Abstract
Photoreceptive inputs to the teleost brain are perceived as image of the visual world and as photo-modulation of neuroendocrine and neuronal signals. The retina and pineal organ are major receptive organs with projections to various parts of the brain, but in the past decades deep brain photoreceptors have emerged as candidates for photoreceptive inputs, either independent or in combination with projections from light sensory organs. This study aimed to test the effects of narrow bandwidth light using light-emitting diodes technology on brain neural activity through putative opsin stimulation in Atlantic salmon. The expression of c-fos, a known marker of neural activity, was compared in situ between dark-adapted salmon parr and following light stimulation with different wavelengths. c-fos expression increased with duration of light stimulation and the strongest signal was obtained in fish exposed to light for 120 minutes. Distinct and specific brain regions were activated following dark to light stimulation, such as the habenula, suprachiasmatic nucleus, thalamus, and hypothalamus. The c-fos expression was overlapping with photoreceptors expressing melanopsin and/or vertebrate ancient opsin, suggesting a potential direct activation by light. Interestingly in the habenula, a distinct ring of vertebrate ancient opsin and melanopsin expressing cells is overlapping with c-fos expression after neural activation. Salmon exposed to different spectra had neural activation in similar brain regions. The most apparent difference was melanopsin expression in the lateral cells of the lateral tuberal nuclus in the hypothalamus, which appeared to be specifically activated by red light. Light-stimulated neuronal activity in the deep brain was limited to subpopulations of neurons, mainly in regions with neuronal modulation activity, retinal and pineal innervations and known presence of nonvisual photoreceptors. The overlapping expression patterns of c-fos and nonvisual opsins support direct light stimulation of deep brain photoreceptors and the importance of these systems in light induced brain activity.
Introduction
Light relays important cues for regulating biological processes and rhythms in animals. Photoreception in teleosts is often related to sensory organs such as the retina and pineal which have innervations to several regions of the brain such as the optic tectum, preoptic area, habenula, thalamus, hypothalamus and tegmentum [1]. However, in the last decade new photoreceptor families have been identified with central expression (e.g., the deep brain) rather than peripheral [2–5]. Studies have shown that the fish brain is packed with photoreceptor elements [2, 4, 6–12], but knowledge about activation of these photoreceptor systems and the regulation of biological processes is limited.
In the past years, the nonvisual opsins have been shown to have specific photosensitive functions in the teleost brain. Zebrafish (Danio rerio) larvae lacking eyes and pineal were shown to display light seeking behavior following exposure to darkness regulated by melanopsin-expressing cells in the preoptic area [8]. Our previous studies have shown that a transient cluster of dual photoreceptors, expressing vertebrate ancient opsin (VA opsin) and melanopsin, in the hindbrain are permissive to the life history transition of hatching in Atlantic halibut (Hippoglossus hippoglossus) embryo with neuroblastic retina [13]. In masu salmon (Oncorhynchus masou), the saccus vasculosus, expressing e.g., short wavelength-sensitive opsin and melanopsin, has been indicated to act as a sensor of seasonal changes in day-length in fish [14] however, the suggestion is debated [15]. Activation of photoreceptors in the brain after light stimulation can be visualized by expression studies of c-fos, known to respond rapidly and transiently to a variety of stimuli such as growth factor stimulation and stimulation of nerve cells [16, 17]. Studies analyzing the neural activity following stimulation with a light pulse in both mammals [18, 19] and fish [13, 20] showed increased expression levels of c-fos. Zebrafish was shown to have a strong response to changing photic conditions, as light-dark transition, and a 30-minute light pulse at night induced c-fos expression in brain regions co-localised with deep brain photoreceptors. The study suggested that elevated level of c-fos expression would reflect a distinct change in neural activity in response to changes in sensory input [20]. Further, our studies in Atlantic halibut showed c-fos activation in the dual photoreceptive hindbrain cluster and hatching glands after light induced hatching. The hindbrain cluster was shown to be imbedded in a neuronal network projecting to the narrow belt of hatching glands in the yolk sac, indicating a direct light control of hatching by neuronal activation [13].
In Atlantic salmon (Salmo salar), the changing seasonal photoperiod strongly influences the physiology and behavior throughout the lifecycle. Photoperiod regulates development and growth, timing of smoltification, migration and maturation [21–26]. Salmon is therefore an interesting species to study the underlying mechanisms of photo-induced brain activity. Narrow bandwidth lights using light-emitting diodes (LED) technology have been used to explore the effects of light spectra on development, growth and survival in a range of species including zebrafish [27], Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) [28]. The present study aimed to determine the effects of narrow bandwidth light on activation of photoreceptive brain regions by using c-fos as a marker of neural activity. To do so, Atlantic salmon parr were exposed to white light and narrow bandwidth light using LED- technology, to explore brain light sensitivity and reveal brain regions that are activated following light stimulation. Our results revealed distinct neural activity after 120 minutes light stimulation in several putative photoreceptive regions of the brain also having retinal and pineal innervations, such as the habenula, suprachiasmatic nucleus, thalamus, and hypothalamus. Salmon exposed to different spectra had neural activation in similar brain regions, however we found melanopsin expressing lateral cells of the lateral tuberal nucleus of hypothalamus which specifically appeared to be activated by red light.
Materials and methods
Animals and sampling
Juvenile Atlantic salmon (Salmo salar) parr (freshwater) (approx. 13 cm) were sourced from ILAB (Industry Laboratory), Bergen, Norway to perform the initial c-fos activation study to determine the optimal stimulation time (Activation time response of c-fos). The use and handling of animals was performed according to Norwegian law and the Norwegian Animal Research Authority (NARA) following procedures at the authorized facility and was given ethical approval by the Norwegian Veterinary Authorities (Application number: 6918). Atlantic salmon parr (approx. 19 cm) used for the narrow bandwidth experiment in Stirling were sourced from the Niall Bromage Freshwater Research Facility (Institute of Aquaculture, University of Stirling, Stirling). The trial was carried out at the University of Stirling temperate freshwater facilities with all experimental procedures conducted in compliance with the Animals Scientific Procedures Act 1986 (Home Office Code of Practice. HMSO: London January 1997) under project license PPL70/7916 “Environmental Regulation of Fish Physiology” in accordance with EU regulation (EC Directive 86/609/EEC). All experimentation performed at the Institute of Aquaculture was subject to an ethical review process carried out by the University of Stirling Animal Welfare and Ethical Review Board prior to the work being conducted. All fishes were euthanized with an overdose of methacaine (MS-222, Sigma, USA) before perfusion fixation.
Activation time response of c-fos
To identify the optimal sampling point after stimulation with light, for the subsequent light activation study using in situ hybridization, a preliminary test sampling was performed. Atlantic salmon parr were acclimatized in tanks for 5 days before being subjected to darkness for 48 hours. Fish were then stimulated for 15, 30, 60 or 120 minutes with two wide spectrum compact fluorescent bulbs (Viva-Lite, Germany). Sampling for in situ hybridization consisted of five fish per treatment (duration of stimulation) euthanized with an overdose of methacaine and fixated by vascular perfusion through the heart with 4% paraformaldehyde. In addition, a dark control (5 fish) was included. The brains were dissected out and prepared for in situ hybridization on cryo-sections as described by [12]. The ISH was carried out under similar conditions for all sampling points.
Narrow bandwidth light activation experiment
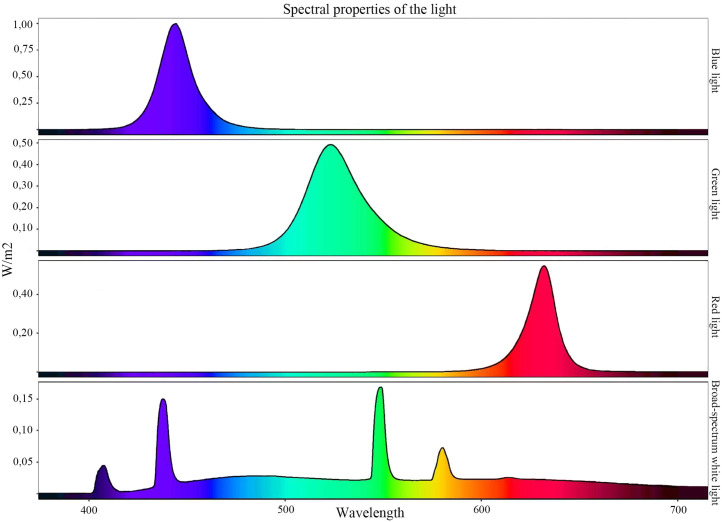
Atlantic salmon (parr) maintained under constant light since hatching were transferred into a recirculating aquaculture system (University of Stirling, UK) and acclimatized in tanks for 5 days before being exposed to darkness for 48 hours. The experiment included thirteen experimental groups held in separate tanks including controls. The experiment was divided in an on-response (dark to light) and an off-response (light to dark). In dark to light groups, fish were exposed to different narrow bandwidth light (Dark/White, Dark/Blue, Dark/Green, Dark/Red and Dark/Dark as the control) for 120 minutes before sampling. In the light to dark groups, dark-adapted fish were exposed to different narrow bandwidth light for 24 hours before returning them to darkness for 120 min (White/Dark, Blue/Dark, Green/Dark, Red/Dark) or not (control) (White/White, Blue/Blue, Green/Green, Red/Red) and then sampling. At each sampling, fish were euthanized and perfused as described above. Narrow bandwidth lights were supplied by Signify using computer-controlled LED units, producing either Blue (444 nm peak), Green (523 nm peak) or Red (632 nm peak) light. The three narrow bandwidth lights were chosen to span different parts of the broad-spectrum white light. The broad-spectrum white light was chosen since it had a broad absorbance spectrum within the range of visible light. Broad-spectrum white light provided for both the test sampling and narrow bandwidth light activation experiment was delivered using two wide spectrum compact fluorescent bulb (Viva-Lite, Germany). Light intensity was measured just below the water surface directly below the lamp using a single sensor channel Watts’s meter (Skye Instruments Ltd, UK) calibrated to National Physics Laboratory (UK) Standards. Intensity in each tank for all light treatments was calibrated to 5 W/m2. See Fig 1 for spectral composition of the experimental light.
Fig 1. Spectral composition of the experimental light.
LED units (Signify) generated narrow bandwidth lights of Blue (444 nm peak), Green (523 nm peak) or Red (632 nm peak). Broad-spectrum white light was delivered using a wide spectrum compact fluorescent bulb (Viva-Lite). Intensity in each tank for all light treatments was calibrated to 5 W/m2.
Identification of c-fos paralogue genes
The salmon database Ssa_ASM_3.6.scaf.fasta at ViroBLAST (http://marineseq.imr.no/salmon2014/viroblast/viroblast.php) was searched with a query sequence of Atlantic halibut c-fos (Accession number: KF941297) giving two relevant hits (ccf1000000124_0–0 and ccf1000000152_0–0). Alignment of the query sequence and subject (contig) indicated two potential c-fos genes. Based on the alignment the exons were predicted and primers located in the potential UTR’s were designed for the two genes. Primers are listed in Table 1.
Table 1. Primers for molecular cloning.
| Primer name | Sequence (5’–3’) |
|---|---|
| cfos124F1 | GGATCACTTGACTTTGACAGC |
| cfos124R1 | TGCGCTGAAGAACAAGTCAAC |
| cfos152F1 | GGGATCACTTGACTTTGACAAC |
| cfos152R1 | GCTTCCTGGTTGTGCGAGTC |
Primers used for cloning of the two c-fos paralogues.
Molecular cloning
Total RNA isolation, DNase treatment and cDNA synthesis were performed according to [29]. The two Atlantic salmon c-fos genes were cloned by amplification of the genes by PCR using a pool of DNase treated cDNA of Atlantic salmon brains. PCR was run with annealing temperature of 63°C for c-fos.1 and 62°C for c-fos.2 and 35 cycles were used. The primer pairs for both genes generated a PCR product of approximately 1500 bp. The PCR products were separated on an agarose gel and the relevant bands were cut out and extracted from the agarose gel by QIAEX II Gel Extraction kit (Qiagen, Germany). The PCR products were ligated into StrataClone PCR cloning vector pSSC-A-amp/kan (Agilent Technologies, USA) and sequenced at the University of Bergen Sequencing Facility. The nucleotide sequences were deposited into GeneBank with the accession number MF685241 for c-fos.1 (ccf1000000124_0–0) and MF685242 for c-fos.2 (ccf1000000152_0–0).
Synthesis of RNA probe for c-fos, melanopsin and VA opsin
For the synthesis of RNA probes, PCR product was used as template for the reaction and primers were designed for c-fos, melanopsin and VA opsin as described by [30]. The primers for c-fos were designed to be specific for both paralogues by making them degenerative, ensuring a probe specific for both genes. For melanopsin, three probes were synthesized and used together, opn4m1a1 (specific for JN210547), opn4x1a (specific for JN210546) and opn4x1b1/2 (specific for both JN210544 and JN210545) ensuring to detect all the functional photopigments of melanopsin. For VA opsin the probe primer was designed based on NM_001123626. Synthesis of DIG-labelled RNA probe for c-fos and the melanopsins and DIG and FITC-labelled RNA probe for VA opsin was done following the manufacturer’s instructions (Roche Diagnostics, Germany). The synthesized probes were precipitated by LiCl and EtOH together with tRNA (Roche Diagnostics, Germany).
In situ hybridization and Nissl staining
Parallel sectioning (10μm) was performed on a Leica CM 3050S cryostat (Leica Biosystems, Germany) and before storage at 20°C, the tissues were air dried for 1 hour at room temperature and for 30 minutes at 65°C. In situ hybridization (ISH) was carried out as described by [12]. One parallel of the sectioned brain was Nissl-stained with 0.5% cresyl fast violet (Chroma-Gesellschaft, Germany), and the other parallel was stained by in situ hybridization.
Results
Activation time response of c-fos
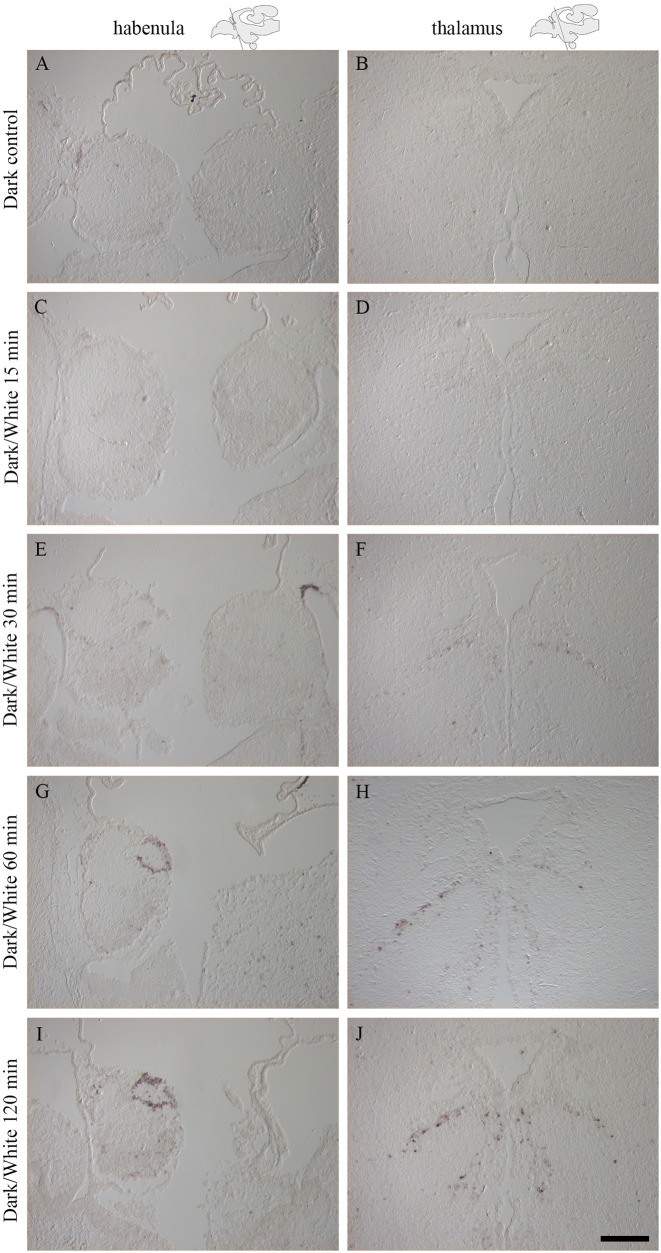
Fish kept in darkness showed no detectable expression of c-fos (Fig 2A and 2B). Stimulation with light for 15 minutes did not result in any detectable c-fos expression by ISH (Fig 2C and 2D). Expression levels of c-fos increased with the duration of the light stimulation (detectable but weak after 30 minutes (Fig 2E and 2F), enhanced after 60 minutes (Fig 2G and 2H) and very strong after 120 minutes (Fig 2I and 2J)). A light stimulation duration of 120 minutes was therefore selected for subsequent experiment and c-fos ISH analyses.
Fig 2. Activation of the immediate early gene c-fos after stimulation with white light was strongest after 120 minutes of light exposure.
In situ hybridization with c-fos is shown in the habenula (A, C, E, G, I) and thalamus (B, D, F, H, J) and schematic drawings indicate the plane of the cryo-section. A-B: Dark control, no c-fos expression detected. C-D: Sampling after 15 minutes exposure to white light, no c-fos expression detected. E-F: Sampling after 30 minutes exposure, weak c-fos expression detected. G-H: Sampling after 60 minutes exposure, strong c-fos expression detected. I-J: Sampling after 120 minutes exposure, the strongest c-fos expression detected. Scale bar of 200 μm.
White light neural activation
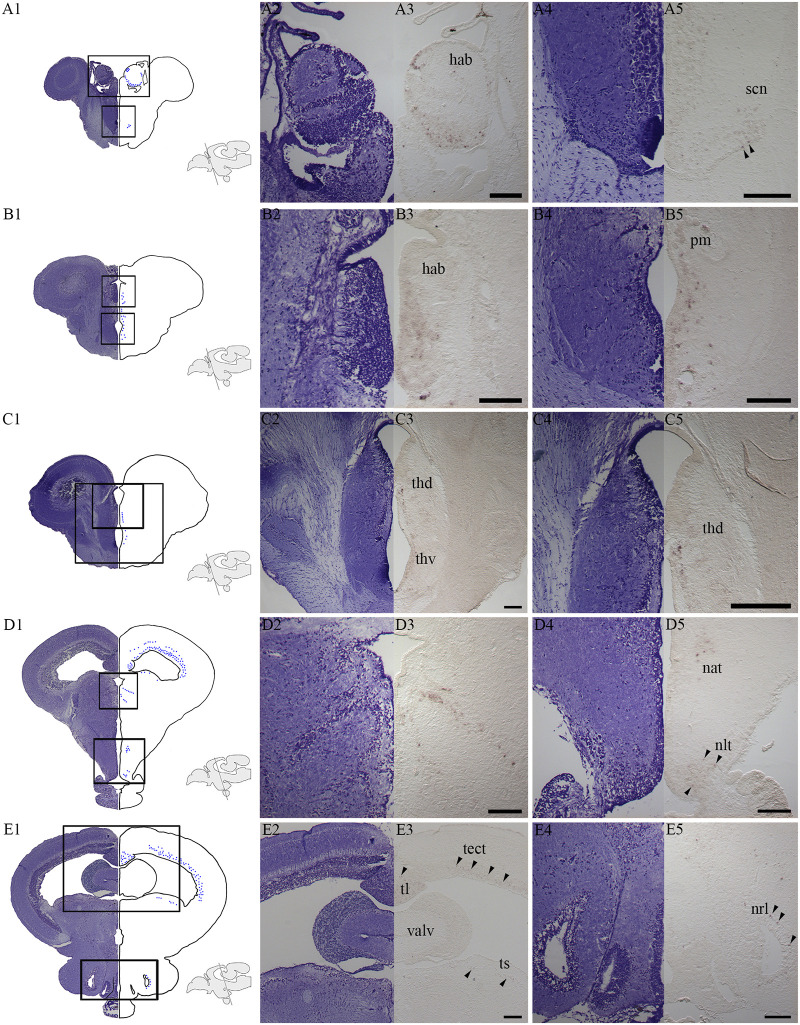
Stimulation with white light (on-response, dark to light) showed c-fos expression in different brain regions (Fig 3). In the diencephalon, a characteristic ring of c-fos positive cells was located in dorsal parts of the left habenula (Fig 3A3). In addition, ventral parts of the habenula (Fig 3A3) and the suprachiasmatic nucleus (SCN) (Fig 3A5) also expressed c-fos. Fig 3B3 shows a cell group expressing c-fos just ventral to the caudal parts of the habenula and in the same section c-fos positive cells can be seen in cells adjacent to the third ventricle including the magnocellular preoptic nucleus (Fig 3B5). Activated cells were also detected in both dorsal and ventral parts of the thalamus (posterior nucleus of thalamus) close to the third ventricle (Fig 3C3 and 3C5). In more caudal parts of thalamus, a characteristic pattern of c-fos expression was observed (Fig 3D3) and in the hypothalamus, c-fos expression was located both in the anterior tuberal nucleus and in the lateral tuberal nucleus (nucleus lateralis tuberis (NLT)) (Fig 3D5). Positive cells have also been localized in the mesencephalic tectum, the longitudinal torus, the semicircular torus (Fig 3E3 and S1 Fig) and the nucleus of the lateral recess (Fig 3E5). Off-response in white light (light to dark) was also studied, resulting in c-fos expression in the same brain regions as for the on-response, although expression was in general weaker. The c-fos expression after stimulaton (White/Dark) was stronger than in the control (White/White) (See S2 and S3 Figs).
Fig 3. Activated brain regions after stimulation with white light for 120 minutes.
A1-E1: Nissl-stained transverse sections at the equivalent level of c-fos expressing cells illustrated by blue dots in salmon, parr. Schematic drawings illustrate the level of the section. A2-E2, A4-E4: The Nissl-stained cell populations of interest with a higher magnification. A3-E3, A5-E5: c-fos expression at the same level and with the same high magnification. The overview illustrations (A1-E1) and the left sided tissues with c-fos expression (A3-E3, A5-E5) are presented by flipping the pictures, resembling a whole brain section. A3: Expression in a dorsal ring in the left habenula (hab) and in ventral parts of the habenula. A5: c-fos expression in the suprachiasmatic nucleus (scn). B3: A cell group expressing c-fos just ventral to the caudal habenula. B5: Expression in cells close to the third ventricle and in the magnocellular preoptic nucleus (pm). C3: Activated cells in the dorsal thalamus (thd) and ventral thalamus (thv) (posterior nucleus of thalamus) close to the third ventricle. C5: Focus on the expression in dorsal thalamus (thd) where the expression was strongest. D3: Expression of c-fos in caudal parts of the thalamus. D5: In the hypothalamus, expression was seen in the anterior tuberal nucleus (nat) and lateral tuberal nucleus (nlt). E3: Cells expressing c-fos were also localized in the mesencephalic tectum (tect), longitudinal torus (tl) and semicircular torus (ts). E5: Expression in the nucleus of the lateral recess (nrl). Arrowheads indicate positive cells. Scale bars of 200 μm.
Narrow bandwidth light neural activation
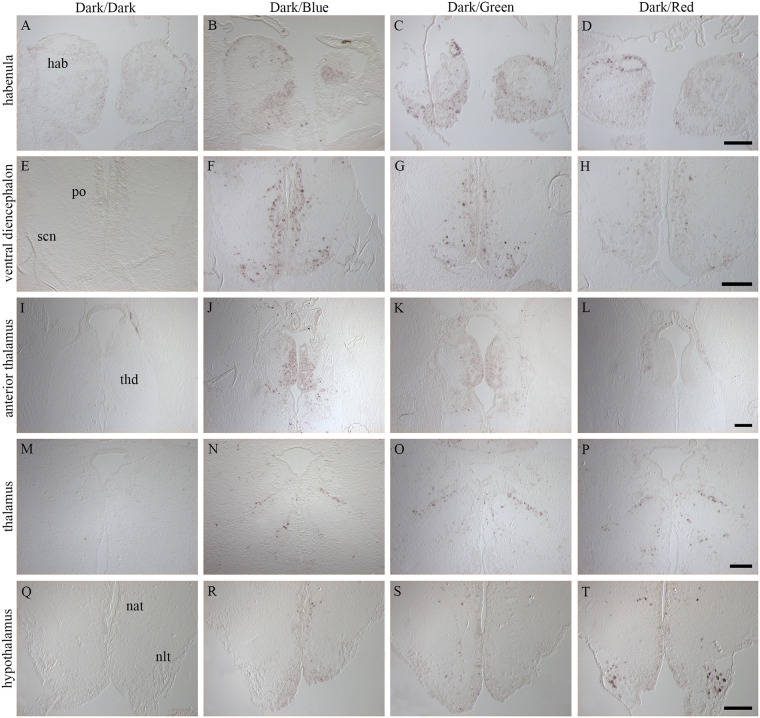
Dark-adapted fish were stimulated for 120 min with narrow bandwidth LED-light (Dark/Blue, Dark/Green and Dark/Red) and c-fos expression was compared against a dark control (Dark/Dark) (Fig 4). Brains from control fish kept in darkness showed no or little c-fos expression (Fig 4A, 4E, 4I, 4M and 4Q). In the left habenula (Fig 4B–4D), the ring of c-fos positive cells was detected in fish exposed to all three light spectra. In the ventral left habenula and right habenula of fishes exposed to Blue and Green light (Fig 4B and 4C), the c-fos expression is more prominent than in fishes exposed to Red light (Fig 4D). In the ventral diencephalon, c-fos expression was detected in the preoptic area and in the SCN for all spectra (Fig 4F–4H) however, the expression is stronger in fishes exposed to Blue and Green light. In the anterior part of the thalamus, expression was detected in the caudal habenula and dorsal thalamus for fish exposed to Blue (Fig 4J) and Green light (Fig 4K), while little expression was detected for Red (Fig 4L). In more caudal parts of the thalamus, the same characteristic expression pattern was observed for all spectra (Fig 4N–4P). In the hypothalamus, expression of c-fos was detected in the anterior tuberal nucleus for all three spectra (Fig 4R–4T) and in fish exposed red light, strong expression was also seen in the NLT (Fig 4T). Off-response in Blue, Green and Red light (light to dark) resulted in c-fos expression in the same brain regions as for the on-response but were in general weaker. In the habenula ring and in the anterior part of the thalamus, little or no staining was detected, and little expression was seen in the lateral cells of NLT for Red/Dark. The c-fos expression after stimulation (Blue/Dark, Green/dark, Red/Dark) was stronger than in the control (Blue/Blue, Green/Green, Red/Red) (See S2 and S3 Figs).
Fig 4. Comparison of c-fos positive signal in the brain of fish exposed to different narrow bandwidth light.
Schematic drawings illustrate the plane of sections. A, E, I, M, Q, U: Control fish kept in darkness (Dark/Dark) showed no or little c-fos expression. B-D: A dorsal ring of c-fos positive cells was detected in the left habenula (hab) for all three spectra. F-H: In the ventral diencephalon, both the preoptic area (po) and superchiasmatic nucleus (scn) displayed c-fos expression. J-L: Expression of c-fos in the caudal habenula and dorsal thalamus (thd) for Blue (J) and Green (K), little expression in Red (L). N-P: Activated cells in caudal parts of the thalamus for all three spectra tested. R-T: In the hypothalamus, expression was detected in the anterior tuberal nucleus (nat) for the three spectra. In addition, a strong expression was detected in the lateral tuberal nucleus (nlt) for fish exposed to red light (T). Scale bars of 200 μm.
Neural activation and deep brain photoreception
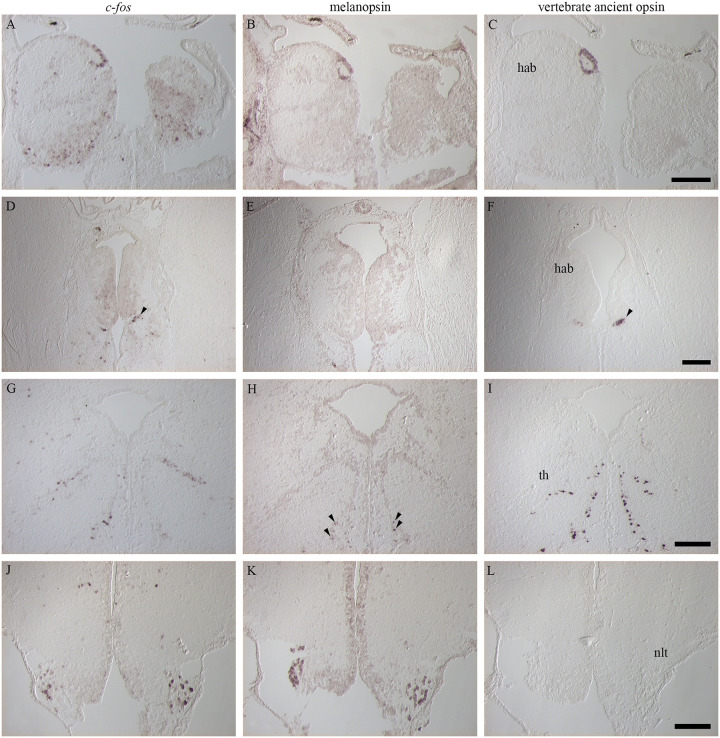
Neural activation and deep brain photoreception were compared (Fig 5) and showed that in the dorsal part of the left habenula, the characteristic ring of c-fos expression (Fig 5A) was also detected for melanopsin (Fig 5B) and VA opsin (Fig 5C). A small cluster of cells just ventral to caudal parts of the habenula was also detected for c-fos (Fig 5D) and VA opsin (Fig 5F) but not for melanopsin (Fig 5E). In caudal parts of the thalamus, the same characteristic expression pattern was observed for c-fos (Fig 5G) and VA opsin (Fig 5I), spreading laterally and ventrally from the ventricle. In addition, low melanopsin signal was detected in the same brain region (Fig 5H), even though the melanopsin expression was limited to the ventral parts. In the hypothalamus, positive cells for both c-fos (Fig 5J) and melanopsin (Fig 5K), but not VA opsin (Fig 5L), was localized in the NLT.
Fig 5. Comparison between activated brain regions, melanopsin and vertebrate ancient opsin (VA opsin) expression following exposure to different narrow bandwidth light treatments.
A-C: In the left habenula (hab) a similar ring of cells was seen for c-fos (A), melanopsin (B) and VA opsin (C). D-F: Ventral of the caudal habenula, a small cluster of cells was detected for c-fos (D) and VA opsin (F) (see arrowheads) but not for melanopsin (E). G-I: In the caudal thalamus (th), a similar expression pattern was seen for c-fos (G) and VA opsin (I) and some melanopsin positive cells (H) (see arrowheads) were also detected. J-L: In the hypothalamus, the lateral cells of the lateral tuberal nucleus (nlt) expressed c-fos (J) after light activation and also melanopsin (K) but not VA opsin (L). Scale bars of 200 μm.
Discussion
In the present study we showed robust c-fos expression in distinct cells of the Atlantic salmon brain after light stimulation that were not seen in fish kept in darkness. The cells are located in brain regions with known photoreceptive capacity that also have innervations from the retina and pineal, indicating that the neural activation can be a result of direct photoreception by deep brain cells or a combination with the retina and pineal. Though narrow bandwidth light stimulation, we showed that red light strongly activated lateral cells of the lateral tuberal nucleus.
Time course of c-fos activation
Expression of c-fos is known to be activated rapidly and transiently as a response to a variety of stimuli. As reviewed in Kovács (2008), it is generally thought that c-fos and its protein are reliable markers for identifying activated cells and central nervous system circuits that respond to physiological (e.g., daily rhythm, sleep⁄wake cycle, oestrus, mating, lactation), environmental (e.g., light noise, predator odor), pharmacological and various stress challenges. Generally, the kinetics of the response to acute stimuli is characterized by a peak of c-fos mRNA after approximately 30 minutes and for protein after 90 to 120 minutes [31]. In accordance with this, mating stimuli in medaka (Oryzias latipes) and pharmacological stimuli in goldfish (Carassius auratus) showed a peak in mRNA level of c-fos after 30 minutes exposure to the stimuli [32, 33]. The time response for c-fos mRNA activation in the present study following light exposure revealed that a good sampling point for getting a strong c-fos positive signal in Atlantic salmon parr was 120 minutes. However, a weak c-fos stimulation was detected after 30 minutes. A similar time course has been described in rainbow trout (Oncorhynchus mykiss) after intraperitoneal administration of kainic acid [34] where c-fos mRNA was detectable after only 30 minutes but reached a peak after 120 minutes. The delay in elevated c-fos expression observed in the present study may be related to temperature (temperate fish species vs. tropical) and/or the type of stimulation as a study in Atlantic salmon has shown strong c-fos expression after acute stress for 30 minutes [35].
Light induced neural activation of the brain
Previous studies performed in adult zebrafish showed that c-fos mRNA expression was induced in specific and discrete brain regions following a 30 minutes light pulse applied at night [20]. Increased gene expression levels of c-fos were detected mainly in the pretectum, suprachiasmatic nucleus, tectum, longitudinal torus, valvula cerebelli, and hypothalamus. In comparison, a study in zebrafish larvae using a combination of zebrafish atlas and immunohistochemical detection of phosphorylated-extracellular signal-regulated kinase as a readout of neural activity, showed activation in subpalladium, tectal neuropil, cerebellum, and hindbrain after a 10 second light pulse. Further, blue light stimulation showed increased activity in vestibular nuclei, habenula, hypothalamus, ventral hindbrain and spinal cord [36]. The present study confirmed activation of distinct and specific brain regions in Atlantic salmon stimulated with light for 120 minutes that were not seen in fish kept in darkness. These regions included the habenula, suprachiasmatic nucleus, thalamus, hypothalamus, tectum, longitudinal torus andsemicircular torus. Importantly, several of these brain regions also contain deep brain photoreceptors, expressing melanopsin and vertebrate ancient opsin, suggesting a potential direct activation of these regions upon light stimulation. However, several of the brain regions expressing c-fos, have retinal and pineal innervations [1] and we cannot exclude that the cells may also be activated as a response to photoreception in the retina or pineal, or in combination. The present results showed no c-fos expression in the pineal organ (see S4 Fig) even though the fish pineal organ is known to contain a photoreceptive circadian oscillator [37].
Habenula
A characteristic dorsal ring of the left habenula showed a similar expression pattern for c-fos, melanopsin and VA opsin, and studies by Sandbakken et al. (2012) have shown that the Xenopus-like melanopsin (Opn4x) cell population in the habenula is co-localized with an asymmetric serotonergic cell group of the left habenula [38]. In salmonids, the left habenula is shown to receive the majority of the innervations from the parapineal organ and the flattened terminal field of the parapineal tract is shown to be located in the dorsal part [39]. Photoreceptors of the left habenula could be related to the assumed photoreceptive function of the parapineal organ as suggested by Sandbakken et al. (2012) that could have a functional relationship also to the limbic system [39]. Interestingly, the neural activity of habenula seen in zebrafish larvae using the phosphorylated-extracellular signal-regulated kinase as a readout of neural activity, is suggested to be linked to a putative visual network, however the activation was seen in the whole left habenula and not in a dorsal ring [36]. Further, using light-sheet microscopy to record activity, reported through the genetically encoded calcium indicator GCaMP5G, showed activity in the left dorsal habenula in zebrafish larvae after red LED flash stimulation. This activity was associated with a visual response linked to the dorsal interpeduncular nuclei of the midbrain that failed to show robust responses in dorsal cells of left habenula after ablation of the eyes [40]. Zebrafish seem to lack the dorsal ring of nonvisual opsins in the left habenula that we find in salmon. Although, we cannot exclude that the neural activation of the dorsal ring of the habenula in salmon is caused by the innervations from retina or pineal, the overlap between melanopsin, VA opsin and c-fos in the distinct ring, supports the idea of a direct neural activation in these photoreceptive cells. Such overlap was also seen in our study in Atlantic halibut, showing c-fos activation in the dual photoreceptive hindbrain cluster expressing both VA opsin and melanopsin [13].
Suprachiasmatic nucleus (SCN)
The SCN is considered to be an integration area for photic information in the teleost brain, receiving both retinal and pineal input [41]. The SCN is also a major source for dopaminergic innervations of the pituitary in Atlantic salmon [42]. It has been shown that Xenopus-like melanopsin positive cells (Opn4x) in the SCN are co-localized with a dopaminergic cell population and it has been suggested that the melanopsin positive cells in the SCN have a role in the dopaminergic regulation of the pituitary function [12]. In accordance with zebrafish exposed to a 30 minutes light pulse at night [20], results from the present study confirmed that this important integration area is also activated in salmon following stimulation with light. The neural activation might be a result of direct photoreception or a combination of activation from the retina and pineal.
Thalamus
In adult zebrafish, the VA opsin positive cells of the thalamus appear from the anterior thalamic nucleus just ventral to the habenula and spread caudally and laterally to the intercalated thalamic nuclei. The VA opsin positive cells have been shown to all be GABAergic as gad67 mRNA co-localize with valop mRNA and it has been suggested that this thalamic population may regulate light-avoidance behavior in zebrafish [10]. Moreover, the thalamic VA opsin population in zebrafish has diurnal rhythmicity and it has been suggested that the thalamic cell group maximizes its photosensitivity diurnally to regulate neuronal activity in the thalamus during dawn and dusk [43]. The thalamic population of VA opsin positive cells in salmon has been shown to have a similar pattern spanning caudally and laterally from the sub-habenula region terminating at the level of the posterior commissure [44]. The present study demonstrated that c-fos is expressed after stimulation with light in the same thalamic regions as VA opsin e.g., anterior thalamus just ventral to the caudal habenula and in posterior parts where the VA opsin positive cells are spread caudally and laterally, although we cannot conclude that the expression is in the same cells. In the caudal thalamus, melanopsin positive cells are also apparent laterally, these are shown to be the mammalian-like (Opn4m) by Sandbakken et al. (2012) revealing both Xenopus-like and mammalian-like melanopsins to be apparent in brain regions with neural activation. In zebrafish, the deep brain VA opsin neurons have been suggested to have a role in time- and light-dependent physiology to adjust to environmental changes [43] and it can therefore be suggested that activated thalamic population in salmon has a similar role. Thalamus is known to be innervated by both the retina and pineal [1] and the neural activation might be a result of direct photoreception or a combination of activation from the retina and pineal.
Lateral cells of the lateral tuberal nucleus (NLT) show neural activation in red light
The hypothalamus is known to be the major source of innervation in the pituitary of teleosts [45–47] and present results revealed that cells in the NLT are activated after light stimulation. Interestingly, lateral cells of the NLT known to express mammalian-like (Opn4m) melanopsin in salmon [12] were activated in dark-adapted salmon stimulated with red light. The neuroendocrine regulation of many of these lateral neurons expressing melanopsin suggests a role in modulating pituitary hormone release and function [12]. Studies in rainbow trout, sea bass (Dicentrarchus labrax) and salmon have shown that the relative transmission of light through the cranium is dependent on the spectral content and that there is a higher penetration towards the red end of the spectrum [48, 49]. Higher transmission of red light through the cranium may explain the presence of photoreceptors detecting red light in the hypothalamus, deep in the brain. Notably, in the present study we have standardized the intensities between light spectra, but wavelength specific differences in cranial absorbance may have resulted in different exposure of light intensity in the brain and pineal. Both the aquatic environment and tissue act as potent filters of the light significantly modifying spectrum and intensity, highlighting the difficulty to dissect light effects in fish. Furthermore, red light has been shown to be less efficient in salmon on suppressing nocturnal melatonin than blue and green light, as the melatonin levels were shown to increase under high intensity of red light reaching 40% of night levels [50]. The light intensity required to suppress diel melatonin production in salmon is higher than for sea bass and cod [49, 50] and both light intensity and spectral composition must be evaluated considering the photoneuroendocrine regulation of the salmon lifecycle. However, the light intensity used in this study is well above the light intensity threshold for suppression of melatonin in Atlantic salmon pineal [49] while the spectral properties of nonvisual opsins in salmon is limited to VA opsin with a λmax of 451 nm [51]. In zebrafish, members of the Opn4m and Opn4x class were shown to have a λmax of 484 nm and 470 nm, respectively [52] and a similar λmax for melanopsin in salmon is likely, contradicting the presumable neural activation of melanopsin positive cells in the NLT. A potential co-expression of a long-wavelength light-sensitive opsin with the melanopsin positive cells of the NLT might be suggested.
In conclusion, this study of light activated neurons in the Atlantic salmon brain, indicates light stimulation of deep brain photoreceptors, by distinct expression of c-fos in photoreceptive brain regions.
Supporting information
(A) Overview of the section with boxes indicating the sections of B-D. (B) Arrowheads indicates c-fos expression in the tectum and in the longitudinal torus. (C) Arrowheads indicates c-fos expression in the semisircularus torus. (D) Higher magnification of some of the cells in C. Scale bars 200 μm.
(TIF)
(A, E, I, M, Q) White/Dark, (B, F, J, N, R) Blue/Dark, (C, G, K, O, S) Green/Dark, (D, H, L, P, T) Red/Dark. In general less or weaker expression of c-fos is detected in the off response then in the on-response. Scale bars 200 μm.
(TIF)
(A, E, I, M, Q) White/White, (B, F, J, N, R) Blue/Blue, (C, G, K, O, S) Green/Green, (D, H, L, P, T) Red/Red. In general little or weaker expression of c-fos is detected in the controls. Scale bars 200 μm.
(TIF)
There are no expression of c-fos in Dark/White stimulated fish. Scale bar 200 μm.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The study was supported by Signify (www.signify.com) to University of Bergen (ME, JVH) and University of Stirling (BGJC, HM). In addition, The Research Council of Norway (www.rcn.no), project number 254894 (ME, JVH), supported the finalization of the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ekström P, Meissl H. The pineal organ of teleost fishes. Rev Fish Biol Fish. 1997;7(2):199–284. [Google Scholar]
- 2.Davies WIL, Tamai TK, Zhen L, Fu JK, Rihel J, Foster RG, et al. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25(11):1666–79. doi: 10.1101/gr.189886.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies WL, Hankins MW, Foster RG. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem Photobiol Sci. 2010;9(11):1444–57. doi: 10.1039/c0pp00203h [DOI] [PubMed] [Google Scholar]
- 4.Fernandes AM, Fero K, Driever W, Burgess HA. Enlightening the brain: linking deep brain photoreception with behavior and physiology. BioEssays. 2013;35(9):775–9. doi: 10.1002/bies.201300034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Fernández JM, Cernuda-Cernuda R, Davies WIL, Rodgers J, Turton M, Peirson SN, et al. The hypothalamic photoreceptors regulating seasonal reproduction in birds: A prime role for VA opsin. Front Neuroendocrinol. 2015;37:13–28. doi: 10.1016/j.yfrne.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 6.Drivenes Ø, Søviknes AM, Ebbesson LO, Fjose A, Seo HC, Helvik JV. Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain. J Comp Neurol. 2003;456(1):84–93. doi: 10.1002/cne.10523 [DOI] [PubMed] [Google Scholar]
- 7.Eilertsen M, Drivenes Ø, Edvardsen RB, Bradley CA, Ebbesson LOE, Helvik JV. Exorhodopsin and melanopsin systems in the pineal complex and brain at early developmental stages of Atlantic halibut (Hippoglossus hippoglossus). J Comp Neurol. 2014;522(18):4003–22. doi: 10.1002/cne.23652 [DOI] [PubMed] [Google Scholar]
- 8.Fernandes António M, Fero K, Arrenberg Aristides B, Bergeron Sadie A, Driever W, Burgess Harold A. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr Biol. 2012;22(21):2042–7. doi: 10.1016/j.cub.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer RM, Fontinha BM, Kirchmaier S, Steger J, Bloch S, Inoue D, et al. Co-expression of VAL- and TMT-Opsins uncovers ancient photosensory interneurons and motorneurons in the vertebrate brain. PLoS Biology. 2013;11(6):e1001585. doi: 10.1371/journal.pbio.1001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hang CY, Kitahashi T, Parhar IS. Localization and characterization of val-opsin isoform-expressing cells in the brain of adult zebrafish. J Comp Neurol. 2014;522(17):3847–60. doi: 10.1002/cne.23645 [DOI] [PubMed] [Google Scholar]
- 11.Hang CY, Kitahashi T, Parhar IS. Neuronal organization of deep brain opsin photoreceptors in adult teleosts. Front Neuroanat. 2016;10:48. doi: 10.3389/fnana.2016.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandbakken M, Ebbesson L, Stefansson S, Helvik JV. Isolation and characterization of melanopsin photoreceptors of Atlantic salmon (Salmo salar). J Comp Neurol. 2012;520(16):3727–44. doi: 10.1002/cne.23125 [DOI] [PubMed] [Google Scholar]
- 13.Eilertsen M, Valen R, Drivenes Ø, Ebbesson LOE, Helvik JV. Transient photoreception in the hindbrain is permissive to the life history transition of hatching in Atlantic halibut. Dev Biol. 2018;444(2):129–38. doi: 10.1016/j.ydbio.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Nakane Y, Ikegami K, Iigo M, Ono H, Takeda K, Takahashi D, et al. The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat Commun. 2013;4(1):2108. doi: 10.1038/ncomms3108 [DOI] [PubMed] [Google Scholar]
- 15.Irachi S, Hall DJ, Fleming MS, Maugars G, Björnsson BT, Dufour S, et al. Photoperiodic regulation of pituitary thyroid-stimulating hormone and brain deiodinase in Atlantic salmon. Mol Cell Endocrinol. 2021;519:111056. doi: 10.1016/j.mce.2020.111056 [DOI] [PubMed] [Google Scholar]
- 16.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296(4):517–30. doi: 10.1002/cne.902960402 [DOI] [PubMed] [Google Scholar]
- 17.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4(4):477–85. doi: 10.1016/0896-6273(90)90106-p [DOI] [PubMed] [Google Scholar]
- 18.Rusak B, Robertson H, Wisden W, Hunt S. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248(4960):1237–40. doi: 10.1126/science.2112267 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz WJ, Takeuchi J, Shannon W, Davis EM, Aronin N. Temporal regulation of light-induced Fos and Fos-like protein expression in the ventrolateral subdivision of the rat suprachiasmatic nucleus. Neuroscience. 1994;58(3):573–83. doi: 10.1016/0306-4522(94)90082-5 [DOI] [PubMed] [Google Scholar]
- 20.Moore HA, Whitmore D. Circadian rhythmicity and light sensitivity of the zebrafish brain. PLoS One. 2014;9(1):e86176. doi: 10.1371/journal.pone.0086176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebbesson LO, Nilsen TO, Helvik JV, Tronci V, Stefansson SO. Corticotropin-releasing factor neurogenesis during midlife development in salmon: genetic, environmental and thyroid hormone regulation. J Neuroendocrinol. 2011;23(8):733–41. doi: 10.1111/j.1365-2826.2011.02164.x [DOI] [PubMed] [Google Scholar]
- 22.Ebbesson LOE, Ebbesson SOE, Nilsen TO, Stefansson SO, Holmqvist B. Exposure to continuous light disrupts retinal innervation of the preoptic nucleus during parr–smolt transformation in Atlantic salmon. Aquaculture. 2007;273(2):345–9. [Google Scholar]
- 23.Handeland SO, Stefansson SO. Photoperiod control and influence of body size on off-season parr–smolt transformation and post-smolt growth. Aquaculture. 2001;192(2):291–307. [Google Scholar]
- 24.Lorgen M, Casadei E, Król E, Douglas A, Birnie MJ, Ebbesson LO, et al. Functional divergence of type 2 deiodinase paralogs in the Atlantic salmon. Curr Biol. 2015;25(7):936–41. doi: 10.1016/j.cub.2015.01.074 [DOI] [PubMed] [Google Scholar]
- 25.McCormick SD, Hansen LP, Quinn TP, Saunders RL. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci. 1998;55(S1):77–92. [Google Scholar]
- 26.Stefansson SO, Nilsen TO, Ebbesson LOE, Wargelius A, Madsen SS, Bjornsson BT, et al. Molecular mechanisms of continuous light inhibition of Atlantic salmon parr-smolt transformation. Aquaculture. 2007;273(2–3):235–45. [Google Scholar]
- 27.Villamizar N, Vera LM, Foulkes NS, Sánchez-Vázquez FJ. Effect of lighting conditions on zebrafish growth and development. Zebrafish. 2014;11(2):173–81. doi: 10.1089/zeb.2013.0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sierra-Flores R, Davie A, Grant B, Carboni S, Atack T, Migaud H. Effects of light spectrum and tank background colour on Atlantic cod (Gadus morhua) and turbot (Scophthalmus maximus) larvae performances. Aquaculture. 2016;450:6–13. [Google Scholar]
- 29.Grassie C, Braithwaite VA, Nilsson J, Nilsen TO, Teien H-C, Handeland SO, et al. Aluminum exposure impacts brain plasticity and behavior in Atlantic salmon (Salmo salar). J Exp Biol. 2013;216(16):3148–55. [DOI] [PubMed] [Google Scholar]
- 30.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3(1):59–69. doi: 10.1038/nprot.2007.514 [DOI] [PubMed] [Google Scholar]
- 31.Kovács KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20(6):665–72. doi: 10.1111/j.1365-2826.2008.01734.x [DOI] [PubMed] [Google Scholar]
- 32.Fujikawa Y, Kozono K, Esaka M, Iijima N, Nagamatsu Y, Yoshida M, et al. Molecular cloning and effect of c-fos mRNA on pharmacological stimuli in the goldfish brain. Comp Biochem Physiol Part D Genomics Proteomics. 2006;1(2):253–9. doi: 10.1016/j.cbd.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 33.Okuyama T, Suehiro Y, Imada H, Shimada A, Naruse K, Takeda H, et al. Induction of c-fos transcription in the medaka brain (Oryzias latipes) in response to mating stimuli. Biochem Biophys Res Commun. 2011;404(1):453–7. doi: 10.1016/j.bbrc.2010.11.143 [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka I, Fuyuki K, Shoji T, Kurihara K. Identification of c-fos related genes and their induction by neural activation in rainbow trout brain. Biochim Biophys Acta. 1998;1395(2):220–7. doi: 10.1016/s0167-4781(97)00164-4 [DOI] [PubMed] [Google Scholar]
- 35.Vindas MA, Gorissen M, Höglund E, Flik G, Tronci V, Damsgård B, et al. How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish. J Exp Biol. 2017;220(8):1524–32. doi: 10.1242/jeb.153213 [DOI] [PubMed] [Google Scholar]
- 36.Randlett O, Wee CL, Naumann EA, Nnaemeka O, Schoppik D, Fitzgerald JE, et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat Methods. 2015;12(11):1039–46. doi: 10.1038/nmeth.3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol. 2010;165(3):469–82. doi: 10.1016/j.ygcen.2009.04.026 [DOI] [PubMed] [Google Scholar]
- 38.Ebbesson LO, Holmqvist B, Ostholm T, Ekström P. Transient serotonin-immunoreactive neurons coincide with a critical period of neural development in coho salmon (Oncorhynchus kisutch). Cell Tissue Res. 1992;268(2):389–92. doi: 10.1007/BF00318807 [DOI] [PubMed] [Google Scholar]
- 39.Yáñez J, Meissl H, Anadón R. Central projections of the parapineal organ of the adult rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 1996;285(1):69–74. doi: 10.1007/s004410050621 [DOI] [PubMed] [Google Scholar]
- 40.Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364(1519):1005–20. doi: 10.1098/rstb.2008.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmqvist BI, Östholm T, Ekström P. Neuroanatomical analysis of the visual and hypophysiotropic systems in Atlantic salmon (Salmo salar) with emphasis on possible mediators of photoperiodic cues during parr-smolt transformation. Aquaculture. 1994;121(1):1–12. [Google Scholar]
- 42.Holmqvist BI, Ekström P. Hypophysiotrophic systems in the brain of the Atlantic salmon. Neuronal innervation of the pituitary and the origin of pituitary dopamine and nonapeptides identified by means of combined carbocyanine tract tracing and immunocytochemistry. J Chem Neuroanat. 1995;8(2):125–45. doi: 10.1016/0891-0618(94)00041-q [DOI] [PubMed] [Google Scholar]
- 43.Hang CY, Kitahashi T, Parhar IS. Brain area-specific diurnal and photic regulation of val-opsinA and val-opsinB genes in the zebrafish. J Neurochem. 2015;133(4):501–10. doi: 10.1111/jnc.13084 [DOI] [PubMed] [Google Scholar]
- 44.Philp AR, Garcia-Fernandez JM, Soni BG, Lucas RJ, Bellingham J, Foster RG. Vertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar). J Exp Biol. 2000;203(Pt 12):1925–36. [DOI] [PubMed] [Google Scholar]
- 45.Anglade I, Zandbergen T, Kah O. Origin of the pituitary innervation in the goldfish. Cell Tissue Res. 1993;273(2):345–55. doi: 10.1007/BF00312837 [DOI] [PubMed] [Google Scholar]
- 46.Ball JN. Hypothalamic control of the pars distalis in fishes, amphibians, and reptiles. Gen Comp Endocrinol. 1981;44(2):135–70. doi: 10.1016/0016-6480(81)90243-4 [DOI] [PubMed] [Google Scholar]
- 47.Terlou M, Ekengren B. Nucleus praeopticus and nucleus lateralis tuberis of Salmo salar and Salmo gairdneri: structure and relationship to the hypophysis. Cell Tissue Res. 1979;197(1):1–21. doi: 10.1007/BF00233550 [DOI] [PubMed] [Google Scholar]
- 48.Gern WA, Greenhouse SS, Nervina JM, Gasser PJ. The Rainbow Trout Pineal Organ: An Endocrine Photometer. In: Ali MA, editor. Rhythms in Fishes. Boston, MA: Springer US; 1992. p. 199–218. [Google Scholar]
- 49.Migaud H, Taylor JF, Taranger GL, Davie A, Cerdá-Reverter JM, Carrillo M, et al. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J Pineal Res. 2006;41(1):42–52. doi: 10.1111/j.1600-079X.2006.00330.x [DOI] [PubMed] [Google Scholar]
- 50.Vera LM, Davie A, Taylor JF, Migaud H. Differential light intensity and spectral sensitivities of Atlantic salmon, European sea bass and Atlantic cod pineal glands ex vivo. Gen Comp Endocrinol. 2010;165(1):25–33. doi: 10.1016/j.ygcen.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 51.Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photoreceptors. Nature. 1998;394(6688):27–8. doi: 10.1038/27794 [DOI] [PubMed] [Google Scholar]
- 52.Davies WI, Zheng L, Hughes S, Tamai TK, Turton M, Halford S, et al. Functional diversity of melanopsins and their global expression in the teleost retina. Cell Mol Life Sci. 2011;68(24):4115–32. doi: 10.1007/s00018-011-0785-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Overview of the section with boxes indicating the sections of B-D. (B) Arrowheads indicates c-fos expression in the tectum and in the longitudinal torus. (C) Arrowheads indicates c-fos expression in the semisircularus torus. (D) Higher magnification of some of the cells in C. Scale bars 200 μm.
(TIF)
(A, E, I, M, Q) White/Dark, (B, F, J, N, R) Blue/Dark, (C, G, K, O, S) Green/Dark, (D, H, L, P, T) Red/Dark. In general less or weaker expression of c-fos is detected in the off response then in the on-response. Scale bars 200 μm.
(TIF)
(A, E, I, M, Q) White/White, (B, F, J, N, R) Blue/Blue, (C, G, K, O, S) Green/Green, (D, H, L, P, T) Red/Red. In general little or weaker expression of c-fos is detected in the controls. Scale bars 200 μm.
(TIF)
There are no expression of c-fos in Dark/White stimulated fish. Scale bar 200 μm.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.