5, H and I). Together, our results have demonstrated that IL-27 does not confer protective immunity against T. gondii infection by directly acting on IECs and that diminished CD8αα+CD4+ IELs in the absence of IEC-derived IL-27 are the major cause of elevated pathogen burdens observed in IEC-KO mice.
Since their initial discovery, the main function of CD8αα+CD4+ IELs has been to promote intestinal tolerance to innocuous commensals and food antigens (Das et al., 2003; Mucida et al., 2013; Sujino et al., 2016). This specific IEL subset exhibits many anti-inflammatory properties similar to what were found in T reg cells. However, it remains unclear why T reg cells convert into CD8αα+CD4+ IELs once they enter the epithelium. One possibility is that the immune system simply evolves to carry this conversion to prevent unwanted inflammation in a tissue microenvironment that is unfavorable for maintaining T reg cell lineage stability, as suggested by a previous study (Sujino et al., 2016). Alternatively, the differentiation/conversion of CD8αα+CD4+ IELs is important for not only limiting intestinal inflammation but also the acquisition of cytotoxic capacities, allowing them to work alongside other cytotoxic IEL subsets (e.g., CD8αβ+ IELs) as a front-line defense against gut-associated pathogens. While the exact effector mechanisms underlying CD8αα+CD4+ IEL-mediated immunity remain to be elucidated, here, we have clearly demonstrated that CD8αα+CD4+ IELs play an immunoprotective role in host defense in the intestinal mucosa. Likewise, even though IL-27 is generally considered to be immunoregulatory in many disease settings, including infections, autoimmune diseases, and cancers (Chihara et al., 2018; Yoshida and Hunter, 2015), our study suggests that this multifunctional cytokine can exert its diverse activities in a context- and cell type–specific manner. Moving forward, as IL-27 and many other cytokines and their receptors have become major targets for immunotherapy, a better understanding of their function in a specific environment at a particular point in time is needed to develop an effective therapeutic strategy targeting IL-27 as well as other cytokines to treat a selected disease.
Materials and methods
Mice
CD11c-cre Il27p28fl/fl mice (Lee et al., 2015) and Foxp3creIl27rafl/fl mice (Do et al., 2017) were described previously. In addition, CD4-cre (Lee et al., 2001), Lysozymecre (Clausen et al., 1999), Vil-cre mice (Madison et al., 2002), and CD45.1+ B6 mice were purchased from the Jackson Laboratory. All mice were bred and housed under specific pathogen–free conditions. 8–12-wk-old mice of both sexes were used as young mice, 6–7-mo-old mice were used as aged mice, and only WT littermates of the same gender served as controls in each experiment. All mice were maintained and handled in accordance with the Institutional Animal Care and Use Guidelines of University of California, San Diego and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Animal Research: Reporting In Vivo Experiments guidelines.
Tissue preparation and cell isolation
Spleen and lymph nodes were mechanically dissociated between frosted glass slides or with the back of a syringe plunger and filtered through a 100-µm nylon mesh to yield single-cell suspensions. Lamina propria (LP) lymphocytes and IELs were isolated as previously described (Lee et al., 2015). Briefly, small intestines were removed and placed in chilled RPMI-1640 media. The intestines were carefully cleaned from the mesentery and flushed of fecal content. Intestines were opened longitudinally and then cut into 1-cm pieces. The intestinal tissue was transferred to a 50-ml Falcon tubes containing 10 ml prewarmed RPMI-1640 complemented with 1% PenStrep (100 U/ml penicilium and 100 μg/ml streptomycin), 0.5% BSA, 5 mM EDTA, 20 mM Hepes, and 1 mM dithiothreitol and shaken at 200 rpm for 20 min at 37°C. The tissue suspension was passed through a stainless steel sieve into 50-ml conical tubes, and the cells were pelleted by centrifugation at 1,500 rpm for 10 min at 4°C. The cell pellet was resuspended in 47% Percoll and centrifuged at 1,500 rpm for 10 min. The upper fraction and the pellet were collected, washed and resuspended in complete RPMI media. These purified upper fraction cells constituted IECs, and the pellet constituted the IEL populations. To isolate LP lymphocytes, the remaining intestinal tissue in the stainless steel sieve was minced and transferred to conical tubes. The minced pieces were resuspended in 10 ml complete RPMI-1640 containing 1% PenStrep, 20 mM Hepes, 0.05 mg/ml Liberase TL (Roche) 0.05%, and DNaseI (Roche) and shaken at 200 rpm for 30 min at 37°C in 50-ml Falcon tubes. The tissue suspension was collected and passed through a 70-µm cell strainer, and the cells were pelleted by centrifugation at 1,200 rpm. The cells were then resuspended and purified by 47% Percoll, centrifugated, and processed as described above for the IEL preparation.
Flow cytometry and antibodies
Cells were stained with Ghost Dye Red 780 (Tonbo Biosciences; 13–0865-T100), followed by surface and intracellular antibody staining for CD4 (Thermo Fisher Scientific; 45–0042-82), CD45 (Thermo Fisher Scientific; 17–0454-82), EpCAM (Thermo Fisher Scientific; 46–5791-82), CD8α (Thermo Fisher Scientific; 25–0081-82), CD8β (BioLegend; 126611), TCRβ (Thermo Fisher Scientific; 48–5961-82), TCRγδ (Thermo Fisher Scientific; 46–5711-82), T-bet (Thermo Fisher Scientific; 12–5825-82), and FOXP3 (Thermo Fisher Scientific; 53–5773-82) at the manufacturer’s recommended concentrations. Fixation and permeabilization of cells were performed with the FOXP3/Transcription Factor Staining Kit (Tonbo Biosciences; TNB-0607). To detect IFNγ (Thermo Fisher Scientific; 17–7311) production, cells were stimulated in a 96-well plate with 50 ng/ml PMA, 0.5 mg/ml ionomycin, and 1 mg/ml brefeldin A (all from Sigma-Aldrich) in complete 5% RPMI media for 4 h at 37°C before staining. To detect IL-10 (Thermo Fisher Scientific; 12–7101) production, cells were stimulated with plate-bound αCD3 (Bio X Cell; BE0001) and αCD28 (Bio X Cell; BE0015), both at 1 µg/ml, plus TGFβ (5 ng/ml) and IL-2 (100 U/ml) for 72 h at 37°C. Upon harvesting, cells were restimulated for 4 h with PMA, ionomycin, and brefeldin A, as described above, before staining. An LSRFortessa or LSRFortessaX20 cell analyzer (BD Biosciences) was used for data collection, and FlowJo software (BD Biosciences) was used for data analysis.
qPCR analysis
For quantification of Il27p28 and Ebi3 expression, CD45−EpCAM+ IECs from IEC-KO and WT mice were first isolated as described in the aforementioned Tissue preparation and cell isolation section and sorted on a FACSAria II cell sorter (BD Biosciences) with a purity of greater than 95%. 1.5 × 105 cells were stimulated with LPS (1 µg/ml) or IFNγ (10 ng/ml) in the 96-well U-bottom plate for 6 h at 37°C followed by RNA isolation using a RNeasy Kit (QIAGEN). Extracted RNA was converted to cDNA with an iScript cDNA Synthesis Kit (Bio-Rad), followed by qPCR reactions using SYBR Select Master Mix (Thermo Fisher Scientific). All real-time reactions were run on a 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific) with the following primers: Il27p28, forward, 5′-CTGAATCTCGATTGCCAGGAGTGA-3′ and reverse, 5′-AGCGAGGAAGCAGAGTCTCTCAGAG-3′; Ebi3, forward, 5′-CGGTGCCCTACATGCTAAAT-3′ and reverse, 5′-GCGGAGTCGGTACTTGAGAG-3′; Il27ra, forward, 5′-CAAGAAGAGGTCCCGTGCTG-3′ and reverse, 5′-TTGAGCCCAGTCCACCACAT-3′; Ido1, forward, 5′-GTTCGAAAGGTGCTGCCCCGC-3′ and reverse, 5′-AGAAGCCCTTGTCGCAGTCCCC-3′; Dmbt1, forward, 5′-TGTTTCCAGTGACCAGCAGC-3′ and reverse, 5′-GGGCGAGGAGTAGGATTGGT-3′.
ELISA assay
For quantification of the production of IL-27 from different cellular sources in small intestine, CD45−EpCAM+ IECs, CD11chiCD11b− cDCs, and CD11c−CD11b+ myeloid cells from IEC-KO and WT mice were sorted on a FACSAria II cell sorter (BD Biosciences) with a purity of greater than 95%. 1.5 × 105 cells were stimulated with LPS (hi: 1 µg/ml; lo: 0.2 µg/ml), Pam3CysSerLys4 (hi: 100 ng/ml; lo: 20 ng/ml), and CpG (hi: 1 µg/ml; lo: 0.2 µg/ml), as suggested by a previous report (Pirhonen et al., 2007), or IFNγ (10 ng/ml) in the 96-well U-bottom plate for 72 h at 37°C. Supernatant were collected and measured by ELISA kits according to the manufacturer’s instructions (BioLegend; 438707). Absorbance was measured at 450 nm with a microplate reader (Molecular Devices).
T. gondii infection
The ME-49 strain of T. gondii was maintained in Swiss Webster and CBA/CaJ mice, and tissue cysts from the brain were used for infection as previously described (Lee et al., 2015). For all studies, 8–12-wk-old or 6-mo-old mice were infected with 40 cysts of ME-49 by oral route and analyzed for parasite burden. To quantify parasite burden, qPCR analysis was performed for DNA isolated from duodenum and liver of infected mice using primers 5′-TCCCCTCTGCTGGCGAAAAGT-3′ (forward) and 5′-AGCGTTCGTGGTCAACTATCGATTG-3′ (reverse) to determine the relative abundance of T. gondii B1 gene to mouse Gapdh gene. The transgenic Pru strain parasites engineered to express tandem dimers of tdTomato-OVA were maintained through serial passage in Swiss Webster and CBA mice (John et al., 2009). For infections, brains of chronically infected mice were mechanically separated by passage through a series of 18-gauge, 20-gauge, and 22-gauge needles; cysts in the brain homogenate were then counted, and ∼50 cysts were delivered orally with a 20-gauge gavage needle. Mice were infected orally with 50 Pru-tdTomato cysts and sacrificed 6 d after infection and Pru-tdTomato–infected cells were detected using flow cytometry.
Adoptive transfer of CD8αα+CD4+ IELs
IELs were first isolated from small intestine of WT CD45.1+ B6 mice as described above in Tissue preparation and cell isolation. Next, CD4+ IELs were further isolated by using Mouse CD4 T Cell Negative Selection kit (BioLegend; 480006). Finally, isolated CD4+ IELs were stained with anti-CD8α-PE antibody, and CD8αα+CD4+ IELs were separated by using anti-PE-beads (BioLegend; 480080). After isolation, 1 × 106 cells were injected i.p. into aged WT or IEC-KO mice. Mice were challenged with 40 cysts of ME-49 4 d after the adoptive transfer of cells and analyzed on day 8 after infection. For the IEL maintenance study, IEC-KO mice receiving 1 × 106 CD45.1+CD8αα+CD4+ IELs cells were harvested at day 10, 20, and 30 after cell transfer.
Statistics
An unpaired, two-tailed Student’s t test (or one-way ANOVA for the CD8αα+CD4+ IEL adoptive transfer studies) was performed using GraphPad Prism 8 software (GraphPad Software).
Online supplemental material
Fig. S1 shows frequencies and FACS analysis of small intestinal CD8αα+CD4+ IELs in young IEC-KO mice and WT littermates. Fig. S2 shows frequencies and FACS analysis of small intestinal T-bet+ T reg cells and IL-10 producing conventional T cells in IEC-KO mice and WT littermates. Fig. S3 shows frequencies and FACS analysis of small intestinal IFNγ+ conventional T cells in DC-KO, Mye-KO, and IEC-KO mice and their corresponding WT littermates during T. gondii infection.
Acknowledgments
We thank all members of our laboratory for discussions.
This work was supported by National Institutes of Health grants AI108651, AI127751, AI163813 (to L.-F. Lu), and AI125247 (to B. Min).
Author contributions: C.-H. Lin and L.-F. Lu conceived and designed the project. C.-H. Lin, M.-C. Chen, and L.-L. Lin performed the experiments. C.-H. Lin and L.-F. Lu analyzed the data. D.A. Christian, B. Min, and C.A. Hunter contributed critical reagents and materials. C.-H. Lin and L.-F. Lu wrote the manuscript.
References
- Awasthi, A., Carrier Y., Peron J.P., Bettelli E., Kamanaka M., Flavell R.A., Kuchroo V.K., Oukka M., and Weiner H.L.. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8:1380–1389. 10.1038/ni1541 [DOI] [PubMed] [Google Scholar]
- Batten, M., Li J., Yi S., Kljavin N.M., Danilenko D.M., Lucas S., Lee J., de Sauvage F.J., and Ghilardi N.. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7:929–936. 10.1038/ni1375 [DOI] [PubMed] [Google Scholar]
- Benson, A., Pifer R., Behrendt C.L., Hooper L.V., and Yarovinsky F.. 2009. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe. 6:187–196. 10.1016/j.chom.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilate, A.M., Bousbaine D., Mesin L., Agudelo M., Leube J., Kratzert A., Dougan S.K., Victora G.D., and Ploegh H.L.. 2016. Tissue-specific emergence of regulatory and intraepithelial T cells from a clonal T cell precursor. Sci. Immunol. 1:eaaf7471. 10.1126/sciimmunol.aaf7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzoni-Gatel, D., Lepage A.C., Dimier-Poisson I.H., Bout D.T., and Kasper L.H.. 1997. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J. Immunol. 158:5883–5889. [PubMed] [Google Scholar]
- Buzoni-Gatel, D., Debbabi H., Moretto M., Dimier-Poisson I.H., Lepage A.C., Bout D.T., and Kasper L.H.. 1999. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J. Immunol. 162:5846–5852. [PubMed] [Google Scholar]
- Chen, Q., Ghilardi N., Wang H., Baker T., Xie M.H., Gurney A., Grewal I.S., and de Sauvage F.J.. 2000. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 407:916–920. 10.1038/35038103 [DOI] [PubMed] [Google Scholar]
- Cheroutre, H., Lambolez F., and Mucida D.. 2011. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11:445–456. 10.1038/nri3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara, N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., Nyman J., Marjanovic N.D., Kowalczyk M.S., Wang C., et al. 2018. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 558:454–459. 10.1038/s41586-018-0206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova, T., Schaeffer M., Han S.J., van Dooren G.G., Nollmann M., Herzmark P., Chan S.W., Satija H., Camfield K., Aaron H., et al. 2008. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 29:487–496. 10.1016/j.immuni.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, B.E., Burkhardt C., Reith W., Renkawitz R., and Förster I.. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277. 10.1023/A:1008942828960 [DOI] [PubMed] [Google Scholar]
- Cox, J.H., Kljavin N.M., Ramamoorthi N., Diehl L., Batten M., and Ghilardi N.. 2011. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J. Exp. Med. 208:115–123. 10.1084/jem.20100410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, G., Augustine M.M., Das J., Bottomly K., Ray P., and Ray A.. 2003. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 100:5324–5329. 10.1073/pnas.0831037100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann, J., Olszak T., Göke B., Blumberg R.S., and Brand S.. 2012. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J. Biol. Chem. 287:286–298. 10.1074/jbc.M111.294355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, J., Kim D., Kim S., Valentin-Torres A., Dvorina N., Jang E., Nagarajavel V., DeSilva T.M., Li X., Ting A.H., et al. 2017. Treg-specific IL-27Rα deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc. Natl. Acad. Sci. USA. 114:10190–10195. 10.1073/pnas.1703100114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan, D., Lambolez F., Attinger A., Wang-Zhu Y., Sullivan B.A., and Cheroutre H.. 2006. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 25:631–641. 10.1016/j.immuni.2006.08.018 [DOI] [PubMed] [Google Scholar]
- Gregg, B., Taylor B.C., John B., Tait-Wojno E.D., Girgis N.M., Miller N., Wagage S., Roos D.S., and Hunter C.A.. 2013. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect. Immun. 81:1635–1643. 10.1128/IAI.01126-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A.O., Beiting D.P., Tato C., John B., Oldenhove G., Lombana C.G., Pritchard G.H., Silver J.S., Bouladoux N., Stumhofer J.S., et al. 2012a. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 37:511–523. 10.1016/j.immuni.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, A.O., Silver J.S., and Hunter C.A.. 2012b. The immunobiology of IL-27. Adv. Immunol. 115:1–44. 10.1016/B978-0-12-394299-9.00001-1 [DOI] [PubMed] [Google Scholar]
- Hansen, S.G., Vieville C., Whizin N., Coyne-Johnson L., Siess D.C., Drummond D.D., Legasse A.W., Axthelm M.K., Oswald K., Trubey C.M., et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299. 10.1038/nm.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, C.A., and Kastelein R.. 2012. Interleukin-27: balancing protective and pathological immunity. Immunity. 37:960–969. 10.1016/j.immuni.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, B., Harris T.H., Tait E.D., Wilson E.H., Gregg B., Ng L.G., Mrass P., Roos D.S., Dzierszinski F., Weninger W., and Hunter C.A.. 2009. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 5:e1000505. 10.1371/journal.ppat.1000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.S., Go H., Akira S., and Chung D.H.. 2011. TLR2-mediated production of IL-27 and chemokines by respiratory epithelial cells promotes bleomycin-induced pulmonary fibrosis in mice. J. Immunol. 187:4007–4017. 10.4049/jimmunol.1101654 [DOI] [PubMed] [Google Scholar]
- Lee, P.P., Fitzpatrick D.R., Beard C., Jessup H.K., Lehar S., Makar K.W., Pérez-Melgosa M., Sweetser M.T., Schlissel M.S., Nguyen S., et al. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 15:763–774. 10.1016/S1074-7613(01)00227-8 [DOI] [PubMed] [Google Scholar]
- Lee, H.M., Fleige A., Forman R., Cho S., Khan A.A., Lin L.L., Nguyen D.T., O’Hara-Hall A., Yin Z., Hunter C.A., et al. 2015. IFNγ signaling endows DCs with the capacity to control type I inflammation during parasitic infection through promoting T-bet+ regulatory T cells. PLoS Pathog. 11:e1004635. 10.1371/journal.ppat.1004635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage, A.C., Buzoni-Gatel D., Bout D.T., and Kasper L.H.. 1998. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J. Immunol. 161:4902–4908. [PubMed] [Google Scholar]
- Liu, J., Guan X., and Ma X.. 2007. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J. Exp. Med. 204:141–152. 10.1084/jem.20061440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod, B.L., Elsaesser H.J., Snell L.M., Dickson R.J., Guo M., Hezaveh K., Xu W., Kothari A., McGaha T.L., Guidos C.J., and Brooks D.G.. 2020. A network of immune and microbial modifications underlies viral persistence in the gastrointestinal tract. J. Exp. Med. 217: e20191473. 10.1084/jem.20191473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison, B.B., Dunbar L., Qiao X.T., Braunstein K., Braunstein E., and Gumucio D.L.. 2002. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277:33275–33283. 10.1074/jbc.M204935200 [DOI] [PubMed] [Google Scholar]
- Mucida, D., Husain M.M., Muroi S., van Wijk F., Shinnakasu R., Naoe Y., Reis B.S., Huang Y., Lambolez F., Docherty M., et al. 2013. Transcriptional reprogramming of mature CD4+ helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat. Immunol. 14:281–289. 10.1038/ni.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, S., Bühler-Jungo M., and Mueller C.. 2000. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J. Immunol. 164:1986–1994. 10.4049/jimmunol.164.4.1986 [DOI] [PubMed] [Google Scholar]
- Pflanz, S., Timans J.C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., et al. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 16:779–790. 10.1016/S1074-7613(02)00324-2 [DOI] [PubMed] [Google Scholar]
- Pirhonen, J., Sirén J., Julkunen I., and Matikainen S.. 2007. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J. Leukoc. Biol. 82:1185–1192. 10.1189/jlb.0307157 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum, S., Paglino J., Eslami-Varzaneh F., Edberg S., and Medzhitov R.. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118:229–241. 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Reis, B.S., Hoytema van Konijnenburg D.P., Grivennikov S.I., and Mucida D.. 2014. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 41:244–256. 10.1016/j.immuni.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer, M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., Ter Horst R., Jansen T., Jacobs L., Bonder M.J., et al. 2016. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell. 167:1125–1136.e8. 10.1016/j.cell.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer, J.S., Silver J.S., Laurence A., Porrett P.M., Harris T.H., Turka L.A., Ernst M., Saris C.J., O’Shea J.J., and Hunter C.A.. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8:1363–1371. 10.1038/ni1537 [DOI] [PubMed] [Google Scholar]
- Sujino, T., London M., Hoytema van Konijnenburg D.P., Rendon T., Buch T., Silva H.M., Lafaille J.J., Reis B.S., and Mucida D.. 2016. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science. 352:1581–1586. 10.1126/science.aaf3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesaki, Y., Setoyama H., Matsumoto S., and Okada Y.. 1993. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 79:32–37. [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H., and Hunter C.A.. 2015. The immunobiology of interleukin-27. Annu. Rev. Immunol. 33:417–443. 10.1146/annurev-immunol-032414-112134 [DOI] [PubMed] [Google Scholar]
- Yoshida, H., Hamano S., Senaldi G., Covey T., Faggioni R., Mu S., Xia M., Wakeham A.C., Nishina H., Potter J., et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 15:569–578. 10.1016/S1074-7613(01)00206-0 [DOI] [PubMed] [Google Scholar]
- Yoshimoto, T., Yoshimoto T., Yasuda K., Mizuguchi J., and Nakanishi K.. 2007. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 179:4415–4423. 10.4049/jimmunol.179.7.4415 [DOI] [PubMed] [Google Scholar]