Abstract
Tyrosinase (TYR) inhibitors are in great demand in the food, cosmetic and medical industrials due to their important roles. Therefore, the discovery of high-quality TYR inhibitors is always pursued. Natural products as one of the most important sources of bioactive compounds discovery have been increasingly used for TYR inhibitors screening. However, due to their complex compositions, it is still a great challenge to rapid screening and identification of biologically active components from them. In recent years, with the help of separation technologies and the affinity and intrinsic activity of target enzymes, two advanced approaches including affinity screening and inhibition profiling showed great promises for a successful screening of bioactive compounds from natural sources. This review summarises the recent progress of separation-based methods for TYR inhibitors screening, with an emphasis on the principle, application, advantage, and drawback of each method along with perspectives in the future development of these screening techniques and screened hit compounds.
Keywords: Tyrosinase inhibitors, natural products, rapid screening, separation methods
1. Introduction
Tyrosinase (TYR), also known as polyphenol oxidase, is a copper-containing metal enzyme that is widely found in different organisms and plays an important role in melanogenesis and enzymatic browning1,2. Therefore, its inhibitors are extensively used in cosmetics and medicinal industries as depigmentation agents and also in food and agriculture industries as antibrowning compounds3. At present, there have been countless studies on TYR inhibitors derived from natural or synthetic sources, most of which were only tested using TYR isolated from the mushroom due to its being readily commercially available. However, the TYRs derived from different sources such as mushroom tyrosinase (mTYR) and recombinant human tyrosinase (hTYR) were shown to have different specificities, causes different reactions of the screened substances on them4,5. Some TYR inhibitors that have been screened based on the mTYR have limited clinical efficacy, which encourages researchers to commit themselves to look for higher quality TYR inhibitors6.
Natural products have been recognised as one of the most excellent pools of lead compounds discovery because of their untapped chemical diversity and biological relevance7,8. However, the components of natural products are very complex, making the rapid discovery of the specific bioactive compounds from them a challenging task. Traditional screening strategies of extraction-isolation-bioassay and bioassay-guided fractionation are time-consuming and labour-intensive for active ingredients discovery9,10. Fortunately, with the continuous development of technology in recent years, some high-throughput screening methods such as ultrafiltration affinity screening, immobilised enzyme affinity, and HPTLC-autography have been established for Tyr inhibitors screening from natural sources (Table 1). By combining these modern separation techniques with the affinity or intrinsic activity of enzymes, affinity screening and inhibition profiling have become the two recent screening strategies. However, most of the current studies only focussed on the introduction of a certain method, the comparisons and applications of these strategies for TYR inhibitors screening have not been summarised yet comprehensively. Considering screening tools may cause confusion, with difficulty in selecting suitable ones due to their considerable diversity, merits and limitations, therefore, this article reviews the screening methods of TYR inhibitors developed in recent years via separation strategies, mainly introducing their principles, applications, advantages and drawbacks as well as the development trends in future.
Table 1.
Applications of modern separation strategies for rapid screening of natural TYR inhibitors.
| Methods | Source | Source of TYR | Active ingredients | References |
|---|---|---|---|---|
| UF-HPLC/MS | Pueraria lobata Ohwi | Unknown | 3′-Hydroxypuerarin1#, Puerarin2, Puerarin-6''-O-xyloside33, Daidzin34, Genistin35, 6''-O-acetyldaidzin36, Daidzein37 | 11 |
| Semen Oroxyli | Mushroom | Oroxin B3, Kaempferide-3-O-β-D-gentiobioside4, Chrysin-7-O-β-D-gentiobioside5, Oroxin A6, Chrysin-7-O-β- D-glucuronid7, Baicalein8, Chrysin9 | 12 | |
| Xanthii fructus | Mushroom | Protocatechuic acqid82, 3,5-Di-O-caffeoylquinic acid57, 1,5-Di-O-caffeoyluinic acid58, Chlorogenic acid59 | 13 | |
| Mulberry leaves | Mushroom | Quercetin-3-O-(6-O-malonyl)-β-D glucopyranoside14, Neochlorogenic acid, Kaempferol-3-O-(6-O-malonyl)-β-D-glucopyranoside15, Chlorogenic acid59, Cryptochlorogenic acid60, 3,4-Dicaffeorylquinic acid61, 3,5-Dicaffeorylquinic acid62, 4,5-Dicaffeorylquinic acid63, Rutin16, Isoquercitrin17, Astragalin18, Kaempferol-3-O-α-L-rhamnopyranosyl-(1-6)-β-D-glucopyranoside19 | 14 | |
| Puerariae lobatae radix | Unknown | Puerarin2, Mirificin38, Daidzin34, Genistinc39 | 15 | |
| UF-HSCCC-HPLC |
Otholobium pubescens (Poir.) J.W. Grimes |
Mushroom | Daidzin34, Isoorientin10, Vitexin11, Isovitexin12, Isoorientin 3′-methyl ether13, Daidzein37, Genistein, 3-(5-Hydroxybenzofuran-6-yl) propanoic acid94 | 16 |
| Gastrodia elata | Mushroom | 12-15 Heneicosadienoic acid, Octadecanoic acid, 2-Hydroxylpropyl esters119, 3-(4-tert-butylphenyl)-2-Methylpropanal120, 4,4'-Dihydroxybenzylsulfid121, Octadecanoic acid, 2-Hydroxy-1-(hydroxymethyl)ethyl ester122, 9-Octadecenamide, (trans-)95, 9-Octadecenamide, (cis-)96, 4,4'-Dihydroxydiphenyl ether64, 4-(1-(4-hydroxyphenyl)phenyl)Phenol65, 2,3-Dihydroxypropyl ester117, 4,4'-Dimethoxydiphenylmethane118, 4,4’-Methylenediphenol66, 2,4-Bis(4-hydroxybenzyl) phenol67,4-Hydroxybenzyl methyl ether68 |
17 | |
| Mango leaves | Mushroom | Gallic acid78, Iriflophenone 3-C-glucoside97, Mangiferin46, Protocatechuic acid82, Iriflophenone 3-C-(2'-O-galloyl)-glucoside98, 6'-O-Galloyl-mangiferin47, Maclurin 3-C-(2'-O- p-hydroxybenzoyl)-glucoside99, Iriflophenone 3-C-(2',6'- di-O-galloyl)-glucoside100, Hyperoside20, Isoquercitrin17, Ethyl gallate79, Iriflophenone 3-C-(2'-O- p-hydroxybenzoyl)-glucoside101, Quercetin-3-O-xyloside21, 3-O-Galloyl shikimic acid75, 3-O-Galloyl quinic acid69, Maclurin 3-C-(2'-O-galloyl)-glucoside102, 3,5-Di-O-galloyl quinic acid70, 5-O-Digalloyl quinic acid71, Digallic acid80, Isomangiferin48, 1,4,6-Tri-O-galloyl glucoside72, Maclurin 3-C-(2',3'- di-O-galloyl)-glucoside, 1,3-Digalleoyl acetone113, 1,3,4,6-Tetra-O-galloyl glucoside73, 6'-O-(p-Hydroxybenzoyl) mangiferin49, Epicatechin gallate76, 1,2,3,4,6-Penta-O-galloyl glucoside74, Iriflophenone 3-C-(2',3',6'- tri-O-galloyl)-glucoside104, Luteolin-7-O-glucoside22, Quercetin-3-O-arapyranoside23, Quercetin-3-O-arafuranoside24, Kaempferol-3-O-glucoside25, Quercetin-3-O-rhamnoside26, Ethyl 2,4-dihydroxy-3-galloyl Oxybenzoate, Ethyl digallate81, 7-O-Methyl quercetin-3- O-rhamnoside27 | 18 | |
| Glycyrrhiza uralensis root | Mushroom | Liquiritin apioside50, Neolicuroside83, Liquiritigenin51, Liquorice saponin G2105, Chrysoeriol52, Dihydrodaidzein53, Formononetin55, Glycyrrhisoflavanone54, Glycyrrhizic acid, Licoarylcoumarin40, Pratensein56 | 19 | |
| IMF-HPLC-MS | Glycyrrhiza uralensis root | Mushroom | Liquiritin apioside50, Neolicuroside83, Liquiritigenin51, Liquorice saponin G2105, Chrysoeriol52, Dihydrodaidzein53, Formononetin55, Glycyrrhisoflavanone54, Glycyrrhizic acid, Licoarylcoumarin40, Pratensein56 | 19 |
| MSPE-HPLC-MS | San-Bai decoction | Mushroom | Gallic acid78, Albiforin106, Paeoniforin107, Liquiritin apioside50, Liquiritin84, Galloylpaeoniflorin, Ononin85, Isoliquiritigenin, Glycyrrhizic acid,Oxypaeoniflora, Benzoylpaeoniflorin, Benzoyloxypaeoniflorin, Mudanpioside C, Paeonolide, Apiopaeonoside | 20 |
| TYR-AHF-HPLC-MS | Pueraria lobata | Mushroom | Puerarin-4′-O-glucoside41, 3′-Hydroxy puerarin42, Puerarin2, Puerarin-6′’-O-xyloside33, 3′-Methoxy puerarin42, Puerarin apioside43,Daidzein37 | 21 |
| Off-line 2 D HPLC-MS/MS | Pueraria lobata | Mushroom | Puerarin2, Puerarin-6′’-O-xyloside33, Mirificin38 | 22 |
| EMMA-CE | 9 kinds of TCMS | Mushroom | –* | 23 |
| 21 kinds of TCMS | Mushroom | – | 24 | |
| IMER-CE | Psoralea corylifolia | Mushroom | – | 25 |
| Folium ginkgo | Mushroom | – | ||
| 19 kinds of natural extracts | Mushroom | – | 26 | |
| (HP)TLC-autography | Glycyrrhiza glabra | Mushroom | Glabridin44 | 27 |
| Ganoderma formosanum | Mushroom | – | 28 | |
| sandalwood oil | Mushroom | α-Santalol112 | 29 | |
| Rhodiola sacra | Mushroom | Naringenin45, 1-O-β-D-glucopyranosyl-4-allylbenzene | 30 | |
| Calamagrostis viridiflavescens (Poir.) | Mushroom | Kojic acid114 | 31 | |
| Cinnamomum cassia essential oil | Mushroom | Cinnamaldehyde115 | 32 | |
| HPLC-MS-EIA | Lavender flowers | Mushroom | 5-Hydroxymethyl-furfural116 | 33 |
| SER | Rheum officinale | Unknown | Emodin111, Veraphenol-4′-O-β-D- glycoside88, 1, 2, 6-Trihydroxy-5-methoxy-7-(3-methylbut-2-enyl) xanthone, 7-Hydroxy-2-(2-hydroxy)propyl-5methyl-benzopyran-γ-one, 2-O-cinnamyl-galloyl glucose89, ω-Hydroxy aloe-emodin, l, 6-Digalloyl-2-cinnamon acyl glucose90, 1, 4, 5, 6-Tetrahydroxy-7, 8-bis(3-methylbut-2-enyl) xanthone30, Emodin methyl ether109 |
34 |
| Salvia miltiorrhiza–Carthamus tinctorius | Agaricus bisporus | Protocatechuic aldehyde91, Hydroxysafor yellow A92, Tanshinone IIA108 | 35 | |
| Morus alba root | Mushroom | Mulberrofuran G93, Kuwanon G31, Kuwanon H32 | 36 |
*: Without active compounds; #: the compound No. in Table 1S.
2. Screening methods based on target enzyme affinity
Modern pharmacological studies show that the first step of most drugs to act is to combine with their targets, which has become the theoretical cornerstone of affinity screening. Currently, affinity screening methods such as ultrafiltration (UF) and immobilisation combined with chromatographic techniques have been widely developed for inhibitors discovery, which has been recognised as one of the most convenient and effective methods for screening active ingredients from complex mixtures. Compared with traditional screening methods, these newly developed methods have higher selectivity and specificity, they not only save time and effort but also allow a small sample37.
2.1. Ultrafiltration affinity screening
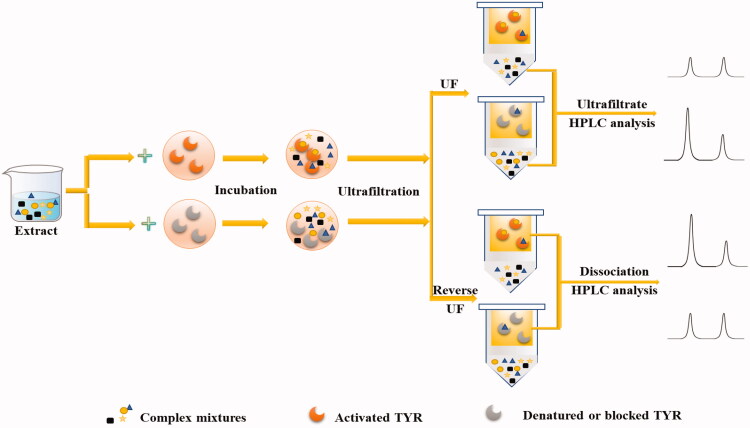
As an affinity selection technology, UF can be used to screen active ingredients from complex mixtures quickly based on the molecular sieve principle of UF membranes38,39. The screening process is mainly to incubate the complex mixtures to be screened with the target enzyme in a mixing chamber containing a UF membrane. While the centrifugal ultrafiltration is performed, the conjugates are trapped and retained on the membrane and the unbound small molecules are eluted, the separation is completed in a flowing state40. The ultrafiltrate was collected and analysed by HPLC-MS. The fingerprint of the extract obtained initially is compared with that obtained from ultrafiltrate. A decrease in the peak areas on the fingerprint of ultrafiltrate indicates that the active compounds specially bind to the TYR. Or use the reverse UF method, that is, wash the retentate repeatedly to clean the remaining unbound small molecule compounds, and then release the active components from the target enzyme by changing the pH of the retentate solution or adding appropriate organic reagents, and then use mass spectrometry (MS) or other methods to identify active compounds. The procedures of these two screening strategies based on UF affinity were shown in Figure 1. This analytical process can maintain the natural conformation of the protein targets, and accurately reflect the physiological conditions for the interaction between the protein targets and small-molecule drugs.
Figure 1.
The procedures of two screening strategies are based on UF affinity.
At present, the UF-LC technology has been extensively used in the screening of TYR inhibitors. For example, the combination of UF-LC and molecular docking has been applied by Zhang et al. 11 and Yin et al. 12 to screen TYR inhibitors in Pueraria lobata Ohwi and Semen Oroxyli, respectively. 7 TYR inhibitors were screened in each experiment at last. Wang et al. 13 developed an Ultrafiltration-high performance liquid chromatography-diode array detector (UF-HPLC-DAD) method to screen and identify TYR inhibitors, and the false negatives were eliminated by reducing the background noise while false-positive results by using blocked TYR instead of enzymes as controls. Before the experiment, in order to obtain the best blocker, the competitive experiments were performed using variously known ligands. Four competitive TYR inhibitors were screened out successfully. Through the selection of optimal conditions including binding conditions, TYR concentration, and incubation time, Yang et al. 14 screened and identify 12 active compounds from mulberry leaves using the established UF-HPLC-DAD-MS method. UF-HPLC-QTOF-MS/MS strategy was applied by Liu et al. 15 to screen TYR inhibitors from Puerariae lobatae Radix, kojic acid was used to blocking the TYR-site to eliminate false positives. The activity results predicted by molecular docking are consistent with that obtained from in vitro activity. High-speed countercurrent chromatography (HSCCC), as a new type of high-efficiency chromatography technology, has been introduced to screen TYR inhibitors now. For example, through the combination of UF, HSCCC, and Pre-HPLC, Zuo et al. 16 screened 10 TYR inhibitors from Otholobium pubescens (Poir.) JW Grimes successfully. And the structure of 8 components was identified by nuclear magnetic resonance (NMR) or other methods. In order to verify the effectiveness of the established method, the in vitro enzyme inhibition activity and kinetics of the isolated compounds were determined finally. HSCCC was also employed to enrich the extract of Gastrodia elata by Wang et al. 17, UF-HPLC was used to screen TYR inhibitors subsequently, 17 ligands with high affinity were identified and separated, 14 of them were characterised by NMR and other methods successfully. In addition to the UF strategy, the efficient reverse UF is also widely used in the screening of TYR inhibitors. Shi et al. 18 proposed a new method that combined reverse UF-HPLC-QTOF-MS/MS with key ion filtering (KIF) strategies to screen TYR inhibitors in mango leaves. First, reverse UF-HPLC-QTOF-MS/MS was used to screen the TYR inhibitors in mango leaves, and then a key ion database was built through the analysis of the screened compounds, the KIF strategy was used to further explore potential TYR inhibitors in mango leaves. Finally, 36 TYR inhibitors were obtained successfully. Similarly, Liu et al. 19 developed a reverse UF-HPLC method and successfully applied it to the screening of TYR inhibitors in Glycyrrhiza uralensis root.
All these previous studies demonstrate that UF coupled with LC-MS can screen and identify active compounds quickly and accurately from a complex mixture. Compared with traditional methods, this strategy displays more advantages such as easy operation, time-saving, and low labour intensity41. However, there are still some drawbacks to this method. One of the most significant limitations is the false positive phenomenon resulting from the non-specific interactions between the target and the candidate screening substances. To reduce the false-positive results, repeated manual washing was commonly required to remove small molecules that are not bound to the active sites of enzyme42. Some components with weak interactions can also be removed. Therefore, this method is inappropriate for investigating the dissociation constant between bioactive components and targets. Alternatively, to eliminate this effect, inactive enzymes or competitive ligands can be used as parallel control samples, but the physical and chemical properties of inactive enzymes may have changed, making it difficult to compare with active enzymes. In addition, the non-specific interaction between components and UF membrane should not be ignored, especially when screening the ones with high lipophilicity. In the future, the competitive test of positive drugs and activity verification of bound ligands will be an option for reducing false results.
2.2. Immobilised target affinity screening
In recent years, significant progress was observed in the application of immobilised enzymes for the discovery of active compounds from complex mixtures43,44. The principle is to immobilise biologically active substances such as enzymes or cells on a specific carrier by physical, chemical, or affinity connection methods and incubate the samples with the carrier under suitable conditions so that the active ingredient can be combined with the biologically active substance, and then the bound ligand is dissociated by a certain method, at last, HPLC-MS/MS method is used to characterise the ligands to the identification of the active compounds screened out.
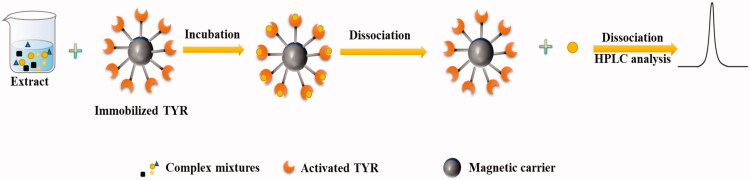
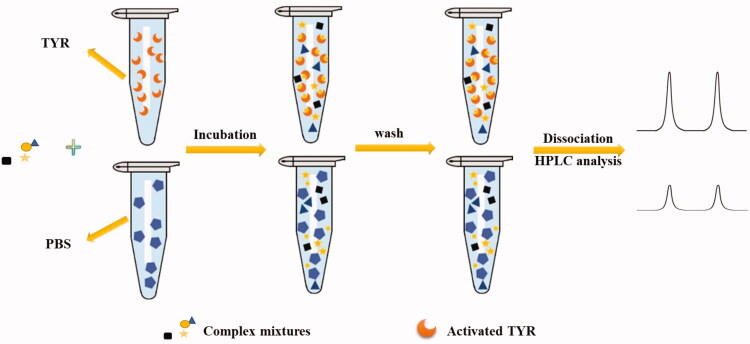
For example, Liu et al. 19 performed the TYR inhibitors ligand fishing from the roots of Glycyrrhiza uralensis based on TYR immobilised magnetic fishing (IMF) coupled with HPLC-DAD-MS/MS (Figure 2). The activity of immobilised TYR was obviously improved, retaining 76.3% after ten consecutive cycles, and over 95% when stored at 4 °C for about two months. Eleven TYR binders were successfully screened and characterised from G. uralensis root. Tao et al. 20 developed a method to analyse and identify TYR inhibitors from San-Bai decoction based on combined magnetic solid-phase extraction (MSPE) combined with HPLC-Q-TOF-MS/MS analysis. Before the experiment, paeoniflorin was used as a model compound to optimise the experimental conditions by response surface design, a total of fifteen TYR binders were identified and their inhibitions on TYR were finally verified by TYR inhibitory assay. Zhao et al. 21 established an adsorption hollow fibre (AHF) immobilised TYR method to screen TYR inhibitors (Figure 3), the reliability of which was firstly verified by kojic acid and ranitidine used as the positive and negative control, respectively. And, repeatability was carried out to confirm the accuracy of the method, seven potential TYR inhibitors were fished out from P. lobata successfully and their chemical structures were tentatively identified by LC-MS/MS, four of them were tested in vitro to verify their inhibitory activities, and molecular docking was performed at last.
Figure 2.
Schematic diagram of TYR inhibitor screening procedure based on TYR immobilised magnetic fishing.
Figure 3.
Schematic diagram of TYR inhibitor screening procedure based on TYR-AHF.
Immobilised strategy can screen and identify TYR inhibitors from complex mixtures rapidly and its combination with LC-MS makes it possible to elaborate the structure of active compounds. Compared with free enzymes UF-HPLC assay, immobilised enzymes not only enhance the stability and durability but also prolong the survival time of the enzymes. Furthermore, the main advantage of immobilisation technology based on magnetic materials is the simple and rapid separation of materials and a mixed solution of samples, eliminating the tedious operation steps such as repeated centrifugation, which can achieve rapid separation and protect biomaterials. While, due to its advantages of simple operation, low cost and reusability, HF has gradually become a common immobilisation material. Despite these successes, limitations persist. As one kind of affinity screening method, immobilisation technology still has the problem of false positives, so how to eliminate non-specific binding is also a major challenge in the screening process. In addition, in the vast majority of cases, separation and analysis are independently done in off-line mode, with that screening is performed first and then the obtained potential active compounds are analysed by chromatography or MS. However, compared with online models, the offline mode could consume more sample and time during sample transfer and showed a much lower efficiency of screening and analysis. Therefore, in the future, efforts need to be made to develop online methods that could couple separation and analysis more efficiently.
2.3. Offline two-dimensional LC/MS affinity screening
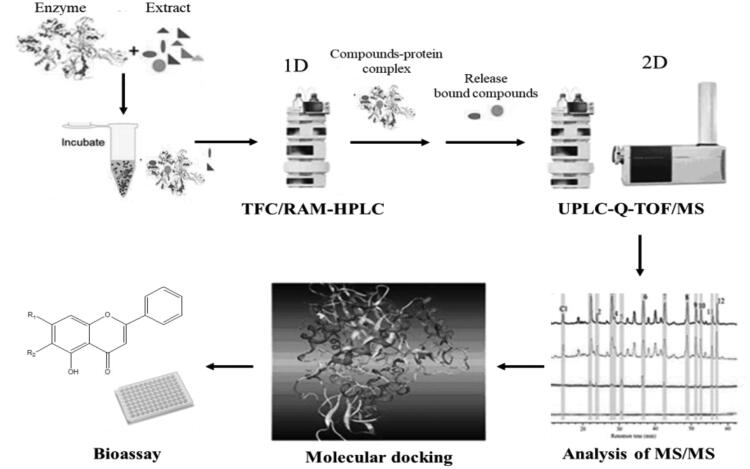
The offline two-dimensional LC/MS technology (2 D LC/MS) includes two independent procedures: the separation of enzyme-ligand conjugates and unbound compounds and the dissociation, analysis, and identification of the bound ligands. Figure 4 showed the scheme of the solution-based free enzyme ligand screening by off 2 D-LC/MS. Firstly, the extract was incubated with an enzyme solution, and then separation of enzyme-ligand conjugates and unbound small molecules was performed by using turbo-flow chromatography (TFC) or restricted-access material (RAM). Based on the different sizes, small molecules can be retained, while the target-ligand conjugates can be quickly eluted. Thereby the rapid separation of conjugates and unbound small molecules was achieved, which is the first-dimensional chromatography. And then use a certain method to dissociate the conjugate and UPLC-MS/MS was used to analyse and identify the active compounds. This is second-dimensional chromatography.
Figure 4.
Schematic representation of the offline two-dimensional LC/MS for TYR inhibitors affinity screening.
This strategy was successfully carried out to discover TYR inhibitors from P. lobata by combing the TFC and LC-MS/MS analysis22. The ultra-fast separation of enzyme-ligand complex and unbound small molecules was achieved in less than 1 min in 1st dimension. Subsequently, the ligands were dissociated from the enzyme-ligand complex with methanol precipitation, and then were injected into the 2ed dimensional LC-MS/MS for ligands characterisation. As a result, three active ingredients were screened successfully and the inhibitory activities of selected TYR inhibitors were verified in vitro, which were consistent with those obtained by UF11 and HF immobilised enzyme affinity screening21, proving that this method was effective for bioactive compounds screening from a complex mixture.
Compared with the immobilised target LC/MS method, this method is a simple and convenient method with good reproducibility. Because enzyme solution is directly used instead of immobilised enzyme to incubate with extract, there is no need to choose a suitable carrier and prepare the immobilised enzyme, and the activity and stability of the enzyme after immobilisation do not need to be considered. While compared with the UF affinity method, the complicated repeated cleaning process can be avoided. However, in the process of achieving the separation of conjugates and small molecules, it is necessary to select a suitable chromatographic column which should be able to retain the unbound components, avoiding the false-positive results caused by its co-elution with the conjugates. At present, this newly developed method is not widely used. However, in terms of its advantages of fast, simple and automatic separation of enzyme-ligand complex and small molecules, the off-line 2 D LC-MS technology will have a good promise for screening bioactive compounds from complex mixtures.
3. Screening method based on the intrinsic activity of the target enzyme
Enzymes have biological activity and can catalyse substrates to produce products, so the activity of enzymes can be evaluated based on the changes in the amount of substrate or product. Combining this characteristic of enzymes with chromatographic technology can screen active ingredients in complex mixtures quickly and accurately.
3.1. Online CE-based methods
The CE-based screening methods combine the enzyme reaction and the separation of capillary electrophoresis (CE) for evaluating the bioactivity of drug candidates. Now the methods commonly used for screening mainly include electrophoretically mediated microanalysis (EMMA) and immobilised enzyme microreactor (IMER) 45. The EMMA screening method is realised by using the different mobility of enzyme and substrate in the capillary, which process is to add enzyme and substrate in the capillary by sequence, then apply voltage. Due to the difference in migration rate, the enzyme can be mixed with the substrate to react, and high voltage is applied to separate them at last, the enzyme activity is determined according to the change of peak area of the product. This EMMA method was first proposed by Bao et al. 46 and is now widely used in the screening of various enzyme inhibitors, more information about EMMA can be found in several previous reviews47–49. For TYR inhibitors screening, Tang et al. 23 developed an EMMA method by integrating the techniques of sandwich mode injection, partial filling, and rapid polarity switching, and by using it, on-line enzyme reaction and the separation of substrate and product were well carried out after optimising the conditions of background electrolyte, mixing voltage, and the incubation time. The inhibitors were directly identified from the reduced peak area of the product compared to those obtained without any inhibitor. As a result, one of nine standard natural compounds, chlorogenic acid, showed inhibitory activity on TYR. Zhang et al. 24 also established an EMMA method using longitudinal diffusion as the mixing technique, the inhibitory activities of 21 TCMs on TYR were determined.
On the other hand, the principle of IMER method is basically the same as EMMA method except that the enzyme is immobilised on the capillary by means of adsorption, bonding, embedding, etc. When screening TYR inhibitors, the complex components are mixed with the substrate solution, and the inhibitory effect of the complex components on the enzyme is detected by comparing the peak area of the substrate without samples. Cheng et al. 25 immobilised TYR at the outlet of the capillary using glutaraldehyde as a cross-linking agent to form IMER of TYR. By adopting a short-end injection procedure, the product and substrate were effectively separated within 2 min. The immobilised TYR could remain 80% active for 30 days at 4 °C. The TYR inhibition rate of 15 standard natural compounds was measured, and the relationship between the screened TYR inhibitors and TYR was studied through molecular docking. Jiang et al. 26 developed an IMRE based on a layer-by-layer assembly for TYR inhibitor screening. TYR was immobilised on the surface of fused silica capillary via ionic binding technique with cationic polyelectrolyte hexadimethrine bromide (HDB). Then, HDB solution was injected again into the capillary to cover the immobilised enzyme by forming HDB–TRS–HDB sandwich-like structure. Using L-Tyrosine as a substrate, the product L-dopa was used for the screening of enzyme inhibitors based on the reduction of its peak area, and finally, the TYR inhibition rate of 19 kinds of traditional Chinese medicine extracts was determined.
As an efficient separation approach, CE possesses many advantages such as less sample consumption, fast analysis speed, high efficiency, easy to realise automation, miniaturisation, etc.50, which can be applied to the screening of multiple enzyme inhibitors due to its multiple separation modes and many types of detectors. The online CE method allows the biochemical assay integrated into the separation process of CE so that avoids the possible pollution caused by the outside world. Among them, the EMMA does not need to modify capillary. But it needs to sample enzyme in each assay, which increases experiment cost especially when enzymes are expensive. The complicated sampling process and mixing in the capillary also result in more measurement errors. As compared with EMMA, the enzymatic reaction inside an IMER avoids these disadvantages. In addition, the IMER not only improves the stability of the enzyme but also can be reused, reducing the consumption of the enzyme and the cost. However, it needs to take a lot of effort to explore how to fix the enzyme on the capillary by a suitable method which not only will not affect the activity of the enzyme but will also increase the frequency of use of IMER. Furthermore, neither EMMA nor IMER, the CE-based methods are only used to perform a preliminary evaluation of enzyme inhibitory activity of screened substances by determining the change of substrate or product, direct screening of single active TYR inhibitors from complex natural products has not been reported yet. In the near future, CE-based enzyme inhibitor screening requires more and more new materials for enzyme immobilisation, new detectors for enhancing the resolution of the products and substrates, ultra-high-throughput screening, more speed analytical techniques, and computer simulation.
3.2. (High-performance) thin-layer chromatography ((HP)TLC)-autography
Among the existing screening technology of active compounds, (HP)TLC-autography is particularly useful51. The target reaction solution was employed as the colour developer, and the active compounds screening was depend on the colour change of the spot. It can not only separate the mixed components but also carry out biological activity detection. The study using the method of TLC-autography to screen enzyme inhibitors can be traced back to 1964, Menn et al. 52 used plasma instead of acetylcholinesterase to screen enzyme inhibitors. Since then, many researchers have carried out in-depth research on this method and the thin-layer chromatography (TLC) bio-autography assay has become very popular in screening for active compounds that may affect enzymes. The main steps are as follows: firstly, drop the mixed components on the thin-layer plate, separate the components with a suitable spreading agent, and then spray appropriate concentration of enzyme solutions and substrates on the thin-layer plate, at last screen out the active ingredients according to the different colours of the spots. This method can not only judge whether the ingredients contain enzyme inhibitors, but also separate the complex mixtures and locate the biologically active ingredients. (HP)TLC-autography is an effective method to screen active compounds in complex mixtures53.
Wangthong et al. 27 developed a TLC post-development technique for the screening of active ingredients of TYR inhibitors in liquorice. Using L-Tyrosine as a substrate, the positive results were could be visualised directly as white spot(s) against a brownish-purple background. The developed method was validated by detecting the TYR inhibitory activity of some known inhibitors including kojic acid, glabridin, arbutin, and was successfully used to analysis the liquorice extract. Similarly, this method was applied to screen the TYR inhibitors from Ganoderma formosanum extracts28 and Sandalwood oil29, respectively. To improve the sensitivity of detection and reduce enzyme consumption, an improved TLC-autographic assay for the discovery of TYR inhibitors was developed with L-DOPA as substrate which has better water solubility than L-Tyrosine. Two TYR inhibitory compounds were isolated from Rhodiola sacra guided by this TLC bioautographic assay30. Commonly, by using the (HP)TLC-autography, TYR inhibitors can be clearly identified as white spots against a dark background in white light remission as well as in white light transmitted through the plate. However, false-positive phenomenon was reported by Taibon et al. 54, that some lipophilic substances in the investigated extracts appeared as white spots in white light remission but were black in white light transmission due to poor wettability of the corresponding spots. To eliminate false-positive results, Triton X-100 was added to the substrate solution and the plate was dried after incubation with a molecular sieve. A variety of plant extracts were screened, and a TYR inhibitor from M. alba was screened and identified by MS successfully at last. When performing the (HP)TLC-autographic assay, the enzymatic activity is very prone to decrease in the process of drying out of the enzyme solution on the silica surface. To overcome this problem, enzyme immobilisation by gel entrapment was verified to be one ideal approach to increase enzyme stability in autographic assays55. García et al. 31,32 developed an autographic assay to detect TYR inhibitors using gel entrapped enzyme, a homogeneous agar gel layer was formed on a normal/reverse phase TLC surface. The applicability of this method was tested by detecting different concentrations of kojic acid and natural product extracts spiked with it.
The (HP)TLC-autographic method combines chromatographic separation and biological activity determination, which can be used to screen TYR inhibitors from complex mixtures simply, rapidly and efficiently, especially suitable for screening comparison among multiple samples simultaneously on a thin-layer plate. The choice of stationary phase and mobile phase is more flexible so it is easy to operate. Besides, this method can save costs as less sample volume and enzymes are required. The solvent can be evaporated quickly so that the enzyme activity is not affected by the solvent, and it is the only chromatographic method that enables the presentation of the results as pictures. However, this method still has some drawbacks, such as poor separation effect and low stability of enzyme. (HP)TLC has weak separation ability, in most cases, the components might be overlapped, so it is difficult to determine whether the biologically active is from a single component or a mixture of different compounds. Therefore, (HP)TLC-autographic method is mostly used as a tool for preliminary screening of active drug candidates. On the other hand, some enzymes have poor stability, drying the enzyme solution on the surface of the thin-layer plate will reduce the enzyme activity and reduce the contrast of the measurement56. How to fix the enzyme solution on the TLC plate without affecting the activity of the enzyme poses a challenge to the (HP)TLC-autographic method. Anyway, as a convenient screening method, the (HP)TLC-autographic is undoubtedly a good alternative for multi-batch and multi-activity comparison57.
As one kind of effect-directed analysis (EDA), TLC bio-autography has the most extensive applications in recent years and has been reviewed comprehensively58–61. In the future, there will be two possible development directions in technology. On the one hand, to overcome the poor separation efficiency of TLC, 2 D HPTLC can be adopted to get a higher peak capacity. On the other hand, for a long time, the lack of hyphenation between TLC and MS always hindered the efficiency of this method. With the commercialisation of some interfaces and the development of in situ detection, the “online” extraction of analytes from the plate prior to electrospray ionisation ESI-MS analysis can be achieved, paving the way for rapid dereplication of bioactive compounds from natural products.
3.3. HPLC-MS coupled with post-column enzyme inhibition assay
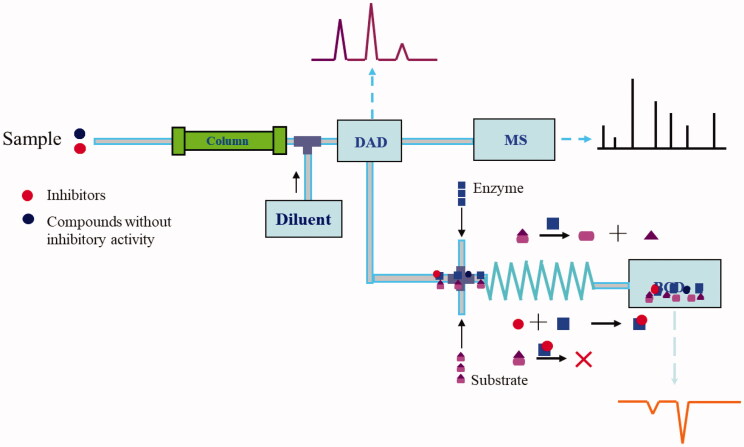
The HPLC-MS coupled with post-column enzyme inhibition assay (EIA) integrates chromatographic separation, compound identification and activity evaluation into one system, the biological activity information and chemical information of the compounds in the complex mixture can be obtained simultaneously through one injection. By using this approach, the complex mixtures are first separated by HPLC, then the compounds are eluted in sequence according to the polarity. The eluent from HPLC system is divided into two streams, one of which enters the MS directly to get a fingerprint, while another strand enters the reaction coil where enzyme and substrate are continuously pumped into. The inhibition profiling of the compounds is recorded by biochemical detection (BCD). If the ingredient has inhibitory activity on an enzyme, the amount of product will decrease, correspondingly, a negative peak will appear in the inhibition profiling (Figure 5). According to the fingerprints and post-column biochemical detection information, active ingredients can be screened out quickly, and the active ingredients can be qualitatively determined by MS62.
Figure 5.
Schematic diagram of enzyme inhibitor screening procedure based on HPLC-MS coupled with post-column bioassay.
Luo et al. 33 established a new method for screening natural TYR inhibitors based on HPLC combined with the post-column EIA. In order to screen active compounds from complex mixtures, the conditions of the enzyme reaction including the amount of enzyme, the enzyme tolerance to organic solvents and reaction time were systematically optimised. The system was verified by the known potent TYR inhibitors, kojic acid, arbutin and hydroquinone, to determine the reliability of the developed method. Finally, the established method was successfully applied to screen bioactive ingredients in lavender flowers. The availability of three TYR inhibitors proves the effectiveness of this method for screening active ingredients in complex mixtures.
As an online method, this strategy is regarded as a more convenient and accurate manner in the screening of active components. Since the separation and analysis can be performed at the same time, the method can provide more intuitive results for the screening results, and real-time monitoring of bioactive information was realised. The method is simple in operation, fast in screening, and does not require purification and separation before activity detection. Compared with the offline experimental assays, this online strategy significantly saves analysis time, improves the accuracy of screening, and reduces the false positive rate63,64. Despite these extraordinary advantages, drawbacks persist. First, as a non-universal screening technique, each enzyme needs to find a suitable substrate for the reaction, facilitating the detection of inhibition profiling with high specificity, sensitivity, and stability and the concentration of the enzyme and the flow rate of the system need to be optimised. Second, the target enzyme needs to be continuously pumped into the reaction coil, which is costly62. Last but not the least, in order to avoid peak diffusion, the enzymatic reaction time should be strictly controlled, usually less than 2 min, which is prone to result from the false-negative results, cause some components with low abundant cannot show their inhibitory activity in such a short time. How to improve the applicability of this method to various enzymes is worthy of in-depth study.
In view of the above-mentioned limitations of HPLC-MS-EIA, there are some developed trends for it: (1) In order to minimalize the consumption of expensive enzymes/substrates, miniaturisation of online screening system is an alternative opinion, which can be carried out by the combination of the micro-scale HPLC elute and high sensitive detectors; (2) To avoid the strict time limits of the online post-column enzymatic reaction, an at-line nanofractionation analytics was proposed by Kool and co-workers65–67. In this approach, the post-column bioassay is a time-course functional assay based on a high-density well-plate, reducing the false-negative results due to the un-sufficient reaction time. (3) Natural products contain many compounds with a wide range of polarity, integration of different separation modes such as hydrophilic interaction chromatography (HILIC), RPLC, and GC with the at-line nanofractionation would be a direction for improving the identification quality of bioactive compounds. Compared to online assays generally requiring considerable method development and intricate implementation, the HPLC-based inhibition profiling in at-line or off-line mode are more versatile, with higher potential for wide application in drug discovery.
3.4. Screening method based on spectrum-effect relationship
The screening method based on spectrum-effect relationship (SER) is to link the biological activity of the sample with its chemical fingerprint68,69. The main step is to analyse the enzyme activity and fingerprints of many samples and establish a prediction model between the enzyme activity and the fingerprint through some data processing techniques such as partial least square (PLS) regression model or canonical correlation analysis. The enzyme activity of the compounds is predicted by the model, and then the selected active compounds are identified by HPLC-MS. Finally, the in vitro activity of the obtained active compound was verified. Exploring the SER may clarify overall therapeutic effects and the relationship between active constituents, which is widely applied in research into the basis for substance effectiveness70.
The screening of TYR inhibitors is more purposeful, is simple and reliable, and saves time and manpower. Liu et al. 34 established a screening method for TYR inhibitors based on the SER. The ethyl acetate extraction part of rhubarb was separated by HPLC, and the activity of the eluate collected at different time periods was measured, and the most active part was selected for UPLC-QTOF (quadrupole time of flight mass spectrometer) analysis to determine the composition. The analysed components were molecularly docked to predict their TYR inhibitory activity, and the predicted results were verified by in vitro activity determination. The results showed that the in vitro verification and prediction were completely consistent. This method can effectively screen TYR inhibitors. Wang et al. 35 employed the spectral effect to screen TYR inhibitors from Salvia miltiorrhiza-safflowe. c through canonical correlation analysis of the in vitro TYR inhibition rate and HPLC fingerprints of Danshen-Safflower mixed in different proportions to predict the chromatographic peaks that have a greater contribution to the inhibition of TYR, and then use HPLC-MS/MS to analyse and identify the compounds. A total of 13 active compounds were found, and in vitro activity measurements were performed on 5 of them to verify the predicted results. Finally, three TYR inhibitors were screened. Kang et al. 36 prepared 42 samples from 6 batches of root bark under 7 different extraction conditions for analysis, established a PLS regression model between fingerprint chromatography and biological activity, and predicted the active ingredients, screened three TYR inhibitors successfully.
This method shows some advantages such as reliability, time-saving capacity, and simple operation. Different from TLC- and HPLC-based EIA methods, the SER method can screen out the active ingredients with a lower content in TCM successfully. However, there are still many problems, such as the poor stability and repeatability of fingerprints of the complex mixture; Each data analysis method has its own advantages and disadvantages, which method needs to be further standardised; The design of efficacy experiment is not comprehensive enough to reflect the process of active substances entering the body. Being an interdisciplinary and cutting-edge science, the spectrum-effect relationship method is a technology integrating analytical chemistry, pharmacodynamics, and chemometrics. In the future, more professional talents need to be introduced to the development of related software engineering, exploring a wider world for the screening of effective compounds.
4. Perspectives of the combination of affinity and inhibition profiles
Though affinity screening and inhibition profiling both have the obvious advantage of the rapid discovery of TYR inhibitors directly from natural sources without any tedious purification procedures, the two approaches are established based on two different, and in some cases complementary, principles. Affinity screening is based on the binding affinity of molecules to the enzyme regardless of their inhibitory potential. However, the false-positive results are commonly caused in affinity screening by the non-specific affinity between compounds and enzymatic non-active sites or the solid support. While, for the mode of inhibition profiling, the inhibitory activity of compounds was measured according to the catalytic properties of the enzyme, some compounds like tannins have also been shown to result in false-positive results because of their non-specific interactions with the enzyme. Instead of the present strategies using either one, the combination of affinity screening and inhibition profiling would provide an opportunity to narrow down the hits with presumed bioactivity, minimising the risk of false-positive results, the proof-of-concept of which was demonstrated by α-glucosidase inhibitors screening from the crude ethyl acetate extract of Ginkgo biloba71. Undoubtedly, the combined approach provides a powerful tool for the screening of specifically target bioactive compounds with both specific affinity and activity.
5. Two important concerns for TYR inhibitors screening
Though the present review mainly focussed on the separation strategies for inhibitors screening, two important concerns for the TYR inhibitors discovery are worth to be noted. On one hand, mTYR rather than hTYR has been commonly used as the target for inhibitors screening due to cheap and commercially available. But, it was reported that inhibitors designed and evaluated against hTYR may have greater efficacy and lead to more effective treatments for pathological hyperpigmentation than those against mTYR4. Therefore, if the condition allows, it is better to use hTYR for inhibitors screening in the future. On the other hand, the previous report demonstrates that many plant-derived inhibitors such as polyphenols are in fact alternative substrates of mTYR and that might cause as well errors during the read-out with MS or UV absorption detection72. Since many polyphenols are found ubiquitously in natural extracts, a detailed understanding of the strengths and limitations of the used techniques is crucial.
6. Inhibitory activity and mechanisms of several screened compounds
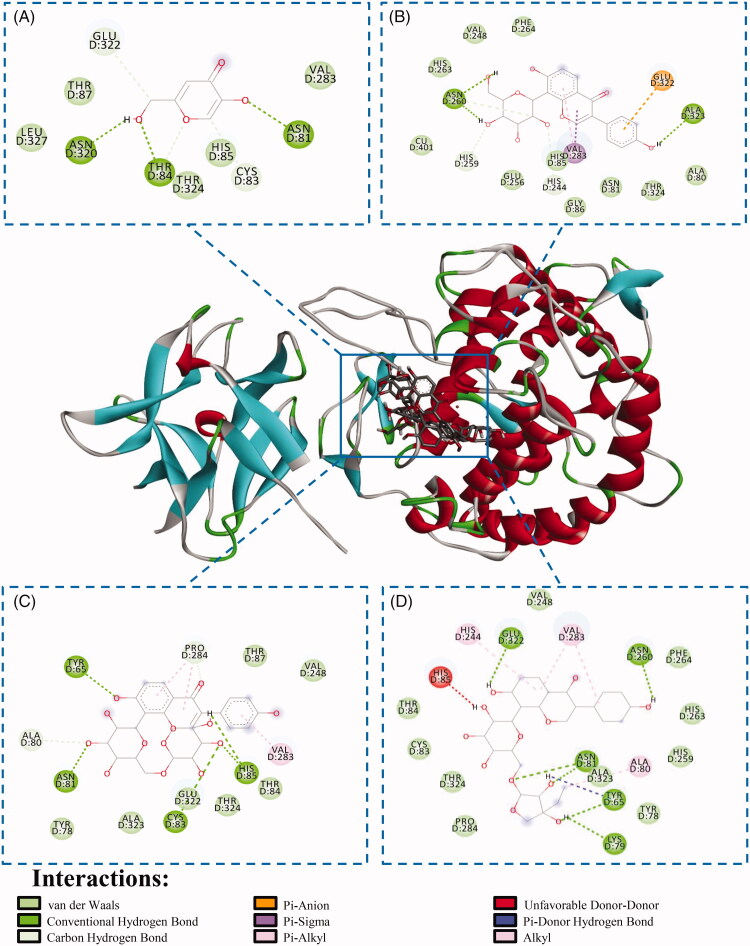
Using the above-described methods, a lot of TYR inhibitors have been found. However, the vast majority of these reports have focussed mainly on the determination of analytical parameters and analysis of samples with very little emphasis on the binding and the mechanism of inhibition. In order to further study the screened TYR inhibitors, enzyme inhibition assay and molecular docking had been usually carried out to explore their inhibitory mechanisms and binding characteristics. Among the TYR inhibitors derived from natural sources (Table 1S, please see the supplementary materials), those containing hydroxyl groups in their structures including flavonoids and polyphenols was turned to be effective for TYR inhibition probably due to chelating copper ions as well as binding critical residues affecting substrate access at the active site pocket, which generally displayed reversible and competitive inhibition types73,74 (Table 2). For example, to confirm the TYR inhibitory activity of hit compounds, four identified compounds from P. lobata extract was tested in vitro, and three of them, namely, puerarin, puerarin-6′’-O-xyloside and puerarin apioside were verified to have good TYR inhibitory activity with IC50 value of 478.5, 513.8, and 877.3 μM, respectively21. In addition, the molecular docking results indicated that these compounds could bind to the amino acid residues in TYR catalytic pocket (Figure 6) via multifarious interactions, including van der Waals, conventional hydrogen bond, carbon-hydrogen bond, Pi-Anion, Pi-Sigma and Pi-Alkyl forces. Similarly, by molecular docking, Zhang et al. 11 and Liu et al. 15 also discovered the importance of hydrogen bonding and π-cation interaction for binding. The more hydrogen bonds that can be formed, the stronger the biological or activity is. Puerarin-6′'-O-xyloside has more hydrogen interactions than puerarin because it has a xylose moiety, resulting in higher biological activity11. The docking results also showed that the four hydroxyl groups in puerarin formed four hydrogen bonds with TYR, and the ring B part of puerarin formed a π-cation interaction with the enzyme, which has a stronger inhibitory effect than the known TYR inhibitor kojic acid11. Liu et al. 15 Ranked the inhibitory activities of discovered hit compounds, which were as follows: puerarin > mirificin > Kojic acid > daidzin≈genistin, the molecular-docking analysis indicated that the addition of glycosyl reduced TYR inhibition due to steric hindrance and changes in polarity.
Table 2.
The IC50, Ki, and inhibition mechanism of some screened compounds.
| Compounds | IC50(mM) | Ki(mM) | Suppression mechanism | References |
|---|---|---|---|---|
| Puerarin2 | 1.23 | / | / | 11 |
| 0.010 | 15 | |||
| 0.479 | 21 | |||
| 0.012 | 22 | |||
| Oroxin A6 | 0.50 | 12 | ||
| Baicalein8 | 0.29 | |||
| Protocatechuic acid82 | / | 9.28 | Competitive inhibitor | 13 |
| 3,5-Di-O-caffeoylquinic acid57 | 0.34 | |||
| 1,5-Di-O-caffeoylquinic acid58 | 1.1 | |||
| Chlorogenic acid59 | 0.9 | Mixed inhibitor | ||
| Quercetin-3-O-(6-O-malonyl)-β-D glucopyranoside14 | 0.268 | / | / | 14 |
| Kaempferol-3-O-(6-O-malonyl)-β-D-glucopyranoside15 | 0.104 | |||
| Mirificin38 | 0.013 | 15 | ||
| Daidzin34 | >500 | |||
| Genistinc39 | >500 | |||
| Vitexin11 | 0.35 | 16 | ||
| Isovitexin12 | 1.73 | |||
| Isoorientin10 | 7.67 | |||
| Isoorientin 3'-methyl ether13 | 8.61 | |||
| Daidzein37 | 1.58 | |||
| Genistein/ | 7.66 | |||
| 3-(5-Hydroxybenzofuran-6-yl) propanoic acid94 | 1.33 | Mixed-type inhibitor | ||
| Gallic acid78 | 0.178 | / | 20 | |
| Albiforin106 | 0.100 | |||
| Paeoniforin107 | 0.102 | |||
| Liquiritin apioside50 | 0.089 | |||
| Liquiritin84 | 0.171 | |||
| Galloylpaeoniflorin/ | 0.036 | |||
| Ononin85 | 0.101 | |||
| Isoliquiritigenin/ | 0.185 | |||
| Glycyrrhizic acid/ | 0.059 | |||
| Oxypaeoniflora/ | 0.083 | |||
| Benzoylpaeoniflorin/ | 0.032 | |||
| Benzoyloxypaeoniflorin/ | 0.040 | |||
| Mudanpioside C/ | 0.083 | |||
| Paeonolide/ | 0.102 | |||
| Apiopaeonoside/ | 0.098 | |||
| Puerarin-6’’-O-xyloside33 | 0.514 | 21 | ||
| Puerarin apioside43 | 0.877 | |||
| Emodin111 | 300mg/ml | 34 | ||
| Protocatechuic aldehyde91 | 0.455 | 35 | ||
| Hydroxysafor yellow A92 | 0.498 | |||
| Tanshinone IIA108 | 1.214 | |||
| Mulberrofuran G93 | 0.018 | 36 | ||
| Kuwanon G31 | >0.2 | |||
| Kuwanon H32 | 0.010 |
Figure 6.
Molecular docking of kojic acid (A), puerarin (B), puerarin-6’’-O-xyloside (C), and puerarin apioside (D) with TYR (Copyrighted from Zhao et al. 21).
The importance of the chemical structure of the compound for biological activity has been confirmed by other studies. For example, dicaffeoylquinic acids showed more potent TYR inhibitory activities than the caffeoylquinic acids that may be attributed to the larger number of catechol moieties, which suggests that compounds with a catechol structure may have higher TYR inhibitory activity14. Tao et al. 20 found that the activity of paeoniflorin was slightly weaker than that of its derivatives, possibly due to the difference in its aromatic substituents. Furthermore, based on the spectrum-efficiency-structure-activity relationship, Liu et al. 34 screened TYR inhibitors in rhubarb. The biological activity of the compound was predicted through the results of molecular docking, and the TYR inhibition experiment was used to verify it. This study also demonstrated the important role of hydrogen bonding for binding. The 6-OH and 8-OH of emodin form hydrogen bonds with TYR, which has strong activity. Veratrol-4′-O-β-D-glucoside, containing meta-dihydroxyl structure, can form hydrogen bonds and complex interaction and also has strong biological activity. 2-O-cinnamyl-galloyl glucose also has a dual role of hydrogen bonding and complexation, which makes it have strong biological activity, but it may be weaker than the former two due to steric hindrance. The docking results of 1, 2, 6-trihydroxy-5-methoxy-7–(3-methylbut-2-enyl) xanthone with o-dihydroxy group shows that it is difficult for the o-dihydroxy group to form a hydrogen bond, so its activity is the weakest. In general, compounds that can form hydroxyl groups have strong biological activity but are also affected by steric hindrance and group polarity.
Undoubtedly, the enzyme inhibition assay and molecular docking will be conducive to understand the mechanism of the inhibitory effect of the hit compounds against TYR and provide the scientific basis for further development of them. However, up to date, the clinical trials with skin whitening agents or formulations hardly had been performed. A clinical study from Korea75 investigated the anti-melanogenesis activity for whitening products containing aerial part of P. lobata extract (APPL). After 4 weeks, subjects with 3% APPL treatment started to improve melanin content and visual evaluation on pigmentation and skin lightness. After 8 weeks of treatment, the Skin colours have been greatly improved. Safety evaluation proved that there was no adverse reaction on the skin upon treatment of products. Natural products have become the potential sources of cosmetics and medication to prevent skin hyperpigmentation. With the progress of screening technology, more and more lead compounds from them have been found, it is expected to carry out more clinical studies to confirm their effectiveness and safety.
7. Conclusion
In recent years, with the wide application of TYR inhibitors, searching for safe, specific, and effective TYR inhibitors has become a concern of researchers. Linking the powerful ability of separation technologies with enzyme characteristics, a series of screening methods have been developed, which have made great contributions to the development and application of TYR inhibitors.
This review summarises the current screening methods for TYR inhibitors developed based on chromatographic technology, introduces the basic principles of various screening methods, the advantages and drawbacks of various methods (Table 3), and their applications in the screening of complex systems such as TCM. In any case, the newly developed methods provide a powerful tool for screening active ingredients in complex compounds and greatly accelerate the speed of drug discovery compared with traditional methods. In the future, simpler, faster, and increasingly accurate modern bioanalytical techniques are expected to develop for the screening and detection of TYR inhibitors in natural products. Besides, more clinical researches are expected to be carried out to develop effective and safe skin whitening agents.
Table 3.
Summary of the advantages and drawbacks of different separation methods for TYR inhibitors screening.
| Separation methods | Advantages | Drawbacks |
|---|---|---|
| UF affinity screening | Easy operation, time-saving | Non-specific binding and false-positive results, repetitive manual operation of reverse UF |
| Immobilised target affinity screening | High stability and durability of the enzymes, low costs, short analysis time | Non-specific binding, possible changes of protein spacial structure, loss of the activities of immobilised enzymes |
| Offline 2 D-LC/MS affinity screening | Ultra-fast separation of enzyme-ligand complex and small molecules, a low false-positive result | Chromatographic column for separation of large and small molecules needs to be optimised |
| Online CE-based methods | High-efficiency separation, short analysis time, minimal sample consumption, high sensitivity, easy to realise automatisation | Poor repeatability, only applicable to the activity evaluation of monomers or extracts |
| (HP)TLC -autography | Rapid and efficient, the capability of simultaneous detection of multiple samples, visualisation of test results | Poor separation effect, low stability of the enzyme, and insufficient sensitivity |
| HPLC-MS-EIA | Automation, simultaneous acquisition of chemical activity information | High cost, false-negative result of low content compounds, more effort to develop the method |
| SER | Less time and solvent consumption, low operating costs, and little pollution to the environment | Poor repeatability of fingerprints, no standardised data processing, the requirement of activity verification |
Author contributions
Xiao-wei Zhang, Guang-li Bian, Pei-ying Kang, Xin-jie Cheng and Kai Yan: Writing – original draft. Yong-li Liu and Yan-xia Gao: Investigation and designation of the framework. De-qiang Li: Conceptualisation and writing – review & editing.
Supplementary Material
Funding Statement
This study was supported by the National Natural Science Foundation of China (81603066), the Natural Science Foundation of Hebei Province of China (H2020206091) and the foundation of the Second Hospital of Hebei Medical University (2HN202101).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Chang TS.An updated review of tyrosinase inhibitors. Int J Mol Sci 2009;10:2440–62475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi KU, Ali AS, Ali SA, Naaz I.. Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem Res Int 2014;2014:854687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isao K, Yoshihiro K, Ikwo K-H.. Tyrosinase inhibitors from Bolivian medicinal plants. J Nat Prod 1995;58:739–43. [DOI] [PubMed] [Google Scholar]
- 4.Mann T, Gerwat W, Batzer J, et al. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J Invest Dermatol 2018;138:1601e1608. [DOI] [PubMed] [Google Scholar]
- 5.Hornyak TJ.Next time, save mushrooms for the Pizza!!. J Invest Dermatol 2018;138:1470–1472. [DOI] [PubMed] [Google Scholar]
- 6.Yan Q, Cao R, Yi W, et al. Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur J Med Chem 2009;44:4235–4243. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, Wu JW, Wang M, et al. 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J Nat Prod 2012;75:82–87. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM.. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016;79:629–661. [DOI] [PubMed] [Google Scholar]
- 9.Sumner LW, Lei Z, Nikolau BJ, Saito K.. Modern plant metabolomics: advanced natural product gene discoveries, improved technologies, and future prospects. Nat Prod Rep 2015;32:212–229. [DOI] [PubMed] [Google Scholar]
- 10.Muhammad S, Han S, Xie X, et al. Overview of online two-dimensional liquid chromatography based on cell membrane chromatography for screening target components from traditional Chinese medicines. J Sep Sci 2017;40:299–313. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Guo XH, Wang SS, et al. Screening and identification of natural ligands of tyrosinase from Pueraria lobata Ohwi by a combination of ultrafiltration and LC-MS. Anal. Methods 2017;9:4858–4862. [Google Scholar]
- 12.Yin XS, Qian ZM, Yin JT, et al. Screening and identification of potential tyrosinase inhibitors from Semen Oroxyli extract by ultrafiltration LC-MS and in silico molecular docking. J Chromatogr Sci 2019;57:838–846. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Hwang SH, Huang B, Lim SS.. Identification of tyrosinase specific inhibitors from Xanthium strumarium fruit extract using ultrafiltration-high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2015;1002:319–328. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Zhang Y, Sun L, et al. An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterising tyrosinase inhibitors from mulberry leaves. Anal Chim Acta 2012;719:87–95. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Zhu Y, Wang T, et al. Enzyme-site blocking combined with optimization of molecular docking for efficient discovery of potential tyrosinase specific inhibitors from Puerariae lobatae radix. Molecules 2018;23:2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuo G, Wang Z, Guillen Quispe YN, et al. Target guided isolation of potential tyrosinase inhibitors from Otholobium pubescens (Poir.) J.W. Grimes by ultrafiltration, high-speed countercurrent chromatography and preparative HPLC. Ind Crop Prod 2019;134:195–205. [Google Scholar]
- 17.Wang Z, Hwang SH, Lim SS.. Comprehensive profiling of minor tyrosinase inhibitors from Gastrodia elata using an off-line hyphenation of ultrafiltration, high-speed countercurrent chromatography, and high-performance liquid chromatography. J Chromatogr A 2017;1529:63–71. [DOI] [PubMed] [Google Scholar]
- 18.Shi F, Xie L, Lin Q, et al. Profiling of tyrosinase inhibitors in mango leaves for a sustainable agro-industry. Food Chem 2020;312:126042. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Shi S, Chen X, Peng M.. Analysis of Tyrosinase binders from Glycyrrhiza uralensis root: evaluation and comparison of tyrosinase immobilized magnetic fishing-HPLC and reverse ultrafiltration-HPLC. J Chromatogr B Analyt Technol Biomed Life Sci 2013;932:19–25. [DOI] [PubMed] [Google Scholar]
- 20.Tao Y, Su D, Du Y, et al. Magnetic solid-phase extraction coupled with HPLC-Q-TOF-MS for rapid analysis of tyrosinase binders from San-Bai decoction by Box–Behnken statistical design. RSC Advances 2016;6:109730–109741. [Google Scholar]
- 21.Zhao CP, Yin SJ, Chen GY, et al. Adsorbed hollow fiber immobilized tyrosinase for the screening of enzyme inhibitors from Pueraria lobata extract. J Pharm Biomed Anal 2021;193:113743. [DOI] [PubMed] [Google Scholar]
- 22.Xu JP, Cheng XJ, Bian GL, et al. Rapid discovery of potential tyrosinase specific inhibitors from Puerariae lobatae radix by off-line 2D LC-MS/MS. Chin. J. Clin. Pharmacol 2020;36:2317–2319. [Google Scholar]
- 23.Tang L, Zhang W, Zhao H, Chen Z.. Tyrosinase inhibitor screening in traditional Chinese medicines by electrophoretically mediated microanalysis. J Sep Sci 2015;38:2887–2892. [DOI] [PubMed] [Google Scholar]
- 24.Zhang WH, Lv ZH, Jiang TF, et al. Screening tyrosinase inhibitors from traditional Chinese medicine by capillary electrophoresis with electrophoretically mediated microanalysis. J. Food. Drug. Anal 2012;20:159–163. [Google Scholar]
- 25.Cheng M, Chen Z.. Screening of tyrosinase inhibitors by capillary electrophoresis with immobilized enzyme microreactor and molecular docking. Electrophoresis 2017;38:486–493. [DOI] [PubMed] [Google Scholar]
- 26.Jiang TF, Liang TT, Wang YH, et al. Immobilized capillary tyrosinase microreactor for inhibitor screening in natural extracts by capillary electrophoresis. J Pharm Biomed Anal 2013;84:36–40. [DOI] [PubMed] [Google Scholar]
- 27.Wangthong S, Tonsiripakdee I, Monhaphol T, et al. Post TLC developing technique for tyrosinase inhibitor detection. Biomed Chromatogr 2007;21:94–100. [DOI] [PubMed] [Google Scholar]
- 28.Hsu KD, Chan YH, Chen HJ, et al. Tyrosinase-based TLC autography for anti-melanogenic drug screening. Sci Rep 2018;8:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra BB, Dey S.. TLC-bioautographic evaluation of in vitro anti-tyrosinase and anti-cholinesterase potentials of sandalwood oil. Nat Prod Commun 2013;8:253–256. [PubMed] [Google Scholar]
- 30.Zhou J, Tang Q, Wu T, Cheng Z.. Improved TLC bioautographic assay for qualitative and quantitative estimation of tyrosinase inhibitors in natural products. Phytochem Anal 2017;28:115–124. [DOI] [PubMed] [Google Scholar]
- 31.Garcia P, Furlan RL.. Multiresponse optimisation applied to the development of a TLC autography for the detection of tyrosinase inhibitors. Phytochem Anal 2015;26:287–292. [DOI] [PubMed] [Google Scholar]
- 32.García P, Ramallo IA, Furlan RLE.. Reverse phase compatible TLC-bioautography for detection of tyrosinase Inhibitors. Phytochem Anal 2017;28:101–105. [DOI] [PubMed] [Google Scholar]
- 33.Luo B.Study on a new screening method of natural tyrosinase inhibitors based on HPLC post-column derivatization [master’s thesis]. Alaer (XJ): Tarim University; 2015. [Google Scholar]
- 34.Liu YJ, Wang Q, Jiang M, et al. Screening of effective components for inhibition of tyrosinase activity in rhubarb based on spectrum-efficiency-structure-activity relationship. Chin Tradit Herb Drugs 2012;43:2120–2126. [Google Scholar]
- 35.Wang YL, Hu G, Zhang Q, et al. Screening and characterizing tyrosinase inhibitors from Salvia miltiorrhiza and Carthamus tinctorius by spectrum-effect relationship analysis and molecular docking. J Anal Methods Chem 2018;2018:2141389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang KB, Lee DY, Kim TB, et al. Prediction of tyrosinase inhibitory activities of Morus alba root bark extracts from HPLC fingerprints. Microchem J 2013;110:731–738. [Google Scholar]
- 37.Zhuo R, Liu H, Liu N, Wang Y.. Ligand fishing: A Remarkable strategy for discovering bioactive compounds from complex mixture of natural products. Molecules 2016;21:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greening DW, Simpson RJ.. A centrifugal ultrafiltration strategy for isolating the low-molecular weight (≤25K) component of human plasma proteome. J Proteomics 2010;73:637–648. [DOI] [PubMed] [Google Scholar]
- 39.Luque-Garcia JL, Neubert TA.. Sample preparation for serum/plasma profiling and biomarker identification by mass spectrometry. J Chromatogr A 2007;1153:259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cieśla Ł, Moaddel R.. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat Prod Rep 2016;33:1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian ZM, Qin SJ, Yi L, et al. Binding study of Flos Lonicerae Japonicae with bovine serum albumin using centrifugal ultrafiltration and liquid chromatography. Biomed Chromatogr 2008;22:202–206. [DOI] [PubMed] [Google Scholar]
- 42.Wei H, Zhang X, Tian X, Wu G.. Pharmaceutical applications of affinity-ultrafiltration mass spectrometry: recent advances and future prospects. J Pharm Biomed Anal 2016;131:444–453. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Wu ZY, Yang YY, et al. Recent applications of immobilized biomaterials in herbal analysis. J Chromatogr A 2019;1603:216–230. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y, Luo J, Qin J, Yang M.. Screening techniques for the identification of bioactive compounds in natural products. J Pharm Biomed Anal 2019;168:189–200. [DOI] [PubMed] [Google Scholar]
- 45.Hai X, Yang B-F, Schepdael AV.. Recent developments and applications of EMMA in enzymatic and derivatization reactions. Electrophoresis 2012;33:211–227. [DOI] [PubMed] [Google Scholar]
- 46.Bao J, Regnier FE.. Ultramicro enzyme assays in a capillary electrophoretic system. J. Chromatogr 1992;608:217–224. [DOI] [PubMed] [Google Scholar]
- 47.Liu DM, Shi YP, Chen J.. Application of capillary electrophoresis in enzyme inhibitors screening. Chinese J. Anal. Chem 2015;43:775–782. [Google Scholar]
- 48.Huang S, Paul P, Ramana P, et al. Advances in capillary electrophoretically mediated microanalysis for on-line enzymatic and derivatization reactions. Electrophoresis 2018;39:97–110. [DOI] [PubMed] [Google Scholar]
- 49.Fan Y, Scriba GKE.. Advances in capillary electrophoretic enzyme assays. J Pharm Biomed Anal 2010;53:1076–1090. [DOI] [PubMed] [Google Scholar]
- 50.Cheng M, Chen Z.. Recent advances in screening of enzymes inhibitors based on capillary electrophoresis. J Pharm Anal 2018;8:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choma IM, Grzelak EM.. Bioautography detection in thin-layer chromatography. J Chromatogr A 2011;1218:2684–2691. [DOI] [PubMed] [Google Scholar]
- 52.Menn JJ, Mcbain JB, Dennis Mj. Detection of naturally occurring chloinesterase inhibitors in several crops by paper chromatography. Nature 1964;202:697–698. [DOI] [PubMed] [Google Scholar]
- 53.Wu T, Cheng Z.. TLC bioautography: high throughput technique for screening of bioactive natural products. Comb Chem High Throughput Screen 2013;16:531–549. [DOI] [PubMed] [Google Scholar]
- 54.Taibon J, Ankli A, Schwaiger S, et al. Prevention of false-positive results: development of an HPTLC autographic assay for the detection of natural tyrosinase inhibitors. Planta Med 2015;81:1198–1204. [DOI] [PubMed] [Google Scholar]
- 55.Salazar MO, Furlan RLE.. A rapid TLC autographic method for the detection of glucosidase inhibitors. Phytochem Anal 2007;18:209–212. [DOI] [PubMed] [Google Scholar]
- 56.Ramallo IA, Zacchino SA, Furlan RL.. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem Anal 2006;17:15–19. [DOI] [PubMed] [Google Scholar]
- 57.Vandana G, Gertrud M.. Effect-directed profiling of Ficus religiosa leaf extracts for multipotent compounds via 12 effect-directed assays. J Chromatogr B 2021;1637:461836. [DOI] [PubMed] [Google Scholar]
- 58.Zang Y, Cheng Z, Wu T.. TLC bioautography on screening of bioactive natural products: an update review. Curr Anal Chem 2020;16:545–556. [Google Scholar]
- 59.Sarah B, Evelyn W.. Recent advances in effect-directed enzyme assays based on thin-layer chromatography. Phytochem Anal 2017;28:74–86. [DOI] [PubMed] [Google Scholar]
- 60.Olivier P, Hamburger M.. Concepts and technologies for tracking bioactive compounds in natural product extracts: generation of libraries, and hyphenation of analytical processes with bioassays. Nat Prod Rep 2013;30:546–564. [DOI] [PubMed] [Google Scholar]
- 61.Marston A.Thin-layer chromatography with biological detection in phytochemistry. J Chromatogr A 2011;1218:2676–2683. [DOI] [PubMed] [Google Scholar]
- 62.Li DQ, Zhao J, Wu D, Li SP.. Discovery of active components in herbs using chromatographic separation coupled with online bioassay. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1021:81–90. [DOI] [PubMed] [Google Scholar]
- 63.Wu SQ, Song HP, Li B, et al. A fast and accurate method for the identification of peroxidase inhibitors from Radix Salvia Miltiorrhizae by on-flow biochemical assay coupled with LC/Q-TOF-MS: comparison with ultrafiltration-based affinity selection. Anal Bioanal Chem 2018;410:4311–4322. [DOI] [PubMed] [Google Scholar]
- 64.Shi S-Y, Zhang Y-P, Jiang X-Y, et al. Coupling HPLC to on-line, post-column (bio)chemical assays for high-resolution screening of bioactive compounds from complex mixtures. TrAC Trends Anal Chem 2009;28:865–877. [Google Scholar]
- 65.Liu R, Kool J, Jian J, et al. Rapid screening α-glucosidase inhibitors from natural products by at-line nanofractionation with parallel mass spectrometry and bioactivity assessment. J Chromatogr A 2021;1635:461740. [DOI] [PubMed] [Google Scholar]
- 66.Mladic M, de Waal T, Burggraaff L, et al. Rapid screening and identification of ace inhibitors in snake venoms using at-line nanofractionation LC-MS. Anal Bioanal Chem 2017;409:5987–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mladic M, Slagboom J, Kool J, et al. Detection and identification of antibacterial proteins in snake venoms using at-line nanofractionation coupled to LC-MS. Toxicon 2018;155:66–74. [DOI] [PubMed] [Google Scholar]
- 68.Li C, Tu C, Che Y, et al. Bioassay based screening for the antiplatelet aggregation quality markers of Polygonum multiflorum with UPLC and chemometrics. J Pharm Biomed Anal 2019;166:264–272. [DOI] [PubMed] [Google Scholar]
- 69.Zhu CS, Lin ZJ, Xiao ML, et al. The spectrum-effect relationship—a rational approach to screening effective compounds, reflecting the internal quality of Chinese herbal medicine. Chin J Nat Med 2016;14:177–0184. [DOI] [PubMed] [Google Scholar]
- 70.Zhuo L, Peng J, Zhao Y, et al. Screening bioactive quality control markers of QiShenYiQi dripping pills based on the relationship between the ultra-high performance liquid chromatography fingerprint and vascular protective activity. J Sep Sci 2017;40:4076–4084. [DOI] [PubMed] [Google Scholar]
- 71.Wubshet SG, Liu B, Kongstad KT, et al. Combined magnetic ligand fishing and high-resolution inhibition profiling for identification of α-glucosidase inhibitory ligands: a new screening approach based on complementary inhibition and affinity profiles. Talanta 2019;200:279–287. [DOI] [PubMed] [Google Scholar]
- 72.Mayr F, Sturm S, Ganzera M, et al. Mushroom tyrosinase-based enzyme inhibition assays are not suitable for bioactivity-guided fractionation of extracts. J Nat Prod 2019;82:136–147. [DOI] [PubMed] [Google Scholar]
- 73.Orhan IE, Khan MT.. Flavonoid derivatives as potent tyrosinase inhibitors – a survey of recent findings between 2008–2013. Curr Top Med Chem 2014;14:1486–1493. [DOI] [PubMed] [Google Scholar]
- 74.Kim YJ, Uyama H.. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 2005;62:1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon CB, Ryu JH.. A clinical evaluation about whitening effect and safety of cosmetic containing aerial part of Pueraria lobata extract. Yakhak HOEJI 2017;61:233–239. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.