Abstract
The ratio between slower and faster frequencies of brain activity may change after stroke. However, few studies have used quantitative electroencephalography (qEEG) index of ratios between slower and faster frequencies such as the delta/alpha ratio (DAR) and the power ratio index (PRI; delta + theta/alpha + beta) for investigating the difference between the affected and unaffected hemisphere poststroke. Here, we proposed a new perspective for analyzing DAR and PRI within each hemisphere and investigated the motor impairment-related interhemispheric frequency oscillations. Forty-seven poststroke subjects and twelve healthy controls were included in the study. Severity of upper limb motor impairment was classified according to the Fugl–Meyer assessment in mild/moderate (n = 25) and severe (n = 22). The qEEG indexes (PRI and DAR) were computed for each hemisphere (intrahemispheric index) and for both hemispheres (cerebral index). Considering the cerebral index (DAR and PRI), our results showed a slowing in brain activity in poststroke patients when compared to healthy controls. Only the intrahemispheric PRI index was able to find significant interhemispheric differences of frequency oscillations. Despite being unable to detect interhemispheric differences, the DAR index seems to be more sensitive to detect motor impairment-related frequency oscillations. The intrahemispheric PRI index may provide insights into therapeutic approaches for interhemispheric asymmetry after stroke.
1. Introduction
Stroke is one of the leading causes of adult disability worldwide [1]. Following a stroke, an imbalance in the interhemispheric cortical interaction may be established [2]. The ability to perform skilled limb movements requires a dynamic interaction between the hemispheres. Maladaptive functioning of this interhemispheric interaction and consequently changes in hemisphere neural activity after stroke are thought to be factors underlying motor impairments and the poorest motor recovery in these patients [3–5].
The abnormal interhemispheric interaction after a brain injury has been mainly investigated by functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and transcranial magnetic stimulation (TMS) [5, 6]. The quantitative electroencephalography (qEEG) may also be a reliable instrument for detecting alterations of interhemispheric interaction poststroke. The presence of low-frequency oscillations (delta and theta rhythm) in the EEG signal is linked to the decline in neuronal integrity [7, 8], whereas the presence of fast-frequency oscillations (alpha, beta, and gamma rhythm) after stroke is associated with the motor recovery and functional outcome following stroke [7, 9]. Thus, the qEEG index of ratio between slower and faster frequencies such as the delta/alpha ratio (DAR) [10], the power ratio index (PRI; delta + theta/alpha + beta) [11, 12], and the theta/beta ratio (TBR) [13, 14] has been largely employed in stroke studies [10, 13, 15–19].
However, to the best of our knowledge, few studies have used PRI and DAR indexes for investigating the difference between affected and unaffected hemisphere in poststroke subjects. In a recent study, Fanciullacci et al. (2017) did not find any significant interhemispheric difference for DAR in subacute poststroke individuals with cortical-subcortical and subcortical lesions. Moreover, Fanciullacci et al. [18] did not consider motor impairment severity, while recent findings using TMS suggested that cortical activity changes within both hemispheres are strongly associated with motor impairments [20].
Our matched controlled study proposed a new perspective for the analysis of these qEEG indexes within each hemisphere. We hypothesized that ratios between slower and faster frequencies in each hemisphere would be more appropriate to study neural activity changes for considering the interhemispheric differences after stroke. Moreover, we expected that such interhemispheric differences of DAR and PRI after stroke might critically depend on motor impairments.
2. Methods
2.1. Participants
Forty-seven poststroke patients were recruited from local rehabilitation clinics. These patients had mild/moderate (25; 52% males; mean age: 59.6) and severe (22; 45.5% males; mean age: 58.2) motor impairment based on the upper limb section of the Fugl–Meyer assessment [21]. It included patients with upper limb hemiparesis resulting from ischemic or hemorrhagic stroke (>3 months) aged from 18 to 75 years old. We excluded patients with cognitive impairment (<18 in the mini mental state examination (MMSE)) or with any psychiatric disorder. As a control study, self-reported healthy volunteers aged and sex-matched with the patients were also included in the study. This study was reviewed and approved by the local human ethical committee. All patients and healthy controls signed the informed consent before participating in the study. Clinical sociodemographic characteristics were collected. Handedness was self-reported.
2.2. Motor Impairment Severity
The upper limb section of the Fugl–Meyer assessment (UL-FMA) with a maximum score of 66 [21] was applied to evaluate the level of motor impairment. Poststroke patients were classified: with mild/moderate (>19 ± 2 points in UL-FMA) and with severe motor impairment (<19 ± 2 points) according to the classification described by Woodbury et al. [22].
2.3. EEG Data Acquisition and Processing
EEG was recorded for 60 seconds in an isolated room with volunteers with eyes opened and seated in a comfortable armchair, as recommended by the American Clinical Neurophysiology Society Guideline [23, 24]. EEG was recorded using a digital EEG equipment (Neuron-Spectrum/Neurosoft, Russia) with nine Ag/AgCl scalp electrodes placed at F3, C3, P3, Fz, Cz, Pz, F4, C4, and P4 and the reference electrodes at A1 and A2 according to the international 10/20 system at a sampling frequency of 500 Hz. The electrode impedances were kept well below 15 kΩ. The signal was filtered with a bandpass (0.5–100 Hz) and a notch filter of 60 Hz.
Offline signal processing was performed by the toolbox EEGLab in the MATLAB® R2014a for Windows. EEG data file was segmented into 30 data points. The artifact analysis was performed in the continuous data by the independent component analysis (ICA) with the RUNICA algorithm. The ICA may be used to remove artifacts embedded in the data (muscle, eye blinks, or eye movement) without removing the affected data position. Following the identification of the artifacts, the rejection was performed by the Multiple Artifact Rejection Algorithm (MARA), considering a cutoff of 50% [25]. Then, the spectral power density (PSD) was computed for each electrode using the Welch estimator, through an approximation of the edge over the Hanning window [26]. The absolute power was calculated for delta (δ) (0.5 to ≤4 Hz), theta (θ) (>4 to ≤8 Hz), alpha (α) (>8 to ≤13 Hz), beta (β) (>13 to ≤30 Hz), and gamma (γ) (<30 a ≤ 45 Hz) [27]. Moreover, the relative band power was calculated by dividing each band's absolute band power with the total power in 0.5-45 Hz. The relative power at each electrode was averaged to obtain global relative power and this global relative power was used to calculate power ratio index (PRI), delta to alpha ratio (DAR), and theta to beta ratio (TBR). PRI was calculated as PRI = (rδ + rθ)/(rα + rβ) [11]. DAR was calculated as DAR = rδ/rα [10]. TBR was calculated as TBR = rθ/rβ [13].
The qEEG index (PRI and DAR) was computed for each hemisphere (intrahemispheric index) and for both hemispheres (cerebral index). The intrahemispheric index was defined as the mean of the global relative power over electrodes within each hemisphere (left side: F3, C3, and P3; right side: F4, C4, and P4). The cerebral index was defined as the mean of the global relative power over all nine electrodes (F3, C3, P3, Fz, Cz, Pz, F4, C4, and P4).
2.4. Data Analysis
Kolmogorov-Smirnov was performed to assess the data normality. First, the independent sample t-test was used to compare the cerebral index between poststroke patients and healthy volunteers. One-way ANOVA repeated measure analysis for each population separately (poststroke and healthy volunteers) was performed for the intragroup comparison among each intrahemispheric index and cerebral index. For poststroke patients, a two-way ANOVA repeated measure with intrahemispheric/cerebral index (affected hemispheric index, unaffected hemispheric index, and cerebral index) as within factor and motor impairment (mild/moderate and severe) as between factors was performed. When necessary, post hoc analysis was performed using t-test. For multiple comparisons, Bonferroni's correction was applied. Mauchly's sphericity was checked, and Greenhouse-Geisser correction was performed when necessary. For all analysis, the observed power of statistical (η2) analysis was calculated; also, the t-test Cohen's D (d) was reported. Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software 20.0 for Windows, adopting a significance level (p) of 0.05.
3. Results
Fifty-six poststroke patients and fourteen healthy volunteers were assessed. Nine poststroke patients and two healthy volunteers had the data missing for EEG acquisition errors. Thus, forty-seven poststroke and twelve healthy volunteers had the data analyzed. Figure 1 shows the flowchart of the volunteers in the study. Table 1 depicts the demographic and clinical characteristics of the participants.
Figure 1.
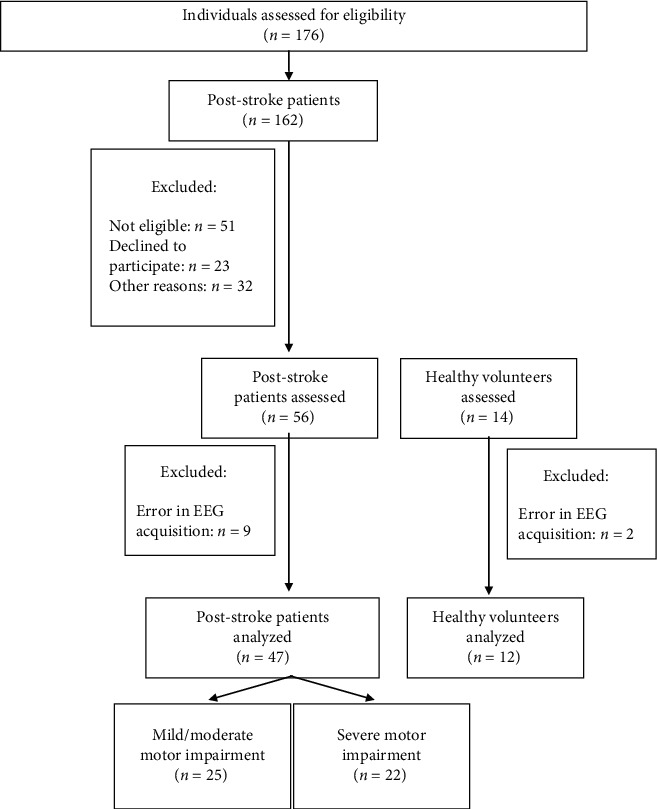
Flowchart of the study.
Table 1.
Demographic and clinical characteristics of participants.
| Poststroke patients | Healthy volunteers (n = 12) |
||
|---|---|---|---|
| Mild/moderate motor impairment (n = 25) |
Severe motor impairment (n = 22) |
||
| Age, mean ± SD (years) | 59.6 ± 8.4 | 58.2 ± 10.0 | 53.3 ± 6.3 |
| Gender (%) | |||
| Male | 52% | 45.5% | 50% |
| Handedness (%) | |||
| Right | 88% | 95.5% | 91.7% |
| UL-FMA, mean ± SD (score) | 38.7 ± 12.5 | 12.2 ± 4.0 | — |
| Hemiparesis (%) | |||
| Left | 60% | 63.6% | — |
| Time since stroke, mean ± SD (months) | 38.3 ± 32.7 | 54.9 ± 40.4 | — |
SD: standard deviation; UL-FMA: upper limb Fugl–Meyer assessment.
3.1. Power Ratio Index
Considering the cerebral index, the independent sample t-test revealed a slowing in brain activity in poststroke patients when compared to healthy volunteers (poststroke: 2.71 ± 0.51; healthy: 2.09 ± 0.71; d = 1.00; t = 3.41; p = 0.001).
The one-way ANOVA showed a significant effect of intrahemispheric/cerebral index for the poststroke patients (F(1.06,48.6) = 10.74; p = 0.02; η2 = 0.91), but not for healthy volunteers (F(1.01, 12.1) = 0.38; p = 0.57; η2 = 0.09). The post hoc analysis revealed an increased intrahemispheric PRI index in the affected hemisphere when compared to the unaffected intrahemispheric (affected hemisphere: 2.75 ± 0.54; unaffected hemisphere: 2.63 ± 0.47; d = 0.23; t = −3.26; p = 0.002) and cerebral index (cerebral index: 2.71 ± 0.51; d = 0.07; t = 2.20; p = 0.03). A decreased intrahemispheric PRI index in the unaffected hemisphere was found when compared to cerebral index (unaffected hemisphere: 2.62 ± 0.47; cerebral index: 2.71 ± 0.51; d = 0.18; t = −3.91; p < 0.001).
The two-way ANOVA showed a significant main effect for intrahemispheric/cerebral index (F(1.06, 47.8) = 12.65; p = 0.001; η2 = 0.95) and for interaction between the two main factors (F(1.06, 47.8) = 5.14; p = 0.026; η2 = 0.62), but not for motor impairment factor (F(1, 45) = 2.72; p = 0.11; η2 = 0.36). In contrast to healthy controls (non-domH: 2.08 ± 0.70; domH: 2.11 ± 0.67; d = 0.04; t = 0.64; p = 0.54) and poststroke patients with mild/moderate motor impairment (mild/moderate-affected hemisphere: 2.60 ± 0.41; mild/moderate-unaffected hemisphere: 2.56 ± 0.41; d = 0.09; t = −1.62; p = 0.12), the post hoc analysis revealed a significant difference between intrahemispheric PRI index of the affected and nonaffected hemisphere in the patients with severe motor impairment (severe-affected hemisphere: 2.91 ± 0.62; severe-unaffected hemisphere: 2.70 ± 0.51; d = 0.36; t = −3.00; p = 0.007). All intrahemispheric PRI index differed to the cerebral index, except for the affected hemisphere of poststroke patients with mild/moderate motor impairment (mild/moderate cerebral index: 2.60 ± 0.41; severe cerebral index: 2.83 ± 0.59; d = 0.45; t = −3.91; p < 0.001). Table 2 shows the intrahemispheric and cerebral power ratio index (PRI) in poststroke patients with upper limb mild/moderate and severe motor impairment and healthy subjects.
Table 2.
Intrahemispheric and cerebral power ratio index (PRI) in poststroke patients with upper limb mild/moderate and severe motor impairment and healthy subjects.
| Intrahemispheric index | Cerebral index | Within-group differences | ||||
|---|---|---|---|---|---|---|
| Affected/non-domH | Unaffected/domH | Affected/non-domH vs. unaffected/domH | Affected/non-domH vs. cerebral index | Affected/domH vs. cerebral index | ||
| Healthy | 2.08 ± 0.70 | 2.11 ± 0.67 | 2.10 ± 0.71 | — | — | — |
| Poststroke patients | 2.75 ± 0.54 | 2.63 ± 0.47 | 2.71 ± 0.51 | t = −3.26; p = 0.002 | t = 2.20; p = 0.03 | t = −3.91; p < 0.001 |
| Mild/moderate | 2.60 ± 0.41 | 2.56 ± 0.41 | 2.60 ± 0.41 | — | — | — |
| Severe | 2.91 ± 0.62 | 2.70 ± 0.53 | 2.83 ± 0.59 | t = −3.00; p = 0.007 | — | — |
non-domH: nondominant hemisphere; domH: dominant hemisphere. Data are mean ± SD. Significant post hoc test values for within-group differences are shown.
3.2. Delta to Alpha Ratio
Similar to cerebral PRI index, the independent sample t-test revealed a statistical slowing in brain activity, as revealed by increased DAR index, in poststroke patients when compared to healthy controls (poststroke: 2.20 ± 0.36; healthy: 1.79 ± 0.50; d = 0.94; t = 3.28; p = 0.02). Table 3 shows the DAR in poststroke and healthy individuals.
Table 3.
Intrahemispheric and cerebral delta to alpha ratio (DAR) in poststroke patients with upper limb mild/moderate and severe motor impairment and healthy subjects.
| Intrahemispheric index | Cerebral index | ||
|---|---|---|---|
| Affected/non-domH | Unaffected/domH | ||
| Healthy | 1.77 ± 0.49 | 1.79 ± 0.48 | 1.79 ± 0.50 |
| Poststroke patients | 2.22 ± 0.32 | 2.20 ± 0.40 | 2.20 ± 0.36 |
| Mild/moderate | 2.06 ± 0.29 | 2.05 ± 0.32 | 2.07 ± 0.31 |
| Severe | 2.41 ± 0.26 | 2.36 ± 0.42 | 2.35 ± 0.36 |
non-domH: nondominant hemisphere; domH: dominant hemisphere. Data are mean ± SD.
The one-way ANOVA showed no significant effect of intrahemispheric/cerebral index for the poststroke patients (F(1.69,77.9) = 0.22; p = 0.80; η2 = 0.08) and for healthy controls (F(1.07,11.8) = 0.19; p = 0.83; η2 = 0.08).
The two-way ANOVA showed a significant effect only for motor impairment factor (F(1, 45) = 13.11; p = 0.01; η2 = 0.94). The post hoc test revealed increased DAR index for poststroke patients with severe motor impairment when compared to the mild/moderate motor impairment for intrahemispheric index (mild/moderate-affected hemisphere: 2.06 ± 0.29; severe-affected hemisphere: 2.41 ± 0.26; d = 1.27; t = −4.30; p < 0.001; mild/moderate-unaffected hemisphere: 2.05 ± 0.32; severe-unaffected hemisphere: 2.36 ± 0.42; d = 0.83; t = −2.90; p = 0.006) and for cerebral index (mild/moderate: 2.07 ± 0.31; severe: 2.35 ± 0.36; d = 0.83; t = −2.87; p = 0.006).
3.3. Theta to Beta Ratio
Even as PRI and DAR, the independent sample t-test revealed an increased TBR index meaning a statistical slowing in brain activity in poststroke patients when compared to healthy controls (poststroke: 5.07 ± 2.18; healthy: 2.09 ± 0.71; d = 1.83; t = 7.85; p < 0.01). Table 4 shows the TBR in poststroke and healthy controls.
Table 4.
Intrahemispheric and cerebral theta to beta ratio (TBR) in poststroke patients with upper limb mild/moderate and severe motor impairment and healthy subjects.
| Intrahemispheric index | Cerebral index | Within-group differences | ||||
|---|---|---|---|---|---|---|
| Affected/non-domH | Unaffected/domH | Affected/non-domH vs. unaffected/domH | Affected/non-domH vs. cerebral index | Affected/domH vs. cerebral index | ||
| Healthy | 2.08 ± 0.70 | 2.11 ± 0.67 | 2.09 ± 0.71 | — | — | — |
| Poststroke patients | 5.39 ± 2.44 | 4.85 ± 2.09 | 5.07 ± 2.18 | t = −2.38; p = 0.02 | t = 2.51; p = 0.01 | t = −2.04; p = 0.04 |
| Mild/moderate | 4.67 ± 1.90 | 4.49 ± 1.78 | 4.54 ± 1.74 | — | — | — |
| Severe | 6.21 ± 2.7 | 5.27 ± 2.37 | 5.66 ± 2.49 | t = −2.35; p = 0.02 | t = 2.33; p = 0.03 | t = −2.17; p = 0.04 |
non-domH: nondominant hemisphere; domH: dominant hemisphere. Data are mean ± SD. Significant post hoc test values for within-group differences are shown.
Similar to the PRI index, the one-way ANOVA showed a statistical difference for intrahemispheric/cerebral index for the poststroke patients (F(1.04,47.99) = 5.60; p = 0.02; η2 = 0.65) and no differences for healthy volunteers (F(1.09,12.08) = 0.38; p = 0.56; η2 = 0.09). The post hoc analysis showed an increased intrahemispheric TBR index in the affected hemisphere when compared to the unaffected intrahemispheric (affected hemisphere: 5.39 ± 2.44; unaffected hemisphere: 4.85 ± 2.09; d = 0.23; t = −2.38; p = 0.02) and cerebral index (cerebral index: 5.07 ± 2.18; d = 0.13; t = 2.51; p = 0.01). In addition, a decreased intrahemispheric TBR index in the unaffected hemisphere was found when compared to cerebral index (d = 0.10; t = −2.04; p = 0.04).
According to the motor impairment analysis, the two-way ANOVA showed a significant main effect for intrahemispheric/cerebral index (F(1.04, 47.07) = 6.32; p = 0.01; η2 = 0.70). However, no interaction was revealed between the two main factors (F(1.04, 47.07) = 2.80; p = 0.09; η2 = 0.38) and for motor impairment factor (F(1, 45) = 3.48; p = 0.06; η2 = 0.44). In addition, the post hoc analysis revealed a significant difference between intrahemispheric TBR index only for the severe motor impairment poststroke patients in the affected and unaffected hemisphere (severe-affected hemisphere: 6.21 ± 2.70; severe-unaffected hemisphere: 5.27 ± 2.37; d = 0.37; t = −2.35; p = 0.02), affected hemisphere and cerebral index (severe cerebral index: 5.66 ± 2.49; d = 0.21; t = 2.33; p = 0.03), and unaffected hemisphere and cerebral index (d = 0.16; t = −2.17; p = 0.04).
4. Discussion
As expected, we found a slowing brain electrical activity of poststroke patients. The presence of low-frequency oscillations (delta and theta) has been associated with brain injuries, as in our poststroke patients [28, 29].
A previous study has investigated the PSD in acute poststroke, showing a higher delta power in poststroke patients when compared to healthy volunteers [10, 30] and in the affected hemisphere of subcortical poststroke patients [18]. The delta oscillations originate in neurons in the thalamus and in deep cortical layers, which may reflect hyperpolarization and inhibition of cortical neurons, resulting in a decrease in neural activity [31]. In addition, it is expected in the poststroke patients, a suppression of high frequency alpha and beta band rhythms [32], since the presence of these rhythms is functionally related to the sensorimotor system, which is activated through motor preparation or execution [33].
In our study, we found an increased intrahemispheric PRI and TBR index in the affected hemisphere when compared to unaffected intrahemispheric and cerebral index and a decreased intrahemispheric PRI and TBR index in the unaffected hemisphere when compared to cerebral index. An increase in these indexes in a specific brain hemisphere supports our hypothesis that the qEEG index for the poststroke patients should be assessed to separate the affected and unaffected hemispheres. PRI seems to be a sensitive index to detect interhemispheric changes in cortical activity.
These findings were in line with previous studies that measure poststroke cerebral and interhemispheric activity by fMRI or TMS [34, 35]. The interhemispheric activity has been used as a surrogate outcome and prognostic factor to predict and monitor stroke progression [15, 34]. In this way, this imbalance could represent the worst prognosis as suggested by Min et al. (2019), which demonstrated slow PSD in the affected hemisphere, while the unaffected hemisphere has a higher PSD.
In contrast to the PRI and TBR indexes, the DAR analysis did not show a difference between the affected and unaffected hemispheres (intrahemispheric DAR index) or in comparison to the cerebral DAR index. Theta band is a potential predictive biomarker for cognitive impairment in patients with cerebral infarcts [36]. The TBR index is also a biomarker of brain processes involved in executive control processes [36], that is, the theta contribution can bring more and valuable information about the poststroke patient. The beta contribution in PRI measure is expected to be increased to determine a better motor recovery outcome (smaller PRI). Indeed, the increase of fast qEEG waves, especially beta rhythm, had been associated with motor recovery in poststroke [7, 9].
Another interesting result is related to the severity of motor impairment. The DAR seems to be a more sensitive index to assessing the severity of the motor impairment in poststroke patients than the PRI index. This trend could be seen for the affected and unaffected hemispheres of the interhemispheric index, as well as for the cerebral index. At the same time, the PRI had detected the difference only at the affected hemisphere. This finding supports the current literature that shows an association between DAR and clinical outcomes [29, 37]. The presence of alpha oscillation indicates neuronal survival [38] and indirectly reflects a decreased DAR. Some studies have demonstrated that the extension of area affected by the infarct is related to motor severity and recovery [39].
Some studies have claimed that an imbalance between interhemispheric connections is related to the severity of the sensorimotor impairment and to the motor recovery post cerebral infarct [2, 6, 20, 37].
Understanding the behavior of the affected and unaffected hemisphere and the severity of motor impairment of the poststroke patients can allow some interventions targeting the individual need, as through neurofeedback by brain-computer interface [14, 19, 40]. With the present study results, we would to suggest a neurofeedback aiming the increase of beta rhythm and decrease of delta for the reduction of the hemispheric PRI in the affected hemisphere.
The main limitation of this study was the limited number of channels in EEG acquisition. We used nine channels that were distributed to capture the cerebral activity of the right and left hemispheres. The extension and lesion location of the affected hemisphere were not controlled. Indeed, evidence suggests that EEG response can vary in patients with stroke depending on lesion [41].
5. Conclusion
The intrahemispheric PRI and TBR indexes are able to find significant interhemispheric differences of frequency oscillations. Despite being unable to detect interhemispheric differences, the DAR index seems to be more sensitive to detect motor impairment-related frequency oscillations. As PRI has the four more usable EEG frequency (delta, theta, alpha, and beta), the intrahemispheric PRI index could provide insights into a precise therapeutic approach through noninvasive brain stimulation or neurofeedback for interhemispheric asymmetry after stroke. These results point out a new perspective of analysis for the cerebral activity in poststroke patients, which could be used as a biomarker of motor recovery and/or as a prognostic measure. Additionally, DAR is the more sensitive index to assessing the severity of motor impairment. This information added to the analysis of each hemisphere's spectral power could guide some interventions as neurofeedback or noninvasive brain stimulations.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001. KMS is supported by CNPq, Grant No. 308291/2015-8.
Data Availability
The data that support the findings of this study are available from the corresponding author (DP) upon reasonable request.
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.WHO. WHO | The Atlas of Heart Disease and Stroke . WHO; 2010. [Google Scholar]
- 2.Nowak D. A., Grefkes C., Ameli M., Fink G. R. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair . 2009;23(7):641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- 3.Min Y. S., Park J. W., Jang K. E., et al. Power spectral density analysis of long-term motor recovery in patients with subacute stroke. Neurorehabilitation and Neural Repair . 2019;33(1):38–46. doi: 10.1177/1545968318818900. [DOI] [PubMed] [Google Scholar]
- 4.van Meer M. P. A., van der Marel K., Wang K., et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. Journal of Neuroscience . 2010;30(11):3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N., Duque J., Mazzocchio R., Cohen L. G. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology . 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 6.Grefkes C., Fink G. R. Connectivity-based approaches in stroke and recovery of function. The Lancet Neurology . 2014;13(2):206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- 7.Thibaut A., Simis M., Battistella L. R., et al. Using brain oscillations and corticospinal excitability to understand and predict post-stroke motor function. Frontiers in Neurology . 2017;8:1–8. doi: 10.3389/fneur.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim B., Winstein C. Can neurological biomarkers of brain impairment be used to predict poststroke motor recovery? A systematic review. Neurorehabilitation and Neural Repair . 2017;31(1):3–24. doi: 10.1177/1545968316662708. [DOI] [PubMed] [Google Scholar]
- 9.Pichiorri F., Petti M., Caschera S., Astolfi L., Cincotti F., Mattia D. An EEG index of sensorimotor interhemispheric coupling after unilateral stroke: clinical and neurophysiological study. European Journal of Neuroscience . 2018;47(2):158–163. doi: 10.1111/ejn.13797. [DOI] [PubMed] [Google Scholar]
- 10.Finnigan S., Wong A., Read S. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clinical Neurophysiology . 2016;127(2):1452–1459. doi: 10.1016/j.clinph.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Nagata K., Tagawa K., Hiroi S., Shishido F., Uemura K. Electroencephalographic correlates of blood flow and oxygen metabolism provided by positron emission tomography in patients with cerebral infarction. Electroencephalography and Clinical Neurophysiology . 1989;72(1):16–30. doi: 10.1016/0013-4694(89)90027-8. [DOI] [PubMed] [Google Scholar]
- 12.Nagata K., Gross C. E., Kindt G. W., Geier M. J., Adey G. R. Topographic electroencephalographic study with power ratio index mapping in patients with malignant brain tumors. Neurosurgery . 1985;17(4):613–619. doi: 10.1227/00006123-198510000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Mane R., Chew E., Phua K. S., Ang K. K., Vinod A. P., Guan C. Quantitative EEG as biomarkers for the monitoring of post-stroke motor recovery in BCI and tDCS rehabilitation. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2018; Honolulu, HI, USA. pp. 3610–3613. [DOI] [PubMed] [Google Scholar]
- 14.Mane R., Chew E., Phua K. S., et al. Prognostic and monitory EEG-biomarkers for BCI upper-limb stroke rehabilitation. IEEE transactions on neural systems and rehabilitation engineering . 2019;27(8):1654–1664. doi: 10.1109/TNSRE.2019.2924742. [DOI] [PubMed] [Google Scholar]
- 15.Bentes C., Peralta A. R., Viana P., et al. Quantitative EEG and functional outcome following acute ischemic stroke. Clinical Neurophysiology . 2018;129(8):1680–1687. doi: 10.1016/j.clinph.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Leon-Carrion J., Martin-Rodriguez J. F., Damas-Lopez J., y Martin J. M. B., Dominguez-Morales M. R. Delta-alpha ratio correlates with level of recovery after neurorehabilitation in patients with acquired brain injury. Clinical Neurophysiology . 2009;120(6):1039–1045. doi: 10.1016/j.clinph.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Saes M., Meskers C. G. M., Daffertshofer A., de Munck J. C., Kwakkel G., van Wegen E. E. H. How does upper extremity Fugl-Meyer motor score relate to resting-state EEG in chronic stroke? A power spectral density analysis. Clinical Neurophysiology . 2019;130(5):856–862. doi: 10.1016/j.clinph.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Fanciullacci C., Bertolucci F., Lamola G., et al. Delta power is higher and more symmetrical in ischemic stroke patients with cortical involvement. Frontiers in Human Neuroscience . 2017;11:1–10. doi: 10.3389/fnhum.2017.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trujillo P., Mastropietro A., Scano A., et al. Quantitative EEG for predicting upper limb motor recovery in chronic stroke robot-assisted rehabilitation. IEEE Transactions on Neural Systems and Rehabilitation Engineering . 2017;27(5):1058–1067. doi: 10.1109/TNSRE.2017.2678161. [DOI] [PubMed] [Google Scholar]
- 20.Veldema J., Bösl K., Nowak D. A. Cortico-spinal excitability and hand motor recovery in stroke: a longitudinal study. Journal of Neurology . 2018;265(5):1071–1078. doi: 10.1007/s00415-018-8802-2. [DOI] [PubMed] [Google Scholar]
- 21.Woytowicz E. J., Rietschel J. C., Goodman R. N., et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Archives of Physical Medicine and Rehabilitation . 2017;98(3):456–462. doi: 10.1016/j.apmr.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodbury M. L., Velozo C. A., Richards L. G., Duncan P. W., Studenski S., Lai S.-M. Longitudinal stability of the Fugl-Meyer assessment of the upper extremity. Archives of Physical Medicine and Rehabilitation . 2008;89(8):1563–1569. doi: 10.1016/j.apmr.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Halford J. J., Sabau D., Drislane F. W., Tsuchida T. N., Sinha S. R. American Clinical Neurophysiology Society guideline 4: recording clinical EEG on digital media. Journal of Clinical Neurophysiology . 2016;33(4):317–319. doi: 10.1097/WNP.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 24.Sinha S. R., Sullivan L., Sabau D., et al. American Clinical Neurophysiology Society guideline 1: minimum technical requirements for performing clinical electroencephalography. Journal of Clinical Neurophysiology . 2016;33(4):303–307. doi: 10.1097/WNP.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 25.Winkler I., Brandl S., Horn F., Waldburger E., Allefeld C., Tangermann M. Robust artifactual independent component classification for BCI practitioners. Journal of Neural Engineering . 2014;11(3, article 035013) doi: 10.1088/1741-2560/11/3/035013. [DOI] [PubMed] [Google Scholar]
- 26.Li Z., Zhang L., Zhang F., Gu R., Peng W., Hu L. Demystifying signal processing techniques to extract resting-state EEG features for psychologists. Brain Science Advances . 2020;6(3):189–209. doi: 10.26599/BSA.2020.9050019. [DOI] [Google Scholar]
- 27.Casson A. J., Abdulaal M., Dulabh M., Kohli S., Krachunov S., Trimble E. Seamless Healthcare Monitoring . Cham: Springer International Publishing; 2018. Electroencephalogram; pp. 45–81. [Google Scholar]
- 28.Freeman W. J., Quiroga R. Q. Imaging brain function with EEG: advanced temporal and spatial analysis of electroencephalographic signals. Imaging Brain Function With EEG: Advanced Temporal and Spatial Analysis of Electroencephalographic Signals . 2013;9781461449:1–248. [Google Scholar]
- 29.Finnigan S., van Putten M. J. A. M. EEG in ischaemic stroke: quantitative EEG can uniquely inform (sub-)acute prognoses and clinical management. Clinical Neurophysiology . 2013;124(1):10–19. doi: 10.1016/j.clinph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Poryazova R., Huber R., Khatami R., et al. Topographic sleep EEG changes in the acute and chronic stage of hemispheric stroke. Journal of Sleep Research . 2015;24(1):54–65. doi: 10.1111/jsr.12208. [DOI] [PubMed] [Google Scholar]
- 31.John E. R., Prichep L. S. The relevance of QEEG to the evaluation of behavioral disorders and pharmacological interventions. Clinical EEG and Neuroscience . 2006;37(2):135–143. doi: 10.1177/155005940603700210. [DOI] [PubMed] [Google Scholar]
- 32.Freeman W. J., Quiroga R. Q. Imaging Brain Function With EEG . New York, NY: Springer New York; 2013. Frequency analysis; pp. 21–36. [Google Scholar]
- 33.Pfurtscheller G., Neuper C. Motor imagery activates primary sensorimotor area in humans. Neuroscience Letters . 1997;239(2–3):65–68. doi: 10.1016/s0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu P., Zeng F., Li Y., et al. Changes of resting cerebral activities in subacute ischemic stroke patients. Neural Regeneration Research . 2015;10(5):760–765. doi: 10.4103/1673-5374.156977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simis M., di Lazzaro V., Kirton A., et al. Étude transversale multicentrique de mesures neurophysiologiques du cortex moteur lese ou non lese dans l'AVC. Neurophysiologie Clinique . 2016;46(1):53–61. doi: 10.1016/j.neucli.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 36.van Son D., De Blasio F. M., Fogarty J. S., Angelidis A., Barry R. J., Putman P. Frontal EEG theta/beta ratio during mind wandering episodes. Biological Psychology . 2019;140:19–27. doi: 10.1016/j.biopsycho.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Finnigan S. P., Walsh M., Rose S. E., Chalk J. B. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clinical Neurophysiology . 2007;118(11):2525–2532. doi: 10.1016/j.clinph.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Alawieh A., Zhao J., Feng W. Factors affecting post-stroke motor recovery: implications on neurotherapy after brain injury. Behavioural Brain Research . 2018;340:94–101. doi: 10.1016/j.bbr.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertolucci F., Chisari C., Fregni F. The potential dual role of transcallosal inhibition in post-stroke motor recovery. Restorative Neurology and Neuroscience . 2018;36(1):83–97. doi: 10.3233/RNN-170778. [DOI] [PubMed] [Google Scholar]
- 40.Kober S. E., Schweiger D., Reichert J. L., Neuper C., Wood G. Upper alpha based neurofeedback training in chronic stroke: brain plasticity processes and cognitive effects. Applied Psychophysiology Biofeedback . 2017;42(1):69–83. doi: 10.1007/s10484-017-9353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park W., Kwon G. H., Kim Y. H., Lee J. H., Kim L. EEG response varies with lesion location in patients with chronic stroke. Journal of Neuro Engineering and Rehabilitation . 2016;13(1):1–10. doi: 10.1186/s12984-016-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (DP) upon reasonable request.


