Abstract
In 2012, Kidney Disease: Improving Global Outcomes (KDIGO) published a guideline on the classification and management of acute kidney injury (AKI). The guideline was derived from evidence available through February 2011. Since then, new evidence has emerged that has important implications for clinical practice in diagnosing and managing AKI. In April of 2019, KDIGO held a controversies conference entitled Acute Kidney Injury with the following goals: determine best practices and areas of uncertainty in treating AKI; review key relevant literature published since the 2012 KDIGO AKI guideline; address ongoing controversial issues; identify new topics or issues to be revisited for the next iteration of the KDIGO AKI guideline; and outline research needed to improve AKI management. Here, we present the findings of this conference and describe key areas that future guidelines may address.
Keywords: acute kidney disease, acute kidney injury, fluid management, nephrotoxicity, renal replacement therapy, risk stratification
In 2012, Kidney Disease: Improving Global Outcomes (KDIGO) published a guideline on the classification and management of acute kidney injury (AKI).1 Since then, new evidence has emerged that has important implications for clinical practice. Large epidemiology studies and risk profiles for AKI have become available in adults and children, such as the AKI–Epidemiologic Prospective Investigation (AKI-EPI) study,2 the 0by25 Initiative,3 the Southeast Asia–AKI (SEA-AKI) study,4 and the Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE)5 and Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN)6 studies. The effectiveness of the KDIGO recommendations in preventing AKI has been confirmed in small single-center randomized controlled trials (RCTs), such as the Prevention of AKI (PrevAKI)7 and the Biomarker Guided Intervention for Prevention of AKI (BigpAK)8 studies. In addition, results of RCTs have provided new data relevant to several facets of preventing and managing AKI, including early resuscitation, fluid therapy, prevention of contrast-associated AKI, and timing of acute renal replacement therapy (RRT).9–15 Finally, there is now evidence from large studies in different countries that the use of KDIGO criteria for AKI, as part of computer decision-support systems, can improve clinical outcomes.16,17 However, there has also been important progress in the development of new tools to diagnose and manage AKI, including biomarkers, decision support programs, and electronic alerts, that go beyond the current KDIGO definition/staging criteria, and these warrant consideration for inclusion in AKI guidelines.17–24
These advances are not without controversy. Adoption of new biomarkers has been heterogenous,25 and there are calls to revise KDIGO AKI staging based on creatinine and urine output,26 and even calls to discard the KDIGO staging completely.27 Thus, in April 2019, KDIGO held a controversies conference entitled Acute Kidney Injury, in Rome, Italy. Participants examined and summarized evidence published since 2012 as it relates to the risk assessment, diagnosis, and management of patients with AKI and provided commentary on areas of controversy and agreement. The ultimate goals were to provide the clinical and research communities with a snapshot of the current state of the art for diagnosis and management of AKI and to prepare for future revision of the 2012 guideline.
NOMENCLATURE AND DIAGNOSTIC CRITERIA
AKI-related definitions
AKI and chronic kidney disease (CKD) are increasingly recognized as related entities representing a continuum of disease. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) 2002 guideline and the 2012 KDIGO AKI guideline defined CKD as measured or estimated glomerular filtration rate (GFR) <60 ml/min per 1.73 m2, or the presence of markers of kidney damage (e.g., albuminuria) for >90 days.1 The 2012 KDIGO guideline defined AKI as an abrupt decrease in kidney function occurring over 7 days or fewer (Table 1).1 To complete the continuum, the 2012 guideline proposed the term acute kidney diseases and disorders (AKD) to define conditions of impaired kidney function not meeting the criteria for either AKI or CKD but having adverse outcomes and requiring clinical care. However, consensus on the exact criteria and indicators of severity is urgently needed.
Table 1 |.
Definitions of AKI, CKD, and AKD1
| Acronym | Functional criteria | Structural criteria |
|---|---|---|
| AKI | Increase in SCr by ≥ 50% within 7 d, OR Increase in SCr by ≥ 0.3 mg/dL (≥26.5 μmol/l) within 48 h, OR Oliguria |
No criteria |
| CKD | GFR < 60 ml/min per 1.73 m2 for > 3 mo | Kidney damage > 3 mo |
| AKD | AKI, OR GFR < 60 ml/min per 1.73 m2 for < 3 mo, OR Decrease in GFR by ≥ 35% or increase in SCr by > 50% for < 3 mo |
Kidney damage < 3 mo |
| NKD | GFR ≥ 60 ml/min per 1.73 m2 Stable SCr without AKI/AKD/CKD |
No damage |
AKD, acute kidney diseases and disorders; AKI, acute kidney injury; CKD, chronic kidney disease; GFR, glomerular filtration rate; NKD, no kidney disease; SCr, serum creatinine.
Because the diagnosis of AKI should be tied to management decisions, and because changing disease definitions may have major implications for disease epidemiology, the case for revising the 2012 KDIGO definition of AKI should be strong before changes are proposed. Furthermore, in the context of an AKI guideline revision, several classification systems in addition to the stages of AKI should be rigorously defined. These relate to the distinctions among persistent, transient, relapsing, and recovered AKI; various etiologies of AKI; and community-onset versus hospital-onset AKI. In addition, there is emerging evidence that markers of structural kidney damage may be associated with clinically relevant outcomes and therefore identify potentially actionable entities. For an AKI guideline revision, the evidence base should be reviewed to determine whether markers of kidney damage constitute risk factors for AKI, define a new entity (such as subclinical or preclinical AKI), or should be incorporated into the AKI definition. Finally, the future guideline should use nomenclature that is precise and patient-centered.
The clinical importance of AKD needs to be further assessed. Retrospective cohort data based only on changes in serum creatinine values and with limited clinical context suggest a relevance for AKD: the population of patients who meet laboratory criteria for AKD but not CKD or AKI is relatively large, and these individuals have increased risks of incident and progressive CKD, kidney failure (formally referred to as “end-stage kidney disease”), and death,28 confirming the need to better define and classify AKD. Furthermore, a revised definition and classification of AKD could be better harmonized with both the definitions and classifications of AKI and CKD and tie to clinical management. As in adults, the AKI/AKD/CKD spectrum should be unified in children, and definitions should be the same for children and adults. A special consideration in children, as well as in adults with low muscle mass, is a reduced serum creatinine concentration, which may impact AKI diagnosis.
The assessment of renal recovery is still controversial, and its definition is essential given the implications for patients and clinicians. Issues related to assessment of recovery include changes in creatinine generation due to reduction in muscle mass, among others.
Advances in diagnosis of AKI
Serum creatinine and urine output continue to be the foundational measures for AKI diagnosis even though their limitations are well known. In the future, kidney damage biomarkers, biopsy, and imaging may be useful for staging AKI, classification of cause, prognosis, and treatment. However, currently there is insufficient information about any of these measures to warrant addition to the AKI definition. Given that the global availability of novel biomarkers is limited, incorporating them into definitions will be challenging. Measurements of real-time or kinetic GFR are research tools at present, and more evidence is needed regarding their clinical applicability (Table 2).
Table 2 |.
Research priorities and questions for investigation in AKI
| Diagnostics |
| 1. Evaluate the clinical applicability of real-time or kinetic GFR. |
| 2. Determine the magnitude of change in serum creatinine concentration that indicates AKI. |
| 3. Explore how differences in body composition (e.g., overweight, fluid overload) affect urine output, and whether these differences need to be considered regarding the thresholds for AKI. |
| Risk stratification |
| 1. Conduct multicenter studies for external validation of AKI risk models as well as standardization and correlation with outcomes. |
| 2. Define the role of kidney biopsy in managing AKI. |
| 3. Identify additional endpoints (beyond mortality, chronic kidney disease, and dialysis dependency) for both clinical management and trials. These could include recovery of function, continued need for dialysis, maximum changes in creatinine concentration, stage of AKI, functional renal reserve, biomarkers, and patient experience assessment (patient-reported outcome measures and patient-reported experience measures). |
| 4. Develop a more accurate definition of recovery and its functional (i.e., filtration, tubular, endocrine) and anatomic/structural dimensions. |
| Fluid management and hemodynamic support |
| 1. Determine the optimal indications and targets for fluid and vasoactive drugs to improve kidney outcomes in acute medical illness and in the perioperative setting. |
| 2. Determine the optimal vasopressor in this context and explore how the indications and targets should be translated to and from resource-limited settings. |
| 3. Investigate the optimal method of administering fluid for preventing or mitigating AKI (route [oral or i.v.]; bolus versus continuous; and rate, volume, and frequency of boluses) and explore whether the optimal methods vary in different contexts where different levels of monitoring are available, including pre-hospital settings and resource-limited settings. |
| 4. Investigate new techniques to detect fluid overload in adults and from this define fluid overload thresholds to guide management decisions; in addition, determine how fluid removal should be optimally accomplished including method, rate, targets, and monitoring. |
| 5. Investigate if there is a confirmed hazard from the use of 0.9% saline compared with balanced solutions on kidney outcomes in adults and identify the mechanism and explore whether there are any subgroups at particular risk. |
| 6. Explore the role for sodium bicarbonate in patients with AKI and metabolic acidosis. |
| Nephrotoxic agents and drugs that affect kidney function |
| 1. Investigate whether biomarkers for risk prediction, surveillance, or diagnostic evaluation (to discriminate between kidney dysfunction and injury) can affect choice of treatment strategy. |
| 2. Determine the role of electronic clinical decision-support systems to proactively identify risk and injury from drugs. |
| 3. Undertake drug burden assessment to determine which nephrotoxic drugs and drug combinations are associated with increasing risk for dysfunction and injury. |
| 4. Conduct studies to guide timing of ACE-I/ARBs discontinuation and re-initiation in AKI/AKD in different clinical contexts such as heart failure, surgery, and sepsis. |
| 5. Undertake research on the role of statins in preventing contrast-associated AKI in stable and acute settings, and study the dose-dependence of effect. |
| RRT |
| 1. Investigate the optimal timing, dose, and modality of RRT and identify the indicators that predict successful discontinuation of RRT. |
| 2. Compare different methods of assessing fluid removal goals and rates of fluid removal with RRT. |
| 3. Develop a registry focused on patients receiving ECLS-RRT. |
| 4. Develop a registry of patients receiving combined ECMO/ECCO2R and RRT. |
| 5. Evaluate whether ECCO2R can be efficiently applied in a system combining RRT and ECCO2R. |
| 6. Conduct clinical studies on respiratory dialysis (ECCO2R and ECMO) with modified dialysis solutions. |
| 7. Compare anticoagulation strategies (including citrate) of the RRT circuit during ECMO/ECCO2R. |
ACE-I, angiotensin-converting enzyme inhibitors; AKD, acute kidney diseases and disorders; AKI, acute kidney injury; ARB, angiotensin-receptor blockers; ECCO2R, extracorporeal carbon dioxide removal; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; RRT, renal replacement therapy.
Both urine output and serum creatinine level should continue to be used29; ideally, the new AKI guideline would provide further clarification as to the role of these measurements. If possible, both should be ascertained. However, if serum creatinine measurements are not immediately available, urine output criteria should be used.
It remains unclear how to best determine baseline kidney function. What constitutes a baseline serum creatinine level is controversial and inconsistently defined. It would be ideal to have prior serum creatinine or GFR measurements widely available through electronic medical records, but this is not current practice in many parts of the world. Prior serum creatinine or GFR measures may also further elucidate the risk of AKI in patients considered at high risk on the basis of either comorbidity or an intervention. There is controversy about whether an acute decrease in serum creatinine level indicates AKI that has already occurred, and more research is needed in this area. For example, small declines in serum creatinine level need to be interpreted with caution because they may be the result of acute changes in creatinine production or volume of distribution. After a timed insult (e.g., coronary angiography, elective surgery, nephrotoxic drug exposure), serum creatinine level should be measured at an appropriate time, allowing for AKI to manifest. After AKI onset, serum creatinine level should be measured during follow-up as necessary for clinical management and care transitions (e.g., transfer to and from intensive care) and for determining changes in AKI staging and classification (AKI vs. AKD), including onset of CKD at 90 days.
How urine output should be evaluated is also an area that needs further investigation to avoid variability in reporting of AKI incidence (i.e., use of actual or ideal body weight, strict time period vs. time-averaged values).30 Future guidelines should address how differences in body composition (overweight, fluid overload) affect the interpretation of urine output, and whether these differences need to be considered in regard to the thresholds for AKI. Similarly, fluid status should be considered when evaluating for AKI. Fluid overload is associated with increased mortality and AKI, and it may impact the diagnosis of AKI through its impact on the volume of distribution of serum creatinine. Although there are research methods to define fluid overload, these are not routinely used in clinical practice, and it is unclear whether there is sufficient evidence to define a clinical threshold for fluid overload. In the next AKI guideline, fluid overload should be defined operationally through a rigorous literature review.
AKI RISK STRATIFICATION AND ASSESSMENT
Risk stratification
In community and hospital settings, risk stratification of patients using a combination of baseline risks and acute exposures is important.31 In the future, risk stratification could incorporate various clinical contexts: geographic region, onset in community or hospital settings, and location within hospitals. Although the 2012 guideline discussed risk models and clinical scores, these were limited to models for cardiothoracic surgery, contrast exposure, and aminoglycoside administration. Many other clinical scenarios and contexts, such as sepsis and cardiac failure, require guidance for risk assessment. In clinical practice, risk models may be tailored for location and context. Multicenter studies are needed for externally validating models as well as standardization and correlation with outcomes. Furthermore, since 2012, biomarkers for AKI risk stratification have been approved by the US Food and Drug Administration (https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN130031.pdf) and integrated in recent guideline recommendations for cardiac surgery.32
Determining cause and prognosis
Determining the etiology of AKI is essential for management; however, this can be difficult, especially in the presence of multifactorial mechanisms. Newer developments related to monitoring and evaluating risk progression include e-alert systems, machine-learning algorithms and artificial intelligence for AKI recognition and monitoring,20,33–36 as well as models based upon the renal angina index,37,38 furosemide stress test (FST),39 or biomarkers.40–43 In revisiting the guideline for AKI, severity of AKI should be based not only upon serum creatinine elevation and urine output, but also upon duration, possibly with the inclusion of biomarkers. The need to increase attention for persistent (>48 hours) AKI should also be considered.44
The 2012 KDIGO guideline suggests performing a kidney biopsy when the cause of AKI is unclear. Potential benefits for biopsy in AKI are controversial and further research is needed.45 Since the 2012 guideline, which recommended ultrasound for assessing kidney size and the presence of an obstruction, new imaging techniques have become available, such as contrast-enhanced ultrasound, doppler ultrasound, and blood oxygenation level—dependent functional magnetic resonance imaging.46–48 The role of these techniques in changing outcomes of AKI is yet to be determined.
The 2012 KDIGO guideline recommended urine sediment analysis for differential diagnosis in patients with AKI, especially when glomerular disease is expected. Meeting participants noted that urine sediment analysis is not routinely performed in many centers despite its potential role in the workup of AKI.49,50 Additionally, the value of urine biochemistry analysis has been challenged, especially in sepsis.51
The FST may be useful for identifying patients with AKI who are likely to have progressive disease and need dialysis.52 There is also evidence that the FST is useful in predicting delayed graft function following deceased donor kidney transplantation.53 This test was not included in the 2012 guideline but should now be considered. Importantly, unregulated diagnostics tests such as FST or urine sediment analysis require careful standardization and quality control. Their introduction into clinical practice should include local evaluation for correct performance and interpretation.
The traditional approach to classifying AKI as pre-renal, renal, and post-renal is still found in many medical text-books. A different framework is needed, because these terms are considered unhelpful, especially the term pre-renal, which is often misinterpreted as “hypovolemic” and may encourage indiscriminate fluid administration. For classifying AKI, it may be more beneficial to distinguish between conditions that reduce glomerular function, conditions that result in injury of tubules and/or glomeruli, and conditions that do both.
Endpoints for clinical trials and quality improvement initiatives for AKI include mortality, new onset or progression of CKD, and dialysis dependency. Additional endpoints are needed for both clinical management and research, and these might include recovery of function, maximum changes in creatinine concentration, stage of AKI/AKD, impact on renal reserve, and patient experience. Additionally, there is a need to better define renal recovery and its functional (filtration, tubular, endocrine) and anatomic/structural dimensions.
Follow-up
Increased risks for mortality, cardiovascular events, and progression of kidney disease are well-documented outcomes of AKI.28,54–56 However, not everyone with AKI has a poor outcome, and predictors of poor outcomes have been identified.57 Follow-up recommendations (Figure 1)31 have been proposed that could be integrated into a KDIGO guideline revision. Although it has been suggested that patients be screened at hospital discharge or seen within 1 month of AKI diagnosis,58 there is no consensus on the optimal strategy and duration of follow-up to improve short- and long-term outcomes.
Figure 1 |. Schematic for acute kidney injury/acute kidney diseases and disorders (AKI/AKD) follow-up.
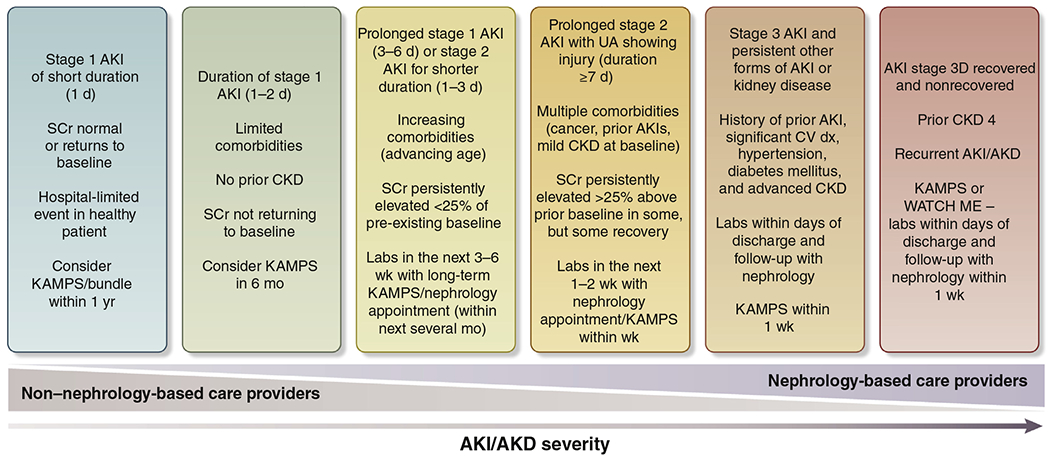
The figure displays a potential paradigm for the care of patients who experience AKI/AKD. The degree of nephrology-based follow-up increases as the duration and severity of AKI/AKD increases. The timing and nature of follow-up are suggestions, as there are limited data to inform this process. Future research efforts should work to clarify the timing of AKI/AKD follow-up and which specific healthcare providers should provide it. The items in each bucket follow the “OR” rule; therefore, each patient should follow the most-severe bucket even if they meet only 1 criterion in that bucket. For example, a patient with CKD G4, regardless of severity of AKI, should be followed by a nephrologist in 1 week. AKI stage 3D, AKI stage 3 treated by dialysis; CKD, chronic kidney disease; CV, cardiovascular; dx, diagnosis; KAMPS, kidney function–advocacy–medications–pressure–sick day protocol; SCr, serum creatinine; UA, urine analysis; WATCH-ME, weight assessment–access–teaching–clearance–hypotension–medications. Reproduced with permission under a Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0) from Acute Dialysis Quality Initiative. Quality improvement goals for acute kidney injury; ADQI XXII. Available at: https://www.adqi.org/Images_Charts-Call.htm. Accessed June 14, 2020.31
FLUID MANAGEMENT AND HEMODYNAMIC SUPPORT
Timing of fluid administration
Ensuring adequate hydration and volume status is essential in preventing and treating AKI. Oral or i.v. fluid may be administered depending on the local environment and clinical context. The administration of i.v. fluids should be guided by hemodynamic assessment for specific indications and contraindications. When deciding on fluid therapy, consideration for the clinical context and history, including timing of the insult, is critical. Table 3 lists clinical contexts in which indications for fluid administration should be balanced against potential coexisting conditions that require a more cautious approach. Because both the physiological response to fluids and the underlying condition related to AKI are dynamic over time, fluid administration should be based on repeated assessment of overall fluid and hemodynamic status and dynamic tests of fluid responsiveness.59,60
Table 3 |.
Clinical contexts for fluid administration in patients with or at risk of AKI
| Clinical context | Reasons for fluid administration and potential benefits | Challenges and risks of fluid administration |
|---|---|---|
| Age and demographics | ||
| Children | • Diseases with volume losses | • Narrow window between hypovolemia and fluid overload • Clear adverse effects of fluid overload |
| Adults | • Indications likely to be context-dependent | • Adverse effects of “one size fits all” approach to fluid management • Uncertain definition of clinically significant fluid overload • In patients with heart disease, poor cardiac reserve to tolerate hypovolemia and hypervolemia • In patients with diastolic dysfunction, risk of potentiating venous hypertension and renal congestion in fluid overload |
| Setting | ||
| Resource-limited | • Specific conditions including diarrheal illness | • Differing spectrum of disease • Potentially delayed presentation to secondary care • Limited range of therapeutic options |
| Pre-hospital Ward/ICU | • Impetus toward early resuscitation • Dynamic phases of illness associated with hypovolemia |
• Lack of advanced hemodynamic monitoring • Inappropriate administration of maintenance fluid • Risk of “fluid creep” leading to insidious fluid overload |
| Comorbid diseases | ||
| CKD | • Inability to conserve salt and water | • Risk of hypovolemia • Inability to handle fluid excess • Predisposition to AKI |
| CHF or severe valvular disease | • Poor cardiac reserve to tolerate hypovolemia | • Potentiation of adverse effects of fluid overload • Potentially pre-existing interstitial edema • Higher CVP associated with worsening kidney function |
| Severe chronic liver disease | • Intravascular hypovolemia despite peripheral edema | • Precipitation of fluid accumulation |
| Acute conditions | ||
| Dehydration | • Acute free water deficit | • Challenges of managing relative water and sodium deficits |
| Hypovolemia | • Salt and water deficit | • Need for consensus on optimal endpoints of resuscitation |
| Hemorrhage | • Acutely impaired oxygen delivery | • Dilution of hemoglobin may offset effects of fluid resuscitation on oxygen delivery |
| Sepsis | • Intravascular hypovolemia | • Endothelial dysfunction, capillary leak, fluid losses to interstitium, and vasodilation • Lack of evidence for goal-directed hemodynamic therapy |
| Cardiogenic shock | • Inability to tolerate hypovolemia • Venodilation due to inotropic drugs |
• Risk of pulmonary edema • Association between high CVP and adverse kidney outcome |
| Major surgery | • Anesthesia-induced venodilation and vasodilation • Perioperative fluid losses |
• Inappropriate administration of maintenance fluid and “fluid creep” leading to insidious fluid overload |
| Nephrotoxic exposure | • Dilution of filtered toxins | • Risk of fluid overload |
| Abdominal compartment syndrome | • Maintenance of visceral and renal perfusion | • Risk of venous hypertension |
| ARDS | • Reduced cardiac preload due to high intrathoracic pressure | • Risk of worsening alveolar edema |
| Rhabdomyolysis/crush injury | • Dilution of myoglobulin • Intravascular hypovolemia due to fluid losses to injured muscle |
• Development/worsening of compartment syndrome |
| Timing | ||
| Biomarker-positive states | • Prevention of progression to overt AKI | • Presence of early renal injury does not signify need for volume replacement |
| AKI stage | • Reversal of early AKI | • Inappropriate attempts to “reverse” established AKI resulting in fluid overload |
| Oliguria/anuria | • Oliguria as an indication of acute compensated hypovolemia | • Multiple etiologies of oliguria beyond hypovolemia • Vicious cycle of fluid overload resulting in worsening kidney function |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CHF, congestive heart failure; CKD, chronic kidney disease; CVP, central venous pressure; ICU, intensive care unit.
There continues to be concern about excessive fluid administration for hypotension, and earlier use of vasoactive medications may be appropriate for some patients.61,62 The effect of these strategies on kidney function is not clearly defined and likely to be context specific.63 Ongoing major multicenter RCTs examining kidney endpoints are evaluating fluid administration and vasoactive medications, and their results are likely to impact AKI treatment recommendations.
Methods of fluid administration
Significant new evidence from several large multicenter RCTs regarding use of protocolized goal-directed fluid therapy in early septic shock has suggested lack of benefits for survival and kidney outcome.64–66 However, there is some evidence to suggest that goal-directed protocols have benefits in perioperative patients.67,68 Therefore, recommendations regarding goal-directed fluid therapy for preventing or treating AKI may emerge to become more context specific. Additionally, clinical fluid therapy targets have evolved to include more dynamic indices, including the passive leg-raising test, pulse/stroke volume variation, and parameters derived from ultrasound. However, there is limited evidence that specific physiological targets for fluid therapy improve kidney outcomes.
Composition of i.v. fluid preparations
Crystalloids.
Evidence of biochemical abnormalities and adverse clinical outcomes associated with 0.9% saline compared with more physiological crystalloids (e.g., lactated Ringer’s) has continued to accumulate since 2012.11,12 Results from two large ongoing multicenter RCTs (NCT02875873, NCT02721654) are awaited. This evidence will require careful evaluation to provide the community with a new consensus regarding the magnitude of risks associated with 0.9% saline in acute illness and surgery, including considerations for resource-limited settings in which alternatives may be limited.
Synthetic colloids.
In recent years, consensus has emerged that due to the increased incidence of kidney dysfunction and mortality, synthetic colloids are harmful in critically ill patients, especially those with sepsis.69,70 However, whether these risks also apply to perioperative patients remains controversial, and this question is being examined in ongoing trials.
Albumin.
In RCTs, the use of albumin (including hyper-oncotic solutions) has not been shown to be harmful to kidney or other outcomes.71,72 However, clear evidence of benefit is also lacking, and any benefits may be limited to specific patient populations.73–75
Fluid removal
Physiological and epidemiologic evidence indicates that volume overload and venous congestion have adverse effects on kidney function and outcomes in both acute and chronic illness.76–78 In children, there is evidence that >10%–15% fluid overload by body weight is associated with adverse outcomes.79,80 However, the method for determining fluid overload and the threshold for clinically significant fluid overload in adults are not well defined, nor is the precise role of timing of fluid removal on kidney function and other outcomes. Therefore, there is a need to develop a consensus around methods and thresholds for fluid overload evaluation in adults and to establish recommendations for its management (Table 2).
NEPHROTOXIC AGENTS AND DRUGS THAT AFFECT KIDNEY FUNCTION
The use of drugs associated with kidney injury or dysfunction is common both in the hospital setting and in the community for patients with chronic illnesses such as hypertension, congestive heart failure, diabetes mellitus, cancer, and CKD. These drugs are often referred to as “nephrotoxic,” although many of them lead to kidney dysfunction without direct glomerular or tubular cell damage. Furthermore, some drugs that may cause a rise in serum creatinine are actually reno-protective and associated with improved outcomes (i.e., angiotensin-converting enzyme inhibitors or sodium-glucose co-transporter-2 inhibitors81 in diabetic nephropathy). Although it would be ideal to propose a simple yet inclusive term to encompass the various mechanisms by which drugs interface with the kidney, meeting participants were unable to identify one. Thus, here the term “nephrotoxic drugs” is retained for consistency with the literature. A new classification should also encompass drugs that are not directly harmful to kidney function but are eliminated by the renal route, and where there is concern about harm from accumulation of parent drug or metabolites in the setting of AKI and AKD. Similarly, failure to increase drug doses and intervals in renal recovery or with enhanced elimination via extracorporeal clearance may lead to therapeutic failure.82
In the past 10 years, significant progress has been made regarding susceptibility, management, and preventive strategies to avoid or ameliorate drug- and drug combination–associated kidney injury and dysfunction more broadly.
Overarching nephrotoxic medication management considerations are as follows:
Patients should receive potentially nephrotoxic medications only if needed and only for as long as needed.
Potentially nephrotoxic agents should not be withheld in life-threatening conditions, owing to concern for AKI, including i.v. contrast.
Kidney function must be monitored in patients who are exposed to agents that are associated with kidney injury or dysfunction, to limit the risk and progression of AKI and AKD.
Patients and clinicians need appropriate and effective education as to the potential for kidney injury and dysfunction from nephrotoxic agents.
Classifying drugs that affect kidney function and/or are nephrotoxic
There are multiple mechanisms by which drugs affect the kidney. They are summarized in 2 major categories: systemic or renal/glomerular hemodynamic effects (i.e., kidney dysfunction); and tubular or structural damage (i.e., kidney injury). Kidney dysfunction can result from drugs that lead to systemic hypotension (e.g., systemic arterial vasodilation) and/or altered intraglomerular hemodynamics (e.g., afferent arteriole constriction, efferent arteriole dilation). As a result, renal perfusion pressure is decreased, and if the decrease is sustained or severe, it can lead to ischemic injury. In comparison, drug-associated kidney injury is characterized by glomerular or tubular cell injury triggered by filtered toxins, tubular obstruction, endothelial dysfunction, or an allergic reaction.83–85 Important to note is that a given drug may lead to both dysfunction and injury.
A useful framework for classifying the mechanisms of drug-induced kidney injury or dysfunction is depicted in a 2x2 table to classify functional, structural, and combined functional/structural AKI86 (Figure 2). Drugs can affect the kidney by each of these mechanisms, and the figure depicts susceptibilities for AKI, as well as accelerants to develop dysfunction or injury and transition to dysfunction and injury. An important aspect of the framework is consideration of risk-mitigation strategies. Currently, there is sufficient evidence to classify drugs that affect kidney function or are nephrotoxic, in a clinically useful way.87,88
Figure 2 |. Classifying drugs that potentially cause acute kidney injury.
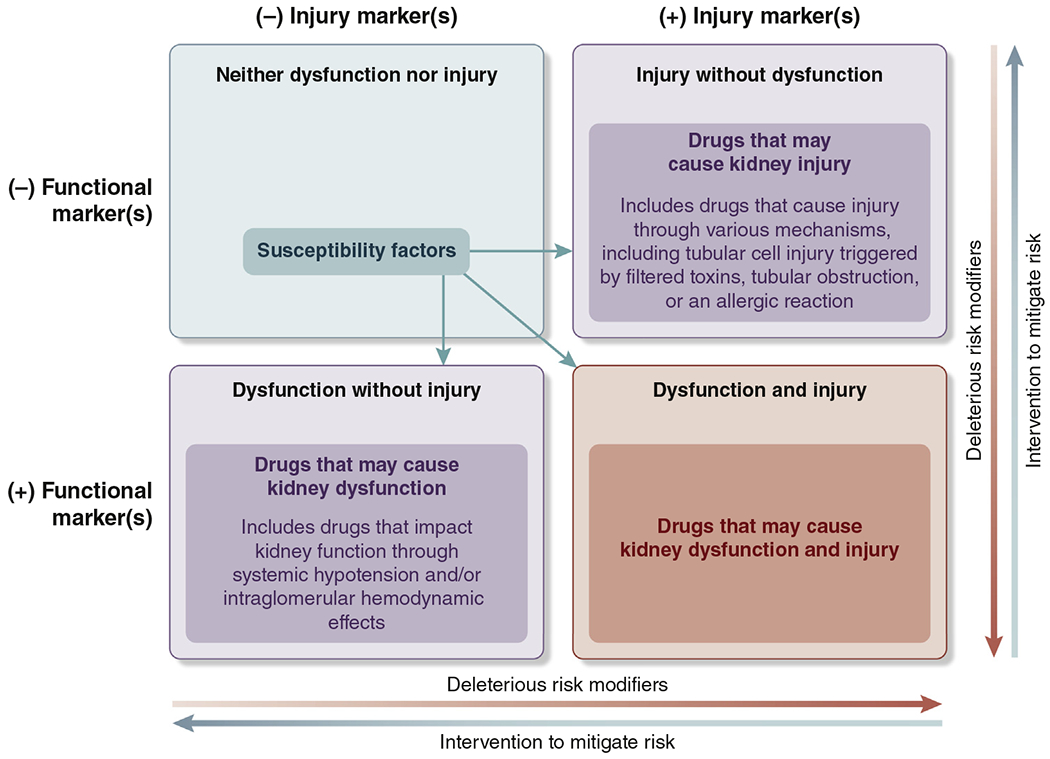
Iterative classification of agents that have potential to cause kidney dysfunction or kidney injury or both. Functional and injury biomarkers have a role in distinguishing among the different pathophysiological processes. Examples of deleterious risk modifiers are duration of therapy, drug burden, hypotension, and pharmacokinetic/pharmacodynamic interactions. Examples of interventions to mitigate risk are daily dynamic prescribing, kidney monitoring, and patient and provider education. Susceptibility factors include those listed in the 2012 Kidney Disease: Improving Global Outcomes Acute Kidney Injury guideline: dehydration or volume depletion; advanced age; female gender; black race; chronic kidney disease; chronic diseases of the heart, lung, or liver; diabetes mellitus; cancer; and anemia.1 Any final impact depends on underlying susceptibility, associated risk factors, clinical context, drug management, and modifying factors. Examples of drugs that correspond to the relevant categories above include trimethoprim, cimetidine (neither dysfunction nor injury); angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers (dysfunction without injury); aminoglycoside, acyclovir, vascular endothelial growth factor antagonists (injury without dysfunction); and nonsteroidal anti-inflammatory drugs (dysfunction and injury).
Preventing and mitigating drug-associated AKI
A number of strategies have emerged for preventing or mitigating drug-associated kidney injury or dysfunction. The most important of these is drug stewardship,21,89,90 with a primary goal of balancing the changing risks and benefits of drug utilization and dosing in AKI/AKD (Table 4).82 Specifically, it is critical to balance the risk of toxicity caused by excessive doses or drug/metabolite accumulation in AKI/AKD versus the risk of therapeutic failure caused by either overly conservative drug avoidance or under-dosing, or the risk of failing to adapt to renal recovery or use of renal replacement therapy (RRT).
Table 4 |.
Strategies for a drug stewardship program focused on AKI/AKD
| • Include a clinical pharmacist for drug stewardship. |
| • Identify patients at risk of AKI/AKD and take into account the risk of AKI/AKD when prescribing. |
| • Assess hydration status. |
| • Assess chronic drugs and their indication for continuation or discontinuation. |
| • Perform medication regimen review and evaluate PK/PD interactions. |
| • Review the use of drugs in patients who develop acute or chronic illnesses that increase the risk of AKI. |
| • Assess the dynamic impact of AKI/AKD on drug PK/PD. |
| • Assess the dynamic impact of renal recovery on drug PK/PD. |
| • Assess concurrent illness on drug PK/PD (e.g., sepsis, heart failure). |
| • Assess the impact of RRT on drug PK/PD. |
| • Undertake dynamic prescription and medication reconciliation at transitions of care. |
AKD, acute kidney diseases and disorders; AKI, acute kidney injury; PD, pharmacodynamics; PK, pharmacokinetics; RRT, renal replacement therapy.
Recent literature has demonstrated that certain drug combinations and overall drug burden are associated with AKI.91 These include the “triple whammy” of renin–angiotensin system inhibitors, diuretics, and nonsteroidal anti-inflammatory drugs, and an increased AKI risk when patients receive 3 or more nephrotoxic drugs daily.92 A single center has utilized electronic health records to identify children exposed to 3 or more nephrotoxic drugs, and the approach has led to a sustained decrease in incidence of AKI.21
Preventing and managing contrast-associated AKI
The only nephrotoxic agent addressed in any detail by the 2012 KDIGO AKI guideline was iodinated radiocontrast media.1 The 2012 guideline included several recommendations to prevent contrast-induced AKI, including use of volume expansion with sodium bicarbonate solutions and oral N-acetylcysteine. Results of the Prevention of Serious Adverse Events Following Angiography (PRESERVE) and POSEIDON trials demonstrated lack of efficacy of these interventions (and instead found improvement using a personalized approach targeting cardiac filling pressures in POSEIDON).93,94 Furthermore, recent evidence suggests that the risks associated with i.v. contrast are far fewer with modern agents and practice patterns, and significant kidney injury is unusual in patients with normal or mildly reduced baseline kidney function.95 I.v. contrast should not be withheld owing to concern for AKI in life-threatening conditions in which the information gained from the contrast study could have important therapeutic implications.
RENAL REPLACEMENT THERAPY
RRT terminology and initiation
In recent years, the suggestion has been made that the English term “renal” should be replaced by “kidney,” because the latter is more familiar to most English speakers. Additionally, the term “replacement” may not be sufficient, and terms such as “support” or “partial replacement” may be more accurate. The implications of changes in nomenclature are not insignificant. Additionally, the distinction between kidney versus renal does not apply in all languages. Accordingly, KDIGO has convened a separate Nomenclature Consensus Conference for the purpose of recommending nomenclature consistent with guidelines for acute and chronic kidney disease.96 Above all, patients should be the focus of all communication and care. Whenever possible, all decisions about treatment should be shared with patients, their families and/or next of kin, and if required, all members of the end-of-life care multidisciplinary team. All communication with patients and their supporting families/friends should be provided in simple lay language at regular intervals, with the awareness that patients may be traumatized. “Life support,” “kidney machine,” or similar words are preferred to the term RRT. If RRT becomes permanent, and the patient enters the chronic dialysis pathway, all relevant medical or nursing personnel should change their language to specify the type of RRT (transplant, hemodialysis, or peritoneal dialysis).
The 2012 KDIGO AKI guideline suggested initiating RRT emergently in the presence of life-threatening changes in fluid, electrolyte, and acid–base balance. Since 2012, data from several RCTs and observational studies have become available.13–15,97–104 However, the optimal timing for acute RRT remains unknown. It has been proposed that initiation of RRT should be considered when metabolic and fluid demands exceed the kidney’s capacity to meet them.105–107 This concept acknowledges the dynamic nature of acute illness and stresses the importance of regular evaluation of the demand and renal capacity relationship. However, the exact methods for determining demand and capacity are unknown. Existing evidence does not support using biomarkers when deciding whether to initiate RRT.13,97,108 Use of a standardized FST can be considered in AKI, to further quantify the likelihood of AKI progression, and integrated into the spectrum of clinical information available when planning for and deciding to initiate RRT.39,52,109,110 In determining whether or not to start RRT, risk of complications, global prognosis, potential for recovery, and patient preferences should be considered (Figure 3). Although some regions of the globe have challenges and constraints in providing universal access to RRT,111 we recommend a similar approach be undertaken for considering for whom and when to start RRT in all regions.112–114 Additionally, a similar approach should be undertaken in both intensive care unit and non–intensive care unit settings.
Figure 3 |.
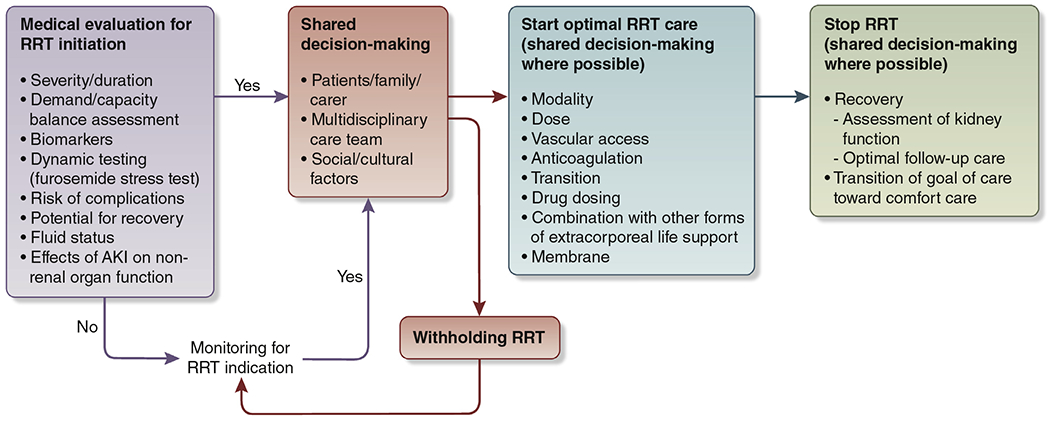
Schematic diagram of renal replacement therapy (RRT) decisions in acute kidney injury (AKI).
Providing RRT
Although the timing of RRT initiation is controversial, the provision of RRT itself has become fairly well established. Patients with AKI requiring RRT have an evolving clinical status and should be supported by the appropriate and available modality. Modality choice should also be tailored to patient clinical status. As suggested in the 2012 KDIGO guideline, in hemodynamically unstable patients, continuous RRT, rather than intermittent hemodialysis, is more physiologically appropriate, but RCTs have not demonstrated better outcomes with continuous RRT.1 Both continuous and intermittent RRT can lead to changes in intracranial pressure, but the risk is higher with intermittent RRT. Selection of modalities should be considered in the context of available resources and expertise of personnel.
An uncuffed non-tunnelled dialysis catheter of appropriate length and gauge should be used to initiate RRT in AKI patients. In patients with expected prolonged indication for RRT, a cuffed catheter can be considered.115 The first choice for site is the right jugular vein or femoral vein, although the femoral site is inferior in patients with increased body mass. The next choices would be left jugular vein followed by subclavian vein. Anticoagulation type should be selected based on local resources and expertise of personnel. The recommendation from 2012 to use regional citrate anticoagulation for continuous RRT in patients who do not have a contraindication remains supported by existing data.116–118 Delivery of RRT must reach the goals of electrolyte, acid–base, solute, and fluid balance for each specific patient.119 When using intermittent or extended RRT, a Kt/V of at least 1.2 per treatment 3 times a week should be delivered.120 For peritoneal dialysis, future studies should focus on dosing in AKI, although currently we suggest a dose of 0.3 Kt/V per session. An effluent volume of 20–25 ml/kg per h should be delivered when continuous RRT is used. This will sometimes require a higher prescription of effluent volume.121,122 The rate of fluid removal for a given patient with fluid overload is controversial,123,124 and more research is needed. Methods to better assess fluid management goals during RRT would also be valuable. Finally, RRT should be discontinued when kidney function has recovered or when RRT becomes inconsistent with shared care goals. Modality transition from continuous RRT to intermittent hemodialysis in intensive care unit patients should be considered when vasopressor support has been stopped, intracranial hypertension has resolved, and positive fluid balance can be controlled by intermittent hemodialysis.
RRT in the context of multi-organ support
The 2012 KDIGO AKI guideline did not address utilization of extracorporeal life support (ECLS) such as extracorporeal membrane oxygenation (ECMO), extracorporeal carbon dioxide removal (ECCO2R), and left or right ventricular assist device. Several issues remain unresolved: the optimal approach to patient selection, techniques, and timing/indications; circuit integration; and monitoring for ECLS and concomitant blood-purification techniques. Several observational studies on this theme warrant analysis and interpretation.125–131
Decisions regarding how to combine RRT with ECLS devices will depend on local expertise, technology, and human resources. Such combined treatment should be based on a multidisciplinary approach to patient care and shared decision-making. More studies are needed to define the best strategy for training and practice.
Although different RRT modalities can be used to support patients during ECLS, and comparative studies are not available, because of hemodynamic status, continuous RRT is more appropriate in this setting. It would be useful to develop a registry focused on patients receiving ECLS-RRT, to understand the epidemiology, technology, indications, and complications associated with current practice.
There is no clear evidence that usual RRT indications should vary according to the presence or absence of an ECMO/ECCO2R circuit. Nonetheless, patients for whom ECMO or ECCO2R is required are very sensitive to fluid overload. Therefore, in patients with versus without ECMO/ECCO2R, earlier RRT may be required for preventing and managing fluid overload. A registry of patients combining ECMO/ECCO2R and RRT could improve understanding of current practice for initiating RRT in patients (adults and children) with ECMO/ECCO2R and fluid management. Respiratory dialysis (ECCO2R and ECMO) with modified dialysis solutions is currently limited to in vitro and experimental studies,132–134 and research focused on this technical aspect is needed.
The anticoagulation of RRT circuits when ECMO/ECCO2R is already running is not standardized. The administration of heparin may depend on patient factors (e.g., risk of bleeding), circuit set-up (e.g., connection to patient or to ECMO), and institutional protocols.128,130,135–141 It is possible to have RRT circuits without dedicated heparin in this setting, unless excessively frequent clotting is observed. Studies are needed to compare different anticoagulation strategies in this setting. Citrate anticoagulation during RRT added to ECMO/ECCO2R is possible.139,140 Its feasibility and performance compared with other forms of anticoagulation remain untested, and thus comparative studies of citrate anticoagulation are recommended.
RRT long-term outcomes and follow-up
Choice of RRT modality and impact on recovery.
The selection of RRT modality does not appear to have a major impact on recovery of kidney function.141–143 Selection of modality of RRT should therefore be based on shared decision-making, local expertise, logistic factors, and patient characteristics. Estimated GFR in conjunction with major adverse kidney events has been used for medium- and long-term assessment but has several limitations. There is uncertainty about the best way to measure renal recovery after RRT in both the short- and medium-term. However, proteinuria is associated with worse long-term outcomes and is easy to measure.
Assessment of kidney function for renal recovery.
In addition to the development of CKD, patient-centered outcomes (quality of life, functional recovery), along with patient experience after AKI, should be a priority and need to be assessed. Post-AKI proteinuria is associated with future loss of kidney function and is regarded as a valuable risk-stratification tool in the post-AKI period.144–146
Optimal follow-up for AKI patients following RRT
Shared decision-making and communication among caregivers, the patient, and family members is crucial to patient recovery. Patients recovering from critical illness and AKI are often discharged to rehabilitation/skilled nursing facilities and need close monitoring to ensure adequate overall recovery to a baseline state of health and well-being. Such patients should receive multidisciplinary, recovery-focused care. Patients with AKI who continue to require RRT at hospital discharge often receive hemodialysis in outpatient dialysis facilities. Patients with congestive heart failure are less likely to recover kidney function.147 Higher ultrafiltration rates and more intradialytic hypotensive episodes are associated with higher risk of non-recovery of kidney function.148,149 To assess for renal recovery, hemodynamic status, intravascular volume, and urine output during dialysis should be carefully monitored.
Quality indicators for acute RRT
The importance of measuring and monitoring the quality of acute RRT provided to critically ill patients with AKI, including the optimal “benchmarking” for acute RRT programs, is receiving great attention.119,150 Quality of acute RRT should be monitored to ensure the effective and safe delivery of care.151 At a minimum, institutions and programs providing RRT should integrate, monitor, and report quality and outcome indicators across all forms of acute RRT therapies.31 These outcome measures should comprise a variety of metrics that incorporate patient survival, patient-centered acute RRT outcomes, safety, AKI survivor–related outcomes, and patient experience. Quality indicators should include shared goals that are patient- and clinically centered.
CONCLUSIONS
Although much of the 2012 KDIGO AKI guideline remains state of the art, advances over the past decade have improved our understanding of best practices. Many of these advances are widely accepted (e.g., nephrotoxic medication stewardship, shared decision making for RRT), but others are more controversial (Table 5). Although some centers and specific programs have embraced new technologies and ways of thinking, others have taken a more conservative, or “wait-and-see” approach. Even among conference participants, there was lack of unanimity for various perspectives, and obvious practice variation continues to exist, even among experts. Perhaps more than any new trial or discovery, this fact provides ample rationale for revisiting the AKI guideline in the near future.
Table 5 |.
Summary points from the Acute Kidney Injury Controversies Conference
| Risk assessment | Diagnosis of AKI | Fluid management | Hemodynamic management | Drug stewardship | RRT | |
|---|---|---|---|---|---|---|
| Consensus | • Need for risk models tailored to clinical context | • Retention of KDIGO criteria but there is need for refinement (i.e., duration, etiology, course, role of biomarkers, definition and staging of AKD) | • Importance of adequate hydration and correction of hypovolemia to prevent and treat AKI | • Importance of adequate renal perfusion to prevent and treat AKI | • Importance of drug stewardship in preventing and managing AKI • Retention of term “nephrotoxic” despite varying effects of drugs on kidney function |
• Need for patient-centered communication • Need to tailor RRT to the condition of the patient |
| Evidence of importance but lack of consensus | • Role of new AKI risk models • Need for additional endpoints beyond mortality and CKD • Impact of renal reserve • Follow-up strategy after AKI |
• Definition of “pre-clinical” AKI • Assessment of baseline kidney function • Evaluation of urine output • Definition of renal recovery • Role of kidney biopsy and new imaging techniques |
• Definition and assessment of fluid overload • Impact of saline in different clinical settings • Role of goal-directed therapy in different clinical scenarios • Strategy of fluid removal |
• Role of goal-directed therapy in different clinical scenarios • Impact of vasopressor type on kidney function |
• Impact of contrast in different clinical settings • Role of ACE-Is/ARBs in AKI |
• Assessment of recovery after RRT, including kidney function and patient-centered outcomes • Combination of RRT and other forms of extracorporeal organ support • Identification of quality indicators for acute RRT |
| Key topics for future research | • Evaluation of AKI risk models • Identification of clinical endpoints |
• Role of real-time or kinetic GFR • Impact of body composition on AKI criteria |
• Determination of optimal indications, fluid type, administration, and clinical endpoints of fluid therapy in AKI • Investigation of fluid-removal strategies |
• Determination of optimal indications, vasopressor type, and clinical endpoints of fluid therapy in AKI | • Development of new classification describing renal effects of drugs • Role of biomarkers and electronic clinical decision-support systems • Management of ACE-Is/ARBs in AKI |
• Determination of optimal fluid-removal strategy • Integration of RRT and other forms of extracorporeal support |
ACE-I, angiotensin-converting enzyme inhibitor; AKD, acute kidney diseases and disorders; AKI, acute kidney injury; ARB, angiotensin-receptor blocker; CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; RRT, renal replacement therapy.
ACKNOWLEDGMENTS
This conference was sponsored by KDIGO and was in part supported by unrestricted educational grants from Akebia Therapeutics, AM-Pharma, Angion, AstraZeneca, Astute Medical, Atox Bio, Baxter, bioMérieux, BioPorto, Boehringer Ingelheim, CytoSorbents, Edwards, Fresenius Medical Care, GE Healthcare, Grifols, Kyowa Kirin, Novartis, NxStage, Outset, and Potrero.
We thank Jennifer King, PhD, for assistance with manuscript preparation. The conference agenda, discussion questions, and plenary session presentations are available on the KDIGO website: https://kdigo.org/conferences/aki-conference/.
DISCLOSURE
MO declared having received consultancy fees from NxStage, speaker honoraria from Fresenius Medical Care, and research support from LaJolla Pharma. RB declared having received speaker honoraria from AbbVie and research support from Baxter. EAB declared having received consultancy fees from AstraZeneca and Fresenius Medical Care, and research support from Fundação de Amparo à Pesquisa do Estado de São Paulo. ZHE declared having received consultancy fees from AstraZeneca, and research support from Health Research Council of New Zealand and National University Hospital Singapore. SLG declared having received stock options from MediBeacon. KDL declared having received consultancy fees from bioMérieux, speaker honoraria from Baxter, and stock options from Amgen. JRP declared having received consultancy fees from MediBeacon, Nikkiso Europe GmbH, and Quark Pharmaceuticals; speaker honoraria from Baxter, Fresenius Medical Care, and Nikkiso Europe GmbH; and research support from bioMérieux. MJ declared having received consultancy fees from Amgen, AstraZeneca, Mundipharma, MSD, and Vifor Fresenius Medical Care Renal Pharma; speaker honoraria from Amgen, Menarini, MSD, and Vifor Fresenius Medical Care Renal Pharma; and research support from Amgen, MSD, and Otsuka. WCW declared having received consultancy fees from Akebia, AstraZeneca, Bayer, Janssen, Merck, Relypsa, and Vifor Fresenius Medical Care Renal Pharma; and research support from the National Institutes of Health. JAK declared having received consultancy fees from Astute Medical, Baxter, bioMérieux, Davita, Fresenius Medical Care, Grifols, NxStage, Potrero, and RenalSense; and research support from Astute Medical, Baxter, bioMérieux, and RenalSense. All the other authors declared no competing interests.
APPENDIX
Other conference participants
Sean M. Bagshaw, Canada; Erin F. Barreto, USA; Azra Bihorac, USA; Ilona Bobek, Hungary; Josée Bouchard, Canada; Jorge Cerdá, USA; Rajasekara Chakravarthi, India; Silvia De Rosa, Italy; Daniel T. Engelman, USA; Lui G. Forni, UK; Ulla Hemmilä, UK; Charles A. Herzog, USA; Eric A. Hoste, Belgium; Sarah C. Huen, USA; Kunitoshi Iseki, Japan; Michael Joannidis, Austria; Kianoush B. Kashani, USA; Jay L. Koyner, USA; Andreas Kribben, Germany; Norbert Lameire, Belgium; Andrew S. Levey, USA; Etienne Macedo, USA; Jolanta Małyszko, Poland; Melanie Meersch, Germany; Ravindra L. Mehta, USA; Irene Mewburn, Australia; Olga Mironova, Russia; Patrick T. Murray, Ireland; Mitra K. Nadim, USA; Jenny S. Pan, USA; Neesh Pannu, Canada; Zhiyong Peng, China; Barbara Philips, UK; Daniela Ponce, Brazil; Patricio E. Ray, USA; Zaccaria Ricci, Italy; Thomas Rimmelé, France; Claudio Ronco, Italy; Edward D. Siew, USA; Paul E. Stevens, UK; Ashita J. Tolwani, USA; Marcello Tonelli, Canada; Suvi T. Vaara, Finland; Marjel van Dam, Netherlands; Anitha Vijayan, USA; Michael Wise, UK; Vin-Cent Wu, Taiwan; Alexander Zarbock, Germany.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2:1–138. [Google Scholar]
- 2.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology’s 0-by-25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. [DOI] [PubMed] [Google Scholar]
- 4.Srisawat N, Kulvichit W, Mahamitra N, et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: a prospective multicentre study. Nephrol Dial Transplant. 2019. pii: gfz087. Accessed June 14, 2020. [DOI] [PubMed]
- 5.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jetton JG, Boohaker LJ, Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocze I, Jauch D, Gotz M, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK Study. Ann Surg. 2018;267:1013–1020. [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA, Chawla LS, Keener C, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389:1312–1322. [DOI] [PubMed] [Google Scholar]
- 11.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315:2190–2199. [DOI] [PubMed] [Google Scholar]
- 14.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. [DOI] [PubMed] [Google Scholar]
- 15.Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jaghbeer M, Dealmeida D, Bilderback A, et al. Clinical decision support for in-hospital AKI. J Am Soc Nephrol. 2018;29:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selby NM, Casula A, Lamming L, et al. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. 2019;30:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tollitt J, Flanagan E, McCorkindale S, et al. Improved management of acute kidney injury in primary care using e-alerts and an educational outreach programme. Fam Pract. 2018;35:684–689. [DOI] [PubMed] [Google Scholar]
- 19.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385:1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolhe NV, Staples D, Reilly T, et al. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One. 2015;10:e0132279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90:212–221. [DOI] [PubMed] [Google Scholar]
- 22.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. [DOI] [PubMed] [Google Scholar]
- 23.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. [DOI] [PubMed] [Google Scholar]
- 25.Guzzi LM, Bergler T, Binnall B, et al. Clinical use of [TIMP-2]*[IGFBP7] biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care. 2019;23:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparrow HG, Swan JT, Moore LW, et al. Disparate outcomes observed within Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury stage 1. Kidney Int. 2019;95:905–913. [DOI] [PubMed] [Google Scholar]
- 27.Barasch J, Zager R, Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389:779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James MT, Levey AS, Tonelli M, et al. Incidence and prognosis of acute kidney diseases and disorders using an integrated approach to laboratory measurements in a universal health care system. JAMA Netw Open. 2019;2:e191795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JC, Gardner DS, Skinner H, et al. Definition of hourly urine output influences reported incidence and staging of acute kidney injury. BMC Nephrol. 2020;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashani K, Rosner MH, Haase M, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755–766. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Baek SH, Ahn S, et al. Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: a quality improvement study. Am J Kidney Dis. 2018;71:9–19. [DOI] [PubMed] [Google Scholar]
- 34.Koyner JL, Carey KA, Edelson DP, et al. The development of a machine learning inpatient acute kidney injury prediction model. Crit Care Med. 2018;46:1070–1077. [DOI] [PubMed] [Google Scholar]
- 35.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, et al. MySurgeryRisk: development and validation of a machine-learning risk algorithm for major complications and death after surgery. Ann Surg. 2019;269:652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomasev N, Glorot X, Rae JW, et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature. 2019;572:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuura R, Srisawat N, Claure-Del Granado R, et al. Use of the Renal Angina Index in determining acute kidney injury. Kidney Int Rep. 2018;3:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu RK, Kaddourah A, Goldstein SL, et al. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health. 2018;2:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol. 2015;26:2023–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr KF, Meisner A, Thiessen-Philbrook H, et al. Developing risk prediction models for kidney injury and assessing incremental value for novel biomarkers. Clin J Am Soc Nephrol. 2014;9:1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JJ, Chi NH, Huang TM, et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care. 2018;22:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64:2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatraju PK, Zelnick LR, Katz R, et al. A prediction model for severe AKI in critically ill adults that incorporates clinical and biomarker data. Clin J Am Soc Nephrol. 2019;14:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. [DOI] [PubMed] [Google Scholar]
- 45.Waikar SS, McMahon GM. Expanding the role for kidney biopsies in acute kidney injury. Semin Nephrol. 2018;38:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hull TD, Agarwal A, Hoyt K. New ultrasound techniques promise further advances in AKI and CKD. J Am Soc Nephrol. 2017;28:3452–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meola M, Nalesso F, Petrucci I, et al. Ultrasound in acute kidney disease. Contrib Nephrol. 2016;188:11–20. [DOI] [PubMed] [Google Scholar]
- 48.Zhou HY, Chen TW, Zhang XM. Functional magnetic resonance imaging in acute kidney injury: present status. Biomed Res Int. 2016;2016:2027370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7:167–174. [DOI] [PubMed] [Google Scholar]
- 50.Bagshaw SM, Haase M, Haase-Fielitz A, et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant. 2012;27:582–588. [DOI] [PubMed] [Google Scholar]
- 51.Bagshaw SM, Bennett M, Devarajan P, et al. Urine biochemistry in septic and non-septic acute kidney injury: a prospective observational study. J Crit Care. 2013;28:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMahon BA, Koyner JL, Novick T, et al. The prognostic value of the furosemide stress test in predicting delayed graft function following deceased donor kidney transplantation. Biomarkers. 2018;23:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawhney S, Mitchell M, Marks A, et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3:e000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James MT, Pannu N, Hemmelgarn BR, et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wald R, Kitchlu A, Harel Z, et al. Care of the acute kidney injury survivor. Nephron. 2017;137:306–309. [DOI] [PubMed] [Google Scholar]
- 59.Monnet X, Teboul JL. Assessment of fluid responsiveness: recent advances. Curr Opin Crit Care. 2018;24:190–195. [DOI] [PubMed] [Google Scholar]
- 60.Cecconi M, Hernandez G, Dunser M, et al. Fluid administration for acute circulatory dysfunction using basic monitoring: narrative review and expert panel recommendations from an ESICM task force. Intensive Care Med. 2019;45:21–32. [DOI] [PubMed] [Google Scholar]
- 61.Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43:155–170. [DOI] [PubMed] [Google Scholar]
- 62.Self WH, Semler MW, Bellomo R, et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med. 2018;72:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–2274. [DOI] [PubMed] [Google Scholar]
- 64.Pro CI, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ARISE Investigators; ANZICS Clinical Trials Group; Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. [DOI] [PubMed] [Google Scholar]
- 66.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. [DOI] [PubMed] [Google Scholar]
- 67.Chong MA, Wang Y, Berbenetz NM, et al. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:469–483. [DOI] [PubMed] [Google Scholar]
- 68.Corcoran T, Rhodes JE, Clarke S, et al. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012;114:640–651. [DOI] [PubMed] [Google Scholar]
- 69.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. [DOI] [PubMed] [Google Scholar]
- 70.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 71.Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. [DOI] [PubMed] [Google Scholar]
- 72.Martensson J, Bihari S, Bannard-Smith J, et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects : The SWIPE randomised clinical trial. Intensive Care Med. 2018;44:1797–1806. [DOI] [PubMed] [Google Scholar]
- 73.Kingeter AJ, Raghunathan K, Munson SH, et al. Association between albumin administration and survival in cardiac surgery: a retrospective cohort study. Can J Anaesth. 2018;65:1218–1227. [DOI] [PubMed] [Google Scholar]
- 74.Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122–134. [DOI] [PubMed] [Google Scholar]
- 75.Martin GS, Moss M, Wheeler AP, et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–1687. [DOI] [PubMed] [Google Scholar]
- 76.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta RL, Bouchard J. Controversies in acute kidney injury: effects of fluid overload on outcome. Contrib Nephrol. 2011;174:200–211. [DOI] [PubMed] [Google Scholar]
- 78.Raimundo M, Crichton S, Martin JR, et al. Increased fluid administration after early acute kidney injury is associated with less renal recovery. Shock. 2015;44:431–437. [DOI] [PubMed] [Google Scholar]
- 79.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. [DOI] [PubMed] [Google Scholar]
- 80.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–258. [DOI] [PubMed] [Google Scholar]
- 81.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 82.Ostermann M, Chawla LS, Forni LG, et al. Drug management in acute kidney disease—Report of the Acute Disease Quality Initiative XVI meeting. Br J Clin Pharmacol. 2018;84:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14:217–230. [DOI] [PubMed] [Google Scholar]
- 84.Radhakrishnan J, Perazella MA. Drug-induced glomerular disease: attention required! Clin J Am Soc Nephrol. 2015;10:1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perazella MA. Drug use and nephrotoxicity in the intensive care unit. Kidney Int. 2012;81:1172–1178. [DOI] [PubMed] [Google Scholar]
- 86.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. [DOI] [PubMed] [Google Scholar]
- 87.Jones M, Tomson C. Acute kidney injury and ‘nephrotoxins’: mind your language. Clin Med (Lond). 2018;18:384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu H, Huang J. Drug-induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Curr Drug Metab. 2018;19:559–567. [DOI] [PubMed] [Google Scholar]
- 89.Karino S, Kaye KS, Navalkele B, et al. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother. 2016;60:3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kane-Gill SL, Goldstein SL. Drug-induced acute kidney injury: a focus on risk assessment for prevention. Crit Care Clin. 2015;31:675–684. [DOI] [PubMed] [Google Scholar]
- 91.Rivosecchi RM, Kellum JA, Dasta JF, et al. Drug class combination-associated acute kidney injury. Ann Pharmacother. 2016;50:953–972. [DOI] [PubMed] [Google Scholar]
- 92.Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603–614. [DOI] [PubMed] [Google Scholar]
- 94.Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 95.McDonald JS, McDonald RJ, Williamson EE, et al. Post-contrast acute kidney injury in intensive care unit patients: a propensity score-adjusted study. Intensive Care Med. 2017;43:774–784. [DOI] [PubMed] [Google Scholar]
- 96.Levey AS, Eckardt K-U, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97:1117–1129. [DOI] [PubMed] [Google Scholar]
- 97.Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88:897–904. [DOI] [PubMed] [Google Scholar]
- 98.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wierstra BT, Kadri S, Alomar S, et al. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaara ST, Reinikainen M, Wald R, et al. Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol. 2014;9:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiao CC, Wu VC, Li WY, et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009;13:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chou YH, Huang TM, Wu VC, et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care. 2011;15:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jun M, Bellomo R, Cass A, et al. Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit Care Med. 2014;42:1756–1765. [DOI] [PubMed] [Google Scholar]
- 104.Bagshaw SM, Adhikari NKJ, Burns KEA, et al. Selection and receipt of kidney replacement in critically ill older patients with AKI. Clin J Am Soc Nephrol. 2019;14:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macedo E, Mehta RL. When should renal replacement therapy be initiated for acute kidney injury? Semin Dial. 2011;24:132–137. [DOI] [PubMed] [Google Scholar]
- 106.Ostermann M, Joannidis M, Pani A, et al. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42:224–237. [DOI] [PubMed] [Google Scholar]
- 107.Wilson FP, Yang W, Machado CA, et al. Dialysis versus nondialysis in patients with AKI: a propensity-matched cohort study. Clin J Am Soc Nephrol. 2014;9:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018;44:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rewa OG, Bagshaw SM, Wang X, et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: a multicenter, prospective, observational study. J Crit Care. 2019;52:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lumlertgul N, Peerapornratana S, Trakarnvanich T, et al. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care. 2018;22:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Obrador GT, Rubilar X, Agazzi E, et al. The challenge of providing renal replacement therapy in developing countries: the Latin American perspective. Am J Kidney Dis. 2016;67:499–506. [DOI] [PubMed] [Google Scholar]
- 112.Ponce D, Buffarah MB, Goes C, et al. Peritoneal dialysis in acute kidney injury: trends in the outcome across time periods. PLoS One. 2015;10:e0126436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.George J, Varma S, Kumar S, et al. Comparing continuous venovenous hemodiafiltration and peritoneal dialysis in critically ill patients with acute kidney injury: a pilot study. Perit Dial Int. 2011;31:422–429. [DOI] [PubMed] [Google Scholar]
- 114.Esezobor CI, Ladapo TA, Lesi FE. Peritoneal dialysis for children with acute kidney injury in Lagos, Nigeria: experience with adaptations. Perit Dial Int. 2014;34:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelly YP, Mendu ML. Vascular access for renal replacement therapy in acute kidney injury: are nontunneled catheters the right choice? Semin Dial. 2019;32:406–410. [DOI] [PubMed] [Google Scholar]
- 116.Gattas DJ, Rajbhandari D, Bradford C, et al. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med. 2015;43:1622–1629. [DOI] [PubMed] [Google Scholar]
- 117.Liu C, Mao Z, Kang H, et al. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41:2098–2110. [DOI] [PubMed] [Google Scholar]
- 119.Bagshaw SM, Chakravarthi MR, Ricci Z, et al. Precision continuous renal replacement therapy and solute control. Blood Purif. 2016;42:238–247. [DOI] [PubMed] [Google Scholar]
- 120.Bouchard J, Macedo E, Mehta RL. Dosing of renal replacement therapy in acute kidney injury: lessons learned from clinical trials. Am J Kidney Dis. 2010;55:570–579. [DOI] [PubMed] [Google Scholar]
- 121.Claure-Del Granado R, Macedo E, Chertow GM, et al. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011;6:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lyndon WD, Wille KM, Tolwani AJ. Solute clearance in CRRT: prescribed dose versus actual delivered dose. Nephrol Dial Transplant. 2012;27:952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Murugan R, Balakumar V, Kerti SJ, et al. Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care. 2018;22:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murugan R, Kerti SJ, Chang CH, et al. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy trial. JAMA Netw Open. 2019;2:e195418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H, Yu RG, Yin NN, et al. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 2014;18:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Allardet-Servent J, Castanier M, Signouret T, et al. Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: The Pulmonary and Renal Support in Acute Respiratory Distress Syndrome Study. Crit Care Med. 2015;43:2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peperstraete H, Eloot S, Depuydt P, et al. Low flow extracorporeal CO2 removal in ARDS patients: a prospective short-term crossover pilot study. BMC Anesthesiol. 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suga N, Matsumura Y, Abe R, et al. A safe procedure for connecting a continuous renal replacement therapy device into an extracorporeal membrane oxygenation circuit. J Artif Organs. 2017;20:125–131. [DOI] [PubMed] [Google Scholar]
- 129.Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7:1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) Group. ASAIO J. 2012;58:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Forster C, Schriewer J, John S, et al. Low-flow CO(2) removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care. 2013;17:R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.May AG, Sen A, Cove ME, et al. Extracorporeal CO2 removal by hemodialysis: in vitro model and feasibility. Intensive Carne Med Exp. 2017;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vu LH, Kellum JA, Federspiel WJ, et al. Carbon dioxide removal using low bicarbonate dialysis in rodents. Perfusion. 2019, 267659119839284. [DOI] [PubMed] [Google Scholar]
- 134.Fanelli V, Cantaluppi V, Alessandri F, et al. Extracorporeal CO2 removal may improve renal function of patients with acute respiratory distress syndrome and acute kidney injury: an open-label, interventional clinical trial. Am J Respir Crit Care Med. 2018;198:687–690. [DOI] [PubMed] [Google Scholar]
- 135.Ostermann M, Connor M Jr, Kashani K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: why, when and how? Curr Opin Crit Care. 2018;24:493–503. [DOI] [PubMed] [Google Scholar]
- 136.Seczynska B, Krolikowski W, Nowak I, et al. Continuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerations. Ther Apher Dial. 2014;18:523–534. [DOI] [PubMed] [Google Scholar]
- 137.Zhou XL, Chen YH, Wang QY. A new approach combining venoarterial extracorporeal membrane oxygenation and CRRT for adults: a retrospective study. Int J Artif Organs. 2017;40:345–349. [DOI] [PubMed] [Google Scholar]
- 138.de Tymowski C, Augustin P, Houissa H, et al. CRRT connected to ECMO: managing high pressures. ASAIO J. 2017;63:48–52. [DOI] [PubMed] [Google Scholar]
- 139.Shum HP, Kwan AM, Chan KC, et al. The use of regional citrate anticoagulation continuous venovenous hemofiltration in extracorporeal membrane oxygenation. ASAIO J. 2014;60:413–418. [DOI] [PubMed] [Google Scholar]
- 140.Husain-Syed F, Ricci Z, Brodie D, et al. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med. 2018;44:1447–1459. [DOI] [PubMed] [Google Scholar]
- 141.Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39:987–997. [DOI] [PubMed] [Google Scholar]
- 142.Truche AS, Darmon M, Bailly S, et al. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med. 2016;42:1408–1417. [DOI] [PubMed] [Google Scholar]
- 143.Liang KV, Sileanu FE, Clermont G, et al. Modality of RRT and recovery of kidney function after AKI in patients surviving to hospital discharge. Clin J Am Soc Nephrol. 2016;11:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Neyra JA, Li X, Yessayan L, et al. Dipstick albuminuria and acute kidney injury recovery in critically ill septic patients. Nephrology (Carlton). 2016;21:512–518. [DOI] [PubMed] [Google Scholar]
- 145.Lim CT, Tan HK, Lau YK. The significance of tubular and glomerular proteinuria in critically ill patients with severe acute kidney injury. Pak J Med Sci. 2014;30:1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsu CY, Chinchilli VM, Coca S, et al. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study. JAMA Intern Med. 2020;180:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thakar CV, Parikh PJ, Liu Y. Acute kidney injury (AKI) and risk of readmissions in patients with heart failure. Am J Cardiol. 2012;109:1482–1486. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, Gallagher M, Li Q, et al. Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence: a systematic review and individual patient data meta-analysis. Nephrol Dial Transplant. 2018;33:1017–1024. [DOI] [PubMed] [Google Scholar]
- 149.De Corte W, Dhondt A, Vanholder R, et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mottes TA, Goldstein SL, Basu RK. Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol. 2019;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rewa OG, Tolwani A, Mottes T, et al. Quality of care and safety measures of acute renal replacement therapy: workgroup statements from the 22nd acute disease quality initiative (ADQI) consensus conference. J Crit Care. 2019;54:52–57. [DOI] [PubMed] [Google Scholar]


