Abstract
Critically ill patients admitted to the intensive care unit require continuous monitoring of vital functions as well as mechanical and pharmacological support, provided through different devices. Chest radiographs play a fundamental role in monitoring the conditions of these patients and assessing the intensive-care devices after their insertion; therefore, the radiologist needs to know their normal appearance and their correct position and should be aware of the possible complications that may occur after their placement. This pictorial review illustrates the radiographic appearance of non-cardiological devices commonly used in clinical practice (central venous catheters, tunneled catheters, Swan-Ganz catheters, chest tubes, endotracheal tubes, and nasogastric tubes), their correct position and the most common complications that may occur after their placement.
Critically ill patients in the intensive care unit require continuous monitoring of vital functions and mechanical and pharmacological support, provided through different devices. Inserting intensive-care devices is a common medical practice but complications may occur and a chest X-ray radiography (CXR) should be performed immediately after placement (1). The widespread availability along with low radiation exposure and low costs, give CXR a decisive role in these settings to assess the position of the device, the response to therapy and the occurrence of any complications (2) (Fig. 1). Radiologists should be aware of the normal appearance of these devices and promptly recognize any abnormal findings.
Figure 1.

A technically correct bedside chest X-ray performed in the intensive care unit. The exam allows to evaluate the position of inserted chest devices (chest tubes, black asterisks; endotracheal tube, white asterisk; pulmonary artery catheter, black arrowhead; nasogastric tube – proximal portion, white arrowhead) and to detect the presence of bilateral pleural effusions (white arrows) and the occurrence of soft tissue emphysema (black arrow). The patient underwent heart surgery and prosthetic valves, median sternotomy wires and external cardiac monitor wires are also present.
This pictorial review illustrates the radiographic appearance of commonly used non-cardiological devices (Table) and the most common complications that may occur after their placement.
Table.
Main noncardiologic intensive-care devices, their insertion route and appropriate position
| Insertion sites | Correct position | |
|---|---|---|
| Central venous catheter | Internal jugular, subclavian, axillary or femoral vein | Distal tip within the superior vena cava, slightly above to the right atrium |
| Tunneled (Tesio) catheter | Internal jugular, subclavian, or femoral vein | Distal tips in the superior vena cava and in the right atrium |
| Pulmonary artery (Swan-Ganz) catheter | Internal jugular, subclavian, or femoral vein | Distal tip in the right or left main pulmonary artery |
| Chest tube | Through the chest wall where the mid-axillary line meets the nipple line in men, or the infra-mammary fold in women. Based on the type of effusion present, and where it accumulates, the insertion site may vary | Distal tip and catheter’s side holes within the pleural space |
| Endotracheal tube | Mouth | Distal tip at least 2 cm and no more than 6 cm above the carina |
| Nasogastric tube | Nostril | Distal tip in the left hypochondrium, at least 10 cm below the gastro-esophageal junction |
Central venous catheter
Central venous catheters (CVC) are used for fluid and/or drug administration and hemodynamic monitoring (3). They are most commonly inserted through the right internal jugular vein, otherwise a subclavian, axillary, or less frequently a femoral vein is used. Peripherally inserted central catheters are small caliber tubes usually positioned through antecubital veins with the advantage that they can be left in place for several months.
The CVC, with the exception of the femoral access, runs supero-inferiorly within the superior vena cava (SVC) towards the heart. The distal end should be parallel to the long axis of the SVC and should not form acute angles with the vessel wall or the heart. The tip should be inside the SVC, just above the right atrium (Fig. 2a), distal to the venous valves (the last of which is usually at the level of the first anterior rib) but still outside the right atrium to avoid cardiac complications like arrhythmias, endocardial damage or pericardial tamponade or perforation (4, 5).
Figure 2. a, b.
Panel (a) shows a central venous catheter (arrow) inserted through the right jugular vein. The distal end is within the SVC, parallel to its long axis. Panel (b) shows a tunneled catheter inserted through the right jugular vein with the distal tips projecting at the right atrium and at the junction of the right atrium and superior vena cava (black arrows).
Tunneled catheters are alternative means of vascular access for temporary hemodialysis in patients with reversible acute kidney injury or awaiting surgical creation of long-term vascular access. They include two 10 F plastic catheters inserted independently into the venous system, preferably through the right internal jugular vein, with the distal tips placed in the right atrium and at the junction of the right atrium and superior vena cava, respectively (6) (Fig. 2b).
Almost a third of CVC are positioned incorrectly, possibly resulting in inaccurate venous pressure measurements and infusion of potentially toxic substances in ectopic sites (7). Misplacements include an abnormal path in a collateral or a contralateral vessel (Fig. 3a, 3b), usually easily detected, or looping, kinking or knotting of the catheter. Pneumothorax occurs in approximately 6% of cases (Fig. 3c), being more frequent with a subclavian vein access (8). Inadvertent arterial puncture is a rare but serious complication that may lead to mediastinal and neck hematoma, which appear as a focal enlargement of the mediastinum (enlarged paratracheal stripe) and a bump in the soft tissues of the neck.
Figure 3. a–c.
Panel (a) shows a malpositioned CVC inserted through the right jugular vein (white arrow): the distal tip enters the ipsilateral subclavian vein (arrowhead). Panel (b) shows a malpositioned CVC, inserted through the right subclavian vein, entered the left subclavian vein (arrow). Panel (c) shows chest X-ray of a patient after insertion of a CVC through the left jugular vein (arrowheads). The catheter is positioned correctly but left apical pneumothorax occurred (arrow).
Late complications include infections, embolization, venous obstruction or vessel perforation (7). Vascular thrombosis usually occurs after a prolonged stay of the catheter and ultrasound or CT are generally needed for diagnosis (9). Vessel perforation and consequent infusion of fluids into ectopic sites is a potentially fatal complication. In this case, the catheter or parts of it appear outside the anatomical projection of the vessel and a widening of the mediastinum, an enlargement of the cardiac silhouette secondary to pericardial tamponade, or a new pleural effusion may occur (4) (Fig. 4). A tip against the vessel wall may be a sign of impending perforation and must be reported (7).
Figure 4. a–e.
SVC perforation (a–c). Panel (a) shows a dialysis patient who had a CVC in place; (b), the CVC was removed and a tunneled catheter was inserted (white arrow). The right mediastinum appears enlarged (black arrow) compared to (a) and the normal mediastinal lines cannot be recognized, findings suspicious for occurrence of mediastinal hematoma. CT image (c) confirms the presence of mediastinal hematoma (black arrow) demonstrating that one of the two tips of the catheter is malpositioned, passing through the right wall of the SVC (white arrow). Right jugular vein perforation (d, e). X-ray image (d) shows a malpositioned CVC, inserted through the right subclavian vein and with the distal end within the right jugular vein. A mild soft tissue swelling can be appreciated (white arrows). CT scan (e) demonstrates a right jugular vein perforated by the catheter (black arrow).
Pulmonary artery catheter
Pulmonary artery catheters (PAC), frequently referred to as Swan-Ganz catheters, are used to detect heart failure or sepsis, monitor therapy and the effects of drugs. Moreover, PACs allow simultaneous measurements including pulmonary arterial pressure, right atrial pressure, right ventricular pressure and pulmonary capillary wedge pressure, which provides an indirect estimate of left atrial pressure. The most common conditions that require their placement are right ventricular failure, pulmonary hypertension, cardiac surgery, or the need to determine whether a pulmonary edema is cardiogenic or noncardiogenic in origin. PACs are two- or three-lumen polyvinyl chloride catheters, 60 to 110 cm long, with a variable caliber (4–8 F). The smallest one is close to the tip and is connected to an inflatable balloon that obstructs temporarily the lumen of the vessel: the pressure measured at the catheter’s tip approximates that of the left atrium (10).
A PAC is inserted through the subclavian or internal jugular vein. The balloon should be inflated only during placement and while measurements are being obtained to reduce the risk of pulmonary infarction. The distal tip should be in the right or left pulmonary artery or in the proximal portion of one of the lobar branches (11) (Fig. 5a). Frequently, the tip is placed too distal, i.e., more than 1 cm lateral to the mediastinal margin, or too proximal, i.e., within the main pulmonary artery or right ventricle (12).
Figure 5. a, b.
Chest X-ray (a) shows a Swan-Ganz catheter inserted through the right internal jugular vein, where the introducer can be recognized (black arrow); the distal tip is in the main pulmonary artery (white arrow). Chest X-ray (b) of another patient shows kinking of the distal end of the Swan-Ganz catheter, with the tip within the left pulmonary artery (white arrows)
Complications ensue in about 10% of cases and are usually minor (1). They include catheter looping, kinking (Fig. 5b), knotting, or pneumothorax (in about 2% of cases) (1). When the catheter is placed too distally, there may be arterial perforation with hemorrhage or lung infarction, which appears on CXR as a wedge-shaped pleural-based pulmonary opacity. When the catheter is too proximal, cardiac complications may occur (3).
Pleural tube
Pleural tubes are used to drain pneumothorax and pleural effusion, therefore are routinely used after thoracic surgeries, traumas, spontaneous pneumothorax or fluid collections (Fig. 6a). They are polyvinyl chloride or silicone tubes, 8–40 F in diameter, equipped with a radio-opaque band and a metal spindle and fenestrated at the apex. Smaller catheters are preferred in case of pneumothorax, while larger tubes (28–40 F) are used for hemorrhagic, lymphatic or serous fluid evacuations (13). They are inserted through the chest wall in the pleural space. In case of pneumothorax or hemothorax, the insertion site is where the mid-axillary line meets the nipple line in men, or the infra-mammary fold in women. For other conditions, the site varies according to where the fluid accumulates. The catheter’s side holes should be within the pleura.
Figure 6. a, b.
Chest X-ray (a) of a patient who had a chest tube inserted (arrow) to drain a massive pneumothorax that caused collapse of the right lung. After insertion of the chest tube, the pneumothorax is drained and the lung is re-expanded. Chest X-ray (b) of a patient who had a chest tube placed to drain a right pleural effusion. Pneumothorax occurred after insertion.
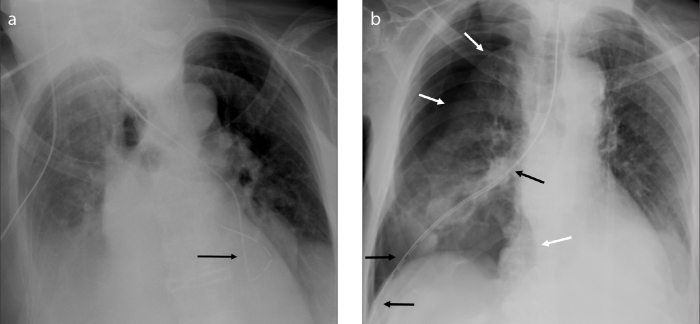
Approximately 10% of tubes are misplaced, therefore a CXR is mandatory to assess their position, the success of drainage and the occurrence of any complications (1). These may be related to fluid drainage, (pneumothorax) (Fig. 6b), insertion of the tube (arterial puncture, bleeding, damage to intercostal nerves, secondary infection of the pleural space, diaphragm perforation, injuries to thoracic or abdominal organs) (Fig. 7a, 7b), or rupture of the tube itself (14) (Fig. 7c). After prolonged stay, a radiotransparent image can sometimes be recognized where the tube was located (“ghost drainage” sign) (15) (Fig. 8).
Figure 7. a–c.
Chest X-ray (a) was performed after insertion of a chest tube to drain a massive right pleural effusion. A thin air crescent can be recognized under the left hemidiaphragm (arrow). CT scan (b) shows laceration of liver parenchyma (arrow) by the malpositioned pleural tube and confirms the presence of sub-diaphragmatic air. Chest X-ray (c) after removal of a chest tube in another patient demonstrates the presence of a ruptured tube fragment (white arrow) and soft tissue emphysema (black arrows).
Figure 8.

“Ghost drainage” sign (arrows). After prolonged stay of a chest tube, a radiotransparent image can sometimes be recognized on chest X-rays where the tube was previously located, particularly in a fibrotic lung.
Endotracheal tube
Endotracheal tubes allow mechanical ventilation of the airways, aspiration of bronchial secretions or prevention of aspiration in unconscious patients. They are flexible plastic tubes with an ample lumen, generally 1 cm maximum for adults, inserted through the mouth in the trachea. When the tube is in place, a low-pressure balloon close to the distal end is inflated to 18–22 mmHg and keeps the tube in its final position, avoids air leaks during ventilation, limits trauma to the tracheal mucosa and protects the airways from blood or saliva aspiration.
On an anteroposterior CXR, the endotracheal tube appears as a vertical radiopaque stripe. The tip should project at least 2 cm and no more than 6 cm above the carina, since the tube can move up to 4 cm with neck flexion or extension. When the carina is not visible, a distal end at the level of T3–T4 is considered well-positioned (3).
Misplacement must be reported if the tip reaches the carina or the main bronchus, which occurs in about 10% of insertions (16) (Fig. 9). When the tube enters a main bronchus, there is hyperinflation of the ipsilateral lung, with the risk of pneumothorax, and partial or complete collapse of the contralateral lung; this occurs more commonly on the right side since the right main bronchus is wider, shorter and more vertical than the left one. Inadvertent extubation or laryngeal damage can occur if the tube is placed too proximal. When it enters the esophagus, the tube projects on an anteroposterior CXR lateral to and not within the trachea, which in turn may be displaced by the balloon cuff, and gastric distension and esophageal air can be observed.
Figure 9.

Malpositioned endotracheal tube: the radiopaque metal marker (white arrow) identifies the distal end of the tube at the origin of the left main bronchus (black arrow, carina)
During insertion maneuvers, the trachea or oropharynx may be inadvertently damaged, especially at the posterior fibromuscular wall. This can be suspected on CXR when the distal portion of the tube is displaced to the right and the balloon is too expanded, eventually with air appearance in the neck and mediastinum. If the rupture communicates freely with the pleural space, pneumothorax may result (17).
A hyperinflated balloon is the main cause of tracheal mucosal ischemia, which, if prolonged, may lead to tracheal stenosis (Fig. 10a) or trachea-esophageal fistulas. However, if underinflated, the balloon may be dislodged or may not provide adequate airway protection leading to aspiration or mechanical ventilation-related pneumonia. Pneumothorax, pneumomediastinum and subcutaneous emphysema are other possible occurrences (18) (Fig. 10b–10d).
Figure 10. a–d.
Chest X-ray (a) showing tracheal stenosis (arrows) due to prolonged endotracheal cannula stay. Chest X-ray (b) of a patient after insertion of an endotracheal tube shows an overdistended distal balloon (long white arrow), pneumomediastinum (short white arrow) and significant right supraclavicular subcutaneous emphysema (black arrow). CT scan (c) confirms these findings (black arrow, subcutaneous emphysema; curved arrow, pneumomediastinum), with the distal balloon measuring about 3.5 cm. Chest X-ray (d) of a patient on mechanical ventilation demonstrates a left heart border more sharply delineated than usual (black arrow); this is a sign of pneumomediastinum.
Nasogastric tube
Nasogastric tubes are inserted to provide nutritional support, decompress and prevent vomiting, treat gastric distension promoting lung expansion, or remove toxic substances from the stomach (19). They are flexible tubes of synthetic material, inserted through a nostril down the esophagus in the stomach. Single-lumen tubes are in plastic or rubber, about 125 cm long, 14–18 F in size, with multiple side holes and circular radiopaque markers in pre-determined positions; double-lumen catheters are made of transparent plastic and are mainly used for gastric decompression.
A correctly positioned nasogastric tube runs vertically within the esophagus, along or near the median line, passing the diaphragm at the level of the gastro-esophageal junction and reaching the stomach (20). The distal tip should be positioned in the left hypochondrium at least 10 cm below the gastro-esophageal junction; if side holes are present, they must be distal to the junction to decrease aspiration risk.
CXR needs to be obtained immediately after insertion and before attempting any feeding (1). Significant misplacement occurs in about 1% of cases (1) (Fig. 11a), one common being a distal end looping and returning upwards within the upper third of the esophagus. If feeding is administered in such a tube there is an increased risk of aspiration, which is also present when the tip is at the gastro-esophageal junction or more proximal. A tip inside the duodenum may lead to malabsorption. The tube must not enter the bronchi to avoid pneumonia or pulmonary injury (Fig. 11b). Esophageal perforation is a possible but rare complication (20).
Figure 11. a, b.
Examples of nasogastric tube malpositions: (a), the distal portion of the nasogastric tube forms a loop in a voluminous gastric hiatal hernia (black arrow); (b), the nasogastric tube follows the right airway (black arrows) and the distal tip ends in the lateral costophrenic angle with laceration of distal airways and occurrence of significant pneumothorax (white arrows).
Conclusion
CXRs represent a fundamental exam to diagnose misplacements, displacements, and possible complications of intensive-care devices in critically ill patients. Radiologists should be familiar with the normal appearance of these devices and promptly recognize any abnormal findings.
Main points.
After placement of invasive devices, chest radiographs play a role in detecting their correct placement.
Inserting invasive devices may result in misplacement and even serious complications.
It is important to promptly recognize and report abnormal findings on radiographies.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Amorosa JK, Bramwit MP, Mohammed T-LH, et al. ACR appropriateness criteria routine chest radiographs in intensive care unit patients. J Am Coll Radiol. 2013;10:170–174. doi: 10.1016/j.jacr.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Henschke CI, Yankelevitz DF, Wand A, Davis SD, Shiau M. Chest radiography in the ICU. Clin Imaging. 1997;21:90–103. doi: 10.1016/0899-7071(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 3.Maffessanti M, Berlot G, Bortolotto P. Chest roentgenology in the intensive care unit: an overview. Eur Radiol. 1998;8:69–78. doi: 10.1007/s003300050342. [DOI] [PubMed] [Google Scholar]
- 4.Rubinowitz AN, Siegel MD, Tocino I. Thoracic imaging in the ICU. Crit Care Clin. 2007;23:539–573. doi: 10.1016/j.ccc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Gibson F, Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333–346. doi: 10.1093/bja/aes497. [DOI] [PubMed] [Google Scholar]
- 6.Perini S, LaBerge JM, Pearl JM, et al. Tesio catheter: radiologically guided placement, mechanical performance, and adequacy of delivered dialysis. Radiology. 2000;215:129–137. doi: 10.1148/radiology.215.1.r00mr43129. [DOI] [PubMed] [Google Scholar]
- 7.Tocino I. Chest imaging in the intensive care unit. Eur J Radiol. 1996;23:46–57. doi: 10.1016/0720-048X(96)88279-9. [DOI] [PubMed] [Google Scholar]
- 8.Gray P, Sullivan G, Ostryzniuk P, McEWEN TAJ, Rigby M, Roberts DE. Value of postprocedural chest radiographs in the adult intensive care unit. Crit Care Med. 1992;20:1513–1518. doi: 10.1097/00003246-199211000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Wechsler RJ, Spirn PW, Conant EF, Steiner RM, Needleman L. Thrombosis and infection caused by thoracic venous catheters: pathogenesis and imaging findings. AJR Am J Roentgenol. 1993;160:467–471. doi: 10.2214/ajr.160.3.8430537. [DOI] [PubMed] [Google Scholar]
- 10.Godoy MCB, Leitman BS, de Groot PM, Vlahos I, Naidich DP. Chest radiography in the ICU: Part 2, evaluation of cardiovascular lines and other devices. AJR Am J Roentgenol. 2012;198:572–581. doi: 10.2214/AJR.11.8124. [DOI] [PubMed] [Google Scholar]
- 11.McLoud TC, Putman CE. Radiology of the Swan-Ganz catheter and associated pulmonary complications. Radiology. 1975;116:19–22. doi: 10.1148/116.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Jain S. A pictorial essay: Radiology of lines and tubes in the intensive care unit. Indian J Radiol Imaging. 2011;21:182. doi: 10.4103/0971-3026.85365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stawicki Stanislaw PA, Kwiatt M, Tarbox A, et al. Thoracostomy tubes: A comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:142. doi: 10.4103/2229-5151.134182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesieme EB, Dongo A, Ezemba N, Irekpita E, Jebbin N, Kesieme C. Tube thoracostomy: complications and its management. Pulm Med. 2012;2012:1–10. doi: 10.1155/2012/256878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzardi G, Bertolaccini L, Terzi A. There is a hole in the lung! J Thorac Dis. 2010;2:253. doi: 10.3978/j.issn.2072-1439.2010.02.04.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marom EM, Goodman PC. Radiologic diagnosis of chest disease. 2nd ed. London: Springer-Verlag; 2001. The intensive care chest. [DOI] [Google Scholar]
- 17.Rollins R, Tocino I. Early radiographic signs of tracheal rupture. AJR Am J Roentgenol. 1987;148:695–698. doi: 10.2214/ajr.148.4.695. [DOI] [PubMed] [Google Scholar]
- 18.Divatia J, Bhowmick K. Complications of endotracheal intubation and other airway management procedures. Indian J Anaesth. 2005;49:308–318. [Google Scholar]
- 19.Christensen M. Bedside methods of determining nasogastric tube placement: a literature review. Nurs Crit Care. 2001;6:192–199. [Google Scholar]
- 20.Hill JR, Horner PE, Primack SL. ICU Imaging. Clin Chest Med. 2008;29:59–76. doi: 10.1016/j.ccm.2007.11.005. [DOI] [PubMed] [Google Scholar]