Abstract
Iron-sulfur proteins are ubiquitous among all living organisms and are indispensable for almost all metabolic pathways ranging from photosynthesis, respiration, nitrogen, and carbon dioxide cycles. The iron-sulfur clusters primarily serve as electron acceptors and donors and transfer electrons to active sites of various enzymes, thus driving the energy metabolism. Prokaryotes like E. coli have ISC and SUF pathways that help in the assembly and maturation of iron-sulfur proteins. These iron-sulfur proteins, especially with [4Fe-4S] clusters, are highly sensitive to molecular oxygen, and it would be advantageous if the de novo proteins and native proteins having iron-sulfur binding sites are expressed and isolated under anaerobic conditions. Bacterially assembled iron-sulfur proteins, when isolated and purified anaerobically, exhibit improved biochemical and biophysical stabilities in comparison to the counterparts expressed and purified aerobically and reconstituted under anaerobic conditions. This protocol outlines the expression and purification of the artificial protein, Coiled-Coil Iron-Sulfur (CCIS). It may be deployed to both natural and artificial [4Fe-4S] proteins when heterologously expressed in E. coli.
Keywords: Iron-sulfur proteins, [4Fe-4S] clusters, Artificial proteins, In vivo biogenesis , Anaerobic expression, Anaerobic purification
Background
Iron-sulfur proteins are ubiquitous to all living organisms and mainly function as electron transporters, routing electrons to the active sites of redox-active enzymes, thereby driving metabolic reactions ( Beinert et al., 1997 ; Beinert, 2000; Johnson et al., 2005 ; Cordes and Giese, 2009). Bacteria have several iron-sulfur proteins and enzymes encoded within their genome and have specialized pathways, such as ISC and SUF, which, along with several metallo-chaperons, help in the biological assembly of these clusters within the iron-sulfur proteins (Yoch and Carithers, 1979; Ayala- Castro et al., 2008 ; Xu and Moller, 2011; Roche et al., 2013 ). Utilizing this bacterial machinery for the biological assembly of iron-sulfur clusters would be highly advantageous for the heterologous expression of native and de novo proteins as it would eliminate additional steps of chemical reconstitution post apo-protein purification ( Kuchenreuther et al., 2010 ; Birrell et al., 2016 ; Jagilinki et al., 2020 ). For successful biological assembly of iron-sulfur clusters, soluble iron and sulfur additives should be added prior to induction of the target protein. Importantly, induction should be performed under strictly anaerobic conditions, promoting an anaerobic fermentative environment ideal for supporting iron-sulfur cluster incorporation within the target proteins (Clark, 1989). By optimizing temperature and total time of anaerobic induction, high yields of in vivo assembled holo-proteins can be achieved.
Here, we describe a protocol to express and purify biologically assembled CCIS, an artificial protein designed to bind [4Fe-4S] clusters ( Jagilinki et al., 2020 ). CCIS has been designed as an artificial electron carrier protein that can be used to transfer electrons to the active sites of oxidoreductases. Some details on troubleshooting and optimization are also described, as we expect this may be instructive in extending the protocol to other proteins.
Materials and Reagents
2 L Erlenmeyer flask with cap (Corning, catalog number: CLS431255-6EA)
1.7 ml Eppendorf tubes (VWR, catalog number: 87003-294)
50 ml Falcon tubes (VWR, catalog number: 21008-940)
Petri plates (VWR, catalog number: 25384-342)
Petri dishes (VWR, catalog number: 25384-164)
10 µl Micropipette tips (VWR catalog number: 37001-162)
200 µl Micropipette tips (VWR catalog number: 53503-606)
1 ml Micropipette tips (VWR catalog number: 83007-376)
10 ml Serological pipette (VWR, catalog number: 89130-910)
Tryptone (BD, catalog number: 211705)
Yeast extract (BD, catalog number: 211929)
Glycerol (Sigma-Aldrich, catalog number: G6279)
Potassium phosphate monobasic (KH2PO4) (BDH, catalog number: BDH0268-2.5KG)
Potassium phosphate dibasic (K2HPO4) (Sigma-Aldrich, catalog number: 60356)
Round media bottles with GL45 caps (Schott Duran, catalog number: 218015455)
DH5α Competent Cells (ThermoFisher, catalog number: 18265017), storing temperature -80°C
BL21(DE3) competent cells (ThermoFisher, catalog number: EC0114), storing temperature -80°C
Kanamycin (Sigma-Aldrich, catalog number: K1377)
-
pET-28a (+) vector (Novagen, catalog number: 69864-3), storing temperature -20°C
Note: This product has been discontinued. Use brown powder instead of green powder.
Protease inhibitor cocktail (Roche, catalog number: 11585916001)
Strep resin (IBA Life Sciences, catalog number: 2-1201-010)
Q Sepharose column (GE Healthcare, catalog number: 17-1153-01)
D-Desthiobiotin (Sigma-Aldrich, catalog number: D1411-1G)
Tobacco Etch Virus (TEV) protease (in house enzyme)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S9763)
TRIS-base (J.T. Baker, catalog number: 410901)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: M7506)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C5670)
Ammonium Chloride (NH4Cl) (Sigma-Aldrich, catalog number: A9434)
Trace metal stock solution (Sigma-Aldrich, catalog number: 92949)
BME Vitamins 100x solution (Sigma-Aldrich, catalog number: B6891)
1 M Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: 11411446001)
Iron-57 Metal Isotope (American Elements, catalog number: FE-M-01-ISO.57)
Luria-Bertani broth (LB) (BD, catalog number: 292438)
Agar (BD, catalog number: 214010)
Ammonium ferric citrate ((NH4)5[Fe(C6H4O7)2]) (Sigma-Aldrich, catalog number: 228966-500G)
L-Cysteine hydrochloride hydrate (Sigma-Aldrich, catalog number: C121800-100G)
D-Glucose (VWR, catalog number: 0188-1KG)
Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma-Aldrich, catalog number: 11411446001)
Terrific broth (TB) (see Recipes)
LB medium (see Recipes)
LB Agar plate (see Recipes)
1 M stock of Isopropyl β-D-1-thiogalactopyranoside (IPTG) (see Recipes)
1 M stock of ammonium ferric citrate ((NH4)5[Fe(C6H4O7)2]) (see Recipes)
1 M L-Cysteine hydrochloride hydrate (see Recipes)
20% D-glucose solution (see Recipes)
Buffer A (see Recipes)
Buffer B (see Recipes)
Buffer C (see Recipes)
Buffer D (see Recipes)
Buffer E (see Recipes)
Buffer F (see Recipes)
Buffer G (see Recipes)
Buffer H (see Recipes)
Equipment
Dry bath (Eppendorf, model: Thermomixer R)
Shaker incubator (Thermo Scientific, model: MaxQ6000)
37°C incubator (Benchmark, MyTempMini)
OD600 spectrophotometer (Eppendorf, BioPhotometer)
Sonicator (Vibra-Cell, Sonics)
Sorvall centrifuge (Thermo Scientific, model: Sorvall LYNX 6000)
Laminar hood
Mini-Centrifuge (Eppendorf, Minispin)
Centrifuge (Eppendorf, model: Centrifuge 5425)
pH meter (Hannah, Edge pH meter)
Anaerobic chamber filled with N2/H2 (95%/5%) (Coy lab, Vinyl anaerobic chamber, type A)
Procedure
The following methods describe the native maturation of iron-sulfur clusters within the CCIS protein and helix 1 of CCIS described as ‘h1’ ( Jagilinki et al., 2020 ). These methods can, in general, be used for any natural and artificial proteins to achieve high yields of biologically assembled [4Fe-4S] clusters.
-
Transformation of CCIS/pET-28a (+) in BL21(DE3) competent cells
Note: Perform Steps A1-A8 under aseptic conditions.
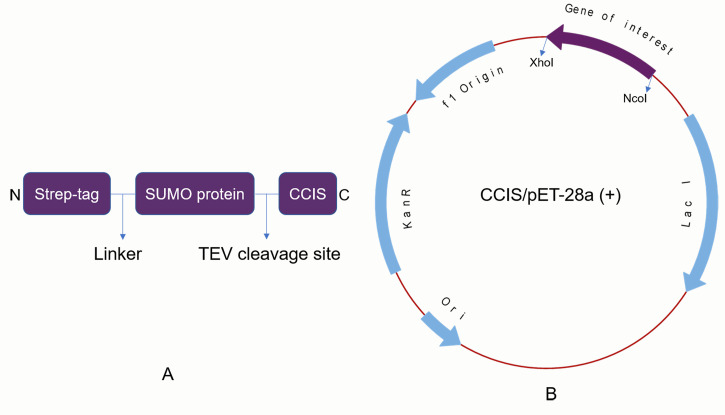
To 20 µl of BL21(DE3) competent cells in a clean Eppendorf tube, add 100 ng of CCIS/pET-28a (+) DNA plasmid and incubate on ice for 40 min. Figure 1A represents the overall layout of the CCIS fusion protein, whereas Figure 1B shows the vector map of the CCIS/pET-28a (+) construct. (weblink to the pET-28a (+) vector map http://wolfson.huji.ac.il/expression/commercial-vectors/pet-28-map.pdf).
During the incubation time, set the dry bath temperature to 42°C.
Following incubation, heat shock the BL21(DE3) competent cells for 90 s in the dry bath at 42°C.
Place the Eppendorf back on ice for 5 min.
Add 800 µl of LB medium to the Eppendorf containing DH5α competent cells inside the laminar hood.
Transfer the competent cell vial to a shaker incubator and incubate at 37°C for 45 min at 200 rpm.
Following incubation, transfer the competent cells inside the laminar hood and add 50 µl of culture onto an LB agar plate containing kanamycin and gently spread the culture using a sterile spreader.
Allow the plate to air dry and incubate the LB agar plate inverted at 37°C for 16 h.
-
Starter culture of BL21(DE3) competent cells harboring CCIS/pET-28a (+)
Note: Perform Steps B1-B3 under aseptic conditions.
Add 10 µl of kanamycin to 10 ml of LB medium in a sterile 50 ml Falcon tube.
Pick an isolated colony from the transformed plate and inoculate into 10 ml of LB broth medium containing kanamycin.
Incubate the culture in a shaker incubator at 37°C overnight.
-
Ideal growth conditions for high yields of in vivo assembled CCIS
Note: Steps C1 to C3 should be performed inside a laminar flow hood.
To 1 L TB medium, add 4 ml of ammonium ferric citrate stock solution (final concentration 4 mM) and add 2 ml of freshly prepared L-Cysteine hydrochloride hydrate (final concentration 2 mM) (see Notes 1 and 2). Similarly, add 25 ml of D-glucose solution (final percentage 0.5%) to the medium (see Note 3).
Add a 1,000-fold dilution of kanamycin (50 mg/ml of stock solution in water) to the culture medium (1 ml in 1 L TB).
Inoculate the 1 L TB medium with a 100-200-fold dilution of overnight grown starter culture (5-10 ml).
Allow the culture to grow in a shaker incubator at 37°C and with 200 rpm agitation until the optical density (OD600) reaches 0.8-2.0, determined using an OD600 spectrophotometer (see Note 4).
Once the desired OD is reached, remove the cap from the culture and transfer the culture flask along with the cap separately inside the anaerobic chamber, and let the culture stay inside the chamber for at least one hour to make the culture anaerobic.
After one hour of incubation inside the chamber, add 2 ml of freshly prepared L-Cysteine hydrochloride hydrate (final concentration 2 mM) and add 500 µl of IPTG solution (0.5 mM) to the culture; then, tighten the GL45 screw cap inside the chamber (see Notes 5 and 6).
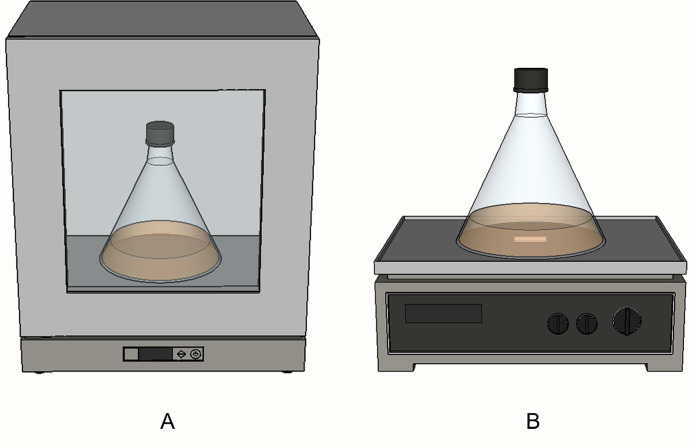
Remove the conical flask containing culture with airtight cap under anaerobic conditions from the anaerobic chamber and allow growth for protein over-expression in a shaker incubator at between 20 and 24°C and 160 rpm of agitation for at least 16 h (Figure 2A).
Alternatively, if inducing at room temperature (~25°C), leave the conical flask inside the anaerobic chamber on a magnetic stirrer at 160 rpm for 16 h with stirrer bar inside the culture (Figure 2B).
After 16 h, transfer the culture inside the anaerobic chamber, pour the culture into the centrifugation bottles, and tighten the cap (see Note 7).
Remove the bottles from the chamber and centrifuge them at 3,075 × g for 15 min at 4°C.
Either freeze the cultures anaerobically or isolate the protein immediately inside the anaerobic chamber.
-
Ideal growth conditions for biologically assembled 57Fe labeled holo-CCIS
Note: Steps D1 to D3 should be performed inside the laminar hood.
To 1 L M9 medium, add 10 mg of 57Fe ferrous sulfate.
Add a 1,000-fold dilution of kanamycin (50 mg/ml of stock solution in water) to the culture medium (1 ml in 1 L M9 medium).
Inoculate the 1 L M9 medium with a 100-200-fold dilution of overnight grown starter culture (5-10 ml).
From step 4 to step 12, refer to steps mentioned in section C. Ideal growth conditions for high yields of in vivo assembled CCIS”.
-
Anaerobic isolation and purification of iron-sulfur proteins
Transfer the bacterial pellet (either fresh or frozen) inside the anaerobic chamber (see Note 8).
Resuspend 1 L bacterial pellet in 20 ml of buffer A additionally containing protease inhibitor cocktail at a 1:1,000 ratio.
Sonicate the culture inside the anaerobic chamber at least for 5 one-minute cycles with intermittent pulses (see Note 9).
Transfer the cell lysate into sorvall tubes and centrifuge at 2,4000 × g for 30 min at 4°C.
Following centrifugation, transfer the sorvall tubes inside the anaerobic chamber and decant the clear supernatant into a clean 50 ml Falcon tube.
Allow the supernatant to flow through (gravity flow) Strep resin, pre-equilibrated with 5 column volumes of buffer A (see Note 10).
Following binding, wash the strep resin with 10 to 20 column volumes of buffer A.
Add TEV protease in 1:200 ratio to perform on-column cleavage. Allow the cleavage for at least 6 h by incubating at room temperature inside the anaerobic chamber. (Weblink of the detailed protocol for the production of ‘in house’ Tobacco Etch Virus (TEV) protease: https://mcl1.ncifcrf.gov/waugh_pubs/64_Waugh.pdf).
The bound tag protein can be stripped off from the strep resin by passing 10 column volumes of buffer B.
Although the strep based affinity chromatography yields high purity of target protein, ion-exchange chromatography is highly recommended to get even better purity as well as more concentrated samples.
For ion-exchange chromatography, dilute the protein sample four times in buffer C so that the final NaCl concentrations are around 50 mM.
Equilibrate the 1 ml Q Sepharose column with 5 ml of buffer D manually using a syringe inside the anaerobic chamber.
Pass the diluted protein through a Q Sepharose column; gradually, the protein starts binding at the top of the column, and a very dark brown color ring appears on the top of the column, indicating the protein is completely bound to the column.
Wash the column with 1 ml of buffer D.
Wash the column with 1 ml of buffer E.
Wash the column with 1 ml of buffer F.
Elute CCIS protein from the column using 1 ml of buffer G.
To regenerate the column, wash the Q Sepharose column with 5 ml of buffer H.
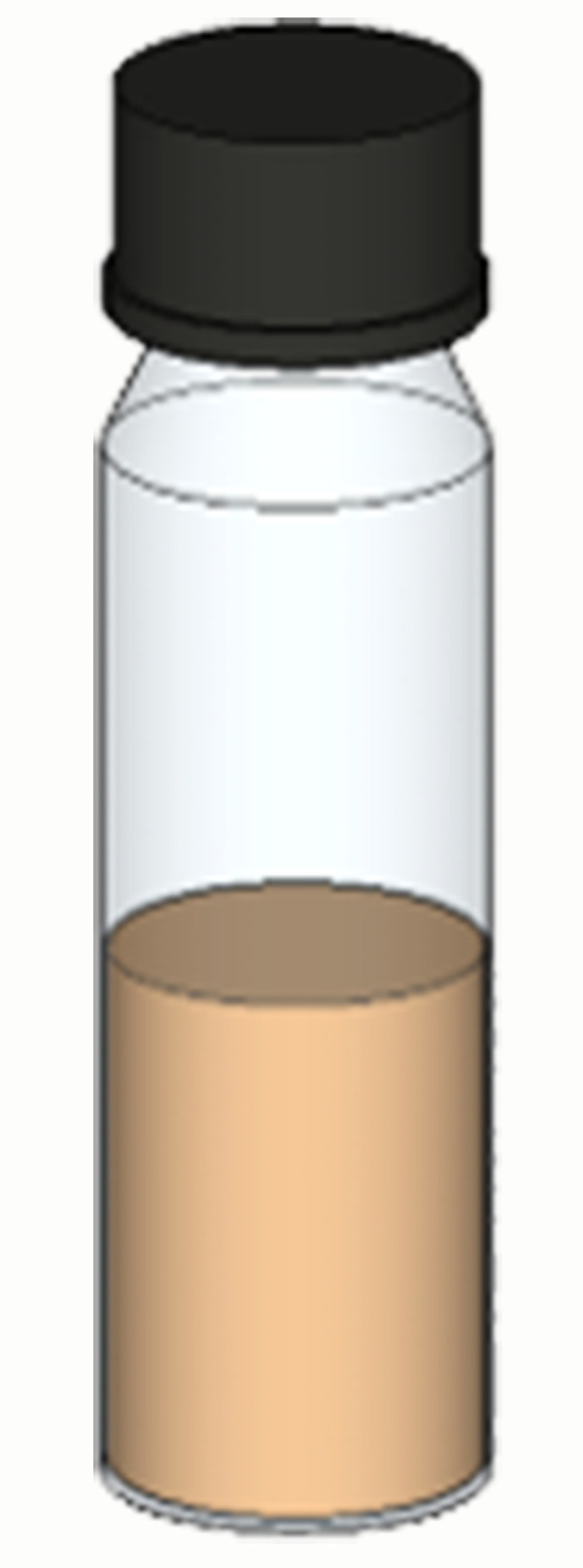
The concentrated holo-CCIS protein should be transferred to an airtight glass vial as shown in Figure 3 and store at 4°C until further use. The strong reddish-brown color of the protein sample is indicative of the presence of iron-sulfur clusters and that the protein can be used in further studies using various biophysical techniques. To determine the purity of the sample, refer to Supplementary Figure 1 (A and B) of Jagilinki et al. (2020) .
Figure 1. CCIS fusion protein layout (A) and vector map of CCIS/pET-28a (+) (B).
The gene of interest is cloned between NcoI and XhoI.
Figure 2. Anaerobic induction of iron-sulfur proteins.
A. Preferred method of anaerobically inducing iron-sulfur proteins inside orbital shaker where both temperature and rotation speed of the shaker can be controlled. B. Alternative method of anaerobically inducing iron-sulfur proteins at room temperature by placing the conical flask on a magnetic stirrer with a stirrer bar inside. This can be performed either inside or outside the anaerobic chamber.
Figure 3. Air-tight glass vial for storing iron-sulfur proteins at 4°C.
Notes
LB medium can be used instead of TB; however, maximum yields of the target protein can be achieved using TB.
L-Cysteine hydrochloride hydrate should be prepared freshly.
Do not autoclave D-glucose solution; only sterile filter it.
Although typical optical density (OD600) values between 0.8 and 1.0 are good, if the solubility of protein is higher, the bacterial culture can be increased to an optical density of 2.0 to achieve extremely high yields of holo-protein.
L-Cysteine is highly sensitive and degrades rapidly; hence it has to be replenished again.
The amount of IPTG must be optimized based on the solubility of the target protein. Higher solubility, higher amounts up to 0.5 mM IPTG can be added. If the solubility of the target protein is reduced in aqueous buffers, add up to 0.1 mM IPTG (i.e., 100 µl of IPTG per 1 L TB medium).
The centrifuge bottles must be transferred inside the anaerobic chamber at least 12 h before usage.
When frozen pellets are being used, allow the bacterial pellet to slowly thaw for at least an hour before resuspending it.
Having a sonicator inside the anaerobic chamber all the time is highly recommended. Otherwise, transfer the sonicator inside the chamber at least 24 h before using it.
Strep tag is highly recommended over other affinity tags for purifying iron-sulfur proteins. Immobilized metal affinity chromatography (IMAC) involving either Ni or Co should be avoided as these metals may interfere with iron-sulfur clusters during purification.
Recipes
-
Terrific broth (TB)
For 1 L medium, add 12 g tryptone, 24 g yeast extract, and 4 ml glycerol in 900 ml double distilled water and allow the solutes to dissolve completely.
A 100 ml salt solution should be prepared by adding 2.312 g of Potassium phosphate monobasic (0.17 M of KH2PO4) and 12.528 g of Potassium phosphate dibasic (0.72 M of K2HPO4).
Autoclave both medium and salt solution in a 2 L conical flask with GL45 screw cap and 500 ml glass bottle, respectively, at 121°C for 20 min at 15 psi.
After autoclaving, when the temperatures of medium and salt solution are under 55°C, add 100 ml salt solution into the 2 L conical flask containing 900 ml medium under sterile conditions inside a laminar hood (see Note 1).
-
LB medium
Add 20 g of LB medium to 1 L water in 2 L conical flask and autoclave.
-
LB Agar plate
To 100 ml of LB medium in a 500 ml conical flask, add 1.25 g of agar and autoclave.
After autoclaving, when the temperature of the LB agar is under 55°C, add 100 µl of kanamycin aseptically inside a laminar hood.
Carefully pour approximately 25 ml of molten LB agar into a clean Petri dish and allow it to solidify.
-
1 M stock of Isopropyl β-D-1-thiogalactopyranoside (IPTG)
Dissolve 2.383 g of IPTG in 10 ml of double distilled water.
-
1 M stock of ammonium ferric citrate ((NH4)5[Fe(C6H4O7)2])
Dissolve 26.5 g of ammonium ferric citrate in 100 ml of double distilled water.
-
1 M L-Cysteine hydrochloride hydrate
Dissolve 0.31524 g of L-Cysteine hydrochloride in 2 ml of double distilled water (see Note 2).
-
20% D-glucose solution
Dissolve 200 g of D-glucose in 1 L of double distilled water and sterile filter the solution into a sterile bottle inside the laminar hood. Keep it refrigerated when not in use (see Note 3).
-
Buffer A
50 mM Tris-HCl pH 8.0
200 mM NaCl
-
Buffer B
50 mM Tris-HCl pH 8.0
200 mM NaCl
2.5 mM D-Desthiobiotin
-
Buffer C
50 mM Tris-HCl pH 8.0
-
Buffer D
50 mM Tris-HCl pH 8.0
50 mM NaCl
-
Buffer E
50 mM Tris-HCl pH 8.0
100 mM NaCl
-
Buffer F
50 mM Tris-HCl pH 8.0
200 mM NaCl
-
Buffer G
50 mM Tris-HCl pH 8.0
300 mM NaCl
-
Buffer H
50 mM Tris-HCl pH 8.0
500 mM NaCl
Acknowledgments
D.N. acknowledges financial support from the Israel Science Foundation petroleum alternatives to transportation grant (GA 2185/17); D.N. and V.N. acknowledge the European Research Council (ERC) consolidator grant (GA615217); D.N., V.N., and B.P.J. were further supported by the NASA Astrobiology Institute Grant 80NSSC18M0093. The anaerobic expression and purification protocol was adapted from previous methods described by Kuchenreuther et al. (2010) and Birrell et al. (2016).
Competing interests
The authors declare no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Ayala-Castro C., Saini A. and Outten F. W.(2008). Fe-S cluster assembly pathways in bacteria. Microbiol Mol Biol Rev 72(1): 110–125., table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beinert H.(2000). Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem 5(1): 2-15. [DOI] [PubMed] [Google Scholar]
- 3.Beinert H., Holm R. H. and Munck E.(1997). Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277(5326): 653-659. [DOI] [PubMed] [Google Scholar]
- 4.Birrell J. A., Laurich C., Reijerse E. J., Ogata H. and Lubitz W.(2016). Importance of Hydrogen Bonding in Fine Tuning the[2Fe-2S] Cluster Redox Potential of HydC from Thermotoga maritima. Biochemistry 55(31): 4344-4355. [DOI] [PubMed] [Google Scholar]
- 5.Clark D. P.(1989). The fermentation pathways of Escherichia coli . FEMS Microbiol Rev 5(3): 223-234. [DOI] [PubMed] [Google Scholar]
- 6.Cordes M. and Giese B.(2009). Electron transfer in peptides and proteins. Chem Soc Rev 38(4): 892-901. [DOI] [PubMed] [Google Scholar]
- 7.Jagilinki B. P., Ilic S., Trncik C., Tyryshkin A. M., Pike D. H., Lubitz W., Bill E., Einsle O., Birrell J. A., Akabayov B., Noy D. and Nanda V.(2020). In Vivo Biogenesis of a De Novo Designed Iron-Sulfur Protein . ACS Synth Biol. [DOI] [PubMed] [Google Scholar]
- 8.Johnson D. C., Dean D. R., Smith A. D. and Johnson M. K.(2005). Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem 74: 247-281. [DOI] [PubMed] [Google Scholar]
- 9.Kuchenreuther J. M., Grady-Smith C. S., Bingham A. S., George S. J., Cramer S. P. and Swartz J. R.(2010). High-yield expression of heterologous[FeFe] hydrogenases in Escherichia coli . PLoS One 5(11): e15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche B., Aussel L., Ezraty B., Mandin P., Py B. and Barras F.(2013). Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim Biophys Acta 1827(3): 455-469. [DOI] [PubMed] [Google Scholar]
- 11.Xu X. M. and Moller S. G.(2011). Iron-sulfur clusters: biogenesis, molecular mechanisms, and their functional significance. Antioxid Redox Signal 15(1): 271-307. [DOI] [PubMed] [Google Scholar]
- 12.Yoch D. C. and Carithers R. P.(1979). Bacterial iron-sulfur proteins. Microbiol Rev 43(3): 384-421. [DOI] [PMC free article] [PubMed] [Google Scholar]