Abstract
In this research, eight local mung bean (Vigna radiata) varieties were analyzed for their performance against two levels of CdCl2 solution (0.3 and 0.5 mM) alone and priming with gibberellic acid (GA3) (100 μM), salicylic acid (SA) (50 μM) and proline (5 mM) solution prior to Cd exposure. Mung bean seedlings were analyzed for disturbance in cytological, morphological, biochemical and enzymatic parameters under cadmium stress. For cytological studies, 48 h grown mung bean seedlings root tips were used to prepare slides and studied for percent mitotic index (MI%) and to calculate percent C-mitosis, laggard, sticky and fragmented chromosomes, pictures were captured by a Nikon camera (DS-Fi 1 Japan) attached with a microscope. One-week grown mung seedlings were studied for growth traits, malondialdehyde (MDA), protein, proline and antioxidant enzymes. ANOVA and DMR test of this research revealed that all the tested mung bean varieties and treatments were significantly different regarding mitotic index and number of chromosomal aberrations. Both the Cd treatments exhibited increased total chromosomal aberrations with different types and a maximum decrease in MI%. In pretreated samples, GA3, SA and proline serve as mitigating agents that reduce mutagenic effects of Cd in mung bean by increasing MI% and decreasing chromosomal aberrations as compared to non-pretreated samples. Both the Cd treatments showed a decrease in all growth traits. Total proteins were also found to be significantly reduced in a dose-dependent manner in all genotypes. Cd treatment increased the activities of all antioxidant enzymes tested. Cd caused oxidative damage as indicated by elevated levels of MDA content in treated samples in comparison to control. Proline content levels were also high in Cd treated seedlings indicating stress. Results demonstrated that pretreatment with phytohormones and proline before Cd were found to improve all morphological parameters, by altering antioxidant enzymes activities along with a decrease in MDA and proline contents as well. It was further noticed that the performance of GA3 was better at 0.3 mM Cd treatment while SA was found to be a good mitigating agent at 0.5 mM Cd stress in all tested mung bean varieties. This research concluded less deleterious effects of Cd on AZRI-2006 while more sensitivity to NM-51 towards Cd. Priming with phytohormones and proline is a user-friendly, economical, and simple mitigation strategy to reduce Cd toxicity in plants and get better yield from contaminated lands.
Introduction
Some metals are found naturally in the earth’s crust and anthropogenic events tend to increase levels of other metals that are contaminating our ecosystem [1]. As micronutrients, these metals have an important role in plant metabolism, but these can be highly toxic for all plants if stored in exorbitant amounts [2]. Plant roots can absorb these metals directly from soil and water or indirectly by foliar application, causing higher accumulation in different plant parts [3]. Poor farming practices and disposal of untreated industrial and urban waste are contaminating agricultural soils in developing countries [4]. The augmentation of heavy metals in the water and soil from various resources can reach us by food chain that is responsible for certain physiological and biochemical anomalies with serious chronic health problems that include itai-itai disease, different cancers, and kidney and liver disorders [5]. Cadmium (Cd) is among the most hazardous mobile element found abundantly in soil, causing growth reduction by altering different processes including photosynthesis, mineral transport, protein and cell membrane damage. They may also cause a disturbance in cell division along with structural alterations in plants, inactivation of enzymes and hormonal imbalance thus reducing crop productivity [6]. Cd interrupts the normal cell cycle thus causing chromosomal abnormalities in cells [7]. Aberrations were resulted due to direct damage to DNA and interference of Cd ions in the transcription and translation process that hinders the synthesis of DNA apparatus. Cd might also disturb enzymes involves in the DNA repair mechanism, either by modifying their structure or by reducing the levels of transcriptomes that can cause aberrations [8]. Studies revealed that Cd treatment in plants can cause growth reduction related to inhibition in mitotic index, by inducing chromosomal and nuclear aberrations in the root meristems [9–11]. ROS production/accumulation in plants is accountable for generating oxidative stress under Cd stress. Plasma membrane permeability was transformed in oxidative stress by restraining ATPase activities, which will assist in ionic homeostasis throughout the cells [12, 13].
Therefore, adopting mitigating strategies is important in reducing the Cd accumulation in plants specifically in edible parts. Remediation techniques have been used previously which were costly, time-consuming and not easy to perform with major side effects as well [14].
Gibberellic acid (GA3) is an endogenous phytohormone that belongs to terpenoids and growth regulator which stimulates hydrolytic enzymes and is helpful in the germination process of seeds [15, 16]. Salicylic acid (SA) is an endogenous signaling molecule and plant growth regulators have major roles in various abiotic stresses like heavy metals in different crops [17]. Under harsh conditions, plant metabolites like amino acids accumulate in different parts; many amino acids like proline have major roles as protein precursors and building blocks, helpful in plant metabolism and development. They act as major osmolytes, a metal chelator, an antioxidant and signaling molecule during different kinds of abiotic stress [9, 18].
Mung bean (Vigna radiata (L.) Wilczek) has been selected as research material as it is very popular in Asia but sensitive to Cd. It is a short-duration, bi-annual, warm seasoned leguminous crop, widely grown for edible purposes. Mung bean has high levels of proteins, folate and iron [19]. It is also a good atmospheric nitrogen fixer at or in the soil that assists in improving soil fertility thus has a major role in the intercropping system [20], thus can reduce the use of expensive fertilizers. Limited research about Cd toxicity in mung beans resulted in slower progress in crop improvement. Thus, one of the reasons for conducting present research was to examine the impact of Cd with or without prior treatment of phytohormones and proline on mung bean genotypes. Morphological parameters such as overall seedling length, fresh and dry weight, relative water content and certain biochemical changes including protein were studied. Stress-induced malondialdehyde (MDA) generation was taken under consideration as a marker for Cd toxicity. Antioxidant defensive molecules such as proline and antioxidant enzymes were analyzed. Furthermore, the mitigating effect of phytohormones and proline on acclimatizing Cd toxicity in different local mung bean germplasms based on mitotic index and percent chromosomal aberrations were analyzed. These strategies may be applied by mung bean farmers to make use of metal-polluted lands for cultivation and to improve mung bean growth and yield under cadmium stress.
Material and methods
Plant material
Seeds of mung bean varieties (NM 2006, NM 19–19, NM 2011, NM 20–21, NM 121–123, AZRI-2006, NM 13–1 and NM-51) were obtained from National Agricultural Research Center (NARC), Islamabad, Pakistan.
Experimental condition
Healthy and uniformed-sized seeds were sterilized by using 1% sodium hypochlorite for 5 minutes, later washed thrice using distilled water (D/W). Priming of seeds was done with D/W (non-pretreated), and GA3 (100 μM), SA (50 μM), and proline (5 mM) solutions (pretreated) for 12 h in 50 ml beakers separately. The experiment was set in CRD with 3 replications. Pretreated and non-pretreated twenty seeds were germinated in each petri dish (6 inches) layered with filter paper moistened with 5 ml of D/W for 24 h at 30°C. Germinated pretreated and non-pretreated seedlings were later given the treatment of 0.3 mM and 0.5 mM CdCl2 for 1 week.
Slides preparation
For slides preparation, three seedlings were selected randomly from all replications at 48 h. Two days old mung bean root tips having 2 cm length were cut with a sharp scalpel. Excess amount of Cd was removed by washing with D/W and placed in Farmer’s fixative (alcohol-acetic acid 3:1) for one day. Fixed root tips were then stained in 1.8% aceto-orcein for 7 days. Hydrochloric acid (1M) was used for 9 min at 60°C to hydrolyze root tips later tapped in 45% glacial acetic acid on clean glass slides [21, 22].
Calculation for mitotic index and chromosomal aberrations
Approximately 500 cells were examined for mitotic divisions and chromosomal aberrations for each slide at an oil immersion lens i.e. 100 X. Photographs were taken by Nikon camera (DS-Fi 1 Japan) attached with a microscope.
Mitotic index and chromosomal aberrations were calculated by the following formula
Morphological traits
At the termination of experiment, seedlings were collected and stored at 4°C to proceed further. Seedling length (8 days old) was measured in centimeters (cm). Fresh weight (FW) was weighed in grams (g) and Dry weight (DW) was calculated by desiccating seedlings in an incubator for 24 h at 70°C. Water content was calculated by the following formula [23].
Protein and enzymatic analysis
Seedlings (0.2 g) were crushed in 50 mM sodium phosphate buffer (pH 7.0) (containing 1% PVP & 0.2 mM ascorbic acid) in an ice-cold mortar and pestle and centrifuge at 10,000 rpm for 30 min in cold conditions [24]. The supernatant was saved at 4°C for further enzymatic analysis and protein estimation [25] using bovine serum albumin (BSA) as a standard.
Antioxidant enzymes estimation
Ascorbate peroxidase (APX) reaction mixture contained 450 μL enzyme, 50 mM sodium phosphate buffer (pH 7.0), 0.1 mM EDTA and 0.5 mM ascorbic acid. Initiation of reaction was done by adding 0.1 mM H2O2 and taking absorbance at 290 nm for 2 min at the interval of 15 sec. The specific activity of APX was calculated by using a formula with E.C 2.8 mM–1 cm–1 [26].
The Reaction mixture for catalase (CAT) consisted of 50 mM potassium phosphate buffer (pH 7.0) and 50 μL enzyme. Enzyme activity was initiated by adding 12.5 mM H2O2 and followed degradation at 240 nm for 2 min (E.C 40 mM–1 cm–1) for calculating specific activity [27].
Guaiacol peroxidase (GPX) activity was determined by taking extracted supernatant (50 μL) added with 150 mM sodium phosphate buffer (pH 5.6), 100 mM guaiacol solution and 176 mM H2O2. Absorbance was taken at 470 nm against the reagent blank. Calculation for the specific activity of GPX was done as followed with E.C 26.6 mM–1 cm–1 [28].
Superoxide dismutase (SOD) activity was assayed by measuring enzyme capability for photochemical reduction of nitro blue tetrazolium (NBT). Reaction mixture consisted of 100 mM potassium phosphate buffer (pH 7.5), 1500 mM sodium carbonate, 200 mM methionine, 2.25 mM NBT, 3 mM EDTA, 1 mL distilled water and 150 μL of enzyme extract incubated at dark for 8 min and 60 μM riboflavin was added at the end. Vortex the reaction mixture and place 30 cm below the light source consisting of 40 W fluorescent lamps for half an hour. Tubes kept in dark were served as a blank, while the control tube was without the enzyme and kept in the light. The absorbance was measured at 560 nm. One unit of activity is the amount of enzymes required to inhibit 50% initial reduction of NBT under light [29].
MDA contents estimation
Lipid peroxidation was determined by estimating the malondialdehyde (MDA) content. Harvested seedlings were extracted in 5% TCA in cold conditions. Homogenate was then spun at 12000 rpm for 15 min. For estimation of MDA, 500 μL homogenate was added in 0.5% TBA in a 20% TCA solution. The reaction mixture was heated at 95°C for 25 min and then the reaction was stopped on ice. Optical density was recorded at 532 and 600 nm for subtracting non-specific absorbance. MDA content was calculated with E.C of 155 mM–1 cm–1 [30].
Proline contents estimation
To estimate free proline contents, seedlings were crushed in 3% sulphosalicylic acid then centrifuged at 10000 rpm for 10 min. An equal volume of extracted material, ninhydrin reagent (1.25 g of ninhydrin in 30 mL of glacial acetic acid and 20 mL of 6 M orthophosphoric acid) and glacial acetic acid were mixed in a test tube. Incubation at 100°C on boiling water bath for 1 h was done; ice was immediately added to stop the reaction. Toluene was added to the solution followed by thorough mixing. The chromophore toluene layer was aspirated from the aqueous phase whose OD was read at 520 nm. Toluene will serve as a blank [31]. Proline was calculated according to the formula mention below
Statistical analysis
A lab experiment was set in petri dishes/treatment/variety as a completely randomized design (CRD) with three replications. All data were subjected to ANOVA by using Computer Program IBM SPSS version 20. DMRT at the P ≤ 0.05 level of significance was performed. Similar alphabets in bar graphs showed non-significant differences between the treatments for each variety. Vertical bars on graphs represent standard errors (n = 3) [32].
Results
Cytological analysis
Highly significant differences among all eight tested varieties and treatments along with a significant interaction between both the factors for mitotic index and chromosomal aberrations were depicted as shown in Table 1.
Table 1. Mean sum of squares for MI% and chromosomal aberrations from mung bean root tips under Cd alone and priming with phytohormones and proline before Cd stress.
| SOV | Df | MS | |||||
|---|---|---|---|---|---|---|---|
| MI% | Stickiness | Laggard | C-mitosis | Fragmentation | Total Aber | ||
| Varieties | 7 | 1202.791** | 4.995** | 6.802** | 8.103** | 5.608** | 80.868** |
| Treatment | 8 | 803.781** | 70.737** | 31.074** | 111.001** | 93.147** | 1142.598** |
| V×T | 56 | 33.227** | 0.749** | 1.313** | 0.995** | 2.05** | 7.884** |
| Error | 144 | 2.329** | 0.116** | 0.083** | 0.162** | 0.144** | 0.329** |
Effect of Cd on mitotic index
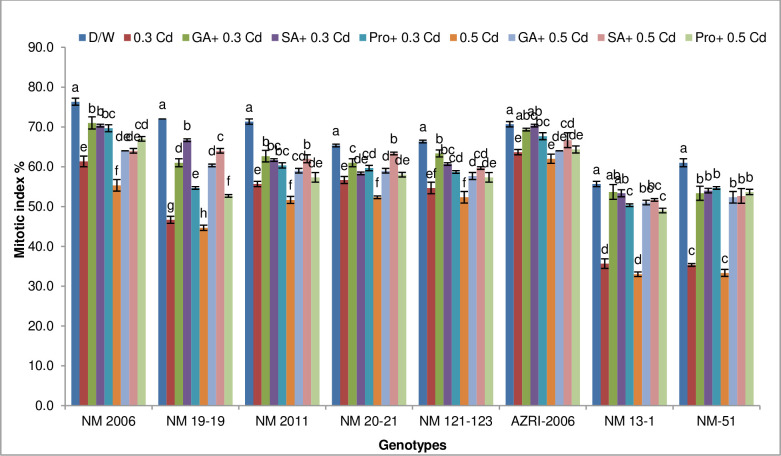
Results of this research indicated that different concentrations of Cd tend to reduce cell division of root cells, indicated by lower values of the mitotic index as compared to control (Fig 1).
Fig 1. Mean mitotic index of mung bean root tips under Cd treatments alone and priming with phytohormones and proline prior to Cd stress.
Different alphabets exhibit significant differences among the treatments for each variety. Vertical bars shows standard error with n = 3 at the P ≤ 0.05 level of significance.
Highest but variable values for MI % in control (D/W) for all mung bean varieties among all treatments were noticed with given hierarchal pattern NM 2006 > NM 19–19 > NM 2011 > AZRI-2006 > NM 121–123 > NM 20–21 > NM-51 > NM 13–1.
During Cd treatment, MI% decreased gradually as Cd concentration was increased. AZRI-2006 performed very well by inhibiting MI% only by 10% at 0.3 mM Cd and 12% at 0.5 mM Cd whereas the highest inhibition was noticed in NM-51 which was 42% at 0.3 mM Cd and 45% at 0.5 mM Cd. However, priming with GA3, SA and proline prior to Cd tends to ameliorate the toxic effect of Cd as indicated in S1 Fig. It was noticed that priming with GA3 was better for 0.3 mM Cd for most of the varieties. However, SA gave a better amelioration effect when applied prior to 0.5 mM Cd for all varieties except for NM 2006. For AZRI-2006, SA pretreatment was best and exhibited 0.5% inhibition at 0.3 mM Cd whereas 6% inhibition in MI% for 0.5 mM Cd. However, for NM-51, priming with proline was more efficient than GA3 and SA in reducing the clastogenic effect of Cd, showing 10% inhibition at 0.3 mM and 12% for 0.5 mM Cd treatment.
Increase in chromosomal aberrations under Cd stress
In this research, various aberrations were visualized under the microscope such as stickiness, laggard, fragmented chromosomes and C-mitosis with variable occurrence for different mung bean varieties caused by Cd treatment in a concentration-dependent manner and negligible aberrations were observed in non-Cd-treated samples (Fig 2).
Fig 2.
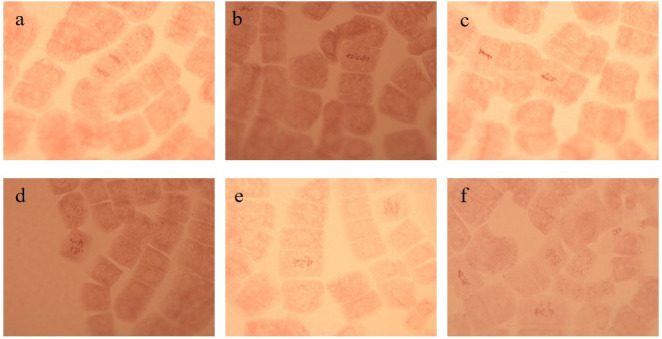
Pictorial demonstration of various normal and aberrated chromosomes observed in root tips of mung beans grown under different treatments (a) Normal Anaphase (b) Normal Metaphase (c) Sticky chromosomes (d) Laggard chromosomes (e) C-Mitosis and (f) Fragmented chromosomes (Scale bars = 200 μm).
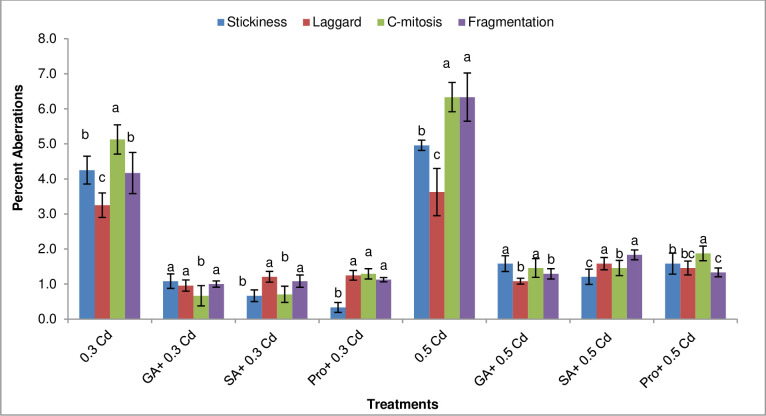
The higher value for C-mitosis (5%) was recorded at 0.3 mM Cd irrespective of variety, followed by stickiness (4%), fragmented (4%) and laggard (3%) chromosomes. Moreover, it was also observed that pretreatment of phytohormones and proline tends to decrease percent aberrations for all varieties at both Cd concentrations (Fig 3).
Fig 3. Percent chromosomal aberrations from mung bean root tips under Cd treatments alone and priming with phytohormones and proline.
Different alphabets in graphs exhibits significant differences among the treatments. Vertical bars shows a standard error with n = 24 at the P ≤ 0.05 level of significance.
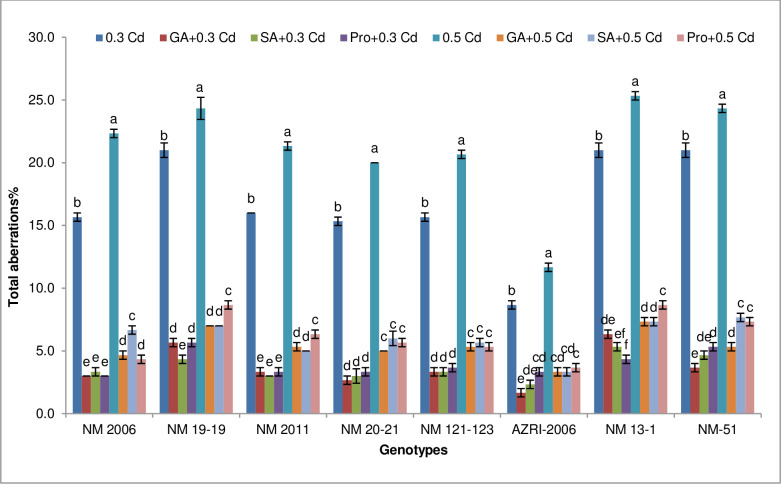
It was revealed that a higher frequency of chromosomal aberrations was found in NM 13–1, NM 19–19 and NM-51 at both Cd treatments (Fig 4). AZRI-2006 revealed the lowest frequency of aberrations 8% and 11% at 0.3 mM and 0.5 mM Cd respectively. However, when seeds were pre-treated with GA3, SA and proline before Cd stress tends to decrease these aberrations in cells of all varieties tested here (Fig 4).
Fig 4. Total aberrations from mung bean root tips under Cd treatments alone and priming with phytohormones and proline prior to Cd stress.
Different alphabets in graphs exhibits significant differences among the treatments for each variety. Vertical bars shows a standard error with n = 3 at the P ≤ 0.05 level of significance.
Effect of Cd on growth traits
After 1 week of Cd treatment seedling length, relative water content, fresh and dry weight was measured. Highly significant differences between genotypes and treatments along with interaction were observed for all growth parameters as indicated in Table 2.
Table 2. Mean sum of squares for morphological parameters of eight mung bean genotypes grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
| SOV | Df | MS | |||
|---|---|---|---|---|---|
| SL | FW | DW | RWC | ||
| Genotypes | 7 | 50.592** | 0.091** | 0.0009** | 34.08** |
| Treatments | 8 | 211.562** | 0.031** | 0.00003** | 9.94** |
| G×T | 56 | 5.912** | 0.003** | 0.00001** | 1.719** |
| Error | 144 | 0.051 | 0.00004 | 0.0000013 | 0.294 |
Results demonstrated that Cd have adverse effects on plant growth as indicated by reduction in seedling length, fresh and dry weight on a concentration-dependent manner. However, the pretreatment with phytohormones (GA3 and SA) and proline prior to Cd alleviated the adverse effects of Cd represented by improvement in these traits (indicated in S2 Fig)
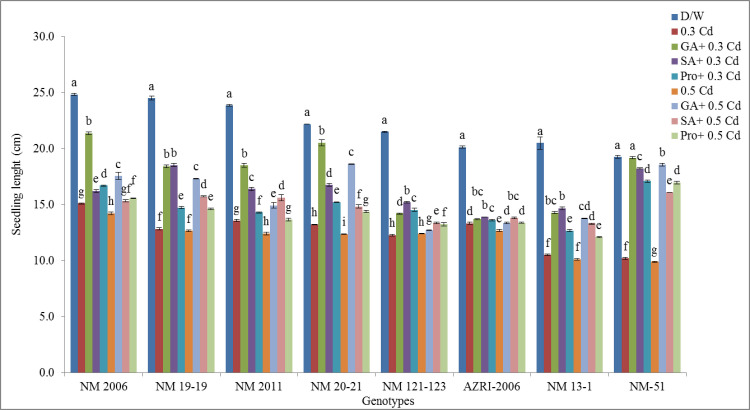
Seedling length
The highest inhibition in seedling length was observed in NM 13–1 (48.76%) followed by NM 19–19 (47.62%) and NM-51(47.09%) at 0.3 mM Cd. Similarly, at 0.5 mM Cd, inhibition in seedling length was highest in NM 13–1 (50.63%), then NM-51 (48.61%) and NM 19–19 (48.37%). Under 0.3 mM Cd and 0.5 mM Cd AZRI-2006 shows the least reduction in seedling length i.e. 33.76% and 36.99% respectively. The lowest inhibition was observed in NM-51 with pre-treatment of GA3 (0.43% and 3.60%), SA (5.36% and 16.50%) and proline (11.21% and 11.93%) at 0.3 and 0.5 mM Cd respectively (Fig 5).
Fig 5. Mean seedling length of mung bean genotypes grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
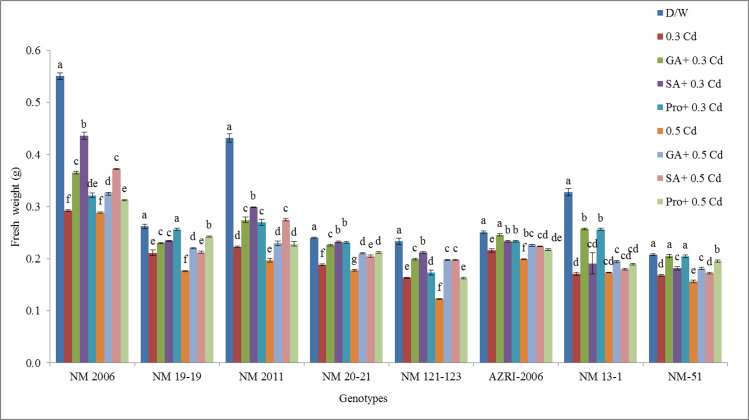
Fresh weight
The highest inhibition in fresh weight was observed in NM 2011 at 0.5 mM Cd with 54.44% inhibition while the lowest inhibition in fresh weight was observed in AZRI-2006 (13.94%) at 0.3 mM Cd as compared to control as shown in S3 Fig. Results also demonstrated that phytohormones and proline were helpful in partially alleviating the negative effects of Cd on mung bean seedlings. Alleviation was more prominent after priming with phytohormones and proline in NM 20–21, AZRI-2006 and NM-51 genotypes as compared to control. It was also worth mentioning that different genotypes have different interactions with both phytohormones and proline during Cd stress (Fig 6).
Fig 6. Mean fresh weight of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
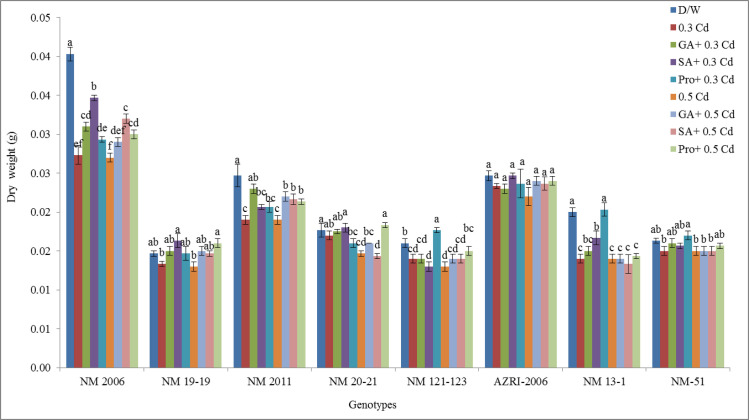
Dry weight
Results indicated that least inhibition in dry weight was observed in NM 20–21 (3.74%) and NM-51 (8.16%) at 0.3 mM Cd (shown in S4 Fig). Surprisingly in genotype AZRI-2006 non-significant difference in dry weight was observed in all treatments with respect to control even SA tends to keep dry weight unchanged (Fig 7).
Fig 7. Mean dry weight of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
The highest inhibition in dry weight was found in NM 2006 (32.20% and 33.10%) at 0.3 mM and 0.5 mM Cd respectively. NM 19–19, NM-51 and AZRI-2006 were least affected by Cd treatment with respect to dry weight. It was further noticed that there was a promotion in dry weight for all priming treatments in genotype NM 19–19, NM 2011 and NM-51 as compared to metal alone. The least alleviation in Cd toxicity was observed in NM 13–1 and NM 2006 under all pretreatments. There was variable response of different pretreatments on genotypes.
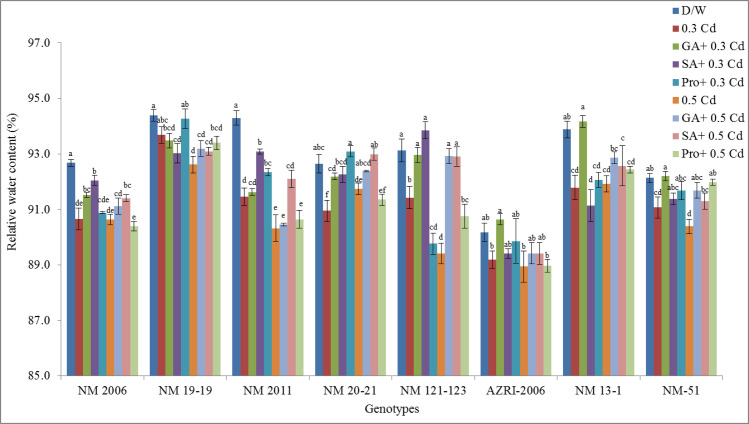
Relative water content
There was a significant decrease in mean relative water content in Cd treated seedlings with respect to control in a concentration-dependent manner (Fig 8).
Fig 8. Mean relative water content of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
While it was clearly observed that pretreatment of phytohormones and proline helped in reducing Cd toxicity on mung bean seedlings, with a variable genotypic response. In NM 20–21 under SA and GA3 pretreatment at 0.3 mM Cd increased in RWC was observed as compared to 0.3 mM Cd alone, however pretreatment with proline, was even higher than in control. The same results were obtained for NM 121–123 under SA pretreatment at 0.3 mM Cd. GA3 at 0.3 mM Cd also tends to increase RWC in NM 13–1 and NM-51. Results showed that relative water contents were severely affected in genotype NM 2011 (2.71% and 5.09%) in both the Cd treatments respectively. Whereas least inhibition in RWC was observed in NM 19–19 at 0.3 mM Cd (0.76%) treatment and in NM 20–21 (0.97%) at 0.5 mM Cd (shown in S5 Fig). Variable response of genotypes under pretreatments was also detected. RWC was found higher than control of few genotypes under some pretreatment at stress.
Alteration in antioxidant enzymes (APX, CAT, GPX, SOD) under stress
Four different antioxidant enzymes activities were analyzed in mung bean seedlings under Cd treatment along priming with phytohormones and proline prior to Cd. Table 3 shows that in all enzymes activities, genotypes and treatments were significantly different. Similarly, the interaction between genotypes and treatments was found to be highly significant (Table 3).
Table 3. Mean sum of squares for antioxidant enzymes, protein, MDA and proline of eight mung bean genotypes grown under Cd and pretreatments of GA3, SA and proline prior to Cd for 1 week.
| SOV | Df | MS | ||||||
|---|---|---|---|---|---|---|---|---|
| APX | CAT | GPX | SOD | Protein | MDA | Proline | ||
| Genotypes | 7 | 29.778** | 0.186** | 10675.649** | 968.489** | 251688.449** | 4052.844** | 24376.923** |
| Treatments | 8 | 12.496** | 0.095** | 1322.377** | 133.224** | 76791.241** | 8364.961** | 6955.175** |
| G×T | 56 | 2.678** | 0.013** | 334.351** | 34.928** | 5077.622** | 276.735** | 473.849** |
| Error | 144 | 0.003 | 0.00002 | 0.376 | 0.059 | 11.718 | 0.246 | 0.238 |
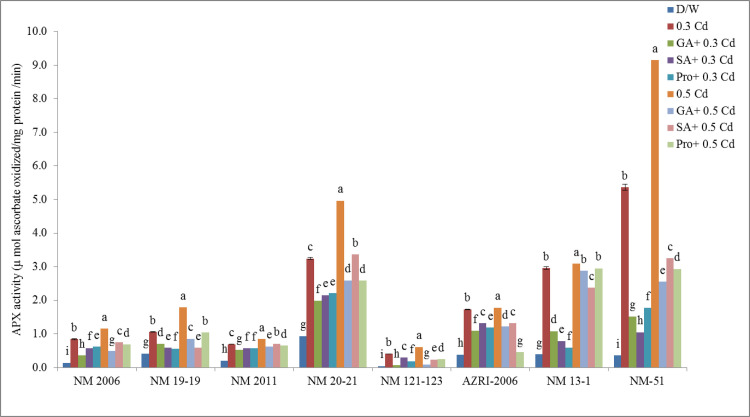
Ascorbate peroxidase
Results revealed that Cd treatment was responsible for the activation of APX enzymes in mung bean seedlings. The induced activity of the APX enzyme was noted in Cd-treated seedlings in a concentration-dependent manner. NM-51 showed the highest activities at both the Cd concentrations while lower activity was observed in NM 121–123 among all genotypes as illustrated in Fig 9.
Fig 9. Specific activity of APX enzyme mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
It was also noticed that pretreatment with GA3, SA and Proline was able to cause a decrease in Cd toxicity by decreasing APX enzyme as compared to Cd but was still higher than in control. The highest promotion in APX activity was observed in NM-51 at 0.5 mM Cd. While NM 19–19 showed the least promotion in APX activities under all pretreatments (shown in S6 Fig).
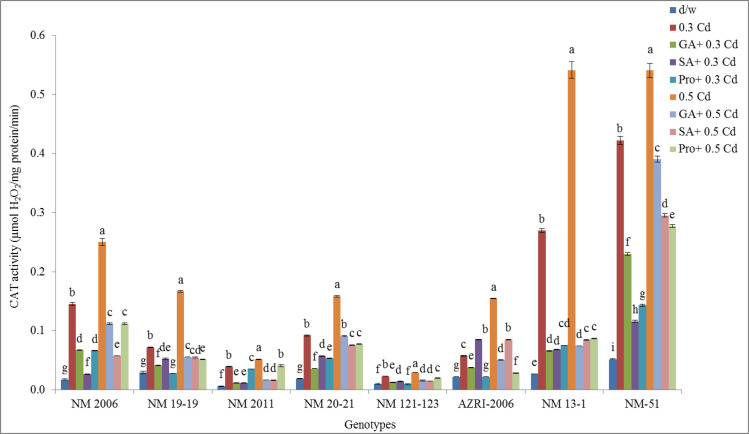
Catalase
It was demonstrated by the graph that CAT was increased by increasing Cd concentration. Among all genotypes, NM-51 and NM 13–1 have higher values of catalase at both the Cd treatments. While lower activities were observed in NM 19–19, NM 121–123 and AZRI-2006. While pretreatment with GA3, SA and proline prior to Cd tends to cause a decrease in CAT activities in all genotypes however these values were still higher than in control (Fig 10).
Fig 10. Catalase activity of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
While higher promotion in CAT activity was observed in NM-51, NM 13–1 and NM 2006 at both the Cd concentrations. The lowest activity for catalase were noticed in NM 121–123. While pretreatment with phytohormones before Cd tends to decrease catalase activity but is still higher than control. In Azri-2006 proline before 0.5 mM Cd was able to maintain this enzyme activity near to control (indicated in S7 Fig).
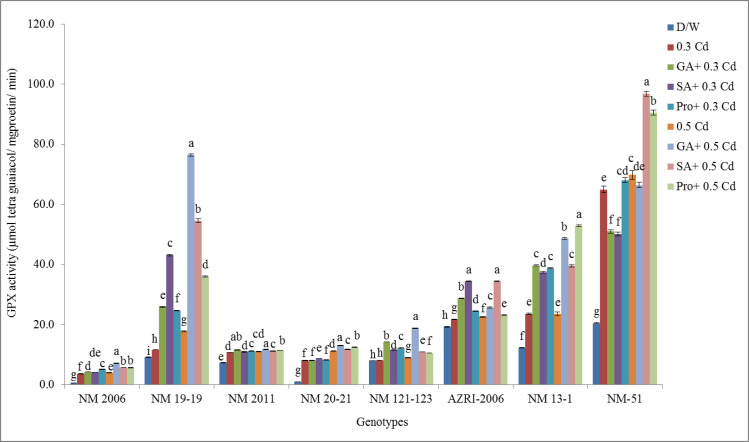
Guaiacol peroxidase
Cd treatment was responsible for increasing GPX activity in a concentration-dependent manner in most of the genotypes. NM 2006 shows the least values for GPX enzymes while higher values were observed in NM-51 under all treatments as well as in control (Fig 11).
Fig 11. Mean values for GPX activity in mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
While all the phytohormones before Cd tend to further promote the GPX activities in most of the genotypes with the non-significant promotion in few genotypes. While highest promotion in GPX activity were observed in pretreated samples of NM 20–21 and NM 2006 as compared to control (indicated in S8 Fig).
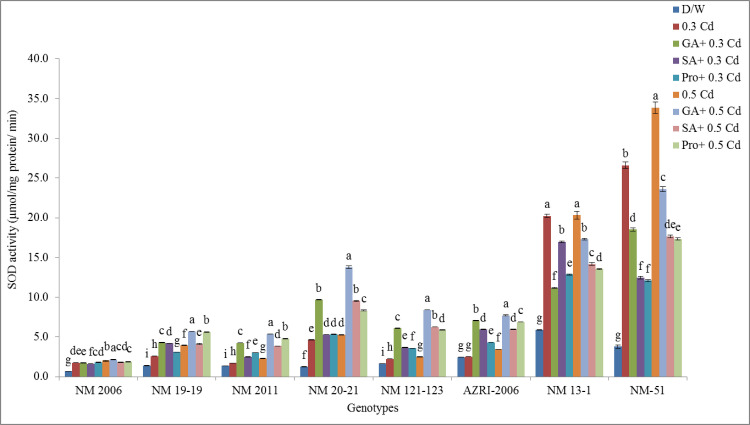
Superoxide dismutase
SOD is an important antioxidative enzyme. SOD activities showed different patterns among eight mung bean genotypes. The lowest activity under control as well as all treatments was measured in NM 2006 while NM 13–1 and NM-51 showed higher values for SOD. It was detected that SOD activity was increased under Cd treatment of all genotypes as compared to control. Pretreatments with phytohormones and proline before Cd showed a decrease in SOD activity of NM 13–1, NM-51 and NM 2006 as compared to metal stress alone. While other genotypes exhibited an increase in SOD activity when pretreated with phytohormones and proline as compared to Cd stress (Fig 12).
Fig 12. Mean SOD activities of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
The lowest promotion in SOD activity was observed in AZRI-2006 for all the treatments. While highest promotion values were observed in NM 20–21 under all the pretreatments (shown in S9 Fig).
Effect of Cd stress on biochemical (Protein, MDA, Proline) attributes
In 1 week Cd exposed mung bean seedlings recorded biochemical parameters were protein, proline, and MDA contents.
Statistical analysis for biochemical parameters showed that all the estimated parameters like protein, MDA, and proline were highly significantly different for genotypes and treatment. Table 3 also demonstrated a highly significant interaction between both factors.
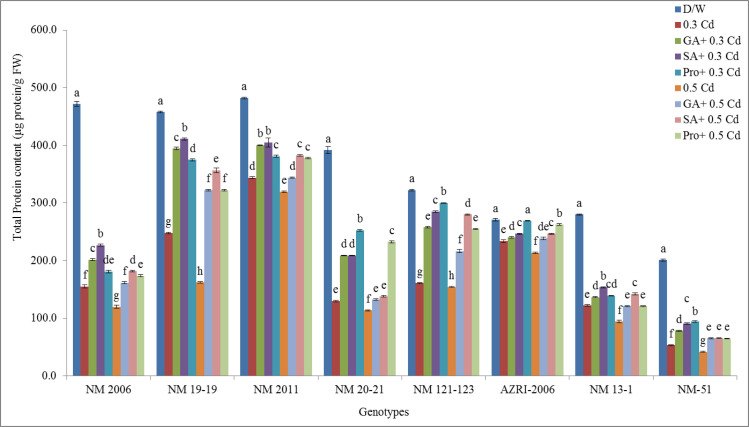
Total protein contents
Total soluble proteins were quantified on UV-Spectrophotometer at 750 nm. It was noticed that the highest protein values were observed in NM 2011 (482.33 μg/g FW) while lowest in NM-51 (201 μg/g FW) under control conditions. While Cd treatment gradually decreases protein contents in a concentration-dependent manner for all genotypes. The lowest value for protein was observed in NM-51 (41.66 μg/g FW) at 0.5 mM Cd treatment. All pretreatments specifically SA and proline were found to be more potent that tend to alleviate the Cd toxicity by increasing protein contents as compared to Cd alone but were still lower than control (Fig 13).
Fig 13. Mean total protein contents of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
The lowest reduction in protein content as compared to control was observed in AZRI-2006 under 0.3 mM and 0.5 mM Cd treatments (13.53% & 21.16% respectively) as well as under all the pretreatments. Whilst highest percent inhibition was observed in NM-51 at 0.3 and 0.5 mM Cd (73.47% and 79.27% respectively) (shown in S10 Fig).
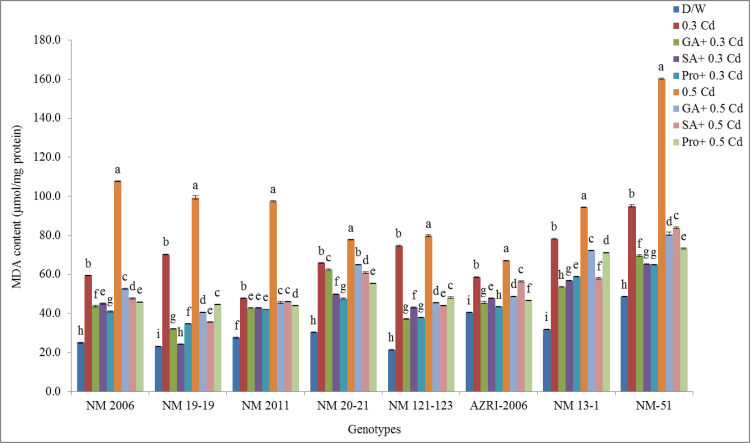
Lipid peroxidation (MDA contents)
It was observed that Cd tends to cause lipid peroxidation in mung bean seedlings that were calculated as MDA content. The highest values for MDA content were found to be in NM-51 at all treatments as well as at 0.3 and 0.5 mM Cd (95.06 and 160.24 μmol/μg protein) respectively. It was indicated from results that pretreatment helped in decreasing lipid peroxidation and membrane damage as indicated by a decrease in MDA values for all genotypes. The lowest values for MDA content were observed in NM 19–19 under all pretreatments at both the Cd concentrations as shown in Fig 14.
Fig 14. MDA contents of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
It was further noticed that proline pretreatment prior to Cd supply was more effective in lowering MDA contents at both the Cd concentrations in most genotypes. NM 121–123 showed the highest promotion (248.48%) while AZRI-2006 showed the lowest (44.80%) at 0.3 mM Cd among all genotypes. More improvement in membrane damage was observed in AZRI-2006 by phytohormones at both the Cd concentrations indicated by lowered MDA content. At a higher concentration of Cd, both NM 2006 and NM 19–19 showed approx. similar promotion values for MDA (329.81 & 329.80%) respectively however least values were found in AZRI-2006 (65.50%) (shown in S11 Fig). The above results showed that Cd was responsible for high membrane damage while pretreatments of phytohormones and proline prior to Cd reduced the membrane damage and protect the cells.
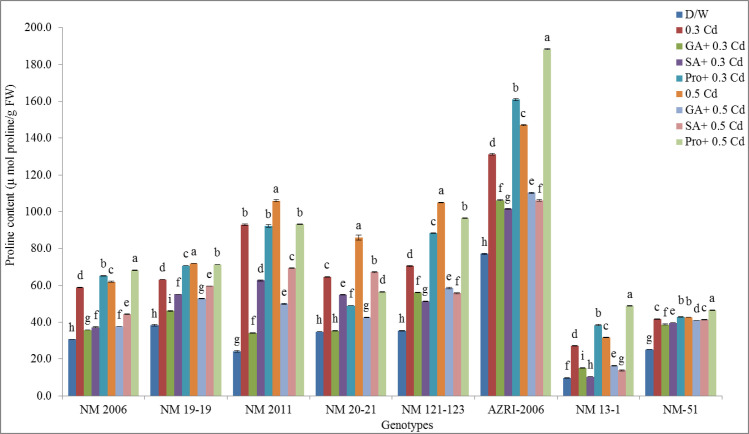
Proline contents
Increased Cd concentration tends to elevate the proline contents in mung bean seedlings as compared to control. Under control treatment, genotypes behave in the same way by producing the lowest proline as compared to any treatment. AZRI-2006 showed the highest proline contents (77.18 μmol/g FW) while NM 13–1 showed the least values (9.74 μmol/g FW) in control among all genotypes. It was noticed that the pretreatments before Cd were able to reduce proline content. However, promotion in proline was seen in all genotypes except NM 20–21 when seeds were imbibed in proline and then treated with Cd (Fig 15).
Fig 15. Mean proline contents of mung bean grown under Cd and pretreatments of GA3, SA and proline prior to Cd.
Proline accumulation under both the Cd treatment was in a dose-dependent manner in all mung bean seedlings. The highest promotion in proline contents was noticed in NM 2011 (284.90% and 338.00% at 0.3 & 0.5 mM Cd respectively) followed by NM 13–1. However, NM 19–19, AZRI-2006 and NM-51 showed a similar pattern for proline percent promotion in all treatments. Surprisingly it was further noticed that in genotype NM 13–1 percent promotion in proline pretreated seedlings was higher among all genotypes (295.10% and 401.80% at 0.3 and 0.5 mM Cd after proline pretreatment respectively) (shown in S12 Fig).
Pearson correlation among different parameters had been tested during this experiment. Morphological parameters (except with DW with RWC), protein, mitotic index and activities were found to be positively correlated among each other. While MDA, antioxidant enzymes and total aberration% were found to be negatively correlated with all growth parameters and protein. Proline negatively correlated with antioxidant enzymes but was non-significant with MDA, while all the antioxidant enzymes and MDA contents were found to have a significant positive correlation as indicated in Table 4.
Table 4. Pearson correlation coefficient among all tested parameters in mung bean seedlings grown under Cd stress with prior application of phytohormones and proline.
| FW | DW | RWC | Proline | MDA | Protein | APX | CAT | GPX | SOD | MI | Aberra | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | 0.56** | 0.30** | 0.37** | -0.38** | -0.56** | 0.44** | -0.40** | -0.32** | -0.15* | -0.32** | 0.61** | -0.65** |
| FW | 0.82** | 0.20** | -0.20** | -0.46** | .044** | -0.40** | -0.31** | -0.40** | -0.46** | 0.60** | -0.42** | |
| DW | -0.38** | 0.16* | -0.27** | 0.22** | -0.31** | -0.20** | -0.38** | -0.42** | 0.60** | -0.29** | ||
| RWC | -0.61** | -0.30** | 0.27** | -0.13 | -0.16* | 0.004 | -0.03 | -0.04 | -0.18** | |||
| Proline | -0.01 | 0.16* | -0.11 | -0.19** | -0.15* | -0.32** | 0.22** | 0.09 | ||||
| MDA | -0.72** | 0.76** | 0.77** | 0.37** | 0.64** | -0.75** | 0.79** | |||||
| Protein | -0.60** | -0.65** | -0.43** | -0.65** | 0.56** | -0.44** | ||||||
| APX | 0.75** | 0.46** | 0.74** | -0.61** | 0.52** | |||||||
| CAT | 0.53** | 0.77** | -0.68** | 0.58** | ||||||||
| GPX | 0.70** | -0.40** | 0.09 | |||||||||
| SOD | -0.70** | 0.30** | ||||||||||
| MI | -0.74** |
Discussion
In developing countries, good quality fertilizers are expensive to be used by the poor farmers so interchangeably they use sewage/industrial wastewater and cheap fertilizers to irrigate their crops which contain a large amount of various heavy metals. These heavy metals may absorb and reside in plants and can cause toxic effects to human beings or farm animals when consumed. On the other hand, heavy metals like Cd exhibited negative effects on the growth of plants which ultimately reduce the yield of the crop. This study was performed to analyze the clastogenic effect of Cd on mung bean seedlings and to find out whether phytohormones and proline help in acclimatizing cytogenetic effects and oxidative stress due to Cd. This study showed that Cd tends to decrease mitotic division and cause different chromosomal disorders in all mung bean varieties. AZRI-2006 showed better values for cell division (MI%) under Cd concentrations as compared to other varieties as indicated in the results section that showed its better genetic potential against Cd. Whereas, NM-51 was found to be the least resistant to Cd at both concentrations. It has been studied that binding of Cd with sulfhydryl group (-SH) of enzymes inhibits mitotic index [33] and decline in MI suggested that the Cd could prevent cells from going into cell division. Similar results regarding mitotic index under cadmium stress were also reported in other crops including Vigna radiata [8, 34], lentils [35], cowpea [36], broad bean [37, 38].
The decrease in MI in Cd-treated root was might be due to disturbances during the process of chromatin formation in the cell cycle that resulted due to interactions between DNA and heavy metals [39]. Reduction in root growth and cell division under cadmium stress was prominent in other plants. It has been described that Cd tends to disturb roots structure by damaging cell nucleoli and inhibits the repair mechanics of DNA [40].
Results of the present work demonstrated various chromosomal abnormalities included C-mitosis, sticky chromosomes, laggards and fragmented that were due to distractions in spindle fibers during cell division of mung bean. Oxidative stress caused by Cd in cowpea is the main cause of damaged DNA that leads to multiple anomalies in chromosomes during cell division, like strand breaks, removal and alterations in nitrogenous basis [36]. Fragments observed in mitotic slides under Cd stress were formed due to the stickiness of chromosomes and chromatids that were not separated at the poles. Cd-treated samples exhibited fragmented chromosomes in all varieties. It was also suggested that ROS tends to cause breakage in a double strand of DNA [41]. The presence of laggard chromosomes in treated samples was formed by instabilities in the spindle apparatus or the centromere. These results are in accordance with [42] who reported that weak C-mitotic effect was responsible for chromosome lagging which may lead to aneuploidy.
There are certain strategies to reduce environmental stresses like heavy metal toxicity in plants, among them an appropriate dose of phytohormones is also a major approach [43]. In this research, we have done priming of seeds with phytohormones (GA3 and SA) and proline prior to Cd stress. Researchers reported that under control conditions, GA3 acts as initiating source to activate loading and transportation of mineral elements within the plants [44]. In many plants, heavy metals may alter the endogenous quantity of phytohormones so an exogenous application of phytohormones helps in reducing metal uptake from the soil that ultimately declines metal toxicity [45], as shown in exogenously supplied GA3 under Cd stress stimulates growth in tomato plants [46]. Interestingly in proposed research, mung bean seeds pretreated with GA3 tend to increase mitotic index grown under Cd stress as compared with non-pretreated seedlings and cause a reduction in chromosomal aberrations. So, it can be deduced that priming with GA3 tends to mitigate the adverse effects of Cd on mung bean cell division. Similarly, other scientists also reported the potentiality of GA3 in alleviating the detrimental effect of metals hence proves its beneficial role in acclimatizing metal stress on Arabidopsis and Lupin [47]. Furthermore, GA3 treatment greatly increased protein as compared to Cd treated plants as reported earlier [48].
Previous research showed the protective role of SA on various crops in signaling under metal toxicity [49]. It has also been suggested that SA facilitates normal growth and has the potential to alleviate Cd toxicity by phytoremediation [50]. Cd tends to decrease mitotic index and increase chromosomal abnormalities in rice, but SA priming of seeds showed an increase in MI% and alleviates Cd adverse effects to promote better growth. Furthermore, it is reported that SA has the potential to increase agriculture production in terms of quality and quantity under various abiotic stresses including metals [51].
Our results showed a decrease in seedling length and biomass with increasing Cd concentration are in accordance with other studies done on mung bean or other crops [52–55]. In our results, AZRI-2006 showed better growth under Cd treatments that can be attributed to its better genetic potential as has been observed in other mung bean cultivars [56]. In our results, the Cd stress to mung bean seedlings seemed to reduce the availability of water to plant, and this may be the reason for the reduction of seedling growth in our experiment supported by other work [57, 58]. In this research, decrease in soluble protein was also recorded in Cd treated seedlings in a concentration-dependent manner, which is associated with reduced plant growth as a result of an imbalance in ion homeostasis [59]. The differential protein expression in spinach reported altered leaf proteome as a defensive mechanism under Cd stress [60]. Metal stress is believed to accelerate protein and starch degradation by increasing the levels of soluble sugars and amino acids [61] that also support our results. The findings of the present study showed that Cd toxicity probably damages the plasma membrane of root cells thereby altering its permeability which affects nutrient uptake by roots. Plants exposed to Cd stress are responsible for altering the nutrient metabolic balances in roots [62]. Current results demonstrated promotion in various antioxidant enzymes in Cd stressed seedlings of all genotypes, supported by other researchers as well [63]. Enhanced activities of APX, GPX and CAT were also reported earlier in Cd resistant genotypes of many cereals, vegetables and legumes [64–66]. An increase in these enzymes activity under Cd treatment suggested their role in minimizing increased levels of lipid peroxidation and peroxide content caused by ROS, helping the seedlings to maintain their normal growth. Most of the work reported showed an increase in antioxidant enzymes activities with few contrary results. Therefore, the exact mechanisms to scavenge excess hydrogen peroxide levels are not only dependent on plant species but also on some external factors, although at least one of these enzymes is found to be in higher levels under stress conditions. Seeds pretreated with phytohormone prior to Cd reduce APX, CAT, and SOD activity, indicating that the toxic effects have probably overcome efficiently by the antioxidant system. An increase in the level of antioxidative defense enzymes can be considered as their defensive potential under stress. Under severe stress, this system failed to reduce the harmful effects more effectively in a few of our genotypes (NM 2006, NM 13–1 & NM-51), or this could be due to their sensitive genotypes itself. Various cytological irregularities were observed during Cd stress in Nigella sativa while post-treatment of SA resulted in a significant increase in MI% with the reduction in chromosomal aberrations. These results are consistent with our findings that observed increase mitotic index and reduced chromosomal aberrations which confirmed ameliorating property of SA under stress [67]. It was reported that Cd tends to reduce the mitotic activity of root cells thus decrease plant growth by shortening G2 phases and prophase that facilitates the abnormal spindle formation while SA pretreatment increased the mitotic index.
Pretreatment with a low concentration of SA before Cd treatment lowered the elevated levels of ROS and enhanced the activities of various antioxidant enzymes thus guarding the plants against oxidative explosion [68]. Our results reported a decline in the activities of the antioxidant enzymes with pretreatment of salicylic acid as compared to Cd stress alone by higher than in control supported by work on rice [69]. There is data on the role played by salicylic acid on plants in signaling and alleviating metal injury [70, 71]. Endogenous and exogenous SA is important in mitigating the uptake of Cd ions and protecting plants against heavy metal stress. Promotion of SA was observed in various Cd-treated plants [72–74]. Particularly, an increase in levels of SA in roots during Cd stress in wheat, might be related to the enhancement of the glutathione cycle, so stimulating antioxidant and metal detoxification systems, which promotes Cd tolerance [75]. The mechanisms that alleviated the Cd toxicity include its uptake reduction and supply in plants, the complex formation and sequestration of Cd, the increase of net photosynthesis and the transpiration rates, to scavenge ROS by enhancing antioxidant defense system to protect membrane [76, 77]. It was suggested earlier that SA is capable of facilitating plant growth and reducing/detoxifying Cd toxicity and could be a promising tool in increasing phytoremediation efficiency. Our results showed that APX and CAT activities decrease in pretreated SA samples while SOD and GPX enzymes further increase in SA pretreatment.
Results obtained from the present investigation revealed that exogenous SA ameliorates the toxic effect of Cd. It was also been demonstrated that the effect of SA not only depends upon its nature, whether it has been provided as acid solely (SA) or as a sodium salt (NaSA). Although the mode of treatment is very crucial as different combinations have variable effects even at the same concentrations [78].
Proline has been cited in the literature as a multi-functional molecule that has a role as an osmoprotectant and a radical scavenger [79]. It is also a helpful stress alleviator that tends to improve plant growth, alter enzyme activity and by increasing MI% under Cd stress [16, 80, 81]. However, our results showed that priming with proline under Cd treatment increases mitotic index and decreases chromosomal aberration caused by Cd. Similar findings were for other abiotic stresses on different crops [82]. Like our results, exogenously supplied proline in cultured tobacco Bright Yellow-2 (BY-2) cells mitigates the Cd-induced inhibitory effects on the growth [83]. This is well-established fact that sugars, amino acids including proline play a major protective role in maintaining osmotic potential or direct detoxification of ROS [84, 85]. Proline accumulated during stress episodes is degraded for energy supply which is utilized to drive growth and relieve the stress, hence helps to continue growth under long‐term stress. Exogenously supplied proline tends to alleviate adverse effects of Cd on growth parameters, antioxidative enzymes capacities, and thus by decreasing MDA and H2O2 contents [86, 87]. In conclusion, there is a great benefit of applying phytohormones and proline prior to Cd treatment that will be helpful in improving metal tolerance of mung bean seedlings.
Conclusion
This research suggested that priming with an appropriate amount of phytohormones and proline is a very cost-effective and feasible strategy to induce an adaptive response in mung bean under Cd stress that significantly enhances the mitotic index and decreasing the total percentage of chromosomal abnormalities as compared to Cd treatment alone. An increase in all growth attributes including protein were observed with an increased level of antioxidant enzymes and decreased in lipid peroxidation. Among phytohormones and proline, GA3 was found to be more promising in mitigating the adverse effects of Cd for all mung bean varieties tested in this research. These findings will be very helpful for farmers to also make use of Cd polluted lands for cultivation of mung beans after pretreatment of seeds with any of the above phytohormones or proline. There is some hope that this mitigating strategy might allow mung bean to grow better even when irrigating with sewage or industrial wastewater. Further research is in progress to understand complete mechanisms that how phytohormones and proline influence other physiological and biological functions in mung bean under Cd stress.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
The authors like to acknowledge NARC for providing mung bean germplasm for this study and also Dr. Tabassum Ara Khanum of NNRC (University of Karachi) for assisting in taking pictures of mitotic slides.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Choppala G, Saifullah Bola N, Bibi S, Iqbal M, Rengel Z, Kunhikrishnan A, et al. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci. 2014; 33: 374–391. [Google Scholar]
- 2.Pajevic S, Borisev M, Nikolic N, Arsenov D, Orlovic S, Zupunski M. Phytoextraction of heavy metals by fast-growing trees. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L, editors. Phytoremediation management of environmental contaminants. Netherlands (NL), Springer; 2016. pp. 29–64. [Google Scholar]
- 3.Galal T. Health hazards and heavy metals accumulation by summer squash (Cucurbitapepo L.) cultivated in contaminated soils. Environ Monit Assess. 2016; 188: 434. doi: 10.1007/s10661-016-5448-3 [DOI] [PubMed] [Google Scholar]
- 4.Kidd PS, Domínguez-Rodríguez MJ, Díez J, Monterroso C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere. 2007; 66: 1458–1467. doi: 10.1016/j.chemosphere.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Zhang W, Yang X, Wang P, McGrath SP, Zhao FJ. Effective methods to reduce cadmium accumulation in rice grain. Chemosphere. 2018; 207: 699–707. doi: 10.1016/j.chemosphere.2018.05.143 [DOI] [PubMed] [Google Scholar]
- 6.Bashir A, Rizwan M, Ali S, Rehman MZ, Ishaque W, Riaz MA, et al. Effect of foliar applied iron complexed with lysine on growth and cadmium uptake in rice under Cd stress. Environ Sci Pollut Res. 2018; 25: 20691–20699. doi: 10.1007/s11356-018-2042-y [DOI] [PubMed] [Google Scholar]
- 7.DalCorso G, Farinati S, Furini A. Regulatory networks of cadmium stress in plants. Plant Signal Behav. 2010; 5: 663–667. doi: 10.4161/psb.5.6.11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muneer S, Qadri TN, Mahmooduzaffar Siddiqi TO. Cytogenetic and biochemical investigations to study the response of Vigna radiata to cadmium stress. Afri J Plant Sci. 2011; 5: 183–92. [Google Scholar]
- 9.Aslam M, Saeed MS, Sattar S, Sajad S, Sajjad M, Adnan M, et al. Specific role of proline against heavy metals toxicity in plants. Int J Pure Appl Biosci. 2017; 5: 27–34. [Google Scholar]
- 10.Gantayat S, Mania S, Pradhan C, Das AB. Ionic stress induced cytotoxic effect of cadmium and nickel ions on roots of Allium cepa L. Cytologia. 2018; 83: 143–148. [Google Scholar]
- 11.Stoyanov IY, Vasileva PL, Popova TP, Slavova BN. The effects of lead and cadmium on cell division and chromosomal structure in Allium cepa test system in vivo. Ecol Balkan. 2018; 10: 73–81. [Google Scholar]
- 12.Cui WT, Li L, Gao ZZ, Wu HH, Xie YJ, Shen WB. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J Exp Bot. 2012; 63: 5521–5534. doi: 10.1093/jxb/ers201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicka-Russak M, Kabala K, Burzynski M. Different effect of cadmium and copper on H+ -ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J Exp Bot. 2012; 63: 4133–4142. doi: 10.1093/jxb/ers097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Liu T, Li Y, Li F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci Total Environ. 2018; 644: 602–610. doi: 10.1016/j.scitotenv.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Rood SB. Application of gibberellic acid to control tillering in early maturing maize. Can J Plant Sci.1985; 65: 901–911. [Google Scholar]
- 16.Hartmann A, Senning M, Hedden P, Sonnewald U, Sonnewald S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2010; 155: 776–796. doi: 10.1104/pp.110.168252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metwally A, Finkemeier I, Georgi M. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Egypt J Agric Res. 2003; 132: 272–81. doi: 10.1104/pp.102.018457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayat S, Hayat Q, Alyemeni M, Ahmad A. Proline enhances antioxidative enzyme activity, photosynthesis and yield of Cicer arietinum L. exposed to cadmium stress. Acta Bot Croat. 2013; 72: 323–335 [Google Scholar]
- 19.Keatinge JDH, Easdown WJ, Yang RY, Chadha M, Shanmugasundaram S. Overcoming chronic malnutrition in a future warming world: the key importance of mung bean and vegetable soybean. Euphytica. 2011; 180: 129–141. [Google Scholar]
- 20.Graham PH, Vance CP. Legumes: importance and constraints to greater use. Plant Physiol. 2003; 131: 872–877. doi: 10.1104/pp.017004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiskesjo G. The Allium test as a standard in environmental monitoring. Hereditas. 1985; 102: 99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Jiang W, Gao X. Effects of cadmium on root growth, cell division and nucleoli in root tips of garlic. Biol Plant. 2003/2004; 47: 79–83. [Google Scholar]
- 23.Sumithra K, Jutur PP, Carmel BD, Reddy AR. Salinity induced changes in two cultivars of Vigna radiata responses of antioxidative and proline metabolism. Plant Growth Regul. 2006; 50: 11–22. [Google Scholar]
- 24.Jiang Y, Huang B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in cool season grasses. J Exp Bot. 2001; 52: 341–349. [PubMed] [Google Scholar]
- 25.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265–275. [PubMed] [Google Scholar]
- 26.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981; 22: 867–880. [Google Scholar]
- 27.Aebi H. Catalase in vitro. Meth Enzymol. 1984; 105: 121–126. doi: 10.1016/s0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 28.Everse J, Johnson MC, Marini MA. Per oxidative activities of hemoglobin derivatives. In: Everse J, Vandegriff KD, Winslow RM editors. Methods in Enzymology. London, Academic Press; 1994. pp. 547–561. doi: 10.1016/0076-6879(94)31038-6 [DOI] [PubMed] [Google Scholar]
- 29.Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44: 276–287. doi: 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- 30.Heath RL, Packer L. Photo peroxidation in isolated chloroplasts. I kinetics and stoichiometry of fatty acid and peroxidation. Arch BiochemBiophys. 1968; 125: 189–198. [DOI] [PubMed] [Google Scholar]
- 31.Bates LS, Waldran RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973; 39: 205–208. [Google Scholar]
- 32.Steel RGD, Torrie JH. Principles and procedures of statistics. New York: McGraw Hill; 1980. [Google Scholar]
- 33.Van Assche F, Clijsters H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990; 13: 195–206. [Google Scholar]
- 34.Sumitra R Nair, Rajani V. Effects of heavy metals on seed germination and protein content of Vigna radiata (L) Wilczek. Int J Adv Res. 2015; 3: 1306–1317. [Google Scholar]
- 35.Kiran Y, Sahin A. The effects of cadmium on seed germination, root development, and mitotic of root tip cells of lentil (Lens culinaris Medik).World J AgriSci. 2006; 2: 196–200. [Google Scholar]
- 36.Amirthalingam T, Velusamy G, Pandian R. Cadmium-induced changes in mitotic index and genotoxicity on Vigna unguiculata (Linn) Walp. J Environ Chem Ecotoxicol. 2013; 5: 57–62. [Google Scholar]
- 37.George NM. Evaluation on mutagenic effects of three heavy metals on Vicia faba. Cytologia. 2000; 65: 75–82. [Google Scholar]
- 38.Arya SK, Mukherjea A. Sensitivity of Allium cepa and Vicia faba towards cadmium toxicity. J Soil Sci Plant Nutr. 2014; 14: 474–458. [Google Scholar]
- 39.Seth CS, Misra V, Chauhan LKS, Singh RR. Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicol Environ Saf. 2008; 71: 711–716. doi: 10.1016/j.ecoenv.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Wang QL, Liu DH, Yue JY. The uptake of cadmium by Allium cepa var agrogarum L. and its effects on chromosome and nucleolar behavior in root tip cells. Phyton-Int J Exp Bot. 2016; 85: 155–161. [Google Scholar]
- 41.Weckx JEJ, Clijsters HMM. Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant PhysiolBiochem. 1997; 35: 405–410. [Google Scholar]
- 42.Fiskesjo G. Assessment of a chemical’s genotoxic potential by recording aberration in chromosomes and cell divisions in root tips of Allium cepa. Environ Toxicol Water Qual. 1997; 9: 235–241. [Google Scholar]
- 43.Li XM, Ma LJ, Bu N, Li YY, Zhang LHF. Effects of salicylic acid pretreatment on cadmium and/or UV-B stress in soybean seedlings. Biol Plant. 2014; 58: 195–199. doi: 10.1016/j.ecoenv.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 44.Aloni B, Dale J, Wyse RE. Enhancement of [14C] sucrose export from source leaves of Vicia faba by gibberellic acid. Plant Physiol. 1986; 82: 962–966. doi: 10.1104/pp.82.4.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poschenrieder C, Gunsé B, Barceló J. Influence of cadmium on water relations, stomatal resistance, and abscisic acid content in expanding bean leaves. Plant Physiol. 1989; 90: 1365–1371. doi: 10.1104/pp.90.4.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khavari-Nejad RA, Najafi F, Ranjbari M. The effects of GA3 application on growth, lipid peroxidation, antioxidant enzymes activities, and sugars levels of cadmium stressed tomato (Lycopersicon esculentum mill. cv. CH) plants. Rom J Biol-Plant Biol. 2013; 58: 51–60. [Google Scholar]
- 47.Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Lin GX, et al. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater. 2012; 239:302–307 doi: 10.1016/j.jhazmat.2012.08.077 [DOI] [PubMed] [Google Scholar]
- 48.Mansour MM, Kamel EA. Interactive effect of heavy metals and gibberellic acid on mitotic activity and some metabolic changes of Vicia faba L. plants. Cytologia. 2005; 70: 275–282. [Google Scholar]
- 49.Belkadhi A, De Haro A, Soengas P, Obregon S, Cartea ME, Chaibi W, et al. Salicylic acid increases tolerance to oxidative stress induced by hydrogen peroxide accumulation in leaves of cadmium-exposed flax (Linumusitatissimum L). J Plant Interact. 2014; 9: 647–654. [Google Scholar]
- 50.Singer AC, Crowley DE, Thompson IP. Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 2003; 21: 123–130. doi: 10.1016/S0167-7799(02)00041-0 [DOI] [PubMed] [Google Scholar]
- 51.Wang LJ, Li SH. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006; 170: 685–694. [Google Scholar]
- 52.Bhardwaj P, Chaturvedi AK, Prasad P. Effect of enhanced lead and cadmium in soil on physiological and biochemical attributes of Phaseolus vulgaris L. Nat Sci. 2009; 7: 63–75. [Google Scholar]
- 53.Zhang F, Zhang H, Xia Y, Wang G, Xu L, and Shen Z. Exogenous application of salicylic acid alleviates cadmium toxicity and reduces hydrogen peroxide accumulation in root apoplasts of Phaseolus aureus and Vicia sativa. Plant Cell Rep. 2011; 30: 1475–1483. doi: 10.1007/s00299-011-1056-4 [DOI] [PubMed] [Google Scholar]
- 54.Abraham K, Sridevi R, Suresh B, Damodharam T. Effect of heavy metals (Cd, Pb, Cu) on seed germination of Arachis hypogeae. L. Asian J Plant Sci Res. 2013; 3: 10–12. [Google Scholar]
- 55.Ashraf MY, Roohi M, Iqbal Z, Öztürk M, Gücel S. Cadmium (Cd) and lead (Pb) induced changes in growth, some biochemical attributes and mineral accumulation in two cultivars of mung bean [Vigna radiata (L.) Wilczek]. Commun Soil Sci Plant Anal. 201; 47: 405–413. [Google Scholar]
- 56.Simonova E, Henselova M, Masarovicova E, Kohanova J. Comparison of tolerance of Brassica juncea and Vigna radiata to cadmium. Biol Plant. 2007; 51: 488–492 [Google Scholar]
- 57.Zaier H, Mudarra A, Kutscher D, De la Campa MRF, Abdelly C, SanzMedel A. Induced lead binding phytochelatins in Brassica juncea and Sesuvium portulacastrum investigated by orthogonal chromatography inductively coupled plasma-mass spectrometry and matrix assisted laser desorption ionization-time of flight-mass spectrometry. Anal Chim Acta. 2010; 671: 48–54. doi: 10.1016/j.aca.2010.04.054 [DOI] [PubMed] [Google Scholar]
- 58.Ali B, Gill RA,Yang S, Gill MB, Farooq MA, Liu D, et al. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE. 2015; 10. doi: 10.1371/journal.pone.0123328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int J Mol Sci. 2012; 13: 6604–6619. doi: 10.3390/ijms13066604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagheri R, Bashir H, Ahmad J, Baig A, Qureshi MI. Effects of cadmium on leaf proteome of Spinacia oleracea (spinach). Int J Agric Food Sci Technol. 2013; 4: 33–36. [Google Scholar]
- 61.Hirve M, Bafna A. Effect of Cadmium exposures on growth and biochemical parameters of Vigna radiata seedlings. Int J Environ Sci.2013; 4: 315–322. [Google Scholar]
- 62.Muneer S, Hakeem KR, Mohamed R, Hyun Lee J. Cadmium Toxicity Induced Alterations in the Root Proteome of Green Gram in Contrasting Response towards Iron Supplement. Int J Mol Sci. 2014; 15: 6343–6355. doi: 10.3390/ijms15046343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smeets K, Cuypers A, Lambrechts A, Semane B, Hoet P, Van Laere A, et al. Induction of oxidative stress and antioxidative mechanisms in Phaeslous vulgaris after cadmium application. Plant PhysiolBiochem. 2005; 43: 437–444. [DOI] [PubMed] [Google Scholar]
- 64.Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci.1997; 127: 139–147. [Google Scholar]
- 65.Wu FB, Zhang GP, Dominy P. Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot. 2003; 50: 67–78. [Google Scholar]
- 66.Anjum SA, Tanveer M, Hussain S, Bao M, Wang LC, Khan I, et al. Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ SciPollut Res. 2015; 22: 17022. doi: 10.1007/s11356-015-4882-z [DOI] [PubMed] [Google Scholar]
- 67.El-Ghamery AA, Mousa MA. 2018. Salicylic acid triggers adaptation cadmium cytogenetic toxicity in roots of Nigella sativa L. Egypt J Bot. 2018; 58: 297–310. [Google Scholar]
- 68.Panda SK, Patra HK. Effect of salicylic acid potentiates cadmium induced oxidative damage in Oryza sativa L. leaves. Acta Physiol Plant. 2007; 29: 567–575. [Google Scholar]
- 69.Choudhury S, Panda SK. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol. 2004; 30: 95–110. [Google Scholar]
- 70.Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, et al. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant PhysiolBiochem. 2009; 47: 224–231. doi: 10.1016/j.plaphy.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 71.Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015; 6: 462. doi: 10.3389/fpls.2015.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, et al. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006; 29: 1532–1544 doi: 10.1111/j.1365-3040.2006.01531.x [DOI] [PubMed] [Google Scholar]
- 73.Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 2008; 165: 920–931. doi: 10.1016/j.jplph.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 74.He J, Ren Y, Pan X, Yan Y, Zhu C, Jiang D. Salicylic alleviates the toxicity effect of cadmium on germination, seedling growth and amylase activity of rice. J Plant Nutr Soil Sci. 2010; 173: 300–305. [Google Scholar]
- 75.Kovacs V, Gondor OK, Szalai G. Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J Hazard Mat. 2014; 280: 12–19. doi: 10.1016/j.jhazmat.2014.07.048 [DOI] [PubMed] [Google Scholar]
- 76.Noriega G, Caggiano E, Lecube ML, Cruz DS, Batlle A, Tomaro M, et al. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals. 2012; 25: 1155–1165. doi: 10.1007/s10534-012-9577-z [DOI] [PubMed] [Google Scholar]
- 77.Wang CX, Tao L, Ren J. The response of maize seedlings to cadmium stress under hydroponic conditions. Russ J Plant Physiol. 2013; 60: 295. [Google Scholar]
- 78.Gondor OK, Pál M, Darkó É, Janda T, Szalai G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS One. 2016; 11: 1–18. doi: 10.1371/journal.pone.0160157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.KaviKishor PB, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2013; 37: 300–311. doi: 10.1111/pce.12157 [DOI] [PubMed] [Google Scholar]
- 80.Hossain MA, Hasanuzzaman M, Fujita M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants. 2010; 16: 259–272. doi: 10.1007/s12298-010-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zouari M, Ben Ahmed C, Elloumi N, Bellassoued K, Delmail D, Labrousse P, et al. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Oleaeuropaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf. 2016; 128: 195–205. doi: 10.1016/j.ecoenv.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 82.Ehsanpour AA, Fatahian N. Effects of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult. 2003; 73: 53–56. [Google Scholar]
- 83.Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, et al. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol. 2009; 166: 1587–1597. doi: 10.1016/j.jplph.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 84.Sharma SS, Dietz KJ. The significance of amino acids and amino acid derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006; 57: 711–726. doi: 10.1093/jxb/erj073 [DOI] [PubMed] [Google Scholar]
- 85.Keunen E, Peshev D, Vangronsveld J, van den Wim E, Cuypers A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013; 36: 1242–1255. doi: 10.1111/pce.12061 [DOI] [PubMed] [Google Scholar]
- 86.Rouina B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. CV Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf. 2016; 128. [DOI] [PubMed] [Google Scholar]
- 87.Hassan M, Mansoor S. Priming seeds with phytohormones alleviates cadmium toxicity in mung bean (Vigna radiata L. Wilczek) seedlings. Pak J Bot. 2017; 49: 2071–2078. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.