Abstract
Members of the NF-κB/RelB family of transcription factors play important roles in the regulation of inflammatory and immune responses. RelB, a member of this family, has been characterized as a transcription activator and is involved in the constitutive NF-κB activity in lymphoid tissues. However, in a previous study we observed an overexpression of chemokines in RelB-deficient fibroblasts. Here we show that RelB is an important transcription suppressor in fibroblasts which limits the expression of proinflammatory mediators and may exert its function by modulating the stability of IκBα protein. Fibroblasts from relb−/− mice overexpress interleukin-1α (IL-1α), IL-1β, and tumor necrosis factor alpha in response to lipopolysaccharide (LPS) stimulation. These cells have an augmented and prolonged LPS-inducible IKK activity and an accelerated degradation which results in a diminished level of IκBα protein, despite an upregulated IκBα mRNA expression. Consequently, NF-κB activity was augmented and postinduction repression of NF-κB activity was impaired in these cells. The increased κB-binding activity and cytokine overexpression was suppressed by introducing RelB cDNA or a dominant negative IκBα into relb−/− fibroblasts. Our findings suggest a novel transcription suppression function of RelB in fibroblasts.
Members of the Rel/NF-κB family of transcription factors play a central role in the regulation of inflammatory and immune responses (for recent reviews, please see (1, 3, 4, 25, 46, 54). In vertebrates, NF-κB consists of homo- or heterodimers of Rel (c-Rel), p65 (RelA), RelB, p50 (NFKB1), and p52 (NFKB2), all of which contain a conserved N-terminal Rel homology domain that contains the DNA-binding and dimerization domains and the nuclear localization signal. In most unstimulated cells, a large portion of NF-κB is retained in the cytoplasm as inactive complexes by a family of inhibitory proteins called IκB that bind to the Rel homology domain and mask the nuclear localization signal. There are at least five distinct IκB proteins, IκBα, IκBβ, IκBɛ, IκBγ, and bcl-3; both the p105 precursor of p50 and the p100 precursor of p52 possess domains that function as IκBs as well. Upon cell stimulation by a wide variety of stimuli, signal-responsive IκB kinases (IKK) α and β are activated and phosphorylate two serine residues in the IκB proteins (14, 19, 36, 42, 43, 61, 63, 64). For IκBα and IκBβ, the inducible phosphorylation sites are serines 32 and 36 and serines 19 and 23, respectively (9, 10, 18, 51). The phosphorylated IκBs are subsequently ubiquitinated and targeted for degradation by the 26S proteosome, releasing the NF-κB dimers to translocate to the nucleus to activate the transcription of genes containing the so-called κB-binding site (2, 26, 48). Among the NF-κB-inducible genes are IκB members, and the newly synthesized IκBs quickly interact with and inactivate NF-κB, leading to an autoregulation of the NF-κB system (11, 45, 50). Both IκBα and IκBβ function not only in the cytoplasm but also in the nucleus to inhibit NF-κB activity; however, only IκBα is required for postinduction repression of NF-κB (5, 52).
RelB shares many common features of the NF-κB family, and is a strong transcriptional activator (7, 8, 21, 44). Unlike other NF-κB members, however, RelB cannot form homodimers and only associates efficiently with p50 and p52 (22). The RelB heterodimers have a much lower affinity for IκBα than other NF-κB complexes do and are less susceptible to inhibition by IκBα (22, 32). As a result, it is predicted that RelB will be located in the nucleus and represent the constitutive NF-κB activity. Indeed, RelB heterodimers represent the major constitutive NF-κB activity in lymphoid tissues and are expressed at high levels in the nuclei of interdigitating dendritic cells, suggesting an important role for RelB in the constitutive expression of κB-regulated genes in lymphoid tissues (13, 32, 33, 40, 56).
The implicated in vivo function of RelB in lymphoid tissues is supported by studies of relb−/− mice (12, 17, 34, 57–60, 62). Mice deficient in RelB have a dramatic reduction in constitutive κB-binding activity and specific defects in lymphoid tissues, including the absence of mature lymphoid dendritic cells, myeloid hyperplasia, and splenomegaly (12, 57). relb−/− mice also have multifocal defects in immune responses and fail to mount inflammatory reactions against a number of pathogens (17, 60). Surprisingly, relb−/− mice spontaneously develop a persistent noninfectious multiorgan inflammatory syndrome (12, 34, 57). This apparent discrepancy suggests additional defects in nonlymphoid tissues in relb−/− mice. In this regard, we showed in our previous report that the multifocal inflammation is due to non-bone-marrow-derived cells, that lipopolysaccharide (LPS)-stimulated relb−/− fibroblasts overexpress chemokines and induce leukocyte recruitment into tissues, and that RelB, while being a transcriptional activator for κB-regulated genes in macrophages, acts as a transcription suppressor in fibroblasts (62). Our findings may provide hints about the role that fibroblasts might play in the initial leukocyte infiltration and how RelB might be involved in the regulation of this process (49, 62). Additional pathological changes, however, must be involved in the development of multiorgan inflammation in relb−/− mice. Cytokines which promote inflammatory effector functions, including tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α), IL-1β, and gamma interferon, are expressed at increased levels in the nonlymphoid organs of relb−/− mice but have either normal or reduced expression in lymphoid tissues; in particular, isolated relb−/− macrophages are impaired in the production of TNF-α (60, 62). Since macrophages are normally the major source of TNF-α production, one wonders what the probable cellular source of TNF-α and other cytokines in the nonlymphoid organs of the relb−/− mice might be, what molecular mechanisms are involved, and, especially, how RelB may function in this process. In this report, we show that RelB is a key transcriptional suppressor of cytokine expression in fibroblasts and that its absence leads to the dysregulation of IL-1α, IL-1β, and TNF-α expression in relb−/− fibroblasts. We further demonstrate that RelB exerts its transcriptional suppressor function through the stabilization of IκBα protein. These data suggest new physiological roles for the NF-κB/Rel factors in the regulation of inflammatory and immune responses and may provide insights to uncover novel intra- and interfamily interactions of the NF-κB/Rel and IκB regulatory molecules.
MATERIALS AND METHODS
Animals.
relb−/− mice were generated and characterized as previously described (12). The mice were generated on an inbred C57BL/6J background and bred onto a B10.D2 background. The control mice were of B10.D2 origin. All experimental procedures were carried out according to the guidelines listed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell culture and in vitro LPS stimulation.
Fibroblasts were isolated from the kidneys of normal control and relb−/− mice as previously described (62) and were cultured in Dulbecco modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS). Stable transfection of fibroblasts with a RelB cDNA construct was performed as described previously (62). For LPS stimulation, cells were serum starved for 24 h and then treated with 1 μg of LPS (List Biological Laboratories, Campbell, Calif.) per ml in DMEM plus 0.5% FBS. The cells were harvested at different time points for RNA and protein extraction.
RNA extraction and RNase protection assay.
Total RNA was isolated from fibroblasts by a single-step method (16). cDNA fragments of mouse IL-1α (bp 172 to 362; accession no. X01450), IL-1β (bp 500 to 672; accession no. M15131), TNF-α (bp 429 to 556; accession no. M11731), RelB (bp 1350 to 1603; accession no. M83380), and IκBα (bp 372 to 632; accession no. U36277) were generated by reverse transcription-PCR or by PCR amplification of cDNA templates. The PCR products were cloned into pGEM4Z (Promega Corp., Madison, Wis.) for the generation of α-32P-incorporated riboprobes. RNase protection assays were performed as previously described (62).
Luciferase assay.
The luciferase reporter plasmids containing the wild-type or mutant TNF promoter were constructed as described by others (23, 27) except that the chloramphenicol acetyltransferase reporter vector was replaced by luciferase reporter vector pGL2-Basic (Promega). The plasmids were transfected into fibroblasts by electroporation with the setting of 280 V, 600 μF and 48 Ω (BTX Electro Cell Manipulator 600; Genetronics, San Diego, Calif.). At 48 h after the transfection, the cells were stimulated with LPS for 12 h and were harvested for the luciferase assay. This assay was performed with a luciferase assay kit (Promega), and the light production was measured with a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, Calif.).
Nuclear extract and EMSA.
Nuclear extracts were prepared by a published procedure (20). Electrophoretic mobility shift assay (EMSA) was performed as described previously (62). Briefly, 2 μg of nuclear extract was incubated for 15 min at 25°C with a 32P-labeled oligonucleotide containing the κB site from the murine intronic κ chain (5′-AGTTGAGGGGACTTTCCCAGG-3′ [the NF-κB consensus DNA binding motif is underlined]; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), and the reaction mixtures were electrophoresed in a 6% polyacrylamide sequencing gel. For supershift assays, the nuclear extract was preincubated with 1 μg of rabbit anti-RelA serum for 20 min at 25°C before the addition of oligonucleotides.
Western blot analysis and ELISA.
The cytoplasm and nuclear proteins from LPS-stimulated fibroblasts (5 μg per sample) were electrophoresed in a NuPAGE gel (Novex, San Diego, Calif.) and electroblotted onto a nitrocellulose membrane. The protein blots were probed with rabbit antibodies against mouse RelA, RelB, IκBα, IκBβ (Santa Cruz), actin (Sigma, St. Louis, Mo.), or NF-κB-inducing kinase (NIK) (Torrey Pines Biolabs, San Marcos, Calif.). The bound antibodies were detected with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody (Pierce, Rockford, Ill.) and the SuperSignal Kit (Pierce). TNF-α protein was measured with a mouse enzyme-linked immunosorbent assay (ELISA) kit (Biosource International, Inc., Camarillo, Calif.).
Metabolic labeling and immunoprecipitation.
Prior to the metabolic labeling, 2 × 106 fibroblasts were treated with 1 μg of LPS per ml for 1 h. The cells were transferred to 1 ml of methionine- and cysteine-free DMEM plus 5% dialyzed FCS for 1 h, and then 0.2 mCi of Tran[35S] (ICN, Costa Mesa, Calif.) was added to the medium. After 30 min, the cells were washed with phosphate-buffered saline and incubated with the regular medium of DMEM plus 10% FBS. The cells were harvested at different time points and were lysed in 20 mM Tris-HCl (pH 7.4)–100 mM NaCl–1 mM EDTA–0.2% Nonidet P-40–0.1% Triton X-100. The cell lysates were incubated with 1 μg of normal rabbit immunoglobulin G (Sigma) at 4°C for 1 h and with 10 μl of protein A-Sepharose (Pharmacia, Piscataway, N.J.) for 30 min and then centrifuged for 10 min. The precleared lysates were reacted with 1 μl of rabbit anti-IκBα antibody (Santa Cruz) at 4°C for 1 h and then with 10 μl of protein A-Sepharose for 8 h. The immune complexes were centrifuged, washed twice in phosphate-buffered saline, electrophoresed in a Nu-PAGE gel (Novex), and visualized by autoradiography.
Production of adenovirus vectors and infection of fibroblasts.
cDNA fragments containing the coding region of the murine IκBα or a dominant negative mutant IκBα (53) were subcloned into the shuttle plasmid pAdv/CMV (55). The resulting plasmids, pAdv/CMV-IκBwt and pAdv/CMV-IκBmut, were each cotransfected with a helper plasmid, pJM17, into 293T cells to generate recombinant adenoviruses by a previously described method (55). Recombinant adenoviruses confirmed by PCR analysis were plaque purified and amplified in 293T cells. Concentrated adenoviruses were prepared by CsCl gradient ultracentrifugation. The titer of adenovirus was determined from DNA content of the viral solution, with 1.0 optical density at 260 nm unit being equivalent to 1.0 × 1012 viral particles/ml. The adenovirus construct Adv/β-gal, containing the β-galactosidase (β-Gal) cDNA, was generated by a similar strategy. Infection of fibroblasts was done by adding adenoviruses to the culture medium to a titer of 5,000 viral particles/cell.
IκBα kinase assay.
The activities of IκB kinases of fibroblasts were determined by an immunokinase assay (19, 39). After 24 h of serum starvation, the fibroblasts were stimulated with LPS at 1 μg/ml of medium and collected at 0, 15, and 30 min and 1 and 4 h. The cells were disrupted in lysis buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.25% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM PNPP, 300 μM Na3VO4, 1 mM benzamidine, 1 mM dithiothreitol, proteinase inhibitors) by repeated aspiration through a 21-gauge needle. The supernatant was incubated with 1.0 μg of anti-mouse IKKα polyclonal antibody (M280; Santa Cruz) at 4°C for 1 h and then with 20 μl of protein A-Sepharose (Pierce) for 8 h. The immunocomplexes were then collected, washed, and suspended in 30 μl of kinase buffer (20 mM HEPES [pH 7.6], 100 mM NaCl, 20 mM β-glycerophosphate, 10 mM MgCl2, 10 mM P-nitrophenyl phosphate, 100 μM Na3VO4, 10 μg of aprotinin per ml, 2 mM dithiothreitol, 20 μM ATP, 5 μCi of [γ-32P]ATP [ICN]) with 5 μg of glutathione S-transferase–IκB-α(1–54) as a substrate (19, 39). The reaction was stopped by adding 10 μl of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer after a 30-min incubation at 30°C. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% polyacrylamide) (Novex) and exposed to a film.
RESULTS
IL-1α, IL-1β, and TNF-α overexpression and TNF-α promoter activation in LPS-stimulated relb−/− fibroblasts.
Normal fibroblasts do not express IL-1α, IL-1β, and TNF-α, even when treated with LPS (Fig. 1A) (31). However, when relb−/− fibroblasts were stimulated with LPS, the mRNA expression of all three cytokines was dramatically induced (Fig. 1A). The induction was readily detectable at 1 h after the LPS stimulation, peaked at 4 to 8 h, and persisted through 24 h. To examine the expression of cytokine proteins, the relb−/− fibroblasts culture medium was analyzed by ELISA. We chose to analyze the TNF-α protein level because its synthesis is regulated at both the transcription and translation levels (6). The expression of TNF-α protein in LPS-stimulated fibroblasts correlated well with its mRNA expression (Fig. 1B).
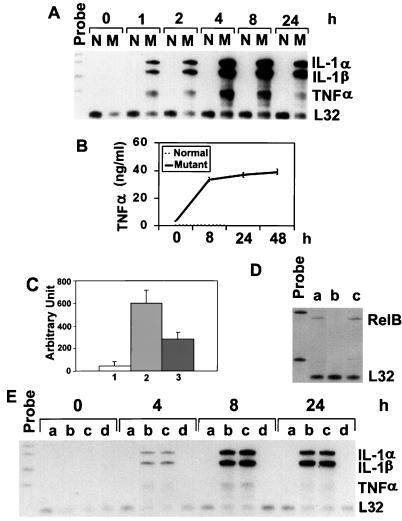
FIG. 1.
Expression of proinflammatory cytokines in normal and relb−/− fibroblasts. (A) IL-1α, IL-1β, and TNF-α mRNA expression in normal (N) and mutant (M) fibroblasts. Total RNA from fibroblasts treated with LPS for the indicated times was analyzed by the RNase protection assay. Riboprobes contain polylinker sequences and are longer than the protected bands. The mouse L32 gene was used as a housekeeping gene. (B) TNF-α protein expression in normal and mutant fibroblasts. Fibroblasts were treated with LPS, and the culture medium was collected at the indicated time points for ELISA analysis. (C) TNF-α promoter activity in fibroblasts as determined by the luciferase assay. Normal and relb−/− fibroblasts were transfected with TNF-α promoter-luciferase plasmids, and the transfected cells were treated with LPS for 12 h and then assayed for luciferase activities (arbitrary units), as described in Materials and Methods. The results are from three independent experiments (means ± standard deviations). Columns 1 and 2, normal and relb−/− fibroblasts transfected with TNFα promoter-luciferase plasmid; column 3, relb−/− fibroblasts transfected with a mutant TNF-α promoter-luciferase plasmid. (D) RelB cDNA-transfection of relb−/− fibroblasts. The expression vector pcDNA, containing a RelB cDNA, was used to transfect relb−/− fibroblasts. Total RNA from positive clones was analyzed for RelB mRNA expression (a). relb−/− fibroblasts transfected with pcDNA vector only (b) and normal fibroblasts (c) were used as controls. (E) Reversal of LPS-induced cytokine overexpression in relb−/− fibroblasts by RelB cDNA transfection. Normal fibroblasts (a), relb−/− fibroblasts (b), relb−/− fibroblasts transfected with pcDNA plasmid (c), and relb−/− fibroblasts transfected with pcDNA vector containing a mouse RelB cDNA fragment (d) were treated with LPS for the indicated times and then analyzed for cytokine expression by the RNase protection assay.
To examine the effect of RelB deficiency on the promoter activity of proinflammatory cytokine genes in fibroblasts, we transfected into normal and relb−/− fibroblasts a reporter plasmid of a luciferase gene under the control of the TNF-α promoter. The TNF-α promoter was chosen because the TNF-α gene is irreversibly silenced in the fibroblasts (6). After treatment with LPS for 12 h, no luciferase activity was detected in the transfected normal fibroblasts; in contrast, a significant level of luciferase activity was induced in the transfected relb−/− fibroblasts (Fig. 1C). A reporter plasmid with a mutation in one of the four κB sites in the TNF-α promoter had less than 50% luciferase activity induced by LPS in relb−/− fibroblasts (Fig. 1C), indicating that the promoter activation was related to NF-κB-mediated transcription upregulation. These results suggest that the RelB-deficient environment has a direct effect on LPS induction of cytokine promoters in relb−/− fibroblasts, and excludes alterations in cytokine genes as the cause of cytokine expression these cells.
Reverse of cytokine overexpression in relb−/− fibroblasts by RelB cDNA transfection.
Since the relb−/− fibroblasts were isolated from the kidneys of relb−/− mice, the dysregulation of proinflammatory cytokine expression in these cells could be the result of developmental changes that were secondary to the RelB deficiency. To verify the casual effect of RelB on the suppression of cytokine expression in fibroblasts, RelB cDNA was transfected into relb−/− fibroblasts. The expression of the transfected RelB in relb−/− fibroblasts (Fig. 1D) completely abolished the overexpression of IL-1α, IL-1β, and TNF-α mRNA induced by LPS (Fig. 1E). This result strongly suggests an indispensable role of RelB in the transcription suppression of proinflammatory cytokines in fibroblasts.
Augmented IκBα mRNA expression in relb−/− fibroblasts.
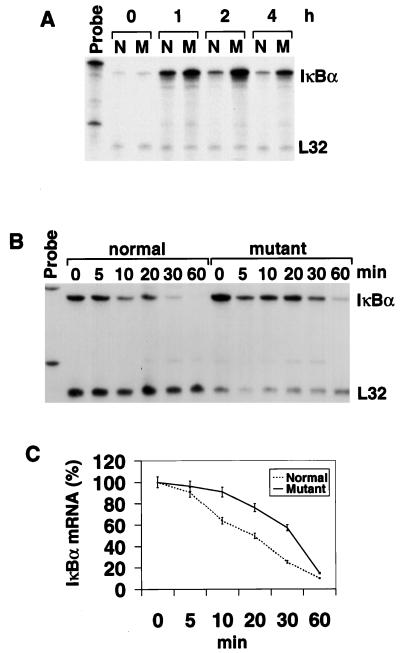
The mutual regulations of NF-κB and IκB activities play a primary role in the control of NF-κB-activated genes. Since NF-κB molecules interact with IκB molecules differently, this could lead to a hierarchy or mutual regulation among different NF-κB members (22). In particular, RelB is relatively resistant to IκBα inhibition and can strongly induce the expression of IκBα. In our previous study, we showed that LPS treatment of relb−/− fibroblasts led to an exaggerated and persistent activation of NF-κB activity that was mainly attributed to RelA/p50 (62). Since IκBα is the major inhibitor of RelA/p50 activity, RelB may exert its transcriptional suppressor function in fibroblasts through the regulation of IκBα activities. The mRNA expression of IκBα in normal and relb−/− fibroblasts was therefore analyzed. While IκBα mRNA was induced in both type of cells by LPS, the induction was significantly augmented in mutants (Fig. 2A), probably the result of enhanced RelA/p50 activity. We then examined the stability of IκBα mRNA but found no significant difference in the half-life of IκBα mRNAs in normal and mutant fibroblasts (Fig. 2B and C). These results indicate that IκBα mRNA expression is not down-regulated by RelB deficiency in relb−/− fibroblasts.
FIG. 2.
Analysis of IκBα mRNA expression in fibroblasts. (A) IκBα mRNA expression in normal (N) and relb−/− (M) fibroblasts treated with LPS for different periods. (B) Analysis of IκBα mRNA stability in LPS-treated fibroblasts. Normal and mutant fibroblasts were treated with LPS for 1 h before actinomycin D was added to stop mRNA synthesis. Cells were harvested at 5, 10, 20, 30, and 60 min after the addition of actinomycin D and analyzed for IκBα mRNA levels by an RNase protection assay. (C) Graphic representation of the IκBα mRNA half-life. The IκBα and L32 bands in panel B were quantitated by phosphorimager scanning. The count of each IκBα band was factored by that of the corresponding L32 band. The final value of each time point is expressed as a percentage of that at time zero.
Rapid degradation of IκBα protein in relb−/− fibroblasts.
Analysis of IκBα protein levels, however, revealed a dramatic difference between normal and relb−/− fibroblasts (Fig. 3A). The down-regulation of IκBα protein in relb−/− fibroblasts in the presence of higher mRNA levels could be due to either translation suppression or a decrease in protein stability. A metabolic labeling experiment was carried out with LPS-stimulated normal and mutant cells to determine the actual mechanism. The level of metabolically labeled IκBα protein in relbsup−/− fibroblasts at the initial time point after a pulse-labeling was comparable to that in normal fibroblasts (Fig. 3B), suggesting that the IκBα mRNA in relb−/− fibroblasts can be translated and that translational suppression is not the primary cause of IκBα protein down-regulation in these cells. However, as early as 30 min after the pulse-labeling, most of the newly synthesized IκBα protein disappeared in the relb−/− fibroblasts (Fig. 3B), indicating a very rapid degradation. In contrast, the observed half-life of IκBα in normal fibroblasts was on the order of 2 to 3 h, significantly longer than that in the mutant cells. This indicated that the accelerated degradation of IκBα protein was responsible for the decreased IκBα protein in relb−/− fibroblasts.
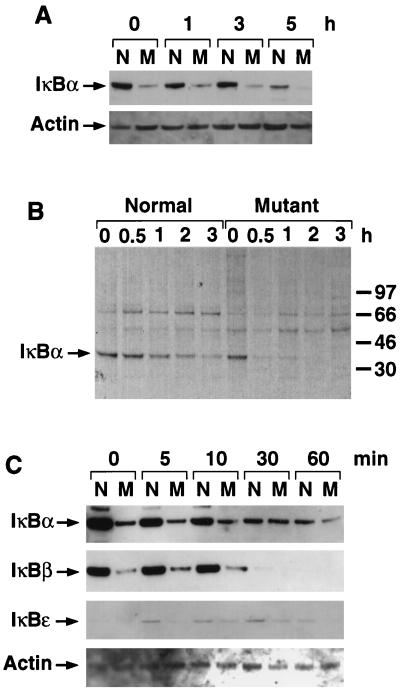
FIG. 3.
Analysis of IκBα protein level in fibroblasts. (A) IκBα protein level in normal (N) and mutant (M) fibroblasts. Fibroblasts were treated with LPS for the indicated times. The cell lysates were analyzed for IκBα protein by Western blotting. A total of 5 μg of protein was used for each sample. The same blot was probed for actin to ensure even loading. (B) Pulse-chase experiment to determine the stability of IκBα in normal and relb−/− fibroblasts. Cells were treated with LPS for 1 h before being pulse-labeled for 1 h with [35S]Met-[35S]Cys. The cells were then chased with cold medium for the indicated times. The cell lysates were immunoprecipitated with anti-IκBα antibody and analyzed as described in Materials and Methods. (C) Western blot analysis of different IκB family members in normal (N) and relb−/− (M) fibroblasts. Cells were treated with LPS for the times shown. The cell lysates were analyzed for IκBα, IκBβ, and IκBɛ protein levels by Western blotting. The same blots were reprobed with anti-actin antibody to show comparable amounts of proteins in different lanes.
We also examined the protein levels of IκBβ and IκBɛ in LPS-stimulated normal and relb−/− fibroblasts. The IκBβ protein level was also down-regulated in relb−/− fibroblasts (Fig. 3C). IκBɛ protein was expressed at low levels in both normal and relb−/− fibroblasts and appeared to be down-regulated in the mutant cells (Fig. 3C). Since there is a functional redundancy between IκBα and IκBβ (15) and since the main functional difference between IκBα and IκBβ is their divergent expression control, we focused the rest of our study on IκBα.
Impaired postinduction repression of NF-κB activity by IκBα in relb−/1− fibroblasts.
One irreplaceable function of IκBα is the postinduction repression of NF-κB activation, whereas its cytoplasmic retention of NF-κB can be compensated for by other IκB proteins (5, 52). Newly synthesized IκBα not only stops cytoplasmic NF-κB from entering the nucleus but also enters the nucleus to dissociate NF-κB binding to DNA and exports the bound NF-κB to the cytoplasm. The accelerated degradation may preempt the IκBα in LPS-stimulated relb−/− fibroblasts from carrying out its function in the postinduction repression, and this may be the underlying cause of the prolonged activation of NF-κB activity and persistent expression of cytokine mRNAs in LPS-stimulated relb−/− fibroblasts. Since LPS-induced NF-κB activity in fibroblasts consists mainly of p50 and RelA, we assessed the pattern of RelA translocation in LPS-stimulated normal and relb−/− fibroblasts by Western blot analysis. In resting normal and mutant fibroblasts, RelA was located in the cytoplasm. LPS stimulation induced a rapid RelA translocation from cytoplasm to nucleus in both types of cells, peaking 30 min after LPS stimulation. At 1 h after LPS stimulation, nuclear RelA in normal fibroblasts was significantly reduced, and 3 h later it was much lower than that in the cytoplasm (Fig. 4A, top). In mutant cell, however, the nuclear localization of RelA in relb−/− fibroblasts was significantly prolonged. A large portion of RelA persisted in the nucleus even at 3 h after the LPS stimulation (Fig. 4A, bottom), indicating an impaired postinduction termination of RelA nuclear localization in the mutant cells.
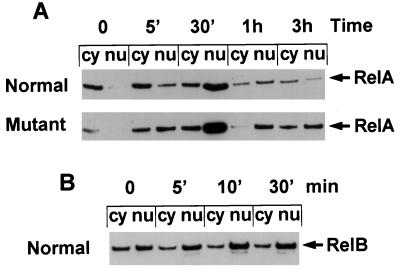
FIG. 4.
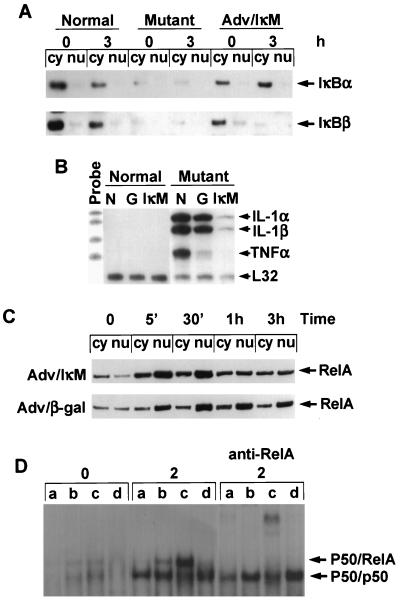
LPS induction and postinduction repression of NF-κB nuclear localization. Normal and relb−/− fibroblasts were treated with LPS for the times shown. Cytoplasmic (cy) and nuclear (nu) extracts were prepared for Western blot analysis of RelA protein (A) or RelB protein (B).
RelB has a low affinity for IκBα and is less susceptible to inhibition by IκBα (22). Compared to RelA, RelB has a very different subcellular distribution (Fig. 4B), with a major portion being located in the nucleus even in unstimulated fibroblasts. LPS stimulation may slightly increase the nuclear translocation of RelB in these cells.
Presence of IκBα in the nucleus of normal fibroblasts.
A similar level of nuclear translocation of RelA during the first 30 min of LPS stimulation of normal and relb−/− fibroblasts suggests a difference in the RelA activity in these cells: the κB-binding of RelA is much lower in the normal cells than in the mutants (62). In the presence of proteosome inhibitor, IκBα has been detected in the nucleus in unstimulated endothelial cells (41). To investigate whether IκBα localizes in the nuclei of unstimulated fibroblasts, we performed Western blot analysis of subcellular fractions of normal fibroblasts. As shown in Fig. 5, IκBα was clearly detectable in the nucleus, albeit at a lower level than in the cytoplasm. To rule out the possibility of contamination of the nuclear fraction by cytoplasm, the same blot was reprobed with antiserum to NIK, a cytoplasmic protein (35). The exclusive localization of NIK in the cytoplasm indicated that our preparation of the nuclear fraction was free of cytoplasmic input. The preexistence of IκBα in the nuclei of normal fibroblasts may account for the low κB-binding activity of RelA in these cells.
FIG. 5.
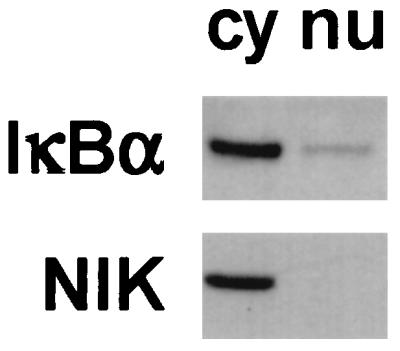
Analysis subcellular distribution of IκBα protein in fibroblasts. Cytoplasmic (cy) and nuclear (nu) extracts were prepared from resting normal fibroblasts and analyzed by Western blotting. IκBα protein can be detected in both the cytoplasmic and nuclear extracts. The same blot was reprobed with anti-NIK antibody to ensure that the nuclear extract was free of cytosolic proteins.
Reverse of cytokine overexpression in relb−/− fibroblasts by a dominant negative mutant of IκBα.
Our data presented above suggested the importance of IκBα stability in the regulation of NK-κB activity in fibroblasts as well as a connection between IκBα destabilization in relb−/− fibroblasts and the overexpression of cytokines in these cells. To further demonstrate the relationship between the IκBα stability and the regulation of cytokine expression in fibroblasts, we used an adenovirus vector carrying a dominant negative mutant of IκBα (Adv/IκM) (53). In this IκBα variant, serines 32 and 36 were substituted with alanines. IκM is therefore resistant to phosphorylation-induced degradation and is a potent and specific inhibitor of NF-κB. Adv/IκM and a control adenovirus vector, Adv/β-Gal, were used to infect normal and relb−/− fibroblasts, and the infected cells were studied for LPS stimulation. Infection of Adv/IκM resulted in a high level of IκBα mRNA in both normal and mutant fibroblasts, indicating that the infection was successful (data not shown). Adv/IκM infection of relb−/− fibroblasts recovered IκBα protein to a level comparable to that in the normal fibroblasts (Fig. 6A). The overexpression of cytokine mRNAs in LPS-stimulated relb−/− fibroblasts was dramatically reversed by Adv/IκM infection, although a weak induction of IL-1α and IL-1β mRNAs was still detectable compared with that in the normal fibroblasts (Fig. 6B). This indicates that the transcription suppression of cytokines by RelB in fibroblasts can be partially compensated by the expression of a stable IκBα.
FIG. 6.
Effects of a dominant negative mutant IκBα (IκM) on cytokine expression and NF-κB activity in fibroblasts. (A) Analysis of IκBα and IκBβ proteins in resting or LPS-treated normal fibroblasts, relb−/− fibroblasts, and Adv/IκM-infected relb−/− fibroblasts. Cells were treated with LPS for 3 h or left untreated. Cytoplasmic (Cy) and nuclear (nu) extracts were prepared and analyzed by Western immunoblotting. (B) Suppression of LPS-induced cytokine overexpression in relb−/− fibroblasts by Adv/IκM infection. Normal and relb−/− fibroblasts with no adenovirus infection (N), Adv/β-Gal infection (G), or Adv/IκM infection (IκM) were treated with LPS for 4 h. IL-1α, IL-1β, and TNF-α mRNA expression in these cells were analyzed by an RNase protection assay. (C) Effect of IκM on RelA nuclear localization. Adv/IκM- or Adv/β-Gal-infected relb−/− fibroblasts were treated with LPS for the indicated times. Cytoplasmic (cy) and nuclear (nu) extracts of these cells were prepared and analyzed for RelA protein by Western blotting. (D) Effect of IκM on NF-κB activity in fibroblasts. Normal fibroblasts (a) relb−/− fibroblasts (b), relb−/− fibroblasts infected with Adv/β-Gal (c), or relb−/− fibroblasts infected with Adv/IκM (d) were treated with LPS for 2 h or left untreated. Nuclear extracts of these cells were analyzed by EMSA or supershift EMSA, as described in Materials and Methods.
To study NF-κB activation and postinduction repression in the infected fibroblasts, we examined RelA nuclear translocation in these cells. Adv/IκM- and Adv/β-Gal-infected relb−/− fibroblasts all showed a 30-min peak of RelA nuclear translocation similar to that observed in normal and relb−/− fibroblasts (compare Fig. 6C and 4A). The RelA nuclear translocation in Adv/IκM-infected relb−/− fibroblasts was probably due to the degradation of endogenous IκBα, while the higher proportion of RelA retained in the cytoplasm in these cells implicated the effect of IκM. Compared with relb−/− fibroblasts and Adv/β-Gal-infected relb−/− fibroblasts, the nuclear portion of RelA was reduced in Adv/IκM-infected relB−/− fibroblasts at 1 and 3 h after LPS stimulation; however, a significant amount of RelA still existed in Adv/IκM-infected relb−/− fibroblasts. We then analyzed the κB-binding activity of NF-κB in these cells by EMSA. The basal and LPS-induced NF-κB activity was increased in relb−/− fibroblasts compared to normal fibroblasts (Fig. 6D), as we reported previously (62). The increase was further augmented in Adv/β-Gal-infect relb−/− fibroblasts, probably in response to adenovirus infection. Supershift experiments showed that the increased DNA-binding activity was mostly attributed to RelA. Adv/IκM infection of relb−/− fibroblasts, on the other hand, reduced DNA-binding activity in these cells (Fig. 6D). Despite the significant presence of RelA in the nuclei of Adv/IκM-infected relb−/− fibroblasts, the κB-binding activity in these cells was suppressed, implicating a function of IκBα in the nucleus of fibroblasts.
Increased IκBα phosphorylation by IκB kinases.
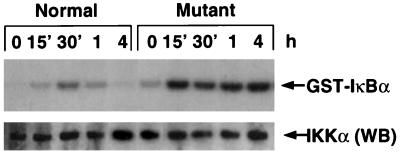
IKK phosphorylation of IκBα is the key step in LPS-induced IκB degradation. To address the potential cause of accelerated degradation of IκBα protein in LPS-treated relb−/− fibroblasts, we compared the expression level and activity of IKKα and IKKβ in normal and mutant cells. We did not detect a difference in IKKα or IKKβ mRNA (data not shown) and protein levels in normal and relb−/− fibroblasts (Fig. 7). In resting cells, the basal IKK activity was low and comparable in normal and mutant cells (Fig. 7). However, after LPS stimulation, the IKK activity was significantly augmented and prolonged in relb−/− fibroblasts compared with normal fibroblasts, causing an increased and persistent IκBα phosphorylation (Fig. 7). This prolonged activation of IKK may explain the observed destabilization of IκBα, IκBβ, and IκBɛ in relb−/− fibroblasts.
FIG. 7.
LPS-induced IKK activity in normal and relb−/− (mutant) fibroblasts. Fibroblasts were stimulated with 1 μg of LPS per ml for the indicated times, and cell lysate was prepared. IκB kinase complex was immunoprecipitated from 400 μg of lysate, and kinase activity was determined by using GST-IκBα (top). The same samples (10-μg portions) were used for Western blot analysis by anti-mouse IKKα polyclonal antibody (bottom).
DISCUSSION
In this study, we have shown that RelB plays an indispensable role in the suppression of cytokine expression in fibroblasts. IL-1α, IL-1β, and TNF-α expression are dysregulated in LPS-stimulated relb−/− fibroblasts, and this dysregulation can be reversed by RelB cDNA transfection. This result is consistent with our previous observation of overexpression of chemokines in activated relb−/− fibroblasts. Together, our findings provide a plausible explanation for the multiorgan inflammation of relb−/− mice. We have also provided evidence that the overexpression of NF-κB-activated genes in relb−/− fibroblasts is a result of IκBα destabilization in these cells, suggesting that RelB exerts its effect in normal fibroblasts by affecting the inhibitory function of IκBα on RelA or other NF-κB molecules. These results ascribe a transcription suppression function to the NF-κB/Rel family of transcription factors previously thought of exclusively as activators, and they suggest a new mode of intra- and interfamily interaction among the NF-κB/Rel and its coevolved IκB families of regulators.
Multiorgan inflammation in relb−/− mice as the result of RelB deficiency-associated defects in lymphoid and nonlymphoid tissues.
By virtue of its low affinity with IκBα (22), RelB is unique among the NF-κB/Rel family of transcription factors in that it is located in the nucleus and has constitutive NF-κB activity in lymphoid tissues (13, 32, 33, 56). As a result, RelB is believed to be responsible for the constitutive expression of NF-κB-regulated genes in the lymphoid organs and to play important housekeeping roles in the immune system. Indeed, when RelB genes are disrupted in transgenic mice, multifocal defects develop in the lymphoid organs of relb−/− mice, including the disappearance of thymic medulla and dendritic cells, dramatically enlarged spleen, and loss of lymph nodes and Peyer’s patches (12, 34, 57). Inflammatory and immune functions are also affected in these mice (17, 58, 60). Interestingly, relb−/− mice develop overwhelming inflammation in multiple nonlymphoid organs (12, 34, 57). The multiorgan inflammation is T-cell dependent and may be caused by autoreactive T-cell clones in these mice (17, 58). However, adoptive transfer and bone marrow chimera studies reveal that the inflammation is not dependent on the defects in relb−/− T cells (17, 62). We then, after careful examination of the lymphoid system, looked into possible defects in nonlymphoid tissues and investigated whether the multiorgan inflammation might be rooted in nonimmune cells.
The finding that relb−/− fibroblasts overexpress chemokines in response to LPS and TNF-α stimulation provided the first hint of RelB deficiency-related defects in nonimmune cells (62). Chemokine overexpression is associated with an exaggerated and prolonged activation of NF-κB activity, attributed mainly to p50 and RelA. This prompted us to examine other NF-κB-regulated genes. Unlike chemokines, IL-1α, IL-1β, and TNF-α are not expressed in fibroblasts, even when stimulated with LPS. Kruys et al. had shown that TNF-α gene locus is extinct in fibroblasts (31). We found that IL-1α, IL-1β, and TNF-α are dramatically and persistently induced in LPS-stimulated relb−/− fibroblasts (Fig. 1A), suggesting an important role of RelB in the extinction of cytokine expression in fibroblasts. The overexpression of both chemokines and cytokines can be reversed by RelB cDNA transfection (Fig. 2) (62), proving that the dysregulation of gene expression in relb−/− fibroblasts is a direct result of RelB deficiency. RelB-mediated gene suppression does not seem to be gene dose dependent. Fibroblasts from heterozygotic relb−/− mice express less RelB but behave in the same manner as wild-type fibroblasts in terms of cytokine expression; in addition, RelB antisense oligonucleotides and anti-RelB antibody to did not change the extinction of IL-1α, IL-1β, and TNF-α expression in these cells (data not shown). It remains to be investigated whether RelB alone can account for the mechanism of TNF extinction in fibroblasts, as demonstrated by Kruys et al. (31); our findings nonetheless provide an example of how such extinction can be altered.
Kruys et al. also anticipated that disruption of proper inactivation of the TNF locus may lead to diseases with inflammatory characteristics (31). Our results seem to substantiate this prediction. In an in vivo leukocyte recruitment analysis (62), LPS-stimulated relb−/− fibroblasts elicited a significantly greater granulocytic infiltrate than did similarly treated normal fibroblasts, suggesting that the overexpression of proinflammatory mediators in relb−/− fibroblasts is physiopathologically relevant. We, along with others, have observed increased cytokine expression in nonlymphoid tissues in relb−/− mice, but the cytokine expression in lymphoid tissue was either normal or reduced and TNF-α production was impaired in relb−/− macrophages (57, 60, 62). The mutant fibroblasts may in part account for the increased expression of cytokines and play a role in multiorgan inflammation in relb−/− mice. Autoreactive (17) or otherwise defective (58) T cells may activate fibroblasts to release chemokines and cytokines. Once activated, the fibroblasts attract more leukocytes into the affected tissues and enter a prolonged activation state until the animals succumb.
RelB modulation of IκBα stability in fibroblasts.
Our studies further suggest that the suppressive effect of RelB on cytokine expression in fibroblasts is mediated, at least in part, by modulating the stability of IκBα protein. When stimulated by LPS, normal and relb−/− fibroblasts have a similar pattern of RelA nuclear translocation (Fig. 4A). The κB-binding activity of RelA in normal fibroblasts, however, is much lower than that in mutant cells (Fig. 6D) (62), perhaps due to preexisting IκBα in the nucleus (Fig. 5). IκBα is the primary inhibitor of NF-κB activity. It has been shown previously that while not efficiently inhibited by IκBα, RelB can strongly induce the expression of IκBα and may inhibit RelA or c-Rel activities by driving the expression of IκBα (22, 24). In normal fibroblasts, a large portion of RelB is located in the nucleus (Fig. 4B). Intuitively, one would suspect the overexpression of proinflammatory mediators in relb−/− fibroblasts to be the result of insufficient IκBα expression in these cells. However, we have found no reduction in the IκBα mRNA level in the mutant cells; in fact, IκBα mRNA expression in LPS-stimulated relb−/− fibroblasts was increased compared with that in similarly treated normal fibroblasts.
Surprisingly, with a normal or increased mRNA level, the IκBα protein level was markedly decreased in relb−/− fibroblasts, indicating either a suppressed translation of IκBα mRNA or an accelerated degradation of IκBα protein in the mutant cells. Pulse-chase experiments revealed that the de novo IκBα protein synthesis was comparable in LPS-treated normal and relb−/− fibroblasts. However, the newly synthesized IκBα protein was very unstable in the mutant cells. The half-life of IκBα in LPS-stimulated normal fibroblasts is on the order of 2 to 3 h, but the half-life in LPS-stimulated mutant cells is less than 30 min. The rapid degradation of IκBα in LPS-stimulated relb−/− fibroblasts not only leads to a dramatic induction of NF-κB activity and RelA nuclear localization but also impairs the postinduction repression of NF-κB activity. The persistent NF-κB activation, rapid IκBα degradation, and overexpression of IL-1α, IL-1β, and TNF-α can be all reversed by RelB cDNA transfection (Fig. 1D and data not shown), strongly implicating a connection between RelB deficiency, IκBα destabilization, and NF-κB-activated cytokine expression in relb−/− fibroblasts. The relationship between IκBα destabilization and overexpression of cytokines in relb−/− fibroblasts is further demonstrated by the introduction of IκM, a dominant negative mutant of IκBα, into relb−/− fibroblasts. IκM is stable in relb−/− fibroblasts and, significantly, is able to reverse the overexpression of cytokines in these cells (Fig. 6B).
The mechanism of IκBα destabilization in relb−/− fibroblast, and therefore its corresponding mechanism of IκBα stabilization by RelB in normal fibroblasts, may be direct or indirect. In normal fibroblasts, both RelB and IκBα exist in the nucleus, and so an interaction between the two proteins is a formal possibility. On the other hand, RelB is known to have a low affinity for IκBα, and it is the inefficient binding with IκBα that allows RelB to enter and remain in the nucleus (22). We have not been able to detect any direct association of IκBα with RelB in fibroblasts. Alternatively, RelB may affect the stability of IκBα indirectly. Enhanced constitutive IκBα degradation has been reported by several groups (37, 47), and a number of different IκBα degradation pathways have been identified (28, 38). Our data from the IκM experiment suggest that IκBα degradation in relb−/− fibroblasts is Ser-32/36 dependent, hence implicating the prototypic IκBα degradation pathway (54). Since unstimulated relb−/− fibroblasts do not express cytokines and chemokines constitutively, an inducible phosphorylation step may be involved in initiating the breakdown of IκBα. Since the inducible phosphorylation of Ser-32/36 of IκBα is triggered by IKKs, the expression level and activities of IKK were examined. We did not detect a difference in IKK mRNA (data not shown) and protein levels in LPS-stimulated normal and relb−/− fibroblasts (Fig. 7), suggesting that IKK up-regulation may not be the cause of accelerated degradation of IκBα in the mutant cells. The basal IKK activity was low and comparable in both normal and mutant cells. However, LPS-induced IKK activity was significantly higher and more prolonged in mutant cells than in normal cells (Fig. 7). The kinetics of LPS-induced IKK activity in relb−/− fibroblasts coincides with the degradation of IκBα (Fig. 3B) and the overexpression of cytokine mRNA (Fig. 1A). This result suggests that the effect of RelB on IκBα stability is mediated at least partially by affecting IKK activity. Since IKK activity in normal fibroblasts was also induced by LPS, the relative importance of the augmented IKK activation in mutant cells to the cytokine overexpression remains to be investigated, perhaps by using kinase-inactive forms of IKKs. The postphosphorylation steps may play important roles in the degradation of IκBα in fibroblasts. RelB may affect these steps indirectly, either by up-regulation of a stabilizing protein(s) that binds to or modifies IκBα or by down-regulation of a protein(s) that participates in the degradation of IκBα (54). It is noteworthy that IκBα in LPS-stimulated relb−/− fibroblasts is unstable despite a significantly increased RelA level, a condition that generally promotes binding and the subsequent stabilization of IκBα (45). Perhaps a factor facilitating the IκBα/RelA interaction is missing in the mutant cells due to the RelB deficiency. Regardless of the actual mechanism of RelB stabilization of IκB, our results suggest that by modulating the protein stability of IκBα, a hierarchy control of one NF-κB by another NF-κB member may be achieved.
Besides the stabilization of IκBα, RelB seems to have additional effects on the suppression of cytokine expression in fibroblasts. While the expression of IκM significantly suppresses cytokine expression in LPS-stimulated relb−/− fibroblasts, the effect is not as complete as RelB cDNA transfection (compare Fig. 1E and 6A). It is also important to note the in vitro nature of our system in this study, and the findings need to be confirmed in vivo, preferably by tissue-specific knockout of the relb gene in mice. Moreover, the developmental programs or pathways that determine how RelB would function have yet to be investigated. We, along with others, have shown that RelB is a transcription activator of chemokines and TNF-κ in macrophages (60, 62). In fibroblasts, however, RelB plays the role of transcription suppressor of these genes. Is RelB differentially modified in different cells? When RAW 264.7 macrophages were fused with NIH 3T3 fibroblasts to generate a stable hybrid, Kruys et al. noted that trans-dominant factors contributed by the fibroblasts act to silence the TNF genes contributed by macrophages (31). What is the nature of such trans-dominant factors? DNA methylation plays a critical role in the extinction of TNF-κ genes in the macrophage-fibroblast hybrid (31). RelB is a key player for κB-dependent gene demethylation in B cells (29, 30) but functions differently in DNA methylation in fibroblasts (61a). What might be the molecular basis for this tissue-specific function of RelB? Our experimental system may provide a tool to address these novel and fundamental issues concerning the NF-κB/Rel family of transcription factors and their regulators, the IκBs. In particular, ours is the first to address the transcription suppression function of the NF-κB molecules. Our data further suggest a new mode of interfamily interaction between the NF-κB/Rel and IκB molecules and a resultant hierarchy structure in the intrafamily regulation of NF-κB activity.
ACKNOWLEDGMENTS
We are grateful to Jiahuai Han for the luciferase assay construct, to Carole Banka and Curtis B. Wilson for helpful suggestions, and to Pauline Pess for secretarial assistance.
This work was supported in part by NIH grants AR40770, DK49832 (L. Feng), AI 38375 (D. Lo), and 5T32 A107244 (S. Chen). Y. Xia was the recipient of a fellowship from the National Kidney Foundation of Southern California.
Footnotes
Publication 12172-IMM from the Department of Immunology, The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P J, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 5.Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Kruys V. Lipopolysaccharide signal transduction, regulation of tumor necrosis factor biosynthesis, and signaling by tumor necrosis factor itself. J Cardiovasc Pharmacol. 1995;25(Suppl. 2):S1–S8. doi: 10.1097/00005344-199500252-00002. [DOI] [PubMed] [Google Scholar]
- 7.Bours V, Azarenko V, Dejardin E, Siebenlist U. Human RelB (I-Rel) functions as a κB site-dependent transactivating member of the family of Rel-related proteins. Oncogene. 1994;9:1699–1702. [PubMed] [Google Scholar]
- 8.Bours V, Burd P R, Brown K, Villalobos J, Park S, Ryseck R P, Bravo R, Kelly K, Siebenlist U. A novel mitogen-inducible gene product related to p50/p105-NF-κB participates in transactivation through a κB site. Mol Cell Biol. 1992;12:685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 11.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκB-α. Proc Natl Acad Sci USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkly L, Hession C, Ogata L, Reilly C, Marconi L A, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco D, Ryseck R P, Bravo R. Expression of relB transcripts during lymphoid organ development: specific expression in dendritic antigen-presenting cells. Development. 1993;118:1221–1231. doi: 10.1242/dev.118.4.1221. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J D, Ryseck R P, Attar R M, Dambach D, Bravo R. Functional redundancy of the nuclear factor κB inhibitors IκBα and IκBβ. J Exp Med. 1998;188:1055–1062. doi: 10.1084/jem.188.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran W L, Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997;158:2558–2566. [PubMed] [Google Scholar]
- 18.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NK-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 20.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrzanski P, Ryseck R P, Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrzanski P, Ryseck R P, Bravo R. Differential interactions of Rel-NF-κB complexes with IκBα determine pools of constitutive and inducible NF-κB activity. EMBO J. 1994;13:4608–4616. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drouet C, Shakhov A N, Jongeneel C V. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-α promoter in primary macrophages. J Immunol. 1991;147:1694–1700. [PubMed] [Google Scholar]
- 24.Ferreira V, Tarantino N, Korner M. Discrimination between RelA and RelB transcriptional regulation by a dominant negative mutant of IκBα. J Biol Chem. 1998;273:592–599. doi: 10.1074/jbc.273.1.592. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, May M J, Kopp E B. NF-κB and rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 26.Grilli M, Chiu J J, Lenardo M J. NF-κB and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Huez G, Beutler B. Interactive effects of the tumor necrosis factor promoter and 3′-untranslated regions. J Immunol. 1991;146:1843–1848. [PubMed] [Google Scholar]
- 28.Imbert V, Rupec R A, Livolsi A, Pahl H L, Traenckner E B, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle P A, Peyron J F. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 29.Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y. A role for nuclear NF-κB in B-cell-specific demethylation of the Igκ locus. Nat Genet. 1996;13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 30.Kistler B, Baumann B, Bergman Y, Wirth T. RelB is a key player for both κB-dependent transcription and demethylation in B cells. Immunobiology. 1997;198:24–34. doi: 10.1016/s0171-2985(97)80024-1. [DOI] [PubMed] [Google Scholar]
- 31.Kruys V, Thompson P, Beutler B. Extinction of the tumor necrosis factor locus, and of genes encoding the lipopolysaccharide signaling pathway. J Exp Med. 1993;177:1383–1390. doi: 10.1084/jem.177.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lernbecher T, Kistler B, Wirth T. Two distinct mechanisms contribute to the constitutive activation of RelB in lymphoid cells. EMBO J. 1994;13:4060–4069. doi: 10.1002/j.1460-2075.1994.tb06723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lernbecher T, Muller U, Wirth T. Distinct NF-κB/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature. 1993;365:767–770. doi: 10.1038/365767a0. [DOI] [PubMed] [Google Scholar]
- 34.Lo D, Quill H, Burkly L, Scott B, Palmiter R D, Brinster R L. A recessive defect in lymphocyte or granulocyte function caused by an integrated transgene. Am J Pathol. 1992;141:1237–1246. [PMC free article] [PubMed] [Google Scholar]
- 35.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 36.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto S, Chiao P J, Verma I M. Enhanced IκBα degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol Cell Biol. 1994;14:3276–3282. doi: 10.1128/mcb.14.5.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto S, Seufzer B J, Shumway S D. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol Cell Biol. 1998;18:19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connell M A, Bennett B L, Mercurio F, Manning A M, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Biol Chem. 1998;273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 40.Pettit A R, Quinn C, MacDonald K P, Cavanagh L L, Thomas G, Townsend W, Handel M, Thomas R. Nuclear localization of RelB is associated with effective antigen-presenting cell function. J Immunol. 1997;159:3681–3691. [PubMed] [Google Scholar]
- 41.Read M A, Neish A S, Gerritsen M E, Collins T. Postinduction transcriptional repression of E-selectin and vascular cell adhesion molecule-1. J Immunol. 1996;157:3472–3479. [PubMed] [Google Scholar]
- 42.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 43.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 44.Ryseck R P, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, Bravo R. RelB, a new Rel family transcription activator that can interact with p50–NF-κB. Mol Cell Biol. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott M L, Fujita T, Liou H C, Nolan G P, Baltimore D. The p65 subunit of NF-κB regulates IκB by two distinct mechanisms. Genes Dev. 1993;7:1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- 46.Sha W C. Regulation of immune responses by NF-κB/Rel transcription factor. J Exp Med. 1998;187:143–146. doi: 10.1084/jem.187.2.143. . (Erratum, 187:661.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shattuck-Brandt R L, Richmond A. Enhanced degradation of I-κBα contributes to endogenous activation of NF-κB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–3039. [PubMed] [Google Scholar]
- 48.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 49.Smith R S, Smith T J, Blieden T M, Phipps R P. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 50.Sun S C, Ganchi P A, Ballard D W, Greene W C. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 51.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran K, Merika M, Thanos D. Distinct functional properties of IκBα and IκBβ. Mol Cell Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 54.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Krushel L A, Edelman G M. Targeted DNA recombination in vivo using an adenovirus carrying the cre recombinase gene. Proc Natl Acad Sci USA. 1996;93:3932–3936. doi: 10.1073/pnas.93.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weih F, Carrasco D, Bravo R. Constitutive and inducible Rel/NF-κB activities in mouse thymus and spleen. Oncogene. 1994;9:3289–3297. [PubMed] [Google Scholar]
- 57.Weih F, Carrasco D, Durham S K, Barton D S, Rizzo C A, Ryseck R P, Lira S A, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 58.Weih F, Durham S K, Barton D S, Sha W C, Baltimore D, Bravo R. Both multiorgan inflammation and myeloid hyperplasia in RelB-deficient mice are T cell dependent. J Immunol. 1996;157:3974–3979. [PubMed] [Google Scholar]
- 59.Weih F, Durham S K, Barton D S, Sha W C, Baltimore D, Bravo R. p50-NF-κB complexes partially compensate for the absence of RelB: severely increased pathology in p50(−/−)relB(−/−) double-knockout mice. J Exp Med. 1997;185:1359–1370. doi: 10.1084/jem.185.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weih F, Warr G, Yang H, Bravo R. Multifocal defects in immune responses in RelB-deficient mice. J Immunol. 1997;158:5211–5218. [PubMed] [Google Scholar]
- 61.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 61a.Xia, Y., and L. Feng. Unpublished observations.
- 62.Xia Y, Pauza M E, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 63.Zandi E, Chen Y, Karin M. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 64.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]