Abstract
Aedes aegypti mosquitoes are the main vectors of many medically relevant arthropod-borne (arbo) viruses, including Zika (ZIKV), dengue (DENV), and yellow fever (YFV). Vector competence studies with Ae. aegypti often involve challenging mosquitoes with an artificial bloodmeal containing virus and later quantifying viral titer or infectious plaque-forming units (PFU) in various mosquito tissues at relevant time points post-infection. However, Ae. aegypti mosquitoes are known to exhibit midgut infection and escape barriers (MIB and MEB, respectively), which influence the prevalence and titer of a disseminated infection and can introduce unwanted variability into studies analyzing tissues such as the salivary glands. To surmount this challenge, we describe herein a protocol for the intrathoracic inoculation of ZIKV in Ae. aegypti. This method bypasses the midgut, which leads to a more rapid and higher proportion of disseminated infections in comparison to oral challenge, and mosquitoes become infected with a consistent dose of virus. Our protocol is advantageous for studies that need a large sample size of infected mosquitoes, need to bypass the midgut, or are analyzing salivary gland infection or escape barriers.
Graphic abstract:
Cartoon depiction of Aedes aegypti intrathoracic inoculation. Figure made with Biorender.com.
Keywords: Aedes aegypti, Mosquito, Zika virus, Arbovirus, Intrathoracic inoculation, Microinjection
Background
Because Aedes aegypti mosquitoes are the main vectors of many medically relevant arthropod-borne (arbo) viruses, understanding the variables that influence the vector’s ability to successfully transmit these viruses will be key in preventing disease. Intrinsic factors governing vector competence are often studied in a laboratory setting, where mosquitoes can be orally infected with arboviruses via an artificial bloodmeal. Artificial membrane feeding involves supplementing defibrinated animal blood with a virus of interest, introducing the blood into a feeding system that is heated to 37°C by a water bath, and allowing the mosquitoes to feed on the blood through a membrane such as parafilm or hog’s gut. This strategy mimics the natural route of infection for a mosquito, albeit without a skin barrier that undoubtedly contains many factors that mediate the infection process. Feeding mosquitoes on infected animals overcomes this disadvantage, but animal models for many arboviruses remain limited ( Reynolds et al., 2017 ) or cannot induce sufficient viremia for infecting mosquitoes.
Not all Ae. aegypti that are fed with virus-containing blood will become infected. Furthermore, not all Ae. aegypti that exhibit midgut infections will be able to transmit the virus to a new host. This is because Ae. aegypti exhibit natural barriers against arbovirus infections, including midgut infection and escape barriers (MIB or MEB, respectively), as well as salivary gland infection and escape barriers (SIB and SEB, respectively) ( Franz et al., 2015 ). Only after a virus has overcome these barriers, disseminated throughout the mosquito, and infected the saliva glands along with saliva can it be transmitted to a new host. The factors that govern these tissue barriers and mediate vector competence continue to be intensely investigated. However, MIB and MEBs can also be seen as barriers against vector competence studies investigating, for example, SIB or SEBs. Indeed, the molecular mechanisms underlying SIB and SEBs are poorly understood in comparison to MIB and MEBs, perhaps because of the difficulty acquiring enough infected salivary glands to perform such studies.
To facilitate our studies of salivary gland infection and escape barriers (Sanchez- Vargas et al., 2021 ), we modified and adapted a protocol originally described in Gubler and Rosen (1976) for the intrathoracic inoculation of ZIKV in Ae. aegypti. This method bypasses the midgut, which leads to a more rapid and higher proportion of disseminated infections in comparison to oral challenge. Mosquitoes also become infected with a consistent dose of virus, as compared to during blood feeding, when the infectious virus titer introduced into the mosquito is governed by the volume of ingested blood. Our protocol is advantageous for studies that need a large sample size of infected mosquitoes, need to bypass the midgut (for example, as a control for MIB studies), require a disseminated infection, or investigate whether a particular virus has the ability to infect a mosquito. This protocol can be adapted and used to intrathoracically inoculate other viruses of interest, including DENV, CHIKV, or even SARS-CoV-2 ( Huang et al., 2020 ) into a wide variety of mosquito vectors.
We do not discuss the generation and quantification of ZIKV stocks in this paper as that has been detailed in Bio-protocol elsewhere ( Freppel et al., 2018 ).
Materials and Reagents
1.5 ml polypropylene Eppendorf tubes (VWR, catalog number: 10025-728)
Pipette tips
3M Permanent double-sided tape ½” (Scotch, catalog number: 34-8507-8235-9)
Plastic Petri dishes (VWR, catalog number: 25389-040)
Glass capillaries, 3.5” (Drummond Scientific Company, catalog number: 3-000-203-G/X)
Glass Petri dishes, 100 × 15 mm (VWR, catalog number: 75845-546)
64 oz. white double poly-coated paper food cup (WebstaurantStore, catalog number: 999SOUP64WB) with pen-sized hole punched out covered with rubber stopper (for mosquito enclosement)
Rubber stopper (VWR, catalog number: 217-0515) (for mosquito enclosement)
30 G × ½” hypodermic needles (BD, catalog number: 305106)
1 ml syringe (BD, catalog number: 309628)
PCR tubes (VWR, catalog number: 20170-010)
Kimwipes (Fisher Scientific, catalog number: 06-666A)
1 oz. translucent plastic souffle cups (WebstaurantStore, catalog number: 301100PC)
2 oz. translucent polystyrene souffle cups (WebstaurantStore, catalog number: 760P200N)
3-4 days old Aedes aegypti female mosquitoes (multiple strains as eggs are available from BEI Resources), see Notes for tips on selecting female versus male mosquitoes
Vero cell (ATCC, catalog number: CCL-81)
Sugar source, such as sugar cubes or raisins
Ice
Ice bucket
Mineral oil (Sigma-Aldrich, catalog number: M5904)
Kendall/Covidien Curity all-purpose sponges, non-sterile, non-woven, 4-Ply, 3" × 3" (7.6 × 7.6 cm) (Model 9023)
White chiffon fabric 8” × 8” (Fabricwholesale.com)
Dulbecco’s Modified Eagle Medium (Corning, catalog number: 10-013-CV) supplemented with 7% heat-inactivated (56°C for 30 min) fetal bovine serum (FBS, Atlas Biologicals, catalog number: F-0500-A), 1% penicillin/streptomycin (Corning, catalog number: 30-001-Cl), 1% glutamine (Corning, catalog number: 25-005-Cl) (DMEM 7% FBS, see Recipes)
Frozen ZIKV (PRVABC59, accession number: KU501215) stock at a concentration of at least 2 × 106-8 × 106 PFU/ml [the minimum concentration for injecting ~100-500 PFU in 69 nl as performed in Williams et al. (2020) and Sanchez-Vargas et al. (2020) ] [see Freppel et al. (2018) for a protocol on quantifying infectious virus titer (see Recipes)]
20% Bleach (Clorox) (see Recipes)
70% Ethanol (see Recipes)
Equipment
Pipettes
Forceps (Dumont, catalog number: 5SF)
Leica GZ4 StereoZoom microscope
Dual Gooseneck Microscope Illuminator (Dolan Jenner Fiber-Lite 180, 181-1 System)
Sutter Instrument Co. Vertical Micropipette Puller (Sutter Instrument, model: P-30)
Nanoject II Auto-Nanoliter Injector (Drummond Scientific Company, catalog number: 3-000-204)
Procedure
-
Prepare intrathoracic inoculation needles and virus injection mix
-
Prepare micropipette needles for injections
Line the diameter of a plastic Petri dish with Scotch double-sided tape. This will be used to store the prepared micropipette needles so that their delicate tips will not be damaged.
Place a single 3.5” glass capillary into the Sutter Instrument Co. Vertical Micropipette Puller.
Set the micropipette puller to heat 999, Pull 003.
Pull needle.
Place the needle on top of the double-sided tape in the previously prepared Petri dish for storage until use.
-
Prepare virus injection mix
Thaw frozen ZIKV stock on ice.
Dilute virus with DMEM 7% FBS in a 200 μl PCR tube. The concentration should be ~2 × 106-8 × 106 PFU/ml with a target injection concentration of 100-500 PFU in 69 nl (the maximum volume for a single injection with the Nanoject II model instrument). See Recipes for relevant calculations.
Keep virus injection mix on ice.
-
Load micropipette with mineral oil
Using forceps, gently break the tip of a pulled needle so that it is wide enough to load injection mix (Figure 1) but not too blunt that it would harm the mosquito. Ideally, the tip size should be 10-30 microns in size. The tip of the needle should be visually inspected under the microscope to ensure it is not overly blunt. Overly blunted needles may cause mosquito mortality after injection.
Fill a 1.5 ml Eppendorf tube with mineral oil.
Using a 30 G × ½” gauge and syringe, backfill the needle by injecting oil into it through the non-pointed side. The Nanoject II will not operate properly without backfilling the needle before attaching it to the injector.
Set the injection volume and speed on the Nanoject II Auto-Nanoliter Injector control panel (Figure 2A) according to manufacturer’s instructions (Figure 2B). The volume and speed are controlled by the positions of the dip switches on the side of the control box. Volumes range from 2.3-69 nl, while speeds are either 23 (slow) or 46 (fast) nl/s. Once the volume has been selected, each time the INJECT button is pressed, the selected volume will be dispensed.
Carefully place a pulled needle through the metal tip of the Nanoject following operating instructions until it stops into place. Give the needle a slight pull to confirm it is securely mounted.
Once the needle is secured, press and hold the EMPTY button until the wire plunger is ejected out ~½” from the end of the needle. Oil that was backfilled will be expelled.
Place the tip of the micropipette into the 1.5 ml Eppendorf tube filled with mineral oil. Be careful not to touch the tip of the needle to the bottom of the tube. Press the FILL button to allow the needle to fill with oil.
-
Load needle with virus injection mix
-
Place the tip of the needle into the virus injection solution.
Approximately 50 μl is the minimum volume for virus preparation in a 200 μl PCR tube. A fully loaded micropipette needle will contain approximately 2.5 μl of virus injection solution. If the injection volume in the PCR tube is too small, the user risks sucking air into the micropipette needle that could generate air bubbles, which could cause inaccurate injection volumes.
Press and hold the FILL button to allow the plunger to retract, drawing in the virus solution. Do not allow air bubbles to form in the needle. These bubbles can cause inaccurate injection volumes. There should be an obvious boundary between the virus injection mix and the mineral oil. The mineral oil layer also prevents the virus from contaminating any equipment.
Load the needle with sufficient virus injection mix to comfortably fill the needle (about halfway up). The amount may depend on one’s injection speed: allowing virus to sit in the needle too long may decrease infectivity as the virus is no longer on ice. The Nanoject II will beep twice when the needle is fully loaded.
Once the needle is loaded, be careful not to damage the tip by touching it to any hard surface. This may blunt the tip. If tip becomes blunted, repeat Steps A3-A4.
-
To ensure that the virus injection mix flows freely from the needle, perform a few test injections onto a Kimwipe (thereafter spray Kimwipe with 70% ethanol solution to eliminate the virus).
Carefully hold the Nanoject II with your dominant hand and gently place the tip of the loaded needle onto the surface of a Kimwipe.
-
Press INJECT. A small pink drop should eject from needle tip. If it doesn’t, click “inject” until virus injection mix flows freely.
Notes:
If virus injection mix does not flow freely after a few test injections, the tip may not be wide enough. If this is the case, eject virus injection mix back into the PCR tube containing the mix. Discard needle in 20% bleach solution and repeat Steps A3 and A4.
If virus injection mix does not flow freely after a few test injections, and the tip is wide enough, the needle may be clogged. If this is the case, discard needle in 20% bleach solution, and repeat Steps A3 and A4. This may occur after intrathoracic inoculation of several mosquitoes.
-
-
-
Perform ZIKV intrathoracic inoculation on Ae. aegypti
-
Prepare mosquitoes for intrathoracic inoculation by anesthetizing them at 4°C
-
Place a glass Petri dish with a lid on ice.
Arrange dual gooseneck microscope illuminator so that the light is centered on the Petri dish.
Adjust stereomicroscope so that the eyepiece is in focus on the Petri dish.
Place a small carton of Ae. aegypti in a 4°C fridge. Do not anesthetize too many at once, depending on one’s injection speed. Leaving mosquitoes on ice for too long (> 30 m) may cause significant mortality.
Once mosquitoes are anesthetized (~5 min), pour them into the inside of the glass Petri placed on ice.
-
Using forceps, arrange a single mosquito on its side so that its target injection point is visible.
Using the microscope, visually inspect the mosquito thorax for the injection point. Upon careful examination, there is a small “V” shaped region of soft tissue that is unprotected from the mosquito cuticle. This is the target injection point. For reference, in Figure 3, a mosquito has been injected with 69 nl purple NuPAGE LDS Sample buffer, and the target injection point is indicated.
-
-
Perform ZIKV intrathoracic inoculation
-
Using your dominant hand, gently place the tip of the loaded needle into the mosquito’s target injection point (Figure 4).
Note: If the needle is properly prepared, the tip should gently slide into target injection point. The needle tip need not be inserted into great depth. If force is required, the mosquito may be damaged, which may cause mortality; if this is the case, repeat Steps A3 and A4.
-
Inject virus injection mix into mosquito target injection point.
Once the needle tip is inserted into target injection point, press INJECT. The mosquito will slowly rise up a little as the injection mix is flowing. Multiple injections (up to 3 injections [= 207 nl total vol.]) can be made by simply pressing INJECT again. Pressing INJECT again before the first injection is complete will not produce a second injection. A beep sound indicates an injection.
-
Place mosquito back into a paper carton.
Place paper carton that acts as a mosquito cage, with a small hole punched out blocked by a rubber stopper, on its side (Figure 5A).
Remove rubber stopper from carton, and very gently wipe the mosquito off the needle at the edge of the carton’s hole using an upward motion (Figure 5B). Replace rubber stopper.
-
-
Leave carton of infected mosquitoes with sugar and water in proper containment
Place sugar source, such as a damp sugar cube or a raisin, on top of the mosquito carton.
Place water source on top of carton. We use a small water-filled plastic cup that is covered with 2 layers of gauze secured around the top by rubber bands.
Place infected mosquito carton in proper containment.
-
Figure 1. Micropipette tip preparation for injection.
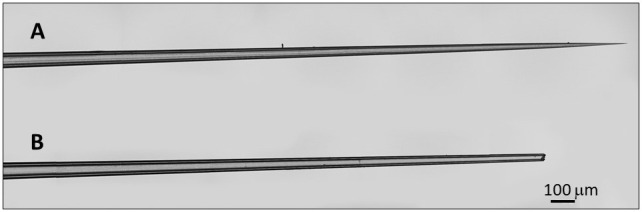
A. Pulled micropipette tip before breaking the tip, unsuitable for intrathoracic inoculation. B. Properly prepared micropipette tip suitable for intrathoracic inoculation.
Figure 2. Nanoject II Auto-Nanoliter Injector control panel settings.

A. Dip switches indicating an injection volume of 50.6 nl and a flow speed of fast. B. Nanoject II instructions for setting injection volume and speed. U = up, D = down.
Figure 3. Ae. aegypti target injection site.
A. female Ae. aegypti mosquito was intrathoracically inoculated with purple NuPage LDS Sample Buffer for visualization purposes. The target injection site, in the context of the mosquito thorax, is denoted by a red arrow.
Figure 4. Intrathoracic inoculation procedure.

A. Mosquitoes are anesthetized on ice while performing intrathoracic inoculation. B. Mosquito will be punctured enough that it becomes stuck to the tip of the needle.
Figure 5. Return infected mosquito into proper containment.
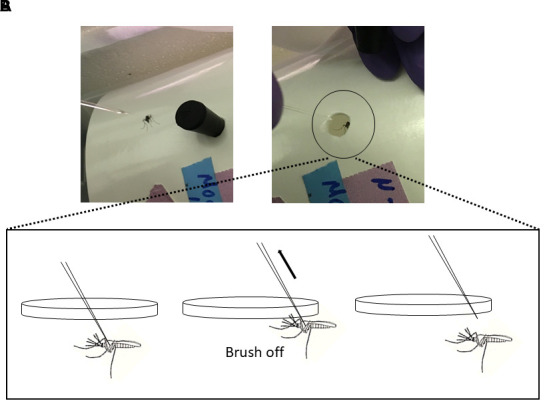
A. A paper carton with a small pen-sized hole serves as a mosquito cage. It is placed on its side. B. The infected mosquito is gently returned into the carton by brushing it off of the needle using an upward motion (depicted in cartoon form). The top of the carton is lined with netting that is secured with a rubber band.
Data analysis
We have found that one hundred percent of Ae. aegypti (Higgs White Eye and Mapastepec strains) that were intrathoracically inoculated with 100-500 PFU ZIKV PRVABC59 become infected at high titers 7-8 days post-injection ( Williams et al., 2020 , Figure 6 left panel labeled “HWE”; Sanchez- Vargas et al., 2020 , Figure 4). All mosquitoes exhibited similar virus titers [4.3 × 105 ± 5.2 × 104 PFU/ml (strain: HWE) and 6.8 × 105 ± 6.5 × 104 PFU/ml (strain: Mapastepec)]. Infection prevalence and infectious virus titers were assessed by Vero cell (ATCC CCL-81) plaque assay. When comparing virus titers between different groups, we use the non-parametric Mann-Whitney U-test for analyzing non-normally distributed data. When comparing the prevalence of infected mosquitoes between different groups, we use a two-tailed Fisher’s exact test. Typically, an n = 30 sample size has sufficient power to reveal any statistical significance.
Figure 6. Female versus male adult Ae. aegypti.
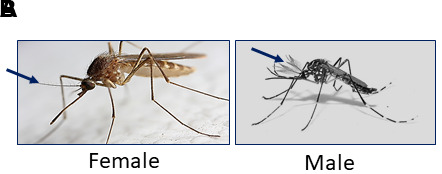
A. Female Ae. aegypti have plain antennae. B. Male Ae. aegypti have feathery antennae and are usually smaller than females. Blue arrows indicate antennae.
Notes
Although both female and male mosquitoes are suitable for inoculation, most studies focus on female mosquitoes because only females transmit arboviruses by an infectious bloodmeal. To differentiate female versus male adult mosquitoes before injection, closely examine their antennae (Figure 6). Male mosquitoes have noticeably “bushy” fine hairs on their antennae and are also smaller than females.
We have found that, given there are no issues with clogging, the same micropipette needle can be used to inoculate approximately 100-120 mosquitoes. A fully loaded micropipette needle will contain approximately 2.5 μl of sample. If the user is injecting 69 nl, approximately 35 mosquitoes can be injected before refilling the needle. However, the same needle can be refilled and reused.
We estimate that 30-100 mosquitoes can be injected per 1 h, depending on one’s skill level and speed.
We advise to check on treated mosquitoes 24-48 h post-injection to record mortality. We have found that mortality is largely dependent on injection technique, but if performed gently, most, if not all, mosquitoes will survive.
We also want to highlight that it should be ensured that the virus injection mix is flowing freely from the injection needle before mosquito intrathoracic inoculation. If the needle tip is not wide enough, the injection mix will not flow. More commonly, the needle tip may become clogged after injecting many mosquitoes, probably due to mosquito debris that may become stuck on the tip. It is sometimes possible to unclog the needle by performing several “pulse” injections to dispel debris. If the needle is still clogged, discard it in 20% bleach, and prepare a new needle for intrathoracic inoculation. If the needle is clogged but intrathoracic inoculation is continued, some mosquitoes will not receive virus, and the resulting data may appear skewed (the mosquitoes that received virus will be highly infected while the ones that did not will be uninfected).
Recipes
-
Dulbecco’s Modified Eagle Medium with 7% heat-inactivated fetal bovine serum (DMEM 7% FBS)
70 ml heat-inactivated fetal bovine serum
Note: FBS can be heat inactivated at 56°C for 30 min.
10 ml 100× Penicillin-Streptomycin/L-Glutamine
920 ml DMEM
-
Injection volume (in nl) for 100 PFU ZIKV for each inoculated mosquito
Divide 100/stock concentration in nl. For example, a virus stock of 8 × 106 PFU/ml is 8 PFU/nl [(8 × 106 PFU/ml)/(1 × 106 nl/ml)]. To obtain 100 PFU/injection, divide 100 PFU/8 PFU/nl to obtain 12.5 nl.
Note, however, that the Nanoject II control panel injection volumes are not adjustable (Figure 2B), and the virus stock in this case would need to be diluted to match an injection volume executed by the Nanoject II. For example, the closest injection volume to 12.5 executed by the Nanoject II is 13.8 nl. The virus injection solution would therefore need to be diluted to obtain 100 PFU/13.8 nl by adding 1.3 nl (13.8 nl-12.5 nl) DMEM 7% FBS media per 12.5 nl stock virus. For example, if starting with 50 μl of 8 × 106 PFU/ml stock (the minimum volume for preparing the injection solution), convert 50 μl to nl by multiplying 50 × 103 = 50,000 nl. Divide 50,000 nl/12.5 nl = 4,000. Then multiply 4,000 × 1.3 nl = 5,200 nl or 5.2 μl additional DMEM 7% FBS media to 50 μl of 8 × 106 PFU/ml stock for a total volume of 55.2 μl.
-
20% Bleach
200 ml Clorox bleach
800 ml tap water
-
70% Ethanol
700 ml Ethanol
300 ml tap water
Acknowledgments
The authors would like to thank Dr. William (Bill) Reid for the photo adapted for use in Figure 3. This research was funded by the NIH, grant number R01 AI130085-02. This protocol was originally described in Williams et al. (2020) and Sanchez-Vargas et al. (2021).
Competing interests
The authors declare no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Franz A. W., Kantor A. M., Passarelli A. L. and Clem R. J.(2015). Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 7(7): 3741-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freppel W., Mazeaud C. and Chatel-Chaix L.(2018). Production, Titration and Imaging of Zika Virus in Mammalian Cells. Bio-protocol 8(24): e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gubler D. J. and Rosen L.(1976). A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am J Trop Med Hyg 25(1): 146-150. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y. S., Vanlandingham D. L., Bilyeu A. N., Sharp H. M., Hettenbach S. M. and Higgs S.(2020). SARS-CoV-2 failure to infect or replicate in mosquitoes: an extreme challenge. Sci Rep 10(1): 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds E. S., Hart C. E., Hermance M. E., Brining D. L. and Thangamani S.(2017). An Overview of Animal Models for Arthropod-Borne Viruses. Comp Med 67(3): 232-241. [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Vargas I., Olson K. E. and Black W. C. 4th (2021). The Genetic Basis for Salivary Gland Barriers to Arboviral Transmission. Insects 12(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams A. E., Sanchez-Vargas I., Reid W. R., Lin J., Franz A. W. E. and Olson K. E.(2020). The Antiviral Small-Interfering RNA Pathway Induces Zika Virus Resistance in Transgenic Aedes aegypti . Viruses 12(11): 1231. [DOI] [PMC free article] [PubMed] [Google Scholar]



