Abstract
Colorectal cancer liver metastases (CRLMs) are common. Treating CRLMs with thermal ablation can prolong survival, but compared to lesions smaller than 3 cm, local control rates and overall survival are relatively worse with larger, intermediate (3–5 cm) lesions. Local recurrence rates range between 1.7%–20.2% and 6.7%–68.9% for CRLMs less than 3 cm and greater than 3 cm, respectively. Worse outcomes are also present when ablating intermediate size hepatocellular carcinoma (HCC) and there are some pathological similarities with CRLMs, namely the presence of micrometastatic disease. Combining ablation with transarterial chemoembolization is more effective in treating intermediate-size HCC than ablation alone. A meta-analysis of robust randomized controlled trials demonstrated long-term improved survival with combination therapy compared to ablation alone (odds ratio at 1, 3 and 5 years of 2.74, 2.77 and 5.23, respectively). There is, however, minimal evidence for combination therapy in CRLMs, limited to a handful of studies that are predominantly retrospective and have heterogeneous inclusion criteria. Given the difficulty in successfully treating intermediate CRLMs, the strong evidence for combination therapy in intermediate HCC and potential pathological similarities, formal evaluation of combination treatment in CRLM is merited. This review highlights existing evidence for treatment of intermediate-size liver lesions and highlights where trials in CRLMs should focus.
Colorectal cancer was the third most common cancer in the world in 2018 with nearly 2 million cases and it had the second highest number of cancer-related deaths (1). Colorectal liver metastases (CRLMs) are present in around a third of patients with colorectal cancer (2). Surgery, aiming for a complete “R0 resection”, is the gold standard treatment of CRLMs in anatomically and clinically appropriate cases (3), yet despite this, only around a quarter of patients with CRLMs achieve hepatic resection (2). In patients with CRLMs that are not amenable to resection primarily or after systemic therapy and in those unfit for surgery, local ablative therapies can be considered. Ablative therapies can also be considered in conjunction with resection (4) and can prolong survival when used in addition to chemotherapy (5).
These local ablative therapies include both local and locoregional treatments. Microwave ablation (MWA) and radiofrequency ablation (RFA) are local thermal treatments and are now commonly used in the treatment of CRLMs. Locoregional interventions include transarterial chemoembolization (TACE) and radioembolization.
Thermal ablative methods can provide effective local control of CRLM, with the lowest rates of local recurrence achieved in lesions smaller than 3 cm (6, 7). There is evidence that combining thermal ablation with TACE in treating larger hepatocellular carcinoma (HCC) improves long-term survival with no increase in complications (8), but data supporting this combination approach (ablation and TACE or bland embolization) for treatment of larger CRLMs are lacking.
This review aims to examine the current published data evaluating treatment of intermediate-size liver tumors (3–5 cm) with thermal ablation alone and combination therapy with TACE and ablation. Outcome data in HCC is summarized and areas for further research in the field of CRLM are highlighted.
Intermediate-size CRLMs treated with thermal ablation alone
There is a vast amount of data regarding the use of thermal ablation in the treatment of CRLMs. Table summarizes the results of studies including at least 50 CRLMs treated with thermal ablation where an assessment of the effect of lesion size on local recurrence rates was made.
Table.
Published studies of thermal ablation in the treatment of at least 50 colorectal liver metastases with an assessment of lesion size on efficacy
| Reference | Description of study | Number of lesions (patients) undergoing ablation and population description | Local recurrence rates (%): a) <3 cm b) >3 cm c) Any size |
Clinical details |
|---|---|---|---|---|
| Tanis et al., 2014 (17) | RFA arm of an RCT | 167 (55) All had prior systemic therapy. Unresectable CRLMs. No extra-hepatic disease |
a) 4/139 (2.9) b) 6/28 (21.4) c) 10/167 (6.0) |
4.7 years median follow-up 91.1% performed open |
| Abitabile et al., 2007 (11) | Case series RFA | 147 (47) Chemotherapy naïve. High surgical risk or unresectable CRLMs. Resectable extra-hepatic disease not a contraindication, but percentage not described |
a) 2/118 (1.7) b) 11/29 (37.8) c) 13/147 (8.8) |
2.8 years median follow-up 67.1% performed open |
| Kingham et al., 2012 (22) | Case series cryotherapy, RFA and MWA | 315 (158) Majority had prior chemotherapy (92%). Indications and presence of extra-hepatic disease not described |
c) 36/315 (11.4) | 1.4 years median follow-up All but one tumor ablated open Recurrence-free survival 92% ≤1 cm and 49% >3 cm |
| Hammill et al., 2011 (14) | Case series RFA | 236 (101) Majority had prior chemotherapy (82%). Resectable and unresectable CRLMs. No extra-hepatic disease |
a) 3/102 (2.9) b) 9/134 (6.7) c) 12/236 (5.1) |
2.6 year median follow-up All laparoscopic |
| Hamada et al., 2012 (20) | Case series RFA | 141 (84) Majority had prior chemotherapy (85%). Unresectable CRLMs or presence of extra-hepatic disease (27.4%) |
a) 15/106 (14.2) b) 24/35 (68.6) c) 39/141 (27.7) |
2.3 years median follow-up All percutaneous > 3 cm independent predictor of worse survival (HR, 2.30; CI, 1.15–4.61) |
| Shady et al., 2016 (25) | Case series RFA | 233 (165) Unclear how many had prior systemic chemotherapy. Resectable and unresectable CRLMs. 61% extra-hepatic disease |
c) 113/233 (48.5) | 4.6 years median follow-up All percutaneous > 3 cm independent predictor of local tumor progression (HR, 2.0; CI, 1.2–3.3) |
| Bale et al., 2012 (12) | Case series stereotactic RFA | 189 (63) Majority had prior chemotherapy (85%). Resectable and unresectable CRLMs. No extra-hepatic disease |
a) 23/130 (17.7) b) 8/59 (13.6) c) 31/189 (16.4) |
2.1 years median follow-up All percutaneous Tumor size no effect on DFS and OS |
| Kennedy et al., 2013 (15) | Case series RFA | 259 (130) Majority had prior chemotherapy (76%). Resectable and unresectable CRLMs. No extra-hepatic disease |
a) 3/84* (3.6) b) 9/46* (19.6) c) 12/130* (9.2) *per patient |
1.8 years median follow-up All laparoscopic > 2.9 cm worse OS (HR, 1.29; CI, 1.04–1.59) |
| Liu et al., 2017 (26) | Case series RFA and MWA | 83 (134) RFA; 18 (27) MWA 43.6% had prior chemotherapy. Resectable and unresectable CRLMs. Unclear if extra-hepatic disease present |
a) 25/124 (20.2) b) 11/19 (57.9) c) 36/143 (25.2) |
1.8 years mean follow-up All percutaneous >3 cm independent predictor of LTP (not the case with HCC— also assessed in this study) |
| Nielsen et al., 2013 (23) | Case series RFA | 282 (128) Unclear how many had prior chemotherapy. Resectable and unresectable CRLMs. Extra-hepatic disease not a contraindication if local control possible (% not described) |
a) 18/200 (9.0) b) 22/49 (44.9) c) 9/20 (45.0) |
3.0 years median follow-up All open Trend towards worse survival > 3 cm (median 37 vs. 45 months, p = 0.118) |
| Vogl et al., 2014 (27) | Case series LITT | 1545 (594) 36% had prior chemotherapy. Resectable and unresectable CRLMs. 23% extra-hepatic disease |
c) 2/594 (2.0)* *per patient |
1.9 years mean follow-up All percutaneous Maximal metastasis diameter associated with worse OS and PFS |
| Qin et al., 2018 (24) | Case series MWA | 411 Majority had prior chemotherapy (74%). Unclear if CRLMs were resectable or unresectable. 25% extra-hepatic disease |
a) 17/398 (4.3) b) 5/13 (38.5) c) 22/411 (5.4) |
1.5 years median follow-up All percutaneous |
| Solbiati et al., 2012 (16) | Case series RFA | 202 (99) All patients received adjuvant chemotherapy. Resectable and unresectable CRLMs. Oligonodular (< 3) lung disease not a contraindication (percentage not described) |
c) 32/99 (32.3)* *per patient |
4.4 years median follow-up All percutaneous No significant effect of tumor size on LTP, new metastases or overall survival (only 11 lesions >3 cm) |
| Valls et al., 2014 (18) | Case series RFA | 91 (59) Unclear how many received chemotherapy. Unresectable CRLMs. Resectable lung metastases not a contraindication (percentage not described) |
a) 4/66 (6.1) b) 13/25 (52.0) c) 17/91 (18.7) |
2.1 years median follow-up All percutaneous and prior hepatic resection Size > 3 cm an independent risk factor for local recurrence |
| Van Tilborg et al., 2011 (19) | Case series RFA | 237 (100) 43% prior chemotherapy. Unresectable CRLMs. 7% had curable extra-hepatic disease |
a) 8/143 (5.6) b) 22/94 (23.4) c) 30/237 (12.7) |
2.4 years mean follow-up 93.2 % performed open Size a predictor of mean survival |
| Veltri et al., 2008 (10) | Case series RFA | 186 (122) Majority had prior chemotherapy (71%). Unresectable CRLMs. 21% extra-hepatic disease |
a) (33.3)* b) (66.7)* c) 49/186 (26.3) *includes residual unablated tumor |
2.0 years mean follow-up 13.0% laparoscopic 21 lesions performed with “ischemic maneuvers” including 5 TACE |
| Veltri et al., 2012 (9) | Case series RFA | 458 (262) Unclear how many had chemotherapy Unresectable CRLMs. 20% extra-hepatic disease |
2.2 years mean follow-up 7.3% laparoscopic Worse survival >3 cm (21.7 months; CI, 16.6–27.4 vs. 41 months; CI, 32.6–49.7) |
|
| Gu et al., 2018 (13) | Case series RFA | 185 (102) Majority had chemotherapy following RFA (64%). Unresectable CRLMs. No extra-hepatic disease |
c) 29/185 (15.7) | All percutaneous > 3 cm worse OS (HR, 3.9; CI, 1.4–8.8) |
An Ovid Medline search was performed with studies limited to English language and at least 50 participating patients. Where boxes are empty, the data are not reported. Recurrence rates are per lesion unless otherwise stated.
RFA, radiofrequency ablation; RCT, randomized controlled trial; CRLMs, colorectal cancer liver metastases; MWA, microwave ablation; HR, hazard ratio; CI, 95% confidence interval; DFS, disease-free survival; OS, overall survival; LTP, local tumor progression; HCC, hepatocellular carcinoma; LITT, laser-induced interstitial thermotherapy; PFS, progression-free survival; TACE, transarterial chemoembolization.
Amongst the 18 identified studies, a total of 5296 CRLMs were ablated. Two studies likely overlap in terms of patients, but report different outcomes so both are included (9, 10). Nine studies treated patients with curative intent (11–19), four studies had a combination of curative and palliative intent (9, 10, 20, 21) and in five studies, the treatment goal was not clear (8, 22–25). Sixteen studies (9–11, 13–15, 17–20, 22–27) indicated a greater local recurrence rate and/or worse survival in patients with lesions larger than 3 cm. The local recurrence rates were 2.0%–48.5% overall and where separately reported, the range of local recurrence rates for lesions smaller than 3 cm and greater than 3 cm was 1.7%–20.2% and 6.7%–68.9%, respectively. Of the two studies that failed to demonstrate such an association, one used stereotactic navigation software (12) and the other included the lowest number of treated lesions larger than 3 cm (16).
Surgical series demonstrated the presence of micrometastatic disease surrounding CRLMs (28, 29). These are tiny foci of tumor adjacent to, but not directly continuous with the main lesion. Micrometastatic disease was present in over 50% of resected CRLMs and its presence was related to the size of the macrometastasis, being identified in 20% of lesions less than 3 cm, but 87% of CRLMs larger than 3 cm (28). The distance of micrometastatic invasion was also related to lesion size; lesions larger than 3 cm showed an invasion depth of 8.0±8.4 mm (mean ± standard deviation) compared with 4.1±2.9 mm with lesions smaller than 3 cm (28). In another series, which included lesions larger than 4 cm, invasion was always less than 10 mm (29). A nodular contour to CRLM specimens was associated with the presence of micrometastases and worse survival in a surgical series (30) and this feature on imaging also independently predicted survival following hepatic resection (31), but the impact on ablation efficacy has not been assessed.
The presence of micrometastatic disease with CRLMs (not appreciable by imaging) explains the importance of achieving an ablation zone margin of at least 10 mm where feasible (6, 25, 32). Predicting ablation zone size is notoriously difficult as manufacturer thermal dosimetry data is often based on ex vivo non-diseased liver (33), and thermal tissue properties vary in disease, with perfusion and with prior treatments. The maximum achievable ablation zone is frequently around 4–5 cm in human clinical studies (33). Given the need to strive for 1 cm margins, it is little surprise that poorer outcomes are achieved when treating lesions greater than 3 cm. Tumors above this size would likely need more than one needle position (but it is difficult to predict ablation zones in this manner) or simultaneous treatment with more than one probe and the use of stereotactic software. Given the elliptical or spherical ablation zones produced, it is challenging to accurately predict ablation zone overlap and the exact size and shape of the overall ablation zone. Many lesions are difficult to visualize with computed tomography (CT) or ultrasound, making planning, delivery and verification of treatment more complex. Augmenting ablative treatment by combining with TACE to increase the volume of treatment and subsequently the size of lesions that can be successfully treated is therefore of interest.
Combination therapy in HCC
The modified Barcelona Clinic Liver Cancer (BCLC) staging is widely used to guide HCC management and indicates ablation as a curative treatment option for very early stage or early stage disease (34). This corresponds to patients with a performance status of 0, preserved liver function and up to 3 tumors at less than 3 cm. TACE is suggested as a non-curative therapy for intermediate stage disease, where there are more than 3 lesions or at least one lesion larger than 3 cm and no portal vein invasion or extrahepatic spread.
The definition of BCLC intermediate stage results in a heterogeneous group of patients and tumors, and efforts have been made to subclassify this group to help clarify which patients are best suited to TACE or other therapies (35). What is clear is that treatment of intermediate-size (3–5 cm) HCC with TACE resulted in worse survival relative to smaller lesions (36) and, paralleling the findings in CRLM, greater rates of local tumor progression (LTP) were seen when ablating HCC larger than 3 cm (37).
Given the limitations of treating intermediate-size HCC, combination therapy with TACE and ablation has been investigated and several prospective studies have evaluated this therapeutic strategy. Randomized controlled trials (RCTs) treating intermediate size (mean tumor size, 3.4–3.7 cm) HCCs in 37–189 patients consistently demonstrated less recurrence (38–40) and also improved survival (39, 40) at long-term follow-up when TACE is added to ablation, compared with ablation alone. A meta-analysis combining these data and those from other RCTs demonstrated improved survival at 1, 3 and 5 years (odds ratio, 2.74, 2.77 and 5.23, respectively) with combination therapy and no difference in complication rates (8). On subgroup analysis, one study demonstrated that tumor number and size predicted overall survival (39).
The ablation procedure can be performed on the same day as the TACE (38) or within 2 weeks (39). Conventional TACE (cTACE) was performed with epirubicin in all studies and carboplatin and mitomycin additionally in two of the studies (39, 40). Interestingly, one study showed that the ablation zones in the combined group were larger with a significant increase in the short-axis (5.8 by 5 cm compared to 5 by 4.1 cm) resulting in a more spherical ablation zone, despite fewer needle insertions (38). These studies do not report on whether there was an effect on the rate of liver transplantation, but this is an interesting area for consideration.
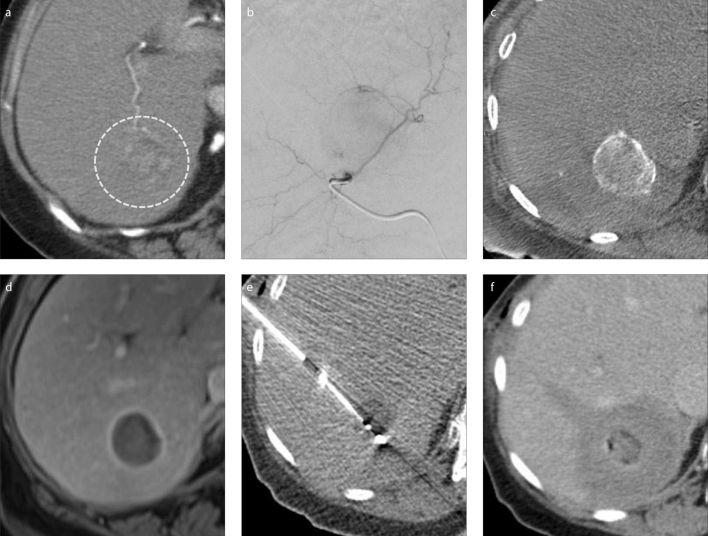
There are several explanations for this clinical benefit. Given that larger ablation zones with combination therapy are demonstrated (38, 41), this may help treat adjacent micrometastatic disease which is also described in HCC (42). Reducing hepatic perfusion via the arterial or portal systems increases the amount of coagulative necrosis achievable (43) and the larger ablation zones achieved with combination therapy may well be due to reduced heat sink from the embolized arterial vasculature. Furthermore, arterio-portal shunting results in embolic material reaching the portal system additionally (44) and could further help combat the heat sink effect. The local delivery of the chemotherapy should further aid in treatment of the tumor and surrounding micrometastatic disease. Finally, performing embolization first may aid in identification and targeting of the tumor during CT-guided ablation. This can be achieved with lipiodol if cTACE is performed or with radiopaque embolic material such as the DC Bead LUMI (BTG) that can also be loaded with chemotherapy. Ablation is generally performed following embolization to take advantage of the reduced perfusion and the potentially improved visualization (45). An example of combination therapy with an intermediate HCC is shown (Fig.).
Figure. a–f.
Preprocedural axial CT (a) shows a 3.5 cm arterialized hepatocellular carcinoma (HCC) (circle). Digital subtraction angiogram (b) indicates the treatment position for transarterial chemoembolization (TACE). Unenhanced cone beam CT performed directly after TACE (c) shows contrast being entrapped between the beads in the target lesion. Follow-up MRI at 1 month (d) shows the lesion to be devascularized. In panel (e), the HCC had shrunk prior to treatment with microwave ablation and the hypoattenuating appearance post TACE helped allow visualization. Immediate post-procedural CT (f) shows an augmented ablation zone following TACE, with an appropriate margin. The patient remains disease-free at long-term follow-up.
Combination therapy in CRLMs
The European Society of Medical Oncology suggests that TACE can be considered for patients with oligometastatic CRLMs that are failing chemotherapy (3). This is most frequently performed with irinotecan-loaded beads. The most robust evidence to support this is obtained from a 2012 RCT that demonstrated improved survival of irinotecan TACE compared to FOLFIRI systemic chemotherapy (median 22 versus 15 months) in patients who had received previous chemotherapy (46).
There are no prospective, randomized trials assessing the addition of chemoembolization to the use of ablation in treating CRLMs. A recent, single-arm prospective phase II trial included 25 patients with 38 CRLMs of mean size 2.2 cm (range, 1.0–4.2 cm) (47). RFA was performed immediately after chemoembolization of a lobar or segmental hepatic artery with degradable starch microspheres (a temporary embolic agent) mixed with mitomycin C. Local tumor progression developed in 4 tumors (10.5%), 3 larger than 3 cm, over nearly a 3 year follow-up period, and overall and recurrence-free survival were 88.0% (95% confidence interval, 75.3%–98.5%) and 63.3% (44.2%–82.5%), respectively. These results are comparable with ablation-only results from other studies with lesions of similar sizes (11–15, 17–19, 22, 24).
A large retrospective series (21) compared patients with chemotherapy resistant CRLMs treated palliatively with selective cTACE (233 patients) to those treated with selective cTACE as a neoadjuvant therapy prior to CT-guided MWA or laser-induced thermotherapy (219 patients and time interval between embolization and ablation not stated). The palliative cTACE-only group had more advanced extrahepatic and nodal disease at baseline. Mean lesion size prior to the first cTACE was 5.1 cm (range, 1.1–15.6 cm) in the cTACE-only group and only 3.1 cm (0.5–13.4 cm) in the combination group, with the latter group shrinking to 2.2 cm (0.5–5.5 cm) prior to ablation. Overall survival (OS) and progression-free survival (PFS) was 12.6 and 5.9 months in the cTACE group compared with 25.8 and 10.8 in the combination group. CRLM size was a prognostic factor for OS and PFS.
Other small series (48–51) of combination therapy (range, 26–44 lesions) treating lesions of 3.3–4.7 cm mean diameter report local recurrence rates of 31.2%–44.4%, but in three of these series, non-colorectal liver metastases make up only 36.9%–57.1% of cases. In one such series, where CRLM outcomes were reported separately, 6 lesions larger than 5 cm had 50% local recurrence at 6 months, compared to 20% with lesions smaller than 5 cm (48).
Given that HCC is typically more vascular than CRLMs, it may be more difficult to perform selective TACE with the latter. The limited data to date for combination therapy describes different approaches to the catheter position for embolization (21, 47). The most widely described protocol for TACE of CRLMs recommends a lobar catheter position (52). This protocol is for more widespread disease than would be suitable for ablation and clearly if there is more focal disease with a well demonstrated arterial supply on angiography, then more targeted embolization could be performed as part of combination therapy, particularly if the goal is to improve visualization for ablation. If the lesion(s) for combination treatment cannot be appreciated on angiography or the vessels cannot be catheterized, then, dependent on hepatic function and treatment history, a more proximal embolization of the arterial territory in question could still be considered, with the theoretical benefits of reduced perfusion and heat sink.
It does, however, remain to be seen whether the success of combination therapy in HCC would be transferable to CRLMs given the different pathogenesis. As stated, there are some pathological similarities in terms of the adjacent micrometastatic disease, but as CRLMs are less arterialized relative to HCC, they may not benefit as much in terms of the reduced heat sink from prior embolization.
One study demonstrated that temporary hepatic or portal vein balloon occlusion was effective in achieving local control with RFA for lesions (the majority were CRLMs) abutting large veins where the hepatic tumors were smaller than 3.5 cm (53). LTP for this group was 11% and was equivalent to RFA only of similar size lesions away from big veins (LTP 9%). Venous occlusion did not affect LTP for peri-venous lesions larger than 3.5 cm (LTP 40%). Reduced arterial inflow and locally administered chemotherapy may therefore provide benefit in treating these larger tumors where venous-mediated heat sink does not appear to be the factor limiting efficacy of ablation.
Alternate options and future directions
Other options to combination therapy to treat intermediate CRLMs should be considered. Placing RFA or MWA probes in several positions to treat larger tumors can be performed, but this lacks precision without stereotactic techniques. Some impressive results are demonstrated with stereotactic ablation (12, 54), but the additional hardware, software and therefore subsequent cost have perhaps limited more widespread use to date. The role of stereotactic radiotherapy and radioembolization in treating intermediate CRLMs should also not be overlooked.
Given the advantages of combination therapy in treating intermediate-size HCC, the potential pathological similarities and the widespread use of TACE to treat CRLMs, combination therapy warrants prospective research. This should initially take the form of a prospective series with well-defined inclusion criteria, combining TACE and ablation in pre-treated, non-surgical candidates with intermediate CRLMs in order to assess safety and efficacy. Following this, prospective RCTs may be justified.
Conclusion
Thermal ablation is an important treatment option for patients who cannot undergo surgery for CRLMs, but above 3 cm the efficacy of ablation is reduced. This is likely due to the limited ablation zone sizes that can be reproducibly achieved and the presence of micrometastatic disease. Reduced efficacy of thermal ablation in treating intermediate-sized HCC is also demonstrated, yet RCTs have shown that combining ablation with TACE in treating intermediate-size HCC reduces recurrence and improves survival. The published evidence regarding combination therapy in CRLMs is very limited. Given the high rates of LTP with thermal ablation of intermediate-size CRLMs and a theoretical benefit of combination therapy in this field, robust prospective evaluation is required in order to assess whether the benefits demonstrated in HCC are transferable. Due to theoretical advantages of improved visualization and reduced heat sink, embolization should be performed before ablation of CRLMs in such prospective studies.
Main points.
Local control and survival are worse when ablating colorectal liver metastases larger than 3 cm.
Combining transarterial chemoembolization with ablation improves outcomes when treating larger hepatocellular carcinomas (3–5 cm).
To date, there is minimal evidence regarding the use of this combination treatment for larger colorectal liver metastases. Prospective studies investigating combination therapy in this setting are justified.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Colon cancer; National Comprehensive Cancer Network [Internet] 2019. [cited June 2019]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 5.Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: Results of a randomized phase II trial. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Futterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases - a meta-analysis. HPB (Oxford) 2017;19:749–756. doi: 10.1016/j.hpb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2013;25:187–194. doi: 10.1097/MEG.0b013e32835a0a07. [DOI] [PubMed] [Google Scholar]
- 9.Veltri A, Guarnieri T, Gazzera C, et al. Long-term outcome of radiofrequency thermal ablation (RFA) of liver metastases from colorectal cancer (CRC): Size as the leading prognostic factor for survival. Radiol Med. 2012;117:1139–1151. doi: 10.1007/s11547-012-0803-3. [DOI] [PubMed] [Google Scholar]
- 10.Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: Small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948–956. doi: 10.1007/s00270-008-9362-0. [DOI] [PubMed] [Google Scholar]
- 11.Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007;33:67–71. doi: 10.1016/j.ejso.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol. 2012;22:930–937. doi: 10.1007/s00330-011-2314-0. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Huang Z, Gu H, et al. Does the site of the primary affect outcomes when ablating colorectal liver metastases with radiofrequency ablation? Cardiovasc Intervent Radiol. 2018;41:912–919. doi: 10.1007/s00270-018-1937-9. [DOI] [PubMed] [Google Scholar]
- 14.Hammill CW, Billingsley KG, Cassera MA, Wolf RF, Ujiki MB, Hansen PD. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol. 2011;18:1947–1954. doi: 10.1245/s10434-010-1535-9. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy TJ, Cassera MA, Khajanchee YS, Diwan TS, Hammill CW, Hansen PD. Laparoscopic radiofrequency ablation for the management of colorectal liver metastases: 10-year experience. J Surg Oncol. 2013;107:324–328. doi: 10.1002/jso.23268. [DOI] [PubMed] [Google Scholar]
- 16.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 17.Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the european organisation for research and treatment of cancer #40004 and #40983. Eur J Cancer. 2014;50:912–919. doi: 10.1016/j.ejca.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Valls C, Ramos E, Leiva D, Ruiz S, Martinez L, Rafecas A. Safety and efficacy of ultrasound-guided radiofrequency ablation of recurrent colorectal cancer liver metastases after hepatectomy. Scand J Surg. 2015;104:169–175. doi: 10.1177/1457496914553147. [DOI] [PubMed] [Google Scholar]
- 19.Van Tilborg AA, Meijerink MR, Sietses C, et al. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: A potentially curative intervention. Br J Radiol. 2011;84:556–565. doi: 10.1259/bjr/78268814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: Prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30:567–574. doi: 10.1007/s11604-012-0089-0. [DOI] [PubMed] [Google Scholar]
- 21.Vogl TJ, Lahrsow M, Albrecht MH, Hammerstingl R, Thompson ZM, Gruber-Rouh T. Survival of patients with non-resectable, chemotherapy-resistant colorectal cancer liver metastases undergoing conventional lipiodol-based transarterial chemoembolization (cTACE) palliatively versus neoadjuvantly prior to percutaneous thermal ablation. Eur J Radiol. 2018;102:138–145. doi: 10.1016/j.ejrad.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Kingham TP, Tanoue M, Eaton A, et al. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19:834–841. doi: 10.1245/s10434-011-2048-x. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen K, van Tilborg AA, Meijerink MR, et al. Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg. 2013;37:1340–1347. doi: 10.1007/s00268-013-1997-6. [DOI] [PubMed] [Google Scholar]
- 24.Qin S, Liu GJ, Huang M, et al. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: A review of a 4-year experience at a single center. Int J Hyperthermia. 2019;36:36–43. doi: 10.1080/02656736.2018.1528511. [DOI] [PubMed] [Google Scholar]
- 25.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: Factors affecting outcomes--a 10-year experience at a single center. Radiology. 2016;278:601–611. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Huang GL, Xu M, et al. Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: A comparison of local therapeutic efficacy. Int J Hyperthermia. 2017;33:446–453. doi: 10.1080/02656736.2017.1278622. [DOI] [PubMed] [Google Scholar]
- 27.Vogl TJ, Dommermuth A, Heinle B, et al. Colorectal cancer liver metastases: Long-term survival and progression-free survival after thermal ablation using magnetic resonance-guided laser-induced interstitial thermotherapy in 594 patients: Analysis of prognostic factors. Invest Radiol. 2014;49:48–56. doi: 10.1097/RLI.0b013e3182a6094e. [DOI] [PubMed] [Google Scholar]
- 28.Nanko M, Shimada H, Yamaoka H, et al. Micrometastatic colorectal cancer lesions in the liver. Surg Today. 1998;28:707–713. doi: 10.1007/BF02484616. [DOI] [PubMed] [Google Scholar]
- 29.Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84:1077–1080. doi: 10.1046/j.1365-2168.1997.02743.x. [DOI] [PubMed] [Google Scholar]
- 30.Yasui K, Hirai T, Kato T, et al. A new macroscopic classification predicts prognosis for patient with liver metastases from colorectal cancer. Ann Surg. 1997;226:582–586. doi: 10.1097/00000658-199711000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagakura S, Shirai Y, Hatakeyama K. Computed tomographic features of colorectal carcinoma liver metastases predict posthepatectomy patient survival. Dis Colon Rectum. 2001;44:1148–1154. doi: 10.1007/BF02234637. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiter SJS, Heerink WJ, de Jong KP. Liver microwave ablation: A systematic review of various FDA-approved systems. Eur Radiol. 2019;29:4026–4035. doi: 10.1007/s00330-018-5842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 36.Hu HT, Kim JH, Lee LS, et al. Chemoembolization for hepatocellular carcinoma: Multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol. 2011;22:917–923. doi: 10.1016/j.jvir.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 38.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: A randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer. 2010;116:5452–5460. doi: 10.1002/cncr.25314. [DOI] [PubMed] [Google Scholar]
- 39.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: A prospective randomized trial. J Clin Oncol. 2013;31:426–432. doi: 10.1200/JCO.2012.42.9936. [DOI] [PubMed] [Google Scholar]
- 40.Yi Y, Zhang Y, Wei Q, et al. Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone. Chin J Cancer Res. 2014;26:112–118. doi: 10.3978/j.issn.1000-9604.2014.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47–53. doi: 10.1055/s-1999-14233. [DOI] [PubMed] [Google Scholar]
- 42.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/S1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 44.Kan Z, Sato M, Ivancev K, et al. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: Experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- 45.Young S, Golzarain J. Locoregional therapies in the treatment of 3- to 5-cm hepatocellular carcinoma: Critical review of the literature. AJR Am J Roentgenol. 2020:1–12. doi: 10.2214/AJR.19.22098. [DOI] [PubMed] [Google Scholar]
- 46.Fiorentini G, Aliberti C, Tilli M, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (debiri) versus intravenous therapy (folfiri) for hepatic metastases from colorectal cancer: Final results of a phase iii study. Anticancer Res. 2012;32:1387–1395. [PubMed] [Google Scholar]
- 47.Yamakado K, Inaba Y, Sato Y, et al. Radiofrequency ablation combined with hepatic arterial chemoembolization using degradable starch microsphere mixed with mitomycin c for the treatment of liver metastasis from colorectal cancer: A prospective multicenter study. Cardiovasc Intervent Radiol. 2017;40:560–567. doi: 10.1007/s00270-016-1547-3. [DOI] [PubMed] [Google Scholar]
- 48.Alexander ES, Mick R, Nadolski GJ, Mondschein JI, Stavropoulos SW, Soulen MC. Combined chemoembolization and thermal ablation for the treatment of metastases to the liver. Abdom Radiol (NY) 2018;43:2859–2867. doi: 10.1007/s00261-018-1536-x. [DOI] [PubMed] [Google Scholar]
- 49.Kan XF, Wang Y, Lin GC, et al. Radiofrequency ablation combined with transarterial chemoembolization for liver metastases from gastrointestinal cancers. J Huazhong Univ Sci Technolog Med Sci. 2016;36:200–204. doi: 10.1007/s11596-016-1566-y. [DOI] [PubMed] [Google Scholar]
- 50.Wu ZB, Si ZM, Qian S, et al. Percutaneous microwave ablation combined with synchronous transcatheter arterial chemoembolization for the treatment of colorectal liver metastases: Results from a follow-up cohort. Onco Targets Ther. 2016;9:3783–3789. doi: 10.2147/OTT.S105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonomo G, Della Vigna P, Monfardini L, et al. Combined therapies for the treatment of technically unresectable liver malignancies: Bland embolization and radiofrequency thermal ablation within the same session. Cardiovasc Intervent Radiol. 2012;35:1372–1379. doi: 10.1007/s00270-012-0341-0. [DOI] [PubMed] [Google Scholar]
- 52.Lencioni R, Aliberti C, de Baere T, et al. Transarterial treatment of colorectal cancer liver metastases with irinotecan-loaded drug-eluting beads: Technical recommendations. J Vasc Interv Radiol. 2014;25:365–369. doi: 10.1016/j.jvir.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 53.de Baere T, Deschamps F, Briggs P, et al. Hepatic malignancies: Percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology. 2008;248:1056–1066. doi: 10.1148/radiol.2483070222. [DOI] [PubMed] [Google Scholar]
- 54.Schullian P, Johnston EW, Putzer D, Eberle G, Laimer G, Bale R. Safety and efficacy of stereotactic radiofrequency ablation for very large (>/=8 cm) primary and metastatic liver tumors. Sci Rep. 2020;10:1618. doi: 10.1038/s41598-020-58383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]