Age-based COVID-19 vaccination prioritizes white people above higher-risk others; geographic prioritization improves equity.
Abstract
COVID-19 mortality increases markedly with age and is also substantially higher among Black, Indigenous, and People of Color (BIPOC) populations in the United States. These two facts can have conflicting implications because BIPOC populations are younger than white populations. In analyses of California and Minnesota—demographically divergent states—we show that COVID vaccination schedules based solely on age benefit the older white populations at the expense of younger BIPOC populations with higher risk of death from COVID-19. We find that strategies that prioritize high-risk geographic areas for vaccination at all ages better target mortality risk than age-based strategies alone, although they do not always perform as well as direct prioritization of high-risk racial/ethnic groups. Vaccination schemas directly implicate equitability of access, both domestically and globally.
INTRODUCTION
Distributing coronavirus disease 2019 (COVID-19) vaccines represents one of the most notable public health challenge in a century, both in the United States (1) and globally (2). U.S. national guidelines issued by the Centers for Disease Control and Prevention (CDC) in December 2020 (3) were consistent with the evidence that the risk of death from COVID-19 increases starkly with age (4). However, the guidelines ignored evidence that the risk of exposure to and subsequent infection from SARS-CoV-2, the causative agent of COVID-19, is substantially higher for Black, Indigenous, and People of Color (BIPOC) (5). As a result, vaccine prioritization based solely on age may have exacerbated racial/ethnic inequities in COVID-19 burden because BIPOC populations are generally younger than the white population, more likely to be infected at younger ages, and at higher risk of dying from COVID-19 at all ages (5–7).
In contrast, prioritizations that consider other dimensions of risk alongside age may more effectively target those at greatest risk of COVID-19 death while reducing racial and ethnic inequities. Yet, not all targeted approaches are feasible in practice. While BIPOC populations have notably higher COVID-19 age-specific mortality, distributing vaccines based on race and ethnicity may not be legally viable (8) or politically tenable (9–11) in the United States. Further, a race-based approach may be perceived as discriminatory, given long-standing medical racism (8, 12). Instead, geographic targeting, using indices of health or COVID-19 mortality, may be more practical, more resistant to legal challenges, and still more equitable than strategies based on age alone (13). Here, we explore the mortality and equity implications of alternative vaccine eligibility schemas based on age alone, age and race, and age and geography.
We analyze four paired sets of alternative vaccination prioritization strategies and evaluate their sociodemographic and health equity implications. The baseline strategy for all comparisons is sequential age-based vaccination that starts with the oldest people and progressively extends eligibility for younger groups; in three of our four comparisons, age-based vaccination proceeds in 5-year age units. The comparison schemas use race/ethnicity or geography alongside age to determine eligibility. To reflect the COVID-19 mortality risk of the general population, we excluded those already prioritized in the U.S. phase 1A vaccine rollout (i.e., long-term care residents and health care workers). We assumed that policymakers and health departments aim to prioritize vaccinations for the groups with the highest COVID-19 mortality risk (14) [rather than with the highest risk of transmission; (15, 16)], in the context of limited vaccine supply. Other COVID-19 vaccine modeling studies consider which age groups to prioritize (17, 18) and various trade-offs between age, comorbidities, and occupations (14, 16, 19–21). Here, we compare strategies for vaccinating the general population based on age, race and ethnicity, and alternative measures of geographic risk.
We evaluate eligibility schemas by how well they match vaccine eligibility to the maximum COVID-19 mortality risk, using the observed COVID-19 mortality in 2020 (i.e., before mass vaccine rollout) as a proxy measure of risk. Our underlying assumptions are that vaccinating individuals prevents some deaths from COVID-19 that would otherwise occur in the context of partial community vaccination (22, 23) and that vaccine supply is smaller than vaccine demand, necessitating choices about whom to prioritize. Given fixed vaccine supply, matching eligibility to the maximum mortality risk should avert the largest number of deaths by directing vaccines to the people at highest risk (14). In addition, maximizing the mortality risk of the eligible also improves equity in the sense that it does not prioritize lower-risk populations above higher-risk populations. We consider an eligibility schema to be inequitable when lower-risk white people are eligible before higher-risk BIPOC people and when lower-risk socioeconomically advantaged people are eligible before higher-risk socioeconomically deprived people. Thus, in contrast to debates about “efficiency versus equity,” (13, 24) our analyses examine whether widely used eligibility rules were simultaneously less equitable and less effective than feasible alternatives.
In analyses, we used individual-level death certificate data from California and Minnesota. These two states are socioeconomically and demographically distinct. They experienced divergent pandemic trajectories and, according to a CDC analysis, differential success at key junctures in vaccinating their most vulnerable residents (25). We can thus compare the health equity implications of the four sets of vaccine prioritization strategies in two different populations, showing how this framework can be flexibly applied across diverse settings.
Our results serve three distinct goals. First, they can be used as a retrospective assessment of decisions made by U.S. states throughout spring 2021. Second, they remain directly relevant for future shortages associated with COVID-19 vaccine policy (e.g., forthcoming booster shots against variants), as well as scarce resource allocation decision-making generally in future disasters, including future pandemics. Third, they help provide a generalizable framework for evaluating vaccine eligibility and equity requiring only data on either areal measures of deprivation or historic COVID-19 mortality. For example, this framework could be used in other settings where vaccine scarcity remains an issue, such as low- or middle-income countries that are not expected to have adequate vaccine supply until at least 2022 (26), or in settings where disadvantaged populations are younger on average than advantaged populations.
RESULTS
Age-based prioritization alone results in substantial racial and ethnic disparities in averted deaths
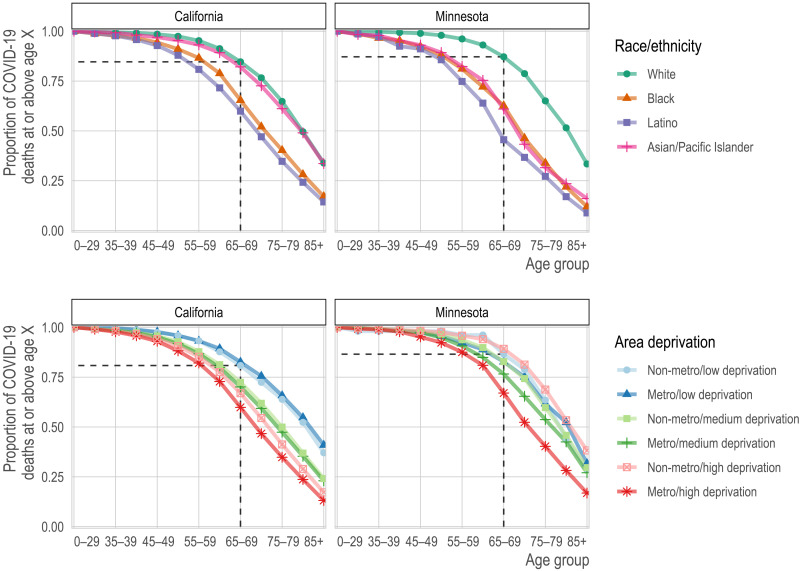
We found that sequential age-based prioritization alone would result in substantial racial/ethnic disparities in deaths averted. For example, vaccinating all people aged 75+ would have prevented nearly two-thirds of white COVID-19 deaths (CA: 65%; MN: 65%). Yet, for California and Minnesota, respectively, this age-based prioritization alone would have prevented only 40 and 33% of Black COVID-19 deaths, 35 and 27% of Latino COVID-19 deaths, and 61 and 32% of Asian and Asian-American COVID-19 deaths (Fig. 1, top row; Native American populations were not analyzed separately because of their small size). These stark differences reflect both that the white population is substantially older than most BIPOC populations and that COVID-19 mortality reaches high levels at substantially younger ages in BIPOC populations (Fig. 2). Age-based prioritization therefore reduces much more of the total risk in white populations compared to BIPOC populations.
Fig. 1. Proportion of COVID-19 deaths by race/ethnicity (top row) or geography (bottom row) and age group (x axis) for each state (columns).
Each line corresponds to the proportion of deaths (y axis) at or above each successive age group (x axis). In the top row, each line corresponds to a racial/ethnic category. For reference, we show the proportion of deaths among non-Hispanic whites ages 65 and older. For nearly all other racial/ethnic groups, the proportion of deaths at age 65 is lower. Correspondingly, for nearly all other racial/ethnic groups, the same proportion of deaths occurs at substantially lower ages. In the bottom row, each line represents a metropolitan area and deprivation level. Darker shades are metropolitan, while lighter shades are non-metropolitan. Blue is low deprivation, green is medium deprivation, and red is high deprivation. The reference lines show the proportion of deaths at ages 65 and above among non-metropolitan, low-deprivation areas.
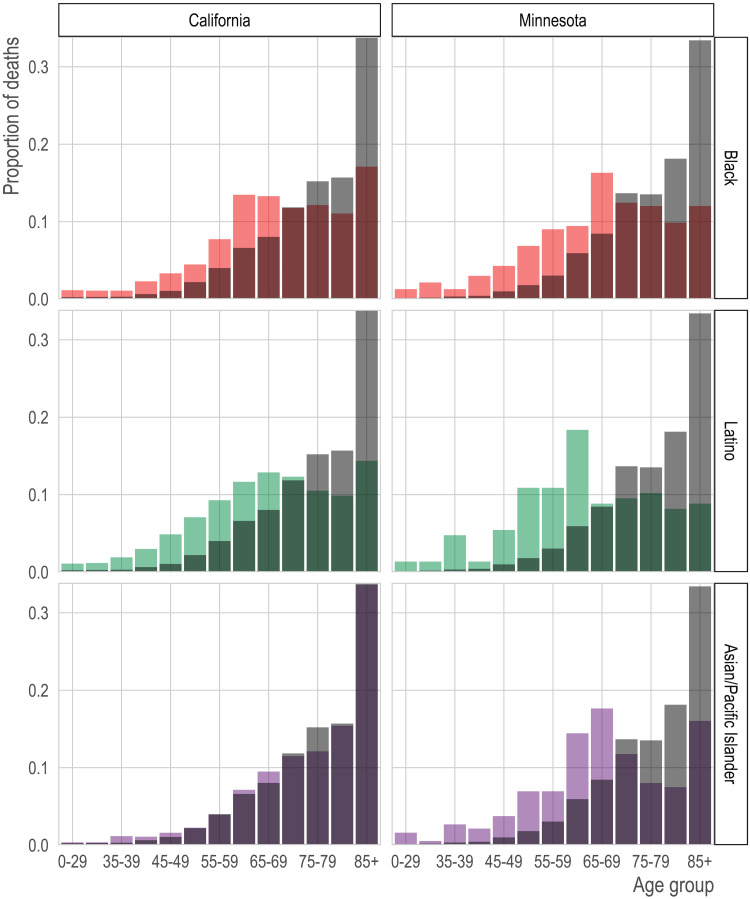
Fig. 2. Age distribution of COVID-19 deaths by race/ethnicity and state.
We show the proportion of COVID-19 deaths (y axis) in each age group (x axis) by race/ethnicity (rows) for California (left column) and Minnesota (right column). The gray bars in the background of each panel show the age distribution of deaths in the non-Hispanic white population for comparison.
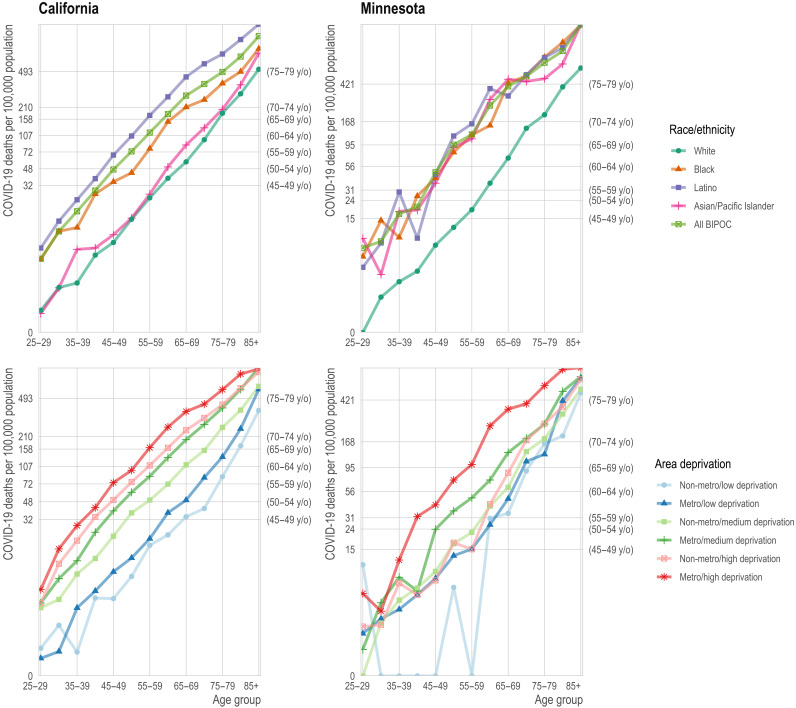
A consequence of this multidimensional COVID-19 mortality risk is that structurally disadvantaged groups often have mortality that exceeds the state aggregate rate for age groups that are 10 or even 15 years older. For example, if mortality at ages 65 to 69 is sufficiently high to merit vaccine priority, the same would be true for (in California) Latinos older than 55 or (in Minnesota) BIPOC as a whole who are older than 50 because their COVID-19 mortality exceeds their state’s aggregate COVID mortality at ages 65 to 69 (Fig. 3, top row).
Fig. 3. Age-specific mortality rate from COVID-19 by race/ethnicity (top row) and geography (bottom row).
Top row: The mortality rate (y axis) by age (x axis) varies by race/ethnicity (colors) with the non-Hispanic white population (dark green) experiencing substantially lower mortality at any age relative to the BIPOC (light green) and Latino (purple) populations. Age-based eligibility rules ignore this variation. The secondary y axis on the right shows the age group corresponding to the state-wide age-specific mortality rate. For example, in California, the age-specific mortality rate for non-Hispanic white 65- to 69-year-olds is 57 per 100,000, close to the state average for 50- to 59-year-olds (secondary y axis) and to 40- to 49-year-old BIPOC and Latinos. Bottom row: The mortality rate (y axis) by age (x axis) varies by area deprivation index (ADI; colors). We divide areas into “Metro” (darker shades) and “Non-metro” (lighter shades). We define “Metro” as the seven counties in the Twin Cities metropolitan area in Minnesota and Los Angeles, San Diego, San Francisco, Santa Clara, and Fresno counties in California. Non-metro areas include all Census tracts outside of the metro category. Low deprivation is defined as an ADI of 1 to 3, medium deprivation is 3.01 to 7.49, and high deprivation is 7.5 to 10.
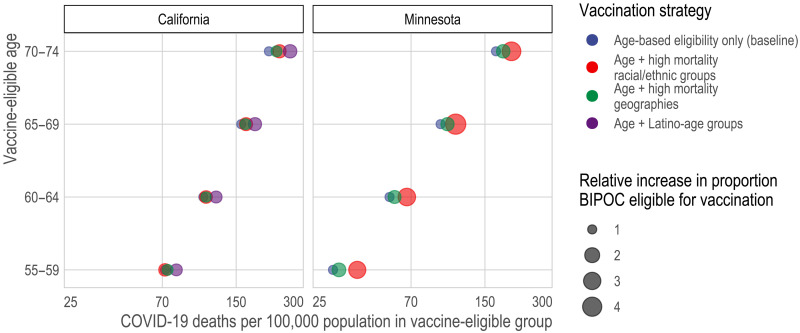
In the first set of paired, alternative vaccination strategies, we compare sequential age-based vaccination (in 5-year age groups) to vaccination schedules that combine the same age thresholds with race/ethnicity-age groups whose COVID-19 mortality exceeds that of the aggregate COVID mortality for the youngest eligible age group (e.g., ages 65 to 69 versus ages 65 to 69 and BIPOC ages 50 to 64 in Minnesota). We found that prioritizing vaccination for race-age groups with the highest risk would better target vaccination to high-risk individuals (Fig. 4 and fig. S1). Yet, the legal, political, and practical barriers to such race-based prioritization motivate the research questions addressed in the remaining three comparison sets, which consider to what extent geographic prioritization can achieve similar ends of targeting high-risk individuals and improving racial equity in vaccination, compared to age-based rules that, in practice, prioritize white populations.
Fig. 4. Death rate from COVID-19 of vaccine-eligible groups under different vaccination scenarios.
Here, we compare the predicted mortality rate from COVID-19 (x axis) of different types of vaccine allocation strategies (color) based on age (y axis). Specifically, we compare age alone (blue), age in combination with racial/ethnic groups (red), age in combination with high mortality locations (green), and, for California (the larger, more diverse state), age in combination with the highest mortality racial/ethnic group, which is Latinos (purple). In all cases and across all ages, incorporating additional, younger but higher-risk groups improves the efficiency of the rollout and reduces racial/ethnic inequities. In California, targeting high-mortality geographies (green) achieves similar efficiency as prioritizing disadvantaged racial/ethnic groups as a whole (red). In Minnesota, incorporating high-mortality racial/ethnic groups always outperforms incorporating high-mortality geographies; however, high-mortality geography still improves the alignment of vaccine allocation with COVID-19 mortality risk.
Geographic prioritization based on area-level deprivation improves equity and averts more deaths
In the second set of alternative vaccination strategies, we compare sequential age-based vaccination to vaccination schedules that also prioritize geography-age groups whose COVID mortality exceeds that of the aggregate for the youngest eligible age group. While age-based prioritization for the 75+ age group alone would have prevented nearly two-thirds of COVID-19 deaths in advantaged neighborhoods (California: 65%; Minnesota: 62%), it would have prevented only 35 and 40% of COVID-19 deaths in deprived neighborhoods in major metropolitan areas in California and Minnesota, respectively (Figs. 1, bottom row, and 3, bottom row).
Compared to age-based prioritization alone, prioritizing by area-level deprivation can better target high-risk groups (Fig. 4 and table S1). In California, geographic prioritization targets mortality about as effectively as prioritizing BIPOC as a whole, although not as well as prioritizing Latinos (the highest-risk racial group) specifically; in Minnesota, geographic prioritization is less effective than prioritizing BIPOC populations. Geographic prioritization also increases racial equity in Minnesota but does so only very modestly in California.
Universal adult vaccination in the highest-mortality neighborhoods can improve equity and avert more deaths
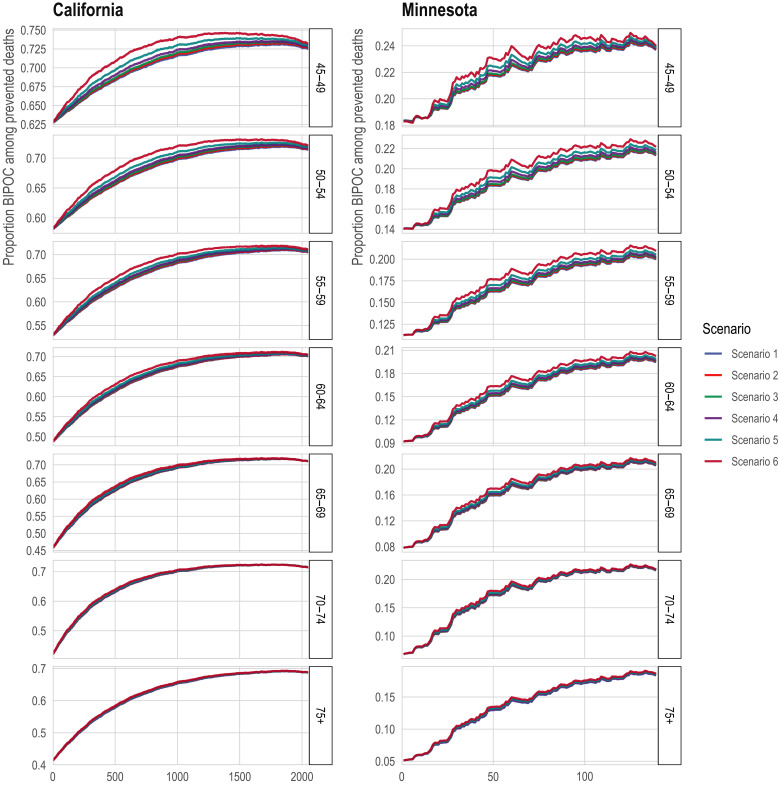
In the third comparison set, an alternative geographic prioritization strategy would directly identify Census tracts with historically higher COVID-19 mortality rather than proxying risk by area deprivation and major metropolitan status. This strategy mirrors one adopted by some states (27). Compared to statewide sequential age-based prioritization alone, adding vaccination for all adults (ages 20+) in the highest mortality tracts would generally improve the targeting of high-mortality groups in contexts where it also improves vaccine uptake among older people in the high-mortality tracts, but not in contexts where vaccinating the high-mortality tracts adds vaccination only for the youngest (not among those who were already eligible due to their age) (Fig. 5; see details in Materials and Methods). Prioritizing high-mortality tracts would also markedly increase vaccine access for BIPOC communities (Fig. 6). These results are qualitatively robust to a sensitivity analysis that assumes that a large portion of “high-mortality tracts” included unidentified long-term care facilities whose deaths should be excluded from the analysis (fig. S5; see details in Materials and Methods).
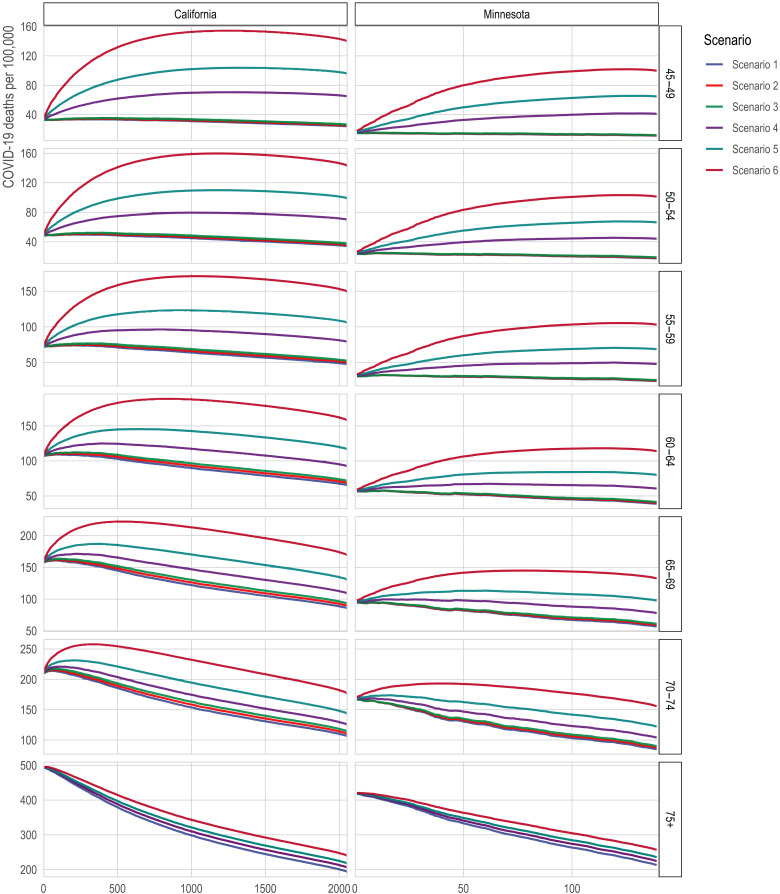
Fig. 5. Death rates from COVID-19 among the eligible with direct targeting of high-mortality Census tracts.
The x axis is the number of tracts in which all adults (ages 20+) are prioritized for vaccination. The y axis is 2020 COVID-19 deaths per 100,000; a higher death rate among the eligible indicates better targeting of vaccines toward high-risk individuals. The lines correspond to alternative scenarios as described in Materials and Methods.
Fig. 6. Proportion of non-white among the eligible with direct targeting of high COVID-19 mortality Census tracts.
The x axis is the number of tracts in which all adults (ages 20+) are prioritized for vaccination. The y axis is the proportion of the state’s eligible population that is non-white. The lines correspond to alternative scenarios as described in Materials and Methods.
For illustration, in California, if prioritizing tracts does not increase vaccine uptake among the oldest tract residents (who would already be eligible by age) but only results in vaccinating younger tract residents, then vaccinating the 500 highest-mortality tracts would decrease the mortality averted by 8% (from 158 to 145 deaths per 100,000) compared to vaccinating the 65- to 69-year-olds alone. The inflection point, where prioritizing all adults in a tract is neutral, occurs at around 220 tracts under the assumption of no improved older-age vaccination; under this assumption, prioritizing fewer than about 220 tracts improves mortality targeting and prioritizing more than about 220 tracts worsens it. However, if prioritizing tracts increases vaccine uptake by 50% among the oldest, already-eligible residents of those tracts, then vaccinating the 500 highest-mortality tracts would increase the averted mortality by 17% (from 158 to 185 deaths per 100,000).
Universally lowering the age of eligibility averts fewer deaths and is less equitable than selectively lowering eligibility age
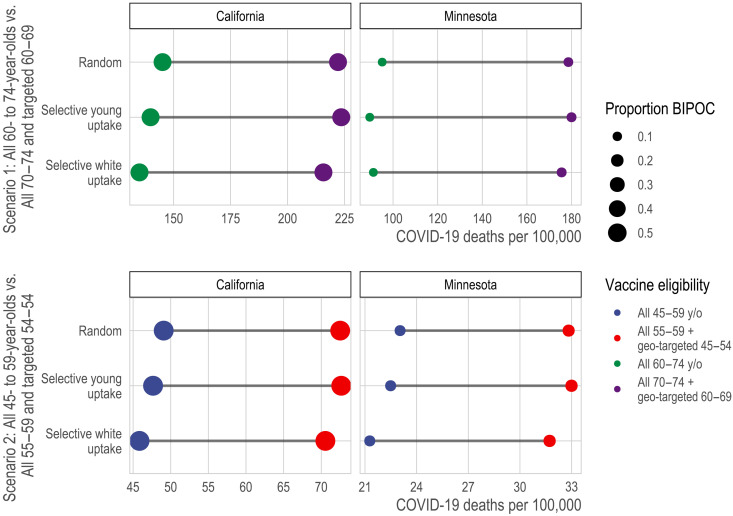
In the fourth comparison, we consider alternative strategies aimed at increasing racial equity in vaccination: substantially lowering age thresholds across the board, as some states have adopted with this motivation (28), versus selectively lowering age thresholds for high-mortality geographies. We compare these strategies at two critical junctures representing “early” and “late” vaccine rollout points: when vaccinating the 70 to 74 age group and when vaccinating the 55 to 59 age group (Fig. 7; see details in Materials and Methods). Compared with universally lowering the age threshold, the benefits of selectively lowering it, for maximizing the extent to which eligibility aligns with those at highest mortality risk, are substantial: For the older ages, selective lowering better targets the aggregate mortality risk of the eligible by 53% (222 versus 145 deaths per 100,000) in California and 88% (179 versus 95 deaths per 100,000) in Minnesota; for the younger ages, selective lowering better targets mortality risk among the eligible by 48% (73 versus 49 deaths per 100,000) in California and 42% (33 versus 23 deaths per 100,000) in Minnesota. However, in California, selective lowering of the age threshold does not meaningfully increase the proportion of vaccine-eligible people who are BIPOC for either early or late rollout. For Minnesota, it increases the proportion of vaccine-eligible who are BIPOC modestly (11% versus 8% for the older ages; 18% versus 14% for the younger ages).
Fig. 7. Death rate from COVID-19 of vaccine-eligible populations under alternative strategies designed to increase equity for BIPOC populations.
Here, we compare the targeted mortality rate from COVID-19 (x axis) of different types of vaccine allocation strategies (color) under alternative assumptions about vaccine uptake (y axis). Specifically, we compare strategies that universally reduce the age at eligibility (blue and green) to strategies that retain a higher age at eligibility but drop to a much younger age for high-risk geographic units, defined by ADI and major metropolitan status. In each panel, the first line assumes that vaccine uptake is random among the eligible; the second assumes that, conditional on eligibility, each successively younger 5-year age group increases uptake by 10% if the age threshold is high and by 25% if the age threshold is low; and the third line assumes that, conditional on eligibility, whites increase uptake by 10% if the age threshold is high and by 25% if the age threshold is low. The second and third lines indicate that a larger eligible group relative to vaccine supply may exacerbate selective uptake of lower-risk eligible people.
An additional shortcoming of broadly lowering age thresholds is obscured by the assumption of random uptake among the eligible: To the extent that the size of the eligible group exceeds the available vaccine supply, broadly lowering the age threshold can exacerbate the differences between groups in vaccine uptake (“selective uptake”), especially of lower-risk individuals. To capture this phenomenon, we compare the mortality risk among the vaccinated and proportion BIPOC among the vaccinated, under varying degrees of selective uptake among whites and selective uptake among younger eligible people. We find that, to the extent that creating a larger eligible population might exacerbate selective uptake by badly outstripping vaccine supply [e.g., white people being 25% more likely than BIPOC to access vaccines when eligible under broad eligibility—a number in line with observed rates (29)—versus only a hypothetical 10% under more restricted eligibility], geographic targeting will be even more effective at targeting high-risk groups and will also produce more equitable vaccination (fig. S8). At these relatively low rates of selective uptake, the difference made by selective uptake is small relative to the differences made by the vaccination schedules even assuming random uptake. Larger rates of selective uptake produce more marked divergences between the schedules (fig. S6).
DISCUSSION
Strict age-based vaccination strategies for COVID-19 disproportionately benefit the white population. For example, in both California and Minnesota, after excluding long-term care populations and health care workers, around two-thirds of white COVID-19 deaths, but well below half of Black and Latino deaths, occurred above age 75. This prioritization might be justifiable if older populations were at higher risk than younger populations, irrespective of race, much as prioritizing residents of long-term care facilities in phase 1A resulted in prioritizing a largely white population at overwhelming risk (30). However, we show that this justification does not apply to age-based vaccination after phase 1A. For example, when state vaccination eligibility was extended from 75+ to 65+, the mortality rate among the newly eligible was lower than the mortality rate among BIPOC groups that are 10 or 15 years younger yet still ineligible for vaccination. These age-ineligible, yet high-risk, BIPOC groups had to wait up to 3 months longer to be eligible for vaccination (31) and, by the time they were eligible, may have been competing for access with the general public. These results underscore the implications of prioritizing vaccine allocation based on the 65+ age threshold, as many states implemented in January 2021. Inequities driven by eligibility rules based on age alone may partly account for continued disparities, such as, in Minnesota, the fact that at the time of writing, a majority of the white population, but only one-third of the Black and Latino populations, are fully vaccinated (32), a disparity also occurring nationwide (33).
Compared to a vaccine eligibility strategy based on age alone, a strategy that combines geographic location based on socioeconomic characteristics with age-based eligibility—such as by extending eligibility to the geographic and age groups with higher mortality than the youngest age-eligible group—better aligns with risk of COVID-19 mortality. The total improvements in risk coverage from this age-geography prioritization are fairly modest (improving the targeting of high-mortality groups by 3 to 9% across age groups and states) because the populations added through geographic prioritization are small relative to the 5-year age groups in each state, so they have only a relatively small effect on aggregate risk among the eligible. However, the small size of the populations that would additionally become eligible also implies that geographic prioritization has a low direct opportunity cost, as only a small number of vaccines need to be allocated to high-risk geographies to achieve the equity gains of targeting.
Our results can reframe some debates about “efficiency versus equity.” In the context of vaccine scarcity, efforts to save the most lives possible and to save lives equitably can be at odds (13, 24). Yet, our results suggest that more equitable approaches can also be more effective at matching vaccines to the people at greatest risk, compared to arguably simpler, less equitable approaches. In particular, our results suggest that, in some cases, directing vaccination efforts at small, high-risk geographic areas without regard to age can improve on efforts to target older ages throughout the state, especially when such geographically targeted efforts improve vaccine uptake among older residents of high-risk areas. These results suggest that states should consider targeting broad swaths of the population (e.g., all adults) in highly specific geographic contexts when—and, from the perspective of directly reducing mortality, perhaps only when—this targeting allows for tactics that allow older residents to be more effectively reached. Such tactics could include home visits (34, 35), walk-in pop-up clinics (36), assigning appointment slots to all residents (37), and other forms of direct outreach that prioritize disadvantaged neighborhoods (38). Such approaches may be especially likely to succeed in increasing uptake among the highest-risk when high-risk populations are vaccine-hesitant but might be more likely to adopt vaccination as others in their networks become vaccinated and to the extent that such approaches increase framing of vaccination as the local default (37). Such direct outreach might be an effective strategy to vaccinate very high-risk populations quickly.
Moreover, broadly prioritizing all adults in the highest-mortality neighborhoods may be even more effective than the results here suggest. To the extent that groups with disproportionately high mortality also have disproportionate incidence of infection (39–42), the mortality-based results here may understate the benefits of better targeting at-risk groups. Because people live in segregated communities, people at heightened risk of COVID-19 death are likely to interact with others at elevated risk. Thus, prioritizing vaccination more effectively by neighborhood can potentially have multiplier effects as vaccinating relatively old residents reduces mortality directly and vaccinating younger residents reduces transmission to high-risk older people (15, 16, 43, 44). Because the analyses in this article do not model transmission dynamics, they do not provide a comprehensive answer to the optimal vaccine eligibility schedule. Instead, they show that, even in the setting most favorable to purely age-based eligibility (i.e., a setting that considers only directly-averted deaths), such eligibility schemas are inferior to those that incorporate multiple dimensions of demographic risk.
Our results show that some strategies designed to increase equity are unlikely to do so, and may result in a poor match of vaccine eligibility to risk. In the period between phase 1A and universal adult eligibility, several states extended age eligibility to age 50+ (45) and even to all adults before universal eligibility was widespread (46, 47), with reductions in the age at eligibility sometimes driven by a recognition that BIPOC people die of COVID-19 at younger ages on average (48). However, large universal drops in the age threshold for eligibility have the consequence of targeting risk quite poorly. We show that, compared to such a strategy, an alternative strategy that incorporates only high-risk geographies at younger ages does substantially better at prioritizing people with higher mortality risk. This is especially true in the context of disproportionate vaccine uptake by the advantaged among the eligible. However, our vaccine uptake simulation results suggest that small to moderate rates of selective uptake make relatively little difference in the extent to which each vaccination strategy succeeds in prioritizing high-risk people, compared to the large difference made by the choice of eligibility schema itself.
Better-optimized vaccination strategies should consider local demographics, intersectional risks, and both large-scale (e.g., large metro areas) and small-scale (e.g., Census tract disadvantage) geographic stratification. For example, in both states, disadvantaged metropolitan Census tracts had distinctly higher COVID-19 mortality than all other geographies. Yet, we found that geographic risk was more stratified by area deprivation index (ADI) in California and more stratified by major Metro status in Minnesota, implying that a one-size-fits-all approach may be suboptimal given vast demographic and geographic heterogeneity across states. Our results underscore the need for each state to individually consider what metrics would be most impactful for vaccine prioritization that simultaneously maximizes the reduction in deaths due to COVID-19 while also ensuring a fair and equitable approach. This lesson—that equitable and effective vaccination approaches require looking flexibly at multiple dimensions of risk in local context—should also extend to countries beyond the United States as they undertake their own vaccination campaigns amid scarcity (49).
This study has several limitations. One set of limitations concerns the data. First, the calculations reported in this analysis are based on mortality data obtained from January to December 2020. Therefore, to the extent that mortality patterns by age, race/ethnicity, and place have changed over the course of the pandemic (e.g., in response to selective shutdowns or social distancing patterns), our results may not reflect future deaths averted by vaccination. To address this, we confirmed that our main results persist when mortality risk is estimated from data for November to December 2020 only. Second, we were only able to evaluate strategies that prioritize on the basis of information included on death certificates, which typically excludes risk factors such as some comorbidities, income, health care access, and immigration status. Third, in some of our analyses of racial equity, we grouped all BIPOC into a single racial/ethnic category. Collapsing across diverse racial/ethnic and Indigenous populations poses challenges with respect to generalizability and implies a universal lived experience that does not exist (50, 51). However, combining groups enabled us to make direct comparisons between states (including a smaller, predominantly white state, Minnesota). Fourth, our data depend on the quality of COVID-19 cause assignments and racial categorization assignments in death certificates (52, 53).
A second set of limitations concerns the scope of the results. First, our study focused on vaccine eligibility; yet, access given eligibility may be as important as eligibility per se in determining equitability in COVID-19 vaccination. We incorporated analyses of how eligibility decisions might interact with differential access to vaccination via two kinds of selective uptake simulations: (i) greater vaccine uptake among white (or younger) eligible people than BIPOC (or older) eligible people, and (ii) highly localized geographic prioritization increases uptake among residents of those geographies who would have been eligible regardless. In both cases, we explored how selective uptake could exacerbate or ameliorate the consequences of eligibility rules. In general, however, our results illuminate only one of several mechanisms of inequitable vaccine access: the portion directly attributable to decisions about who is eligible to be vaccinated. Moreover, inequity in vaccination is only one route to inequity in COVID-19 outcomes that also stems from differences in transmission (39–42) and vulnerability (54). Second, some strategies are easier to implement than others. Geographic prioritization strategies require states to leverage data to determine where to target, whether broad indices of risk such as the ADI or direct measures of where deaths have been concentrated in the state. Strategies that prioritize active outreach in small, high-risk areas require coordination, other resources, and, to be effective, staff with linguistic competence and community connections that health departments may lack. Last, vaccination strategies that are not widely perceived as legitimate can undermine social solidarity and increase efforts to flout the rules (55), and we did not evaluate whether geographic prioritization is likely to be widely perceived—or can be made to be widely perceived—as fair.
A central argument for age-based vaccination schedules is that they may minimize administrative burdens that may undermine more targeted schedules by preventing the eligible people who are at highest risk from accessing the vaccine. For example, targeting comorbidities may inadvertently exclude people without primary care doctors (56). Geographic prioritization strategies, such as those explored here, may chart a middle path between, on the one hand, broad eligibility criteria that minimize administrative burden and, on the other, highly targeted criteria that aim to direct vaccines at groups with the highest mortality risk. Geographic prioritization is not free of administrative burden, particularly for those without secure housing, who need to be reached with alternative strategies. In particular, since few individuals know their Census tract, the prioritization strategies considered here would require individuals to check the eligibility of their addresses (e.g., through an online system or over the phone) or to be proactively contacted by state health systems; merely placing vaccination sites in high-risk neighborhoods does little to ensure that residents of those neighborhoods will be the people vaccinated (57). On the other hand, tract boundaries typically conform well to local contextual knowledge of neighborhood boundaries (58, 59), suggesting that eligibility can be defined in meaningful terms for the public by communicating street boundaries. The feasibility of tract-based eligibility is an important consideration since the benefits shown here for targeting Census tracts may not extend to geographic units that are larger and not defined with regard to meaningful social boundaries, such as the zip code targeting employed in California’s eligibility rules (60, 61). Our results suggest that, in the U.S. vaccine rollout, discussion should have turned earlier to how best to make prioritization feasible at highly localized geographic levels.
In many spheres of service provision, there are strong arguments in favor of universalist systems that minimize the burdens of demonstrating eligibility (62). Yet, the vaccine rollout is a unique context in which, during the crucial early months in the United States—and likely for some time to come in most of the world—the supply has been inflexibly scarce, making a truly universal approach untenable. Given this, strategies that prioritize residents of the neighborhoods where risk of dying of COVID-19 has been heavily concentrated could protect people whom age-based strategies exclude, despite their heightened risk of death.
MATERIALS AND METHODS
Mortality data
We used death certificate data provided by the California and Minnesota Departments of Public Health to identify all deaths due to COVID-19 from 1 January 2020 to 31 December 2020 (California, N = 33,311; Minnesota, N = 5803). We excluded deaths occurring in 2021 to limit distortion from vaccinations, since our goal is to estimate the mortality risk of various groups were they unvaccinated. Deaths are considered to be COVID-19 deaths if any mention of ICD code U07.1 appears on the death certificate, whether as the underlying or as a contributing cause. To reflect the underlying COVID-19 mortality risk that would be observed in the general population, we excluded decedents who would be eligible for phase 1A of the vaccine. Specifically, we removed COVID-19 deaths that occurred in long-term care facilities or nursing home residents (N = 4959 for California; N = 3070 for Minnesota) and, in California, deaths that occurred in hospice (N = 161), which were (somewhat ambiguously) included in California’s phase 1A (hospice deaths were not included in Minnesota’s phase 1A). Deaths that occurred in long-term care facilities were identified in California by, first, using place of death, as indicated on the death certificate and, second, matching the location of deaths to a comprehensive list of long-term care facilities. In Minnesota, these deaths were identified by the death certificate (see Supplementary Materials for details and sensitivity analyses). In addition, we removed deaths among health care workers in California (N = 1494); however, we were unable to remove health care worker deaths in Minnesota (N = 28, per communication with the Minnesota Department of Health). Last, we excluded those who do not reside in the state in which they died or who could not be successfully geolocated (California, N = 1817; Minnesota, N = 162). Our final analytic samples are N = 24,880 geolocated deaths in California and N = 2584 geolocated deaths in Minnesota.
We limited analyses of specific racial groups to non-Hispanic White, non-Hispanic Black, Latino, and Asian/Asian-American populations because they are the largest populations in both states, and we have greater confidence that death certificate racial assignments match population racial categorizations. Latino or Hispanic identity took precedence over racial group assignment. In Minnesota numerators and in both state denominators, the Asian group includes Pacific Islanders; in California, those are coded as “other-race” deaths (N = 564 for the full other-race category), resulting in a small undercount of the COVID-19 death rates for Asians in California. We treat all non-white populations (including those recorded as “Other race” on death certificates) as the BIPOC group.
A sensitivity analysis on the main results found that they are qualitatively robust to defining mortality risk using data for November to December 2020 only instead of data for all of 2020 (see Supplementary Materials; figs. S2 to S4).
Population data
Official 2020 population estimates are not yet available. We projected 2020 population estimates by race/ethnicity and age using historical population counts. Specifically, we used the Census Bureau July 1 population estimates by race, sex, and single year of age for 2019 and the number of deaths by race, sex, birth cohort, and age that took place between 1 July 2019 and 30 June 2020 in each state. Assuming zero net migration for each state during this 12-month period, we implemented the cohort-component method for each sex and race to estimate the July 1 population estimates by race, sex, and single year of age for 2020. These calculations were performed after redistributing deaths of “Other” races, including multiple races (i.e., other than “non-Hispanic white,” “non-Hispanic Black,” “non-Hispanic Native American,” “non-Hispanic Asian,” and “Hispanic”) proportionately over the other races for each sex and within each age group. Similarly, we redistributed deaths of unknown ages proportionately over all known ages within each race, sex, and age group category. We verified that our population estimates for 1 July 2020 were consistent with past trends in the state (using Census Bureau July 1 population estimates for 2010 through 2019) (63) for each combination of race, sex, and 5-year age group.
To estimate Census tract-specific populations for each race-, sex-, and age-specific group, we used the 2013–2018 National Historical Geographic Information System (NHGIS) estimates (64), which are the most recent available, as a baseline measure. The NHGIS estimates are in 10-year age bands. To produce 5-year age bands, we used the single-year age projections of the 2020 population to estimate the proportion of each race- and sex-specific 10-year age interval that is in the older or younger 5-year group in that interval. Last, we scaled the resulting Census tract-specific estimates up to the projected 2020 population size using the ratio of the 2020 population to the 2013–2018 NHGIS estimates for each race-, sex-, and 5-year age-specific population. This procedure assumes that differential population growth across geographic areas in each state is proxied by the resident demographics.
Geographic disadvantage
We define geographic disadvantage using the ADI and metropolitan status. For each Census block group, the ADI provides a score ranging from 1 (low deprivation) to 10 (high deprivation) based on 17 area-level measures of education, employment, housing quality, and poverty (65); the scores represent deciles of the state distribution of multidimensional deprivation. We use the ADI rather than the widely used social vulnerability index (SVI) because the SVI includes the racial composition of geographic areas as a component of vulnerability (25), whereas our goal is to evaluate facially race-neutral geographic targeting. For each Census tract, we took the population-weighted mean ADI score and categorized tracts as low deprivation (≤3), medium deprivation (3.01 to 7.49), and high deprivation (≥7.5). The asymmetry in the cut points for advantage and disadvantage reflects that there are extremely few COVID-19 deaths in areas with ADI < 2.5 in Minnesota, producing unstable age-specific mortality rates without including slightly less advantaged tracts. In addition, we categorized tracts by metropolitan status. In Minnesota, metropolitan tracts were the seven counties in the Twin Cities metropolitan area (collectively representing about 56% of the state population). In California, metropolitan tracts were those in Los Angeles, San Diego, San Francisco, Santa Clara, and Fresno counties (collectively representing about 44% of the state population). Non-metro areas include all Census tracts outside of the metro categorization.
Statistical analysis
Our analysis is based on estimating the COVID-19 mortality risk of the eligible population under various eligibility schemes. Mortality is an unadjusted ratio of observed COVID-19 deaths per 100,000 population. We used COVID-19 mortality in 2020 to proxy COVID-19 mortality risk in 2021 in the absence of vaccination, thus ignoring changes from selective mortality, improved treatment, and evolving patterns of risk. We do not assume that risk in 2021 in the absence of vaccination would equal risk in 2020, but we do assume that, across groups, 2021 risk in the absence of vaccination would be proportional to 2020 risk. This assumption allows us to compare the aggregate risk of the eligible under various vaccination strategies. As a sensitivity analysis, to see whether evolving patterns of risk would alter our results, we also used only November to December 2020 mortality to estimate risk (see the Supplementary Materials) instead of 2020 as a whole.
Thus, the risk to each subpopulation at age group a and group i (which can be a racial group or geographic group), Mi,a, represents that subpopulation’s aggregate COVID-19 mortality across 2020. As expressed in deaths per 100,000 people, this risk is given by
| (1) |
where Di,a is a count of COVID-19 deaths in 2020 in group i and age interval a, and Ni,a is the estimated 2020 population size in the same subpopulation. Weight wi,a is set to 1 for all subpopulations in the main analysis, reflecting that our main results assume vaccine uptake is random among the eligible group, and thus all eligible subpopulations are weighted equally. However, we also compared prioritization schedules in the context of differential vaccine uptake among the eligible by race or by age (to varying degrees), reflecting that the available vaccine supply exceeds demand among the eligible. These simulations are described in detail below. They are accomplished mechanically by up-weighting the mortality of the populations assumed to have selective uptake (e.g., white people) to estimate a counterfactual mortality risk of a population of vaccine receivers that are a nonrandom draw from the eligible population. We implemented code review procedures for all of the statistical analyses (66).
Census tract estimates (comparison set 3)
To assess schedules that include high-mortality Census tracts directly, we rank tracts in each state by their COVID-19 mortality. We limit the tract ranking to tracts with at least 1000 residents and at least five COVID-19 deaths (which means that the ranking is limited to relatively high-mortality tracts; N = 2055 tracts in California; N = 139 tracts in Minnesota) to reduce the inclusion of tracts that had high mortality for idiosyncratic reasons in 2020 that would not have applied in 2021. A remaining danger is that some included tracts may contain unidentified long-term care facilities, which would mean that the true inflection point (at which prioritizing all adults in high-mortality tracts flips from improving to worsening mortality targeting) would occur at a lower number of tracts than our analysis suggested. This possibility is explored in a sensitivity analysis described in the Supplementary Materials (fig. S5).
We consider tract inclusion under six scenarios, ranked by the extent to which vaccinating all adults in the tract would prioritize high-risk individuals
1) “No tract benefit for older people”: It assumes that older people in prioritized tracts, who are eligible by age and tract, would be vaccinated at the same rate as their age group in the rest of the state and gain no additional likelihood of vaccination from tract priority;
2) “Tract benefit only for youngest old (smaller benefit)”: It assumes that the youngest older people in prioritized tracts, who have just recently become eligible by age and tract, would become 25% more likely to be vaccinated when their tract is prioritized, but older people who have been eligible longer would be vaccinated at the same rate as their age group in the rest of the state and gain no additional likelihood of vaccination from tract priority;
3) “Tract benefit only for youngest old (larger benefit)”: It assumes that the youngest older people in prioritized tracts, who have just recently become eligible by age and tract, would become 50% more likely to be vaccinated when their tract is prioritized, but older people who have been eligible longer would be vaccinated at the same rate as their age group in the rest of the state and gain no additional likelihood of vaccination from tract priority;
4) “Tract benefit for all older people (smaller benefit)”: It assumes that all older people in prioritized tracts, who are eligible by age and tract, would become 25% more likely to be vaccinated when their tract is prioritized;
5) “Tract benefit for all older people (larger benefit)”: It assumes that all older people in prioritized tracts, who are eligible by age and tract, would become 50% more likely to be vaccinated when their tract is prioritized;
6) “Tract benefit regardless of age eligibility”: It assumes that all older people in prioritized tracts, who are eligible by age and tract, would become twice as likely to be vaccinated when their tract is prioritized (thus benefiting from the same absolute increase as younger people in the tract).
All six scenarios additionally assume that people in prioritized tracts who are too young to be otherwise eligible become vaccinated at the same rate as age-eligible people outside prioritized tracts. Mechanically, the selective uptake assumptions amount to setting the wi,a weighting term in Eq. 1 equal to some factor greater than 1 for those who are assumed to experience selective uptake, and wi,a = 1 for all eligible others. For example, scenario 4 weights people who are eligible by virtue of both their age and their tract at wi,a = 1.25, while those who are eligible by virtue of age or tract, but not both, are weighted at wi,a = 1.
Analysis of alternative equity strategies: Substantially lowering the age threshold universally versus selectively (comparison set 4)
In these analyses, we compared the strategy of substantially lowering the age threshold for eligibility universally to the strategy of keeping the age threshold at a higher level but selectively lowering it for high-risk geography-age groups. High-risk geography-age groups are defined in the same way as in comparison set 2: Geographies are stratified by area deprivation and metropolitan status, and geography-age groups are declared eligible when their mortality exceeds that of the state aggregate for the youngest eligible age group.
In these analyses, we compared these strategies at two points:
1) Scenario 1/early vaccination: setting statewide eligibility at age 60 versus setting statewide eligibility at age 70 while additionally prioritizing high-risk geographies as young as 60.
2) Scenario 2/late vaccination: setting statewide eligibility at age 45 versus setting statewide eligibility at 55 while additionally prioritizing high-risk geographies as young as 45 (California) or 40 (Minnesota).
We selected these age targets to match comparisons across states at “early” and “late” vaccination ages. In both California and Minnesota, the statewide 70 to 74 age group’s mortality is exceeded by residents of deprived Metro areas beginning at age 60 (in California, the 70 to 74 age group’s mortality is also exceeded by residents of deprived non-Metro areas beginning at age 65). In California, the statewide 55 to 59 age group’s mortality is exceeded by residents of deprived Metro areas beginning at age 45; in Minnesota, this age group’s mortality is exceeded by residents of deprived Metro areas beginning at age 40 (the 55 to 59 age group’s mortality is also exceeded by residents of deprived non-Metro areas in California and medium-deprivation Metro areas in Minnesota, beginning at age 50). Thus, scenario 2’s comparison statewide threshold of age 45 is conservative for Minnesota in the sense that a schedule aiming to include the most deprived neighborhoods would need to incorporate age 40, not just 45.
In the baseline analysis of these scenarios, we assumed that vaccine uptake was random among the eligible (wi,a = 1 for all subpopulations). In the selective uptake simulations (described more fully in the Supplementary Materials), we considered what would happen if markedly increasing the size of the eligible group (e.g., by dropping the statewide age threshold by 10 years of age) increased the selective uptake of white people, or of younger people, among the eligible from 10 to 25% (i.e., if having a vaccine-eligible population whose size greatly exceeds supply results in wwhite,a increasing from 1.1 to 1.25, while wBIPOC,a remains fixed at 1). These numbers were chosen to be roughly calibrated against data, suggesting about 25% selective uptake among white people 6 weeks into eligibility at 65+ (29).
Additional information
This study was deemed exempt from full review by the University of Minnesota institutional review board (STUDY00012527) and was approved by the California Health and Human Services institutional review board (project number: 2020-109).
Acknowledgments
We thank E. Hernandez, M. Glymour, J. Goldstein, M. Lipsitch, E. Murray, M. Niemann, G. Persad, and M. Plummer for helpful discussion. We thank the California Department of Public Health and Minnesota Department of Health for access to death certificate data. Funding: This work is supported in part by the National Institutes of Health. E.W.-F., A.R.R., M.B., and D.V.R. are supported in part by the National Institute on Aging (E.W.-F. and D.V.R.: P30AG066613; A.R.R.: T32AG049663; M.B.: P30AG012839 and R03G058110). E.W.-F., D.V.R., and J.P.L. are supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P2CHD041023). M.V.K. is supported in part by the National Institute on Drug Abuse (K99DA051534). In addition, E.W.-F. is supported by a Sustainable Development Goals Rapid Response Grant, a College of Liberal Arts Seed Grant, and, during initial data processing stages, was supported by the Fesler-Lampert Chair of Aging Studies at the University of Minnesota. This research also relied on data and research support from the NHGIS, which is supported by the National Institutes of Health (R01HD057929) and National Science Foundation (1825768). Last, we thank the Berkeley Workshop in Formal Demography [supported by the National Institutes of Health (R25HD083136, P30AG012839, and P2CHD073964)], which facilitated the initial connection between the California-based and Minnesota-based research teams. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NSF. Author contributions: E.W.-F. and M.V.K. conceived and designed the study. E.W.-F., M.V.K., Y.-H.C., M.B., D.V.R., J.P.L., A.R.R., K.B.-D., and J.P.L. acquired, analyzed, and interpreted the data. E.W.-F., M.V.K., A.R.R., E.C.M., M.B., K.A.D., and J.P.L. drafted the initial version of the manuscript. All authors provided critical revisions. All authors approve of the final manuscript and take final responsibility for the decision to submit for publication. E.W.-F. and M.V.K. had direct access to and verified the underlying data. In addition, J.P.L. had full access to the Minnesota death certificate data, and Y.-H.C. had full access to the California death certificate data. A.R.R. had full access to and verified the analytic data. Competing interests: E.W.F. is a member of the Steering Committee of the Seward Vaccine Equity Project, an unpaid volunteer position. All authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The data can be downloaded from a permanent repository at https://osf.io/tzeq3/?view_only=191ff8cfbd104e3aa2e76518af554943.
Supplementary Materials
This PDF file includes:
Sensitivity Analysis
Figs. S1 to S6
Table S1
REFERENCES AND NOTES
- 1.Centers for Disease Control and Prevention (CDC) , Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly Rep. 60, 619–623 (2011). [PubMed] [Google Scholar]
- 2.World Health Organization, “Inside the mammoth undertaking of global vaccine distribution” (2021); www.who.int/news-room/feature-stories/detail/inside-the-mammoth-undertaking-of-global-vaccine-distribution.
- 3.Dooling K., Marin M., Wallace M., McClung N., Chamberland M., Lee G. M., Talbot H. K., Romero J. R., Bell B. P., Oliver S. E., The advisory committee on immunization practices’ updated interim recommendation for allocation of COVID-19 vaccine—United states, December 2020. MMWR Morb. Mortal. Wkly Rep. 69, 1657–1660 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow N., Fleming-Dutra K., Gierke R., Hall A., Hughes M., Pilishvili T., Ritchey M., Roguski K., Skoff T., Ussery E., Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 382–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackey K., Ayers C. K., Kondo K. K., Saha S., Advani S. M., Young S., Spencer H., Rusek M., Anderson J., Veazie S., Smith M., Kansagara D., Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann. Intern. Med. 174, 362–373 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persad G., Peek M. E., Emanuel E. J., Fairly prioritizing groups for access to COVID-19 vaccines. JAMA 324, 1601–1602 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Bassett M. T., Chen J. T., Krieger N., Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: A cross-sectional study. PLoS Med. 17, e1003402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt H., Gostin L. O., Williams M. A., Is it lawful and ethical to prioritize racial minorities for COVID-19 vaccines? JAMA 324, 2023–2024 (2020). [DOI] [PubMed] [Google Scholar]
- 9.G. Persad, “Allocating medicine fairly in an unfair pandemic” (SSRN Scholarly Paper ID 3699769, Social Science Research Network, Rochester, NY, 2021); https://papers.ssrn.com/abstract=3699769.
- 10.S. Cwiek, “The social vulnerability index, COVID-19 vaccines, and why it makes some Republicans mad” (2001); www.michiganradio.org/post/social-vulnerability-index-covid-19-vaccines-and-why-it-makes-some-republicans-mad.
- 11.J. Ross, “When a Texas County tried to ensure racial equity in COVID-19 vaccinations, It didn’t go as planned,” Time (2021); https://time.com/5942884/covid-19-vaccine-racial-inequity-dallas/.
- 12.Jagannathan, Meera, “Should Black and Latino people get priority access to a COVID-19 vaccine?,” MarketWatch (2020).
- 13.Bibbins-Domingo K., Petersen M., Havlir D., Taking vaccine to where the virus is—Equity and effectiveness in coronavirus vaccinations. JAMA Health Forum 2, –e210213 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Bubar K. M., Reinholt K., Kissler S. M., Lipsitch M., Cobey S., Grad Y. H., Larremore D. B., Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 371, 916–921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matrajt L., Eaton J., Leung T., Brown E. R., Vaccine optimization for COVID-19: Who to vaccinate first? Sci. Adv. 7, eabf1374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner J. H., Chowell G., Springborn M. R., Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. medRxiv, 2020.09.22.20199174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein J. R., Cassidy T., Wachter K. W., Vaccinating the oldest against COVID-19 saves both the most lives and most years of life. Proc. Natl. Acad. Sci. U.S.A. 118, e2026322118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein J. R., Cassidy T., Mahmud A. S., Lives saved from age-prioritized covid-19 vaccination. medRxiv, 2021.03.19.21253991 (2021). [Google Scholar]
- 19.Chapman L. A. C., Shukla P., Rodríguez-Barraquer I., Shete P. B., León T. M., Bibbins-Domingo K., Rutherford G. W., Schechter R., Lo N. C., Comparison of COVID-19 vaccine prioritization strategies in the United States. medRxiv, 2021.03.04.21251264 (2021). [Google Scholar]
- 20.Adams M. L., Katz D. L., Grandpre J., Population based estimates of comorbidities affecting risk for complications from COVID-19 in the US. medRxiv, 2020.03.30.20043919 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulberry N., Tupper P., Kirwin E., McCabe C., Colijn C., Vaccine rollout strategies: The case for vaccinating essential workers early. medRxiv, 2021.02.23.21252309 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilishvili T., Fleming-Dutra K. E., Farrar J. L., Gierke R., Mohr N. M., Talan D. A., Krishnadasan A., Harland K. K., Smithline H. A., Hou P. C., Lee L. C., Lim S. C., Moran G. J., Krebs E., Steele M., Beiser D. G., Faine B., Haran J. P., Nandi U., Schrading W. A., Chinnock B., Henning D. J., LoVecchio F., Nadle J., Barter D., Brackney M., Britton A., Marceaux-Galli K., Lim S., Phipps E. C., Dumyati G., Pierce R., Markus T. M., Anderson D. J., Debes A. K., Lin M., Mayer J., Babcock H. M., Safdar N., Fischer M., Singleton R., Chea N., Magill S. S., Verani J., Schrag S.; Vaccine Effectiveness Among Healthcare Personnel Study Team , Interim estimates of vaccine effectiveness of Pfizer-BioNTech and Moderna COVID-19 vaccines among health care personnel—33 U.S. Sites, January–March 2021. MMWR Morb. Mortal. Wkly Rep. 70, 753–758 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating, Dan and Shapiro, Leslie, The unseen covid-19 risk for unvaccinated people. Washington Post (2021).
- 24.Committee on Equitable Allocation of Vaccine for the Novel Coronavirus, Board on Health Sciences Policy, Board on Population Health and Public Health Practice, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine, Framework for Equitable Allocation of COVID-19 Vaccine (National Academies Press, 2020); www.nap.edu/catalog/25917. [PubMed]
- 25.Hughes M. M., Wang A., Grossman M. K., Pun E., Whiteman A., Deng L., Hallisey E., Sharpe J. D., Ussery E. N., Stokley S., Musial T., Weller D. L., Murthy B. P., Reynolds L., Gibbs-Scharf L., Harris L. T., Ritchey M. D., Toblin R. L., County-level COVID-19 vaccination coverage and social vulnerability—United States, December 14, 2020–March 1, 2021. MMWR Morb. Mortal. Wkly Rep. 70, 431–436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.So A. D., Woo J., Reserving coronavirus disease 2019 vaccines for global access: Cross sectional analysis. BMJ 371, m4750 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S. Cline, G. Flaccus, Under new program, some Oregon centers can vaccinate anyone, in AP NEWS (2021); https://apnews.com/article/portland-coronavirus-pandemic-oregon-5ed46270d0da623dd453189d1bc48e2e.
- 28.Alaska Department of Health and Social Services, State of Alaska COVID-19 Vaccine Allocation Guidelines (Washington, D.C., 2021); http://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/HumanCoV/COVIDvaccine_AlaskaAllocationGuidelines.pdf.
- 29.C. Richert, D. Kraker, State data shows disparities in race, ethnicity of who’s getting COVID-19 vaccine, in MPR News (2021); www.mprnews.org/story/2021/03/05/state-data-shows-disparities-in-race-ethnicity-of-whos-getting-vaccinated.
- 30.Painter E. M., Ussery E. N., Patel A., Hughes M. M., Zell E. R., Moulia D. L., Scharf L. G., Lynch M., Ritchey M. D., Toblin R. L., Murthy B. P., Harris L. Q., Wasley A., Rose D. A., Cohn A., Messonnier N. E., Demographic characteristics of persons vaccinated during the first month of the COVID-19 vaccination program—United states, December 14, 2020–January 14, 2021. MMWR Morb. Mortal. Wkly Rep. 70, 174–177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minnesota Department of Health, Minnesota Guidance for Allocating and Prioritizing COVID-19 Vaccine—Phases 1b, 1c, 2 (2021), pp. 1–7.
- 32.Minnesota Department of Health, Vaccine Data; https://mn.gov/covid19/vaccine/data/index.jsp.
- 33.Nambi Ndugga; Olivia Pham; Latoya Hill; Samantha Artiga; Raisa Alam; Noah Parker, Latest Data on COVID-19 Vaccinations Race/Ethnicity. Kaiser Family Foundation (2021); www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/.
- 34.Spectrum News Staff, “Councilman Levine says door-to-door vaccine delivery should be an option,” Spectrum News NY1 (2021); www.ny1.com/nyc/all-boroughs/politics/2021/02/17/councilman-mark-levine-says-door-to-door-vaccine-delivery-should-be-an-option.
- 35.A. Pinsker, MCHD Launching Vaccination For Homebound Hoosiers. News - Indiana Public Media; https://indianapublicmedia.org/news/mchd-launching-vaccination-for-homebound-hoosiers.php.
- 36.J. Lai, L. McCrystal, “Philly started allowing walk-ins at FEMA vaccine site to improve equity. It might be working,” The Philadelphia Inquirer (2021); www.inquirer.com/news/fema-vaccine-racial-equity-walk-ins-appointment-20210320.html.
- 37.K. L. Milkman, M. S. Patel, L. Gandhi, H. Graci, D. Gromet, H. Ho, J. Kay, T. Lee, M. Akinola, J. Beshears, J. Bogard, A. Buttenheim, C. Chabris, G. B. Chapman, J. J. Choi, H. Dai, C. R. Fox, A. Goren, M. Hilchey, J. Hmurovic, L. John, D. Karlan, M. Kim, D. Laibson, C. Lamberton, B. C. Madrian, M. N. Meyer, M. Modanu, J. Nam, T. Rogers, R. Rondina, S. Saccardo, M. Shermohammed, D. Soman, J. Sparks, C. Warren, M. Weber, R. Berman, C. Evans, C. Snider, E. Tsukayama, C. Van den Bulte, K. Volpp, A. Duckworth, “A mega-study of text-based nudges encouraging patients to get vaccinated at an upcoming doctor’s appointment” (SSRN Scholarly Paper ID 3780267, Social Science Research Network, Rochester, NY, 2021); doi: 10.2139/ssrn.3780267. [DOI]
- 38.Parker W. F., Persad G., Peek M. E., Fair allocation at COVID-19 mass vaccination sites. JAMA Health Forum 2, e210464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogedegbe G., Ravenell J., Adhikari S., Butler M., Cook T., Francois F., Iturrate E., Jean-Louis G., Jones S. A., Onakomaiya D., Petrilli C. M., Pulgarin C., Regan S., Reynolds H., Seixas A., Volpicelli F. M., Horwitz L. I., Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw. Open 3, e2026881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price-Haywood E. G., Burton J., Fort D., Seoane L., Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 382, 2534–2543 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rentsch C. T., Kidwai-Khan F., Tate J. P., Park L. S., King J. T., Skanderson M., Hauser R. G., Schultze A., Jarvis C. I., Holodniy M., Lo Re V., Akgün K. M., Crothers K., Taddei T. H., Freiberg M. S., Justice A. C., Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med. 17, e1003379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelner J., Trangucci R., Naraharisetti R., Cao A., Malosh R., Broen K., Masters N., Delamater P., Racial disparities in coronavirus disease 2019 (COVID-19) mortality are driven by unequal infection risks. Clin. Infect. Dis. 72, e88–e95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Z. McLaren, “Why a staggered vaccine rollout is better than first come, first served,” in FiveThirtyEight (2021); https://fivethirtyeight.com/features/how-do-we-get-back-to-normal-fastest-prioritize-access-to-the-vaccine/.
- 44.Ferranna M., Cadarette D., Bloom D. E., COVID-19 vaccine allocation: Modeling health outcomes and equity implications of alternative strategies. Engineering, S2095809921001934 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser Family Foundation, State COVID-19 Data and Policy Actions, in KFF State Vaccine Rollout (2021); www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/.
- 46.J. J. Cooper, Arizona opens vaccine appointments to everyone 16 and older. Associated Press (2021); https://apnews.com/article/pandemics-health-arizona-coronavirus-pandemic-43da1a9af71bfece7fd30c0bda6ccb1a.
- 47.K. B. Harper, Texas opens COVID-19 vaccine to everyone 16 and older on March 29, in Texas Tribune (2021); www.texastribune.org/2021/03/23/texans-eligible-covid-vaccine/.
- 48.R. DeBrock, Pritzker lowers vaccine distribution age to 65, in The Telegraph (2021); www.thetelegraph.com/news/article/Pritzker-lowers-vaccine-distribution-age-to-65-15850887.php.
- 49.Goralnick E., Kaufmann C., Gawande A. A., Mass-vaccination sites—An essential innovation to curb the Covid-19 pandemic. N. Engl. J. Med. 384, e67 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Chen Y.-H., Glymour M. M., Catalano R., Fernandez A., Nguyen T., Kushel M., Bibbens-Domingo K., Excess mortality in California during the coronavirus disease 2019 pandemic, March to August 2020. JAMA Intern. Med. 181, 705–707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wrigley-Field E., Garcia S., Leider J. P., Robertson C., Wurtz R., Racial disparities in COVID-19 and excess mortality in minnesota. Socius. 6, 2378023120980918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGivern L., Shulman L., Carney J. K., Shapiro S., Bundock E., Death certification errors and the effect on mortality statistics. Public Health Rep. 132, 669–675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (CDC), “The validity of race and hispanic-origin reporting on death certificates in the United States: An update” (DHHS Publication No. 2016–1372). Vital and Health Statistics (2016). [PubMed]
- 54.Wiemers E. E., Abrahams S., AlFakhri M., Hotz V. J., Schoeni R. F., Seltzer J. A., Disparities in vulnerability to complications from COVID-19 arising from disparities in preexisting conditions in the United States. Res. Soc. Stratif. Mobil. 69, 100553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Working Group on Readying Populations, for COVID-19 Vaccine, “The public’s role in COVID-19 vaccination: Planning recommendations informed by design thinking and the social, behavioral, and communication sciences” (2020), p. 47.
- 56.K. Kennedy, “Critics: Doctor’s note for vaccine unfairly penalizing poor.” (AP) The Philadelphia Inquirer (2021); www.usnews.com/news/us/articles/2021-03-18/critics-doctors-note-for-vaccine-unfairly-penalizing-poor.
- 57.J. Wick, Vaccine access codes for hard-hit Black, Latino communities improperly used in other L.A. areas, in Los Angeles Times (2021); www.latimes.com/california/story/2021-02-22/vaccine-access-codes-for-hard-hit-communities-of-color-circulate-widely-in-affluent-l-a.
- 58.Krieger N., A century of census tracts: Health & the body politic (1906–2006). J. Urban Health 83, 355–361 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krieger N., Waterman P., Chen J. T., Soobader M.-J., Subramanian S. V., Carson R., Zip code caveat: Bias due to spatiotemporal mismatches between zip codes and US census–defined geographic areas—The public health disparities geocoding project. Am. J. Public Health 92, 1100–1102 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.J. Snow, California’s “Equity” algorithm could leave 2 million struggling californians without additional vaccine supply, in ACLU NorCal (2021); www.aclunc.org/blog/californias-equity-algorithm-could-leave-2-million-struggling-californians-without-additional.
- 61.Grubesic T. H., Matisziw T. C., On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int. J. Health Geogr. 5, 58 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.P. Herd, D. P. Moynihan, Administrative Burden: Policymaking by Other Means (Russell Sage Foundation, 2019).
- 63.Census Bureau, “Annual County resident population estimates by age, sex, race, and Hispanic origin: April 1, 2010 to July 1, 2019” (CC-EST2019-ALLDATA); https://www2.census.gov/programs-surveys/popest/datasets/2010-2019/counties/asrh/cc-est2019-alldata.csv.
- 64.Manson, Steven, Schroeder, Jonathan, Van Riper, David, Kugler, Tracy, Ruggles, Steven, National Historical Geographic Information System: Version 15.0 (2020), doi: 10.18128/D050.V15.0. [DOI]
- 65.Kind A. J. H., Buckingham W. R., Making neighborhood-disadvantage metrics accessible—The neighborhood atlas. N. Engl. J. Med. 378, 2456–2458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vable A. M., Diehl S. F., Glymour M. M., Code review as a simple trick to enhance reproducibility, accelerate learning, and improve the quality of your team’s research. Am. J. Epidemiol. kwab092 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity Analysis
Figs. S1 to S6
Table S1