Abstract
Mutant isocitrate-dehydrogenase 1 (mIDH1) synthesizes the oncometabolite 2-hydroxyglutarate (2HG), which elicits epigenetic reprogramming of the glioma cells’ transcriptome by inhibiting DNA and histone demethylases. We show that the efficacy of immune-stimulatory gene therapy (TK/Flt3L) is enhanced in mIDH1 gliomas, due to the reprogramming of the myeloid cells’ compartment infiltrating the tumor microenvironment (TME). We uncovered that the immature myeloid cells infiltrating the mIDH1 TME are mainly nonsuppressive neutrophils and preneutrophils. Myeloid cell reprogramming was triggered by granulocyte colony-stimulating factor (G-CSF) secreted by mIDH1 glioma stem/progenitor-like cells. Blocking G-CSF in mIDH1 glioma–bearing mice restores the inhibitory potential of the tumor-infiltrating myeloid cells, accelerating tumor progression. We demonstrate that G-CSF reprograms bone marrow granulopoiesis, resulting in noninhibitory myeloid cells within mIDH1 glioma TME and enhancing the efficacy of immune-stimulatory gene therapy.
Mutant IDH1 gliomas are infiltrated by nonsuppressive immature myeloid cells, resulting in enhanced response to immunotherapy.
INTRODUCTION
Mutation in isocitrate dehydrogenase (mIDH) is a common genetic lesion in glioma patients (1). Approximately 90% of IDH1 mutations occur in exon 4 at codon 132, resulting in a change of a single amino acid from arginine to histidine (R132H). Less common IDH2 mutations occur in an analogous codon at position R172 (2). Although IDH1/2 mutations are always heterozygous, they exert a dominant gain-of-function enzymatic activity, which leads to the production of 2-hydroxyglutarate (2HG). Excessive 2HG production causes DNA hypermethylation via inhibition of methylcytosine dioxygenase TET2 (3) and also promotes histone hypermethylation through competitive inhibition of α-ketoglutarate (αKG)–dependent Jumonji-C histone demethylases (4). This leads to epigenetic reprogramming of the transcriptome within the mIDH1 glioma cells (4, 5).
Several studies suggested that mIDH1 may play a critical role in shaping the immunological landscape of the tumor microenvironment (TME) (6–9). Glioma samples from patients with mIDH1 have decreased Programmed death-ligand 1 (PD-L1) expression (due to hypermethylation of the CD274 promoter) (7, 10), reduced levels of inflammation, and reduced levels of infiltrating immune cells (6, 8). Myeloid cells represent the major immune cells infiltrating the glioma microenvironment (11, 12). It has recently been shown that the TME, from a genetically engineered mouse model (GEMM) of mIDH1 glioma (PDGFB/shP53/Ink4a/Arf−/−/mIDH1), exhibits fewer tumor-infiltrating macrophages, dendritic cells (DCs), and neutrophils compared to the wild-type IDH1 counterpart (6). Nevertheless, the impact of mIDH1 on tumor-infiltrating myeloid cells’ phenotype and function, as well as the impact of mIDH1 in glioma on the response to immunotherapies, has not been explored, particularly in the context of concurrent inactivation mutations in ATRX and TP53 (1, 13).
In this context, the lack of therapeutic efficacy of immunotherapies in patients with glioma in the clinical arena can be attributed, in part, to the immunosuppressive properties elicited by glioma-infiltrating immune cells (14). Myeloid-derived suppressor cells (MDSCs) have emerged as one of the dominant types of immunosuppressive cells that directly interfere with the efficacy of immunotherapy (12, 15). In patients with glioma, it has been demonstrated that granulocytic MDSCs [also known as polymorphonuclear (PMN)–MDSCs] are a major subset that expand during glioma progression, which negatively correlates with patients’ survival (16–18).
Here, we demonstrate that gliomas harboring mIDH1 in the context of ATRX and TP53 deficiency exhibit an enhanced therapeutic response to immune-stimulatory gene therapy (herpes simplex virus 1–thymidine kinase/Feline McDonough sarcoma (Fms)–like tyrosine kinase 3 ligand: TK/Flt3L) when compared to wtIDH1 tumors. MDSC depletion enhanced TK/Flt3L therapeutic efficacy in wtIDH1 glioma– but not in mIDH1 glioma–bearing mice. We report that in genetically engineered mIDH1 glioma mouse models, there is an expansion of tumor-infiltrating granulocytes when compared to wtIDH1 glioma. Upon phenotypic and functional characterization, we uncovered that tumor-infiltrating granulocytes in mIDH1 glioma, counter to the tumor-infiltrating granulocytes in wtIDH1, did not exhibit immune-suppressive properties. Single-cell sequencing coupled with mass cytometry analysis revealed that these granulocytes are heterogeneous and composed of three distinct clusters, i.e., neutrophils, preneutrophils, and a smaller cluster of bona fide immunosuppressive PMN-MDSCs. Moreover, primary human gliomas showed a higher frequency of cells exhibiting the PMN-MDSC gene signature in wtIDH1 tumors when compared to mIDH1 glioma. Our data demonstrate that the mechanism by which mIDH1 in glioma mediates the expansion of nonimmunosuppressive granulocytes involves epigenetic reprogramming. This leads to enhanced expression of granulocyte colony-stimulating factor (G-CSF) by stem-like mIDH1 glioma cells. Blocking G-CSF restored the inhibitory potential of PMN-MDSCs and accelerated tumor progression. Our results also demonstrate that systemic administration of recombinant G-CSF (rG-CSF) prolonged the median survival (MS) of wtIDH1 glioma–bearing mice and rendered tumor-infiltrating MDSCs nonimmunosuppressive. Consistent with that, low-grade glioma (LGG) astrocytoma with mIDH1 is the sole tumor cohort within all The Cancer Genome Atlas (TCGA) datasets in which high CSF3 gene expression correlates with favorable patients’ outcome. Our data provide mechanistic insights related to the impact of mIDH1 on the phenotypic and functional properties of myeloid cells in the glioma TME. This study reveals an important role of mIDH1 on reprogramming the phenotypic and functional diversity of myeloid cells in glioma TME, a feature that can be harnessed to enhance the efficacy of immunotherapies in patients with glioma.
RESULTS
Immune stimulatory gene therapy eradicates mIDH1 tumor irrespective of Ly6G+ myeloid cell depletion
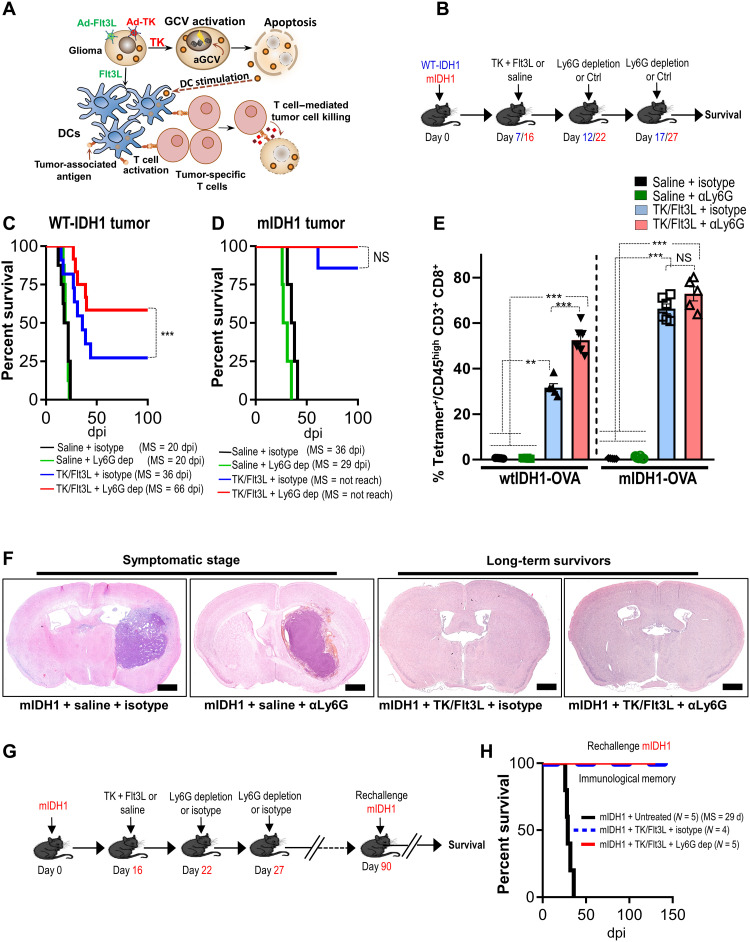
The role of IDH1 mutation in shaping the immune landscape and response to immunotherapy in glioma is poorly understood. We have recently shown that the PMN-MDSCs are the major immune cells infiltrating the high-grade glioma with wtIDH1, and they determine the efficacy of immunotherapy (12). To uncover the impact of IDH1R132H in the context of ATRX and TP53 deficiency on glioma-infiltrating PMN-MDSCs and the response to immunotherapy, we generated a mIDH1 GEMM using the Sleeping Beauty (SB) transposon system (19–21). Two groups of implantable neurospheres were generated using the SB method; each contains a combination of genetic lesions commonly encountered in astrocytoma: the mIDH1 group [shp53/shATRX/mIDH1R132H (NPAI)] and the control wtIDH1 group [shp53/shATRX/wtIDH1 (NPA)] (fig. S1A). Similar to the human data, the mIDH1 tumor–bearing mice have a longer MS compared to the wtIDH1 group [wtIDH1 MS = 21 days postimplantation (dpi) and mIDH1 MS = 33 dpi] (fig. S1B). Using this model, we tested the efficacy of immune stimulatory gene therapy TK/Flt3L that induces a robust antitumor T cell response leading to tumor regression, long-term survival, and immunological memory in glioblastoma models (12, 22) (Fig. 1A). The efficacy of TK/Flt3L in mIDH1 was tested in combination with PMN-MDSC depletion using the αLy6G antibody. The depletion was timed to coincide with the peak of TK/Flt3L-induced T cell response occurring at ~7 days after immune-mediated gene therapy (Fig. 1B) (12, 22, 23). Administration of α Lymphocyte antigen 6 complex locus G6D (Ly6G) antibody as monotherapy to wtIDH1 or mIDH1 glioma–bearing mice did not confer survival benefit (Fig. 1, C and D). However, treatment wtIDH1 tumor–bearing mice with TK/Flt3L significantly prolonged their survival (P < 0.05; Fig. 1C). Unexpectedly, TK/Flt3L gene therapy resulted in a much greater survival benefit in the mIDH1 tumor–bearing animals as compared to those with wtIDH1 tumor (Fig. 1D). Approximately 85% of mIDH1 tumor–bearing animals survived long term when treated with TK/Flt3L gene therapy (Fig. 1D). Combining TK/Flt3L gene therapy with Ly6G depletion confers survival benefit in the wtIDH1 but not in the mIDH1 tumor–bearing mice (P < 0.05; Fig. 1, C and D). We confirmed the tumor eradication in the mIDH1 groups treated with either TK/Flt3L + isotype or TK/Flt3L + αLy6G by hematoxylin and eosin staining, which showed no signs of tumor in the long-term survivors (Fig. 1F). In contrast, the mIDH1 groups treated with saline or saline + αLy6G displayed tumor burden (Fig. 1F). To test whether these mice developed an immunological memory response, we rechallenged the long-term survivors from the TK/Flt3L + isotype and TK/Flt3L + αLy6G groups with mIDH1 mouse neurospheres implanted in the contralateral hemisphere at day 90 after initial tumor implantation (Fig. 1G). These animals remained tumor free without further treatment, whereas control mice implanted with mIDH1 neurospheres succumbed because of tumor burden (Fig. 1H). These results validate the effectiveness of TK/Flt3L against mIDH1 tumors regardless of myeloid cell depletion. We concluded that glioma harboring mIDH1 exhibit an improved response to immunotherapy and that Ly6G depletion does not improve the therapeutic response to TK/Flt3L.
Fig. 1. mIDH1 tumor models have enhanced response to immune-stimulatory gene therapy irrespective of myeloid cell depletion.
(A) Schematic showing the mechanism by which TK/Flt3L gene therapy recruits and activates a tumor-specific T cell response. (B) Schematic illustrating the treatment strategy of the TK + Flt3L gene therapy in combination with Ly6G depletion in wtIDH1 or mIDH1 tumor–bearing mice. WT, wild type. (C and D) Kaplan-Meier survival curves of implanted mice-bearing wtIDH1 or mIDH1 tumors, treated with TK + Flt3L, Ly6G depletion, or combination therapy. dpi, days postimplantation; NS, not significant. (E) Tumor-specific CD8 T cell frequency within the TME of wtIDH1 or mIDH1 tumors treated with TK + Flt3L, Ly6G depletion, or combination therapy was analyzed by staining for SIINFEKL-Kb tetramers. (F) Hematoxylin and eosin staining of brain sections from mIDH1 tumors treated with saline, αLy6G, TK/Flt3L, and TK/Flt3L + αLy6G. (G) Schematic illustrating the treatment strategy of the TK + Flt3L gene therapy in combination with Ly6G depletion in mIDH1 tumor–bearing mice. Most mIDH1 tumor–bearing mice (90%) survived long term in response to TK/Flt3L gene therapy regardless of Ly6G depletion. The long-term survived mice were rechallenged at day 90 after implantation. (H) Kaplan-Meier survival plot for rechallenged long-term survivors from mIDH1 + TK/Flt3L + isotype (N = 4), mIDH1 + TK/Flt3L + Ly6G dep (N = 5), or control (mIDH1 + untreated) (N = 5). Data were analyzed using the log-rank (Mantel-Cox) test. Scale bars, 1 mm. **P < 0.01 and ***P < 0.005, One-way analysis of variance (ANOVA).
Depletion of myeloid cells in combination with immunotherapy enhances antitumor CD8 T cell response in wtIDH1 but not in mIDH1 glioma
To provide a mechanistic basis for the different responses to immunotherapy observed in our glioma models, we examined the effect of PMN-MDSC depletion in combination with immune-stimulatory TK/Flt3L gene therapy in wtIDH1 and mIDH1 model harboring the surrogate tumor antigen, ovalbumin (OVA). We sought to examine the impact of treatment on the quantity and quality of the antitumor T cell response (fig. S1C). Using OVA expressing wtIDH1 or mIDH1 neurospheres, we were able to quantify tumor-specific CD8+ T cells by making use of the SIINFEKL-H2Kb expressing CD8+ T cells. TK/Flt3L treatment increased the frequency of total CD8+ T cells infiltrating both wtIDH1 and mIDH1 glioma, with no differences between isotype or Ly6G depletion (fig. S1, D and E). Combining TK/Flt3L with Ly6G depletion increased the frequency of tumor-specific CD8+ T cells in the TME (~1.6-fold) compared to the TK/Flt3L + isotype (P < 0.05) in the wtIDH1 group but not in the mIDH1 group (Fig. 1E). The mIDH1 tumor–bearing mice showed more than twofold increase in the percentage of tumor-specific T cells in response to the gene therapy (TK/Flt3L) with no difference between the Ly6G depletion and control (Fig. 1E).
To test the impact of PMN-MDSCs on the cytotoxicity of CD8+ T cells in the TME, we investigated the granzyme B (GzB) expression in CD8+ T cells. TK/Flt3L gene therapy resulted in a more than twofold increase in the frequency of GzB+ CD8+ T cells compared to saline-treated animals (fig. S1, F and G). This was further enhanced in the presence of Ly6G depletion in wtIDH1 but not in the mIDH1 group (fourfold, P < 0.05; fig. S1, F and G). Overall, these data suggest that mIDH1 tumors have an enhanced response to immunotherapies regardless of Ly6G depletion, suggesting an altered functional property of the PMN-MDSCs infiltrating the mIDH1 glioma.
mIDH1 glioma in the context of ATRX and TP53 inactivation causes expansion of the granulocytic myeloid cells
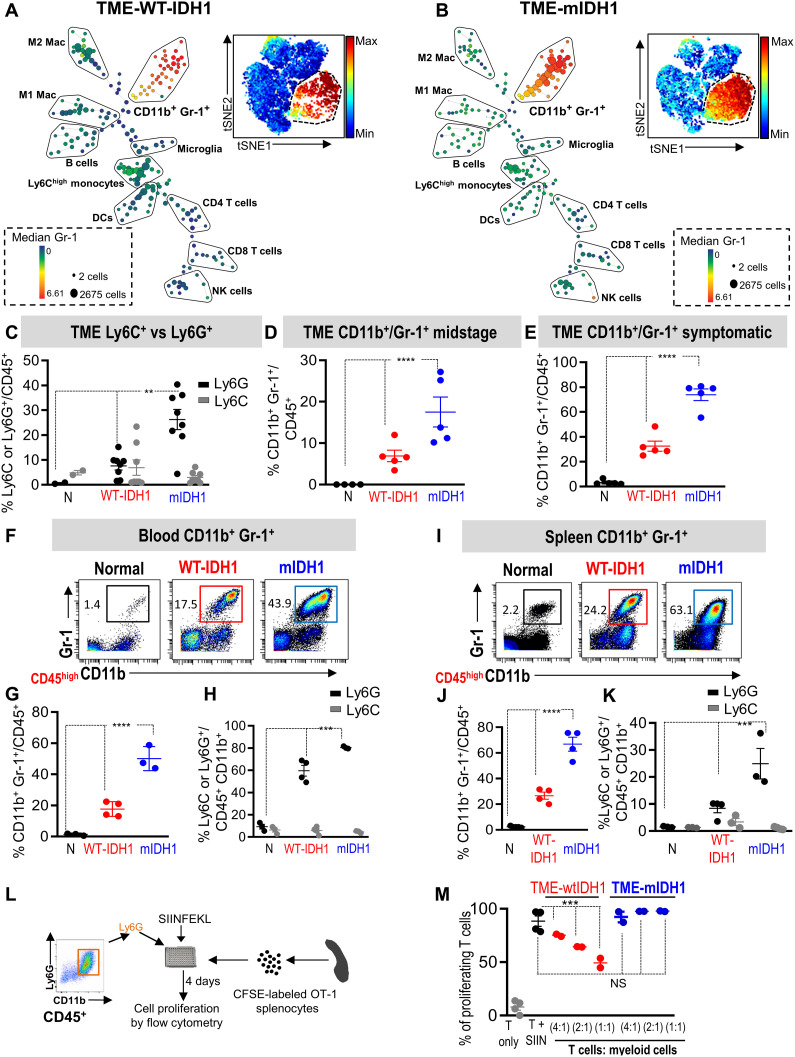
To study the impact of mIDH1 on the phenotype of myeloid cells, we used the merit of mass cytometry by time of flight (CyTOF) (table S1), which measures 37 parameters simultaneously, to analyze immune cell composition within the TME. Using Spanning-tree progression analysis for density-normalized events (SPADE) analysis (24), we found that the glioma TME contains several immune cell types including macrophages, DCs, B cells, natural killer (NK) cells, CD8+ T cells, and CD4+ T cells (Fig. 2, A and B). There was an expansion of cells (depicted by the large red/yellow nodes) characteristic for MDSCs (CD45high/CD11b+/Gr-1+) in the TME of mIDH1 glioma compared to wtIDH1 glioma (Fig. 2, A and B). Similarly, visual inspection of viSNE plots, which represent high-dimensional CyTOF output in two dimensions (25), showed a higher percentage of Gr-1+ cells in the TME from mIDH1 glioma compared to those from wtIDH1 glioma (Fig. 2, A and B, insets).We confirmed that this difference is statistically significant using a χ2 test (P < 0.0001). The Gr-1 antibody can detect both the granulocytic (CD45high/CD11b+/Ly6G+/Ly6Clow) and monocytic (CD45high/CD11b+/Ly6G−/Ly6Chigh) MDSC populations. We found that most of the expanded myeloid cell populations in the mIDH1 tumor belonged to the granulocytic population, with no changes in the frequency of the monocytic population (Fig. 2C). To further validate the abundance of the Ly6G population in the TME of the mIDH1 tumor, we used the CITRUS algorithm (cluster identification, characterization, and regression) to identify abundant populations in wtIDH1 versus mIDH1 tumors (26). The relative frequencies of these clustered populations were compared between the two groups. Results showed that two major node clusters were different between the TME from wtIDH1 versus the TME from mIDH1 (fig. S2A; red nodes). The first population expresses mainly Ly6G, which was abundant in the mIDH1 group (clusters 1 to 9) (fig. S2, B to K). In contrast, the TME from wtIDH1 tumors has higher abundance of macrophage clusters (clusters 12, 27, and 17 to 25) (fig. S3, A to M). These results showed that the mIDH1 tumors have a higher frequency of granulocytic myeloid cells but a lower frequency of macrophages.
Fig. 2. mIDH1 glioma models have high expansion of granulocytic myeloid cell population (CD45high/CD11b+/Ly6G+).
(A and B) SPADE analysis of mass cytometry (CyTOF) data represents immune cell composition within the TME of SB-induced wtIDH1 (A) or mIDH1 (B) tumors. Insets are viSNE visualizations of high-dimensional mass cytometry data; color intensity represents the Gr-1 expression level. (C) Phenotypic characterization of CD45high/CD11b+/Gr-1+ population as granulocytic or monocytic based on the expression of Ly6G or Ly6C at symptomatic stage. Most of the CD45high/CD11b+/Gr-1+ in the mIDH1 glioma TME are CD45high/CD11b+/Ly6G+ (granulocytic) cells. (D and E) Flow cytometry analysis of the frequency of MDSCs (CD45high/CD11b+/Gr-1+) within the TME at the midstage of tumor implantation (D) and at the symptomatic stage (E). The percentage of CD45high/CD11b+/Gr-1+ cells was higher in mIDH1 tumors compared to wtIDH1 tumors. (F and G) CyTOF analysis of CD45high/CD11b+/Gr-1+ cells in blood from mice with no tumor (normal) and SB-induced wtIDH1 or mIDH1 glioma. (H) Phenotypic characterization of circulating CD45high/CD11b+/Gr-1+ cells according to expression of Ly6C or Ly6G. (I and J) CyTOF analysis of CD45high/CD11b+/Gr-1+ cells from the spleens of mice with no tumor and SB-induced wtIDH1 or mIDH1 glioma. (K) Phenotypic characterization of splenic CD45high/CD11b+/Gr-1+ cells according to expression of Ly6C or Ly6G. (L) Schematic figure of the in vitro T cell proliferation assay. (M) Flow analysis of the inhibitory potential of CD45high/CD11b+/Ly6G+ cells from TME of wtIDH1 or mIDH1 tumor. N = normal, **P < 0.01, ***P < 0.005, and ****P < 0.0001, ANOVA.
In addition to the increase in granulocytes, mIDH1 tumors showed a decreased frequency of DCs (~4-fold) with no significant change in the frequency of lymphocytes (including NK cells, B cells, and CD8+ and CD4+ T cells) as compared to wtIDH1 tumors (fig. S4, A to H). Notably, there was no significant difference in the percentage of CD45+ cells between wtIDH1 and mIDH1 glioma (fig. S4H). To test whether the increase in myeloid cells is associated with the tumor growth, we analyzed the frequency of CD45high/CD11b+/Gr-1+ population at the midstage (12 days after implantation) and at the symptomatic stage of tumor development (Fig. 2, D and E). In both time points, we found that the mIDH1 tumor group had an increased frequency of CD45high/CD11b+/Gr-1+ cells compared to the wtIDH1 group (Fig. 2, D and E).
The expansion of CD45high/CD11b+/Ly6G+ cells was not limited to the tumor site; in both the circulation and the spleen of mIDH1 tumor–bearing animals, there was a higher number and percentage of CD45high/CD11b+/Ly6G+ (Fig. 2, F to K, and figs. S5 and S6). Moreover, in both the circulation and spleen from tumor-bearing animals, there was no significant difference in the M-MDSCs between wtIDH1 and mIDH1 glioma–bearing mice (figs. S5 and S6). However, mIDH1 exhibited a decreased number of macrophages in the circulation and spleen, with no differences in the DCs or lymphocytes (figs. S5 and S6).
We validated our findings using two additional glioma mouse models. First, we used the glioma model induced by Replication-Competent Avian leukemia virus Splice acceptor expressing platelet-derived growth factor β (PDGFB), shTP53, and mIDH1 or wtIDH1 in NTva_Ink4a/Arf−/− mice (19, 27). Neurospheres cultured from these tumors were engineered to encode shATRX, as described in Materials and Methods, to generate glioma cells with the following molecular alterations: PDGFB/shTP53/shATRX/Ink4a/Arf−/−/mIDH1 or PDGFB/shTP53/shATRX/Ink4a/Arf−/−/wtIDH1 (19). Second, we used the SB-generated mouse model that harbors the following genetic lesions: CDKN2A−/−/shP53/shATRX/wtIDH1 (CPA) or CDKN2A−/−/shP53/shATRX/mIDH1 (CPAI). In both models, we confirmed that the mIDH1 tumor–bearing animals exhibit an expanded CD45high/CD11b+/Gr-1+ population with a high predominance of the CD45high/CD11b+/Ly6G+ population (fig. S7). Collectively, these results suggest that the mIDH1 tumor causes activation of pathways favoring granulocytic myeloid cells’ expansion.
Granulocytic myeloid cells infiltrating mIDH1 glioma are not immunosuppressive
Immunosuppressive CD45high/CD11b+/Ly6G+ cells are characterized by their ability to suppress T cell functions. Therefore, we examined the influence of tumor-infiltrating CD45high/CD11b+/Ly6G+ myeloid cells on antigen-specific T cell proliferation. We isolated granulocytes by flow cytometry from gliomas expressing wtIDH1 or mIDH1 and cocultured them with carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled splenocytes from OT-1 mice (Fig. 2L). Isolated splenocytes were stimulated with the cognate OVA peptide, SIINFEKL (SIIN), to induce OT-1–specific T cell proliferation (Fig. 2L). In response to SIIN, T cell proliferation spiked to ~90% in the control condition (T + SIIN) (Fig. 2M). When CD45high/CD11b+/Ly6G+ myeloid cells from the TME of wtIDH1 tumors were added to the culture at different ratios (T cells:myeloid cells; 1:1, 2:1, and 4:1), the percentage of proliferating T cells was reduced to ~48, 64, and 75%, respectively (Fig. 2M). Unexpectedly, CD45high/CD11b+/Ly6G+ cells isolated from the TME of mIDH1 gliomas failed to suppress antigen-specific T cell proliferation at any coculturing ratio (Fig. 2M). The effect of mIDH1 on the function of tumor-infiltrating CD45high/CD11b+/Ly6G+ cells was validated using the two additional mouse models described above. In both models, we found that CD45high/CD11b+/Ly6G+ cells isolated from the mIDH1 TME do not inhibit T cell proliferation (fig. S8). Notably, granulocytes isolated from spleens of wtIDH1 or mIDH1 tumor–bearing mice failed to inhibit antigen-specific T cell proliferation (fig. S9). Together, these results suggest that the expanded granulocytes in mIDH1 TME are not inhibitory. To further investigate the effect of granulocytes on T cell activation, we isolated T cells from the spleen of tumor-free mice and incubated them with beads loaded with anti-CD3 and anti-CD28 (Dynabeads) (fig. S10A). Tumor-infiltrating CD11b+/Ly6G+ myeloid cells isolated from wtIDH1 or mIDH1 tumors were added to the culture, and T cell activation was analyzed by CD69 expression (fig. S10A). Results showed that granulocytes from wtIDH1 markedly decreased CD69 expression in a ratio-dependent manner, whereas the granulocytes from mIDH1 did not change CD69 expression in CD8+ T cells at any coculturing ratio (fig. S10, B to E).
To investigate whether a tumor-derived factor is responsible for remodeling granulocytes in mIDH1, we performed an ex vivo MDSC differentiation assay. We isolated bone marrow (BM) cells from naïve mice and cultured them in media containing 10% fetal bovine serum (FBS), with wtIDH1 neurospheres or with mIDH1 neurospheres (2:1, BM cells:neurospheres) (fig. S11A). After 7 days, we analyzed the frequency of granulocytes formed in each condition (fig. S11A). Consistent with the in vivo data, there was a higher percentage of CD45high/CD11b+/Ly6G+ cells expressing lower levels of Arg1, PD-L1, and CD80 in the mIDH1 coculture condition (fig. S11, B to E). In contrast to granulocytes generated from wtIDH1 coculture, CD45high/CD11b+/Ly6G+ cells generated from mIDH1 culture failed to inhibit antigen-specific T cell proliferation in vitro (fig. S11, F and G). Collectively, these data validate that a tumor-derived factor is responsible for developing the nonimmunosuppressive myeloid cells in mIDH1 glioma.
mIDH1 glioma development results in profound remodeling of BM hematopoiesis
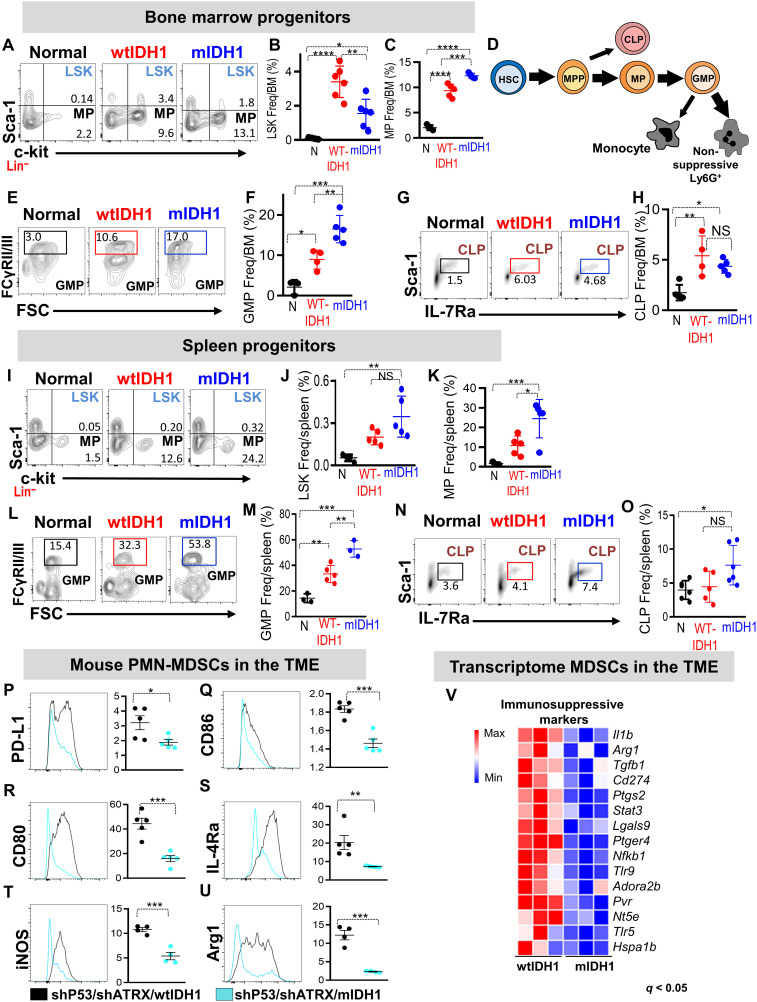
The expansion of the granulocytic myeloid cells within the TME, blood, and spleen suggested that mIDH1 tumor growth induces profound alterations in the BM hematopoiesis. Therefore, we examined the changes in hematopoiesis within the BM and spleen of the SB-induced murine glioma model. We found that within the lin− population, the frequency of LSK (Lin−/c-Kit+/Sca-1+) was decreased in the BM from mIDH1 tumor–bearing mice (2.2-fold, P < 0.05; Fig. 3, A and B). In contrast, BM from mIDH1 tumors contained higher frequencies of myeloid progenitors (MPs; %MP ~13%, P < 0.05;Fig. 3, A and C). In the spleen, there were no differences in the frequency of LSK between the wtIDH1 and mIDH1 tumor–bearing animals (Fig. 3, I and J). However, spleens from mIDH1 tumor–bearing animals showed more than twofold increase in MPs compared to wtIDH1 tumors (P < 0.05; Fig. 3, I and K). There was also a higher frequency (twofold) of granulocyte-macrophage precursors (GMPs) in both the BM and spleen of mIDH1 tumor–bearing animals (P < 0.05, Fig. 3, E, F, L, and M). Despite the increase in MPs and GMPs, there was no significant change in the frequency of common lymphocyte progenitors (CLPs) between the two groups (Fig. 3, G, H, N, and O). The enhanced production of granulocytes, MPs, and GMPs in BM and spleen indicates the activation of the granulocytic myeloid differentiation pathway in mIDH1 tumor–bearing animals (Fig. 3D).
Fig. 3. Phenotypic and molecular characterization of myeloid cell lineages in mIDH1 glioma.
(A to C) Representative flow cytometry plots and quantification of the percentage of (B) LSK (Lin−/c-Kit+/Sca-1+) and (C) MP (Lin−/c-Kit+/Sca-1−) in BM from normal mice (N), and mice implanted with wtIDH1 or mIDH1 neurospheres. (D) Schematic diagram representing the shift in myelopoeisis within mIDH1 tumor–bearing mice. Thick arrows represent predominant developmental pathways in mIDH1 tumor–bearing mice. (E to H) Flow cytometry analysis of the frequency of (E and F) GMPs (Lin−/IL-7Rα−/c-Kit+/Sca-1−/CD34+/FcyRII/IIIhigh) and (G and H) CLPs (c-Kitlow/Sca-1low/Lin−/IL-7Rα+) in the BM from normal and wtIDH1 or mIDH1 tumor–bearing mice. (I to K) Representative flow cytometry plots and quantification of the percentage of (J) LSK and (K) MPs in spleen from normal and wtIDH1 or mIDH1 tumor–bearing mice. (L and M) Representative flow cytometry plot and quantitation analysis showing the frequency of common GMPs in spleens from normal mice and wtIDH1 and mIDH1 tumor–bearing animals. (N and O) Representative flow cytometry plot and quantitation analysis showing the frequency of CLPs in spleens from normal mice, wtIDH1 and mIDH1 tumor–bearing mice. (P to U) Flow cytometry analysis of immunosuppressive/costimulatory markers in the CD45high/CD11b+/Ly6G+ population within TME of wtIDH1 (black) or mIDH1 (blue). (V) Heatmap showing the normalized expression of genes related to PMN-MDSC immunosuppressive signature in myeloid cells (CD11b+/ Gr-1+) from wtIDH1 or mIDH1 TME. *P < 0.05, **P < 0.01, ***P < 0.005, and ****P < 0.0001, one-way ANOVA.
Molecular and phenotypic characterization of mIDH1 tumor–infiltrating granulocytes
We next investigated the molecular characteristics of the expanded CD45high/CD11b+/Ly6G+ cells in mIDH1 tumors. First, we examined the surface expression of T cell–suppressive/costimulatory molecules commonly expressed by PMN-MDSCs. In the CD45high/CD11b+/Ly6G+ cells from mIDH1 TME, the expression level of immunosuppressive and costimulatory molecules, such as PD-L1, CD86, CD80, interleukin-4Rα (IL-4Rα), inducible nitric oxide synthase, and arginase 1, was lower compared to these within wtIDH1 TME (Fig. 3, P to U). To characterize the molecular differences between mIDH1 and wtIDH1 tumor–infiltrating myeloid cells, we purified CD45high/CD11b+/Gr-1+ cells from tumor-bearing animals and performed transcriptome analysis. Compared to wtIDH1, myeloid cells isolated from mIDH1 tumors have decreased expression of all immunosuppressive genes associated with the PMN-MDSC signature, such as Il1β, Arg1, Tgfb1, CD274, and Stat3 (Fig. 3V).
Single-cell transcriptome analysis reveals that neutrophils and preneutrophils are the major granulocytic lineages in mIDH1 TME
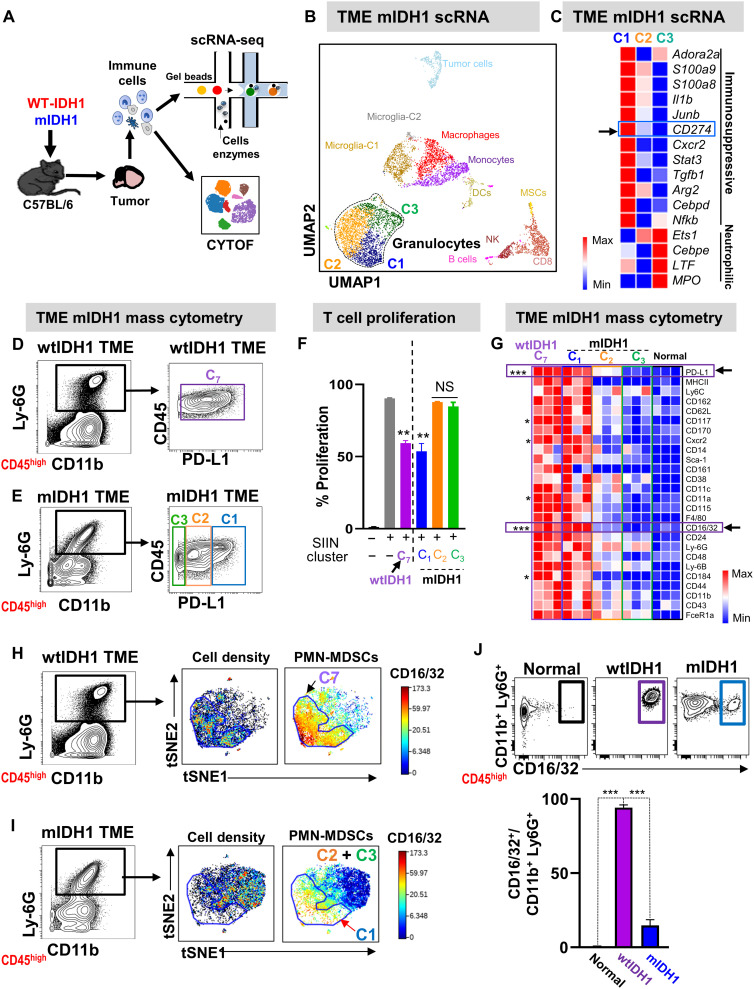
To compare the molecular differences of TME-derived granulocytes on an individual cell basis, we used single-cell RNA sequencing (scRNA-seq) coupled with mass cytometry (Fig. 4A). Two biological replicates of purified immune cells from TME of mIDH1 mice (8881 cells) and TME of wtIDH1 mice (7408 cells) were sequenced at an average depth of ~20,000 reads per cell. After filtering cells with low overall expression and/or high mitochondrial gene expression, we performed dimensionality reduction and unsupervised cell clustering, using methods implemented in the Seurat software suite, independently for mIDH1 and wtIDH1 datasets to identify distinct cell populations (28) (fig. S12, A and B). In mIDH1 tumors, we identified 14 distinct cell clusters expressing known markers of major immune cell types (Fig. 4B). Granulocytes formed the largest population and consisted of three clusters, which we refer to as C1, C2, and C3 (Fig. 4B and fig. S12, B and D). C1 displayed high expression of PMN-MDSC–related genes such as Il1b and Arg2, two major immunosuppressive factors previously used to define PMN-MDSCs in cancer models (29) (Fig. 4C and fig. S12, B and D). C2 constitutes the largest granulocytic cluster and expressed high levels of S100a8, inflammatory cytokines involved in the recruitment of CD8+ T cells (Ccl3 and Ccl4), and cell cycle genes such as Cdc20 and G0s2 (fig. S12D) (30). C3 expressed genes involved in neutrophil maturation (Cebpe and Mpo; Fig. 4C and fig. S12D). These results suggest that C1 belongs to the immunosuppressive PMN-MDSC population within the TME of mIDH1 tumors, whereas C2 and C3 correspond to preneutrophils and neutrophils, respectively. In wtIDH1, there was only one cluster of granulocytes (C7; fig. S12, A and C). This cluster expressed PMN-MDSC–related genes such as Il1b and Arg2, similar to cluster C1 in TME-mIDH1 (Fig. 4C and fig. S12, A and C).
Fig. 4. Nonsuppressive neutrophils and preneutrophils are the major granulocyte population in mIDH1 tumors.
(A) Schematic overview of single-cell sequencing and CyTOF analysis of immune cells infiltrating wtIDH1 and mIDH1 tumors. (B) Combined Seurat analysis of immune cells from mIDH1 shown in Uniform Manifold Approximation and Projection (UMAP) projection results in various distinct clusters of immune cells (N = 2). (C) Heatmap of differentially expressed immunosuppressive and neutrophil-related genes between the three granulocytic clusters in mIDH1 TME. (D and E) Mass cytometry analysis of granulocytes from TME of (D) wtIDH1 or (E) mIDH1 tumors. (F) T cell proliferation analysis to assess the inhibitory potential of myeloid cells cluster (C7) from wtIDH1 tumors and C1, C2, and C3 myeloid cell clusters isolated from mIDH1 tumors. (G) Heatmap representing normalized expression level of myeloid biomarkers within granulocytes from the TME of normal mice, mice implanted with wtIDH1 tumors, and C1, C2, and C3 clusters from the TME of mIDH1 tumors. (H and I) Unsupervised clustering and viSNE visualization of granulocytes from wtIDH1 (H) or mIDH1 (I) tumors using the biomarker shown in (G). (J) Flow plots and quantitation analysis of the proportion of bona fide PMN-MDSCs (CD45high/CD11b+/Ly6G+/CD16/32+) infiltrating normal mice and wtIDH1 or mIDH1 tumors. *P < 0.05, **P < 0.01, ***P < 0.005, ANOVA.
To confirm that granulocytes in wtIDH1 tumor (C7) are similar to C1 granulocytes in the mIDH1 tumor, we integrated the wtIDH1 and mIDH1 tumor–infiltrating immune cell datasets using functionality available in the Seurat package (31). Jointly clustering wtIDH1 and mIDH1 tumor–infiltrating immune cells showed that the granulocytic population formed three clusters corresponding to the C1, C2, and C3 clusters found in the TME of mIDH1 tumors (fig. S13, A to C). Most of the granulocytes from the TME of the wtIDH1 tumor clustered with the C1 cluster of granulocytes from mIDH1 tumors (fig. S13, B to D), suggesting that these cells share a common origin and have a similar function.
To further determine whether C1 represents the immunosuppressive PMN-MDSCs within TME-mIDH1, we looked for a cell surface marker differentially expressed in C1 that would allow us to isolate cells belonging to this cluster and assess their function. Among the top 50 differentially expressed genes in cluster C1, CD274 (PD-L1) was the only cell surface marker that showed high expression in C1 compared to moderate and low expression in C2 and C3, respectively (Fig. 4C and table S4). On the basis of CD274 expression, we reclassified the granulocytic populations of both TMEs. We found that there was one granulocyte population in the wtIDH1 tumors and three distinct granulocytic populations in the mIDH1 tumors, corresponding to C1, C2, and C3 (Fig. 4, D and E). We FACS (fluorescence-activated cell sorting)–sorted each population based on the expression of PD-L1 (see fig. S14 for gating strategy) and determined their inhibitory potential by performing a T cell proliferation assay. Similar to the results obtained with granulocytes from wtIDH1 TME (C7 cluster), coculturing with C1 cells from mIDH1 glioma resulted in ~40% inhibition of T cell proliferation (P < 0.05), whereas C2 and C3 from mIDH1 TME did not inhibit T cell proliferation (Fig. 4F). These results validate the inhibitory potential of C1 as the immunosuppressive PMN-MDSCs cluster in mIDH1 glioma TME.
CD16/32 is a specific marker that defines bona fide immunosuppressive PMN-MDSCs
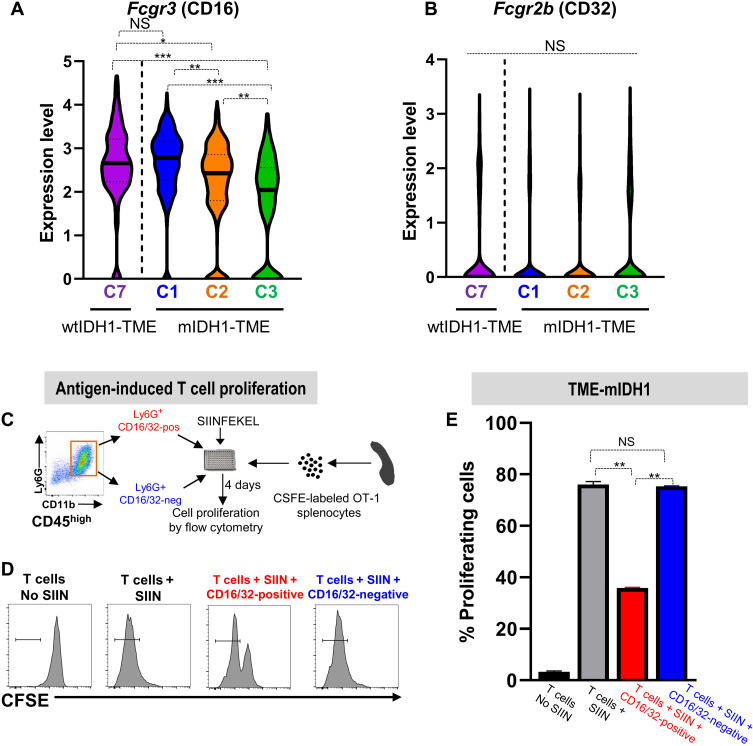
Our next goal was to identify a specific myeloid marker that would allow us to distinguish the inhibitory PMN-MDSCs from the noninhibitory granulocytes. To identify a unique marker, we used a myeloid-specific antibody panel (table S1) that measures ~30 myeloid-specific parameters simultaneously and used it to perform CyTOF analysis of the granulocytic clusters found in the mIDH1 and wtIDH1 glioma TME. We found that granulocytes from wtIDH1 and the C1 cluster from mIDH1 TME have similar expression of most of the myeloid biomarkers, with the FC-gamma receptor family (CD16/32) up-regulated in all inhibitory PMN-MDSCs (Fig. 4G). CD16/32 is expressed on macrophages and granulocytes, and its expression is correlated with maturation and function (32, 33). To confirm the correlation between PMN-MDSC phenotype and CD16/32 expression, we performed unsupervised clustering and dimensionality reduction that showed that granulocytes from mIDH1 TME clustered differently than granulocytes from the wtIDH1 TME (Fig. 4, H and I). We observed high CD16/32 expression exclusively in all granulocytes from wtIDH1 and only in C1 from mIDH1 tumors (Fig. 4, H and I). The majority of granulocytes from wtIDH1 TME (~95%) express high levels of CD16/32, compared to ~12% of granulocytes in mIDH1 glioma TME (P < 0.05, Fig. 4J). To investigate which gene (CD16 or CD32) is more predominantly expressed by immunosuppressive PMN-MDSCs, we analyzed the difference in expression at the transcriptome level between Fcgr3 (gene encodes for CD16) and Fcgr2b (gene encodes for CD32). Transcriptome analysis showed that Fcgr3 was the highest in both C7 from wtIDH1 TME and C1 from mIDH1 TME (PMN-MDSCs) compared to levels in C2 and C3 mIDH1 TME (Fig. 5A). In contrast, Fcgr2b was not differentially expressed between any cluster (Fig. 5B).
Fig. 5. CD16/32 is a specific marker that defines bona fide immunosuppressive PMN-MDSCs.
(A and B) Violin plot showing the expression of the Fcgr3 (A) or Fcgr2b (B) gene in each granulocytic cluster infiltrating the wtIDH1 tumor (C7) or mIDH1 tumors (C1, C2, and C3). Fcgr3, but not Fcgr2b, is expressed at high level in both immunosuppressive granulocytic MDSCs clusters (C7 and C1). (C) Schematic of the in vitro T cell proliferation assay to analyze immune suppressive properties of CD45high/CD11b+/Ly6G+/CD16/32−/+. Sorted CD45high/CD11b+/Ly6G+/CD16/32-positive or CD45high/CD11b+/Ly6G+/CD16/32-negative cells were cocultured with CFSE-labeled splenocytes from Rag2/OT-1 transgenic mouse. Cultures were stimulated with 100 nM SIINFEKL peptide for 4 days, after which proliferation was analyzed by flow cytometry. (D) Representative flow plots showing CFSE staining of unstimulated splenocytes (T only), splenocytes undergoing rapid proliferation in response to SIINFEKL (T + SIIN), and the effect of SIINFEKL-induced T cell proliferation in the presence of CD45high/CD11b+/Ly6G+/CD16/32-negative or CD45high/CD11b+/Ly6G+/CD16/32-positive cells from the TME of mIDH1 tumors. (E) Quantitation analysis of the inhibitory potential of CD45high/CD11b+/Ly6G+/CD16/32-negative or CD45high/CD11b+/Ly6G+/CD16/32-positive cells sorted from TME of mIDH1 tumor. CD45high/CD11b+/Ly6G+/CD16/32-positive cells inhibit T cell proliferation, whereas CD45high/CD11b+/Ly6G+/CD16/32-negative cells did not suppress T cell proliferation. *P < 0.05, **P < 0.01, and ***P < 0.005, one-way ANOVA.
To further validate our finding, we examined the suppressive capacity of tumor-infiltrating CD45high/CD11b+/Ly6G+/CD16/32+ or CD45high/CD11b+/Ly6G+/CD16/32− myeloid cells on antigen-specific T cell proliferation. Isolated granulocytes were cocultured with CFSE-labeled splenocytes from OT-1 mice as described before (Fig. 5C). Results showed that only CD45high/CD11b+/Ly6G+/CD16/32+ myeloid cells from the TME of mIDH1 tumors blocked T cell proliferation ~36 ± 3.2% (P < 0.001; Fig. 5, D and E). Together, these results establish CD16/32 as a robust and generalizable cell surface marker for immunosuppressive PMN-MDSCs.
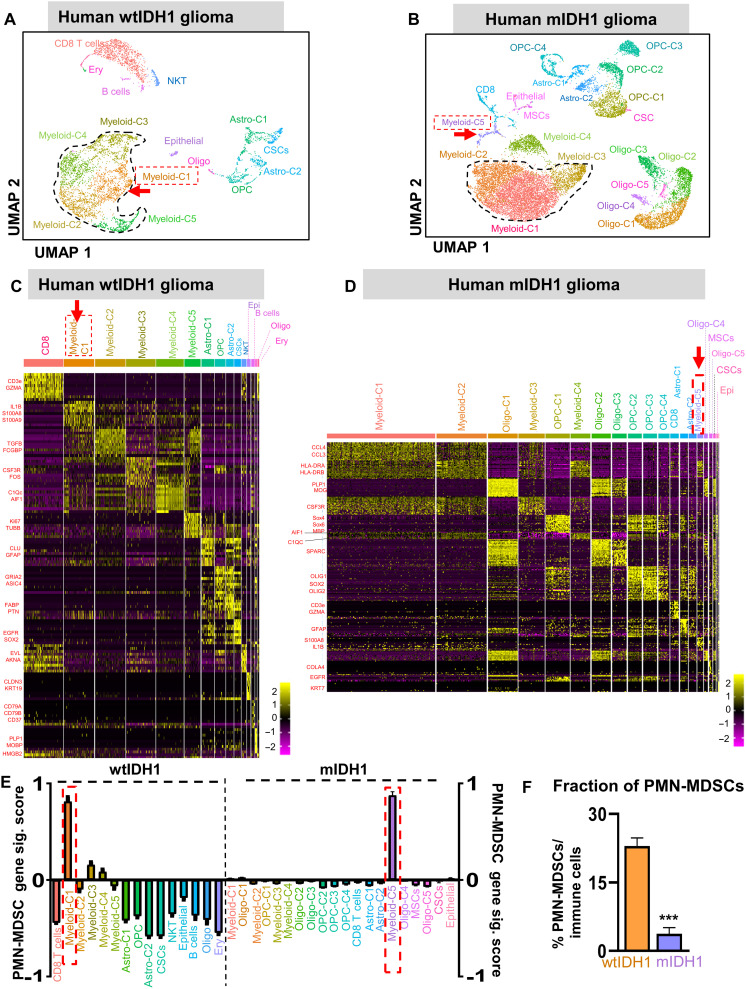
PMN-MDSC gene signature is expressed in a higher proportion of tumor-infiltrating immune cells in human wtIDH1 glioma
We then performed scRNA-seq of immune cells isolated from primary tumor samples of patients with wtIDH1 and mIDH1 glioma. We collected 18 patients’ glioma samples (8 samples from wtIDH1 tumors and 10 samples from mIDH1 tumors). All patients’ clinical data are listed in table S5. After performing immune cell purification and applying quality controls, a total of 9765 cells from wtIDH1 tumors and 17,452 from mIDH1 tumors were analyzed. wtIDH1 and mIDH1 datasets were analyzed separately using Seurat to reproduce cell-type labels (Fig. 6, A and D). Myeloid clusters were the most abundant immune cells infiltrating both the wtIDH1 and mIDH1 human gliomas (Fig. 6, A to D). In both groups, there was a total of five clusters of myeloid cells (myeloid C1 to C5) (Fig. 6, A to D); only one cluster (C4) expressed high microglial genes (C1Qc and AIF1) (Fig. 6, C and D). Similar to the mouse data, the largest cluster in mIDH1 (myeloid-C1) differentially expressed CCL3 and CCL4 (Fig. 6, B and D). Moreover, myeloid-C2 expressed antigen presentation-associated genes (HLA-A and HLA-DRA), whereas myeloid-C3 expressed granulocyte differentiation genes (CSF3R and NEAT1; Fig. 6D). We then investigated the expression of the PMN-MDSC gene signature (IL1β, S100a8, S100a9, ARG1, and TGFβ1) within each cluster in wtIDH1 and mIDH1 myeloid cells, separately. The highest PMN-MDSC gene signature was expressed by cells in myeloid-C1 and myeloid-C5 in wtIDH1 and mIDH1 tumors, respectively (Fig. 6E). Upon quantification, nearly 23% of all immune cells in wtIDH1 tumors were PMN-MDSCs, whereas, in the mIDH1 glioma TME, PMN-MDSCs were only 3.75% of the total immune cells (Fig. 6F). These results are consistent with data from our preclinical model that showed that PMN-MDSCs are present at a higher fraction in the TME of wtIDH1 glioma than in the TME of mIDH1 glioma.
Fig. 6. PMN-MDSC gene signature is expressed in a higher proportion of tumor-infiltrating immune cells in human wtIDH1 glioma.
Seurat analysis of immune cells from (A) wtIDH1 or (B) mIDH1 primary tumor samples, shown in UMAP projection, results in various distinct clusters. (C and D) Heatmaps showing the differentially expressed genes in each cluster of (C) wtIDH1 tumor and (D) mIDH1 tumor samples. (E) Bar plots showing the relative PMN-MDSC gene signature score in each cluster of primary human samples. (F) Quantification of the tumor-infiltrating PMN-MDSCs/total immune cells calculated from scRNA-seq data. ***P < 0.001, Student’s t test.
G-CSF is the major cytokine that is epigenetically regulated in mIDH1 glioma
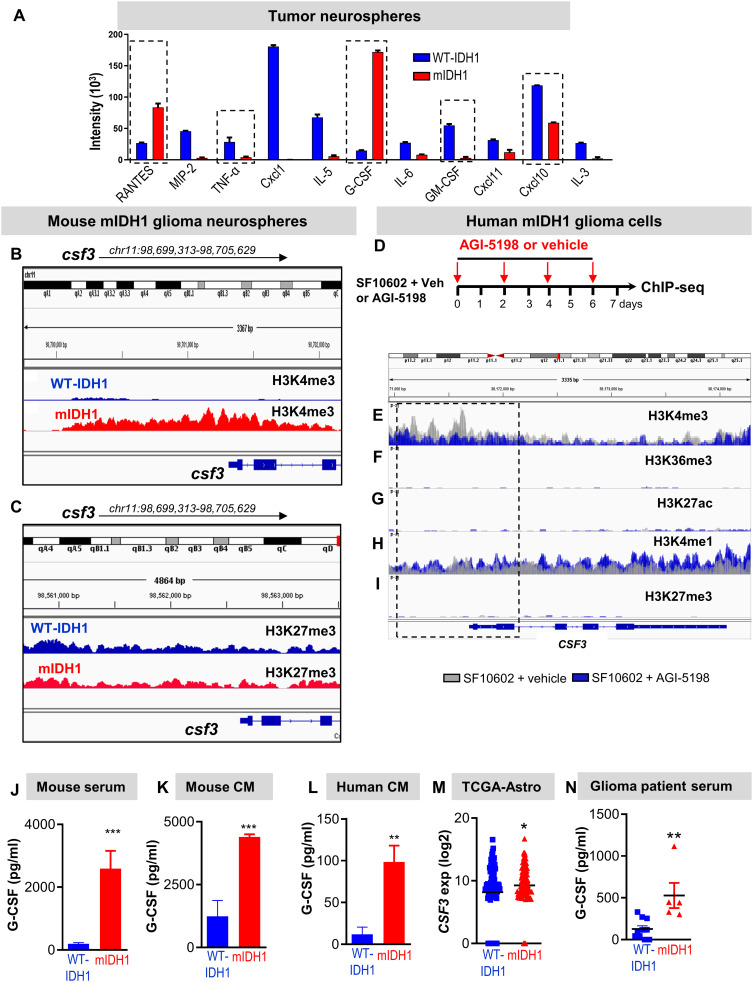
Our data demonstrate that most of the granulocytes found in mIDH1 TME are noninhibitory granulocytes. We hypothesized that the change in granulocytes’ phenotype and function is due to epigenetic reprogramming, which affects cytokine expression in the mIDH1 glioma cells. Therefore, we profiled cytokines known to influence myeloid differentiation in conditioned media collected from wtIDH1 or mIDH1 cultured mouse glioma neurospheres. GM-CSF, CXCL1, CXCL10, IL-5, macrophage inflammatory protein 2, IL-6, and tumor necrosis factor–α were among the downregulated cytokines in mIDH1 conditioned media (Fig. 7A and fig. S15). However, G-CSF, Regulated on activation, normal T cell expressed and secreted (RANTES) (CCL5), IL-33, and Stem Cell Factor (SCF) were the only cytokines that were up-regulated in mIDH1-conditioned media (fig. S15). To investigate whether these cytokines were epigenetically regulated by mIDH1, we used a chromatin immunoprecipitation sequencing (ChIP-seq) dataset that we have recently reported [National Center for Biotechnology Information (NCBI) Omnibus identifier: GSE99806], which was obtained using SB-generated neurospheres with mIDH1 and wtIDH1 (19). Gene promoters enriched for H3K4me3 peaks are generally associated with transcriptional activation. Among the genes encoding the cytokines that were up-regulated in conditioned media from mIDH1, only Csf3 showed a marked change in the peak enrichment for H3K4me3 mark around the promoter region (Fig. 7, B and C). To uncover the epigenetic mechanisms of CSF3 regulation in mIDH1 glioma, we performed ChIP-seq analysis on SF10602 (patient-derived glioma cells expressing mIDH1, inactivating mutations in ATRX and TP53) after treatment with vehicle or 5 μM of the mIDH1 inhibitor (AG1-5198) for 7 days (Fig. 7D). At the CSF3 locus, we observed a down-regulation of H3K4me3 deposition upstream of the transcriptional start site at the gene promoter region in SF10602 treated with mIDH1 inhibitor (AGI-5198, blue) compared to untreated SF10602 (gray) (Fig.7, E to I). There was no significant deposition of H3K6me3, H3K27ac, and H3K27me3 in both groups (Fig. 7, F, G, and I). Moreover, there was no difference in the H3K4me1 deposition around the CSF3 promoter region between SF10602 treated with vehicle versus AGI-5198 (Fig. 7H). These results suggest that mIDH1 is responsible for the epigenetic regulation of the CSF3 expression.
Fig. 7. G-CSF is a major epigenetically regulated cytokine expressed by glioma stem-like cells.
(A) Cytokine array analysis performed on conditioned media from wtIDH1 and mIDH1 neurospheres. (B) H3K4me3 and (C) H3K27me3 occupancy at specific genomic regions of Csf3 gene. (D) Experimental design for ChIP-seq analysis of mIDH1 patient-derived neurospheres (SF10602) treated with vehicle or the mIDH1 inhibitor (AGI-5198). (E to I) Histone marks occupancy at specific genomic regions of CSF3 gene in SF10602 treated with mIDH1 inhibitor (AGI-5198, blue) compared to untreated cells (gray). (J to L) Quantitative ELISA of the G-CSF level in mouse serum of tumor-bearing animals (J), conditioned media from cultured mouse neurospheres (K), and conditioned media from cultured human neurospheres (L) expressing wtIDH1 or mIDH1. (M) Analysis of TCGA data for CSF3 gene expression in mIDH1 glioma patients harboring TP53 and ATRX mutation (n = 99) and wtIDH1 (n = 82). (N) Quantitative ELISA of serum G-CSF from glioma patients with wtIDH1 or mIDH1 (astrocytoma). *P < 0.05, **P < 0.01, and ***P < 0.005, Student’s t test.
To demonstrate the increase in G-CSF in mIDH1 glioma, we performed quantitative enzyme-linked immunosorbent assay (ELISA) in the mouse serum of tumor-bearing animals, as well as in conditioned media from cultured mouse and human neurospheres. In all cases, the level of G-CSF was increased in mIDH1 compared to wtIDH1 (~5-fold, P < 0.05; Fig. 7, J to L). Of note, the levels of G-CSF in the conditioned media were not altered when mIDH1 neurospheres were cultured in FBS-containing media (fig. S16). In addition, transcriptome analysis of patients with astrocytoma in TCGA revealed that CSF3 gene expression was significantly up-regulated in patients with mIDH1 glioma harboring ATRX and TP53 mutations (Fig. 7M). The increased level of G-CSF in mIDH1 could explain the significant expansion of granulocytes in mIDH1 tumor–bearing mice as well as the enrichment of the cytokine signaling pathway triggered by G-CSF receptor expressed on myeloid cells. To further validate the enhanced G-CSF secretion in mIDH1 glioma, we collected serum samples from 16 glioma patients with wtIDH1 or mIDH1 glioma and analyzed the G-CSF levels by quantitative ELISA. All patients’ clinical information is shown in table S5. Of note, none of the patients had any prior treatments with Temozolomide (TMZ) or commercially available rG-CSF. Results showed that the G-CSF level was higher in patients with glioma with mIDH1 compared to those with wtIDH1 (Fig. 7N).
Glioma mIDH1 stem/progenitor-like cells are the major source of G-CSF expression
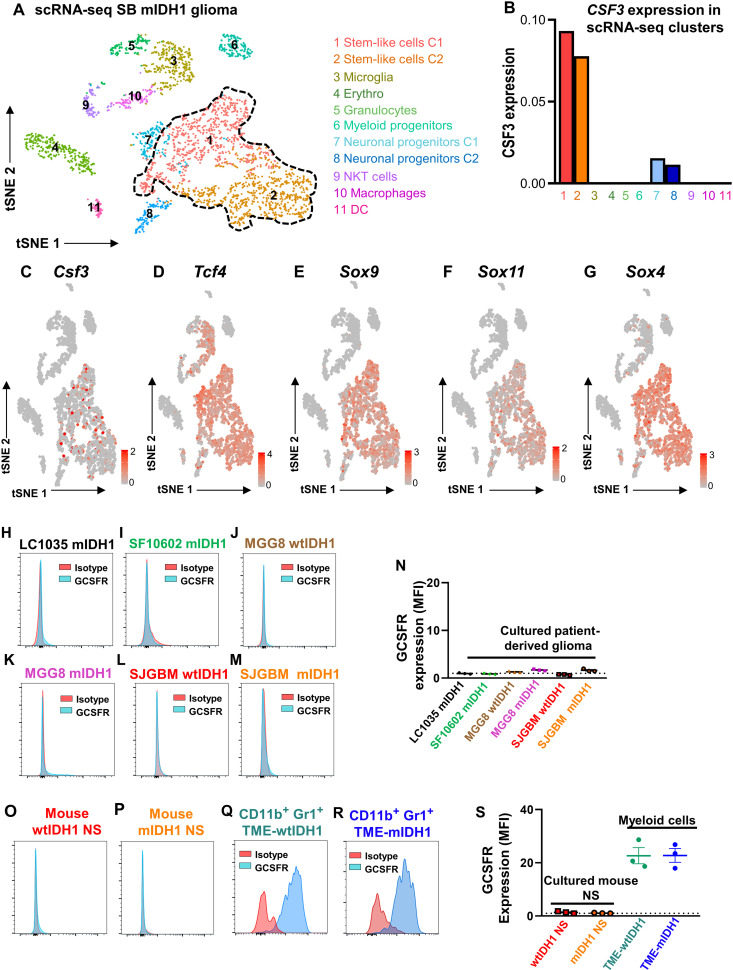
To uncover the source of G-CSF within mIDH1 tumors, we performed scRNA-seq of whole tumors (~6000 cells) isolated from the SB-induced mIDH1 glioma model. Results show that most of the Csf3 were expressed by the major tumor cell clusters, which belong to stem-like cells (Fig. 8, A to C). These cells express genes such as Tcf4, Sox9, Sox11, and Sox4 (Fig. 8, D to G). This suggests that the undifferentiated glioma cells from mIDH1 tumors express high levels of G-CSF, resulting in the phenotypic remodeling of the granulocytic myeloid cells.
Fig. 8. Glioma mIDH1 stem-like cells are the major source of G-CSF expression.
(A and B) Combined Seurat analysis shown in tSNE projection of whole tumor from mIDH1 GEMM of glioma resulted in various distinct clusters of cells. The expression of CSF3 was analyzed between the clusters. Stem-like cells were the major clusters that have the highest CSF3 expression (N = 2). (C to G) Feature plots represent the expression of (C) csf3, (D) tcf4, (E) sox9, (F) sox11, and (G) sox4. (H to M) Flow cytometry analysis of the GCSFR expression in glioma patient-derived cells with wtIDH1 or mIDH1. (N) Quantification of GCSFR expression in cultured human glioma cells with wtIDH1 or mIDH1. MFI, Mean fluorescence intensity. (O and P) Flow cytometry analysis of the GCSFR expression in mouse neurospheres developed by the SB model with wtIDH1 or mIDH1. (Q and R) Flow cytometry analysis of the GCSFR expression on CD11b+ Gr-1+ derived from the TME of wtIDH1 or mIDH1 tumor–bearing mice. (S) Quantification of GCSFR expression in conditions (O) to (R).
G-CSF is a growth factor that promotes the survival and differentiation of early MP cells into neutrophils by binding to its primary receptor GCSFR. To examine the GCSFR expression on glioma cells, we analyzed its expression by flow cytometry using multiple patient-derived glioma cell cultures including SJ-GBM2-wtIDH1, SJ-GBM2-mIDH1, MGG8-wtIDH1, MGG8-mIDH1, LC1035-mIDH1, and SF10602-mIDH1 cells. Data showed that all human-derived cell cultures do not express GCSFR (Fig. 8, H to N). Similarly, cultured mouse neurospheres do not express GCSFR, whereas the majority of GCSFR expression was on myeloid cells (CD11b+ Gr-1+) within the TME of both wtIDH1 and mIDH1 glioma–bearing mice (Fig. 8, O to S).
G-CSF neutralization restores the immunosuppressive properties of CD45high/CD11b+/Ly6G+ in mIDH1 TME
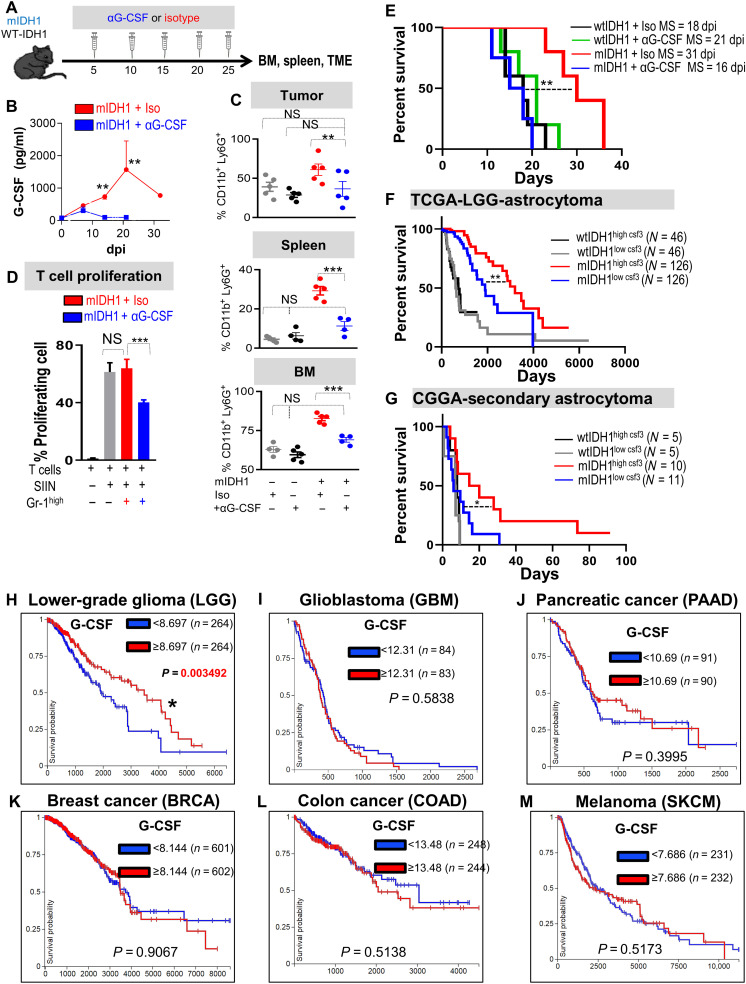
If the CD45high/CD11b+/Ly6G+ phenotype in mIDH1 is dependent on G-CSF expression, blocking G-CSF should reverse their immune-permissive phenotype. We depleted G-CSF in the mIDH1 tumor–bearing animals using αG-CSF neutralizing antibody (Fig. 9A). Treatment with αG-CSF significantly reduced the serum levels of G-CSF in mIDH1 tumor–bearing mice (~86 and ~92% decrease in day 14 dpi and 21 dpi, respectively; Fig. 9B, P < 0.01) and decreased the frequency of granulocytes in the TME, spleen, and BM (~40, ~63, and ~10%, respectively, P < 0.05; Fig. 9C). G-CSF neutralization remodeled the CD45high/CD11b+/Ly6G+ compartment within the TME of mIDH1 tumor–bearing animals to an inhibitory phenotype, which resulted in ~35% inhibition of T cell proliferation (Fig. 9D). Moreover, G-CSF neutralization shortened the MS of mIDH1 (but not the wtIDH1) tumor–bearing animals (P < 0.05; Fig. 9E). This demonstrates that the noninhibitory properties of granulocytes in the mIDH1 glioma–bearing mice are driven by high secretion of G-CSF by glioma cells.
Fig. 9. G-CSF neutralization restores the immunosuppressive properties in myeloid cells and enhances the efficacy of immunotherapy.
(A) Schematic showing experimental design of G-CSF neutralization in wtIDH1 and mIDH1 tumor–bearing mice. (B) Quantitative ELISA of serum G-CSF from mIDH1 glioma-bearing mice treated with either isotype (red) or αG-CSF (blue). (C) Flow analysis of CD45high/CD11b+/Ly6G+ cells from tumor, spleen, and BM of tumor-bearing mice treated with either isotype or αG-CSF. (D) T cell proliferation assay of CD45high/CD11b+/Ly6G+ infiltrating mIDH1 tumor treated with isotype or αG-CSF. (E) Kaplan-Meier survival analysis of mice implanted with either wtIDH1 or mIDH1 neurospheres treated with isotype or αG-CSF. (F) Kaplan-Meier survival analysis of TCGA-LGG astrocytoma wtIDH1 or mIDH1 patients with high and low CSF3 expression. (G) Kaplan-Meier survival analysis of Chinese Glioma Genome Atlas (CGGA)–secondary astrocytoma wtIDH1 or mIDH1 patients with high and low levels of CSF3 expression. (H to M) Kaplan-Meier survival analysis of the different types of tumors in the TCGA data according to CSF3 expression (high or low). LGG is the sole tumor within the TCGA database in which patients who express a high level of CSF3 have a favorable prognosis compared to patients with low CSF3 expression. *P < 0.05, **P < 0.01, and ***P < 0.005, ANOVA.
CSF3 expression is associated with a favorable outcome only in patients with glioma harboring mIDH1
Tumor-derived G-CSF has been shown to promote tumor progression and enhance metastasis in several tumor types (34, 35). To evaluate the effect of G-CSF in patients with glioma, we turned to the TCGA data and analyzed the MS of all tumor types available in the context of G-CSF (CSF3) expression (i.e., MS of patients expressing high versus low levels of CSF3). Out of 24 tumor types in the TCGA portal, we found that lower-grade glioma with mIDH1 was the only tumor type in which a significant difference existed between the MS of patients with high versus low CSF3 expression (Fig. 9, H to M, and fig. S17). Moreover, when we stratified the patients with astrocytoma according to mIDH1 and CSF3 expression, we found that enhanced MS related to high CSF3 expression was solely observed in patients with mIDH1 glioma tumors (Fig. 9F). We validated these results using the Chinese Glioma Genome Atlas in which we found that in secondary Glioblastoma (GBM), patients with mIDH1 tumors expressing high CSF3 level have a better prognosis compared to patients with low CSF3 (Fig. 9G). In both datasets, there were no significant differences in MS between high versus low expression of other cytokines responsible for PMN-MDSC development such as CSF1, IL4, IL1β, TNFα, and IL6 in patients with mIDH1 (fig S18). These results suggest a unique correlation between G-CSF expression in patients with mIDH1 glioma and patient prognosis.
Glioma-derived G-CSF induces the expansion of neutrophils and enhances the efficacy of TK/Flt3L immunotherapy
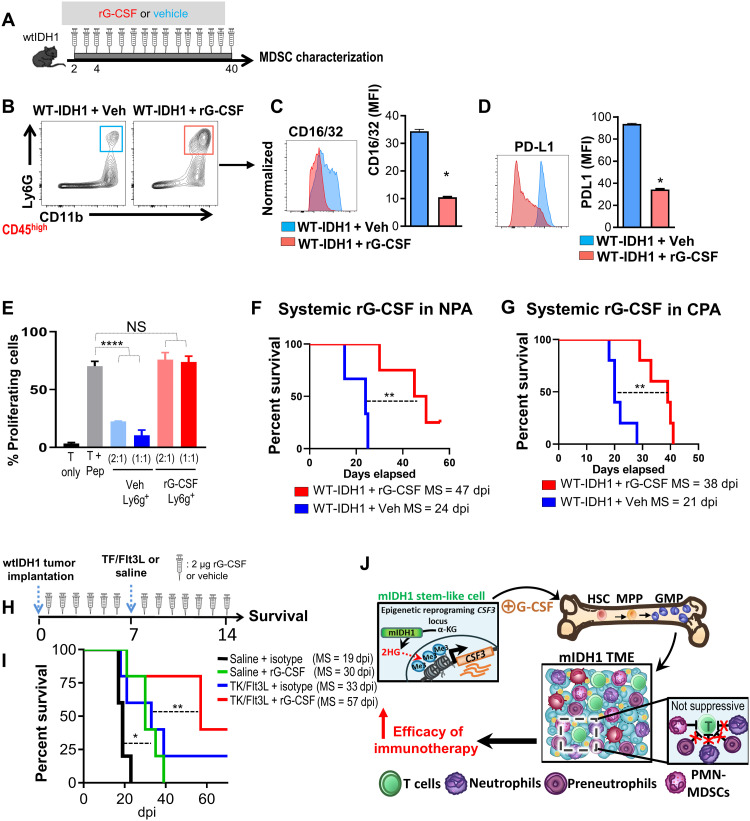
To uncover whether G-CSF alone is sufficient to induce the expansion of nonimmunosuppressive neutrophils, we dosed wtIDH1 tumor–bearing mice with recombinant G-CSF (Fig. 10A). We used rG-CSF (2 μg/day) to achieve steady-state serum G-CSF concentrations comparable to levels observed in mIDH1 tumor–bearing mice (Fig. 10A) and similar to reported doses (36). Compared to vehicle-treated animals, wtIDH1 tumor–bearing animals treated with rG-CSF showed expansion of CD45high/CD11b+/Ly6G+ cells in the TME (Fig. 10B). These granulocytes expressed lower levels of CD16/32 and PD-L1 compared to granulocytes from vehicle-treated control and did not inhibit T cell proliferation (Fig. 10, C to E). rG-CSF administration significantly prolonged the MS of tumor-bearing animals in two different wtIDH1 glioma models (Fig. 10, F and G). These results supported the hypothesis that G-CSF leads to the expansion of nonimmune suppressive neutrophils, validated the association between increased G-CSF secretion, and enhanced prognosis in glioma. To address the exact effects of G-CSF on other immune cells within the wtIDH1 TME, we performed high-dimensional immune profiling using CyTOF analysis of the tumor-infiltrating immune cells from wtIDH1 tumor–bearing animals treated with either rG-CSF or vehicle as described in Fig. 10A (fig. S19A). Results showed that rG-CSF treatment results in a significant increase in CD45high/CD11b+/Ly6G+ cells in the TME (P < 0.005; fig. S19B). Beside the decrease in macrophages in the rG-CSF group (P < 0.05; fig. S19C), there was no significant difference in other immune cells tested (fig. S19, D to I). To validate the source of G-CSF, wtIDH1 neurospheres were stably transduced with lentivirus encoding G-CSF (lenti–G-CSF) or with an empty lentivirus vector (lenti-vector). Successfully transduced neurospheres were cultured in vitro for 14 days before they were used for implantation (fig. S20A). The enhanced G-CSF expression was confirmed by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and by ELISA in conditioned media of cultured neurospheres (fig. S20, B and C). As expected, results showed that animals implanted with wtIDH1–lenti–G-CSF neurospheres had increased CD45high/CD11b+/Ly6G+ cells within the tumor, spleen, and BM compared to mice implanted with wtIDH1–lenti-vector neurospheres (fig. S20, D to F). Collectively, these data demonstrate the effect of tumor-derived G-CSF on the phenotype and function of granulocytes in mIDH1 glioma.
Fig. 10. G-CSF induces the expansion of nonsuppressive neutrophils and enhances the efficacy of TK/Flt3L immunotherapy in wtIDH1 glioma.
(A) Experimental design of rG-CSF or vehicle administration in wtIDH1 tumor–bearing animals. (B) Flow analysis of granulocytes infiltrating wtIDH1 tumors after treatment with vehicle or rG-CSF. (C and D) Flow analysis of CD16/32 and PD-L1 expression on granulocytes isolated from wtIDH1 tumor–bearing animals treated with vehicle (blue) or rG-CSF (red). (E) Flow analysis of the inhibitory potential of CD45high/CD11b+/Ly6G+ cells isolated from TME of wtIDH1 tumor–bearing mice treated with vehicle (blue) or with rG-CSF (red). (F and G) Kaplan-Meier survival analysis of mice bearing wtIDH1 tumors (F) (NPA) or (G) (CPA) treated with either isotype (blue) or rG-CSF (red). (H) Schematic diagram illustrates the treatment strategy of the TK + Flt3L gene therapy in combination with rG-CSF in wtIDH1 tumor–bearing mice. (I) Kaplan-Meier survival curves of mice bearing wtIDH1 tumors, treated with TK + Flt3L, rG-CSF, or combination therapy. (J) Proposed model of aberrant granulocyte differentiation in the mIDH1 tumor. *P < 0.05, **P < 0.01, and ****P < 0.0001, ANOVA.
Since rG-CSF results in expansion of nonimmunosuppressive granulocytes, we asked whether rG-CSF would synergize with the TK/Flt3L gene therapy and prolong mice survival in a high-grade glioma model with wtIDH1. Neurospheres with wtIDH1 were implanted in mice, rG-CSF was administered in a daily dose for 14 consecutive days, and TK/Flt3L was given on day 7 after implantation (Fig. 10H). TK/Flt3L enhanced the MS of wtIDH1 tumor–bearing mice, but combination therapy (TK/Flt3L + rG-CSF) further prolonged the MS of mice with wtIDH1 tumor (P < 0.05, Fig. 10I). These results suggest that rG-CSF further enhanced the efficacy of TK/Flt3L gene therapy.
The data presented here elucidate new potential therapeutic approaches that can enhance antiglioma immunotherapy. A model figure of the proposed molecular mechanism by which mIDH1 remodels tumor-infiltrating granulocytes to noninhibitory neutrophils is shown in Fig. 10J. Through epigenetic reprogramming caused by the presence of 2HG, mIDH1 stem-like cells express a high level of G-CSF that shifts the hematopoiesis in BM and spleen toward the expansion, differentiation, and mobilization of granulocytic myeloid cells. The granulocytes recruited to the TME in mIDH1 tumors are mainly neutrophils and preneutrophils, with a small fraction of PMN-MDSCs. Overall, the expanded granulocytes in mIDH1 TME do not suppress T cell function and enhanced the efficacy of immunotherapy.
DISCUSSION
Advances in genomics and epigenomics revealed the molecular and epigenetic identity of mIDH1 glioma, which is elicited mainly by 2HG-mediated inhibition of the methylcytosine dioxygenase TET2 and αKG-dependent histone demethylases. The resulting genome-wide hypermethylation phenotype influences both glioma development and the TME (37).
Gliomas are characterized by a profound immunosuppressive milieu that is responsible, at least in part, for hindering the efficacy of immunotherapies in clinical settings (14, 38). Previous studies have shown that patients with glioblastoma have elevated levels of circulating MDSCs, the majority of which (~82%) belong to PMN-MDSCs and are associated with poor prognosis (17, 39, 40). We have recently shown that the PMN-MDSCs are the predominant immunosuppressive myeloid cells that, upon depletion, significantly enhanced the efficacy of immunotherapy and prolonged MS in wtIDH1 glioma–bearing mice (12). Our data illustrated that myeloid cells infiltrating the mIDH1 glioma, counter to these infiltrating the wtIDH1 gliomas, express low levels of immunosuppressive genes and did not inhibit T cell function. This explains the significant differences in the efficacy of immunotherapy (TK/Flt3L) between wtIDH1 and mIDH1 glioma–bearing mice. In line with these results, myeloid depletion further enhances the efficacy of TK/Flt3L in the wtIDH1, but not in the mIDH1 gliomas, highlighting the immune permissive microenvironment in mIDH1 glioma.
Gliomas are highly heterogeneous tumors, having both intertumor and intratumor heterogeneity at the cellular and genomic levels (41–43). The mechanism by which genetic heterogeneity influences the tumor immune microenvironment remains elusive (44, 45). The use of scRNA-seq technologies has now provided additional granularity that enables charting of subtypes of immune cellular infiltrates within the glioma microenvironment. This technology enabled us to identify the developmental hierarchies within the glioma-infiltrating myelopoietic cell clusters, uncovering novel drivers and revealing the infiltrating-immune surveillance/evasion cell types relevant to glioma with specific genetic lesions (43, 46, 47). Transcriptome profiling at the single-cell level uncovered the heterogeneity of infiltrating myeloid lineages. We found that the mIDH1 glioma–infiltrating granulocytes are composed of three distinct cell clusters: C1, C2, and C3. Differential gene expression analysis between the three clusters identified that cluster C1 was enriched in immunosuppressive PMN-MDSC signature marker genes such as Tgfb, Il1b, and Arg2. Cluster C2 expressed S100a8, Ccl3, Ccl4, and cell cycle genes such as Cdc20 and G0s2, indicating proliferating neutrophils (preneutrophils), whereas C3 expressed neutrophil maturation genes such as Cebpe and Mpo. In contrast, in the wtIDH1 glioma model, the tumor-infiltrating granulocytes are composed of single-cluster expressing genes similar to granulocyte cluster C1 in the mIDH1 group.
Since a hallmark of MDSCs is their inhibition of antigen-specific CD8+ T cell proliferation, we next aimed to isolate the three newly identified granulocytic clusters encountered within the mIDH1 TME for functional characterization. An ideal marker would be expressed on the cell surface and have different expression levels between the three clusters. Interrogation of the differentially expressed genes between C1, C2, and C3 revealed that CD274 (PD-L1) was expressed at a high level in PMN-MDSCs (cluster C1) compared to intermediate and low levels in the preneutrophils (cluster C2) and neutrophils (cluster C3), respectively. This allowed us to isolate each cluster and examine their T cell inhibitory potential. Using this approach, we found that in the mIDH1 group, cells in cluster C1 were suppressive, whereas the cells in cluster C2 and C3 were nonimmunosuppressive. In the wtIDH1 group, granulocytes expressing PD-L1 formed one single-cell cluster (C7), which inhibited CD8+ T cell proliferation. Last, we performed in-depth high-dimensional mass cytometry characterization and phenotypic analysis of the three granulocytic clusters found in the mIDH1 TME. An advantage of this approach is that automated clustering is done on data reduced to two tSNE dimensions, which allows visual verification of cluster boundaries at the single-cell resolution. Combined clustering of granulocytes infiltrating the TME of mIDH1 or wtIDH1 GEMMs of glioma revealed that most of the granulocytes from the wtIDH1 tumor coclustered with the C1 cluster of the mIDH1 group. CyTOF analysis also led to the identification of CD16/32 as a unique and reproducible myeloid marker that can be used to distinguish immunosuppressive PMN-MDSCs from nonimmunosuppressive granulocytes.
Consistent with results from our GEMMs of glioma, we found that most of the immune cells infiltrating human gliomas expressing mIDH1 exhibit characteristic myeloid cells’ gene signatures, all of which express the early granulocytic receptor GCSFR. The largest myeloid cluster in the human mIDH1 glioma group expressed genes (CCL3 and CCL4) similar to cluster C2 in our mIDH1 GEMM of glioma. Moreover, using a unique PMN-MDSC gene signature, our data elucidated that human mIDH1 gliomas exhibit a lower frequency of tumor-infiltrating bona fide PMN-MDSCs when compared to the patients with wtIDH1 glioma. Therefore, the molecular and phenotypic features of mIDH1 on tumor-infiltrating PMN-MDSCs are largely conserved between our mouse model and human samples.
We also show that G-CSF was up-regulated in the conditioned media of cultured human and mouse mIDH1 NS. This increase was also significant in the serum from patients with mIDH1 astrocytoma and mIDH1 tumor–bearing mice. We demonstrated that the increased G-CSF expression in mIDH1 neurospheres was due to epigenetic reprogramming of the tumor cells’ transcriptome, in which the CSF3 gene showed an enrichment in the deposition of the H3K4me3 mark around the promoter region. We demonstrated that in our mIDH1 GEMM of glioma, tumor cells express a higher level of Sox2 and are less differentiated (19); this is consistent with earlier reports indicating that IDH1R132H suppresses cellular differentiation (4, 48). scRNA-seq revealed that glioma stem-like cells that express Sox2 and Sox4 are the major source of G-CSF expression in mIDH1 tumors. Therefore, the enhanced production of G-CSF is due to the presence of a higher proportion of less-differentiated tumor cells in mIDH1 glioma. In line with this, studies showed that within the glioma niche, glioma stem-like cells are found to be coenriched with myeloid-infiltrating immune cells (49).
The impact of G-CSF on tumor biology is still a point of debate. rG-CSF (Filgastim) is a commercially available product indicated for chemo-induced neutropenia to reverse myelosuppression in patients with cancer, and it has been shown to reduce inflammation and reverse cognitive impairment in patients with Alzheimer (50, 51). In addition, increased G-CSF secretion has been implicated in the recruitment and infiltration of immunosuppressive MDSCs in breast cancer (34) and cervical cancer (35). In contrast, in mice bearing MCA203 sarcomas, G-CSF induced CD11b+/Gr-1hi cells that were nonsuppressive (52). It has also been shown that the administration of G-CSF leads to the formation of nonimmunosuppressive CD45+/CD11b+/Gr-1+ cells in vivo (52) and in vitro (53). Our data in mIDH1 glioma show that the increased production of G-CSF by mIDH1 glioma stem-like cells caused the expansion of CD45high/CD11b+/Ly6G+ cells that exhibit a nonimmunosuppressive phenotype. We also showed that rG-CSF administration to mice harboring wtIDH1 glioma expands nonsuppressive myeloid cells, leading to enhanced efficacy of immunostimulatory gene therapy TK/Flt3L in glioma mice with wtIDH1. In summary, our data demonstrate that mIDH1 tumor–derived G-CSF reprograms myelopoiesis in glioma, which promotes the production of nonimmunosuppressive myeloid cells, a feature that can be harnessed to enhance immunotherapeutic efficacy in patients with mIDH1 LGG.
MATERIALS AND METHODS
Human subjects
All patients gave informed consent for collection of clinical correlates, tissue collection, and research testing under Institutional Review Board–approved protocols (HUM00057130, UMCC 2015.024). Patient clinical information is listed in table S5. Patient studies were conducted according to the Declaration of Helsinki, the Belmont Report, and the U.S. Common Rule.
Experimental model and subject details
Six- to 8-week-old wild-type C57BL/6 female mice (Taconic) were housed under standard specific pathogen–free conditions. All animal procedures were carried out in accordance with the University of Michigan’s Institutional Animal Care and Use Committee. All animals were housed in an American Association for Accreditation of Laboratory Animal Care (AAALAC)–accredited animal facility.
Study design
To study the impact of glioma IDH1R123H on myeloid cell phenotype and function, we generated a genetically engineered animal model injecting SB plasmids encoding NRA G12V, shATRX, and shp53 with or without IDH1R132H (wtIDH1 and mIDH1, respectively) into the lateral ventricle of neonatal mice (19–21). Sample size and any data inclusion/exclusion were defined individually for each experiment. We also used an animal model generated by intracranial implantation of glioma NS (wtIDH1 and mIDH1) derived from our genetically engineered animal model (19). In addition, we used two alternative animal models that do not encode RAS-activating mutations [(PDGFB/shP53/shATRX/Ink4a/Arf−/− wtIDH1-NS or mIDH1-NS) and (CDKN2A−/−/shP53/shATRX/wtIDH1-NS or mIDH1-NS)]. We performed pan characterization of all immune cells by flow cytometry and mass cytometry and identified each immune cell population using unique biomarkers. Detailed information regarding the source of antibodies and immune cells’ gating are listed in tables S1, S2, and S3. We also performed scRNA-seq to define the PMN-MDSC cell population in both mouse and human glioma tissue derived from patients harboring IDH1R132H or wtIDH1. We validated PMN-MDSCs by functional analysis of T cell’s inhibitory characteristics. All sequencing datasets have been deposited in NCBI’s Gene Expression Omnibus with identifier (GSE152277). CyTOF data are deposited in Immport (accession number: SDY1774). Materials and Methods related to tumor implantation, RNA-seq, T cell proliferation assay, ChIP-seq, cytokine analysis, RT-qPCR, and scRNA-seq are described in detail in the Supplementary Materials.
Statistical analysis
Sample sizes were selected on the basis of preliminary data from pilot experiments and previously published results in the literature and our laboratory. Unpaired Student’s t test or one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons posttest, was utilized for comparing experimental groups with controls from flow cytometry analysis, cytokine analysis, mass cytometry analysis, and T cell functional assays. RNA-seq data were processed using the Tuxedo Suite, and differentially expressed genes were considered when false discovery rate ≤ 0.05 and fold change ≥ ±1.5. All single-cell sequencing data were processed by Cell Ranger Pipeline version 3.1.0. Filtered digital gene expression matrix file matrices containing the number of unique molecular identifier counts per gene per cell were analyzed using the Seurat R package version 3.1.4. Statistically significant principal components were determined using the jackstraw function (28, 31). All quantitative data are presented as the means ± SEM from at least three independent samples. ANOVA and two-sample t tests were used to compare continuous outcomes between groups. Survival curves were analyzed using the Kaplan-Meier method and compared using Mantel-Cox tests; the effect size is expressed as MS. Differences were considered significant if P < 0.05. All analyses were conducted using GraphPad Prism software (version 8.00) or R (version 3.4). The statistical tests used are indicated in each figure legend.
Acknowledgments
We thank K. Verbal and A. Gunawan for all the help. We thank the immunology core, UMICH CyTOF core, and the University of Michigan advanced genomic core.
Funding: This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) grants (R37-NS094804, R01-NS105556, and R21-NS107894, and Rogel Cancer Center Scholar Award to M.G.C.); NIH/NINDS grants (R01-NS076991, R01-NS082311, and R01-NS096756 to P.R.L.); the Department of Neurosurgery, the Pediatric Brain Tumor Foundation, Leah’s Happy Hearts Foundation, Ian’s Friends Foundation (IFF), Chad Tough Foundation, Pediatric Brain Tumor Foundation, and Smiles for Sophie Forever Foundation (to M.G.C. and P.R.L.); NIH/NCI T32-CA009676 Post-Doctoral Fellowship (to M.S.A.); and American Brain Tumor Association Basic Research Fellowship “in Memory of Bruce and Brian Jackson” (to M.B.G.-F.).
Author contributions: M.S.A., P.R.L., and M.G.C. conducted and designed the experiments, analyzed the data, and wrote the manuscript. M.S.A., B.L.M., R.P.A., R.T., C.S.H., S.C., M.V., M.B.G.-F., S.H., A.T., N.K., L.Z., S.M.F., and F.J.N. helped conduct the experiments and data analysis. W.N.A., D.O., J.H., P.G.P., and K.E. provided clinical patient samples and patient information. S.H.-J. provided glioma patient–derived cells. S.D.M. and G.N. assisted with study design and analysis. P.J.U., J.W., C.G., and J.L. assisted with the single-cell RNA sequencing analysis. D.H. donated the glioma mouse models using the RCAS system. P.R.L. and M.G.C. directed the research and generated the funding. All authors read and edited the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The RNA-seq and sc-RNA-seq datasets have been deposited in NCBI’s Gene Expression Omnibus with identifiers GSE152277. CyTOF data are deposited in Immport (accession number: SDY1774). All plasmids described in this study have been deposited in Addgene (table S6). Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, MGC (mariacas@med.umich.edu).
Supplementary Materials
This PDF file includes:
Supplementary Materials and Methods
Figs. S1 to S20
Tables S1 to S6
References
REFERENCES AND NOTES
- 1.Ceccarelli M., Barthel F. P., Malta T. M., Sabedot T. S., Salama S. R., Murray B. A., Morozova O., Newton Y., Radenbaugh A., Pagnotta S. M., Anjum S., Wang J., Manyam G., Zoppoli P., Ling S., Rao A. A., Grifford M., Cherniack A. D., Zhang H., Poisson L., Carlotti C. G. Jr., Tirapelli D. P. C., Rao A., Mikkelsen T., Lau C. C., Yung W. K. A., Rabadan R., Huse J., Brat D. J., Lehman N. L., Barnholtz-Sloan J. S., Zheng S., Hess K., Rao G., Meyerson M., Beroukhim R., Cooper L., Akbani R., Wrensch M., Haussler D., Aldape K. D., Laird P. W., Gutmann D. H., Noushmehr H., Iavarone A., Verhaak R. G. W., Anjum S., Arachchi H., Auman J. T., Balasundaram M., Balu S., Barnett G., Baylin S., Bell S., Benz C., Bir N., Black K. L., Bodenheimer T., Boice L., Bootwalla M. S., Bowen J., Bristow C. A., Butterfield Y. S. N., Chen Q. R., Chin L., Cho J., Chuah E., Chudamani S., Coetzee S. G., Cohen M. L., Colman H., Couce M., D’Angelo F., Davidsen T., Davis A., Demchok J. A., Devine K., Ding L., Duell R., Elder J. B., Eschbacher J. M., Fehrenbach A., Ferguson M., Frazer S., Fuller G., Fulop J., Gabriel S. B., Garofano L., Gastier-Foster J. M., Gehlenborg N., Gerken M., Getz G., Giannini C., Gibson W. J., Hadjipanayis A., Hayes D. N., Heiman D. I., Hermes B., Hilty J., Hoadley K. A., Hoyle A. P., Huang M., Jefferys S. R., Jones C. D., Jones S. J. M., Ju Z., Kastl A., Kendler A., Kim J., Kucherlapati R., Lai P. H., Lawrence M. S., Lee S., Leraas K. M., Lichtenberg T. M., Lin P., Liu Y., Liu J., Ljubimova J. Y., Lu Y., Ma Y., Maglinte D. T., Mahadeshwar H. S., Marra M. A., McGraw M., McPherson C., Meng S., Mieczkowski P. A., Miller C. R., Mills G. B., Moore R. A., Mose L. E., Mungall A. J., Naresh R., Naska T., Neder L., Noble M. S., Noss A., O’Neill B. P., Ostrom Q. T., Palmer C., Pantazi A., Parfenov M., Park P. J., Parker J. S., Perou C. M., Pierson C. R., Pihl T., Protopopov A., Radenbaugh A., Ramirez N. C., Rathmell W. K., Ren X., Roach J., Robertson A. G., Saksena G., Schein J. E., Schumacher S. E., Seidman J., Senecal K., Seth S., Shen H., Shi Y., Shih J., Shimmel K., Sicotte H., Sifri S., Silva T., Simons J. V., Singh R., Skelly T., Sloan A. E., Sofia H. J., Soloway M. G., Song X., Sougnez C., Souza C., Staugaitis S. M., Sun H., Sun C., Tan D., Tang J., Tang Y., Thorne L., Trevisan F. A., Triche T., van den Berg D. J., Veluvolu U., Voet D., Wan Y., Wang Z., Warnick R., Weinstein J. N., Weisenberger D. J., Wilkerson M. D., Williams F., Wise L., Wolinsky Y., Wu J., Xu A. W., Yang L., Yang L., Zack T. I., Zenklusen J. C., Zhang J., Zhang W., Zhang J., Zmuda E., Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164, 550–563 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan H., Parsons D. W., Jin G., McLendon R., Rasheed B. A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G. J., Friedman H., Friedman A., Reardon D., Herndon J., Kinzler K. W., Velculescu V. E., Vogelstein B., Bigner D. D., IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa M. E., Abdel-Wahab O., Lu C., Ward P. S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H. F., Tallman M. S., Sun Z., Wolniak K., Peeters J. K., Liu W., Choe S. E., Fantin V. R., Paietta E., Löwenberg B., Licht J. D., Godley L. A., Delwel R., Valk P. J. M., Thompson C. B., Levine R. L., Melnick A., Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu C., Ward P. S., Kapoor G. S., Rohle D., Turcan S., Abdel-Wahab O., Edwards C. R., Khanin R., Figueroa M. E., Melnick A., Wellen K. E., O’Rourke D. M., Berger S. L., Chan T. A., Levine R. L., Mellinghoff I. K., Thompson C. B., IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward P. S., Patel J., Wise D. R., Abdel-Wahab O., Bennett B. D., Coller H. A., Cross J. R., Fantin V. R., Hedvat C. V., Perl A. E., Rabinowitz J. D., Carroll M., Su S. M., Sharp K. A., Levine R. L., Thompson C. B., The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amankulor N. M., Kim Y., Arora S., Kargl J., Szulzewsky F., Hanke M., Margineantu D. H., Rao A., Bolouri H., Delrow J., Hockenbery D., Houghton A. M. G., Holland E. C., Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 31, 774–786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mu L., Long Y., Yang C., Jin L., Tao H., Ge H., Chang Y. E., Karachi A., Kubilis P. S., de Leon G., Qi J., Sayour E. J., Mitchell D. A., Lin Z., Huang J., The IDH1 mutation-induced oncometabolite, 2-hydroxyglutarate, may affect DNA methylation and expression of PD-L1 in Gliomas. Front. Mol. Neurosci. 11, 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berghoff A. S., Kiesel B., Widhalm G., Wilhelm D., Rajky O., Kurscheid S., Kresl P., Wöhrer A., Marosi C., Hegi M. E., Preusser M., Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol. 19, 1460–1468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino P. J., Zager M., McFerrin L., Wirsching H. G., Bolouri H., Hentschel B., von Deimling A., Jones D., Reifenberger G., Weller M., Holland E. C., Multidimensional scaling of diffuse gliomas: Application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol. Commun. 5, 39–39 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadiyala P., Carney S. V., Gauss J. C., Garcia-Fabiani M. B., Haase S., Alghamri M. S., Núñez F. J., Liu Y., Yu M., Taher A., Nunez F. M., Li D., Edwards M. B., Kleer C. G., Appelman H., Sun Y., Zhao L., Moon J. J., Schwendeman A., Lowenstein P. R., Castro M. G., Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice. J. Clin. Invest. 131, e139542 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Feng X., Herting C. J., Garcia V. A., Nie K., Pong W. W., Rasmussen R., Dwivedi B., Seby S., Wolf S. A., Gutmann D. H., Hambardzumyan D., Cellular and molecular identity of tumor-associated macrophages in glioblastoma. Cancer Res. 77, 2266–2278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamran N., Kadiyala P., Saxena M., Candolfi M., Li Y., Moreno-Ayala M. A., Raja N., Shah D., Lowenstein P. R., Castro M. G., Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol. Ther. 25, 232–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis D. N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W. K., Ohgaki H., Wiestler O. D., Kleihues P., Ellison D. W., The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 131, 803–820 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Kamran N., Alghamri M. S., Nunez F. J., Shah D., Asad A. S., Candolfi M., Altshuler D., Lowenstein P. R., Castro M. G., Current state and future prospects of immunotherapy for glioma. Immunotherapy 10, 317–339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang A. L., Miska J., Wainwright D. A., Dey M., Rivetta C. V., Yu D., Kanojia D., Pituch K. C., Qiao J., Pytel P., Han Y., Wu M., Zhang L., Horbinski C. M., Ahmed A. U., Lesniak M. S., CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 76, 5671–5682 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raychaudhuri B., Rayman P., Huang P., Grabowski M., Hambardzumyan D., Finke J. H., Vogelbaum M. A., Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J. Neurooncol 122, 293–301 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Dubinski D., Wölfer J., Hasselblatt M., Schneider-Hohendorf T., Bogdahn U., Stummer W., Wiendl H., Grauer O. M., CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol. 18, 807–818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gielen P. R., Schulte B. M., Kers-Rebel E. D., Verrijp K., Petersen-Baltussen H. M. J. M., ter Laan M., Wesseling P., Adema G. J., Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. J. Neuropathol. Exp. Neurol. 74, 390–400 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Nunez F. J., Mendez F. M., Kadiyala P., Alghamri M. S., Savelieff M. G., Garcia-Fabiani M. B., Haase S., Koschmann C., Calinescu A.-A., Kamran N., Saxena M., Patel R., Carney S., Guo M. Z., Edwards M., Ljungman M., Qin T., Sartor M. A., Tagett R., Venneti S., Brosnan-Cashman J., Meeker A., Gorbunova V., Zhao L., Kremer D. M., Zhang L., Lyssiotis C. A., Jones L., Herting C. J., Ross J. L., Hambardzumyan D., Hervey-Jumper S., Figueroa M. E., Lowenstein P. R., Castro M. G., IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 11, eaaq1427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koschmann C., Calinescu A.-A., Nunez F. J., Mackay A., Fazal-Salom J., Thomas D., Mendez F., Kamran N., Dzaman M., Mulpuri L., Krasinkiewicz J., Doherty R., Lemons R., Brosnan-Cashman J. A., Li Y., Roh S., Zhao L., Appelman H., Ferguson D., Gorbunova V., Meeker A., Jones C., Lowenstein P. R., Castro M. G., ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci. Transl. Med. 8, 328ra328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calinescu A.-A., Núñez F. J., Koschmann C., Kolb B. L., Lowenstein P. R., Castro M. G., Transposon mediated integration of plasmid DNA into the subventricular zone of neonatal mice to generate novel models of glioblastoma. J. Vis. Exp 2015, 52443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali S., King G. D., Curtin J. F., Candolfi M., Xiong W., Liu C., Puntel M., Cheng Q., Prieto J., Ribas A., Kupiec-Weglinski J., van Rooijen N., Lassmann H., Lowenstein P. R., Castro M. G., Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 65, 7194–7204 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghulam Muhammad A. K., Candolfi M., King G. D., Yagiz K., Foulad D., Mineharu Y., Kroeger K. M., Treuer K. A., Nichols W. S., Sanderson N. S., Yang J., Khayznikov M., Van Rooijen N., Lowenstein P. R., Castro M. G., Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: Humoral and cellular immunity lead to tumor regression. Clin. Cancer Res. 15, 6113–6127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendall S. C., Simonds E. F., Qiu P., Amir E. D., Krutzik P. O., Finck R., Bruggner R. V., Melamed R., Trejo A., Ornatsky O. I., Balderas R. S., Plevritis S. K., Sachs K., Pe’er D., Tanner S. D., Nolan G. P., Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amirel A. D., Davis K. L., Tadmor M. D., Simonds E. F., Levine J. H., Bendall S. C., Shenfeld D. K., Krishnaswamy S., Nolan G. P., Pe’er D., viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31, 545–552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruggner R. V., Bodenmiller B., Dill D. L., Tibshirani R. J., Nolan G. P., Automated identification of stratifying signatures in cellular subpopulations. Proc. Natl. Acad. Sci. 111, E2770–E2777 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hambardzumyan D., Amankulor N. M., Helmy K. Y., Becher O. J., Holland E. C., Modeling adult gliomas using RCAS/t-va technology. Transl. Oncol. 2, 89–95 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R., Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alshetaiwi H., Pervolarakis N., Intyre L. L. M., Ma D., Nguyen Q., Rath J. A., Nee K., Hernandez G., Evans K., Torosian L., Silva A., Walsh C., Kessenbrock K., Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 5, eaay6017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honey K., CCL3 and CCL4 actively recruit CD8+ T cells. Nat. Rev. Immunol. 6, 427–427 (2006). [Google Scholar]
- 31.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W. M. III, Hao Y., Stoeckius M., Smibert P., Satija R., Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elghetany M. T., Surface antigen changes during normal neutrophilic development: A critical review. Blood Cell. Mol. Dis. 28, 260–274 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Lambert C., Preijers F. W. M. B., Yanikkaya Demirel G., Sack U., Monocytes and macrophages in flow: An ESCCA initiative on advanced analyses of monocyte lineage using flow cytometry. Cytometry B Clin. Cytom. 92, 180–188 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Casbon A. J., Reynaud D., Park C., Khuc E., Gan D. D., Schepers K., Passegué E., Werb Z., Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl. Acad. Sci. U.S.A. 112, E566–E575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawano M., Mabuchi S., Matsumoto Y., Sasano T., Takahashi R., Kuroda H., Kozasa K., Hashimoto K., Isobe A., Sawada K., Hamasaki T., Morii E., Kimura T., The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci. Rep. 5, 18217 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer M. A., Baer J. M., Knolhoff B. L., Nywening T. M., Panni R. Z., Su X., Weilbaecher K. N., Hawkins W. G., Ma C., Fields R. C., Linehan D. C., Challen G. A., Faccio R., Aft R. L., DeNardo D. G., Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 9, 1250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons D. W., Jones S., Zhang X., Lin J. C.-H., Leary R. J., Angenendt P., Mankoo P., Carter H., Siu I. M., Gallia G. L., Olivi A., Lendon R. M., Rasheed B. A., Keir S., Nikolskaya T., Nikolsky Y., Busam D. A., Tekleab H., Diaz L. A. Jr., Hartigan J., Smith D. R., Strausberg R. L., Marie S. K. N., Shinjo S. M. O., Yan H., Riggins G. J., Bigner D. D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V. E., Kinzler K. W., An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Fabiani M. B., Ventosa M., Comba A., Candolfi M., Candia A. J. N., Alghamri M. S., Kadiyala P., Carney S., Faisal S. M., Schwendeman A., Moon J. J., Scheetz L., Lahann J., Mauser A., Lowenstein P. R., Castro M. G., Immunotherapy for gliomas: Shedding light on progress in preclinical and clinical development. Expert Opin. Investig. Drugs 29, 659–684 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Raychaudhuri B., Rayman P., Ireland J., Ko J., Rini B., Borden E. C., Garcia J., Vogelbaum M. A., Finke J., Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 13, 591–599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan Y., Zhou X., Niu X., Li J., Wang T., Zhang H., Yang Y., Liu Y., Mao Q., Neutrophil/lymphocyte ratio is an independent prognostic factor in elderly patients with high-grade gliomas. World Neurosurg. 127, e261–e267 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Hu B., Hu X., Kim H., Squatrito M., Scarpace L., deCarvalho A. C., Lyu S., Li P., Li Y., Barthel F., Cho H. J., Lin Y.-H., Satani N., Martinez-Ledesma E., Zheng S., Chang E., Sauvé C.-E. G., Olar A., Lan Z. D., Finocchiaro G., Phillips J. J., Berger M. S., Gabrusiewicz K. R., Wang G., Eskilsson E., Hu J., Mikkelsen T., DePinho R. A., Muller F., Heimberger A. B., Sulman E. P., Nam D.-H., Verhaak R. G. W., Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhaak R. G., Hoadley K. A., Purdom E., Wang V., Qi Y., Wilkerson M. D., Miller C. R., Ding L., Golub T., Mesirov J. P., Alexe G., Lawrence M., O’Kelly M., Tamayo P., Weir B. A., Gabriel S., Winckler W., Gupta S., Jakkula L., Feiler H. S., Hodgson J. G., James C. D., Sarkaria J. N., Brennan C., Kahn A., Spellman P. T., Wilson R. K., Speed T. P., Gray J. W., Meyerson M., Getz G., Perou C. M., Hayes D. N.; Cancer Genome Atlas Research Network , Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neftel C., Laffy J., Filbin M. G., Hara T., Shore M. E., Rahme G. J., Richman A. R., Silverbush D., Shaw M. K. L., Hebert C. M., Dewitt J., Gritsch S., Perez E. M., Castro L. N. G., Lan X., Druck N., Rodman C., Dionne D., Kaplan A., Bertalan M. S., Small J., Pelton K., Becker S., Bonal D., Nguyen Q.-D., Servis R. L., Fung J. M., Mylvaganam R., Mayr L., Gojo J., Haberler C., Geyeregger R., Czech T., Slavc I., Nahed B. V., Curry W. T., Carter B. S., Wakimoto H., Brastianos P. K., Batchelor T. T., Stemmer-Rachamimov A., Martinez-Lage M., Frosch M. P., Stamenkovic I., Riggi N., Rheinbay E., Monje M., Rozenblatt-Rosen O., Cahill D. P., Patel A. P., Hunter T., Verma I. M., Ligon K. L., Louis D. N., Regev A., Bernstein B. E., Tirosh I., Suvà M. L., An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pombo Antunes A. R., Scheyltjens I., Duerinck J., Neyns B., Movahedi K., van Ginderachter J. A., Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. eLife 9, e52176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doucette T., Rao G., Rao A., Shen L., Aldape K., Wei J., Dziurzynski K., Gilbert M., Heimberger A. B., Immune heterogeneity of glioblastoma subtypes: Extrapolation from the cancer genome atlas. Cancer Immunol. Res. 1, 112–122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venteicher A. S., Tirosh I., Hebert C., Yizhak K., Neftel C., Filbin M. G., Hovestadt V., Escalante L. E., Shaw M. K. L., Rodman C., Gillespie S. M., Dionne D., Luo C. C., Ravichandran H., Mylvaganam R., Mount C., Onozato M. L., Nahed B. V., Wakimoto H., Curry W. T., Iafrate A. J., Rivera M. N., Frosch M. P., Golub T. R., Brastianos P. K., Getz G., Patel A. P., Monje M., Cahill D. P., Rozenblatt-Rosen O., Louis D. N., Bernstein B. E., Regev A., Suvà M. L., Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 355, eaai8478 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel A. P., Tirosh I., Trombetta J. J., Shalek A. K., Gillespie S. M., Wakimoto H., Cahill D. P., Nahed B. V., Curry W. T., Martuza R. L., Louis D. N., Rozenblatt-Rosen O., Suvà M. L., Regev A., Bernstein B. E., Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Modrek A. S., Golub D., Khan T., Bready D., Prado J., Bowman C., Deng J., Zhang G., Rocha P. P., Raviram R., Lazaris C., Stafford J. M., LeRoy G., Kader M., Dhaliwal J., Bayin N. S., Frenster J. D., Serrano J., Chiriboga L., Baitalmal R., Nanjangud G., Chi A. S., Golfinos J. G., Wang J., Karajannis M. A., Bonneau R. A., Reinberg D., Tsirigos A., Zagzag D., Snuderl M., Skok J. A., Neubert T. A., Placantonakis D. G., Low-grade astrocytoma mutations in IDH1, P53, and ATRX cooperate to block differentiation of human neural stem cells via repression of SOX2. Cell Rep. 21, 1267–1280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otvos B., Silver D. J., Mulkearns-Hubert E. E., Alvarado A. G., Turaga S. M., Sorensen M. D., Rayman P., Flavahan W. A., Hale J. S., Stoltz K., Sinyuk M., Wu Q., Jarrar A., Kim S. H., Fox P. L., Nakano I., Rich J. N., Ransohoff R. M., Finke J., Kristensen B. W., Vogelbaum M. A., Lathia J. D., Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells 34, 2026–2039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Ramos J., Song S., Sava V., Catlow B., Lin X., Mori T., Cao C., Arendash G. W., Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience 163, 55–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B., Gonzalez-Toledo M. E., Piao C. S., Gu A., Kelley R. E., Zhao L. R., Stem cell factor and granulocyte colony-stimulating factor reduce β-amyloid deposits in the brains of APP/PS1 transgenic mice. Alzheimers Res. Ther. 3, 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolcetti L., Peranzoni E., Ugel S., Marigo I., Fernandez Gomez A., Mesa C., Geilich M., Winkels G., Traggiai E., Casati A., Grassi F., Bronte V., Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40, 22–35 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Marigo I., Bosio E., Solito S., Mesa C., Fernandez A., Dolcetti L., Ugel S., Sonda N., Bicciato S., Falisi E., Calabrese F., Basso G., Zanovello P., Cozzi E., Mandruzzato S., Bronte V., Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32, 790–802 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Baker G. J., Castro M. G., Lowenstein P. R., Isolation and flow cytometric analysis of glioma-infiltrating peripheral blood mononuclear cells. J. Vis. Exp. 2015, 53676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curtin J. F., Liu N., Candolfi M., Xiong W., Assi H., Yagiz K., Edwards M. R., Michelsen K. S., Kroeger K. M., Liu C., Muhammad A. K. M. G., Clark M. C., Arditi M., Comin-Anduix B., Ribas A., Lowenstein P. R., Castro M. G., HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLOS Med. 6, e10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamran N., Li Y., Sierra M., Alghamri M. S., Kadiyala P., Appelman H. D., Edwards M., Lowenstein P. R., Castro M. G., Melanoma induced immunosuppression is mediated by hematopoietic dysregulation. Onco. Targets. Ther. 7, e1408750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu P., Simonds E. F., Bendall S. C., Gibbs K. D. Jr., Bruggner R. V., Linderman M. D., Sachs K., Nolan G. P., Plevritis S. K., Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macosko E. Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., Tirosh I., Bialas A. R., Kamitaki N., Martersteck E. M., Trombetta J. J., Weitz D. A., Sanes J. R., Shalek A. K., Regev A., McCarroll S. A., Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Figs. S1 to S20
Tables S1 to S6
References