Abstract
Purpose
Severe cases of coronavirus disease 2019 develop ARDS requiring admission to the ICU. This study aimed to investigate the ultrasound characteristics of respiratory and peripheral muscles of patients affected by COVID19 who require mechanical ventilation.
Materials and methods
This is a prospective observational study. We performed muscle ultrasound at the admission of ICU in 32 intubated patients with ARDS COVID19. The ultrasound was comprehensive of thickness and echogenicity of both parasternal intercostal and diaphragm muscles, and cross-sectional area and echogenicity of the rectus femoris.
Results
Patients who survived showed a significantly lower echogenicity score as compared with those who did not survive for both parasternal intercostal muscles. Similarly, the diaphragmatic echogenicity was significantly different between alive or dead patients. There was a significant correlation between right parasternal intercostal or diaphragm echogenicity and the cumulative fluid balance and urine protein output. Similar results were detected for rectus femoris echogenicity.
Conclusions
The early changes detected by echogenicity ultrasound suggest a potential benefit of proactive early therapies designed to preserve respiratory and peripheral muscle architecture to reduce days on MV, although what constitutes a clinically significant change in muscle echogenicity remains unknown.
Keywords: Muscular ultrasound, Grayscale analysis, Covid19, ARDS
Abbreviations: MV, mechanical ventilation; ARDS, acute respiratory distress syndrome; COVID 19, the novel coronavirus disease 2019; ICU, Intensive Care Unit; RFCSA, rectus femoris cross sectional area; NIV, non-invasive ventilation
1. Introduction
Up to 30% of the patients affected by the novel coronavirus disease 2019 (COVID19) may develop an acute respiratory distress syndrome (ARDS), which requires in the majority of patients a respiratory support with non-invasive mechanical ventilation and, very often, intubation [1,2]. Recent evidence is growing in describing the characteristics of this disease, with main focus on lung morphology [[3], [4], [5]]. Therapeutic strategies implemented for their management may often lead to short-term muscular and functional alterations resulting in ICU-Acquired weakness. These lead to long-term disabilities expressing through dependence and quality of life impairment of survivors. Indeed, few data are still available regarding the peripheral and respiratory muscular characteristics, mainly because this investigation is usually confined in more advance stages of disease [6,7]. Muscular ultrasonography allows visualization and classification of muscle characteristics [8], which may be described besides muscles' thickness with their echogenicity. In fact, lean muscle tissue has a low echogenicity, whereas intramuscular fat and connective tissue are characterized by a high echogenicity [9]. Using a greyscale analysis, the total muscle echo-intensity may in fact be quantified. The assumption is that the higher the mean pixel intensity of a muscle region of interest, the lower the muscle quality (i.e., more intramuscular fat or connective tissue) and thus its inhomogeneity [10]. Muscle echogenicity has already been investigated during critical illness, although its modifications over the time and its associated histopathological characteristics remain to be determined [11,12]. In fact, inflammation and infection as well as fluid shifts may substantially contribute to the increment in muscular inhomogeneity [13,14]. Few data are available regarding the characteristics of respiratory muscle ultrasound quality during critical illness, as most of the study restricted this methodology to the analysis of peripheral muscles in the detection of ICU acquired weakness [15]. Recently, the parasternal intercostal muscles and the diaphragm have been investigated together in the critical care setting as they represent the easiest accessible respiratory muscles to be investigated by ultrasound [[16], [17], [18]]. We hypothesized that a combined assessment of respiratory (i.e., intercostal and diaphragm) and peripheral (i.e., quadriceps) muscles quantity (as measured by thickness) and quality (as assessed by greyscale analysis), would reflect the severity of illness. Thus, we performed this study to assess if the quality characteristics of parasternal intercostal, diaphragm and quadriceps muscles of ICU COVID19 mechanically ventilated patients influenced the outcomes and are correlated with other variables, such as fluid or protein balance, or indexes of inflammation.
2. Material and methods
We conducted a prospective physiologic study on 32 consecutive intubated patients admitted to the general ICU of ASST Santi Paolo e Carlo, San Paolo Hospital, Milan, Italy. We included intubated patients with confirmed infection by SARS COV 2 and diagnosis of ARDS, deeply sedated and paralyzed. Exclusion criteria were age <18 years, history of severe chronic obstructive pulmonary disease, pregnancy, failure to perform respiratory muscle ultrasound. The study was approved by the Institutional Review Board (Comitato Etico Interaziendale Milano Area 1, 2020/ST/178).
2.1. Ultrasound examination
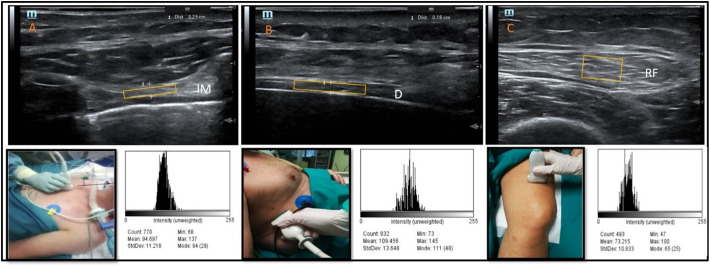
Ultrasound was performed by a single operator (PF) with 10 years of ultrasound experience and qualifications in respiratory ultrasound. B-mode images were obtained utilizing a 6–14 MHz linear array on a Mindray TE-7 machine (Shenzhen Mindray Bio-Medical Electronics Co. Ltd. Shenzen, China) with the participant at 45° degrees. Depth and gain were standardized using the same image presets between patients as previously described [19]. Images were taken of the 2nd parasternal intercostal muscles bilaterally in the sagittal plane at end-tidal expiration with a window visualizing the 2nd/3rd ribs, respectively (Fig. 1 , panel A). End-tidal expiration diaphragm thickness was assessed in the zone of apposition of the diaphragm to the rib cage. The linear probe was placed above the right 10th rib in the mid-axillary line, as previously described [20,21] (Fig. 1, panel B). Cross-sectional area of the rectus femoris (RFCSA) and its echogenicity were measured with the rested leg supported in passive extension. B-mode ultrasonography using an 8 MHz 5.6 cm linear transducer array (MindRay TE-7, Shenzhen Mindray Bio-Medical Electronics Co. Ltd. Shenzen, China) was applied, similar to the method previously described [22]. Briefly, the probe was placed three-fifths of the distance from the anterior superior iliac spine to the superior patellar border, transversely to the muscle for thickness measurements and cross-sectional area (Fig. 1, panel C) [23,24]. RFCSA was outlined by a movable cursor on a frozen and calculated as an average of three consecutive measurements within 10%; muscle thickness in centimeters was selected between the inner and outermost echogenic layer of the muscle fascial borders. Images were saved in JPEG format and echogenicity was quantified using a greyscale histogram analysis of the images. The analysis was performed with ImageJ software (https://imagej.nih.gov/ij/index.html; NIH, Bethesda, MD, USA), using the square method to define the region of interest for analysis using the histogram function, selecting a free-form area devoid of artefacts [25,26]. Images were reviewed by a second reader (VC) who was not directly involved in imagine acquisition.
Fig. 1.
Ultrasound of respiratory (parasternal muscle and diaphragm) and peripheral (rectus femoris).
The figure depicts the ultrasound investigation of respiratory and peripheral muscles. Panel A: Parasternal intercostal ultrasound thickness and greyscale histogram; images were taken of the 2nd parasternal intercostal muscles bilaterally in the sagittal plane at end-tidal inspiration (IM-intercostal muscle). Panel B: Diaphragm thickness and greyscale histogram; image was taken of end-tidal expiration in the right anterior axillary line between the 8th and 9th ribs in the coronal plane (D- diaphragm). Panel C: Rectus femoris, CSA and echogenicity; image was taken of approximately 15 cm above the superior border of the patella (RF-rectus femoris). The yellow rectangles delineate examples of muscles' areas (excluding the pleural and peritoneal membranes, and aponeurosis). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
All patients were deeply sedated and mechanically ventilated at ICU admission. The clinical management of patients was standardized according to local and regional suggestion [27]. More specifically, all patients received muscle relaxants during the first week of ICU stay (4.5 ± 1.2 days), as well as a 10-day course of i.v. dexamethasone 6 mg/day [28]. No other immunomodulatory agents were administered, nor were antiviral drugs.
Fluid balance was evaluated daily during ICU stay. Fluid intake included intravenous fluids, total parenteral and enteral nutrition, blood products, and intravenous medications. Fluid output included urine, feces, blood loss, output from drains and other body cavities, and gastric aspirate [29,30]. Other fluid losses, such as sweat and respiratory evaporation, were excluded because of lack in agreement of computation. All variables were derived directly from computerized clinical records (Digistat Intensive Care Unit (ICU), Ascom, Baar, Switzerland). Urinary urea nitrogen was measured from 24 h collected urine and protein output was calculated as described elsewhere [31].
2.2. Statistical analysis
Given the lack of similar investigations in COVID-19 patients, a priori sample size calculation was not performed. The sample size was pragmatically based on a 2-month time frame as well as recently published literature on similar topics [26,32]. Thirty-two consecutive patients were enrolled. We compared the variables in patients who did and did not survive the ICU stay. Comparisons between normally distributed variables were performed by Student's t-test, while non-normally distributed variables were compared by Wilcoxon signed rank test. Normality was tested by the Shapiro-Wilk test. Normally distributed data are indicated as mean ± SD, while median and interquartile range are used to report non-normally distributed variables. Association between two variables was assessed by linear regression or Spearman regression coefficient, as appropriate. A level of p value of less than 0.05 (two-tailed) was considered as statistically significant. Statistical analyses were performed by Stata/SE 12.0 (StataCorp, College Station, TX USA).
3. Results
3.1. Patients' characteristics
Thirty-two patients underwent ultrasound examinations within 24 h after ICU admission, intubated after a trial of NIV performed in the emergency department or similar. The characteristics of the patients enrolled are shown in Table 1 . Patients were 63.9 ± 7.4 years old and comorbidities were few. Five patients had mild, 18 patients moderate, and nine severe ARDS. The onset of symptoms prior to the hospital admission was 10.3 ± 7 days, while the intubation was performed 12.5 ± 6 days after the onset of symptoms. During this period, the median duration of the NIV trial was 2.2 ± 2.5 days. ICU mortality was 53%. There were no statistically significant differences in the setting of PEEP and FiO2 between patients who did and did not survive the ICU stay. Within the first 24 h of ICU stay, there was a significant difference in fluid balance (847 ± 531 vs. 1344 ± 567 ml/day, p = 0.016) and urinary protein output (73 ± 37.6 vs. 101 ± 35.9 g/day, p = 0.044) between patients who went on to die or to survive. There are no statistical differences in severity scores (SAPS II and SOFA; 31 ± 10, 33 ± 11, p = 0.552 and 6.2 ± 2.5, 6.4 ± 2.5, p = 0.872) between the two groups, and no statistical correlation with echogenicity scores.
Table 1.
Main patients' characteristics.
| Global (n = 32) | Alive (n = 15) | Dead (n = 17) | p Value | |
|---|---|---|---|---|
| Male sex (n°, %) | 25 (78) | 9 (36) | 16 (64) | |
| Age (years) | 63.9 ± 7.4 | 61.4 ± 7.0 | 66.1 ± 7.2 | 0.077 |
| BMI (kg/m2) | 27.2 ± 5.6 | 27.7 ± 6.7 | 26.9 ± 4.6 | 0.705 |
| SAPS II (points) | 32 ± 10 | 31 ± 10 | 33 ± 11 | 0.552 |
| SOFA (points) | 6.3 ± 2.4 | 6.2 ± 2.5 | 6.4 ± 2.5 | 0.872 |
| Onset to hospital (days) | 10.3 ± 7 | 9.9 ± 8 | 10.7 ± 6.1 | 0.762 |
| NIV duration (days) | 2.2 ± 2.5 | 2.4 ± 3.1 | 1.9 ± 1.8 | 0.609 |
| Onset to ETI (days) | 12.5 ± 6 | 11.9 ± 9.1 | 11 ± 7.2 | 0.832 |
| PEEP (cmH2O) | 9.8 ± 1.4 | 9.8 ± 1.9 | 9.8 ± 0.8 | 0.975 |
| FiO2 | 0.75 ± 0.2 | 0.74 ± 0.2 | 0.75 ± 0.2 | 0.805 |
| ETCO2/PaCO2 | 0.72 ± 0.11 | 0.75 ± 0.07 | 0.70 ± 0.12 | 0.174 |
| PaO2/FiO2 (mmHg) | 117.6 ± 39.9 | 127.7 ± 51.4 | 107.5 ± 26.1 | 0.458 |
| C reactive protein (mg/L) | 116 ± 88 | 113 ± 95 | 119 ± 85 | 0.842 |
| Fluid balance (ml/day) | 1111 ± 598 | 847 ± 531 | 1344 ± 567 | 0.0164 |
| Urinary protein output (g/day) | 88.3 ± 38.7 | 73.7 ± 37.6 | 101.1 ± 35.9 | 0.0441 |
3.2. Respiratory and peripheral muscular ultrasound
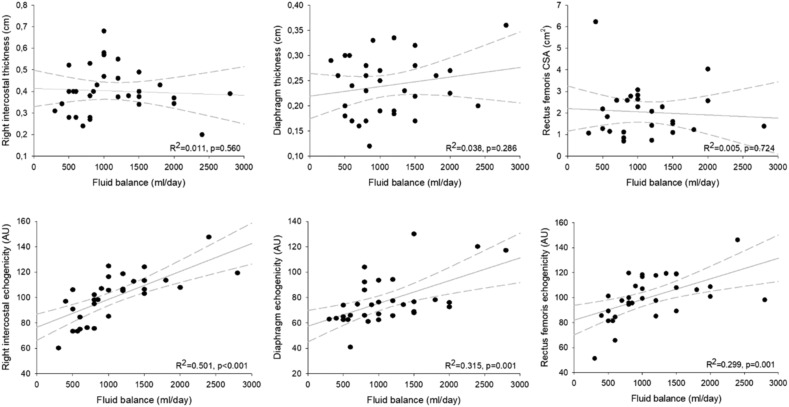
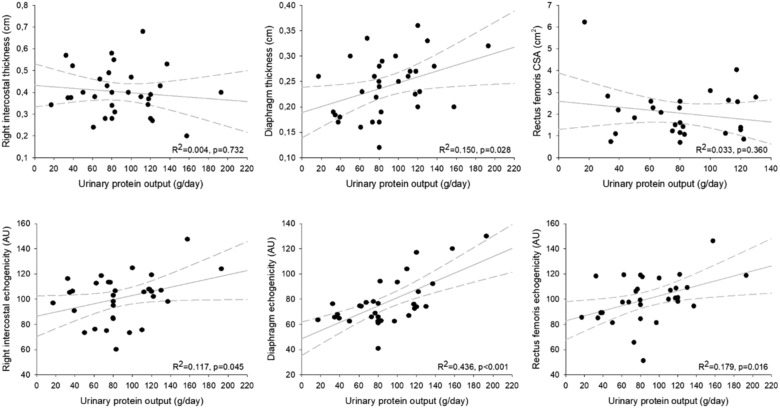
Parasternal intercostal muscles were visualized in all patients. The main results are showed in Table 2 . Intercostal thickness was 0.40 [0.30; 0.44] cm for the second left parasternal intercostal and 0.39 [0.34; 0.47] cm for second right intercostal. Muscle thickness was not different between survivors and non-survivors (0.37 [0.28; 0.40] vs 0.40 [0.36; 0.48], p = 0.189 for right parasternal muscle, and 0.34 [0.30; 0.40] vs 0.40 [0.29; 0.50], p = 0.30, for left one. Echogenicity varied between sites, with a small range of values (106.4 [93.4; 109.4] and 105.6 [86.7; 113.2] AU for left and right parasternal muscle, respectively). Patients who survived showed a significantly lower echogenicity score as compared with those who did not survive for both parasternal intercostal muscles (91 [75.1; 98.3] vs 108 [106.0; 117.6] AU, p = 0.0002 for right parasternal muscles, and 99.2 [77.4; 103.2] vs 108.4 [106.5; 1117.5] AU, p = 0.0003 for left muscles). The right hemidiaphragm was imaged in cross-section in the zone of apposition as mentioned above. The average thickness of the diaphragm was 0.25 [0.19; 0.28] cm, and it was similar in patients who did and did not survive (0.20 [0.17; 0.28] cm vs 0.26 [0.22; 0.30] cm, p = 0.053, respectively). However, the echogenicity was significantly different between alive or dead patients (65 [62.6; 68] vs 77 [74.2; 94], p = 0.0002, respectively). There was a fair correlation between right intercostal echogenicity and both diaphragm (R2 = 0.3225, p = 0.001) and rectus femoris (R2 = 0.6323, p < 0.001) echogenicity. Moreover, we observed a significant correlation between both right parasternal intercostal and diaphragm echogenicity, and the cumulative fluid balance (R2 = 0.501, p < 0.001, R2 = 0.315, p = 0.001, respectively; Fig. 2 ). Rectus femoris architectural characteristics was similar at the admission in ICU survivors and non-survivors, except for echogenicity, which was significantly higher in non-survivors (89.3 [81.4; 95.8] vs 108.8 [101.1; 118.6] AU, p < 0.0001). Interestingly, these values showed a significant correlation with fluid balance (R2 = 0.299, p = 0.001; Fig. 1) as previously described for respiratory muscles. We also observed a good correlation between both respiratory and peripheral muscles echogenicity and urine protein output (R2 = 0.117, p = 0.045, R2 = 0.436, p < 0.001 and R2 = 0.179, p = 0.016), not showed for their thickness (Fig. 2).
Table 2.
Ultrasonographic variables of respiratory and peripheral muscles within 24 h of ICU admission.
| Ultrasound variables | Global (n = 32) | Alive (n = 15) | Dead (n = 17) | p Value |
|---|---|---|---|---|
| Right intercostal thickness (cm) | 0.39 [0.34;0.47] | 0.37 [0.28;0.40] | 0.40 [0.36;0.48] | 0.1881 |
| Right intercostal echogenicity (AU) | 105.6 [86.7; 113.2] | 91 [75.1;98.3] | 108 [106; 117.6] | 0.0002 |
| Left intercostal thickness (cm) | 0.40 [0.30;0.44] | 0.34 [0.30;0.40] | 0.40 [0.29;0.50] | 0.3023 |
| Left intercostal echogenicity (AU) | 106.4 [93.4; 109.4] | 99.2 [77.4; 103.2] | 108.4 [106.5; 117.5] | 0.0003 |
| Diaphragm thickness (cm) | 0.25 [0.19;0.28] | 0.20 [0.17;0.28] | 0.26 [0.22;0.30] | 0.0592 |
| Diaphragm echogenicity (AU) | 74.1 [65.1;84.0] | 65 [62.6;68] | 77 [74.2;94] | 0.0002 |
| Rectus femoris thickness (cm) | 0.59 [0.56;0.69] | 0.69 [0.60;0.75] | 0.56[0.52;0.57] | 0.0283 |
| Rectus femoris CSA (cm2) | 1.83 [1.2;2.6] | 1.83 [1.1;2.6] | 1.84 [1.3;2.7] | 0.9967 |
| Rectus femoris echogenicity (AU) | 99.7 [89.3;115] | 89.3 [81.4;95.8] | 108.8 [101.1; 118.6] | <0.0001 |
AU: arbitrary units; CSA: cross-sectional area.
Fig. 2.
Correlation between respiratory and peripheral muscular characteristics and fluid balance.
The figure shows correlations between respiratory and rectus fermoris muscular thickness and echogenicity and fluid balance. Right parasternal intercostal, diaphragm and rectus femoris echogenicity were expressed in arbitrary units (AU), the cumulative fluid balance was expressed in ml/day.
4. Discussion
The main findings of these preliminary observations are: 1) the echogenicity of both parasternal intercostal muscles and diaphragm is significantly higher in patients who died, and the echogenicity is significantly correlated with fluid balance and urine protein output; 2) echogenicity of rectus femoris is higher in non-survivors, and correlates better with indices of catabolism as compared with muscle thickness or CSA; 3) greyscale analysis of both respiratory and peripheral muscles could be a better ultrasound predictor of outcome (Fig. 3 ).
Fig. 3.
Correlation between respiratory and peripheral muscular characteristics and urinary protein output.
The figure shows the correlations between intercostal, diaphragm and rectus femoris and urinary protein output. The echogenicity of respiratory and peripheral muscles is also showed. Right parasternal intercostal, diaphragm and rectus femoris echogenicity were expressed in arbitrary units (AU), the urinary protein output was expressed in g/day.
Quantitative musculoskeletal ultrasound has been proposed as an alternative imaging modality to provide estimates of muscle quality based on tissue composition [33]. Conventional greyscale B-mode ultrasound provides information about the echogenicity or reflective properties of a tissue, as well as the architectural characteristics of a structure. Keeping the principles of image generation in mind, it is important to understand that conventional greyscale can only provide two types of information. Firstly, it informs about the echogenicity or reflective properties of a tissue, which is an indicator of its composition or makeup (such as the quantity of collagen or fluid it contains); secondly, it provides information about the architecture (i.e., internal structure, size, and shape). As such, changes in muscle size, although influenced by muscle activity, are a merger of many factors. These are ranging from the resting state of the muscle, the extensibility and structure of the musculotendinous unit, the type of contraction taking place, the presence of competing forces, factors associated with interpreting ultrasound images, and factors associated with imaging technique. The assessment of muscle quality via echogenicity measures has been used to discriminate among individuals with and without muscle abnormalities [8,34]. Even if small data are available in critical illness, a diminished muscle composition due to fluid or adipose tissue infiltration or increased fibrotic tissue is associated with impaired muscle mechanics that may contribute to functional deficits [35,36]. Previous studies demonstrated that admission to intensive care causes a reduction in muscle size in the thigh, with loss of muscle thickness occurring within the first 5 days of admission [14,37,38]. Further loss of muscle thickness has also been related with loss of muscle function at ICU discharge [39]. The large majority of COVID-19 ICU survivors developed ICU acquired limb muscle weakness and more than 40% of patients with limb weakness still had severely limited function 1-month post weaning [40]. Unfortunately, we do not know if the echogenicity values are indicative also of a reduction in muscle function, as we did not test any functional scale. Given the narrow time-course of the present study, it is not particularly surprising that we did not observe any correlation between thickness or CSA both of respiratory and peripheral muscles and outcome or fluid balance and urine protein output, as these features likely take few days to be altered. In fact, it has been showed how later biopsy may have revealed some evidence of fiber shift and/or type-specific atrophy that cannot be captured by ultrasound [41]. Even if we did not follow over the time the ultrasound variables, we found that earlier echogenicity of both respiratory and peripheral muscles was associated with outcome. This could be related to the positive fluid balance perpetuated over the days before admission in the ICU and/or due to the inflammation/septic status (as suggested by C-reactive protein) [41]. This result may also explain the difference in diaphragmatic thickness observed in patients who went on to die as compared with survivors, in contrast to previous observations [38,42]. An overall positive fluid balance is not unusual in critical illness. Moreover, independently of such changes, alterations in factors such as oncotic pressure and microvascular permeability may alter fluid distribution between the vascular and tissue compartments. As we observed a relationship between fluid balance and change in echogenicity, we may consider the fluid balance a crude surrogate for fluid state, which does not distinguish between intravascular, intracellular, third spaces and fluid shifts between them. It still remains a mirror of illness severity, even if we did not observe a significant difference in term of SAPS II between the survivor or not. This assumption comes from previous studies based on the biochemical analysis which showed how echogenicity was significantly associated with intramuscular liquid and adipose tissue, rather than fibrotic tissue [9,41]. In fact, a direct correlation between muscle wasting and both inflammation and acute lung injury was described. In addition, all our patients were confined to bed for days before ICU admission, assisted with oxygen and NIV not sufficient to overcome the pulmonary disease. All these factors could be associated with muscle wasting in varying degree [43]. Moreover, echogenicity measures have demonstrated value in the assessment of neuromuscular diseases and may be an important factor in observed muscle performance insufficiencies [[44], [45], [46]]. Edema, neutrophil infiltration, and fibrin deposition dominated the early phase of critical illness, with macrophage-rich cellular fasciitis extending deeper within the muscle fascicles, while the myofiber necrosis and muscle regeneration appeared later. It has already been shown how an increased ultrasound echogenicity during the first week of critical illness predict the presence of myofiber necrosis [47]. We found that a higher muscle echogenicity (indicating poorer muscle quality) negatively correlated with survival in this clinical scenario of COVID19-related ARDS. Importantly, changes in muscle quantity and quality reflect the disease severity and has potential biomarker utility in longitudinal observational and interventional COVID19 related-disease studies.
The strength of the positive correlation between intercostal muscle and diaphragm echogenicity and outcome was similar to that between quadriceps rectus femoris echogenicity and outcome. Previous histological studies have shown that parasternal intercostal muscles undergo remodeling in chronic pulmonary disease [48]. To our knowledge, there are no data looking at imaging correlates of these changes. Eventually, even though severe COVID19-related ARDS has been observed to principally affect the respiratory system, neurological involvements have already been reported [49]. Among different neurological manifestations also skeletal muscle injury and peripheral nerve may be implicated [50]. Signs of skeletal muscle damage often associated with liver and kidney involvement have already been noted [51]. Muscle enzymes have been showed highly elevated in the symptomatic patients, but the exact mechanism of muscle damage has not been established [52]. Thus, we cannot speculate if there could be a direct effect of viral infection to muscular structures that may be exacerbated by inflammatory disease.
This study has several important limitations. The sample size precludes meaningful exploration of the association of wasting with specific disease entities. However, homogeneity of muscle loss with stratification by fluid balance and urine protein output suggests that the specific disease state may not be the most significant driver of muscle loss during the acute phase. Although these data may be relevant to COVID19 disease-related patients during the acute stages of illness, expanded disease-specific studies are needed. Then, the first day of ICU admission does not necessarily reflect the first day of critical illness. However, although we were unable to quantify physiological derangement prior to admission, the median time from hospital to ICU admission was 1 week, in which this phenomenon is likely. Moreover, we did not follow over the time the US variation. As a result, we may have missed those patients who lose muscle far more slowly or rapidly. Even if we did not measure the intraclass correlation for repeated measures (intra-rater reliability), this has been already recently tested [11,19,26]. Eventually, the quantitative estimation of echogenicity in clinical settings is often performed through greyscale histogram analysis. This imaging analysis technique involves the construction of a plot featuring the number of pixels associated with a given ROI within intervals determined by intensity level [53,54]. To our knowledge, data regarding the use of this methodology in critical illness are scarce, especially when considering the recent pandemic related with COVID19 disease.
5. Conclusions
Our preliminary data show the feasibility of performing quantitative measurements on intercostals, diaphragm and quadriceps muscles ultrasound in COVID19 critically ill patients and suggests that early changes in the muscle parameters may potentially predict the survival rate. The early changes detected by ultrasound suggest a potential benefit of proactive early therapies designed to preserve respiratory muscle architecture to reduce days on MV, although what constitutes a clinically significant change in muscle echogenicity remains unknown. Small sample size precludes definite interpretation of trends seen in our study. Larger studies are needed to define parameters of serial intercostal and diaphragm muscle changes on ultrasound in COVID19 patients on MV.
References
- 1.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L., Meissner K., Marini J.J. The baby lung and the COVID-19 era. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albarello F., Pianura E., Di Stefano F., Cristofaro M., Petrone A., Marchioni L., et al. 2019-novel coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis. 2020;93:192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin C.E., Bersten A.D. Alterations in respiratory and limb muscle strength and size in patients with sepsis who are mechanically ventilated. Phys Ther. 2014;94:68–82. doi: 10.2522/ptj.20130048. [DOI] [PubMed] [Google Scholar]
- 7.Tuinman P.R., Jonkman A.H., Dres M., Shi Z.-H., Goligher E.C., Goffi A., et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46:594–605. doi: 10.1007/s00134-019-05892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillen S., van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011;33:1016–1024. doi: 10.1179/1743132811Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 9.Reimers K., Reimers C.D., Wagner S., Paetzke I., Pongratz D.E. Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med. 1993;12:73–77. doi: 10.7863/jum.1993.12.2.73. [DOI] [PubMed] [Google Scholar]
- 10.Mayans D., Cartwright M.S., Walker F.O. Neuromuscular ultrasonography: quantifying muscle and nerve measurements. Phys Med Rehabil Clin N Am. 2012;23:133–148. doi: 10.1016/j.pmr.2011.11.009. xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright M.S., Kwayisi G., Griffin L.P., Sarwal A., Walker F.O., Harris J.M., et al. Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve. 2013;47:255–259. doi: 10.1002/mus.23525. [DOI] [PubMed] [Google Scholar]
- 12.Grimm A., Teschner U., Porzelius C., Ludewig K., Zielske J., Witte O.W., et al. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care. 2013;17:R227. doi: 10.1186/cc13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moukas M., Vassiliou M.P., Amygdalou A., Mandragos C., Takis F., Behrakis P.K. Muscular mass assessed by ultrasonography after administration of low-dose corticosteroids and muscle relaxants in critically ill hemiplegic patients. Clin Nutr. 2002;21:297–302. doi: 10.1054/clnu.2001.0532. [DOI] [PubMed] [Google Scholar]
- 14.Gruther W., Benesch T., Zorn C., Paternostro-Sluga T., Quittan M., Fialka-Moser V., et al. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med. 2008;40:185–189. doi: 10.2340/16501977-0139. [DOI] [PubMed] [Google Scholar]
- 15.Formenti P., Umbrello M., Coppola S., Froio S., Chiumello D. Clinical review: peripheral muscular ultrasound in the ICU. Ann Intensive Care. 2019;9:57. doi: 10.1186/s13613-019-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dres M., Dube B.-P., Goligher E.c., Vorona S., Demiri S., Brochard L.j., et al. C76. Chest wall, respiratory muscles: Neural and ventilatory control. American Thoracic Society; 2018. Intercostal muscle ultrasound activity: A feasibility and physiological study in mechanically ventilated patients; p. A5863. [DOI] [Google Scholar]
- 17.Umbrello M., Formenti P., Lusardi A.C., Guanziroli M., Caccioppola A., Coppola S., et al. Oesophageal pressure and respiratory muscle ultrasonographic measurements indicate inspiratory effort during pressure support ventilation. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Dres M., Demoule A. Beyond ventilator-induced diaphragm dysfunction. New evidence for critical illness-associated diaphragm weakness. Anesthes. 2019;131:462–463. doi: 10.1097/ALN.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 19.Wallbridge P., Parry S.M., Das S., Law C., Hammerschlag G., Irving L., et al. Parasternal intercostal muscle ultrasound in chronic obstructive pulmonary disease correlates with spirometric severity. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umbrello M., Formenti P., Longhi D., Galimberti A., Piva I., Pezzi A., et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care. 2015;19:161. doi: 10.1186/s13054-015-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueki J., De Bruin P.F., Pride N.B. In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax. 1995;50:1157–1161. doi: 10.1136/thx.50.11.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruin P.F., Ueki J., Watson A., Pride N.B. Size and strength of the respiratory and quadriceps muscles in patients with chronic asthma. Eur Respir J. 1997;10:59–64. doi: 10.1183/09031936.97.10010059. [DOI] [PubMed] [Google Scholar]
- 23.Bemben M.G. Use of diagnostic ultrasound for assessing muscle size. J Strength Cond Res. 2002;16:103–108. [PubMed] [Google Scholar]
- 24.Ema R., Wakahara T., Mogi Y., Miyamoto N., Komatsu T., Kanehisa H., et al. In vivo measurement of human rectus femoris architecture by ultrasonography: validity and applicability. Clin Physiol Funct Imaging. 2013;33:267–273. doi: 10.1111/cpf.12023. [DOI] [PubMed] [Google Scholar]
- 25.Harris-Love M.O., Seamon B.A., Teixeira C., Ismail C. Ultrasound estimates of muscle quality in older adults: reliability and comparison of Photoshop and ImageJ for the grayscale analysis of muscle echogenicity. PeerJ. 2016;4 doi: 10.7717/peerj.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coiffard B., Riegler S., Sklar M.C., Dres M., Vorona S., Reid W.D., et al. Diaphragm echodensity in mechanically ventilated patients: a description of technique and outcomes. Crit Care. 2021;25:64. doi: 10.1186/s13054-021-03494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foti G., Giannini A., Bottino N., Castelli G.P., Cecconi M., Grasselli G., et al. Management of critically ill patients with COVID-19: suggestions and instructions from the coordination of intensive care units of Lombardy. Minerva Anestesiol. 2020;86:1234–1245. doi: 10.23736/S0375-9393.20.14762-X. [DOI] [PubMed] [Google Scholar]
- 28.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox P. Insensible water loss and its assessment in adult patients: a review. Acta Anaesthesiol Scand. 1987;31:771–776. doi: 10.1111/j.1399-6576.1987.tb02662.x. [DOI] [PubMed] [Google Scholar]
- 30.Schneider A.G., Baldwin I., Freitag E., Glassford N., Bellomo R. Estimation of fluid status changes in critically ill patients: fluid balance chart or electronic bed weight? J Crit Care. 2012;27 doi: 10.1016/j.jcrc.2011.12.017. 745.e7–12. [DOI] [PubMed] [Google Scholar]
- 31.Tessari P. In: Cachexia and wasting: A modern approach. Mantovani G., Anker S.D., Inui A., Morley J.E., Fanelli F.R., Scevola D., et al., editors. Springer Milan; Milano: 2006. Nitrogen balance and protein requirements: Definition and measurements; pp. 73–79. [DOI] [Google Scholar]
- 32.Mayer K.P., Thompson Bastin M.L., Montgomery-Yates A.A., Pastva A.M., Dupont-Versteegden E.E., Parry S.M., et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24:637. doi: 10.1186/s13054-020-03355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sipilä S., Suominen H. Muscle ultrasonography and computed tomography in elderly trained and untrained women. Muscle Nerve. 1993;16:294–300. doi: 10.1002/mus.880160309. [DOI] [PubMed] [Google Scholar]
- 34.Whittaker J.L., Teyhen D.S., Elliott J.M., Cook K., Langevin H.M., Dahl H.H., et al. Rehabilitative ultrasound imaging: understanding the technology and its applications. J Orthop Sports Phys Ther. 2007;37:434–449. doi: 10.2519/jospt.2007.2350. [DOI] [PubMed] [Google Scholar]
- 35.Maly M.R., Calder K.M., Macintyre N.J., Beattie K.A. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahemi H., Nigam N., Wakeling J.M. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface. 2015;12:20150365. doi: 10.1098/rsif.2015.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turton P., Hay R., Taylor J., McPhee J., Welters I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care – an observational study using ultrasound. BMC Anesthesiol. 2016;16 doi: 10.1186/s12871-016-0269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goligher E.C., Dres M., Fan E., Rubenfeld G.D., Scales D.C., Herridge M.S., et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 39.Parry S.M., El-Ansary D., Cartwright M.S., Sarwal A., Berney S., Koopman R., et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30 doi: 10.1016/j.jcrc.2015.05.024. 1151.e9–14. [DOI] [PubMed] [Google Scholar]
- 40.Medrinal C., Prieur G., Bonnevie T., Gravier F.-E., Mayard D., Desmalles E., et al. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021;21:64. doi: 10.1186/s12871-021-01274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puthucheary Z.A., Phadke R., Rawal J., McPhail M.J.W., Sidhu P.S., Rowlerson A., et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43:1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 42.Goligher E.C., Laghi F., Detsky M.E., Farias P., Murray A., Brace D., et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med. 2015;41:642–649. doi: 10.1007/s00134-015-3687-3. [DOI] [PubMed] [Google Scholar]
- 43.Kress J.P., Hall J.B. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370:1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 44.Ismail C., Zabal J., Hernandez H.J., Woletz P., Manning H., Teixeira C., et al. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front Physiol. 2015;6:302. doi: 10.3389/fphys.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukumoto Y., Ikezoe T., Yamada Y., Tsukagoshi R., Nakamura M., Mori N., et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112:1519–1525. doi: 10.1007/s00421-011-2099-5. [DOI] [PubMed] [Google Scholar]
- 46.Zaidman C.M., Seelig M.J., Baker J.C., Mackinnon S.E., Pestronk A. Detection of peripheral nerve pathology: comparison of ultrasound and MRI. Neurology. 2013;80:1634–1640. doi: 10.1212/WNL.0b013e3182904f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puthucheary Z.A., McNelly A.S., Rawal J., Connolly B., Sidhu P.S., Rowlerson A., et al. Rectus Femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am J Respir Crit Care Med. 2017;195:136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine S., Nguyen T., Friscia M., Zhu J., Szeto W., Kucharczuk J.C., et al. Parasternal intercostal muscle remodeling in severe chronic obstructive pulmonary disease. J Appl Physiol. 2006;101:1297–1302. doi: 10.1152/japplphysiol.01607.2005. [DOI] [PubMed] [Google Scholar]
- 49.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahiri D., Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12 doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillen S., van Dijk J.P., Weijers G., Raijmann W., de Korte C.L., Zwarts M.J. Quantitative gray-scale analysis in skeletal muscle ultrasound: a comparison study of two ultrasound devices. Muscle Nerve. 2009;39:781–786. doi: 10.1002/mus.21285. [DOI] [PubMed] [Google Scholar]
- 54.Steffel C.N., Brown R., Korcarz C.E., Varghese T., Stein J.H., Wilbrand S.M., et al. Influence of ultrasound system and gain on Grayscale median values. J Ultrasound Med. 2019;38:307–319. doi: 10.1002/jum.14690. [DOI] [PMC free article] [PubMed] [Google Scholar]