Abstract
Women may experience new-onset or worsening depressive disorders during pregnancy and the postpartum. If untreated, there may be detrimental consequences to the health and wellbeing of the woman and to her baby. There is a need for improved tools and approaches that can be easily and broadly implemented to effectively detect depression during the perinatal period. Early identification of depression during pregnancy is an important first step towards connecting women to treatment and preventing continued depression into the postpartum or beyond. This report provides preliminary findings from a pilot study of a digital screening app for perinatal depression expiring potential for app reach, engagement, and user demographics and mental health symptoms. With mainly passive recruitment efforts, we collected cross-sectional mental health data on over 700 women during the perinatal period, including women across over 30 countries. We report on mean depression scores among women during pregnancy and the postpartum as well as on constructs that are commonly comorbid with depression, including anxiety, sleep dysregulation, and perceived stress. Over half of the women during pregnancy and over 70% of women in the postpartum had a depression score indicative of clinical depression. Future research directions for this work and potential for public health impact are discussed, including longitudinal data collection and analyses of symptomology over time and embedding evidence-based digital therapeutics into the app as a means to increase access to mental health services.
Introduction
Mood and anxiety disorders are common in women, affecting approximately twice as many women as men [1]. Mood disorders in women can emerge or worsen with pregnancy and postpartum, with the postpartum period presenting a time of increased risk for the onset or recurrence of affective illness [2]. Up to 85% of women experience some type of postpartum mood disturbance, and it can be difficult to predict who is at greatest risk of serious postpartum psychiatric illness and to disseminate information about risk factors [3, 4]. For most women, the symptoms are transient and relatively mild (i.e., postpartum blues); however, 10–15% of women experience a more disabling and persistent form of mood disorder (i.e., postpartum depression) [5–7]. In a widely cited prospective study of postpartum depression, O’Hara and colleagues describe rates of postpartum major and minor depression combined as equal to 10% [6].
Abundant literature suggests that postpartum depression places the mother at risk for recurrent or refractory illness, and there is evidence of long-term negative effects of postpartum depression on child development and behavior [8]. Given the prevalence of this disorder and its potential to cause significant morbidity in both the child and the mother, there is a need for studies to characterize perinatal depression and to identify risk factors in populations at highest risk for this disorder. While it is difficult to predict which women in the general population will develop postpartum depression, certain risk factors have been identified, including recent stressful life events, inadequate social support, and marital discord or dissatisfaction. A personal history of depression increases one’s vulnerability to peripartum and postpartum depression, and depression during pregnancy is one of the most robust risk factors for depression after delivery [9]. Identification of depression in pregnancy may therefore be an important step towards connecting women with treatments to prevent postpartum depression.
One of the key limiting factors in the study of perinatal depression has been the lack of detailed data on symptomology over time. The current standard of care in screening consists of self-administered depression scales, most commonly the Edinburgh Postnatal Depression Scale (EPDS), an instrument initially designed to be given approximately 6–8 weeks postpartum, although is often used more broadly during the perinatal period [10–13]. More granular data, collected remotely on a daily or weekly basis, could better monitor the trajectory of psychopathology in women who are at high risk during pregnancy and into the postpartum [4, 14]. The feasibility of screening for perinatal depression in both developed and low- and middle-income countries has been explored. The ideal instrument, which has psychometric properties with sufficient sensitivity but also specificity to afford scaled use in large populations with limited treatment capacity, has yet to be identified. For example, the previously mentioned Edinburgh Postnatal Depression Scale (EPDS) has been used in both original and abbreviated forms, and results suggest that both long and short versions have good sensitivity, but the specificity frequently does not exceed 75% [15–18].
A systematic review based on studies performed in low- and middle-income countries (LMICs) has shown specificity of screening tools, such as the EPDS, to be highly variable [19]. The study of perinatal mental health on a global scale is also an area warranting further attention; While estimates of the prevalence of perinatal mood disorders have been on the order of 10–15% in developed countries, the estimated prevalence of these disorders in low resource environments has been substantially greater with sparse proportions of those affected being identified or receiving treatment [20–23]. Relevant questions with respect to screening and treating perinatal depression in LMIC have included the extent to which existing primary care staff and community health workers can both screen for psychiatric disorders such as depression and then deliver treatment, as well as whether available screening tools are valid across populations with different cultural and ethnographic characteristics [19, 24]. This is in addition to the stigma linked to asking for mental health provisions, especially for new mothers who often feel guilty or ashamed of being afflicted with a disorder that is out of their control. Improving the specificity of detection measures may support the rationalized allocation of resources in both constrained and less constrained settings, and leveraging big data and technology may help improve such identification of depression and possibly provide connection to virtual sources of screening and support.
The last few years have seen an unprecedented growth in the number, and technological sophistication, of mobile phones worldwide. The ubiquity of mobile phones makes them highly unobtrusive, and given their general presence, they are significantly less likely to interfere with natural psychosocial behaviors than laboratory-based monitoring devices. Mobile phone applications, with their immediate availability and widespread popularity, allow longitudinal and frequent monitoring of mood states in women who are at a high risk for postpartum depression. Patients might also be more likely to divulge sensitive information when asked in a written (or in app) format and comfortable setting (e.g., at home) as opposed to an interview with a clinician. While there has been recent research exploring the use of mobile apps to screen for perinatal mental health [e.g., 25, 26], these studies have had sample sizes of a couple hundred women. Capturing big data on thousands of women will provide us the granular data needed to better identify and treat perinatal depression in high-risk groups of women. Furthermore, an evolution of the use of apps for screening would lend itself to a potentially seamless introduction to electronically delivered referrals and treatment options. Virtual mental health care has been identified as a likely scenario for delivery of at least a proportion of future mental health care and could be utilized to reduce disparities in access to care [27].
To that end, we developed and pilot-tested a mobile phone application that incorporates various measures to assess psychiatric symptomatology, with a focus on depressive symptoms, in pregnancy and the postpartum period. This application, the MGH Perinatal Depression Scale (MGHPDS), will eventually allow us to collect big data on perinatal women and longitudinally assess risk factors that contribute to depression during this period, and will enable us to do so in a minimally invasive manner. The measures that were selected to be included as part of the MGHPDS were informed by research and clinical experience indicating that perceived stress, sleep disruptions, and anxiety drive risk for perinatal depression [28–32], with the ultimate aim of identifying key items from these measures that are robust predictors for perinatal depression. The present article reports on the first version of the MGHPDS which, which we plan to refine prior to future phases of research and testing. Ultimately, a goal of this work is to narrow down questions being asked in future versions of the app, to reduce participant burden, and to streamline screening for perinatal psychiatric disorders. Perinatal anxiety is understudied and thus a secondary aim of the app is to better understand the longitudinal course of perinatal anxiety and clarify its relationship with perinatal depression. In this current article, we report on the findings from a preliminary test of the first version of the MGHPDS to explore potential for reaching perinatal women on a global scale with the app as well as user engagement with the app over time. We also explore demographics and mental health symptom profile among app users. Findings from this initial exploratory pilot study will inform refinement of the app and data collection methods.
Methods
Participants
This study was approved by the Institutional Review Board (IRB) of Mass General Brigham. Subjects were recruited from the Departments of Psychiatry, Primary Care, Obstetrics at MGH, and from MGH-affiliated community health centers. Recruitment efforts from within the hospital and affiliated sites included posting of flyers in hospital-approved areas, placement of flyers and brochures in waiting rooms with authorization from the clinic director, and circulation of emails through the All-User system. We also recruited from our website, www.womensmentalhealth.org and its affiliated listserv. Clinicians who inquired about the study were encouraged to tell their patients about the study and suggest that patients contact our research personnel. Subjects were also recruited from the community by posting flyers in communal areas (e.g., community board posting sites) and placing advertisements in community newsletters and newspapers. We also promoted this study during presentations at national meetings for psychiatrists, obstetricians, and family practitioners. Subjects could also join the study if they searched for or came across the applications within Apple or Google application stores. Anyone who wishes to download and use the app is able to do so free of charge.
Inclusion criteria for this preliminary analysis were women who were: 1) pregnant or up to 12 months postpartum, 2) between the ages of 18–45, 3) able to provide informed consent, 4) willing and able to fill out questionnaires about their mood, sleep, anxiety, and stress levels using a mobile phone application for at least one time point. Exclusion criteria consisted of women who were not pregnant or within 12 months of childbirth or did not complete at least one timepoint assessment (participants were included if there was some missing data from the timepoint assessment).
Procedures
Using the MGHPDS
By downloading the MGHPDS, users may enroll in the research study. However, downloading the app does not automatically enroll a user in the study. Only users who provide consent to participate in the study will be enrolled and their responses to questionnaires will be included as study data. Upon downloading the application, subjects are directed to a screen containing an online consent form that details study participation requirements. This consent form, adapted from standard online survey consent forms, details study goals, the nature of data collection, protection of privacy, and the subject’s right to remove her data from the study at any point in time if she so wishes. Contact information for study personnel is provided in the consent form in the event potential subjects have questions about study participation prior to enrolling. After reading through this consent form screen, subjects were provided with a button to click that states “I agree to participate in this research.” If the subject agrees to consent to the study, a copy of the consent form was emailed to subjects for future reference. If a patient declines to consent for research, she may still use the app.
After obtaining consent, subjects were asked to complete a number of questionnaires, including demographic characteristics (i.e., ethnicity, age, etc.), medical history and psychiatric history, cigarette and alcohol use. Data used to quantify mood, anxiety, sleep, and stress levels across pregnancy and the postpartum period were collected using the mobile phone application. Women completed the battery of questionnaires each time they logged in to the application, although the demographics information was only collected at the first time point. The baseline assessment battery takes approximately 10 minutes to complete. The app is programmed to send participants push notifications (if the participant allows in their phone settings) to complete the questionnaires at the following time points: at 24–26 weeks pregnant, 1–2 weeks postpartum, 4–6 weeks postpartum, and 12 weeks postpartum. Although participants could use the app at multiple timepoints, we report on a cross-sectional analysis in this manuscript for participants who completed the assessment battery for an initial assessment.
Included measures
The MGHPDS includes the following measures:
Edinburgh Postnatal Depression Scale (EPDS). The primary outcome variable of interest was the EPDS, a 10-item self-report questionnaire developed to identify women who have postpartum depression. A score of ≥12 was used to signal probable depression [15]. The Cronbach’s alpha for this study was 0.90.
Patient Health Questionnaire-8-item scale (PHQ-8). The PHQ-8 is an 8-item self-report questionnaire that assesses mood and depressive symptoms ranging from 0 (not at all) to 4 (nearly every day); it has been identified as a useful depression measure for population-based studies, and a cut point ≥ 10 can be used for defining current depression [33]. The Cronbach’s alpha for this study was 0.88.
Generalized Anxiety Disorder-7-item scale (GAD-7). The GAD-7 is a 7-item self-report questionnaire that assesses anxiety symptoms with a total score for the seven items that can from 0 to 21. When used as a screening tool for anxiety disorders, further evaluation is recommended when the score is 10 or greater [34]. The Cronbach’s alpha for this study was 0.90.
Insomnia Severity Index (ISI). The ISI is a 7-item self-rated questionnaire used to assess the severity of initial, middle, and late insomnia. A 5-point Likert scale is used to rate each item (e.g., 0 = no problem; 4 = very severe problem), yielding a total score ranging from 0 to 28. The total score is interpreted as follows: absence of insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28) [35]. The Cronbach’s alpha for this study was 0.89.
Perceived Stress Scale (PSS). The PSS is a 14-item self-report questionnaire that assesses the severity of perceptions of stress; it measures the degree to which situations in one’s life are appraised as stressful, including perceptions of how unpredictable, uncontrollable, and overloaded respondents find their lives. Individual scores on the PSS can range from 0 to 40 with higher scores indicating higher perceived stress [36]. The Cronbach’s alpha for this study was 0.90.
The scoring guidelines used are made accessible to the user by 1) the described scoring guidelines feature accessible within the application, 2) a copy of the consent form, which delineates the guidelines, and is accessible directly through the application, and 3) a copy of the consent form which is emailed to subjects following enrollment. Please see S1 Appendix for the enumerated scoring guidelines for these questionnaires. We used descriptive statistics to summarize the included participants demographic information and average outcome measure values.
Results
Participants and utilization of the app
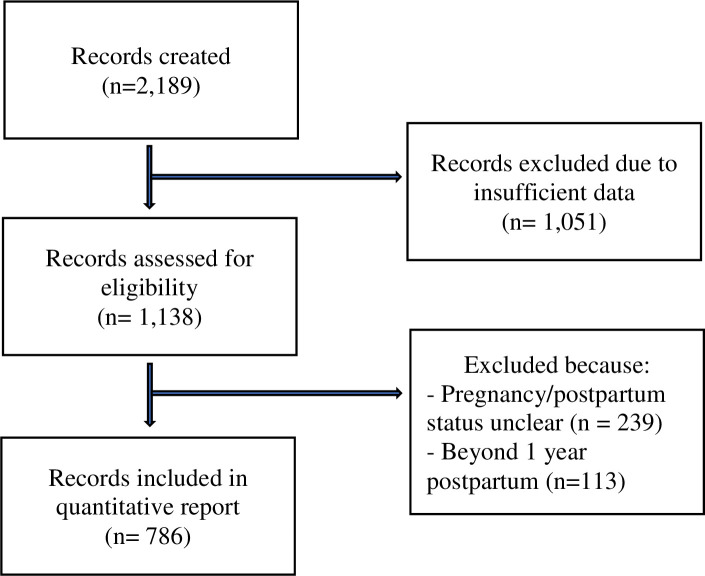
Between, August 2017 and July 2020, there were 2,189 records (i.e., downloads) of the app. Of those, 1,051 records did not complete one timepoint (missing data allowed) of the assessment battery or did not provide study consent and were thus removed. We removed other records where women reported being outside the perinatal time period or did not provide pregnancy/postpartum status. See Fig 1 for a summary of the 786 participants included in this analysis. See Table 1 for a summary of demographics for the participants included.
Fig 1. Flow of included participant records.
Table 1. Sample demographics (N = 786).
| Age (years), M (SD) | 30.30 (5.98) |
| Race, n (%) | |
| Caucasian | 617 (78.5%) |
| Asian | 40 (5.1%) |
| African American | 32 (4.1%) |
| American/Indian/Alaska Native | 6 (0.8%) |
| Native Hawaiian/Pacific Islander | 1 (0.1%) |
| Other | 56 (7.1%) |
| More than 1 race selected | 18 (2.3%) |
| Decline to answer | 16 (2.0%) |
| Ethnicity, n (%) | |
| Non-Hispanic/Non-Latina | 649 (82.6%) |
| Hispanic/Latina | 77 (9.8%) |
| Decline to Answer/Missing Data | 60 (7.6%) |
| Marital status, n (%) | |
| Single, never married | 124 (15.8%) |
| Married or domestic partnership | 637 (81.0%) |
| Widowed | 4 (0.5%) |
| Divorced | 16 (2.0%) |
| Separated | 5 (0.6) |
| Highest education, n (%) | |
| No schooling | 7 (0.9%) |
| Some high school, no diploma | 22 (2.8%) |
| High school graduate, diploma | 163 (20.7%) |
| Vocational training | 36 (4.6%) |
| Associate’s Degree | 68 (8.7%) |
| Bachelor’s Degree | 230 (29.3%) |
| Master’s Degree | 189 (24.0%) |
| Doctoral Degree | 70 (8.9%) |
| Missing Data | 1 (0.1%) |
| Pregnancy/Postpartum status | |
| Pregnant, n (%) | 308, (39.2%) |
| Weeks, m (SD) | 21.97 (11.15) |
| Postpartum, n (%) | 478, (60.8%) |
| Weeks, m (SD) | 16.22 (14.28) |
| Pregnancy History, n (%) | |
| >1 prior pregnancy | 522 (66.4%) |
| Never pregnant before | 262 (33.3%) |
| Missing Data | 2 (0.3%) |
| Smoking, n (%) | |
| Never | 563 (71.6%) |
| Former | 175 (22.3%) |
| Current | 47 (6.0%) |
| Missing Data | 2 (0.1%) |
| Current Alcohol Use, n (%) | |
| Yes | 191 (24.3%) |
| No | 594 (75.6%) |
| Missing Data | 1 (0.1%) |
See Table 1 for a summary of demographics for the participants included. Most participants were from the United States of America (n = 433), followed by Canada (n = 46), Australia (n = 10), and the United Kingdom (n = 9). There were 1–3 participants from the following countries: Angola, Anguilla, Belgium, Bermuda, Brazil, Chile, China, Cyprus, Ecuador, Germany, Greece, Iceland, Ireland, Israel, Japan, Mexico, New Zealand, Pakistan, Panama, Philippines, Puerto Rico, Romania, Serbia, Singapore, South Africa, Switzerland, Turkey, United Arab Emirates. There were 245 participants who did not report their country.
Mental health measures
See Table 2 for a summary of mean scores for the mental health variables of interest by sample overall and broken down by women who were pregnant and women who were within 1 year postpartum. Just over half (51%) of women in pregnancy and 71.7% of women in the postpartum had EPDS scores ≥ 12.
Table 2. Mental health outcomes (sample overall, and by pregnancy status).
| Outcome Variables | Overall | Pregnant Women | Postpartum Women |
|---|---|---|---|
| (n = 786) | (n = 308)* | (n = 478)** | |
| Depression | |||
| PHQ-8 | 9.51 (6.03) | 8.42 (5.70) | 10.21 (6.13) |
| EPDS | 14.01 (6.40) | 12.43 (6.60) | 15.02 (6.10) |
| Anxiety | |||
| GAD-7 | 9.22 (5.81) | 7.95 (5.70) | 10.04 (5.74) |
| Sleep | |||
| ISI | 10.29 (6.30) | 9.85 (6.40) | 10.58 (6.22) |
| Perceived Stress | |||
| PSS | 31.31 (10.85) | 28.36 (10.54) | 33.22 (9.31) |
Notes: * N ranged from 304 to 308 for these measures due to missing data;
** N ranged from 473 to 478 for these measures due to missing data.
Discussion and conclusions
This report provides preliminary findings from a pilot study which explored the potential for reaching and engaging perinatal women on a global scale with the MGHPDS app over time. We also explore demographics and mental health symptom profile among app users. There is a need for improved tools and approaches that can be easily and broadly implemented to effectively detect depression during the perinatal period. Early identification of depression during pregnancy is an important first step towards connecting women to treatment and preventing continued depression into the postpartum. Leveraging the power of big data and technology can enable us to fine tune what we know regarding predictors of perinatal depression. Furthermore, the development and dissemination of an easy-to-use screening app could aid with detection of perinatal depression among hard-to-reach patients, such as those living in rural or low-resourced locations, nationally and internationally. In resource-limited settings, the risks of using screening tools with inadequate specificity include overwhelming already stretched resources by generating referrals to treatment of false-positive cases. Improving the specificity of detection measures may thus support the rationalized allocation of resources in both constrained and less constrained settings.
With mainly passive recruitment efforts we collected mental health data on over 700 women during the perinatal period, reaching women across over 30 countries. Such speaks to the promise of digital screening and how, with greater dissemination efforts, we could aim to collect data from a larger and more diverse sample on a global scale. The increased use of digital technology for both identification and treatment across disease states, including mental health disorders, provides an opportunity to develop an abbreviated perinatal depression screening tool with both high sensitivity and specificity exceeding what is currently available. The use of mobile cellular phones provides an opportunity to screen very large samples of subjects for perinatal depressive symptoms, as well as commonly comorbid symptoms such as anxiety, sleep dysregulation, and perceived stress. The large sample would naturally allow for powered analysis of symptoms when determining best screening practices. This can then be coupled with real time instrument scoring and electronic data capture, providing an opportunity to refine currently available screening tools and to develop digital platforms that can be used in both developed and developing countries.
From a global perspective, this app could be of great interest to low and middle income (LMIC) countries where resources and perinatal mental health tools and services are limited. To date, use of a mobile phone application to screen for perinatal depression has been demonstrated to be feasible in a low resource environment in South Africa where community health workers administered the EPDS using mobile phones [19]. A 4-item screening tool for symptoms of common mental disorders and suicidality has been developed, validated and found to be effective at identifying pregnant women with symptoms of common mental disorders across commonly spoken languages and cultures in Cape Town, South Africa [37, 38]. A recent review of the literature on digital technology for treating and preventing mental disorders in LMIC concluded that, while continued research is needed, findings across 49 studies were promising and demonstrated potential effectiveness of online, text-messaging, and telephone support interventions [39]. Further research is needed to assess 1) the extent to which existing primary care staff and community health workers can both screen for perinatal psychiatric disorders and then deliver treatment, 2) whether available screening tools are valid across populations with different cultural and ethnographic characteristics, and 3) whether self-reported measures delivered by an app can yield greater engagement in treatment [19, 24]. Clearly, accurate screening and reliable diagnosis of perinatal depression will be an essential first step to the ultimate hurdle, which is to build the bridge from detection to delivery of effective evidence-based treatments to those suffering from depression where substantial mental health service resources are limited or simply do not exist.
The majority of the users reported clinically significant levels of depression (51% pregnant women, 72% postpartum women); although, one likely limitation may be selection bias in the findings in that women who are experiencing more depressive symptoms seek out and more actively engage with the app. However, this also suggests that women experiencing depressive symptoms are seeking support services and informational resources through apps. Thus, there is an opportunity to leverage this app and connect women who screen above a specified threshold to an intervention or resources. Furthermore, detection of women experiencing depression in pregnancy could serve as a means to prevent postpartum depression, if these women are then able to be connected to evidence-based depression treatment. Digital technology, including mobile health (mHealth), has been identified as a promising strategy for increasing access to evidence-based intervention for mental health due to factors such as the constant availability, equity of availability, immediate support, low cost, and fact that digital technology can overcome geographic barriers or regional shortages of mental health providers [40]. A systematic review of the effectiveness of mobile apps for monitoring and management of mental health symptoms or disorders concluded that there is support for the potential of apps to effectively reduce mental health symptoms, yet further robust research is needed to develop and test these evidence-based app programs [41]. Specific to the perinatal population, a recent meta-analysis provides support for the efficacy and acceptability of internet-delivered interventions for perinatal women, and highlights the limited number of interventions utilizing technology specifically for this population, and particularly for mental health symptoms during pregnancy [42]. Given low rates of treatment seeking, preferences for care in non-specialty settings, and limited services targeting depression during pregnancy, directing users of the MGHPDS whose scores indicate possible depression to a digital therapeutic intervention could be a highly impactful service [43].
We have identified several future additional directions for this work utilizing the MGHPDS. First, we aim to increase engagement for longitudinal data collection. Engagement with the app over time was minimal; we report only on cross-sectional data as only a small percentage of participants returned to the app for a second time to fill out measures. We will focus efforts further on user engagement over time for mobile applications to develop features and strategies that would encourage participants to return to the app and complete the measures longitudinally at multiple time points over pregnancy and the postpartum. This would enable us to examine the predictors of depression across time in pregnancy and the postpartum.
Relatedly, we hope to explore more about how to optimize the user experience with the MGHPDS for future versions of the app. We will be adding a brief optional survey in the app to obtain a better understanding of what brought women to the app, what their experiences were with using the app, and what they did with the information and scores provided through the app. This knowledge will enable us to fine tune the app to better meet the needs of women in the perinatal period and to improve their satisfaction with using the app. There is also an opportunity to explore in which types of environments women are more likely to report their symptoms of depression or seek treatment. For example, future research could explore differences in reporting depression symptoms when assessed over an app in the privacy of their home compared to an office and with a healthcare provider. Another area of research could be pertaining to the uptake of treatment when screening is conducted via an app and connected to a digital therapeutic. Furthermore, as the trajectory of perinatal anxiety is understudied, we intend to build upon this work as it relates to screening for anxiety, its longitudinal course, and its relationship with elevated depressive symptoms.
As with any data collected through a widely accessible app or survey, an area of concern is with regards to the quality and validity of the data. We plan to conduct a validation study for this screening tool with a sample of participants where we administer a structured psychiatric diagnostic interview (e.g., using the MINI [44]) that will serve as the gold-standard for depression diagnosis. Ultimately, we aim to leverage “big data” to modify available perinatal depression screening tools by potentially including symptoms such as anxiety, sleep dysregulation, and perceived stress with which perinatal depression is so often associated. Longer term aims will include exploring options for embedding evidence-based digital therapeutics into the app as a means to increase access to mental health services. The use of digital technology with an app that allows for both substantial sample size and statistical power as well as accurate scoring, and ultimately a version of the app which is brief, accurate, and can be easily administered (e.g., by community health workers or non-specialist providers) is a significant step forward for the field.
Supporting information
(DOCX)
Data Availability
The data underlying this study contain sensitive and potentially identifying information and cannot be shared publicly. Per the MGB Institutional Review Boards Policy, IRB approval must be obtained and a data use agreement must be established for all outgoing Limited Data Sets containing human subjects data. Data requests should be routed to the MGB IRB (irb@partners.org) or the corresponding author.
Funding Statement
The sources of funding include departmental and philanthropic funds for the Center of Women’s Mental Health within the Psychiatry Department of Massachusetts General Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. Epub 1993/10/01. doi: 10.1016/0165-0327(93)90026-g . [DOI] [PubMed] [Google Scholar]
- 2.Kendall KE, Schnurr PP. The effects of vitamin B6 supplementation on premenstrual symptoms. Obstet Gynecol. 1987;70(2):145–9. Epub 1987/08/01. . [PubMed] [Google Scholar]
- 3.Pitt B. ’Maternity blues’. Br J Psychiatry. 1973;122(569):431–3. Epub 1973/04/01. doi: 10.1192/bjp.122.4.431 . [DOI] [PubMed] [Google Scholar]
- 4.Kennerley H, Gath D. Maternity blues reassessed. Psychiatr Dev. 1986;4(1):1–17. Epub 1986/01/01. . [PubMed] [Google Scholar]
- 5.Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. Epub 1993/07/01. doi: 10.1192/bjp.163.1.27 . [DOI] [PubMed] [Google Scholar]
- 6.O’Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol. 1990;99(1):3–15. Epub 1990/02/01. doi: 10.1037//0021-843x.99.1.3 . [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35–47. Epub 1984/01/01. doi: 10.1192/bjp.144.1.35 . [DOI] [PubMed] [Google Scholar]
- 8.Zou R, Tiemeier H, van der Ende J, Verhulst FC, Muetzel RL, White T, et al. Exposure to Maternal Depressive Symptoms in Fetal Life or Childhood and Offspring Brain Development: A Population-Based Imaging Study. Am J Psychiatry. 2019;176(9):702–10. Epub 2019/05/06. doi: 10.1176/appi.ajp.2019.18080970 . [DOI] [PubMed] [Google Scholar]
- 9.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. Jama. 2006;295(5):499–507. Epub 2006/02/02. doi: 10.1001/jama.295.5.499 . [DOI] [PubMed] [Google Scholar]
- 10.Watson JP, Elliott SA, Rugg AJ, Brough DI. Psychiatric disorder in pregnancy and the first postnatal year. Br J Psychiatry. 1984;144:453–62. Epub 1984/05/01. doi: 10.1192/bjp.144.5.453 . [DOI] [PubMed] [Google Scholar]
- 11.Whiffen VE. Is postpartum depression a distinct diagnosis? Clinical Psychology Review. 1992;12(5):485–508. 10.1016/0272-7358(92)90068-J. [DOI] [Google Scholar]
- 12.Lamb ME. Patterns of attachment: A psychological study of the strange situation. Mary D. Salter Ainsworth, Mary C. Blehar, Everett Waters, and Sally Wall. Hillsdale, N.J., Erlbaum, 1978[distributor, Halsted(Wiley), New York]. xviii, 392 pages, $24.95. Infant Mental Health Journal. 1980;1(1):68–70. . [DOI]
- 13.O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. Epub 1991/02/01. doi: 10.1037//0021-843x.100.1.63 . [DOI] [PubMed] [Google Scholar]
- 14.Pitt B. "Atypical" depression following childbirth. Br J Psychiatry. 1968;114(516):1325–35. Epub 1968/11/01. doi: 10.1192/bjp.114.516.1325 . [DOI] [PubMed] [Google Scholar]
- 15.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. Epub 1987/06/01. doi: 10.1192/bjp.150.6.782 . [DOI] [PubMed] [Google Scholar]
- 16.Kabir K, Sheeder J, Kelly LS. Identifying postpartum depression: are 3 questions as good as 10? Pediatrics. 2008;122(3):e696–702. Epub 2008/09/03. doi: 10.1542/peds.2007-1759 . [DOI] [PubMed] [Google Scholar]
- 17.Buist A, Condon J, Brooks J, Speelman C, Milgrom J, Hayes B, et al. Acceptability of routine screening for perinatal depression. J Affect Disord. 2006;93(1–3):233–7. Epub 2006/05/02. doi: 10.1016/j.jad.2006.02.019 . [DOI] [PubMed] [Google Scholar]
- 18.Thombs BD, Benedetti A, Kloda LA, Levis B, Riehm KE, Azar M, et al. Diagnostic accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for detecting major depression in pregnant and postnatal women: protocol for a systematic review and individual patient data meta-analyses. BMJ Open. 2015;5(10):e009742. Epub 2015/10/22. doi: 10.1136/bmjopen-2015-009742; PubMed Central PMCID: PMC4620163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai AC, Scott JA, Hung KJ, Zhu JQ, Matthews LT, Psaros C, et al. Reliability and validity of instruments for assessing perinatal depression in African settings: systematic review and meta-analysis. PLoS One. 2013;8(12):e82521. Epub 2013/12/18. doi: 10.1371/journal.pone.0082521; PubMed Central PMCID: PMC3858316 of the PLoS Medicine Editorial Board. This does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90(2):139G–49G. Epub 2011/11/24. doi: 10.2471/BLT.11.091850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. International Review of Psychiatry. 1996;8(1):37–54. doi: 10.3109/09540269609037816 [DOI] [Google Scholar]
- 22.Steel Z, Chey T, Silove D, Marnane C, Bryant RA, van Ommeren M. Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: a systematic review and meta-analysis. Jama. 2009;302(5):537–49. Epub 2009/08/06. doi: 10.1001/jama.2009.1132 . [DOI] [PubMed] [Google Scholar]
- 23.Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, et al. The epidemiological modelling of major depressive disorder: application for the Global Burden of Disease Study 2010. PLoS One. 2013;8(7):e69637. Epub 2013/08/08. doi: 10.1371/journal.pone.0069637; PubMed Central PMCID: PMC3726670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V, Weobong B, Weiss HA, Anand A, Bhat B, Katti B, et al. The Healthy Activity Program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet. 2017;389(10065):176–85. Epub 2016/12/19. doi: 10.1016/S0140-6736(16)31589-6 ; PubMed Central PMCID: PMC5236064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kola L, Abiona D, Adefolarin AO, Ben-Zeev D. Mobile Phone Use and Acceptability for the Delivery of Mental Health Information Among Perinatal Adolescents in Nigeria: Survey Study. JMIR Ment Health 2021;8(1):e20314). doi: 10.2196/20314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Porte LM, Kim JJ, Adams MG, Zagorsky BM, Gibbons R, Silver RK. Feasibility of perinatal mood screening and text messaging on patients’ personal smartphones. Arch Womens Ment Health. 2020Apr;23(2):181–188. doi: 10.1007/s00737-019-00981-5 Epub 2019 Jun 15. . [DOI] [PubMed] [Google Scholar]
- 27.Giacco D, Amering M, Bird V, Craig T, Ducci G, Gallinat J, et al. Scenarios for the future of mental health care: a social perspective. Lancet Psychiatry. 2017;4(3):257–60. Epub 2016/11/07. doi: 10.1016/S2215-0366(16)30219-X . [DOI] [PubMed] [Google Scholar]
- 28.McEvoy KM, Rayapati D, Washington Cole KO, Erdly C, Payne JL, Osborne LM. Poor Postpartum Sleep Quality Predicts Subsequent Postpartum Depressive Symptoms in a High-Risk Sample. J Clin Sleep Med. 2019;15(9):1303–10. doi: 10.5664/jcsm.7924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okun ML, Mancuso RA, Hobel CJ, Schetter CD, Coussons-Read M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J Behav Med. 2018;41(5):703–10. Epub 2018/07/22. doi: 10.1007/s10865-018-9950-7 ; PubMed Central PMCID: PMC6192841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farr SL, Dietz PM, O’Hara MW, Burley K, Ko JY. Postpartum anxiety and comorbid depression in a population-based sample of women. J Womens Health (Larchmt). 2014;23(2):120–8. Epub 2013/10/29. doi: 10.1089/jwh.2013.4438 ; PubMed Central PMCID: PMC7469256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow A, Dharma C, Chen E, Mandhane PJ, Turvey SE, Elliott SJ, et al. Trajectories of Depressive Symptoms and Perceived Stress From Pregnancy to the Postnatal Period Among Canadian Women: Impact of Employment and Immigration. Am J Public Health. 2019;109(S3):S197–s204. Epub 2019/06/27. doi: 10.2105/AJPH.2018.304624 ; PubMed Central PMCID: PMC6595522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park ER, Psaros C, Traeger L, Stagg A, Jacquart J, Willett J, et al. Development of a Postpartum Stressor Measure. Matern Child Health J. 2015;19(10):2094–101. Epub 2015/02/16. doi: 10.1007/s10995-015-1731-0 . [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–73. Epub 2008/08/30. doi: 10.1016/j.jad.2008.06.026 . [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. Epub 2006/05/24. doi: 10.1001/archinte.166.10.1092 . [DOI] [PubMed] [Google Scholar]
- 35.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. Epub 2001/07/05. doi: 10.1016/s1389-9457(00)00065-4 . [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–96. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 37.Abrahams Z, Schneider M, Field S, Honikman S. Validation of a brief mental health screening tool for pregnant women in a low socio-economic setting. BMC Psychol. 2019;7(1):77. Epub 2019/12/11. doi: 10.1186/s40359-019-0355-3; PubMed Central PMCID: PMC6902551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Heyningen T, Myer L, Tomlinson M, Field S, Honikman S. The development of an ultra-short, maternal mental health screening tool in South Africa. Glob Ment Health (Camb). 2019;6:e24. Epub 2019/10/31. doi: 10.1017/gmh.2019.21; PubMed Central PMCID: PMC6796322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naslund JA, Aschbrenner KA, Araya R, Marsch LA, Unützer J, Patel V, et al. Digital technology for treating and preventing mental disorders in low-income and middle-income countries: a narrative review of the literature. Lancet Psychiatry. 2017;4(6):486–500. Epub 2017/04/24. doi: 10.1016/S2215-0366(17)30096-2 ; PubMed Central PMCID: PMC5523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olff M. Mobile mental health: a challenging research agenda. Eur J Psychotraumatol. 2015;6:27882. Epub 2015/05/23. doi: 10.3402/ejpt.v6.27882; PubMed Central PMCID: PMC4439418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K, Varma DS, Prosperi M. A systematic review of the effectiveness of mobile apps for monitoring and management of mental health symptoms or disorders. J Psychiatr Res. 2018;107:73–8. Epub 2018/10/23. doi: 10.1016/j.jpsychires.2018.10.006 . [DOI] [PubMed] [Google Scholar]
- 42.Loughnan SA, Joubert AE, Grierson A, Andrews G, Newby JM. Internet-delivered psychological interventions for clinical anxiety and depression in perinatal women: a systematic review and meta-analysis. Arch Womens Ment Health. 2019;22(6):737–50. Epub 2019/05/19. doi: 10.1007/s00737-019-00961-9 . [DOI] [PubMed] [Google Scholar]
- 43.Flynn HA, Henshaw E, O’Mahen H, Forman J. Patient perspectives on improving the depression referral processes in obstetrics settings: a qualitative study. Gen Hosp Psychiatry. 2010;32(1):9–16. Epub 2010/02/02. doi: 10.1016/j.genhosppsych.2009.07.005 ; PubMed Central PMCID: PMC2818112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59Suppl 20:22–33;quiz 4–57. Epub 1999/01/09. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data underlying this study contain sensitive and potentially identifying information and cannot be shared publicly. Per the MGB Institutional Review Boards Policy, IRB approval must be obtained and a data use agreement must be established for all outgoing Limited Data Sets containing human subjects data. Data requests should be routed to the MGB IRB (irb@partners.org) or the corresponding author.