Abstract
The amygdala has been implicated in processing threat and learning fear. However, the amygdala also responds to motivationally relevant stimuli even in the absence of explicit emotional content. We investigated the relationship among amygdala activation, cognitive and emotional factors, and fMRI task data in participants from the Young Adult Human Connectome Project. We expected to see variation in amygdala activation that corresponded with variation in traits that could affect the salience of task related stimuli (i.e. internalizing symptoms and fearful faces). We found no relationship between amygdala activation during face viewing and emotion related traits. However, amygdala activation under working memory load was negatively correlated with fluid intelligence and reading level. There was also a negative relationship between task performance and activation in the amygdala. The observed relationship suggests that the role of amygdala is not limited to the processing of emotional content of incoming information, but is instead related to salience, which can be influenced by individual differences.
Introduction
The amygdala has a well-established link to fear processing. Activation in the amygdala has been correlated with responses to threatening or fearful stimuli in numerous studies and settings (Costafreda, Brammer, David & Fu, 2008, Phan, Wager, Taylor & Liberzon, 2002). In addition, amygdala activation is heightened when viewing fearful faces, and greater amygdala activity has been linked to higher levels of vigilance to facilitate the detection of those faces (Bishop, Duncan, Brett, & Lawrence, 2004, Morris et al., 1996, Holland & Gallagher, 1999, Sabatinelli et al., 2011). This has led to the suggestion that the amygdala is a relay to pass on a danger signal (Ledoux, 2003). As has been shown in additional research however, threat is only part of the function of the amygdala (Scott, Yan & Rolls, 1995). The present study aims to examine alternative functions linked to the amygdala, such as salience or relevance detection, using fMRI tasks and behavioral traits.
We look beyond the danger signal hypothesis because there are results in the literature that are inconsistent with the hypothesis that the amygdala is specialized for processing fear or threat related stimuli (Phan, Wager, Taylor, and Liberzon 2004). The amygdaloid and basal forebrain region showed activation for both positive and negative stimuli, though not neutral (Liberzon, Phan, Decker & Taylor, 2003, Hamann, Ely, Hoffman & Kilts, 2002, Garavan et al., 2001, Costa, Lang, Sabatinelli, Versace, and Bradley 2010). It has been proposed that amygdala serves the more general role of supporting vigilance for the presence of motivationally relevant or salient stimuli (Scott, Yan & Rolls, 1995).
What is salient to a person can vary across individuals and depend on task demands. Accordingly, activation in the amygdala can vary with the given goal in the task, suggesting that amygdala function may also depend on the relevant context, or the specific stimuli that should be salient in the current context. For example, when participants were supposed to focus on negative characteristics of a stimulus, their amygdala activity covaried with their negative ratings (Cunningham & Brosch, 2012). In contrast, the amygdala activity covaried with positive ratings when participants were told to focus on positive characteristics (Cunningham & Brosch, 2012). Thus, activity in the amygdala varied based on what was salient to a person at a given time. Another way to examine whether the amygdala responds to salience is by examining individual differences in traits that may influence what is salient to a person. For example, people with more anxious behavior show increased reactivity of the amygdala to fearful stimuli. Further, levels of activation in the amygdala while viewing fearful faces have been related to anxious traits (Etkin et al., 2004). However, importantly, such trait level relationships are not limited to fearful stimuli. Amygdala response to viewing neutral faces is increased in those with higher levels of reported anxiety (Somerville, Kim, Johnstone, Alexander, & Whalen, 2004), potentially because anxious participants search for potentially threatening information in the expressionless faces. Further, activation for neutral faces in right amygdala correlated with the severity of anxious traits among those with Social Anxiety Disorder, but did not in healthy controls (Cooney, Atlas, Joormann, Eugene & Gotlib, 2006). Altogether, this pattern suggests that individual differences in anxious traits will relate to the degree of amygdala activation during tasks that invoke the emotional processing of facial expressions.
Consistent with a broader role in detecting salient stimuli, there are associations with amygdala activity that extend beyond threat related traits such as associations with odor intensity, but not valence (Anderson et al., 2003, Bonnet, Comte, Tatu, Millot, Moulin & de Bustos 2015). Another example can be found in callous-unemotional traits, such as reduced empathy and emotional response. In both adolescents and adults callous traits are associated with a reduced amygdala response to emotional faces (Marsh et al., 2008). This association may be present because those with callous traits do not find faces emotionally salient (Marsh et al., 2008). In another study, activation in the amygdala in response to happy faces was positively correlated with the degree of extraversion. This finding is consistent with the hypothesis that amygdala plays a role in detecting salient stimuli given that happy faces may be socially relevant to people with extraversion, a trait associated with valuing social interaction (Canli, Sivers, Whitfield, Gotlib & Gabrieli, 2002). Amygdala function has also been connected to cognitive traits, such as individual differences in working memory performance during a working memory task. Faster performance on a high cognitive load working memory task was associated with greater amygdala activation (Schaefer et al., 2006). This would suggest an effect wherein participants who are most vigilant for relevant stimuli to the task are able to recognize and respond more quickly. However, some studies indicate that less activation in the amygdala correlates with improved performance in a working memory task (Yun, Krystal & Mathalon, 2010, Morgan, Terberg, Thornton, Stein, & van Honk, 2012). It has been proposed that the amygdala and prefrontal cortex are in competition, such that the amygdala has an automatic response to potentially relevant environmental stimuli (even in the absence of emotional stimuli) that the prefrontal cortex (thought to support cognitive control and task representation) must overcome to allocate resources for a cognitive task, like working memory (Morgan et al., 2012). If so, then good performance on a WM task would be facilitated by successful reallocation or inhibition of the automatic amygdalar attentional process and be associated with less amygdala activation. Several cognitive traits, including fluid intelligence (defined as the ability to flexibly detect and apply novel task demands) are related to working memory performance, and thus may predict amygdala activation during WM. Further, fluid intelligence is also related to attention control (Unsworth, Fukuda, Awh & Vogel, 2014) which makes it a useful trait to examine amygdala activity during a cognitive task.
To further evaluate whether amygdala activation relates specifically to fear processing, or more generally to salience processing, we examined the relationships between individual differences in amygdala activation during face viewing and a working memory task to individual differences in emotional and cognitive traits. If the detection of threat is the primary function of amygdala, we predicted that amygdala activity would be associated with traits that are thought to be associated with vigilance for threat in the environment (e.g., Anxiety, Depression). Further, these correlations may be strongest for amygdala activity associated with fearful and threatening stimuli as compared to stimuli with no emotional content. In contrast, if the amygdala plays a more general role in detecting and responding to salient stimuli, then we might see broader associations between amygdala activity and traits related to that specific activity. For example, we might see associations between individual differences in anxiety and amygdala activity to neutral faces, because of the potential for socially relevant information even in neutral faces. We also predicted that we would see associations between individual differences in cognitive abilities and amygdala activation during a WM task. As described above, there is some evidence that successful inhibition of amygdala activity during cognitive processing is associated with better performance. If so, we may see that cognitive traits associated with better WM performance would be associated with less amygdala activation during WM. To test these hypotheses, we examined data from the Human Connectome Project. Specifically, we examined activity in the amygdala during face viewing (emotional and neutral) and a working memory task. These contrasts contain information which should be salient to people who vary along different trait dimensions. We correlated amygdala activity during these task conditions with traits measuring emotional and cognitive performance, which might affect the salience of the information in the tasks.
Methods
Participants
Participants for the present study were selected from among those who had completed all measures of interest for these hypotheses in the S1200 release of the Human Connectome Project (HCP) young adult (Van Essen et al., 2013). To avoid the potential of increased false positives due to heritable effects that are not accounted for in analyses (Winkler, Webster, Vidaurre, Nichols & Smith, 2015) participants were divided into separate lists comprised of unrelated participants (i.e., no participant had siblings within the same list). We utilized the largest two lists: List 1 (N= 319, 170 males, mean age = 28.5, stdev= 3.57) and List 2 (N= 256, 109 males, mean age = 28.9 stdev = 3.55). A third list of yet more siblings was available but underpowered, with an N of 149.
Behavioral
The HCP analyzed variability in brain and behavior in a large and representative healthy sample (Barch et al., 2013). Data collection in the Human Connectome Project occurred over 2 days. All emotion-related and cognitive items were selected for their potential to evaluate our hypothesis. Personality was measured by the 60-item version of the Costa and McRae Neuroticism/Extroversion/Openness Five Factor Inventory (NEO-FFI) (McCrae and Costa, 2004), and symptomatology was measured by the Achenbach Adult Self-Report (ASR) for ages 18–59 (Achenbach, 2009). The NIH toolbox (http://www.nihtoolbox.org) is a battery with comprehensive cognitive, emotion, and motor domains. Toolbox measures were computer or tester administered, and for most participants, administered in one behavioral session. Tasks in the cognitive domain were Dimensional Change Card Sort, Flanker Task, Picture Sequencing (working memory), List Sort (working memory), Processing Speed, Picture Vocab, and Oral Reading and Recognition. All domains of emotion in the NIH Toolbox were included and were self-report (Negative affect, Psychological well-being, Social relationships, Stress and self-efficacy) (Barch 2013). For a complete list of emotion measures see Table 1. Additional measures were collected in the University of Pennsylvania Computerized Neuropsychological Testing module. This includes the Variable Short Penn Line Orientation (spatial orientation), the Short Penn Continuous Performance test (sustained attention), Penn Progressive Matrices, Penn Word Memory test and Delayed Discounting, as more representation of cognitive traits (Gur et al., 2010). To clean the data, all variables were tested for skewness using the R package e1071. Any variable with a skew above 1 or below −1 was transformed with a cubed root or squared, respectively. Then outliers were removed using the Outliers package from R.
Table 1.
Factor structure
| Factors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Internalizing | Pos. Affect & Life Satisfaction | Fluid Intelligence | Externalizing | Toolbox Cognitives | Reading Level | Delayed Discounting | ||
| Cognitive Domain | Picture Sequencing Age Adjusted | 0.13 | 0.27 | |||||
| CardSort Age Adj usted | 0.69 | |||||||
| Flanker Age Adjusted | 0.67 | |||||||
| Penn Progressive Matrices: Number of Correct Responses | 0.70 | 0.11 | 0.17 | |||||
| Penn Progressive Matrices: Median Reaction Time for Correct Responses | −0.11 | 0.71 | ||||||
| Reading Level Age Adjusted | 0.11 | 0.68 | ||||||
| Picture Vocab Age Adjusted | 0.10 | 0.72 | ||||||
| Processing Speed Age Adjusted | 0.54 | −0.15 | ||||||
| Delayed Discounting: Area Under the Curve $200 | 0.86 | |||||||
| Delayed Discounting: Area Under the Curve $40K | 0.77 | |||||||
| Variable Short Penn Line Orientation Test: Total Correct | 0.52 | 0.22 | 0.21 | |||||
| Variable Short Penn Line Orientation: Median Reaction Time Divided by Expected Number of Clicks for Correct | 0.54 | −0.25 | −0.11 | |||||
| Variable Short Penn Line Orientation: Total Positions Off for All Trials | −0.10 | −0.54 | −0.21 | −0.26 | −0.10 | |||
| Short Penn Continuous Performance Test: True Positives | 0.12 | 0.11 | ||||||
| Short Penn Continuous Performance Test: Median Response Time for True Positive Response | 0.24 | −0.22 | 0.23 | |||||
| Penn Word Memory Test: Total Number of Correct Responses | 0.19 | 0.13 | 0.14 | −0.23 | 0.11 | 0.23 | ||
| Penn Word Memory Test: Median Reaction Time for Correct Responses | −0.10 | 0.17 | −0.22 | −0.27 | 0.23 | |||
| List Sort Age Adjusted | −0.12 | 0.20 | 0.17 | 0.20 | ||||
| Emotion | Anger Affect Unadjusted | 0.60 | 0.30 | −0.11 | ||||
| Anger Hostility Unadjusted | 0.33 | −0.27 | −0.12 | 0.50 | ||||
| Anger Aggression Unadjusted | −0.20 | −0.10 | −0.13 | 0.10 | ||||
| Fear Affect Unadjusted | 0.84 | 0.14 | 0.18 | −0.12 | ||||
| Fear Somatic Unadjusted | 0.39 | 0.13 | ||||||
| Sadness Unadjusted | 0.69 | −0.17 | ||||||
| Life Satisfaction Unadjusted | −0.22 | 0.50 | 0.13 | |||||
| Mean Purpose Unadjusted | 0.64 | −0.13 | ||||||
| Positive Affect Unadjusted | −0.21 | 0.57 | 0.11 | −0.11 | ||||
| Friendship Unadjusted | 0.70 | −0.11 | ||||||
| Lonliness Unadjusted | 0.32 | −0.55 | 0.14 | |||||
| Percieved Hostility Unadjusted | 0.19 | −0.14 | 0.21 | 0.51 | ||||
| Perceived Rejection Unajusted | 0.15 | −0.41 | 0.41 | |||||
| Emotional Support Unadjusted | 0.70 | −0.25 | ||||||
| Instrumental Support Unadjusted | 0.11 | 0.58 | 0.10 | |||||
| Perceived Stress Unadjusted | 0.60 | −0.17 | −0.12 | 0.19 | ||||
| Self Efficacy Unadjusted | −0.51 | 0.24 | 0.11 | 0.19 | ||||
| ASR Internalizing Raw Score | 0.71 | −0.13 | −0.11 | 0.12 | ||||
| ASR Externalizing Raw Score | 0.30 | −0.15 | 0.50 | 0.10 | ||||
| ASR Thought and Other Problems | 0.57 | −0.10 | 0.31 | −0.17 | 0.18 | |||
| Personality | NEO-FFI Factor Summary Score: Agreeableness | 0.31 | −0.30 | 0.10 | 0.14 | |||
| NEO-FFI Factor Summary Score: Openness | 0.18 | 0.38 | 0.10 | |||||
| NEO-FFI Factor Summary Score: Consientiousness | −0.27 | −0.24 | −0.24 | |||||
| NEO-FFI Factor Summary Score: Neuroticism | 0.71 | −0.14 | −0.11 | |||||
| NEO-FFI Factor Summary Score: Extraversion | 0.56 | 0.24 | 0.16 | 0.17 | 0.10 | |||
Factor analysis of behavioral measures
Factor scores were generated in an exploratory factor analysis as a form of data reduction, to simplify the analysis of the many behavioral variables related to cognition and emotion. Analyses were conducted in R statistical software using the ‘psych’ package (Factor method: OLS, rotation: oblimin, Pearson correlations) (Revelle, 2018). All components included are listed in Table 1. First, a parallel factor analysis was conducted on List 2 to estimate the number of factors in the data. Then, an exploratory factor analysis was run, and factor loadings were generated from List 2. Finally, the factor weightings derived from List 2 were used to generate factor scores by transforming data from participants in List 1, resulting in a single score on each factor for each participant. The transformation of the second list was to make the analysis more robust, so the transformation was not applied to the list it was based upon, as explained in the ‘psych’ package (Revelle, 2018). The scores in List 1 were used for all subsequent analyses.
Emotion and Working Memory fMRI tasks
For the present study, we focused on the Emotion and the Working Memory fMRI tasks. The Emotion task was a simple matching task adapted from the Hariri task (Hariri, Tessitore, Mattay, Ferra, & Weinberger, 2002, Barch et al., 2013). The following task specifications are taken from Barch and colleagues (2013): “The participants are presented with blocks of trials that ask them to decide either which of two faces presented on the bottom of the screen match the face at the top of the screen, or which of two shapes presented at the bottom of the screen match the shape at the top of the screen. The faces have either angry or fearful expressions. Trials are presented in blocks of 6 trials of the same task (face or shape), with the stimulus presented for 2 s and a 1 s ITI. Each block is preceded by a 3 s task cue (“shape” or “face”), so that each block is 21 s including the cue. Each of the two runs includes 3 face blocks and 3 shape blocks.” It should be noted that although the authors have chosen to focus on the amygdala for this specific study, the region is by no means responsible for all brain activation related to emotion processing. The complexity of emotion processing cannot be narrowed to functions supported by only one brain region. Indeed, group-level activation for emotional faces versus shapes was also present in bilateral medial and lateral orbital frontal cortices, hippocampus, and other regions. However, the proportion of participants showing activation in this task was particularly high in bilateral amygdala, fusiform gyrus, and visual cortex (Barch et al., 2013). This pattern supports the idea that the amygdala is one of the brain regions most strongly related to the processing of emotional face stimuli.
The Working Memory task included several categories of stimuli in blocks: faces, places, body parts, and tools, which have been shown to be reliable yet diverse stimuli (Downing, Jiang, Shuman & Kanwisher 2001, Barch et al., 2013). The following are the task design specifications as taken from Barch et al., 2013. “…we embedded the category specific representations component within the working memory task, by presenting blocks of trials that consisted of pictures of faces, places, tools and body parts. Within each run, the 4 different stimulus types are presented in separate blocks within the run. Within each run, 1/2 of the blocks use a 2 back working memory task (respond ‘target’ whenever the current stimulus is the same as the one two back) and 1/2 use a 0 back working memory task (a target cue is presented at the start of each block, and the person must respond ‘target’ to any presentation of that stimulus during the block). A 2.5 s cue indicates the task type (and target for 0 back) at the start of the block. Each of the two runs contains 8 task blocks (10 trials of 2.5 s each, for 25 s) and 4 fixation blocks (15 s each). On each trial, the stimulus is presented for 2 s, followed by a 500 ms ITI. Each block contains 10 trials, of which 2 are targets, and 2–3 are non-target lures (e.g., repeated items in the wrong n-back position, either 1-back or 3-back). The inclusion of lures is critical to ensure that the participants are using an active memory approach to the task and allows one to assess conflict related activity as well as error related activity.” All faces in the Working Memory task had a neutral expression.
From the emotion task, we examined the activation estimates for the contrast between fearful / angry faces versus simple shape stimuli (i.e., FACES – SHAPES). This contrast should be sensitive to the processing of emotional faces, but less sensitive to processes that are similar for the two conditions. From previous research, we expect amygdala activation during emotional faces to be sensitive to emotional processes, but it is important to note that this contrast will also be sensitive to face processing in general. From the working memory task, we examined the activation viewing neutral (expressionless) faces versus the average activation of all other stimuli in the task (i.e., FACE – AVG). This contrast compared faces (collapsed across 2 back and 0 back conditions) minus the average of other stimuli (also collapsed across 2 back and 0 back conditions). The resulting estimate should be maximally sensitive to neutral face activation, while subtracting out general effects of working memory load. We also examined working memory load, collapsing all categories of stimuli in the 2 back condition, minus all categories of stimuli in the 0 back condition. This estimate should be most representative of the high cognitive load associated with working memory.
Working Memory Task Performance
In post-hoc analyses, we examined performance on all types (faces, places, body parts, tools) of the 2 back trials of the working memory task. 2 back accuracy is the percentage of 2 back correct responses divided by total number of 2 back trials. We also looked at median correct reaction time (in msec) of all types of 2 back trials.
MRI acquisition and preprocessing
Whole brain images were acquired at Washington University on the customized Siemens “Connectome Skyra” 3T scanner with a 32-channel head coil (Ugurbil et al., 2013). Functional MRI scans were collected using multiplexed EPI (Feinberg et al., 2010) with a multi-band acceleration factor of 8 (TR = 720 ms, TE = 33.1 ms, flip angle = 52, BW = 2290 Hz/Px, in-plane FOV = 208 × 180 mm, 72 slices, 2.0 mm isotropic voxels). Two runs of each fMRI task were collected, with alternating phase encoding directions (left-to-right and right-to-left).
Minimal preprocessing was completed including gradient unwarping, motion correction, fieldmap-based EPI distortion correction, brain-boundary-based registration of EPI to structural T1-weighted scan, FNIRT registration into MNI152 space, and grand-mean intensity normalization. In the HCP grayordinate-based preprocessing, smoothing of subcortical voxels was constrained by gray matter parcel boundaries to avoid smoothing across white matter and different subcortical regions (Glasser et al., 2013). The CIFTI grayordinate time series image was smoothed by a total of FWHM=4mm (Barch et al., 2013).
Activation estimates were extracted from the left and right amygdala subcortical regions in the CIFTI group-average template. The amygdala regions reflect the automatically segmented amygdala defined by FreeSurfer for participants from the S900 release, after co-registration to a standard subcortical template in CIFTI grayordinate space. We used the average of the two amygdala regions for subsequent analyses (the amygdala is separated into right and left results in the Supplement).We regressed individual differences in head motion estimates out of the task activation estimates, to remove this potential confound from estimates of amygdala activation. For each participant the motion estimates for the right and then left amygdala were obtained. Both mean and SD of relative RMS were run through a regression for activation on all contrasts in the analysis, and the residual activation was saved and used in the further analysis.
Data Analysis
As presented below in Table 1, the factor analysis identified individual difference factors related to emotional/interpersonal characteristics and those related to cognitive function. We started by examining the relationship between the emotional/interpersonal factors and amygdala activation to emotional faces during the emotion task, and to neutral faces during the working memory task. We used partial correlations controlling for gender and age, and corrected for multiple comparisons using False Discovery Rate (Benjamini & Hochberg, 1995). Next, we examined the relationship between the cognitive factors and amygdala activation during the comparison of 2 back and 0 back on the working memory task. We again used partial correlations controlling for gender and age, as well as corrected for multiple comparisons using False Discovery Rate (Benjamini & Hochberg, 1995).
We also correlated the performance metrics in the 2 back trials of the working memory task, accuracy and median reaction time, with cognitive factors and amygdala activation during the 2 back-0 back contrast of the working memory task. We again controlled for age (in years) and gender, and 4 participants’ data were excluded from this analysis due to missing data (N=314). Finally, we corrected for multiple comparisons using the False Discovery Rate.
Results
Factor analysis
Parallel analysis suggested seven factors. The composition and loading of the factors are detailed in Table 1. We interpreted the first factor to be “Internalizing” because many of its components are common to internalizing disorders, such as depression and anxiety. The second factor was termed “Positive Affect and Life Satisfaction” because it loaded strongly on positive outcome measures from the NIH toolbox emotion domain. The third factor appears to index “Fluid Intelligence” due to the high loadings on correct items and median response time on the Penn Matrix Reasoning Test, as well as the scoring items from the Variable Short Line test. The fourth factor was interpreted as “Externalizing” due to the positive loadings on Anger Affect, Perceived Hostility and Rejection, and ASR Externalizing score. The fifth factor was termed “Toolbox Cognitive” and consisted of the Cardsort, Flanker, and Processing Speed tasks from the NIH Toolbox. The sixth factor was named “Reading Level” due to the strong loadings on the Reading Level and Picture Vocab scores from the NIH Toolbox Cognitive domain. The seventh factor was named “Delayed Discounting”. It loaded positively on the area under the curve measures from the Delayed Discounting task, which reflect reduced discounting of delayed rewards. Thus, this factor analysis identified three factors involving emotional/interpersonal characteristics (Internalizing, Positive Affect and Life Satisfaction, and Externalizing) and four factors related to cognitive characteristics (Fluid Intelligence, Reading Level, Toolbox Cognitive, and Delayed Discounting).
Relationships of emotional/interpersonal factors to amygdala activity when viewing emotional and neutral faces
As shown in Table 2, there were no significant partial correlations (controlling for gender and age) between any of the three emotional/interpersonal factors and amygdala activation in the faces-shapes contrast during the Emotion task. Supplemental Figure S1 illustrates the relationship between viewing those emotional faces and Internalizing factor scores, and shows that a limited range in the variance of amygdala activation during this contrast might be contributing to these null results. A one sample t-test of the emotional face contrast was found to be significant: t(318) = 33.3, p < .001, indicating that activation at the group level was significant
Table 2.
Correlation of Emotion Related Factors with Amygdala Activation
| Control Variables | Internalizing | Positive Affect | Externalizing | ||
|---|---|---|---|---|---|
|
| |||||
| Age in Years & Gender | Amygdala: Emotion Faces- Shapes | Correlation | −0.016 | −0.023 | 0.019 |
| 2-tailed Significance | 0.771 | 0.687 | 0.729 | ||
| FDR corrected 2-tailed Significance | 0.771 | 0.771 | 0.771 | ||
| Amygdala: WM Neutral Face-Avg | Correlation | 0.104 | −0.089 | −0.018 | |
| 2-tailed Significance | 0.064 | 0.115 | 0.744 | ||
| FDR corrected 2-tailed Significance | 0.345 | 0.345 | 0.771 | ||
|
| |||||
| df = 315 | |||||
A trend of positive partial correlation was found between Internalizing symptoms and amygdala activation during the WM neutral face-average contrast (neutral faces, Supplemental Figure S2) (r = .104; p < .06, uncorrected), though it did not meet significance. A one sample t-test of the neutral face contrast was significant t(318) = 16.3, p<.001, indicating that activation at the group level was significant. Still, there were no significant partial correlations of any of the three emotional/interpersonal factors with amygdala activation during the neutral face vs average WM task contrast.
Relationships of cognitive factors to amygdala activity during the 2 back condition of the working memory task
A one sample t-test showed significance for the 2 back- 0 back contrast t(318) = −9.82, p<.001, indicating that deactivation at the group level was significant. As shown in Table 3, there was a significant negative partial correlation after FDR correction between Fluid Intelligence and amygdala activation on the WM 2 back-0 back contrast (high cognitive load, Figure 1)(r = −.154; p < .05). The partial correlation between amygdala activation for the same contrast and the Reading Level factor was also significant after FDR correction (high cognitive load, Figure 2)(r = −.167; p < .05). Other cognitive factors were not significantly correlated with amygdala activity.
Table 3.
Correlation of Cognitive Related Factors with Amygdala Activation
| Control Variables | Fluid Intelligence | Toolbox Cognitives | Reading Level | Delayed Discounting | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Age in Years & Gender | Amygdala: WM 2back-0back | Correlation | −0.154 | −0.073 | −0.167 | −0.014 |
| 2-tailed Significance | 0.006** | 0.197 | 0.003** | 0.799 | ||
| FDR corrected 2-tailed Significance | 0.012* | 0.263 | 0.012* | 0.799 | ||
|
| ||||||
| df = 315 | ||||||
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Figure 1:
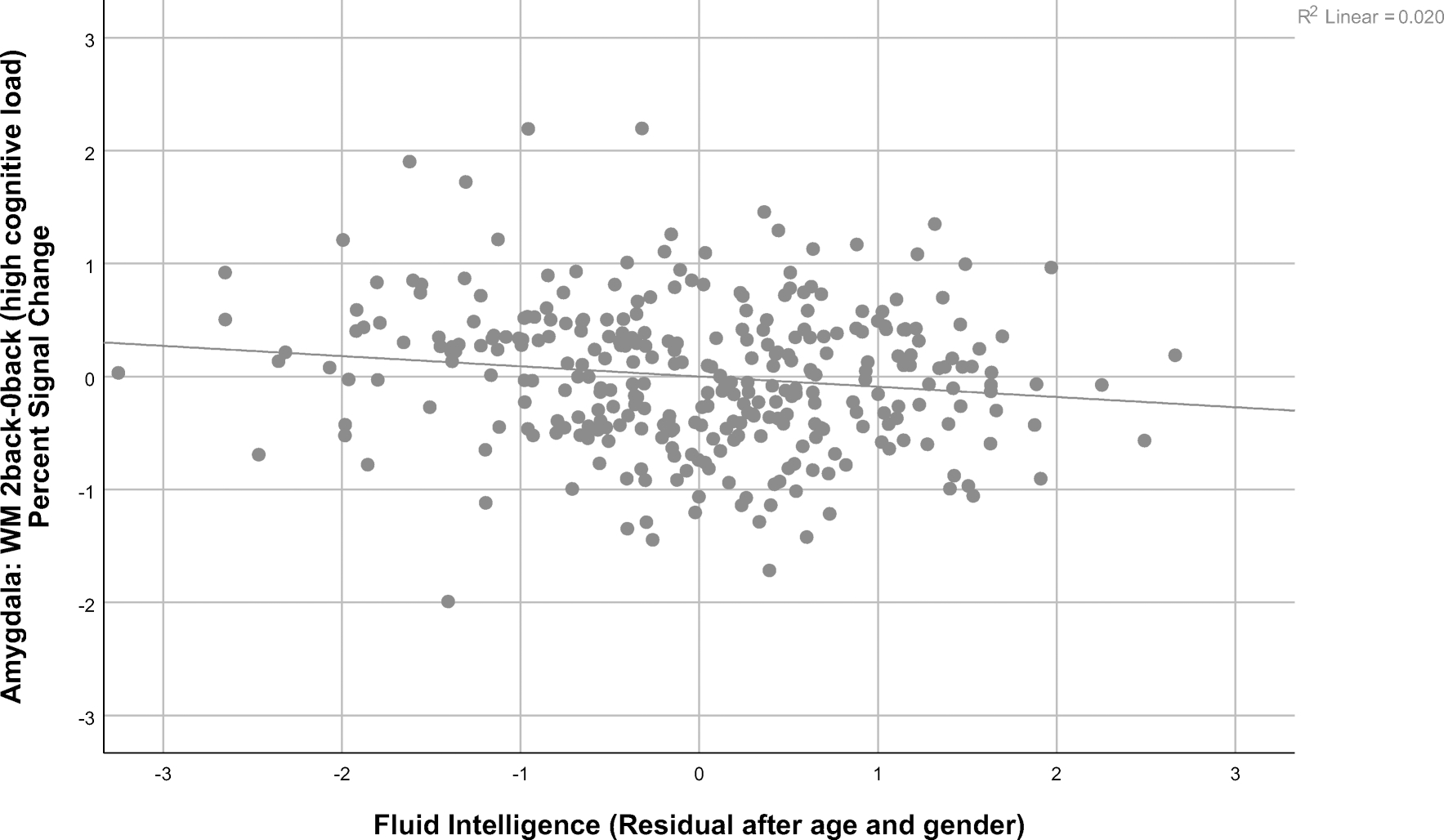
Graph illustrating the relationship between the Fluid Intelligence factor residuals after regressing out age and gender and amygdala activation during the 2 back versus 0 back contrast of the working memory task in the Human Connectome Project
Figure 2:
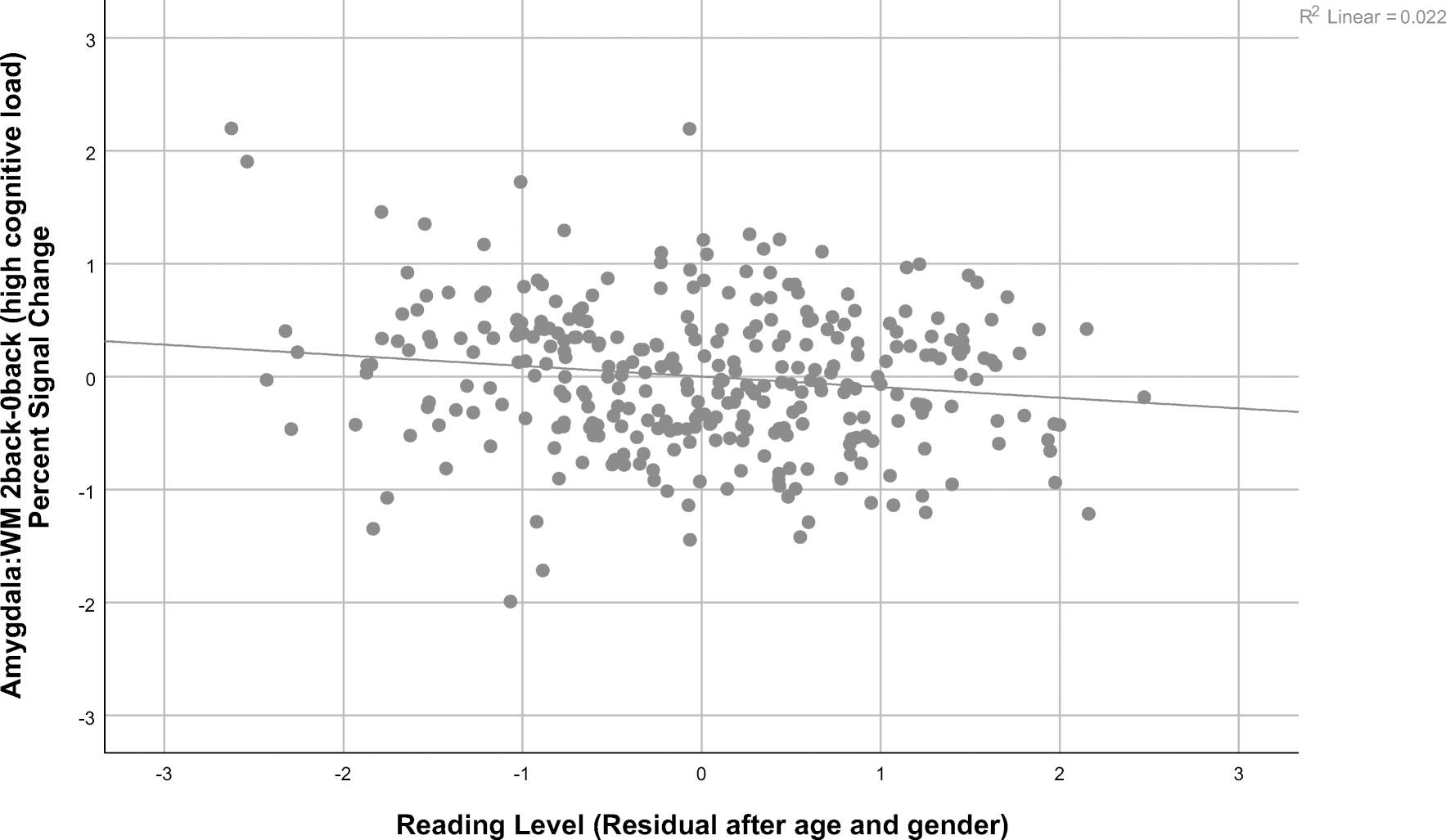
Graph illustrating the relationship between the Reading factor residuals after regressing out age and gender and amygdala activation during the 2 back versus 0 back contrast of the working memory task in the Human Connectome Project
Additional analyses
The analyses presented above demonstrated a relationship between the Fluid Intelligence and Reading factors and less amygdala activation during WM. As described in the introduction, we hypothesized that this reflected a stronger ability to inhibit automatic attention allocation of amygdala activity to potentially relevant environmental stimuli. To further test this hypothesis, we examined the relationship between performance (accuracy and reaction time) on the WM tasks in the 2 back condition, and cognitive factors Fluid Intelligence, Reading Level and Toolbox Cognitives, controlling age and gender. As shown in Table 4, all 3 factors: Fluid Intelligence, Reading Level and Toolbox Cognitive were significantly positively correlated with accuracy on the WM tasks after FDR correction. Median reaction time was correlated with Toolbox Cognitive only. Further, as shown in Table 4, accuracy on the WM task was negatively correlated with amygdala activation in the 2 back - 0 back contrast, consistent with the pattern of correlations for Fluid Intelligence and Reading Level during this contrast of amygdala activation.
Table 4.
Working Memory Performance Correlations with Cognitive Factors and Amygdala Activation
| Control Variables | Fluid Intelligence | Reading Level | Toolbox Cognitives | Amygdala WM: 2back-0back | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Age in Years & Gender | Working Memory Task Accuracy: 2back | Correlation | 0.292 | 0.374 | 0.421 | −0.147 |
| 2-tailed Significance | 0.000** | 0.000** | 0.000** | 0.009** | ||
| FDR corrected 2-tailed Significance | 0.000** | 0.003** | 0.002** | 0.014* | ||
| Working Memory Task Median RT: 2back | Correlation | 0.079 | −0.026 | −0.297 | −0.057 | |
| 2-tailed Significance | 0.160 | 0.649 | 0.000** | 0.312 | ||
| FDR corrected 2-tailed Significance | 0.213 | 0.649 | 0.001** | 0.356 | ||
|
| ||||||
| df = 314 | ||||||
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Discussion
The current analyses examined whether the role of the amygdala was limited to responding to threat and fearful stimuli, or if amygdala played a more general role in responding to salient stimuli, as others have begun to demonstrate (Ousdal et al., 2008). Where prior studies have used experimental manipulations of emotional valence, we examined variation in activation in the amygdala related to individual differences in cognitive and emotional behavioral traits. The hypothesis that amygdala is involved in more than fear or negative emotion predicted that amygdala activation should correlate with emotional traits during emotional task conditions, and that amygdala activation may correlate with non-emotional traits such as cognitive ability, when the task is relevant to those traits.
We found correlations between amygdala activation during the high cognitive load in the WM task and the behavioral factors of Fluid Intelligence and Reading Level. We also saw a nonsignificant correlation between amygdala activation during the neutral face contrast of the WM task, and Internalizing. Interestingly, we found no relationship between amygdala activation during the Emotion task with any of behavioral factors that we examined. These findings support a more general role of the amygdala, perhaps in processing the salience of stimuli rather than the emotional content.
The observed relationship of activation in the amygdala with Reading Level and Fluid Intelligence is consistent with the hypothesis of a broad role of the amygdala. Those with higher fluid intelligence scores showed lower amygdala activation during the working memory task. Working memory is related to fluid intelligence, although they are not one and the same (Ackerman, Beier & Boyle, 2005). Part of working memory performance is managing a large cognitive load and distributing attention toward relevant (or salient) stimuli and suppressing any non-relevant stimuli (Kane & Engle, 2000). The relevance of the amygdala to working memory is indicated by a lesion study finding that a selective lesion in the amygdala improved working memory performance in humans (Morgan et al., 2012). Further, lower amygdala activation has been linked to better working memory performance in past studies (Yun et al., 2010). One explanation that has been proposed is the successful suppression of amygdala activity happens as resources are routed to prefrontal regions for cognitive tasks via top-down inhibition, as proposed in Yun et al., 2010. However, to test that theory, more analysis would be required to establish a causal relationship between behavioral traits, amygdala function and prefrontal function. In line with our findings, the control of attention to stimuli in cognitive tasks has been linked to fluid intelligence (Dempster, 1991). As such, participants with higher fluid intelligence may have been more successful in overriding an automatic amygdala attentional allocation to potentially relevant environmental stimuli. This interpretation is consistent with the other significant correlation, the negative relationship of the Reading Level factor and amygdala activation on the 2 back contrast. Reading level is a component of crystalized intelligence measures, which is closely related to fluid Intelligence (Cattell, 1963). Therefore, the association between better reading and lower amygdala activation may reflect similar processes to those that support the association between fluid intelligence and reduced amygdala activity.
Our post hoc correlations with WM task performance were consistent with this interpretation. Fluid Intelligence and Reading Level were both positively correlated with accuracy on the working memory task, and better accuracy on the 2 back WM task was associated with lower amygdala activation in the 2 back versus 0 back WM contrast. Notably, there was not a significant correlation between amygdala activation during the 2 back - 0 back WM contrast and the Toolbox Cognitive factor. It was not clear why we did not see a similar correlation with the amygdala as found for Fluid Intelligence and Reading, as this factor was as strongly correlated with task performance on the 2 back WM task. However, the correlation between the Toolbox Cognitive factor and amygdala activation was in the same direction (higher scores associated with less amygdala activation), and thus are generally consistent with the overall pattern of better cognitive function being associated with less amygdala activation during high WM load. The lack of correlation with the Delayed Discounting factor could be attributed to the nature of Delayed Discounting, in that it is not an entirely ‘cognitive task’. There are elements of reward processing that are represented as well. However, the authors grouped Delayed Discounting with the cognitive rather than emotional variables, because it requires consideration of quantitative elements like time and money.
Surprisingly, we did not find any relationship between Internalizing symptoms, such anxiety and depression, and amygdala activation either in response to faces with emotional expressions during the Emotion task or in response to faces with neutral expressions during the WM task. This contrasts with prior studies, which have demonstrated that higher anxiety or depressed participants demonstrated higher amygdala activation to fearful faces compared to healthy controls (Bishop, Duncan & Lawrence, 2004, Sheline et al., 2001). These null findings could be influenced by the fact that this was a sample of relatively healthy individuals, given that people with a documented history of treatment for depression and anxiety were excluded. Thus, the range of scores did not approach clinical levels, as a number of prior studies have. Interestingly, the only correlation that did approach significance was association between Internalizing and the neutral face viewing contrast, which was in the expected direction (higher internalizing, higher activation for neutral faces). While not significant, this correlation is consistent with the hypotheses that amygdala activation is not only related to the emotional content of a stimulus, but whether it is salient for the viewer. Another consideration is the task used for the contrast of viewing emotional faces, which is adapted from the Hariri emotion task (Hariri et al., 2002). Low within-subject reliability was found for this task (Plichta et al., 2012, Elliott et al., 2019). This could explain the lack of correlation between any behavioral factor and the emotion viewing contrast.
Limitations
Our analysis did have several limitations. As mentioned above, we used data from a large healthy sample of participants (HCP 1200 subjects release). While we were able to include a large number of datasets, the exclusionary criteria of the study prevented clinical levels of variation in anxiety and depression and may have contributed to our null findings on emotional factors. Also because of our use of HCP data, we were unable to control exactly which tasks and measures we could use, and this may have prevented us from probing examining other types of emotional processing or salience tasks that were not included in the HCP. For example, Because our analysis of the amygdala activation for emotional faces is based on a contrast of faces vs shapes, there is a possibility that the within subject activation for faces and shapes is very similar, and therefore the contrast may have lower reliability. This could be addressed by examining only the activation for faces, but the design of the task used in the HCP does not allow for separate examining of faces or shapes against a baseline (Barch et al., 2013). Due to the lack of fixation blocks, there is no consistent baseline condition, and the implicit baseline may vary arbitrarily across participants. Consequently, the FACES-baseline contrast will be problematic as an individual difference measure.
In addition, although statistically significant even after FDR correction, the effect sizes for the relationships between individual differences in cognition and amygdala activation were relatively small. These small effect sizes may seem surprising when compared to effect sizes previously reported in the literature. However, evidence is emerging from larger neuroimaging studies such as ABCD and HCP that the magnitude of brain-behavior correlations may be quite small (Marek et al., 2020). The larger effect sizes reported in previous neuroimaging studies may have arisen in part from the tendency to find inflated correlations in studies with smaller samples (Marek et al, 2020; Yarkoni, 2009).
Larger neuroimaging studies such as ABCD and HCP provide increased numbers of measures to consider multivariate methods such as CCA (Canonical Correlation Analysis; Smith et al. 2015) and increased numbers of participants to utilize cross-validation procedures (Yarkoni and Westfall, 2017) to avoid overfitting and to evaluate generalization of observed findings. It might be possible that those procedures could increase detectable effect sizes and confidence in the replicability of those effects. However, some initial attempts to utilize CCA with neuroimaging data have found small to moderate effect sizes for the brain-behavior relationship in withheld replication samples (Marek et al. 2020, Feinberg et al. 2015).
In sum, our results showed a pattern of variation in amygdala activation related to cognitive traits during a WM task. These findings are consistent with the hypotheses that amygdala processing may extend beyond reactivity to fear-evoking stimuli. More specifically, the fact that cognitive traits correlate with amygdala activation during a WM task is consistent with the hypothesis that amygdala plays a more general role in reacting and directing attention toward individually salient stimuli.
Supplementary Material
Acknowledgments:
This study was supported by grants NIH 1U54MH091657 and NIMH U01 MH109589
Footnotes
The data and materials for all analyses are available at humanconnectomeproject.org, but none of the analyses were preregistered.”
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Achenbach TM (2009). The Achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications. University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Ackerman PL, Beier ME, & Boyle MO (2005). Working memory and intelligence: The same or different constructs?. Psychological bulletin, 131(1), 30. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, & Damasio AR (1995). Fear and the human amygdala. Journal of Neuroscience, 15(9), 5879–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, … & Sobel N (2003). Dissociated neural representations of intensity and valence in human olfaction. Nature neuroscience, 6(2), 196–202. [DOI] [PubMed] [Google Scholar]
- Armony JL, & Dolan RJ (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia, 40(7), 817–826. [DOI] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, … & Nolan D (2013). Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage, 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros DM, Pereira P, Medina JH, & Izquierdo I (2002). Modulation of working memory and of long-but not short-term memory by cholinergic mechanisms in the basolateral amygdala. Behavioural pharmacology, 13(2), 163–167. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bishop S, Duncan J, Brett M, & Lawrence AD (2004). Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature neuroscience, 7(2), 184. [DOI] [PubMed] [Google Scholar]
- Bonnet L, Comte A, Tatu L, Millot JL, Moulin T, & Medeiros de Bustos E (2015). The role of the amygdala in the perception of positive emotions: an “intensity detector”. Frontiers in behavioral neuroscience, 9, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Lang PJ, Sabatinelli D, Versace F, & Bradley MM (2010). Emotional imagery: assessing pleasure and arousal in the brain’s reward circuitry. Human brain mapping, 31(9), 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, & Fu CH (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain research reviews, 58(1), 57–70. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, & Gabrieli JD (2002). Amygdala response to happy faces as a function of extraversion. Science, 296(5576), 2191–2191. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, & Gotlib IH (2006). Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral?. Psychiatry Research: Neuroimaging, 148(1), 55–59. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, & Brosch T (2012). Motivational salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–59. [Google Scholar]
- Dempster FN (1991). Inhibitory processes: a negleted dimension of intelligence. Intelligence, 15(2), 157–173. [Google Scholar]
- Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, … & Hariri AR (2019). Poor test-retest reliability of task-fMRI: New empirical evidence and a meta-analysis. bioRxiv, 681700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, & Hirsch J (2004). Individual differences in trait anxiety predict the response of thebasolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043–1055. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, … & Yacoub E (2010). Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS one, 5(12), e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, & Risinger RC (2001). Amygdala response to both positively and negatively valenced stimuli. Neuroreport, 12(12), 2779–2783. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, … & Van Essen DC (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, … & Gur RE (2010). A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. Journal of neuroscience methods, 187(2), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Holland PC, & Gallagher M (1999). Amygdala circuitry in attentional and representational processes. Trends in cognitive sciences, 3(2), 65–73. [DOI] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2002). The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic bulletin & review, 9(4), 637–671. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2003). The emotional brain, fear, and the amygdala. Cellular and molecular neurobiology, 23(4–5), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, & Taylor SF (2003). Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology, 28(4), 726. [DOI] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. (2020). Towards Reproducible Brain-Wide Association Studies. bioRxiv, 32, 2020.08.21.257758. 10.1101/2020.08.21.257758 [DOI] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, … & Blair RJR (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165(6), 712–720. [DOI] [PubMed] [Google Scholar]
- McCrae RR, & Costa PT Jr (1999). A five-factor theory of personality. Handbook of personality: Theory and research, 2(1999), 139–153. [Google Scholar]
- Morgan B, Terburg D, Thornton HB, Stein DJ, & van Honk J (2012). Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS One, 7(6), e38116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, & Dolan RJ (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383(6603), 812. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, & Andreassen OA (2008). The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience, 156(3), 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, & Liberzon I (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage, 16(2), 331–348. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, … & Colman P (2012). Test–retest reliability of evoked BOLD signals from a cognitive–emotive fMRI test battery. Neuroimage, 60(3), 1746–1758. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. [Google Scholar]
- Rauch SL, Shin LM, & Wright CI (2003). Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences, 985(1), 389–410. [DOI] [PubMed] [Google Scholar]
- Revelle W (2018). Psych: Procedures for Psychological, Psychometric, and Personality Research. R package version 1.8.12. https://CRAN.R-project.org/package=psych. [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, … & Jeffries J (2011). Emotional perception: meta-analyses of face and natural scene processing. Neuroimage, 54(3), 2524–2533. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, & Gray JR (2006). Individual differences in amygdala activity predict response speed during working memory. Journal of Neuroscience, 26(40), 10120–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TR, Yan J, & Rolls ET (1995). Brain mechanisms of satiety and taste in macaques. Neurobiology (Budapest, Hungary), 3(3–4), 281–292. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, & Mintun MA (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological psychiatry, 50(9), 651–658. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, & Whalen PJ (2004). Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological psychiatry, 55(9), 897–903. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Fukuda K, Awh E, & Vogel EK (2014). Working memory and fluid intelligence: Capacity, attention control, and secondary memory retrieval. Cognitive psychology, 71, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D, Smith S, Barch DM, Behrens T, Yacoub E, and Ugurbil K, for the WU-Minn HCP Consortium. (2013). The WU-Minn Human Connectome Project: Progress and Prospects. Neuroimage, 80, 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, & Jenike MA (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18(1), 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage. 2015;123:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T (2009). Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power-Commentary on Vul et al. (2009). Perspectives on Psychological Science : a Journal of the Association for Psychological Science, 4(3), 294–298. 10.1111/j.1745-6924.2009.01127.x [DOI] [PubMed] [Google Scholar]
- Yun RJ, Krystal JH, & Mathalon DH (2010). Working memory overload: fronto-limbic interactions and effects on subsequent working memory function. Brain imaging and behavior, 4(1), 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


