Abstract
PURPOSE
Hepatocellular carcinoma (HCC) usually occurs accompanied by portal hypertension. Transcatheter arterial chemoembolization (TACE) is recommended as an effective treatment in HCC. Recent studies had conflicting results regarding the effectiveness and safety of TACE for HCC in patients with transjugular intrahepatic portosystemic shunt (TIPS). This meta-analysis aimed to evaluate the influence of TIPS on the effectiveness and safety of TACE for patients with HCC.
METHODS
A comprehensive search of studies among PubMed, Web of Science and Cochrane Library was conducted, from the earliest publishing date to January 27th, 2020. Statistical analyses were all performed using the Stata 13.0 software. I2 index statistic was used to assess heterogeneity.
RESULTS
Six studies with a total of 536 patients with HCC were included in the analysis. The pooled response rate was 51% (95% CI: 25% to 77%) with a significant heterogeneity (I2=93.3%, p < 0.001). The TACE + TIPS group had an inferior response rate than the non-TIPS group, but the difference had no statistical significance (p = 0.171) and heterogeneity was low (I2=0.00%, p = 0.490). Pooled hepatic failure rate was 8.8% (95% CI: 5.2% to 12.4%) with low heterogeneity (I2=0.0%, p = 0.747). But the pooled hepatic failure rate increased to 12.7% (95% CI: 5.7% to 19.7%) with low heterogeneity (I2=11.5%, p = 0.323) if the patients who received TIPS after TACE were excluded.
CONCLUSION
TIPS does not influence the effectiveness of TACE, but attention should be paid to the risk of hepatic failure.
Hepatocellular carcinoma (HCC) usually occurs accompanied by liver cirrhosis, and the relationship between them is close (1–3). For patients with liver cirrhosis, the transjugular intrahepatic portosystemic shunt (TIPS) can be used to relieve portal hypertension and clinical symptoms when it is combined with various medical conditions (4–6). Transarterial chemoembolization (TACE) has been recommended as the standard treatment strategy for intermediate-stage HCC (7, 8). However, as TIPS disrupts the arterial supply of liver and divert portal venous flow, there is concern for the risks of hepatic damage and transient portal pressure increase after TACE combined with TIPS (9). In this context, HCC patients with TIPS in need of locoregional therapy or those with portal hypertension are complicated cases waiting to be solved.
In recent years, there have been conflicting results regarding the effectiveness and safety of TACE for HCC in patients with existing TIPS or waiting to receive TIPS because of portal hypertension (10–12). No randomized controlled trial comparing TACE + TIPS vs. non-TIPS exists. It is difficult to estimate the procedural feasibility, risks and long-term outcomes of this combined treatment strategy, because most of the evidence derives from retrospective cohort studies, case reports, or series. The present study aimed to explore hepatotoxicity, objective reaction rate, and overall survival using meta-analysis and a systematic review.
Methods
Search strategy
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations (13). Because of the study design, institutional review board exemption was approved at Sun Yat-sen University Cancer Center. A comprehensive search of PubMed, Web of Science and Cochrane Library was carried out. The combination of MeSH terms and free-text words used were as follows: “([transjugular intrahepatic portosystemic shunt] or TIPS) AND ([transcatheter arterial chemoembolization] or TACE) AND ([liver cancer] or [hepatocellular carcinoma] or [HCC])”. To avoid neglecting eligible studies, these terms were combined differently and were expanded to relevant topics. The search included literature published until February 27, 2020, with no lower date limit. No language restrictions were applied.
Study selection and eligibility criteria
Two authors screened the titles and abstracts of the literature from the databases independently. Publications were accepted if they complied with the following criteria: (i) prospective and retrospective cohort studies and randomized-controlled trials including at least ten patients; (ii) HCC diagnosed clearly by magnetic resonance imaging (MRI), computed tomography (CT) or pathology; (iii) full-text or abstract published; (iv) published trials which included patients receiving TACE and TIPS either before or after TACE (+/− control group which did not receive TIPS); (v) clearly described parametric data (such as tumor response, overall survival rate or hepatotoxicity effects); (vi) studies approved by the institutional review board and patients provided written informed consent.
The exclusion criteria were as follows: (i) study protocols, reviews, comments or case reports; (ii) limitation to animals or cells.
Data extraction
A standardized form was used to perform data extraction. Two reviewers collected information regarding characteristics of the patients (including sex, age, liver function), study characteristics (including name of author, publication year, sample size, study design, length of follow-up), intervention methods (such as cases with or without TIPS). A third reviewer checked the extracted data. Abdominal MRI or CT before and after treatment were applied to evaluate tumor response. On the basis of the modified Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for HCC, tumor response rates would be recorded after the evaluation. Complete response (CR) was defined if the lesion was completely cleared after intervention therapy; partial response (PR) was defined if there was ≥30% decrease in the summed diameters of the tumor; stable disease (SD) was defined if there was <30% decrease or <20% increase in the summed diameters; progressive disease (PD) was defined as ≥20% increase in the summed diameters. The smallest value was taken as the reference when it was recorded since treatment. The total number of CR and PR was defined as the response rate (14).
Quality assessment
Two authors assessed the quality of the selected studies independently. Because most studies included were observational, the Newcastle-Ottawa Scale was used to perform quality assessment of the included studies (15). A study can be rated from 0 to 9 stars on the basis of the criteria. A low risk of bias was considered if >7 stars, a moderate risk of bias was considered if 4 to 6 stars, and a high risk of bias was considered if <4 stars.
Statistical analysis
The aim of this analysis was to determine the effect of TIPS on clinical effectiveness and safety of TACE for HCC patients. All parametric data were provided as dichotomous variables. The proportion of dichotomous variables, the relative risk ratio (RR) and their 95% confidence interval (CI) were calculated. We also evaluated the pooled risk ratio and proportions. The data model was estimated by heterogeneity (I2). In I2 statistics, serious heterogeneity needs further investigation (70% to 100%); moderate heterogeneity is worth investigating (50% to 70%); low heterogeneity (0 to 50%) does not need investigation. Egger’s test was utilized to quantitatively analyze publication bias (16); it had to explore the source of bias in subgroup analyses if there was a significant risk of bias. We conducted all of the statistical analyses and data manipulations of this study using the Stata software (version 13.0).
Results
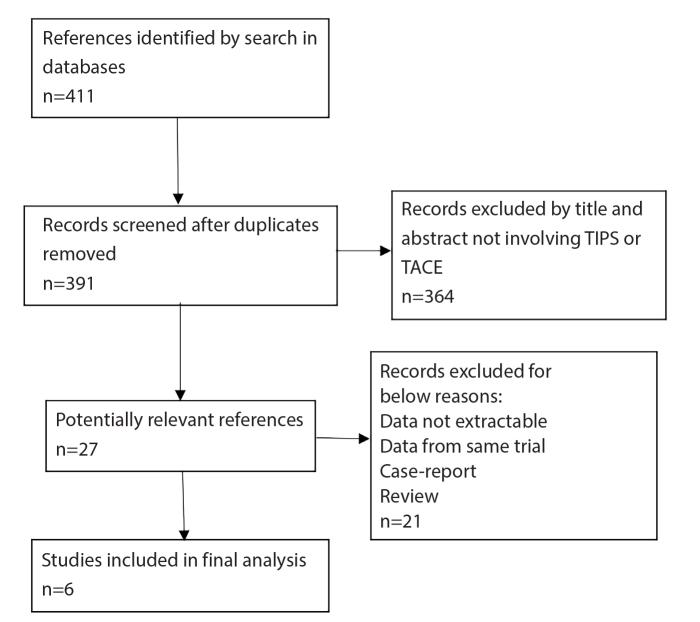
Results of the article search are shown as a flowchart in Fig. 1. A total of 411 articles were initially identified, of which 27 were eligible for full-text review, and 6 matched our standards (11, 17–21). Overall, 536 patients with HCC were included in the studies. The articles were published from 2012 to 2019 and all of them were observational cohorts with or without control groups. The patients in these studies received therapy of TIPS combined with TACE. The main characteristics of the studies are provided in the Table. As shown, two studies were performed in China, three in the United States of America, and one in Korea. Two studies included patients who received TIPS procedure before or after TACE (17, 20). In the remaining four studies, all patients received TIPS before TACE. In one of the studies, the intervention for HCC treatment included radiofrequency ablation (RFA) as well (20), while the other five studies included TACE only. Child–Pugh class and mean of model for end-stage liver disease (MELD) score were collected. According to quality assessment by the Newcastle Ottawa scale, all included studies received five stars or above.
Figure 1.
Identification of eligible studies from the databases.
Table.
Characteristics of clinical trials
| Author | Year | Country | Design | Interventional therapies | Number of patients | Male (number) | Age (mean) | Child-Pugh class (A/B/C) (n) | MELD score (mean) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Luo et al. (20) | 2019 | China | Retrospective study | TACE+TIPS/ TACE | 212/136 | 191 | 46.32/44.79 | 54/129/34; 33/83/20 | 10.21/ 11.37 |
| 2 | Miura et al. (19) | 2015 | USA | Retrospective study | TACE+TIPS | 16 | 12 | 60.5 | 2/12/2 | 12.5 |
| 3 | Wang et al. (18) | 2014 | China | Retrospective study | TACE+TIPS | 19 | 17 | 54 | NA | 13.37 |
| 4 | Kuo et al. (11) | 2013 | USA | Retrospective study | TACE+TIPS/ TACE | 10/23 | 28 | 59/58 | NA | 14/12 |
| 5 | Ruohoniemi et al. (21) | 2020 | USA | Retrospective study | TACE+TIPS/ TACE | 25/25 | 37 | 60/61 | NA | 13/9 |
| 6 | Kang et al. (17) | 2012 | Korea | Retrospective study | TACE+TIPS | 20 | 15 | 56.6 | 7/11/2 | NA |
TACE, transcatheter arterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt; MELD, model for end-stage liver disease; NA, not available.
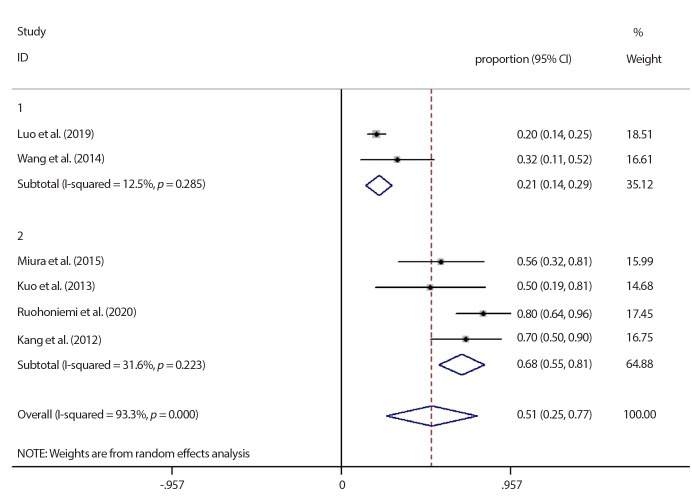
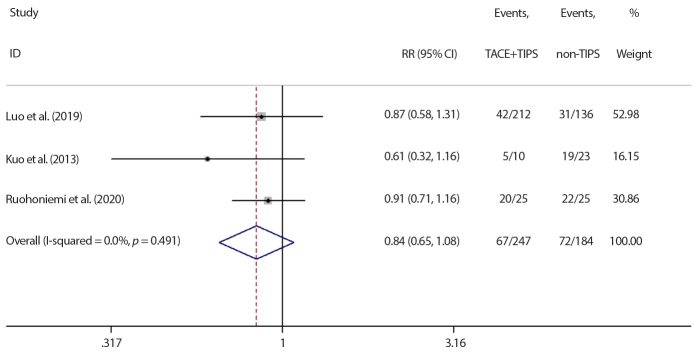
The response rate in all studies followed the RECIST criteria after therapy. All six studies compared the response rate. The pooled rate was 51% (95% CI: 25% to 77%) with significant heterogeneity among all studies (I2=93.3%, p < 0.001), as shown in Fig. 2. A subgroup analysis of studies divided according to the country was performed in order to determine the sources of heterogeneity: two studies were conducted in China (18, 20) (I2=12.5%, p = 0.285) and four in other nations (11, 17, 19, 21) ( I2=31.6%, p = 0.223). No heterogeneity was detected in either group. The result indicated that the nationality contributed the most to study heterogeneity. Moreover, the pooled rate of Chinese patients was lower than patients from other nations (21%, 95% CI: 14% to 29%) vs. (68%, 95% CI: 55% to 81%). Three studies had control groups; the patients in control group (non-TIPS) who did not receive TIPS before or after TACE were all selected during the same time period as in combined group. No heterogeneity was detected among the three studies with control groups (I2=0.00%, p = 0.490) (11, 20, 21). The combined TACE+TIPS group had an inferior response rate compared with the control group (RR= 0.839, 95%CI: 0.652 to 1.079), but the difference had no statistical significance (p = 0.171), as shown in Fig. 3.
Figure 2.
Subgroup analysis results of response rate according to country. First group represents China, second group represents the other nations. CI, confidence interval.
Figure 3.
Meta-analysis result of comparison between TACE+TIPS and non-TIPS group on response rate. Significance test of RR = 1: ( z = 1.37 p = 0.171). RR, risk ratio; CI, confidence interval; TACE, transarterial chemoembolization; TIPS, transjugular intrahepatic portosystemic shunt.
One-year overall survival rates were reported in four studies (17–20) and ranged as 77%–89%; two-year survival rates ranged as 50%–79%, and three-year survival rates ranged as 29%–68%.
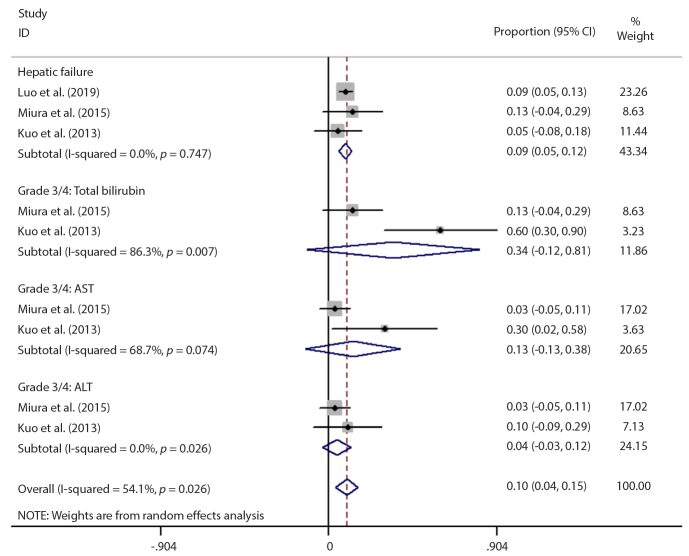
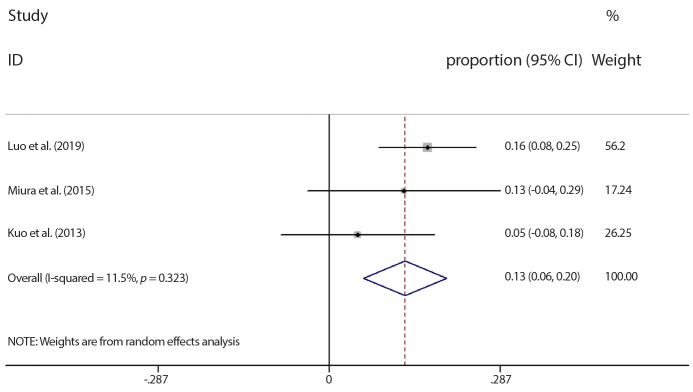
The most common hepatotoxic effects were hepatic failure, high total bilirubin (grade 3 to 4), and high AST/ALT (grade 3 to 4). The hepatotoxicity rate in each trial was summarized in Fig. 4. There was significant heterogeneity between the studies (I2=54.1%, p = 0.026). On the other hand, no heterogeneity was detected between the studies in the hepatic failure subgroup (I2=0.0%, p = 0.747) and pooled hepatic failure rate was calculated as 8.8% (95% CI: 5.2% to 12.4%). However, in one of the studies exploring hepatic failure rate, patients who received TIPS after TACE were also included (20) and hepatic failure rate differed based on the order of TACE and TIPS procedures (TIPS before TACE or TIPS after TACE). If the patients who received TIPS after TACE were excluded, the pooled hepatic failure rate increased to 12.7% (95% CI: 5.7% to 19.7%) with low heterogeneity (I2=11.5%, p = 0.323), as shown in Fig. 5.
Figure 4.
Meta-analysis results of hepatotoxicity. CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Figure 5.
Meta-analysis results of hepatic failure. CI, confidence interval.
The publication bias was determined by an Egger’s regression test. Results showed that publication bias was not obvious for one-year survival rate in the literature (p = 0.190, 95% CI: −2.290 to 0.860 for one-year survival rate).
Discussion
Since TIPS has been proven to be an effective and minimally invasive procedure for the treatment of portal hypertension and its complications (22, 23), it has also been established as an effective treatment for portal hypertension with HCC (20, 24). Because portal hypertension and HCC are severe complications or malignant development of advanced liver cirrhosis, it is not uncommon that patients suffer from both. Choi et al. (25) conducted a retrospective study showing that clinically relevant portal hypertension (grade 2) after TACE was associated with poor outcome. Results from some other studies also indicated that pathophysiological alterations of portal hypertension can potentially affect the outcome of TACE (26, 27).
Considering the venous and arterial flow alterations before and after TIPS creation, the effectiveness and toxicity of TACE deserve particular attention when it is combined with the TIPS procedure. Because of hepatic dual blood supply, hepatic arteries which feed the tumor are embolized after TACE, while normal hepatocytes still possess the preserved portal venous flow (28, 29). Patients may not be ideal candidates for TACE if they have compromised portal venous flow. A patent TIPS not only alters hepatic portal venous flow but also decompresses portal venous flow into the systemic circulation (30). For these reasons, TACE is considered a relative contraindication for HCC patients who have TIPS, and it demands careful consideration. Furthermore, in patients with HCC and portal hypertension, there are also limitations in the application of TIPS procedure. The effect of portal venous flow diversion by TIPS might cause hepatic ischemia after TACE. Another study showed that portal venous contribution can be observed in some HCCs even though blood supply of HCC is primarily derived from hepatic arteries (31). Based on this hypothesis, patients with TIPS and TACE might have more extensive tumor necrosis and better prognosis. However, this analysis did not support this idea. Although the TIPS group had a lower response rate compared with the non-TIPS group, there was no statistically significant difference between the two groups (p = 0.171). This difference in the response rate might be explained by artery-to-portal vein (arterioportal) shunting in liver parenchyma. In a study conducted by Itkin et al. (32), 30% of the flow through the shunt of TIPS attributed to arterioportal shunting directly was detected by measuring flow in the shunt and portal vein after TIPS creation.
In the subgroup meta-analysis, study nation contributed the most to study heterogeneity. This may be mainly associated with different etiology of HCC in different nations. In China, the main cause of HCC is hepatitis B infection, which demands antiviral and immune modulating therapy. Further clinical studies on HCC etiology are needed to confirm this (33). For HCC patients without TIPS, prior studies have demonstrated that after TACE, median overall survival ranged from 15 to 18 months (34, 35). According to the data we collected from the six studies included in this analysis, one-year overall survival of patients receiving TACE is not influenced by TIPS. Because TIPS decreased portal venous flow through the disruption of the arterial vasculature, TACE may theoretically lead to further hepatic dysfunction. When the liver parenchyma which is nutrient-deprived is subjected to chemoembolization, hepatic damage in the form of hyperbilirubinemia, hepatic failure, or ascites can follow. Tesdal et al. (9) treated six HCC patients who had prior TIPS with TACE using epirubicin. The study indicated that TACE and some other locoregional anti-tumor therapies could be safe for patients with TIPS, under the condition that liver function was in a good state. Coincidentally, several prior case series have shown that in patients with HCC and TIPS, TACE as well as percutaneous ethanol injection can be utilized safely, although the small numbers of patients were the limitation of these studies (36, 37). In this study, we conducted a subgroup analysis of hepatotoxic effects and found the pooled hepatic failure rate to be 8.8%, with no heterogeneity. However, if the patients who received TIPS after TACE were excluded, the pooled hepatic failure rate increased to 12.7% with low heterogeneity. This result indicates that the order of execution of these two procedures might influence the rate of hepatic failure. Increased hepatic failure rate might also be explained by the disrupted blood supply of liver after TIPS. Thus, more studies are needed to explore the underlying mechanism behind this.
The limitation of this meta-analysis was the relatively small sample size that included only six studies globally and unstandardized therapy (most with TIPS before TACE and some with TIPS after TACE, no specific treatment protocol). Additionally, there were no randomized controlled trials; all six studies included were retrospective cohorts and three studies had no control group.
In conclusion, the effectiveness and one-yearoverall survival of patients receiving TACE is not influenced by TIPS. However, if the patient’s liver function is not adequate, the risk of liver failure will be high when the two therapeutic strategies are combined.
Main points.
In this study we found that the effectiveness of TACE is not significantly influenced by TIPS.
We also determined that one-year overall survival of patients receiving TACE is not influenced by TIPS.
The risk of liver failure will be high when the two therapeutic strategies are combined.
Acknowledgments
We are grateful to David Waldman, M.D., Ph.D., Department of Imaging Sciences, University of Rochester Medical Center, for manuscript editing and review.
Footnotes
Financial disclosure
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81571780 to Fei Gao), High-level health team project of Zhuhai Municipal Health Bureau (Zhuhai HLHPTP201705) and Science and Technology Planning Project of Guangdong Province (CN) (Grant No.2017B020210004). All authors contributed to and approved the final version of this manuscript.
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 3.Thiele M, Gluud LL, Fialla AD, Dahl EK, Krag A. Large variations in risk of hepatocellular carcinoma and mortality in treatment naïve hepatitis B patients: systematic review with meta-analyses. PloS One. 2014;9:e107177. doi: 10.1371/journal.pone.0107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudler M, Poynard T, Thabut D. Early use of TIPS for cirrhosis and variceal bleeding. New Engl J Med. 2010;363:1376. doi: 10.1056/NEJMc1008663. [DOI] [PubMed] [Google Scholar]
- 5.Sankar K, Moore CM. Transjugular intrahepatic portosystemic shunts. JAMA. 2017;317:880. doi: 10.1001/jama.2016.20899. [DOI] [PubMed] [Google Scholar]
- 6.Riggio O, Ridola L, Angeloni S, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267–272. doi: 10.1016/j.jhep.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–535. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 8.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 9.Tesdal IK, Wikström M, Flechtenmacher C, Filser T, Dueber C. Percutaneous treatment of hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. Cardiovasc Intervent Radiol. 2006;29:778–784. doi: 10.1007/s00270-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 10.Kohi MP, Fidelman N, Naeger DM, LaBerge JM, Gordon RL, Kerlan RK. Hepatotoxicity after transarterial chemoembolization and transjugular intrahepatic portosystemic shunt: do two rights make a wrong? J Vasc Interv Radiol. 2013;24:68–73. doi: 10.1016/j.jvir.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Kuo YC, Kohi MP, Naeger DM, et al. Efficacy of TACE in TIPS patients: comparison of treatment response to chemoembolization for hepatocellular carcinoma in patients with and without a transjugular intrahepatic portosystemic shunt. Cardiovasc Intervent Radiol. 2013;36:1336–1343. doi: 10.1007/s00270-013-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu B, Zhao MF, Yue ZD, et al. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol. 2015;21:12439–12447. doi: 10.3748/wjg.v21.i43.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JW, Kim JH, Ko GY, Gwon DI, Yoon HK, Sung KB. Transarterial chemoembolization for hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt. Acta Radiol. 2012;53:545–550. doi: 10.1258/ar.2012.110476. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Zhang H, Zhao H, et al. Repeated transcatheter arterial chemoembolization is safe for hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt. Diagn Interv Radiol. 2014;20:487–491. doi: 10.5152/dir.2014.13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura JT, Rilling WS, White SB, et al. Safety and efficacy of transarterial chemoembolization in patients with transjugular intrahepatic portosystemic shunts. HPB (Oxford) 2015;17:707–712. doi: 10.1111/hpb.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo SH, Chu JG, Huang H, Yao KC. Safety and efficacy of transjugular intrahepatic portosystemic shunt combined with palliative treatment in patients with hepatocellular carcinoma. World J Clin Cases. 2019;7:1599–1610. doi: 10.12998/wjcc.v7.i13.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruohoniemi DM, Taslakian B, Aaltonen EA, et al. Comparative analysis of safety and efficacy of transarterial chemoembolization for the treatment of hepatocellular carcinoma in patients with and without pre-existing transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2020;31:409–415. doi: 10.1016/j.jvir.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 23.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Bettinger D, Knüppel E, Euringer W, et al. Efficacy and safety of transjugular intrahepatic portosystemic shunt (TIPSS) in 40 patients with hepatocellular carcinoma. Aliment Pharmacol Ther. 2015;41:126–136. doi: 10.1111/apt.12994. [DOI] [PubMed] [Google Scholar]
- 25.Choi JW, Chung JW, Lee DH, et al. Portal hypertension is associated with poor outcome of transarterial chemoembolization in patients with hepatocellular carcinoma. Eur1 Radiol. 2018;28:2184–2193. doi: 10.1007/s00330-017-5145-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347–352. doi: 10.3350/kjhep.2010.16.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakiri Y. Pathophysiology of portal hypertension. Clinics in liver disease. 2014 May;18:281–291. doi: 10.1016/j.cld.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493–497. doi: 10.1148/radiology.178.2.1846240. [DOI] [PubMed] [Google Scholar]
- 29.Matsui O, Miyayama S, Sanada J, et al. Interventional oncology: new options for interstitial treatments and intravascular approaches: superselective TACE using iodized oil for HCC: rationale, technique and outcome. J Hepatobiliary Pancreat Sci. 2010;17:407–409. doi: 10.1007/s00534-009-0234-z. [DOI] [PubMed] [Google Scholar]
- 30.Bannas P, Roldán-Alzate A, Johnson KM, et al. Longitudinal monitoring of hepatic blood flow before and after TIPS by using 4D-flow MR imaging. Radiology. 2016;281:574–582. doi: 10.1148/radiol.2016152247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kudo M, Hatanaka K, Inoue T, Maekawa K. Depiction of portal supply in early hepatocellular carcinoma and dysplastic nodule: value of pure arterial ultrasound imaging in hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):60–67. doi: 10.1159/000315232. [DOI] [PubMed] [Google Scholar]
- 32.Itkin M, Trerotola SO, Stavropoulos SW, et al. Portal flow and arterioportal shunting after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2006;17:55–62. doi: 10.1097/01.RVI.0000191362.75969.F6. [DOI] [PubMed] [Google Scholar]
- 33.Brouwer WP, Chan HLY, Lampertico P, et al. Genome-wide association study identifies genetic variants associated with early and sustained response to (pegylated) interferon in chronic hepatitis B patients: the GIANT-B study. Clin Infect Dis. 2019;69:1969–1979. doi: 10.1093/cid/ciz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiades CS, Liapi E, Frangakis C, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:1619–1624. doi: 10.1097/01.RVI.0000236608.91960.34. [DOI] [PubMed] [Google Scholar]
- 35.Brown DB, Chapman WC, Cook RD, et al. Chemoembolization of hepatocellular carcinoma: patient status at presentation and outcome over 15 years at a single center. AJR Am J Roentgenol. 2008;190:608–615. doi: 10.2214/AJR.07.2879. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi H, Uchida H, Maeda M, et al. Combined transjugular intrahepatic portosystemic shunt and segmental Lipiodol hepatic artery embolization for the treatment of esophagogastric varices and hepatocellular carcinoma in patients with cirrhosis: preliminary report. Cardiovasc Intervent Radiol. 1995;18:9–15. doi: 10.1007/BF02807348. [DOI] [PubMed] [Google Scholar]
- 37.Serafini FM, Zwiebel B, Black TJ, Carey LC, Rosemurgy AS., 2nd Transjugular intrahepatic portasystemic stent shunt in the treatment of variceal bleeding in hepatocellular cancer. Dig Dis Sci. 1997;42:59–65. doi: 10.1023/A:1018876803292. [DOI] [PubMed] [Google Scholar]