Abstract
Background
Over the course of the COVID-19 pandemic, the care of patients with COVID-19 has changed and the use of extracorporeal membrane oxygenation (ECMO) has increased. We aimed to examine patient selection, treatments, outcomes, and ECMO centre characteristics over the course of the pandemic to date.
Methods
We retrospectively analysed the Extracorporeal Life Support Organization Registry and COVID-19 Addendum to compare three groups of ECMO-supported patients with COVID-19 (aged ≥16 years). At early-adopting centres—ie, those using ECMO support for COVID-19 throughout 2020—we compared patients who started ECMO on or before May 1, 2020 (group A1), and between May 2 and Dec 31, 2020 (group A2). Late-adopting centres were those that provided ECMO for COVID-19 only after May 1, 2020 (group B). The primary outcome was in-hospital mortality in a time-to-event analysis assessed 90 days after ECMO initiation. A Cox proportional hazards model was fit to compare the patient and centre-level adjusted relative risk of mortality among the groups.
Findings
In 2020, 4812 patients with COVID-19 received ECMO across 349 centres within 41 countries. For early-adopting centres, the cumulative incidence of in-hospital mortality 90 days after ECMO initiation was 36·9% (95% CI 34·1–39·7) in patients who started ECMO on or before May 1 (group A1) versus 51·9% (50·0–53·8) after May 1 (group A2); at late-adopting centres (group B), it was 58·9% (55·4–62·3). Relative to patients in group A2, group A1 patients had a lower adjusted relative risk of in-hospital mortality 90 days after ECMO (hazard ratio 0·82 [0·70−0·96]), whereas group B patients had a higher adjusted relative risk (1·42 [1·17−1·73]).
Interpretation
Mortality after ECMO for patients with COVID-19 worsened during 2020. These findings inform the role of ECMO in COVID-19 for patients, clinicians, and policy makers.
Funding
None.
Introduction
Clinical guidelines for the management of patients with COVID-19 have evolved.1 The earliest studies of extracorporeal membrane oxygenation (ECMO) for COVID-19 reported prohibitively high mortality rates in small cohorts.2 Yet, informed by existing evidence from non-COVID-19 patients with the acute respiratory distress syndrome,3, 4 international medical organisations still recommended, early in the pandemic, that ECMO should be considered if conventional treatment was not successful.5, 6, 7
Subsequent multicentre observational cohort studies,8, 9, 10, 11 including one from the voluntary international registry of the Extracorporeal Life Support Organization (ELSO), reported outcomes for patients with COVID-19 receiving ECMO that were comparable to ECMO-supported patients with non-COVID-19-related acute respiratory distress syndrome.3 The ELSO Registry study reported outcomes for 1035 patients with COVID-19 who were started on ECMO on or before May 1, 2020. The estimated cumulative incidence of in-hospital mortality 90 days after starting ECMO was 37·4% (95% CI 34·4–40·4).8
As the pandemic progressed, there were substantial changes in the management of COVID-19,12, 13 which might have affected the profile of patients progressing to ECMO support.14, 15 At the same time, there was expansion in the use and in the number of centres providing ECMO support to patients with COVID-19.16 Both of these trends could have altered outcomes.
The reported experiences8, 11 of previous patients with COVID-19 might play a role in clinicians' bedside estimation of the appropriateness of ECMO for individual patients. Therefore, we aimed to compare patients with COVID-19 who were treated with ECMO on or before May 1, 2020, against patients treated after this date, by examining baseline patient characteristics, treatments used, characteristics of the centres providing ECMO support, and patient outcomes. We used two complementary comparisons. First, we compared care and outcomes in a fixed group of early-adopting centres and analysed changes between earlier and more recent patients in the same hospitals. Second, we studied whether the care and outcomes of patients at centres that began using ECMO for COVID-19 later in the pandemic were the same as those of contemporaneous patients at early-adopting centres.
Research in context.
Evidence before this study
We did not perform a formal literature review. The impact of extracorporeal membrane oxygenation (ECMO) support on COVID-19 survival is uncertain because no randomised clinical trials have compared invasive mechanical ventilation without ECMO versus treatment with ECMO support in COVID-19. Outcomes of ECMO started for COVID-19 early in the pandemic (on or before May 1) were reported in several observational studies. A 2021 systematic review and meta-analysis of ECMO in adults with COVID-19 included 22 studies with 1896 patients and reported a pooled in-hospital mortality rate of 37·1% (95% CI 32·2–42·0). Subsequent studies have reported mortality rates of over 50%. It is unclear from these studies whether the mortality of ECMO-supported patients has changed over time and, if so, why such changes might have occurred.
Added value of this study
This study of 4812 patients from 349 sites in 41 countries showed that, over 2020, mortality after ECMO support in patients with COVID-19 increased by about 15% and the median duration of ECMO support increased by 6 days (groups A1 vs A2). Compared with patients with COVID-19 who received ECMO earlier in the pandemic, patients who received ECMO after May 1, 2020, were more commonly treated with corticosteroids. Factors appearing to affect outcomes included that patients who received ECMO after May 1 had a higher likelihood of treatment-refractory disease despite similar conventional risk factors, and that centres with less experience providing ECMO support for COVID-19 were more likely to have a higher mortality rate.
Implications of all the available evidence
It is unknown how ECMO support affects survival in COVID-19; however, mortality after ECMO and the duration of ECMO support are not static over time. This study showed that mortality worsened over the course of the pandemic, an evolution that requires continued surveillance of ECMO outcomes in COVID-19. Furthermore, given that ECMO is a finite resource and that 25% of patients with COVID-19 received 5 weeks or more of ECMO support, centres should consider developing local policies that guide the ethical allocation of ECMO when resources are constrained.
Methods
Data source and participants
We considered patients (aged ≥16 years) who were diagnosed with COVID-19 via positive PCR test and supported with ECMO in 2020 (Jan 1 to Dec 31). Follow-up data were last updated on June 9, 2021. We divided patients into three groups based on the time and centre at which ECMO was started. First, group A1, which included patients with COVID-19 who started ECMO on or before May 1, 2020, at so-called early-adopting centres. An early-adopting centre was one that reported using ECMO support for patients with COVID-19 on or before May 1. This time frame was identical to the previous study of the ELSO Registry COVID-19 data; however, it was updated to include patients entered into the registry after final data lock (June 9, 2021) in that previous study.8 Second, group A2, which included patients who started ECMO support at an early-adopting centre between May 2, and Dec 31, 2020, the second time period. Third, group B, which included patients who started ECMO between May 2 and Dec 31, 2020, in so-called late-adopting centres, defined as centres that only provided ECMO for COVID-19 after May 1, 2020. Compared with earlier in the pandemic, there were substantial changes in the management of COVID-19,12, 13 which might have affected the characteristics of patients progressing to ECMO support. We demarcated earlier care as on or before May 1, 2020, because it was early in the pandemic and was the time period used in the previous study of ELSO Registry COVID-19 data.8
The University of Michigan Institutional Review Board deemed this study of existing, de-identified data exempt from human participant review. The ELSO Registry characteristics, data entry procedures, and COVID-19 diagnostic criteria have been described previously,8 and are summarised in the appendix (p 3). We followed STROBE reporting guidelines for observational studies.
Outcomes
The primary outcome was in-hospital mortality in a time-to-event analysis assessed 90 days after ECMO started. Secondary outcomes were the median duration of ECMO support, median duration of hospital stay, the patients' final dispositions, and complications experienced during ECMO. The ELSO Registry observes patient outcomes while they are at an ELSO member centre. If a patient is discharged to another institution, the ELSO Registry records their outcome before discharge but does not observe the outcome after discharge from the ELSO member centre.
Statistical analysis
We estimated the cumulative incidence of mortality using the Aalen-Johansen cumulative incidence estimator as previously reported.8, 17 To address temporal changes, we compared in-hospital mortality 90 days after ECMO in patients treated in early-adopting centres either on or before May 1, 2020 (group A1) or after May 1, 2020 (group A2). Next, to address the compositional changes in centres providing ECMO support between May 2 and Dec 31, 2020, we compared patients receiving ECMO at early-adopting centres (group A2) to patients receiving ECMO support for COVID-19 at late-adopting centres (group B). To assess whether the relative risks for mortality, also called hazard ratios (HRs), were similar over time, we fit separate Cox proportional hazards models to groups A1 and A2, adjusting for an identical set of covariates in each model and calculating the estimated HR for each covariate in each model. Each patient's follow-up period was defined as the start of ECMO to the patient's death. Any other event (eg, patient discharged to home, long-term acute care, acute rehabilitation, or another hospital) was treated as a censoring event. If no event was reported, follow-up was censored at the patient's most recent update that was before June 9, 2021.
We estimated the relative risks between characteristics and mortality using Cox models that included all those from a previously reported model for ECMO-supported patients with COVID-19,8 except for race. Specifically, we included age, sex, chronic cardiac disease, body-mass index (BMI), cancer, immunocompromised state (appendix p 3), diabetes, pre-existing cardiac disease, chronic respiratory disease (excluding asthma), asthma, cardiac arrest before starting ECMO, presence of co-infection, duration of pre-ECMO endotracheal intubation, partial pressure of arterial carbon dioxide (PaCO2), the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2:FiO2), diagnosis of acute kidney injury, and initial ECMO mode (venovenous vs venoarterial or veno-venoarterial). We also added geographically determined ELSO Chapters to the model (see appendix p 3 for a list of countries in each ELSO Chapter). We excluded race because adjusting for race might excuse racially biased practices or prognoses that reinforce structural inequalities, and there is no known physiological basis for expecting differential racial outcomes of ECMO support.18 We tested that the proportional hazards assumptions for Cox models were met and incorporated time-varying effects into our final models to better satisfy these assumptions (appendix pp 6–8, 18–20).
To assess whether differences in the type or mix of patients treated within early-adopting centres resulted in differences in expected mortality, we calculated the cross-validated distribution of relative risk19 in each group as predicted by the aforementioned model fit to group A1. A patient's relative risk is interpreted as their multiplicative increase in mortality rate, taking into account their pre-ECMO characteristics, relative to a common reference patient. We used this model of earlier-treated patients only to predict the relative risk and to determine whether any observed increased mortality in patients treated after May 1 could be explained by a change in pre-ECMO factors. The process for calculating each patient's cross-validated relative risk is described in the appendix (p 4). We used permutation tests to assess differences in the distribution of relative risks for patients in groups A1 and A2 (appendix p 5).
To evaluate contextual changes in distribution of risk among patients who were more recently cared for at early-adopting versus late-adopting centres, we fit a single Cox proportional hazards model to the patients from group A2 and group B combined using all of the same model factors described above. In addition, we hypothesised that early experience providing ECMO for COVID-19 might reduce the relative risk of mortality in subsequent ECMO-supported patients with COVID-19.
We calculated each hospital's experience providing ECMO support for COVID-19 on or before May 1, 2020. We categorised the annualised COVID-19 case volume using previously reported annual ECMO volume categories.20 We also accounted for the adult hospital ECMO volume in 2019 and, separately, 2019 adult hospital venovenous ECMO volume,14 using the same previously reported annual ECMO volume categories (appendix p 3).20 To quantify the effect of early COVID-19 experience on the relative risk of mortality later in the pandemic, we did a similar cross-validated assessment. Namely, we refit the model after leaving out a single centre from group A2 or group B, then we predicted the risk for each ECMO case in the centre that was withheld. We repeated this analysis for all centres in both groups (detailed in appendix pp 4–5).
To estimate the relative risk of mortality between groups after adjusting for patient and centre-level characteristics, a Cox proportional hazard model was fit to all patients (appendix pp 7–8, 20).
We estimated the distribution of duration of hospital stay, defined as time from admission to ELSO centre to discharge for any reason, using Kaplan-Meier methodology.21 Any runs for which the time of discharge was not reported were censored at the last reported update. Multiple imputation was used to account for missing values in the predictor variables using fully specified chained equations in the R package (version 4.1.0; appendix pp 8, 11).22
Role of the funding source
There was no funding source for this study.
Results
Between Jan 1 and Dec 31, 2020, 5211 ECMO-supported patients with COVID-19 were reported to the ELSO Registry. This number was reduced to 5098 after excluding the following: five patients who started ECMO before being diagnosed with COVID-19, 85 patients younger than 16 years, 20 patients who did not have PCR confirmation of SARS-CoV-2, and three patients without follow-up. 286 patients were excluded because there was not a completed COVID-19 addendum, leaving 4812 ECMO-supported patients from 349 centres across 41 countries (appendix pp 12, 21). On or before May 1, 2020, ECMO support for COVID-19 was provided to 1182 patients at 236 early-adopting centres (group A1). Between May 2 and Dec 31, 2020, an additional 2824 patients received ECMO support at one of the early-adopting centres (group A2), whereas 806 patients received ECMO for COVID-19 at one of 113 late-adopting centres between these dates (group B; appendix pp 13–14, 21).
First, considering only patients managed at early-adopting centres, we compared group A1 and A2. Group A1 patients had a similar median BMI, sex distribution, and rate of pre-ECMO acute heart failure and cardiac arrest, relative to group A2 patients (table 1 ). Diabetes, pre-existing heart disease, immunocompromised status, bacterial pneumonia, and bloodstream co-infections were more common in patients in group A2 than in group A1. Overall, risk factors for mortality had similar estimates (HRs) on or before May 1 (group A1) and after May 1 (group A2; appendix pp 9, 22–23).
Table 1.
Patient characteristics among three cohorts receiving ECMO for COVID-19
| Group A1 | Group A2 | Group B | p value: A1 vs A2 | p value: A2 vs B | ||
|---|---|---|---|---|---|---|
| Participants | 1182 | 2824 | 806 | .. | .. | |
| Age, years | 50 (42–57) | 51 (42–58) | 49 (40–58) | 0·004 | 0·030 | |
| Body-mass index, kg/m2 | 31 (27–36) | 32 (28–38) | 32 (28–37) | 0·40 | 0·60 | |
| Sex | ||||||
| Male* | 876 (74%) | 2049 (73%) | 598 (74%) | 0·39 | 0·36 | |
| Female | 304 (26%) | 775 (27%) | 208 (26%) | .. | .. | |
| Pre-ECMO comorbidities | ||||||
| Any | 768 (65%) | 2066 (73%) | 547 (68%) | 0·002 | 0·088 | |
| Cancer | 15 (1%) | 58 (2%) | 9 (1%) | 0·058 | 0·080 | |
| Immunocompromised | 27 (2%) | 124 (4%) | 29 (4%) | 0·002 | 0·32 | |
| Diabetes | 287 (24%) | 923 (33%) | 217 (27%) | <0·001 | 0·002 | |
| Pre-existing heart disease | 31 (3%) | 139 (5%) | 28 (3%) | 0·004 | 0·15 | |
| Pre-existing lung disease | 36 (3%) | 127 (4%) | 27 (3%) | 0·23 | 0·22 | |
| Pre-existing renal insufficiency | 33 (3%) | 109 (4%) | 12 (1%) | 0·06 | <0·001 | |
| Frailty | 6 (0·5%) | 20 (0·7%) | 7 (0·9%) | 0·45 | 0·64 | |
| Asthma | 128 (11%) | 292 (10%) | 72 (9%) | 0·60 | 0·35 | |
| Pregnancy | 24 (2%) | 62 (2%) | 14 (2%) | 0·88 | 0·40 | |
| Acute heart failure | 58 (5%) | 190 (7%) | 39 (5%) | 0·052 | 0·12 | |
| Acute kidney injury | 367 (31%) | 758 (27%) | 214 (27%) | <0·001 | 0·91 | |
| Pre-ECMO cardiac arrest† | 51 (4%) | 140 (5%) | 43 (5%) | 0·72 | 0·66 | |
| Co-infections‡ | ||||||
| No co-infection | 697 (59%) | 1413 (50%) | 456 (57%) | <0·001 | 0·13 | |
| Bacterial pneumonia | 346 (29%) | 1062 (38%) | 250 (31%) | <0·001 | 0·10 | |
| Co-viral infection | 102 (9%) | 195 (7%) | 66 (8%) | 0·57 | 0·61 | |
| Bloodstream infection | 164 (14%) | 471 (17%) | 98 (12%) | 0·012 | 0·052 | |
| Urinary tract infection | 52 (4%) | 229 (8%) | 54 (7%) | 0·002 | 0·38 | |
Data are median (IQR) or n (%). Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO between May 2 and Dec 31, 2020, at early-adopting centres. Group B patients started ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020. ECMO=extracorporeal membrane oxygenation. p values derived from ECMO centre-based permutation tests of the null hypothesis that there is no difference in the medians (for quantitative) or proportions (for binary) between the two specified columns. Denominators for percentages are given in the footnotes when they differ from the participant row.
Group A1 n=1180.
Group A1 n=1167, group A2 n=2796, and group B n=794.
Group A1 n=1180 and group A2 n=2823.
Second, considering only patients who received ECMO more recently (after May 1), we compared patients in groups A2 and B. Although ECMO-supported patients in groups A2 and B were generally similar (table 1), patients in group A2 were more likely to have diabetes or pre-existing renal insufficiency. After adjusting for COVID-19 ECMO experience early in the pandemic, the predicted risk of mortality was similar between groups A2 and B (p=0·50; appendix p 24).
The majority of early-adopting and late-adopting centres were located in North America. Early-adopting centres were more likely to be in Europe, China, South Korea, or Australia, and less likely to be in south and west Asia, Africa, or Latin America (p<0·001; appendix pp 13–14), than late-adopting centres. The median 2019 ECMO adult case volume was higher at early-adopting centres (22 [IQR 2–60]) than at late-adopting centres (nine [0–24], p<0·001; appendix p 13).
Among patients at early-adopting centres, the pre-ECMO treatment differed over 2020. Group A2 patients were more likely to have received non-invasive ventilation before ECMO and had a shorter median duration of invasive ventilation before ECMO than did group A1 patients (table 2 ). Additionally, use of corticosteroids or remdesivir was more common in group A2 than in group A1. Patients in groups A1 and A2 started ECMO at similar pre-ECMO ventilator settings, PaCO2, and PaO2:FiO2 (table 2).
Table 2.
Treatment and support among three cohorts receiving ECMO for COVID-19
| Group A1 | Group A2 | Group B | p value: A1 vs A2 | p value: A2 vs B | ||
|---|---|---|---|---|---|---|
| Participants | 1182 | 2824 | 806 | .. | .. | |
| Pre-intubation non-invasive ventilation* | 689 (58%) | 2139 (76%) | 564 (70%) | <0·001 | 0·16 | |
| Bilevel positive airway pressure | 202 (17%) | 939 (33%) | 313 (39%) | <0·001 | 0·18 | |
| Continuous positive airway pressure | 158 (13%) | 385 (14%) | 73 (9%) | 1·00 | 0·19 | |
| High-flow nasal cannula | 420 (36%) | 1463 (52%) | 341 (42%) | <0·001 | 0·036 | |
| More than one non-invasive support | 83 (7%) | 592 (21%) | 151 (19%) | <0·001 | 0·47 | |
| Prone positioning† | 700 (60%) | 1684 (60%) | 405 (51%) | 0·96 | 0·022 | |
| Neuromuscular blockade‡ | 845 (73%) | 2090 (74%) | 506 (63%) | 0·80 | 0·016 | |
| Any vasoactive support§ | 715 (61%) | 1721 (61%) | 455 (57%) | 0·91 | 0·23 | |
| Pre-ECMO endotracheal intubation, days¶ | 4·0 (1·7–6·3) | 3·1 (0·9–6·3) | 2·7 (0·8–5·9) | <0·001 | 0·20 | |
| Pre-ECMO conventional ventilation‖ | 1086 (98%) | 2498 (96%) | 650 (97%) | 0·018 | 0·47 | |
| PaCO2, mm Hg** | 60 (50–74) | 61 (50–76) | 60 (50–74) | 0·48 | 0·46 | |
| PaO2:FiO2, mm Hg†† | 72 (60–94) | 71 (58–92) | 70 (56–93) | 0·44 | 0·49 | |
| PEEP, cm of H2O‡‡ | 14 (12–16) | 14 (10–16) | 14 (10–16) | 1·00 | 1·00 | |
| PIP, cm of H2O§§ | 33 (30–38) | 34 (30–38) | 34 (30–38) | 0·87 | 1·00 | |
| PEEP, cm of H2O at ECMO hour 24¶¶ | 10 (10–14) | 10 (10–12) | 10 (10–12) | 1·00 | 1·00 | |
| PIP, cm of H2O at ECMO hour 24‖‖ | 25 (21–28) | 25 (21–28) | 25 (22–29) | 1·00 | 1·00 | |
| COVID-19 therapies | ||||||
| Any | 914 (77%) | 2590 (92%) | 644 (80%) | <0·001 | <0·001 | |
| Glucocorticoids | 511 (43%) | 2196 (78%) | 583 (72%) | <0·001 | 0·20 | |
| Remdesivir | 103 (9%) | 1598 (57%) | 404 (50%) | <0·001 | 0·28 | |
| Chloroquine or hydroxychloroquine | 627 (53%) | 180 (6%) | 63 (8%) | <0·001 | 0·45 | |
| Venovenous ECMO | 1110 (94%) | 2623 (93%) | 762 (95%) | 0·39 | 0·94 | |
| ECMO support type | ||||||
| Respiratory support | 1140 (96%) | 2686 (95%) | 777 (96%) | 0·060 | 0·24 | |
| Cardiac support | 29 (2%) | 110 (4%) | 27 (3%) | .. | .. | |
| ECPR | 13 (1%) | 28 (1%) | 2 (0·2%) | .. | .. | |
Data are median (IQR) or n (%). Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO between May 2 and Dec 31, 2020, at early-adopting centres. Group B patients started ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020. The mode of mechanical ventilation, PaCO2, PaO2:FiO2, PEEP, and PIP are the measures given nearest to ECMO starting (within the previous 6 h). PEEP at ECMO hour 24 and PIP at ECMO hour 24 are measured closest to 24 h after ECMO started (within 18–30 h after ECMO started). p values derived from ECMO centre-based permutation tests of the null hypothesis that there is no difference in the medians (for quantitative) or proportions (for binary) between the two specified columns. ECMO=extracorporeal membrane oxygenation. ECPR=extracorporeal cardiopulmonary resuscitation. PaCO2 =partial pressure of arterial carbon dioxide. PaO2 :FiO2 =ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen. PEEP=positive end-expiratory pressure. PIP=peak inspiratory pressure. Denominators for percentages are given in the footnotes when they differ from the participant row.
Group A1 n=1180.
Group A1 n=1167, group A2 n=2813, and group B n=793.
Group A1 n=1163, group A2 n=2813, and group B n=801.
Group A1 n=1163, group A2 n=2813, and group B n=798.
Group A1 n=1055, group A2 n=2452, and group B n=658.
Group A1 n=1107, group A2 n=2595, and group B n=669.
Group A1 n=1020, group A2 n=2358, and group B n=625.
Group A1 n=984, group A2 n=2245, and group B n=602.
Group A1 n=993, group A2 n=2317, and group B n=604.
Group A1 n=785, group A2 n=1892, and group B n=484.
Group A1 n=1041, group A2 n=2516, and group B n=681.
Group A1 n=908, group A2 n=2211, and group B n=493.
When comparing patients in groups A2 and B, a higher proportion of patients in group A2 received prone positioning and neuromuscular blockade, but other reported pre-ECMO treatments were similar.
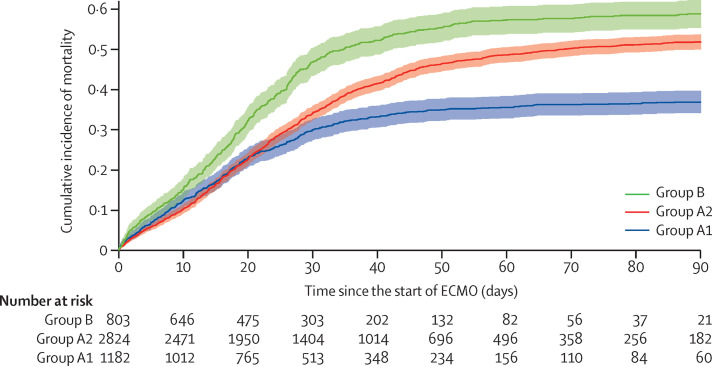
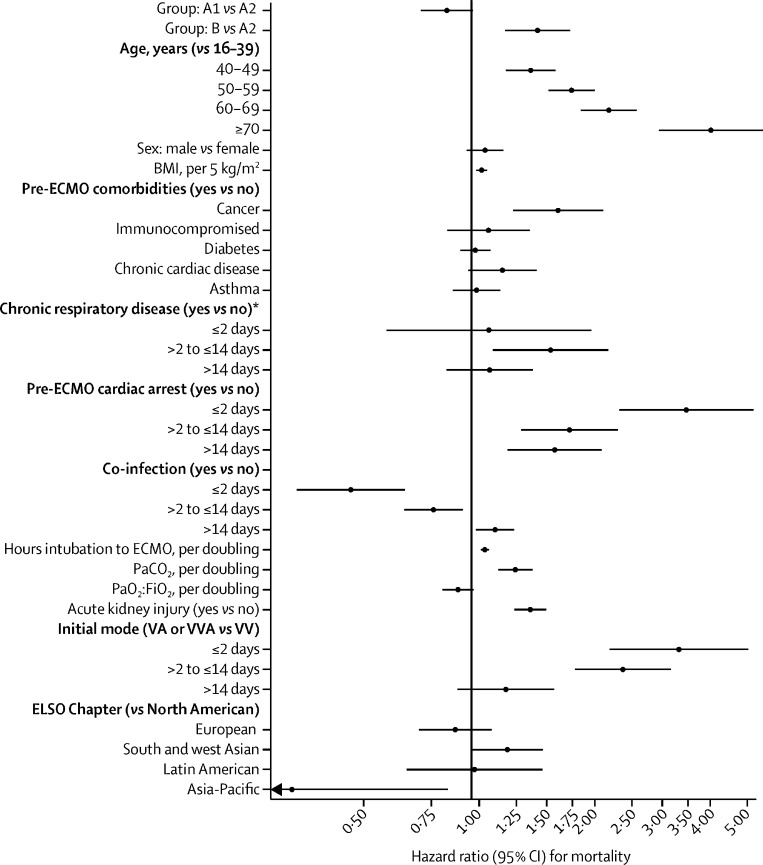
Among early-adopting centres, the cumulative incidence of in-hospital mortality 90 days after starting ECMO was 36·9% (95% CI 34·1–39·7) in group A1 versus 51·9% (50·0–53·8) in group A2 (figure 1 ). At late-adopting centres (group B), the cumulative incidence of in-hospital mortality 90 days after ECMO initiation was 58·9% (55·4–62·3). These differences persisted after adjusting for patient-level and centre-level characteristics. Relative to group A2, group A1 patients had a lower adjusted relative risk of in-hospital mortality 90 days after ECMO (HR 0·82 [95% CI 0·70–0·96]). Conversely, patients in group B had a higher adjusted relative risk (HR 1·42 [1·17–1·73]) than those in group A2 (figure 2 ). Advanced age, cancer, cardiac arrest before starting ECMO, and acute kidney injury were associated with an increased relative risk of mortality (figure 2; appendix p 22).
Figure 1.
Cumulative incidence of mortality after ECMO initiation
ECMO=extracorporeal membrane oxygenation. Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO between May 2 and Dec 31, 2020, at early-adopting centres,. Group B patients received ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020.
Figure 2.
Relative risk of mortality at early-adopting centres (on or before May 1 and after May 1) and at late-adopting centres (after May 1)
Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO between May 2 and Dec 31, 2020, at early-adopting centres. Group B patients received ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020. BMI=body-mass index. ECMO=extracorporeal membrane oxygenation. PaCO2=partial pressure of arterial carbon dioxide. PaO2:FiO2=ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen. VA=venoarterial. VV=venovenous. VVA=veno-venoarterial. *Chronic respiratory disease is a pre-ECMO comorbidity.
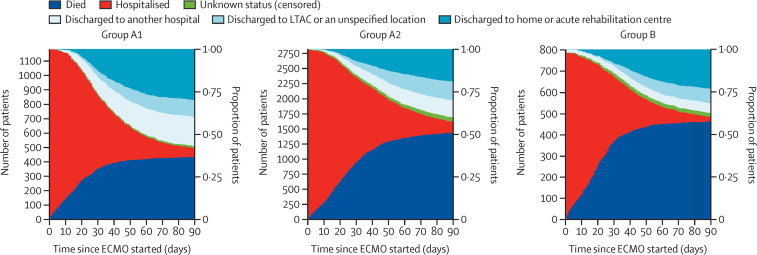
The final disposition of ECMO-supported patients was different (table 3 ; figure 3 ; appendix pp 9, 15, 21). Patients in group A1 were more likely to be discharged home or to an acute rehabilitation centre and were more likely to be transferred to another hospital than were patients in group A2 (permutation test p=0·01).
Table 3.
ECMO outcomes among three cohorts with COVID-19
| Group A1 | Group A2 | Group B | |||
|---|---|---|---|---|---|
| Last known patient status | 1182 | 2824 | 806 | ||
| Discharged | |||||
| To home or acute rehabilitation | 376 (32%) | 623 (22%) | 190 (24%) | ||
| To long-term acute care or unspecified location | 128 (11%) | 329 (12%) | 71 (9%) | ||
| To another hospital | 212 (18%) | 301 (11%) | 47 (6%) | ||
| Remain in the hospital, discharged from ICU | 2 (<1%) | 5 (<1%) | 1 (<1%) | ||
| Remain in the ICU | 16 (1%) | 78 (3%) | 22 (3%) | ||
| In-hospital death | 448 (38%) | 1488 (53%) | 475 (59%) | ||
| Select complications* | 494 (45%) | 1233 (52%) | 363 (54%) | ||
| CNS infraction | 7 (1%) | 53 (2%) | 8 (1%) | ||
| CNS haemorrhage | 68 (6%) | 196 (7%) | 42 (5%) | ||
| Haemolysis | 53 (5%) | 219 (8%) | 30 (4%) | ||
| Pump failure | 10 (1%) | 29 (1%) | 12 (2%) | ||
| Oxygen failure | 108 (9%) | 370 (13%) | 66 (8%) | ||
| Circuit change | 161 (14%) | 469 (17%) | 71 (9%) | ||
Data are n (%). Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO between May 2 and Dec 31, 2020, at early-adopting centres. Group B patients started ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020. Denominators for percentages are given in the footnotes when they differ from the last known patient status row. ECMO=extracorporeal membrane oxygenation. ICU=intensive care unit.
Group A1 n=1157, group A2 n=2767, group B n=782.
Figure 3.
Stacked bar plots of disposition for ECMO for COVID-19 among three cohorts
Group A1 patients started ECMO on or before May 1, 2020, at early-adopting centres. Group A2 patients started ECMO after May 1 at early-adopting centres. Group B patients received ECMO at late-adopting centres, which only provided ECMO for COVID-19 after May 1, 2020. Unknown status (censored) refers to patients who, as of June 9, 2021, did not meet one of the following three criteria: died, discharged alive, or survived at least 90 days after ECMO started. Hospitalised patients are those who, as of June 9, 2021, are still in the hospital where ECMO support was started. ECMO=extracorporeal membrane oxygenation. LTAC=long-term acute care centre.
Among early-adopting centres, the median duration of ECMO support was 14·1 days (IQR 7·9–24·1) in group A1 and 20·0 days (9·7–35·1) in group A2 (p<0·001). The Kaplan-Meier median duration of hospital stay after ECMO started at the ELSO centre was 27·1 days (15·8–44·2) in group A1 versus 30·7 days (17·6–50·7) in group A2.
The proportion of patients with CNS haemorrhage and haemolysis was similar between groups A1 and A2, but CNS infarction and mechanical ECMO complications were more common in group A2 (table 3; appendix p 16). Complication rates were generally similar for groups A1 versus A2 and for groups A2 versus B when normalised for duration of ECMO support (complication rates per 1000 h of ECMO support; appendix p 17). The prevalence and rate per 1000 h of ECMO support of CNS infarction, CNS haemorrhage, and haemolysis were similar between group A2 and group B (table 3; appendix pp 16–17). However, mechanical complications were reported less commonly in group B (table 3; appendix pp 16–17).
A higher volume of centre experience providing ECMO support to patients with COVID-19 was associated with lower in-hospital mortality 90 days after ECMO initiation. Early-adopting centres caring for at least nine patients with COVID-19 on or before May 1, 2020, had a risk-adjusted mortality rate of 0·56 (95% CI 0·43–0·75) relative to centres without such experience (appendix pp 25–26).
Discussion
At early-adopting centres, the cumulative incidence of in-hospital mortality 90 days after ECMO initiation for COVID-19 increased by about 15% (on an absolute basis) for patients who started ECMO between May 2 and Dec 31, 2020, compared with patients who started on or before May 1. This difference was not attributable to differences in known risk factors at baseline. We also saw a difference in outcomes among ECMO-supported patients who were in group A2 versus group B.
There are several potential explanations for the increased mortality seen in group A2 relative to group A1. These fall into three broad categories: patient selection, patient treatment, and the final disposition of patients.
First, patient selection might have changed between stages of the pandemic. It is possible that earlier in the pandemic, clinicians only started ECMO in patients with a high likelihood of survival, since there was initial concern that critical care resources might be overwhelmed in many areas.2, 5, 23, 24 However, this hypothesis might not explain the results because our model, which was based on established risk factors for COVID-19 and ECMO,25 suggests patients in group A1 had a higher (rather than lower) risk of mortality.8 These data suggest that patient selection on the basis of established risk factors was probably not an important contributor to the differential mortality observed. Nonetheless, we cannot exclude the possibility that unmeasured patient factors contributed to the differences in mortality.
Second, there were pre-ECMO changes in patients' COVID-19 treatment over time. At early-adopting centres, patients who were treated after May 1 (group A2) more frequently received non-invasive forms of ventilation before endotracheal intubation, potentially selecting for a cohort with greater patient self-inflicted lung injury relative to group A1.26 Patients in groups A1 and A2 received similar rates of prone positioning and use of neuromuscular blockade before ECMO; however, patients in group A2 more commonly received dexamethasone and remdesivir, which is consistent with evolving recommendations for COVID-19.1, 7, 12, 13 Together, these differences in treatment suggest that a higher proportion of patients supported with ECMO after May 1 had treatment-refractory disease than did those treated earlier, and thus might represent a different clinical phenotype with a worse overall prognosis after ECMO, despite few clear differences in established risk factors.
Third, patients' final disposition changed over time. Early-adopting centres caring for patients on or before May 1 transferred 18% of patients to other hospitals; however, after May 1, the same centres only transferred 11% of patients. Some transferred patients might have later died without the ELSO Registry recording the death;8 however, this possibility does not fully explain our results. Under the extreme assumption that all transferred patients died after transfer, the difference in mortality would remain significantly worse (appendix p 9).
Early-adopting centres had several different characteristics from late-adopting centres that might have contributed to differences in patient outcomes. For example, early-adopting centres had a higher volume of adult ECMO cases in 2019 than did late-adopting centres. However, the reasons that some centres used ECMO later in the pandemic are unknown. It is possible that they did not admit patients who required ECMO for COVID-19 early in the pandemic (due to lockdowns or patterns of spread), or that resources were unavailable either to offer ECMO or to submit cases to the ELSO Registry. We found that after adjusting for patient risk and centre volume, mortality for patients who started ECMO after May 1 was comparable for early-adopting (group A2) and late-adopting centres (group B). However, both had worse mortality than for those who started ECMO on or before May 1 (group A1). This finding is consistent with the existence of a volume–outcome relationship in ECMO for COVID-19.
The initial published experience of ECMO use for COVID-19 from the ELSO Registry reported an estimated cumulative incidence of in-hospital mortality 90 days after ECMO starting of 37·4% (95% CI 34·4–40·4),8 which is comparable to outcomes after ECMO for acute respiratory distress syndrome unrelated to COVID-19.3 This finding implied that health-care teams could make similar clinical judgments about the role of ECMO in patients with refractory acute respiratory distress syndrome whether or not they had COVID-19. However, this no longer appears to be true. Relative to patients who started ECMO on or before May 1, 2020, those supported after May 1 required ECMO for longer and had an absolute increase in mortality of about 15% such that the majority of these ECMO-supported patients died. In those with specific risk factors—such as advanced age, cancer, cardiac arrest before starting ECMO, and acute kidney injury—the expected mortality after ECMO could be significantly higher, but this risk would also be expected in these patients without the use of ECMO. This study was not a randomised clinical trial, and it remains unclear precisely which patients should be supported with ECMO. However, based on the data from this study, any clinical assessment of the risks versus potential benefits of starting ECMO in COVID-19 must factor in these evolving trends, particularly in resource-constrained settings.
The changing mortality risk of patients with COVID-19 supported with ECMO suggests that health-care centres need to track institutional and regional outcomes. Centres also need to develop local policies to guide allocation of ECMO during local or regional resource constraints.23, 24, 27 The increasingly longer patterns of ECMO support increase this possibility.
The association between lower mortality in centres with greater experience providing ECMO support to patients with COVID-19 suggests that there is an opportunity to improve the distribution of knowledge and resources.27 Networks providing ECMO support during COVID-19 have coordinated referrals and shared equipment and transportation resources, demonstrating the feasibility of coordinated sharing even in these sickest of patients and amid the challenges of a pandemic.14
Our study has limitations. First, data entered into the ELSO Registry depend on voluntary participation by centres worldwide. Centres submitting data during the pandemic might have differed from those that did not submit data. Second, submitted cases are not externally validated, and there is no confirmation that all cases were submitted. However, ELSO does have quality control standards; all ELSO site data managers pass a data entry exam, there is point-of-entry data assessment for error and validity, and full-record validation on submission ensures all mandatory fields are completed.8, 28 Third, we used a fixed demarcation point of May 1, 2020, largely based on a previous publication.8 However, analysing the data with this fixed time frame might decrease the sensitivity to detect differences. Finally, our study was not a randomised clinical trial. We cannot, therefore, speculate on what might have happened to patients had they not received ECMO support.
In summary, mortality for ECMO-supported patients with COVID-19 significantly worsened worldwide over the course of the pandemic, and duration of ECMO support increased. These dynamic outcomes compel continued surveillance, facilitating awareness of local and regional mortality rates, duration of ECMO support, and resource constraints. Our findings have important implications, both in setting expectations with patients and surrogate decision makers, and for informing clinicians and policy makers with respect to the most appropriate ECMO initiation criteria.29 Furthermore, centres should consider developing local policies that guide the ethical allocation of ECMO when resources are constrained.
For the data dictionary see https://www.elso.org/Registry/DataDefinitions,Forms,Instructions.aspx
For ELSO policies see https://www.elso.org/AboutUs/ELSOPolicies.aspx
Data sharing
The data dictionary and ELSO policies are available online. The participant data collected for this study are available, as a limited data set, to member centres conditional on approval from ELSO's Scientific Oversight Committee, but it is not publicly available. ELSO Scientific Oversight Committee approved data requests and the date of data release for research are listed online at https://www.elso.org/Registry/ApprovedDataRequests.aspx.
Declaration of interests
RPB is the ELSO Registry chair. GM, MLP, CMS, and DB are on the ELSO board of directors. MLP is the current president of ELSO and DB is the president-elect of ELSO. DB also chairs the executive committee for the International ECMO Network. PSB has received funding from ELSO for statistical analysis related and unrelated to this study. CMS receives payment from ELSO in her role as chief executive officer of ELSO. RPB, CA, and EF are members of the ELSO Steering Committee. RL and AC are past members of the European ELSO Steering Committee. ASS chairs the Scientific Oversight Committee of the International ECMO Network. RPB reports grants from the US National Institutes of Health (R01 HL153519, R01 HD015434, and K12 HL138039). AC reports speaking fees from Baxter, Getinge, and Fresenius, unrelated to the submitted work. EF reports consulting fees from ALung Technologies and Vasomune; speaking fees from Getinge; and fees for Data Safety Monitoring or Advisory Board participation from Baxter and Boehringer-Ingelheim. KH reports unpaid consulting work with Abbott Vascular. RL reports research support from Getinge; consulting fees from Getinge, Livanova, and Medtronic; and honoraria for lectures from Getinge and Livanova, unrelated to the submitted work. TJI is employed by the US Government. ASS reports consulting fees from Baxter and Xenios, unrelated to the submitted work. DB reports grants from ALung Technologies, and medical advisory board relationships with Baxter (past), Xenios, Abiomed, Cellenkos, Medtronic, and Hemovent, unrelated to the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors and ELSO thank front-line health-care workers throughout the world for providing heroic and humanistic care to patients during the pandemic despite clear personal risks. We are also very grateful to our colleagues for providing data to the ELSO Registry, while simultaneously tending to clinical responsibilities, to advance science in the interests of all of our patients.
Contributors
RPB, GM, and DB conceived the study. PSB conceived the analytic plan. RPB, GM, PSB, AC, TJI, ASS, and DB designed the study and drafted the initial manuscript. All authors analysed and interpreted the results, critically edited the manuscript, approved the final work, and agree to be accountable for the accuracy and integrity of the work. RPB and PSB had full access to and verified all the data. RPB, GM, and DB had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.National Institutes of Health; COVID-19 Treatment Guidelines Panel Coronavirus disease 2019 (COVID-19) treatment guidelines. August 4, 2021. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 2.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett RH, Ogino MT, Brodie D, et al. Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Clinical management of COVID-19: interim guidance. May 27, 2020. https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf
- 8.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Hajage D, Lebreton G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaefi S, Brenner SK, Gupta S, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care. 2021;25:211. doi: 10.1186/s13054-021-03634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broman LM, Eksborg S, Coco VL, De Piero ME, Belohlavek J, Lorusso R. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. 2021;9:e80–e81. doi: 10.1016/S2213-2600(21)00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorusso R, Combes A, Coco VL, et al. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47:344–348. doi: 10.1007/s00134-020-06272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141–150. [Google Scholar]
- 18.Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396:1125–1128. doi: 10.1016/S0140-6736(20)32076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction. Springer; New York, NY: 2009. Chapter 7: model assessment and selection. [Google Scholar]
- 20.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric Estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 23.Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518–526. doi: 10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 25.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 27.Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorusso R, Alexander P, Rycus P, Barbaro R. The Extracorporeal Life Support Organization Registry: update and perspectives. Ann Cardiothorac Surg. 2019;8:93–98. doi: 10.21037/acs.2018.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supady A, Curtis JR, Abrams D, et al. Allocating scarce intensive care resources during the COVID-19 pandemic: practical challenges to theoretical frameworks. Lancet Respir Med. 2021;9:430–434. doi: 10.1016/S2213-2600(20)30580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data dictionary and ELSO policies are available online. The participant data collected for this study are available, as a limited data set, to member centres conditional on approval from ELSO's Scientific Oversight Committee, but it is not publicly available. ELSO Scientific Oversight Committee approved data requests and the date of data release for research are listed online at https://www.elso.org/Registry/ApprovedDataRequests.aspx.