OBJECTIVES:
Ketamine is increasingly being used for analgosedation, but its effect on delirium remains unclear. We compared delirium risk variables and ketamine analgosedation use between adults who developed incident delirium and those who did not, evaluated whether ketamine analgosedation increases delirium risk, and compared ICU delirium characteristics, treatments, and outcomes between ketamine and nonketamine patients with delirium.
DESIGN:
Secondary, subgroup analysis of a cohort study.
SETTING:
Single, 36-bed mixed medical-surgical ICU in the Netherlands from July 2016 to February 2020.
PATIENTS:
Consecutive adults were included. Patients admitted after elective surgery, not expected to survive greater than or equal to 48 hours, admitted with delirium, or where delirium occurred prior to ketamine use were excluded.
INTERVENTION:
None.
MEASUREMENTS AND MAIN RESULTS:
Trained ICU nurses evaluated patients without coma (Richmond Agitation Sedation Scale. –4/–5) every 8 hours with the Confusion Assessment Method ICU; a delirium day was defined by greater than or equal to1 + Confusion Assessment Method ICU and/or scheduled antipsychotic use. Among 11 variables compared between the delirium and nondelirium groups (Baseline: age, Charlson Comorbidity score, cognitive impairment, admission type, and Acute Physiology and Chronic Health Evaluation-IV score, daily ICU [until delirium occurrence or discharge]: Sequential Organ Failure Assessment score, coma, benzodiazepine, opioid, and ketamine use) and total ICU days, 7 (age, Charlson score, Sequential Organ Failure Assessment score, coma, benzodiazepine, opioid, and ketamine use) were significantly different and were entered, along with delirium occurrence, in a logistic regression model. A total of 332 of 925 of patients (36%) developed delirium. Ketamine use was greater in patients with delirium (54 [16%] vs 4 [0.7%]; p < 0.01). Ketamine use (adjusted odds ratio, 5.60; 95% CI, 1.09–29.15), age (adjusted odds ratio, 1.03; 95% CI, 1.01–1.06), coma (adjusted odds ratio, 2.10; 95% CI, 1.15–3.78), opioid use (adjusted odds ratio, 171.17; 95% CI, 66.45–553.68), and benzodiazepine use (adjusted odds ratio, 34.07; 95% CI, 8.12–235.34) were each independently and significantly associated with increased delirium. Delirium duration, motoric subtype, delirium treatments, and outcomes were not different between the ketamine and nonketamine groups.
CONCLUSIONS:
Ketamine analgosedation may contribute to increased ICU delirium. The characteristics of ketamine and nonketamine delirium are similar. Further prospective research is required to evaluate the magnitude of risk for delirium with ketamine use.
Keywords: delirium, intensive care, ketamine, risk factor
Ketamine, an anesthetic with both analgesic and sedative properties, is increasingly being used in both surgical and medical critically ill adults for analgosedation (1–3). Delirium, a common sequela of critical illness, is associated with poor outcomes (4). Medications, including benzodiazepines (5) and opioids (6), are well-established risk factors for delirium in the ICU. Ketamine, an N-methyl-d-aspartate receptor antagonist, results in a dissociative state clinically manifesting as catatonia and amnesia, and is associated in a dose-dependent fashion with hallucinations and psychosis (7). Similarities exist between the clinical effects of ketamine and the symptoms of delirium (4).
One randomized trial (8) and four cohort studies (9–12) have evaluated delirium in critically ill adults managed with ketamine analogosedation. In the randomized trial (8), the ketamine dose administered was low and fixed, and compared with the placebo group, the ketamine group received fewer opioids, an important dose-related risk for delirium (6). None of the ICU cohort studies reported a difference in delirium with ketamine use (9–12). However, none of these studies considered the presence of other potential baseline and ICU delirium risk factors nor ensured delirium occurred after ketamine was started. The risk for ICU delirium with ketamine analgosedation and the characteristics and outcomes of delirium that occur after its initiation remain poorly characterized. We compared variables, including ketamine analgosedation use, between critically ill adults who developed ICU incident delirium and those who did not, evaluated whether ketamine analgosedation increases delirium risk, and compared delirium characteristics, treatments, and outcomes between ketamine and nonketamine patients who developed delirium.
MATERIALS AND METHODS
Design, Setting, and Patients
This cohort study is a post hoc, secondary, subgroup analysis of the Monitoring cOnsequeNces of InTensive care fOR Intensive Care patients (MONITOR-IC) study (ClinicalTrials.gov NCT03246334) (13, 14). The multicenter, MONITOR-IC cohort study prospectively compared patient self-reported cognitive, psychologic, and physical health statuses before and 1 year after ICU admission in 2,345 critically ill adults in the Netherlands (13, 14). The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed throughout the study (15).
Consecutive patients enrolled in the MONITOR-IC study between July 2016 and February 2020 who were admitted to the 36-bed mixed medical-surgical ICU at RadboudUMC, Nijmegen, NL, were included in our analysis. Patients admitted after elective surgery or not expected by the ICU attending physician to survive greater than or equal to 48 hours were excluded. Risk factors for delirium are different between elective surgery and critically ill adults admitted to the ICU (16, 17). At RadboudUMC, ketamine use after elective surgery is usually administered at a low dose as part of multimodal analgesia and is rarely administered at the higher doses used for analgosedation (1, 2, 4). Patients with delirium at ICU admission, who developed ICU delirium prior to or on the same day as ketamine initiation, or who received ketamine for a nonanalgosedation indication (e.g., status asthmaticus) were also excluded. Although use of the “A” for Assessment, Prevention, and Manage pain; “B” for Both Spontaneous Awakening Trials and Spontaneous Breathing Trials; “C” for Choice of Analgesia and Sedation; “D” for Delirium Assess, Prevent, and Manage; “E” for Early Mobility and Exercise; and “F” for Family Engagement and Empowerment (ABCDEF) bundle is established the RadboudUMC ICU (18), sedative choice, including the use of ketamine for analgosedation, is not protocolized. This study was approved by the ethics committee of the RadboudUMC (2016-2724). Each participant, or their legal representative, provided written informed consent.
Outcomes
Baseline demographic data were obtained from the Monitor-IC study database. All remaining patient data were extracted from the RadboudUMC Epic (Verona, WI) electronic health record by trained research personnel. Data on daily ICU ketamine use, including the average daily infusion dose (mg/kg/hr), were collected. Well-trained ICU nurses evaluated all patients without coma (Richmond Agitation Sedation Scale [RASS] score, –4/–5) (19) for delirium every 8 hours using the Confusion Assessment Method-ICU (CAM-ICU) (20). When possible, delirium assessments were conducted when the patient was maximally awake. A day with delirium was defined by greater than or equal to 1 positive CAM-ICU assessment or scheduled antipsychotic use (21). This combined delirium definition is based on the frequent use of haloperidol/quetiapine for ICU delirium treatment at RadboudUMC and concerns the sensitivity of the CAM-ICU to detect delirium by bedside clinicians, even after training, is not as high as that seen with research personnel (22).
The duration of ICU stay before incident delirium occurrence (or discharge) and data for n = 9 potential ICU delirium risk factors (Pre-ICU: age, Modified Charlson Comorbidity score, presence of cognitive impairment [Cognitive Failure Questionnaire Score ≥ 43]; ICU baseline: admission type [medical or urgent surgery], Acute Physiologic and Chronic Health Evaluation [APACHE] IV score; and daily ICU [until delirium occurrence or discharge]: average Sequential Organ Failure Assessment [SOFA] score, coma days, daily ICU benzodiazepine use [≥ 5-mg IV midazolam equivalents], and opioid use [≥ 10 IV morphine milligram equivalents, MME]) were collected. Each of these factors is well established in the literature as risk factors for delirium in critically ill adults (4–6, 17, 23). For patients who developed incident delirium, the motoric subtype was categorized as either hyperactive (+CAM-ICU with RASS > 0 or any scheduled antipsychotic use), hypoactive (+CAM-ICU with RASS ≤ 0), or mixed (mix of both hyperactive and hypoactive) (23). Data on the daily ICU use of opioids (≥ 10 IV MME)/d, scheduled nonopioid analgesics (i.e., acetaminophen, pregabalin, and gabapentin), sedatives (i.e., benzodiazepines [≥ 5 mg IV midazolam equivalents/d], IV clonidine, dexmedetomidine, or propofol), antipsychotics (IV haloperidol and quetiapine), and restraints were also collected. Data on ICU days spent with delirium and mechanical ventilation, ICU length of stay, and ICU mortality were also collected.
Analysis
Incident ICU delirium was the primary end point of our analysis; ketamine use was the independent variable and incident delirium the dependent variable. We compared the nine potential delirium risk factors, ICU ketamine use, and ICU days (before delirium or discharge) between the delirium and no-delirium groups. With the exception of the baseline APACHE-IV score, we included all variables significantly different between the delirium and no-delirium groups, ketamine use, and delirium occurrence in a logistic regression model. With only 58 (6.3%) of the study cohort exposed to ketamine and concerns about model overfitting, we eliminated baseline APACHE-IV score as a model variable because daily ICU SOFA score is a more robust estimate of severity of illness during the ICU stay. We, therefore, included six delirium risk variables in the model (i.e., age, modified Charlson Comorbidity Score, and ICU SOFA score, coma, and opioid and benzodiazepine use prior to delirium occurrence [or ICU discharge]). To address our second objective, we compared relevant baseline and ICU variables between ketamine and nonketamine patients who developed ICU incident delirium. Days of medication (e.g., haloperidol) or nonmedication interventions (e.g., restraints) after delirium occurrence were not considered. Dichotomous variable data were presented as n (%) and compared using the chi-square test. All continuous variable data were deemed not to be normally distributed and, thus, were presented as a median (interquartile range [IQR]) and compared using the Wilcoxon rank-sum test. Random data missingness was low (< 5%) for all variables. A p value of less than 0.05 was deemed significant. All analyses were performed using R Version 4.0.3 (R Foundation for Statistical Computing, 2020).
RESULTS
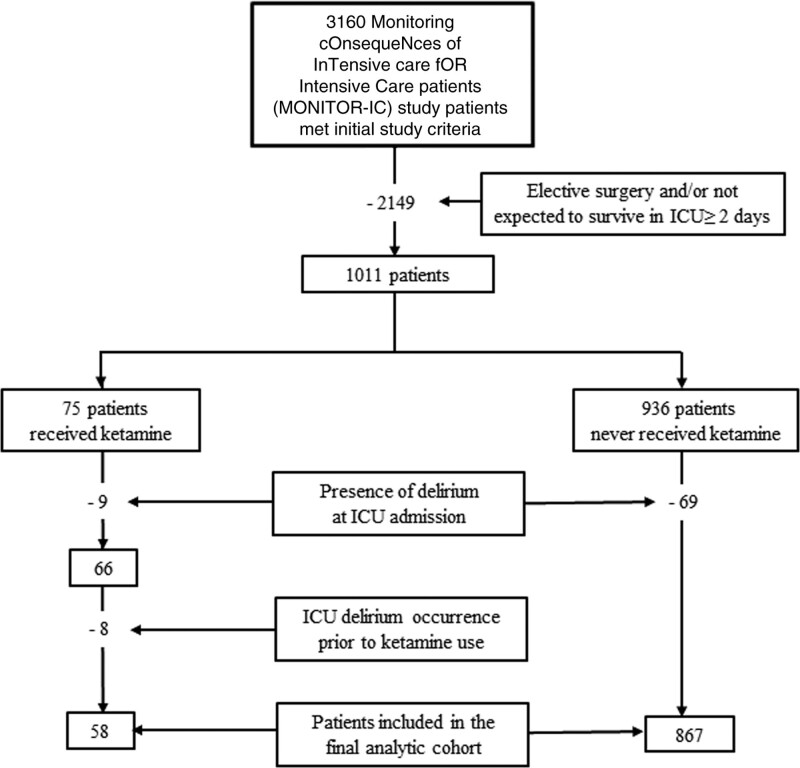
As outlined in Figure 1, among 3,160 MONITOR-IC trial patients screened for participation in the analysis, 2,149 were initially excluded. Among the 1,011 remaining patients, 75 received ketamine and 936 did not. After excluding patients admitted to the ICU with delirium or who developed delirium prior to ketamine use, 925 (58 ketamine and 867 nonketamine) patients were included in the final analysis. No patient received ketamine for a nonanalgosedation indication. Delirium occurred in 332/925 patients (56%) for a median (IQR) 2 (1–5)days.
Figure 1.
Study flowchart.
Univariate analysis revealed age, Modified Charlson Comorbidity score, APACHE-IV score, ketamine use, SOFA score, coma, opioid use, and benzodiazepine use to be significantly higher/greater in the delirium group (Table 1). Baseline cognitive impairment, ICU admission for urgent surgery (vs a medical condition), and days spent in the ICU were not different between delirium and nondelirium groups. The logistic regression analysis revealed ketamine use (adjusted odds ratio [aOR], 5.56; 95% CI, 1.09–28.65; p = 0.04), coma (aOR, 2.10; 95% CI, 1.15–3.78), opioid use (aOR, 171.17; 95% CI, 66.45–553.68), and benzodiazepine use (aOR, 34.07; 95% CI, 8.12–235.34) each to be independently and significantly associated with greater incident ICU delirium (Table 2).
TABLE 1.
Comparison of Potential Delirium Risk Factors Between Patients With and Without ICU Delirium
| Variables | All Patients (n = 925) | Incident Delirium Occurrence | ||
|---|---|---|---|---|
| Yes (n = 332) | No (n = 593) | p | ||
| Pre-ICU admission variables | ||||
| Age, median (IQR) | 62 (51–71) | 64 (53–72) | 60 (49–70) | < 0.01 |
| Modified Charlson Comorbidity Score, median (IQR) | 2 (1–4) | 3 (2–4) | 2 (1–3) | 0.03 |
| Cognitive impairment, n (%) | 39 (4.2) | 17 (5.1) | 22 (3.7) | 0.33 |
| ICU baseline variables | ||||
| Admission type, n (%) | ||||
| Medical | 594 (64.2) | 208 (62.7) | 386 (65.1) | 0.50 |
| Urgent surgery | 331 (35.8) | 124 (37.3) | 207 (34.9) | 0.50 |
| Acute Physiologic and Chronic Health Evaluation IV score, median (IQR) | 67 (52–83) | 76 (62–95) | 61 (47–77) | < 0.01 |
| ICU clinical variablesa | ||||
| Ketamine exposure, n (%) | 58 (6.3) | 54 (16.3) | 4 (0.7) | < 0.01 |
| Sequential Organ Failure Assessment score, median (IQR) | 6.0 (3.6–8.3) | 8.2 (6.0–10.5) | 4.7 (2.9–6.8) | < 0.01 |
| Presence of coma, n (%) | 346 (37.4) | 221 (66.6) | 125 (21.1) | < 0.01 |
| Opioid exposure (IV or oral), n (%)b | 263 (28.4) | 258 (77.7) | 5 (0.8) | < 0.01 |
| Benzodiazepine exposure (IV or oral), n (%)c | 171 (18.5) | 169 (50.9) | 2 (0.3) | < 0.01 |
| ICU days, median (IQR) | 2.0 (1.0–3.8) | 2.8 (0.8–4.8) | 2.0 (1.0–3.0) | 0.30 |
IQR = interquartile range.
aPrior to delirium (or ICU discharge).
bAverage IV morphine equivalent dose ≥ 10 mg/d.
cAverage midazolam equivalent dose ≥ 5 mg/d.
TABLE 2.
Association Between Ketamine Use and Other Variables and ICU Delirium Occurrence
| Variables | Delirium Occurrence | |
|---|---|---|
| Adjusted OR (95% CI) | p | |
| Pre-ICU admission variables | ||
| Age | 1.03 (1.01–1.06) | < 0.01 |
| Modified Charlson Comorbidity Score | 1.08 (0.94–1.22) | 0.26 |
| ICU clinical variablesa | ||
| Ketamine exposure | 5.56 (1.09–28.65) | 0.04 |
| Sequential Organ Failure Assessment score | 1.09 (0.99–1.20) | 0.06 |
| Presence of coma | 2.10 (1.15–3.78) | 0.01 |
| Opioid exposure (IV or oral)b | 171.19 (66.45–553.68) | < 0.01 |
| Benzodiazepine exposure (IV or oral)c | 34.07 (8.12–235.34) | < 0.01 |
OR = odds ratio.
aPrior to delirium (or ICU discharge).
bAverage IV morphine equivalent dose ≥ 10 mg/d.
cAverage midazolam equivalent dose ≥ 5 mg/d.
Boldface entry indicates the primary result of this study.
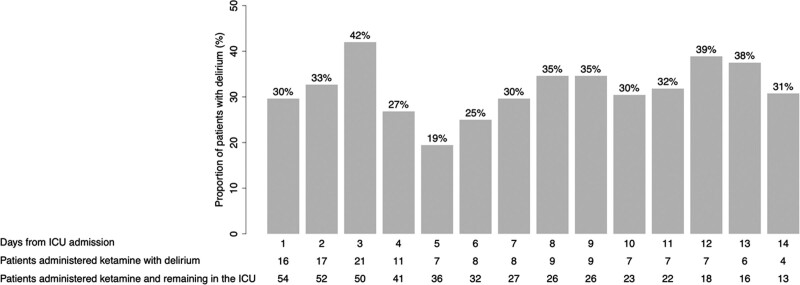
The median (IQR) ketamine dose was more than four times higher in the delirium (vs no delirium) group, but this difference did not reach statistical significance (0.50 mg/kg/hr [0.17–0.83 mg/kg/hr] vs 0.12 mg/kg/hr [0.06–0.29 mg/kg/hr]; p = 0.10). Daily ICU delirium prevalence for ketamine-exposed patients, as a proportion of patients remaining in the ICU on each day, is presented in Figure 2. The results of the comparison between the n = 54 ketamine and n = 278 nonketamine patients who developed delirium are presented in Table 3. The ketamine (vs nonketamine) delirium groups were more likely to be admitted to the ICU for/after urgent surgery and administered an opioid or IV clonidine prior to delirium. However, the average daily opoid dose (in IV MME) on days of opioid exposure prior to delirium occurrence was not different between the two groups. No patient received an antipsychotic prior to delirium occurrence. After delirium occurrence, use of opioids, nonopioid analgesics, sedatives, and antipsychotics were not different between ketamine and nonketamine delirium groups. The duration of ICU delirium and its motoric subtypes were similar between the two groups. Outcomes, including duration of mechanical ventilation, ICU length of stay, and ICU mortality, were also not different between the two groups.
Figure 2.
Daily ICU delirium prevalence for those patients administered ketamine who developed incident delirium (n = 54) based on the patients remaining in the ICU each day.
TABLE 3.
Comparison of Ketamine and Nonketamine Patients Who Developed Incident Delirium
| Variable | Patients With Incident Delirium (n = 332) | p | |
|---|---|---|---|
| Ketamine (n = 54) | Nonketamine (n = 278) | ||
| Baseline | |||
| Age, median (IQR) | 64 (56–71) | 64 (53–72) | 0.95 |
| Modified Charlson Comorbidity Score, median (IQR) | 2 (2–3) | 3 (2–4) | 0.12 |
| Admission type, n (%) | |||
| Urgent surgical | 29 (54) | 95 (34) | 0.01 |
| Medical | 25 (46) | 183 (66) | 0.01 |
| During ICU admission until delirium occurrence | |||
| Sequential Organ Failure Assessment score, median (IQR) | 8 (6–10) | 8 (6–11) | 0.38 |
| Days with coma, median (IQR) | 1 (0–5) | 1 (0–3) | 0.76 |
| Opioid use (IV or oral), n (%)a | 49 (91) | 209 (75) | 0.02 |
| Average daily dose (mg), median (IQR)a | 21 (13–45) | 18 (10–90) | 0.29 |
| Nonopioid analgesic use, n (%) | |||
| Paracetamol (IV or oral) | 46 (85) | 229 (82) | 0.76 |
| Pregabalin | 2 (4) | 10 (4) | 1.00 |
| Gabapentin | 1 (2) | 3 (1) | 0.51 |
| Sedative use, n (%) | |||
| Propofol | 46 (85) | 214 (77) | 0.25 |
| Benzodiazepine (IV or oral)b | 32 (59) | 137 (49) | 0.23 |
| Clonidine (IV) | 18 (33) | 55 (20) | 0.04 |
| Dexmedetomidine | 4 (7) | 33 (12) | 0.47 |
| Antipsychotic use, n (%) | |||
| Haloperidol (IV) | 0 (0) | 0 (0) | - |
| Quetiapine | 0 (0) | 0 (0) | - |
| During ICU admission after delirium occurrence | |||
| Days with delirium, median (IQR) | 2 (1–3) | 2 (1–5) | 0.21 |
| Delirium subtype, n (%) | |||
| Hypoactive | 30 (56) | 138 (50) | 0.52 |
| Hyperactive | 8 (15) | 36 (13) | 0.88 |
| Mixed | 16 (30) | 104 (37) | 0.35 |
| Opioid use, n (%)a | 32 (59) | 159 (57) | 0.90 |
| Nonopioid analgesic use, n (%) | |||
| Paracetamol (IV or oral) | 45 (83) | 241 (87) | 0.66 |
| Pregabalin | 4 (7) | 10 (4) | 0.26 |
| Gabapentin | 0 (0) | 3 (1) | 1.00 |
| Sedative use, n (%) | |||
| Propofol | 30 (56) | 150 (54) | 0.95 |
| Clonidine (IV) | 23 (43) | 109 (39) | 0.75 |
| Benzodiazepine (IV or oral)b | 17 (31) | 64 (23) | 0.25 |
| Dexmedetomidine | 11 (20) | 84 (30) | 0.19 |
| Antipsychotic use, n (%) | |||
| Haloperidol (IV) | 38 (70) | 169 (61) | 0.24 |
| Quetiapine | 5 (9) | 36 (13) | 0.60 |
| Days with physical restraint, median (IQR) | 4 (0–11) | 6 (1–14) | 0.39 |
| During entire ICU admission | |||
| Days with mechanical ventilation, median (IQR) | 5 (2–11) | 7 (3–14) | 0.41 |
| Days in the ICU, median (IQR) | 7 (3–13) | 9 (4–17) | 0.38 |
| Mortality, n (%) | 7 (13) | 36 (11) | 0.19 |
IQR = interquartile range.
aAverage IV morphine equivalent dose ≥ 10 mg/d.
bAverage midazolam equivalent dose ≥ 5 mg/d.
DISCUSSION
Our results suggest ketamine, when administered to critically ill surgical and medical patients requiring analgosedation, may increase the risk for ICU incident delirium. Age, coma, opioid use, and benzodiazepine use were also each independently associated with a greater delirium risk. With the exception that patients admitted to the ICU for urgent surgery were more likely to develop delirium with ketamine and more ketamine patients with delirium received opioid and IV clonidine therapy, other delirium risks, motorotic subtypes, treatments, and ICU outcomes were similar between the ketamine and nonketamine patients who developed delirium.
Ketamine, with its lack of effect on respiratory drive and low propensity to cause hypotension or coma, has advantages over opioids and propofol in critically ill adults requiring analgosedation (1, 2). Ketamine is increasingly being used to optimize ICU patient comfort and safety (3) despite the current lack of a large, multicenter, controlled trial evaluating its analgosedation safety and efficacy (3). The pharmacology of ketamine is complex, and dose-dependent: analgesic effects usually predominate at lower doses (0.1–0.4 mg/kg/hr) and sedative effects at higher doses (0.4–1.0 mg/kg/hr) (1, 2). The ketamine patients in our analysis who developed delirium received a median ketamine infusion dose of 0.5 mg/kg/hr—a dose similar to that reported in other ketamine analgosedation studies (9, 11, 12). The undesirable psychiatric symptoms of ketamine including psychosis and hallucinations are most common at doses greater than or equal to 1 mg/kg/hr (7).
The lower frequency of delirium reported in the ketamine group of Perbert et al's (11) trial compared to our results may be due to the fact ketamine was administered as a fixed-dose infusion 2.5 times lower in their trial than the average dose administered to the patients in our study who developed delirium. The results of our prospective cohort analysis are challenging compared with retrospective, cohort studies where the rigor of delirium assessment was unclear and important ICU risk factors for delirium including daily severity of illness, coma occurrence, and opioid and benzodiazepine exposure were not considered (1, 2, 8–10).
The reason(s) for the very high prevalence of incident delirium we report in the ketamine-exposed patents in our cohort remains unclear. We carefully considered common ICU delirium risk factors and compared delirium characteristics, treatments, and outcomes between the ketamine and no-ketamine patients who developed ICU incident delirium. Although more ketamine-treated patients received opioid therapy prior to delirium, ICU opioid exposure prior to delirium occurrence was included as a variable in our regression model. Although we were not able to evaluate all delirium symptoms, some of which may be directly related to the known clinical response to ketamine therapy (e.g., hallucinations), the motoric subtypes of delirium we report and use of treatments for delirium including restraints, antipsychotics, and dexmedetomidine were similar. Although data on the occurrence of severe agitation were not able to be collected, the use of sedatives (including IV clonidine and IV haloperidol) and restraints was similar between the ketamine and nonketamine delirium groups.
Our study has important strengths. We evaluated close to 1,000 adults who were critically ill (median APACHE-IV score = 67), prospectively collected data on both ketamine use and common delirium risk variables, rigorously evaluated both delirium and coma tid, and ensured patients with delirium prior to ketamine initiation were excluded. We then evaluated the risk of ketamine for incident delirium in a regression model that accounted for delirium risk variables that were different between the delirium and nondelirium groups (4).
Our study also has limitations. The number of patients in our cohort who received ketamine was low; ketamine analgosedation is most often used in patients with high sedative needs. The wide CIs that surround the odds ratios for delirium risk we present weaken the precision of our findings. A consistent approach for ketamine analgosedation prescribing was not in place throughout the study; the specific prescribing rationale for ketamine initiation and titration was not known. The high proportion of ketamine patients who developed incident delirium precluded determining whether the ketamine dose or infusion duration affects this risk. Delirium risk factors not included in our analysis may have affected our results. For patients having delirium prior to or on the same day of ketamine initiation, the contributory effect of ketamine to delirium occurrence remains unknown.
We were not able to consider whether the time from ICU admission to ketamine initiation affects delirium risk. The results of our single-hospital analysis may be different in ICUs where ketamine prescribing practices are different, patients have different delirium risks, or delirium prevention and treatment strategies are different or unavailable (e.g., IV clonidine). Some of the delirium treatment strategies may have been used for nondelirium treatment indications (e.g., haloperidol for postoperative nausea and vomiting). We did not collect data on daily ABCDEF bundle use. Although all ICU variables were collected and considered only before ICU delirium occurrence (if it occurred), formal time-dependent analytic approaches were not used, and thus, residual confounding may have occurred (5, 6). Finally, the presence of psychiatric symptoms known to be associated with ketamine use but not detected by the CAM-ICU (e.g., hallucinations and psychosis) was not collected (4, 7, 20).
CONCLUSION
In conclusion, our study suggests ketamine analgosedation in critically ill surgical and medical adults may contribute to an increased risk for incident ICU delirium. Despite the extensive comparative analyses conducted between the ketamine and nonketamine groups who developed delirium, the mechanisms for delirium in patients receiving ketamine analgesedation remain unclear. Future, well-designed, prospective, randomized trials are needed to carefully evaluate the magnitude of delirium risk associated ketamine analgosedation and whether psychiatric symptoms observed during ketamine therapy truly represent delirium.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hurth KP, Jaworski A, Thomas KB, et al. The reemergence of ketamine for treatment in critically ill adults. Crit Care Med. 2020; 48:899–911 [DOI] [PubMed] [Google Scholar]
- 2.Manasco AT, Stephens RJ, Yaeger LH, et al. Ketamine sedation in mechanically ventilated patients: A systematic review and meta-analysis. J Crit Care. 2020; 56:80–88 [DOI] [PubMed] [Google Scholar]
- 3.Magee C, Hammond D, Rech M, et al. Ketamine in critically ill adult patients: Use, perceptions, and barriers. Crit Care Med. 2021; 49:457 [Google Scholar]
- 4.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 5.Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015; 41:2130–2137 [DOI] [PubMed] [Google Scholar]
- 6.Duprey MS, Dijkstra-Kersten SMA, Zaal IJ, et al. Opioid use increases the risk of delirium in critically ill adults independently of pain. Am Respir Crit Care Med. 2021; 204:566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen KG. Psychiatric side effects of ketamine in hospitalized medical patients administered subanesthetic doses for pain control. Acta Neuropsychiatr. 2014; 26:230–233 [DOI] [PubMed] [Google Scholar]
- 8.Perbet S, Verdonk F, Godet T, et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: A randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018; 37:589–595 [DOI] [PubMed] [Google Scholar]
- 9.Pruskowski KA, Harbourt K, Pajoumand M, et al. Impact of ketamine use on adjunctive analgesic and sedative medications in critically ill trauma patients. Pharmacotherapy. 2017; 37:1537–1544 [DOI] [PubMed] [Google Scholar]
- 10.Groetzinger LM, Rivosecchi RM, Bain W, et al. Ketamine infusion for adjunct sedation in mechanically ventilated adults. Pharmacotherapy. 2018; 38:181–188 [DOI] [PubMed] [Google Scholar]
- 11.Garber PM, Droege CA, Carter KE, et al. Continuous infusion ketamine for adjunctive analgosedation in mechanically ventilated, critically ill patients. Pharmacotherapy. 2019; 39:288–296 [DOI] [PubMed] [Google Scholar]
- 12.Shurtleff V, Radosevich JJ, Patanwala AE. Comparison of ketamine- versus nonketamine-based sedation on delirium and coma in the intensive care unit. J Intensive Care Med. 2020; 35:536–541 [DOI] [PubMed] [Google Scholar]
- 13.Geense W, Zegers M, Vermeulen H, et al. MONITOR-IC study, a mixed methods prospective multicentre controlled cohort study assessing 5-year outcomes of ICU survivors and related healthcare costs: A study protocol. BMJ Open. 2017; 7:e018006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geense WW, Zegers M, Peters MAA, et al. New physical, mental, and cognitive problems 1 year after ICU admission: A prospective multicenter study. Am J Respir Crit Care Med. 2021; 203:1512–1521 [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epid. 2008; 61:344–349 [DOI] [PubMed] [Google Scholar]
- 16.Gosselt AN, Slooter AJ, Boere PR, et al. Risk factors for delirium after on-pump cardiac surgery: A systematic review. Crit Care. 2015; 19:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaal IJ, Devlin JW, Peelen LM, et al. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015; 43:40–47 [DOI] [PubMed] [Google Scholar]
- 18.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001; 29:1370–1379 [DOI] [PubMed] [Google Scholar]
- 21.van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. ; REDUCE Study Investigators. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018; 319:680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 23.Rengel KF, Hayhurst CJ, Jackson JC, et al. Motoric subtypes of delirium and long-term functional and mental health outcomes in adults after critical illness. Crit Care Med. 2021; 49:e521–e532 [DOI] [PMC free article] [PubMed] [Google Scholar]