Abstract
Acute myeloid leukemia (AML) is as a highly aggressive and heterogeneous hematological malignancy. MiR-20a-5p has been reported to function as an oncogene or tumor suppressor in several tumors, but the clinical significance and regulatory mechanisms of miR-20a-5p in AML cells have not been fully understood. In this study, we found miR-20a-5p was significantly decreased in bone marrow from AML patients, compared with that in healthy controls. Moreover, decreased miR-20a-5p expression was correlated with risk status and poor survival prognosis in AML patients. Overexpression of miR-20a-5p suppressed cell proliferation, induced cell cycle G0/G1 phase arrest and apoptosis in two AML cell lines (THP-1 and U937) using CCK-8 assay and flow cytometry analysis. Moreover, miR-20a-5p overexpression attenuated tumor growth in vivo by performing tumor xenograft experiments. Luciferase reporter assay and western blot demonstrated that protein phosphatase 6 catalytic subunit (PPP6C) as a target gene of miR-20a-5p was negatively regulated by miR-20a-5p in AML cells. Furthermore, PPP6C knockdown imitated, while overexpression reversed the effects of miR-20a-5p overexpression on AML cell proliferation, cell cycle G1/S transition and apoptosis. Taken together, our findings demonstrate that miR-20a-5p/PPP6C represent a new therapeutic target for AML and a potential diagnostic marker for AML therapy.
Introduction
Acute myeloid leukemia (AML), as a highly aggressive and heterogeneous hematological malignancy, is characterized by abnormal growth of bone marrow stromal cells and undifferentiated myeloid progenitor cells in the bone marrow and peripheral blood [1, 2]. At present, the principal therapeutic approaches, including chemotherapy, targeted therapy, and hematopoietic stem cell transplantation, have greatly improved the therapeutic outcomes of patients with AML [3, 4]. Unfortunately, the prognosis and outcomes remain still poor in AML patients at advanced stage, which might be correlated with major problems, such as serious infection and bleeding [5–7]. Therefore, it is of great importance to elucidate the molecular mechanisms responsible for the progression of AML.
MicroRNAs (mRNAs/miRs) are small noncoding RNA molecules consisting of 18–24 nucleotides that function as post-transcriptional regulator on gene expression by targeting mRNAs via binding to their 3′-untranslated region (3′-UTR) [8]. Increasing evidence suggests that miRNAs are frequently aberrantly expressed in malignancies and function as tumor suppressors or oncogenes by participating in diverse biological functions, including cell proliferation, apoptosis and differentiation [9, 10]. In recent years, miR-20a-5p, a 23-nucleotides-length non-coding RNA, has been reported to be involved in carcinogenesis. For instance, Bai et al [11] showed that miR-20a-5p was highly expressed in triple-negative breast cancer and promoted the cell proliferation through targeting runt-related transcription factor 3 (RUNX3). MiR-20a-5p overexpression contributed to hepatocellular carcinoma (HCC) cell proliferation and migration through reducing the translation of RUNX3 [12]. Similarly, Huang et al [13] demonstrated that miR-20a-5p promoted radio-resistance in nasopharyngeal cancer cells via repression of Rab27B. In addition to act as an oncogene, the suppressive role of miR-20a-5p was also manifested in endometrial cancer cells by targeting STAT3 [14], neuroblastoma cells by targeting ATG7 [15], as well as multi-drug resistance in osteosarcoma by cells targeting KIF26B [16] and SDC2 [17]. What interests us most is that a recent study by Ping et al [18] reported that knockdown of circ_0009910 inhibited AML cell proliferation and induced apoptosis through sponging miR-20a-5p. Nevertheless, the clinical significance and regulatory mechanisms of miR-20a-5p in AML cells have not been fully understood and needs to be further explored.
Protein phosphatase 6 catalytic subunit (PPP6C/PP6C) is a human ortholog of the yeast protein Sit4 and conserved phosphatase among eukaryotes from yeast to humans, which has been shown to regulate mitotic spindle formation by dephosphorylating Aurora A bound to its activator TPX2 and be required for normal cell cycle G1 to S transition [19]. PPP6C protein expression was significantly increased in glioma tissues [20]. Similarly, Zhu et al [21] reported that PPP6C expression was increased and directly regulated by miR-335 in cervical cancer. Functionally, loss of PPP6C in mouse keratinocytes increases susceptibility to ultraviolet-B-induced carcinogenesis [22]. NF-κB activation enhances keratinocyte proliferation by inhibiting PPP6C expression directly through the induction of miR-31 [23]. Our online bioinformatic prediction identified that PPP6C as a target gene of miR-20a-5p. However, the functional role of PPP6C and miR-20a-5p/PPP6C axis in AML cell proliferation have not been investigated.
In the present study, we first determined the expression of miR-20a-5p in bone marrow from AML patients and healthy controls. The correlation between miR-20a-5p and clinicopathological variables and survival prognosis was analyzed in AML patients. The in vitro experiments, including CCK-8 assay and flow cytometry and in vivo tumor xenograft experiments were performed to investigate the role of miR-20a-5p on AML tumorigenesis. Moreover, the association between miR-20a-5p and PPP6C was validated in AML cells.
Materials and methods
Patients and specimen collection
All specimens were collected through bone marrow aspiration from patients diagnosed as AML (n = 61) and age matched healthy volunteers (n = 61) in the Henan Provincial People’s Hospital (Henan, China) between September 2017 and October 2019. None of the patients received anti-tumor therapies, including chemotherapy or radiotherapy before sample collection. All obtained specimens were immediately kept at −80°C until further analysis. Some basic clinicopathological variables of AML patients with survival information were recorded. All patients were followed-up every 2–4 months for at least 5 years and the overall survival was defined as the period of time from diagnosis to death. This study was performed in accordance with the declaration of Helsinki and Ethic Committee of Henan Provincial People’s Hospital.
Cell culture and transfection
Four AML cell lines (Kasumi-1, THP-1, U937 and HL-60) and a normal human bone marrow stromal cell line HS-5 were provided by American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were cultured in RPMI-1640 medium (Gibco, CA, USA) with 10% FBS (Gibco) at 37°C in a humidified incubator containing 5% CO2. Oligonucleotides, including miR-20a-5p mimics, negative control (miR-NC), small interfering RNA targeting PPP6C (si-PPP6C), si-NC and PPP6C overexpression plasmids were purchased from RiboBio Co., Ltd. (Guangzhou, China). THP-1 or U937 cells were seeded into six-well plates and transfected with the above synthetic oligonucleotides for 48 h according to experimental requirements using lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA).
RNA isolation and quantitative real time PCR
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed with SuperScript™ IV Reverse Transcriptase (ThermoFisher Scientific). Quantification of miR-20a-5p was performed using Hairpin‐it™ miRNA qPCR Quantitation Kit (GenePharma, China) with the condition: 95°C for 3 min, and 39 circles of 95°C for 10s and 60°C for 30 s. The specific primers used in this study were as follows: miR-20a-5p, forward: 5′-GTAAAGTGCTTATAGTGCAG-3′ and reverse: 5′-GTCGTATCCAGTGCGTGTCG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′, reverse, 5′-AACGCTTCACGAATTTGCGT-3′. Relative quantification of miR-20a-5p expression was achieved using the 2−ΔΔCt method by normalization against endogenous control U6.
Cell proliferation analysis
Cell proliferation was assessed using Cell Counting Kit-8 assay (Dojindo Laboratories). In brief, approximately 0.5 × 104 transfected cells were seeded in each well of 96-well plates and cultured overnight. After incubation for 24, 48, 72 and 96 h, respectively, cells in each well were incubated with 10 μl CCK-8 reagents for another 1 h before the optical density (OD) value was measured at 450 nm by a microplate reader.
Cell cycle analysis
For cell cycle analysis, approximately 2 × 106 transfected cells were harvested by trypsin treatment and centrifugation at 300 g for 5 min. After washed with PBS twice, cells were fixed with 75% ethanol overnight at 4°C, rehydrated with PBS at room temperature and stained with 500 μL propidium iodide (PI) solution for 15 min in darkness. Finally, cell cycle distribution was analyzed by a BD FACSCanto II (BD Biosciences, San Jose, CA).
Apoptosis analysis
After 48 h transfection, cells were collected and seeded into 24-well plates at a density of 2 × 105 cells per well. After washed twice with PBS, cells were resuspended in 500 μl binding buffer containing 10 μl fluorescein isothiocyanate (FITC) Annexin V and 10 μl PI solution (Sigma‐Aldrich) for 10 min, followed by apoptotic analysis through a BD FACSCanto II.
Luciferase reporter assay
Based on the predicted binding sites between miR-20a-5p and PPP6C on TargetScan7.1 (http://www.targetscan.org), the sequences of PPP6C 3’UTR mRNA containing either wild-type (WT) or mutant (MUT) miR-20a-5p binding fragments were synthesized by GeneScript (Nanjing, China), which were then inserted into pmirGLO dual-luciferase miRNA target expression vector (Promega, Madison, WI, USA). Constructed recombinant plasmids, including PPP6C-WT or PPP6C-MUT were transfected into THP-1 or U937 cells with miR-20a-5p mimics or miR-NC by lipofectamine 3000 following the manufacturer’s instructions. After 48 h, luciferase activity was measured using a Dual Luciferase Reporter Assay system (Promega). Relative luciferase activities were calculated by normalizing Firefly luciferase activity to Renilla luciferase activity.
Western blot analysis
Total protein sample was extracted using a RIPA lysis kit (Beyotime, Shanghai, China) and protein concentration was determined by BCA protein assay (Beyotime). Approximately 30 μg of protein sample was separated on 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). Block of membranes was performed with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 2 h, the membranes were incubated with primary antibodies against PPP6C and GAPDH (Abcam, Cambridge, MA, USA) overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized with an enhanced chemiluminescence kit (Millipore).
Tumor xenograft experiments
Four‐week‐old male BALB/c nude mice were purchased from the Animal Center of Nanjing Medical University and kept in specific pathogen-free cages with standard rodent chow. A total of 3 × 106 miR-20a-5p mimics or miR-NC transfected THP-1 cells in 200 μl PBS were subcutaneously injected into the right posterior flank of equal nude mice. The tumor size was measured at 3, 7, 14 and 28 day, respectively by a caliper after cell implantation. Accordingly, tumor volume was calculated by the formula: volume (cm3) = (length × width 2)/2. On 28 day, the mice were sacrificed and tumor tissues were removed for weighing and miR-20a-5p expression analysis. All procedures of animal experiments were conducted in accordance with the Declaration of Helsinki and approved by the Animal Care Committee of the Xi’an Jiaotong University.
Statistical analysis
After statistical analysis by GraphPad Prism (Version 6.0), the quantitative data were expressed as mean ± SD from three independent experiments. The association of miR-20a-5p expression with clinicopathological variables of patients with AML was analyzed by Chi-square test. The overall survival rate estimates survival time were calculated using the Kaplan-Meier method with log-rank test. The differences among two groups were estimated by Student’s t-test or one-way ANOVA with Tukey post hoc test for multiple groups. The values of p less than 0.05 were considered to be statistically significant.
Results
MiR-20a-5p was downregulated and correlated with worse prognosis in AML patients
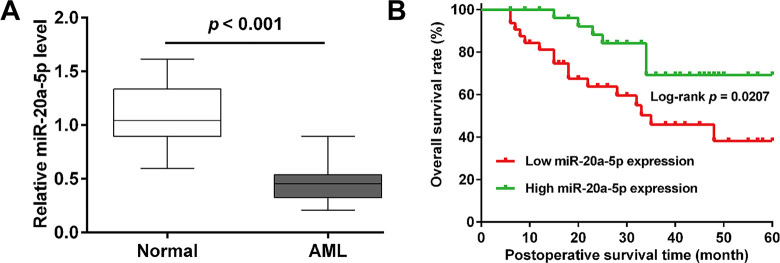
To explore the potential role of miR-20a-5p in AML development, the expression of miR-20a-5p was first analyzed by quantitative real time PCR in bone marrow from 61 AML patients and healthy controls. As shown in Fig 1A, miR-20a-5p expression was significantly downregulated in AML patients compared with matched healthy volunteers. Next, all AML patients were allocated to high and low miR-20a-5p expression group based on the median value, which were applied to research the correlation between miR-20a-5p expression and clinical features. As illustrated in Table 1, miR-20a-5p expression was associated with risk status, but not correlated with age, gender, FAB subtypes and relapse. Moreover, we further analyzed the relationship between miR-20a-5p levels and survival time in AML patients. The Kaplan-Meier survival cure showed that the patients with low miR-20a-5p expression had shorter overall survival rate than those with high miR-20a-5p expression (Fig 1B). These data indicated that decreased miR-20a-5p expression might predict worse prognosis in AML patients.
Fig 1. The expression and prognosis of miR-20a-5p in AML patients.
(A) Expression of miR‐20a-5p in bone marrow from AML (n = 61) and age matched healthy volunteers (n = 61) were detected by quantitative real time PCR analysis. (B) Kaplan–Meier analysis of overall survival rate of AML patients with low or high miR-20a-5p expression.
Table 1. The association between miR-20a-5p expression levels and clinicopathological variables of AML patients.
| Variable | miR-20a-5p | P-values | |
|---|---|---|---|
| Low expression (n = 32) | High expression (n = 29) | (chi-square test) | |
| Age (year) | 0.088 | ||
| < 50 | 14 | 19 | |
| ≥ 50 | 18 | 10 | |
| Gender | 0.104 | ||
| Male | 22 | 14 | |
| Female | 10 | 15 | |
| Risk status | 0.030* | ||
| Poor/Intermediate | 25 | 15 | |
| Better | 7 | 14 | |
| FAB subtypes | 0.536 | ||
| M1 | 5 | 4 | |
| M2 | 8 | 6 | |
| M3 | 10 | 8 | |
| M4 | 7 | 6 | |
| M5 | 2 | 5 | |
| Relapse | 0.621 | ||
| Yes | 19 | 19 | |
| No | 13 | 10 | |
Note
*p < 0.05. Abbreviations: AML, acute myeloid leukemia; FAB, French-American-British classification
MiR-20a-5p overexpression suppressed cell proliferation, induced G0/G1 phase arrest and apoptosis in AML cells
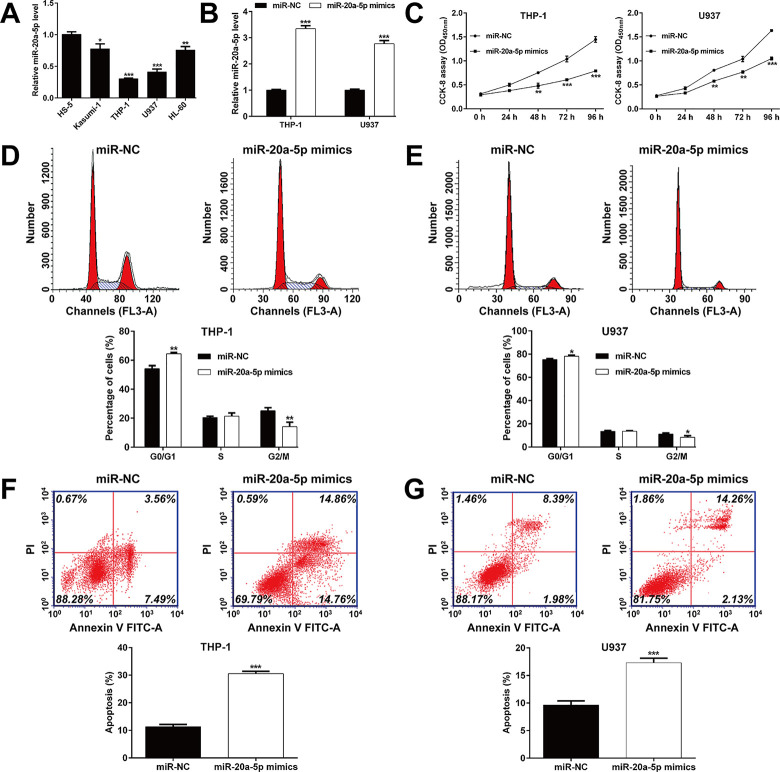
Consistently, we found that miR-20a-5p was significantly decreased in four AML cell lines (Kasumi-1, THP-1, U937 and HL-60) compared with a normal human bone marrow stromal cell line HS-5 (Fig 2A). Then, two AML cell lines (THP-1 and U937) were selected for gain-of-function assays, which owned lowest miR-20a-5p expression level among four AML cell lines, demonstrating better typicality and representation. Quantitative real time PCR analysis confirmed that the expression of miR-20a-5p was significantly elevated in THP-1 and U937 cells after miR-20a-5p mimics transfection compared with miR-NC transfection (Fig 2B). Results of CCK‐8 showed that upregulated miR-20a-5p inhibited the proliferation of THP-1 and U937 cells at 48, 72 and 96 h, respectively (Fig 2C). Flow cytometry was utilized to further analyze the effects of upregulated miR-20a-5p on cell cycle progression and apoptosis status. Cell cycle analysis showed that overexpression of miR-20a-5p presented a significant increase in the percentage of cells at G0/G1 phase while a significant decrease in the percentage of cells at G2/M phase in THP-1 (Fig 2D) and U937 (Fig 2E) cells, which indicated that cell cycle G0/G1 phase arrest was induced by miR-20a-5p overexpression. In addition, apoptosis analysis showed that miR-20a-5p mimics transfection remarkably promoted cell apoptosis in both THP-1 (Fig 2F) and U937 (Fig 2G) cells.
Fig 2. Effects of miR-20a-5p overexpression on cell proliferation, cell cycle progression and apoptosis in AML cells.
(A) Expression of miR‐20a-5p in four AML cell lines (Kasumi-1, THP-1, U937 and HL-60) and a normal human bone marrow stromal cell line HS-5 were detected by quantitative real time PCR analysis. (B) Expression of miR‐20a-5p was determined in THP-1 and U937 cells after transfection with miR-20a-5p mimics or miR-NC. (C) CCK-8 assay was performed to assess the proliferation of transfected THP-1 and U937 cells. (D-E) The percentage of cells at G0/G1, S and G2/M phase was analyzed by flow cytometry in transfected THP-1 and U937 cells. (F-G) Apoptotic cells were quantified in transfected THP-1 and U937 cells by flow cytometry analysis. Data are presented as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, compared with miR-NC.
MiR-20a-5p negatively regulated PPP6C expression by targeting its 3’UTR
The online public bioinformatics tool TargetScan was used to predict the potential target of miR-20a-5p. Among these predicted target genes, PPP6C, associated with cell cycle and tumor formation/progression, was selected as a potential target of miR-20a-5p for further analysis. To test the effect of miR-20a-5p on PPP6C, WT and MUT PPP6C-3’UTR sequences were cloned into reporter plasmids (Fig 3A). The results of luciferase reporter assay showed that ectopic miR-20a-5p expression significantly decreased the luciferase activities of PPP6C-WT in THP-1 (Fig 3B) and U937 (Fig 3C) cells. Western blot analysis further demonstrated that miR-20a-5p mimics transfection obviously downregulated the expression of PPP6C protein in THP-1 and U937 cells compared with miR-NC transfection (Fig 3D). These data suggested that miR-20a-5p mediated PPP6C expression by directing targeting its 3’UTR, which may be involved in the suppressive role in AML.
Fig 3. MiR-20a-5p negatively regulated PPP6C expression by targeting its 3’UTR.
(A) The complementary sequences between wild-type or mutant human PPP6C 3’-UTRs mRNA and human miR-20a-5p. (B) THP-1 and (C) U937 cells were co-transfected with miR-20a-5p mimics or miR-NC and the luciferase reporter construct containing the wild-type or mutant PPP6C 3′-UTR. For each experiment, the results were normalized to the luciferase activity detected in the cells transfected with the control vector. Data are presented as mean ± SD of three independent experiments. **p < 0.01, compared with miR-NC; (D) The expression of PPP6C protein was detected in THP-1 and U937 cells after transfection with miR-20a-5p mimics or miR-NC.
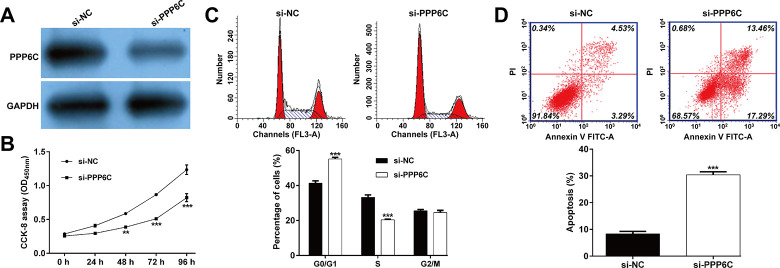
Knockdown of PPP6C suppressed cell proliferation, induced G0/G1 phase arrest and apoptosis in AML cells
Since PPP6C was negatively regulated by miR-20a-5p in AML cells, we then performed loss-of-function assay in AML cells by transfection with si-PPP6C or si-NC into THP-1 cells. As shown in Fig 4A, the protein level of PPP6C was obviously downregulated in THP-1 cells after si-PPP6C transfection. Consistent with the effects of miR-20a-5p in AML cells, we observed that knockdown of PPP6C markedly suppressed cell proliferation (Fig 4B), induced cell G0/G1 phase arrest (Fig 4C) and promoted apoptosis (Fig 4D) in THP-1 cells. These data indicated that PPP6C played a positive role in AML cell growth and proliferation.
Fig 4. Effects of PPP6C knockdown on cell proliferation, cell cycle progression and apoptosis in AML cells.
THP-1 cells were transfected with si-PPP6C or si-NC, respectively. (A) Western blot analysis was performed to measure the protein level of PPP6C in transfected THP-1 cells. (B) Cell proliferation was assessed by CCK-8 assay in transfected THP-1 cells. Flow cytometry assay was applied to determined cell cycle distribution (C) and apoptotic rate (D) in transfected THP-1 cells. Data are presented as mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001, compared with si-NC.
PPP6C overexpression reversed the effects of miR-20a-5p on AML cell proliferation, G1/S transition and apoptosis
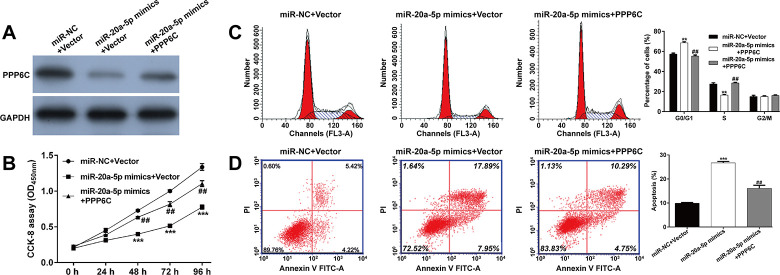
Next, we performed rescue experiments to confirm whether PPP6C was the downstream regulator involved in miR-20a-5p regulating cell functions in AML cells. THP-1 cells were co-transfected with miR-20a-5p mimics and PPP6C. As shown in Fig 5A, the expression of PPP6C protein was decreased after miR-20a-5p + Vector, compared with miR-NC + Vector transfection, which was recovered after co-transfection with miR-20a-5p mimics and PPP6C. CCK-8 assay showed that increased PPP6C expression partially restored the impaired proliferation induced by miR-20a-5p overexpression in THP-1 cells (Fig 5B). Moreover, PPP6C overexpression reversed the accelerative effects of miR-20a-5p on G0/G1 arrest (Fig 5C) and apoptosis (Fig 5D) in THP-1 cells.
Fig 5. PPP6C overexpression reversed the effects of miR-20a-5p on AML cell proliferation, G1/S transition and apoptosis.
THP-1 cells were co-transfected with miR-20a-5p mimics and PPP6C or vector. (A) The expression of PPP6C protein was detected in transfected THP-1 cells. (B) CCK-8 assay was performed to assess the proliferation of transfected THP-1 cells. (C) Flow cytometry with PI staining was applied to analyze cell cycle distribution in transfected THP-1 cells. (D) Apoptotic rate was determined in transfected THP-1 cells. Data are presented as mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001, compared with miR-NC +Vector; ##p < 0.01, compared with miR-20a-5p mimics +Vector.
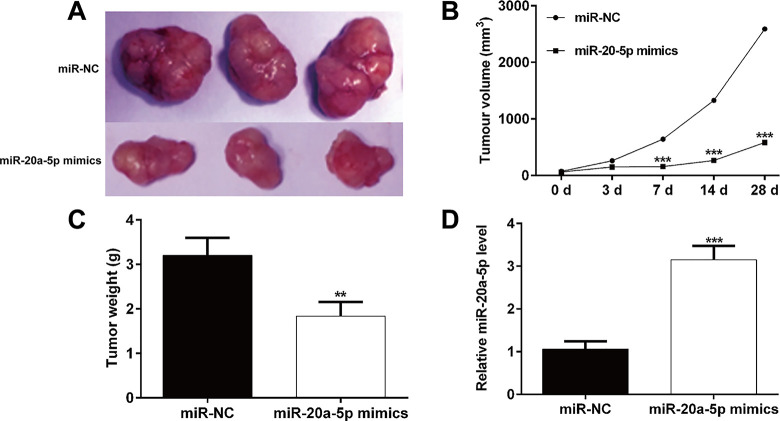
MiR-20a-5p inhibited tumor growth in mice xenograft models
To reveal the in vivo tumor suppressive effects of miR-20a-5p, THP-1 cells transfected with miR-20a-5p mimics or miR-NC were injected into the flanks of nude mice to generate tumors ectopically. As depicted in Fig 6A, mice injected with miR-20a-5p mimics transfected-THP-1 cells generated smaller tumor size compared with those with miR-NC transfected cells. After injections of miR-20a-5p mimics at consecutive 28 days, tumor growth was significantly lower than that following injection of miR-NC (Fig 6B). Moreover, the formed tumor weight was significantly decreased in miR-20a-5p mimics group, in comparison with miR-NC group (Fig 6C). In addition, quantitative real time analysis confirmed that miR-20a-5p expression was upregulated in miR-20a-5p mimics-transfected tumors when compared with miR-NC-transfected tumors (Fig 6D). Taken together, these results clearly demonstrate that miR-20a-5p functioned as a tumor suppressor in vivo.
Fig 6. MiR-20a-5p inhibited tumorigenicity in vivo.
(A) Representative photographs of tumors were obtained from the different groups of nude mice (n = 5 per group) transfected with miR-20a-5p‐mimics, and miR‐NC. Tumors were observed by (B) tumor volume and (C) average weight. (D) Expression of miR-20a-5p was detected by quantitative real time PCR. Data are presented as mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001, compared with miR-NC.
Discussion
In this study, we found that miR-20a-5p was significantly decreased in bone marrow from AML patients, compared with that in healthy controls. Moreover, decreased miR-20a-5p expression was correlated with risk status and poor survival prognosis in AML patients. Consistently, the expression level of miR-20a-5p was reported to be downregulated along with clinical staging of neuroblastoma progression [15], in drug-resistant pancreatic cancer cells [24], breast tumors [25, 26] and endometrial cancer tissues [14]. Downregulation of miR-20a-5p was correlated with the malignancy and poor prognosis of glioma patients [27]. HCC patients with lower level of miR-20a-5p had significantly shorter OS and PFS survivals after surgical resection [28]. Contrary to our results, miR-20a-5p was upregulated in lung squamous cell carcinoma (SCC) tissues and serum exosomes with considerable clinical value in the diagnosis of male lung SCC patients [29]. The upregulation of miR-20a-5p was associated with advanced clinical stages of astrocytoma [30]. Notably, the downregulation of miR-20a-5p identified by Ping et al [18] confirmed our data on the decreased expression of miR-20a-5p and its correlation with worse prognosis in AML patients.
Functionally, we further demonstrated that overexpression of miR-20a-5p significantly suppressed cell proliferation, induced cell cycle G0/G1 phase arrest and apoptosis in two AML cell lines (THP-1 and U937), as well as inhibited tumor growth in mice xenograft models. These findings indicated the tumor suppressive role of miR-20a-5p in AML progression. In line with our data, miR-20a-5p inhibited cell proliferation and promoted apoptosis in SH-SY5Y cells [15]. Forced expression of miR-20a-5p counteracted osteosarcoma cell chemoresistance in both cell culture and tumor xenografts in nude mice [16]. Ping et al [18] further confirmed that miR-20a-5p inhibitors reversed circ_0009910 siRNA-mediated cell proliferation inhibition, cell cycle arrest and cell apoptosis in AML5 cells. Different from the study from Ping et al, our study used two AML cell lines (THP-1 and U937) and performed gain-of-function assay to consistently demonstrated the suppressive effects of miR-20a-5p in AML cell proliferation and tumor growth. On the contrary, some studies described the oncogenic role of miR-20a-5p in other tumor cells, including colorectal cancer [31], HCC [12] and radio-resistance in nasopharyngeal cancer [13]. These differences in the regulatory role of miR-20a-5p might be ascribed to different tissue resources, cell types and different culture conditions.
As our best knowledge, miRNAs bind to the 3’-UTR of their target mRNAs, leading to degradation of mRNA or inhibiting mRNA translation. Herein, PPP6C was a bioinformatics target of miR-20a-5p, and we further validated miR-20a-5p directly targeted PPP6C and reduced the expression of PPP6C protein. And indeed, PPP6C has been demonstrated to be a target gene of several miRNAs, such as miR-31 [32], miR-373 [33] and miR-335 [21]. Our data additionally indicated that PPP6C knockdown imitated, while overexpression reversed the effects of miR-20a-5p overexpression on AML cell proliferation, cell cycle G1/S transition and apoptosis, which suggested that miR-20a-5p regulated AML cell proliferation, G1/S transition and apoptosis via PPP6C repression. In fact, PPP6C protein expression was significantly upregulated in glioma [20] and cervical cancer [21]. The pro-survival effects of PPP6C have been manifested in ultraviolet-B-induced carcinogenesis [22] and keratinocyte proliferation [23]. By searching the members of the protein serine/threonine phosphatase family, we found PPP5C could significantly promote the tumor cell proliferation and survival, including HCC [34], ovarian cancer [35] and prostate cancer [36]. Interestingly, knockdown of PPP5C suppressed the proliferation ability, and led to G0/G1 phase arrest, induced cell apoptosis in leukemic cell line U937 cells [37], which is line with our data showed that PPP6C promoting AML cell proliferation.
Conclusions
In summary, we demonstrated that miR-20a-5p was significantly downregulated and correlated with worse prognosis in AML patients. MiR-20a-5p overexpression impaired AML cell proliferation, induced cell cycle G0/G1 phase arrest, apoptosis in vitro and tumor growth in vitro. Moreover, we confirmed that miR-20a-5p negatively regulated PPP6C expression by binding to its 3’-UTR and further confirmed that PPP6C was the downstream regulator involved in miR-20a-5p regulating AML cell functions. The results of this study indicate that the miR-20a-5p/PPP6C axis closely correlates with the malignant progression of AML, which might be of potential value as novel therapeutic targets for AML treatment.
Supporting information
(TIF)
Data Availability
The data are not publicly available due to data contain potentially identifying or sensitive patient information. Please contact the ethics committee of Henan Provincial People’s Hospital (Henan, China; Tel: +86-0371-65964325; Email: 404957522@qq.com) to which data requests may be sent.
Funding Statement
This work was supported by the Science and Technology Project of Health Department of Henan Province (No. 201602221).
References
- 1.Khaled S et al. : Acute Myeloid Leukemia: Biologic, Prognostic, and Therapeutic Insights. Oncology, 2016, 30(4):318–329. [PubMed] [Google Scholar]
- 2.Short NJ, Ravandi F: Acute Myeloid Leukemia: Past, Present, and Prospects for the Future. Clinical lymphoma, myeloma & leukemia, 2016, 16Suppl:S25–29. [DOI] [PubMed] [Google Scholar]
- 3.Dombret H, Gardin C: An update of current treatments for adult acute myeloid leukemia. Blood, 2016, 127(1):53–61. doi: 10.1182/blood-2015-08-604520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnett A et al. : Therapeutic advances in acute myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2011, 29(5):487–494. doi: 10.1200/JCO.2010.30.1820 [DOI] [PubMed] [Google Scholar]
- 5.Uchida H et al. : New therapeutic approaches to acute myeloid leukemia. Expert opinion on drug discovery, 2008, 3(6):689–706. doi: 10.1517/17460441.3.6.689 [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T: Treatment of acute myeloid leukemia under relapsed/refractory conditions or in older adults. [Rinsho ketsueki] The Japanese journal of clinical hematology, 2016, 57(10):1934–1943. doi: 10.11406/rinketsu.57.1934 [DOI] [PubMed] [Google Scholar]
- 7.Saultz JN, Garzon R: Acute Myeloid Leukemia: A Concise Review. Journal of clinical medicine, 2016, 5(3). doi: 10.3390/jcm5030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004, 116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 9.Iwakawa HO, Tomari Y: The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends in cell biology, 2015, 25(11):651–665. doi: 10.1016/j.tcb.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y et al. : Oncogenic and tumor suppressive roles of microRNAs in apoptosis and autophagy. Apoptosis: an international journal on programmed cell death, 2014, 19(8):1177–1189. doi: 10.1007/s10495-014-0999-7 [DOI] [PubMed] [Google Scholar]
- 11.Bai X et al. : MiRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 2018, 103:1482–1489. doi: 10.1016/j.biopha.2018.04.165 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y et al. : MicroRNA-20a-5p targets RUNX3 to regulate proliferation and migration of human hepatocellular cancer cells. Oncol Rep, 2016, 36(6):3379–3386. doi: 10.3892/or.2016.5144 [DOI] [PubMed] [Google Scholar]
- 13.Huang D et al. : MiR-20a-5p promotes radio-resistance by targeting Rab27B in nasopharyngeal cancer cells. Cancer cell international, 2017, 17:32. doi: 10.1186/s12935-017-0389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Yang N: MicroRNA-20a-5p inhibits epithelial to mesenchymal transition and invasion of endometrial cancer cells by targeting STAT3. International journal of clinical and experimental pathology, 2018, 11(12):5715–5724. [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y et al. : MiR-20a-5p suppresses tumor proliferation by targeting autophagy-related gene 7 in neuroblastoma. Cancer cell international, 2018, 18:5. doi: 10.1186/s12935-017-0499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pu Y et al. : MiR-20a-5p represses multi-drug resistance in osteosarcoma by targeting the KIF26B gene. Cancer cell international, 2016, 16:64. doi: 10.1186/s12935-016-0340-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao F et al. : MiR-20a-5p represses the multi-drug resistance of osteosarcoma by targeting the SDC2 gene. Cancer cell international, 2017, 17:100. doi: 10.1186/s12935-017-0470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ping L et al. : Silencing of circ_0009910 inhibits acute myeloid leukemia cell growth through increasing miR-20a-5p. Blood cells, molecules & diseases, 2019, 75:41–47. doi: 10.1016/j.bcmd.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 19.Zeng K et al. : Protein phosphatase 6 regulates mitotic spindle formation by controlling the T-loop phosphorylation state of Aurora A bound to its activator TPX2. The Journal of cell biology, 2010, 191(7):1315–1332. doi: 10.1083/jcb.201008106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J et al. : AEG-1 expression correlates with CD133 and PPP6c levels in human glioma tissues. Journal of biomedical research, 2014, 28(5):388–395. doi: 10.7555/JBR.28.20140015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y et al. : Hsa_circ_103973 Acts as a Sponge of miR-335 to Promote Cervical Cancer Progression. OncoTargets and therapy, 2020, 13:1777–1786. doi: 10.2147/OTT.S215736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato H et al. : Loss of protein phosphatase 6 in mouse keratinocytes increases susceptibility to ultraviolet-B-induced carcinogenesis. Cancer letters, 2015, 365(2):223–228. doi: 10.1016/j.canlet.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 23.Yan S et al. : NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nature communications, 2015, 6:7652. doi: 10.1038/ncomms8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H et al. : MiR-20a-5p regulates gemcitabine chemosensitivity by targeting RRM2 in pancreatic cancer cells and serves as a predictor for gemcitabine-based chemotherapy. Bioscience reports, 2019, 39(5). doi: 10.1042/BSR20181374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvano Filho CM et al. : Triple-negative and luminal A breast tumors: differential expression of miR-18a-5p, miR-17-5p, and miR-20a-5p. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, 2014, 35(8):7733–7741. [DOI] [PubMed] [Google Scholar]
- 26.Meng Q et al: Deletion of HNF1A-AS1 Suppresses the Malignant Phenotypes of Breast Cancer Cells In Vitro and In Vivo Through Targeting miRNA-20a-5p/TRIM32 Axis. Cancer biotherapy & radiopharmaceuticals, 2020. [DOI] [PubMed]
- 27.Yang BY et al: Long non-coding RNA SNHG16 contributes to glioma malignancy by competitively binding miR-20a-5p with E2F1. Journal of biological regulators and homeostatic agents, 2018, 32(2):251–261. [PubMed] [Google Scholar]
- 28.Liu DL et al. : miR-17-5p and miR-20a-5p suppress postoperative metastasis of hepatocellular carcinoma via blocking HGF/ERBB3-NF-κB positive feedback loop. Theranostics, 2020, 10(8):3668–3683. doi: 10.7150/thno.41365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L et al: A three-microRNA signature for lung squamous cell carcinoma diagnosis in Chinese male patients. Oncotarget, 2017, 8(49):86897–86907. doi: 10.18632/oncotarget.19666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhi F et al. : Identification of 9 serum microRNAs as potential noninvasive biomarkers of human astrocytoma. Neuro-oncology, 2015, 17(3):383–391. doi: 10.1093/neuonc/nou169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng D et al. : MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4. Oncotarget, 2016, 7(29):45199–45213. doi: 10.18632/oncotarget.9900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X et al. : MicroRNA-31 Regulates Immunosuppression in Ang II (Angiotensin II)-induced Hypertension by Targeting Ppp6C (Protein Phosphatase 6c). Hypertension, 2019, 73(5):e14–e24. doi: 10.1161/HYPERTENSIONAHA.118.12319 [DOI] [PubMed] [Google Scholar]
- 33.Wu N et al. : MicroRNA-373, a new regulator of protein phosphatase 6, functions as an oncogene in hepatocellular carcinoma. The FEBS journal, 2011, 278(12):2044–2054. doi: 10.1111/j.1742-4658.2011.08120.x [DOI] [PubMed] [Google Scholar]
- 34.Feng L et al. : Knockdown of PPP5C inhibits growth of hepatocellular carcinoma cells in vitro. Applied biochemistry and biotechnology, 2015, 175(1):526–534. doi: 10.1007/s12010-014-1281-8 [DOI] [PubMed] [Google Scholar]
- 35.Zheng X et al. : Knockdown of protein phosphatase 5 inhibits ovarian cancer growth in vitro. Oncology letters, 2016, 11(1):168–172. doi: 10.3892/ol.2015.3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv JM et al. : PPP5C promotes cell proliferation and survival in human prostate cancer by regulating of the JNK and ERK1/2 phosphorylation. OncoTargets and therapy, 2018, 11:5797–5809. doi: 10.2147/OTT.S161280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li GY et al. : Knockdown of protein phosphatase 5 (PPP5C) suppresses the growth of leukemic cell line U937. Cellular and molecular biology (Noisy-le-Grand, France), 2016, 62(11):27–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
The data are not publicly available due to data contain potentially identifying or sensitive patient information. Please contact the ethics committee of Henan Provincial People’s Hospital (Henan, China; Tel: +86-0371-65964325; Email: 404957522@qq.com) to which data requests may be sent.