Abstract
Background
Autologous whole blood or platelet‐rich plasma (PRP) injections are commonly used to treat lateral elbow pain (also known as tennis elbow or lateral epicondylitis or epicondylalgia). Based on animal models and observational studies, these injections may modulate tendon injury healing, but randomised controlled trials have reported inconsistent results regarding benefit for people with lateral elbow pain.
Objectives
To review current evidence on the benefit and safety of autologous whole blood or platelet‐rich plasma (PRP) injection for treatment of people with lateral elbow pain.
Search methods
We searched CENTRAL, MEDLINE, and Embase for published trials, and Clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal for ongoing trials, on 18 September 2020.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs comparing autologous whole blood or PRP injection therapy to another therapy (placebo or active treatment, including non‐pharmacological therapies, and comparison between PRP and autologous blood) for lateral elbow pain. The primary comparison was PRP versus placebo. Major outcomes were pain relief (≥ 30% or ≥ 50%), mean pain, mean function, treatment success, quality of life, withdrawal due to adverse events, and adverse events; the primary time point was three months.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 32 studies with 2337 participants; 56% of participants were female, mean age varied between 36 and 53 years, and mean duration of symptoms ranged from 1 to 22 months. Seven trials had three intervention arms. Ten trials compared autologous blood or PRP injection to placebo injection (primary comparison). Fifteen trials compared autologous blood or PRP injection to glucocorticoid injection. Four studies compared autologous blood to PRP. Two trials compared autologous blood or PRP injection plus tennis elbow strap and exercise versus tennis elbow strap and exercise alone. Two trials compared PRP injection to surgery, and one trial compared PRP injection and dry needling to dry needling alone. Other comparisons include autologous blood versus extracorporeal shock wave therapy; PRP versus arthroscopic surgery; PRP versus laser; and autologous blood versus polidocanol.
Most studies were at risk of selection, performance, and detection biases, mainly due to inadequate allocation concealment and lack of participant blinding.
We found moderate‐certainty evidence (downgraded for bias) to show that autologous blood or PRP injection probably does not provide clinically significant improvement in pain or function compared with placebo injection at three months. Further, low‐certainty evidence (downgraded for bias and imprecision) suggests that PRP may not increase risk for adverse events. We are uncertain whether autologous blood or PRP injection improves treatment success (downgraded for bias, imprecision, and indirectness) or withdrawals due to adverse events (downgraded for bias and twice for imprecision). No studies measured health‐related quality of life, and no studies reported pain relief (> 30% or 50%) at three months.
At three months, mean pain was 3.7 points (0 to 10; 0 is best) with placebo and 0.16 points better (95% confidence interval (CI) 0.60 better to 0.29 worse; 8 studies, 523 participants) with autologous blood or PRP injection, for absolute improvement of 1.6% better (6% better to 3% worse). At three months, mean function was 27.5 points (0 to 100; 0 is best) with placebo and 1.86 points better (95% CI 4.9 better to 1.25 worse; 8 studies, 502 participants) with autologous blood or PRP injection, for absolute benefit of 1.9% (5% better to 1% worse), and treatment success was 121 out of 185 (65%) with placebo versus 125 out of 187 (67%) with autologous blood or PRP injection (risk ratio (RR) 1.00; 95% CI 0.83 to 1.19; 4 studies, 372 participants), for absolute improvement of 0% (11.1% lower to 12.4% higher).
Regarding harm, we found very low‐certainty evidence to suggest that we are uncertain whether withdrawal rates due to adverse events differed. Low‐certainty evidence suggests that autologous blood or PRP injection may not increase adverse events compared with placebo injection. Withdrawal due to adverse events occurred in 3 out of 39 (8%) participants treated with placebo versus 1 out of 41 (2%) treated with autologous blood or PRP injection (RR 0.32, 95% CI 0.03 to 2.92; 1 study), for an absolute difference of 5.2% fewer (7.5% fewer to 14.8% more). Adverse event rates were 35 out of 208 (17%) with placebo versus 41 out of 217 (19%) with autologous blood or PRP injection (RR 1.14, 95% CI 0.76 to 1.72; 5 studies; 425 participants), for an absolute difference of 2.4% more (4% fewer to 12% more).
At six and twelve months, no clinically important benefit for mean pain or function was observed with autologous blood or PRP injection compared with placebo injection.
Authors' conclusions
Data in this review do not support the use of autologous blood or PRP injection for treatment of lateral elbow pain. These injections probably provide little or no clinically important benefit for pain or function (moderate‐certainty evidence), and it is uncertain (very low‐certainty evidence) whether they improve treatment success and pain relief > 50%, or increase withdrawal due to adverse events. Although risk for harm may not be increased compared with placebo injection (low‐certainty evidence), injection therapies cause pain and carry a small risk of infection. With no evidence of benefit, the costs and risks are not justified.
Plain language summary
Autologous blood or PRP injection for lateral elbow pain
Background
Lateral elbow pain, also known as tennis elbow or lateral epicondylitis, is a degenerative (age‐related structural change of tissue) tendon disease at the site where forearm extensor muscles attach to the outer part of the elbow. It is a common cause of elbow pain and disability, typically in middle‐aged people.
Autologous blood and platelet‐rich plasma (PRP) injections have been suggested to improve tendon healing. Autologous blood is derived from the person's own venous blood sample (blood taken from a vein), and PRP is a concentrate of plasma and platelets isolated from autologous blood.
This study aimed to review evidence regarding the benefits and harms of autologous blood or PRP injection for treatment of lateral elbow pain.
Study characteristics
We searched MEDLINE, CENTRAL, Embase, Clinicaltrials.gov, and WHO trials registries, unrestricted by date or language, on 18 September 2020.
We included 32 trials with 2337 participants. Mean age varied between 36 years and 53 years, and mean duration of symptoms ranged from 1 month to 22 months. Of 21 studies that reported gender, 56% of participants were female. Among the included studies, three studies were funded by manufacturers of the PRP centrifugation system; two studies were provided PRP kits; and one study received funding from PRP kit manufacturers.
Key findings
Comparison with placebo at three months revealed the following.
Pain (lower scores mean less pain) (8 studies, 523 participants; moderate‐certainty evidence).
Pain improved by 2% (3% worse to 6% better), or by 0.16 points on a zero to 10 scale.
• People who had placebo rated their pain as 3.7 points.
• People who had autologous blood or PRP injection rated their pain as 3.9 points.
Function (0 to 100; lower scores mean better function or less disability) (8 studies, 502 participants; moderate‐certainty evidence).
Function improved by 2% (5% better to 1% worse), or by 2 points on a zero to 100 scale.
• People who had placebo rated their function as 27 points.
• People who had autologous blood or PRP injection rated their function as 29 points.
Treatment success (4 studies, 372 participants; very low‐certainty evidence).
0% more people rated their treatment a success (11% fewer to 12% more), or zero more people out of 100.
• 65 out of 100 people considered treatment as successful after placebo injection.
• 67 out of 100 people considered treatment as successful after autologous blood or PRP injection.
Health‐related quality of life (higher scores mean better quality of life).
None of the studies measured this outcome.
Pain relief (> 30% or > 50%).
None of the studies reported this outcome at three months.
Withdrawals due to adverse events (1 study, 80 participants; very low‐certainty evidence).
5% fewer people withdrew from the study because of an adverse event (7.5% fewer to 14.8% more), or 5 fewer people out of 100.
• 7 out of 100 people withdrew from the study due to an adverse event after placebo injection.
• 2 out of 100 people withdrew from the study due to an adverse event after autologous blood or PRP injection.
Adverse events (typically transient injection site pain) (5 studies, 425 participants; low‐certainty evidence).
2% more people had adverse events (4% fewer to 11% more), or 2 more people out of 100.
• 17 out of 100 people reported adverse events after placebo injection.
• 19 out of 100 people reported adverse events after autologous blood or PRP injection.
Certainty of the evidence
For people with lateral elbow pain, moderate‐certainty evidence (downgraded for bias, i.e. methodological shortcomings in the included studies) shows that autologous blood or PRP injection probably provides little or no clinically important benefit for pain or function compared with placebo injection, and low‐certainty evidence (downgraded due to risk of bias, i.e. methodological shortcomings; and imprecision, i.e. too few data to estimate the precise difference) suggests that autologous blood or PRP injection may not cause higher risk for adverse events. We are uncertain whether autologous blood or PRP injection is associated with a higher proportion of people reporting treatment success, or if this treatment increases withdrawals due to adverse events.
Summary of findings
Summary of findings 1. Autologous blood or PRP versus placebo at 3 months' follow‐up.
| Autologous blood or PRP versus placebo at 3 months' follow‐up | ||||||
| Patient or population: lateral elbow pain Setting: outpatient Intervention: autologous blood or PRP injection Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with autologous blood or PRP injection | |||||
|
Pain (VAS, PRTEE) translated to 0 to 10, where 0 is no pain Follow‐up: 3 months |
Mean pain in the placebo group was 3.7 pointsa | Mean pain was 0.16 points better (0.60 better to 0.29 worse) | ‐ | 523 participants (8 studies) |
⊕⊕⊕⊝ Moderateb |
PRP probably provides little to no benefit for pain. Absolute benefit 1.6% better (6% better to 3% worse); relative benefit 2.3% better (9% better to 4% worse).c Not clinically significant |
|
Function (PRTEE, DASH, MMCPIE, Roles‐Maudsley), translated to 0 to 100, where 0 is best function, or no disability Follow‐up: 3 months |
Mean function in placebo was 27.5 pointsd | Mean function was 1.86 points better (4.97 better to 1.25 worse) |
‐ | 502 participants (8 studies) | ⊕⊕⊕⊝ Moderated |
PRP probably provides little to no benefit for function. Absolute benefit 1.9% better (5% better to 1% worse); relative benefit 4% (11% better to 3% worse).e Not clinically significant |
|
Treatment success (> 25% improvement in pain or function) Follow‐up: 3 months |
650/1000 | 670/1000 (582 to 765) | RR 1.0 (0.83 to 1.19) | 372 participants (4 studies) |
⊕⊝⊝⊝ Very lowb,e,f |
We are uncertain whether PRP provides better treatment success. Absolute benefit 0% higher (11.1% lower to 12.4% higher); relative benefit 0% higher (17% lower to 19% higher) |
|
Health‐related quality of life Not measured |
See comment | See comment | ‐ | (0 studies) | See comment | Not measured in any of the included studies |
|
Pain relief ≥ 30% or ≥ 50% Not measured at 3 months |
See comment | See comment | ‐ | (0 studies) | See comment | Not reported in any of the included studies at 3 months |
|
Withdrawal due to adverse events |
77/1000 | 24/1000 (2 to 225) | RR 0.32 (0.03 to 2.92) |
80 participants (1 study) | ⊕⊝⊝⊝ Very lowb,g |
We are uncertain whether PRP results in more people withdrawing due to adverse events. Absolute change 5.2% less (7.5% less to 14.8% more); relative change 68% less (97% less to 192% more) |
|
Adverse events (pain and swelling at injection site and limitation of elbow movement following injection) Follow‐up: 12 months |
168/1000 | 192/1000 (128 to 290) | RR 1.14 (0.76 to 1.72) |
425 participants (5 studies) | ⊕⊕⊝⊝ Lowb,f |
PRP may not increase the number of people reporting adverse events. Absolute change 2.4% more (4% less to 12% more); relative change 14% more (24% less to 72% more) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DASH: Disabilities of the Arm, Shoulder and Hand; MMCPIE: Modified Mayo Clinic Performance Index for Elbow; OR: odds ratio; PRP: platelet‐rich plasma; PRTEE: Patient‐Rated Tennis Elbow Evaluation; RR: risk ratio; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence. High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMedian pain value from placebo groups in the included studies (excluding Mishra 2014, which reported percentage improvement).
bDowngraded one level for risk of bias in the included studies.
cRelative changes calculated relative to baseline in control group (i.e. mean difference divided by mean at baseline in the placebo group) (from Montalvan 2015 ‐ value for pain was 7 points on a 0 to 10 scale; for function from Krogh 2013 ‐ value was 47 points on a 0 to 100 scale). Absolute change calculated as mean difference divided by scale of the instrument, expressed as percentage.
dMedian function from placebo groups at 3 months' follow‐up.
eDowngraded one level for indirectness, as none of the studies measured global participant‐reported success directly but measured pain or function improvement cutoff values.
fDowngraded one level for imprecision due to 95% CIs including both effect and no effect.
gDowngraded evidence by two levels because of a small number of events leading to very wide confidence intervals, which overlap relative risk estimates of 0.75 and 1.25.
Background
Description of the condition
Lateral elbow pain is described by many analogous terms in the literature, including tennis elbow, lateral epicondylitis (or epicondylosis), rowing elbow, tennis elbow, lateral epicondylitis, tendonitis of common extensor origin, extensor tendinopathy, and peritendinitis of the elbow. For the purposes of this review, and in keeping with previous Cochrane systematic reviews for this condition, we will use the term 'lateral elbow pain'.
Lateral elbow pain is a common condition that causes pain in the lateral elbow and forearm. It affects 1% to 3% of the general population and up to 15% of workers in at‐risk industries, and is a common sports injury (Hume 2006; Ranney 1995; Walker‐Bone 2004). Men and women appear to be affected equally. The annual incidence in general practice is 4 to 7 per 1000 person‐years, with an incidence of 11 per 1000 person‐years in the 40 to 60‐year age group ‐ the age group most affected (Bot 2005).
Lateral elbow pain is thought to be an overuse injury at the common extensor origin at the lateral epicondyle. Histological studies have identified the presence of angiofibroblastic hyperplasia (fibroblast proliferation, vascular hyperplasia, and disorganised collagen) (Nirschl 1979). Although no histological studies of acute lesions are available, the presence of typical inflammatory symptoms such as night pain and early morning stiffness suggests there may be an early inflammatory component. In spite of the title 'tennis elbow', tennis is a direct cause in only 5% of cases. Other risk factors include repetitive wrist turning and hand gripping. People in strenuous occupations that involve repetitive use are at increased risk.
People with lateral elbow pain typically present with insidious onset of worsening pain and tenderness over the lateral epicondyle. Repetitive movement, lifting, and gripping often aggravate the pain. Examination findings include localised tenderness over the common extensor origin at the lateral epicondyle and elicitation of pain on resisted dorsiflexion of the wrist, middle finger, or both.
Acute lateral elbow pain usually lasts 6 to 12 weeks and often results in work absence (Mallen 2009). For most, it is a self‐limiting condition, but some episodes may persist for up to two years. One study found that 80% of patients with elbow pain already lasting longer than four weeks recovered after one year without any specific treatment (Bisset 2006). Prognostic factors at least moderately associated with poorer outcomes at one year include previous occurrence, high physical strain at work, a manual job, high baseline levels of pain and/or distress, and less social support. Depression and ineffective coping skills have also been found to strongly predict disability (Alizadehkhaiyat 2007). An ultrasound study determined that the presence of a lateral collateral ligament tear or of large (≥ 6 mm) intrasubstance tears was associated with poorer outcomes, but no relationship between tendon thickness or neovascularity and outcomes was seen (Clarke 2010).
Although lateral elbow pain is generally a self‐limiting condition, it results in significant disability, increased healthcare utilisation, lost productivity, and increased costs (Silverstein 2006). Therefore, treatment that shortens the duration of symptoms and disability has the potential to be of significant value in terms of reduced morbidity and costs to both the individual and the community.
Although many treatments are available for lateral elbow pain, the optimal evidence‐based treatment remains unclear. Currently used treatments include topical and oral non‐steroidal anti‐inflammatory drugs (Pattanittum 2013), orthotic devices (Borkholder 2004; Struijs 2002), physiotherapy modalities such as deep friction massage, exercise, and laser and ultrasound therapy (Bisset 2005; Bjordal 2008; Herd 2008; Kohia 2008; Smidt 2003), glucocorticoid injection (Assendelft 1996; Coombes 2010; Smidt 2002b), extracorporeal shock wave therapy (Buchbinder 2005), acupuncture (Green 2002), and surgery (Buchbinder 2011; Lo 2007). Less than 10% of patients with lateral epicondylitis undergo surgery (Nirschl 1979).
Description of the intervention
Autologous whole blood injection involves collection of the patient's blood, which is then injected directly back into the area of tendinopathy. Platelet‐rich plasma (PRP) injection, sometimes referred to as autologous conditioned plasma (ACP), or platelet concentrate, is a treatment by which platelet‐rich centrifuged blood is injected into the affected tendon (Kampa 2010). Autologous conditioned serum (ACS) is another type of autologous blood preparation that can be used. ACS differs from PRP in that it has a higher concentration of anti‐inflammatory cytokines, particularly naturally occurring interleukin‐1 receptor antagonists (IL‐1Ras), rather than platelets (Evans 2016).
No standardised nomenclature or method of preparation has been adopted for autologous blood products. Different classification systems have been proposed for comparison between different PRP preparations. One of the most widely reported is the PAW (Platelets, Activation, White cells) classification system, which is based on (1) absolute numbers of platelets, (2) the manner in which platelet activation occurs, and (3) the presence or absence of white cells in the injectable product (DeLong 2012). More recent classification systems incorporate additional measures, including concentration of red blood cells, the preparation method, and use of imaging‐guided injection (Lana 2017).
Little consensus has been reached on the optimal preparation process for autologous blood products. Centrifugation time and speed can vary, as can the volume of blood extracted and injected back into the body, as well as platelet and white blood cell content (Bennell 2017; Mautner 2015). PRP can be injected into the tendon without further treatment immediately after spinning, or it can be frozen and stored for later use (Kampa 2010). Frozen storage of PRP provides convenience when serial injections are used, but the act of freezing and thawing may have physiological effects on the blood product that alter its efficacy (Bennell 2017). Other modifications of the intervention include the addition of activating factors such as calcium to further enhance the release of cytokines and growth factors (Wehling 2007), or dry needling to cause fresh injury to the tendon.
The procedure is simple to perform, and theoretically at least, adverse effects, such as temporary pain or stiffness following the injection, should be minor (Kampa 2010).
How the intervention might work
Autologous whole blood or PRP injection has been proposed as treatment for chronic non‐healing tendon injuries including lateral epicondylitis. The rationale of action is based upon the hypothesis that platelets would release high concentrations of platelet‐derived growth factors and cytokines to stimulate angiogenesis and healing (Edwards 2003; Engebretsen 2010; Samson 2008; Suresh 2006).
Although platelets have traditionally been thought to be involved exclusively with haemostasis at sites of vascular injury, they are now known to play a role in tissue regeneration and healing through release of an abundant array of cytokines and growth factors such as transforming growth factor‐beta, vascular endothelial growth factor, platelet‐derived growth factor, and epithelial growth factor (Eppley 2004). These growth factors are known to be important in tissue regeneration and healing (Lee 2013). One study showed that injection of autologous blood into rabbit patellar tendons resulted in significantly stronger tendons than with non‐injection, although no histological differences were identified after 12 weeks (Taylor 2002).
Why it is important to do this review
Autologous whole blood and PRP have been used for over 20 years in a variety of surgical situations to reduce blood loss (Carless 2011); recently these modalities have been used to promote wound and bone healing (Griffin 2012; Martinez‐Zapata 2012; Martinez‐Zapata 2013; Samson 2008), as well as to treat chronic tendinopathy (Bell 2013; De Vos 2010). However, few rigorous controlled trials have been reported.
Based on a review of the procedure in 2009, the UK National Institute for Health and Clinical Excellence (NICE) stated that current evidence on the safety and efficacy of autologous blood injection for tendinopathy is inadequate in quantity and quality (NICE 2013). This statement was reiterated in a systematic reviews of the evidence (De Vos 2010; Kampa 2010), and in a 2010 International Olympics Committee consensus paper on use of PRP in sports medicine (Engebretsen 2010).
Several randomised studies have compared autologous blood or PRP injection with various treatments, with conflicting results. These products are used increasingly despite the lack of sound evidence supporting their efficacy and safety. This review is timely in seeking to determine whether further research is needed, and in assessing the value of these therapies for this condition.
Objectives
To review current evidence on the benefit and safety of autologous whole blood or platelet‐rich plasma (PRP) injection for treatment of people with lateral elbow pain.
Methods
Criteria for considering studies for this review
Types of studies
We included studies described as randomised controlled trials (RCTs) and trials describing quasi‐randomised methods of participant allocation. We included studies reported as full text, those published as abstract only, and unpublished data. We used no language or date restrictions.
Types of participants
We included adult participants (> 16 years) with lateral elbow pain as defined by trial authors. These criteria may include clinical features such as pain that is maximal over the lateral epicondyle and pain that is reproduced by tests including palpation of the lateral epicondyle or the common extensor origin of the elbow, gripping, resisted wrist, or second or third finger extension (dorsiflexion), as well as imaging results such as ultrasound or magnetic resonance imaging (MRI) showing the presence of focal hypo‐echoic areas or frank tears or alterations in the normal fibrillary pattern in the common extensor origin. However, studies that did describe particular features of lateral elbow pain were still eligible for inclusion.
In addition, we included participants with tendonitis at other sites, provided lateral elbow pain results were presented separately, or at least 90% of participants in the trial had lateral elbow pain.
We excluded participants with lateral elbow pain due to acute traumatic injury.
Types of interventions
Interventions: autologous whole blood, platelet‐rich plasma (PRP), or other autologous blood products including autologous conditioned serum.
-
Comparators included:
placebo;
no treatment;
exercise and other physical therapy interventions including braces and orthotics;
other injections (including glucocorticoid injection, hyaluronic acid injection, or cell‐based therapies such as stem cell therapy);
surgical interventions;
drug therapy (including analgesics and non‐steroidal anti‐inflammatory drugs); and
supplements and complementary therapies.
Co‐interventions were eligible for inclusion provided they were applied equally in all treatment groups.
Trials that assess the additional benefit of platelet‐rich plasma or other autologous blood products in a surgical procedure compared to surgery alone will be excluded.
Types of outcome measures
There is considerable variation in the outcome measures reported in clinical trials of interventions for pain. However, there is general agreement that outcome measures of greatest importance to patients should be considered, and people with lateral elbow pain typically suffer from pain as suggested by the name of the condition.
The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has published consensus recommendations for determining clinically important changes in outcome measures in clinical trials of interventions for chronic pain (Dworkin 2008). Reductions in pain intensity ≥ 30% and ≥ 50% reflect moderate and substantial clinically important differences, respectively, and it is recommended that the proportion of patients that respond with these degrees of pain relief should be reported.
NICE has recommended that trials of tendinopathy include functional and quality of life outcomes with minimum follow‐up of one year (NICE 2013).
Major outcomes
Participant‐reported pain relief: proportion reporting pain relief of 30% or greater, or 50% or greater
Mean pain or mean change in pain score on a visual analogue scale or a numerical rating scale, or subscore of a total function score, or other measure
Function/disability as measured by disease‐specific disability measures such as the Patient‐Rated Tennis Elbow Evaluation (PRTEE) questionnaire (Rompe 2007), or the upper limb‐specific Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire (Gummesson 2003), or other measure
Participant's perception of overall effect or success, as measured by a global rating of treatment satisfaction such as the Patient Global Impression of Change (PGIC) scale, or of overall treatment success, as defined in the trials (e.g. includes proportion without elbow pain; proportion with 25% pain or disability reduction)
Health‐related quality of life as measured by either generic measures (such as components of Short Form‐36 (SF‐36)) or disease‐specific tools
Proportion of withdrawals due to adverse events
Proportion with any adverse event
Minor outcomes
Other pain measures including proportion achieving pain score below 30/100 mm on a visual analogue scale (VAS); PGIC in pain much or very much improved
Grip strength (preferably pain‐free maximum grip strength)
Proportion with serious adverse events (defined as adverse events that are fatal, are life‐threatening, or require hospitalisation)
Timing of outcome assessment
For the purpose of this review, if multiple time points were reported, we grouped outcomes up to three weeks, greater than three weeks and up to six weeks, over six weeks to three months, over three months to six months, over six months to a year, and more than a year. If trials included outcomes at more than one time point within these time periods, we extracted the latest time point. Adverse event data were extracted at the end of the trials. Our primary time point was over six weeks to three months.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, unrestricted by date or language, on 18 September 2020.
Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid) (Appendix 1).
MEDLINE (Ovid 1946 to present) (Appendix 2).
Embase (Ovid 1947 to present) (Appendix 3).
Clinical trials registers such as ClinicalTrials.gov (http://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/), for ongoing trials (Appendix 4Appendix 5).
Searching other resources
We screened reference lists of retrieved review articles and trials to identify potentially relevant studies.
Data collection and analysis
Selection of studies
Two review authors (TK, SC) independently reviewed the search results to identify trials that appeared to fulfil our inclusion criteria. All articles selected by at least one of the review authors were retrieved for closer examination. Review authors were not blinded to the journal nor the authors. Disagreement about inclusion or exclusion of individual studies was resolved by consensus, or if consensus was not reached, by a third review author (RJ).
Data extraction and management
Two review authors (TK, SC) extracted the following data from the included trials and resolved any differences by consensus.
Trial characteristics including size and location of the trial, and source of funding.
Characteristics of the study population including age and comorbidities.
Characteristics of autologous whole blood or PRP injection therapy such as dose and frequency of injections, schedule of treatment, total number of treatment sessions.
Characteristics of autologous blood product preparation and injection protocols, including a description of the centrifugation protocol (speed and time) and the number of centrifugations, use and type of activating agents, use of frozen or fresh PRP, leukocyte rich or poor, and injection characteristics (such as volume injected, frequency and total number of injections, injection approach, use of local anaesthetic and imaging such as ultrasound).
Characteristics of control interventions.
Risk of bias domains as outlined in Assessment of risk of bias in included studies.
Outcome measures: measurement scale and direction of the scale, mean and standard deviation, number of participants per treatment group for continuous outcomes (such as mean pain, function, quality of life), number of events and number of participants per treatment group for dichotomous outcomes (such as proportion with 30% or greater pain relief, treatment success, withdrawal due to adverse events, adverse events), as outlined in Types of outcome measures.
We noted in the Characteristics of included studies tables whether outcome data were not reported in a form suitable for meta‐analysis, and when missing data were calculated or estimated from a graph or were imputed.
Our a priori decision rules to extract data in the event of multiple outcome reporting in trials are as follows.
When trialists report both final values and change from baseline values for the same outcome, we extracted final values.
When trialists report both unadjusted and adjusted‐for‐baseline values for the same outcome, we extracted adjusted values.
When trialists reported data analysed based on the intention‐to‐treat (ITT) sample and another sample (e.g. per‐protocol, as‐treated), we extracted ITT‐analysed data.
For cross‐over RCTs, we extracted data from the first period only.
When trials did not include a measure of overall pain but included one or more other measures of pain, for the purpose of pooling data, we combined overall pain with other types of pain in the following hierarchy: unspecified pain; pain with activity; daytime pain. For disability, the hierarchy was Patient‐Rated Tennis Elbow Evaluation (PRTEE) questionnaire (Rompe 2007), followed by upper limb‐specific Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire (Gummesson 2003), then other measures. When studies used scales in the opposite direction to PRTEE (0 = worst function), we changed the direction of scores to ensure consistency in interpretation of results.
When multiple time points were reported within our time frames (up to six weeks; over six weeks to three months; over three months to six months; over six months to a year; over one year), we extracted the latest time point (e.g., if data were reported at four weeks, five weeks, three months, and six months, we extracted outcomes at five weeks, three months, and six months).
Assessment of risk of bias in included studies
Two review authors (TK, SC) assessed the risk of bias of each included trial and resolved any disagreements by consensus, and if consensus was not reached, by consultation with a third review author (RJ).
We assessed the following methodological domains, as recommended by Cochrane (Higgins 2017c).
Sequence generation (to determine if the method of generating the randomisation sequence was adequate, such as random‐number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling of cards, and drawing of lots).
Allocation sequence concealment (to determine if adequate methods were used to conceal allocation, such as central randomisation and use of sequentially numbered, sealed, opaque envelopes).
Blinding of participants and personnel.
Blinding of outcome assessors for subjective self‐reported outcomes such as pain and function.
Blinding of outcome assessors for objective outcomes.
Incomplete outcome data.
Selective outcome reporting.
Other potential threats to validity, such as inappropriate analysis in cross‐over trials, baseline imbalance, inappropriate administration of an intervention (or co‐intervention), contamination, inappropriate interim analysis.
Each of these criteria was explicitly judged as having low risk of bias, high risk of bias, or unclear risk of bias (either lack of information or uncertainty over the potential for bias). We considered blinding of objective outcomes separately from blinding of subjective participant‐reported outcomes (e.g. pain, function). We presented figures generated by the risk of bias tool to provide summary assessments of the risk of bias.
Measures of treatment effect
When possible, analyses were based on ITT data (outcomes provided for every randomised participant) from individual trials. For each trial, we presented outcome data as point estimates with mean and standard deviation for continuous outcomes, and as risk ratios (RRs) with corresponding 95% confidence intervals (CIs) for dichotomous outcomes.
For continuous data, results were presented as mean differences (MDs). However, when different scales were used to measure the same outcome or concept, standardised mean differences (SMDs) were used. SMD was re‐expressed as a mean difference on a typical scale (e.g. 0 to 10 for mean pain) by multiplying by a typical among‐person standard deviation (e.g. standard deviation of the control group at baseline from the most representative trial) (Schünemann 2017b). We entered data presented as a scale with a consistent direction of effect across studies.
In the Effects of interventions results section and the 'Comments' column of the 'Summary of findings' table, we provided absolute and relative per cent differences and the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH) (NNTB or NNTH was provided only when the outcome showed a clinically significant difference). For dichotomous outcomes, NNTB or NNTH was calculated from the control group event rate, and relative risk using the Visual Rx NNT calculator (Cates 2008). NNTB or NNTH for continuous measures was calculated using the Wells calculator (available at the Cochrane Musculoskeletal Group (CMSG) Editorial Office) (http://musculoskeletal.cochrane.org/).
For dichotomous outcomes, the absolute per cent change was calculated from the difference in risks between intervention and control groups using GRADEpro (GRADEpro GDT 2015), and was expressed as a percentage. The relative per cent change for dichotomous data was calculated as risk ratio ‐ 1 and was expressed as a percentage. For continuous outcomes, the absolute difference was calculated as the mean difference between intervention and control groups in original measurement units, and was also expressed as a percentage (percentage of the measurement scale); the relative difference was calculated as the absolute change (MD) divided by the baseline mean of the control group from a representative trial. We assumed a minimal clinically important difference (MCID) of 1.5 points on a 10‐point continuous pain scale, and 10 points on a 100‐point scale, for function or disability for input into the calculator (Gummesson 2003).
Unit of analysis issues
When multiple trial arms were reported in a single trial, we included only the relevant arms but reported that there were multiple trial arms in the Characteristics of included studies table. If two comparisons from a three‐arm trial (e.g. PRP regimen 1 versus PRP regimen 2) were combined in the same meta‐analysis, we combined the two treatment groups if both regimens were relevant, and we compared the combined treatment group to the placebo group in the usual way.
If we identified trials that injected both forearms but trialists reported outcomes per participant without accounting for the bilateral correlation, we planned to report results from one arm when possible. If we were unable to obtain the data for a single arm, or to adjust the outcome data, we planned to include data as reported by trialists and to comment on the validity of such analyses, and to assess the effects of including such data by performing sensitivity analyses. For a cross‐over design, we planned to include data only from the first treatment episode.
If two comparisons (e.g. autologous whole blood versus placebo and PRP versus placebo) from one trial were combined in the same meta‐analysis, we halved the placebo group to avoid double‐counting.
Dealing with missing data
When data were missing or incomplete, we sought further information from the study authors.
In cases where individuals were missing from the reported results, we assumed the missing values to have a poor outcome. For dichotomous outcomes that measured adverse events (e.g. number of withdrawals due to adverse events), we calculated the withdrawal rate by using the number of patients who received treatment as the denominator. For dichotomous outcomes that measured benefits (e.g. proportion of subjects with 30% or greater reduction in pain), we calculated the proportion using the number of randomised subjects as the denominator. For continuous outcomes (e.g. pain), we calculated MD or SMD based on the number of patients analysed at the time point. If the number of patients analysed was not presented for each time point, we used the number of randomised patients in each group at baseline.
When possible, we computed missing standard deviations from other statistics such as standard errors, confidence intervals, or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions. If standard deviations could not be calculated, they were imputed (e.g. from other studies in the meta‐analysis) (Higgins 2017a; Higgins 2017b).
Assessment of heterogeneity
We assessed clinical and methodological diversity in terms of participants, interventions, outcomes, and study characteristics for the included studies to determine whether a meta‐analysis would be appropriate. We did this by observing these data from the data extraction tables. We assessed statistical heterogeneity by visually inspecting the forest plot to assess for obvious differences in results between studies, and by using I² and Chi² statistical tests. As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017), interpretation of an I² value of 0% to 40% might 'not be important'; 30% to 60% may represent 'moderate' heterogeneity; 50% to 90% may represent 'substantial' heterogeneity; and 75% to 100% represents 'considerable' heterogeneity. As noted in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), we considered that the importance of I² depends on (1) magnitude and direction of effects and (2) strength of evidence for heterogeneity. The Chi² test with a P value ≤ 0.10 was interpreted as indicating evidence of statistical heterogeneity. If we identified substantial heterogeneity, we reported this and investigated possible causes by following the recommendations provided in Section 9.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Assessment of reporting biases
To determine whether reporting bias was present, we determined whether the protocol of the trial was published before patients were recruited for the study. For studies published after 1 July 2005, we screened the WHO ICTRP search portal, as described in Electronic searches. We checked trial protocols against published reports to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
We planned to create and examine a funnel plot to explore possible small‐study biases and to examine the different possible reasons for funnel plot asymmetry, as outlined in Section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions and to relate this to review results (Sterne 2017). We compared the fixed‐effect estimate against the random‐effects model in the primary analyses to assess the possible presence of small‐sample bias in the published literature (i.e. in which the intervention effect is more beneficial in smaller studies). In the presence of small sample bias, the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate. We planned to undertake formal statistical tests to investigate funnel plot asymmetry when more than 10 studies were included in a single meta‐analysis.
Data synthesis
For studies with similar participant and intervention characteristics and a common comparator, we pooled outcomes in a meta‐analysis using the random‐effects model as a default, and we performed a sensitivity analysis with the fixed‐effect model.
Because all blood products contain similar active biological factors although in different concentrations, it is likely that the mode of action is similar. We therefore elected to combine data in a single comparison, irrespective of whether the trial evaluated autologous blood or PRP. However, we did perform subgroup analyses to compare results for different blood products (as below).
Our main comparison was autologous or PRP versus placebo. Other comparisons included the following.
Autologous blood or PRP injection versus glucocorticoid injection.
PRP and dry needling versus dry needling alone.
PRP versus autologous blood.
Autologous blood or PRP versus extracorporeal shock wave therapy (ESWT).
PRP versus surgery.
Autologous blood plus tennis elbow strap versus exercise and tennis elbow strap.
PRP versus laser.
Autologous blood versus polidicanol injection.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses to assess whether pain and function differ between the following groups at the primary time point of three months.
Participants who receive whole blood compared to those who receive PRP or autologous conditioned serum.
Participants with a lateral collateral ligament tear or a large (≥ 6 mm) intrasubstance tear compared to participants without these tears.
Use of freshly prepared versus frozen autologous blood product.
Use of leukocyte‐rich versus leukocyte‐depleted autologous blood product.
We used the formal test for subgroup interactions in Review Manager (RevMan 2014), and we applied caution in interpreting subgroup analyses, as advised in Section 9.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We compared the magnitude of effects between subgroups by assessing the overlap of CIs of the summary estimate. Non‐overlap of CIs indicated statistical significance.
Sensitivity analysis
We conducted a sensitivity analysis to assess the robustness of treatment effects of pain and function with regard to selection and detection biases, by excluding trials with potential for selection (inadequate or unclear random sequence generation or allocation concealment) and detection (unclear or inadequate participant blinding) bias from the meta‐analysis at the primary time point (three months for placebo; six weeks and six months for glucocorticoid comparisons).
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in a 'Summary of findings' table, which provides key information concerning quality of evidence, magnitude of effect of interventions examined, and the sum of available data on outcomes (proportion reporting pain relief ≥ 30% or ≥ 50%; mean (or mean change in) pain; function; treatment success; health‐related quality of life; withdrawals due to adverse events; proportion of participants with adverse events). The comparison in the 'Summary of findings' table shows autologous blood or PRP injection versus placebo at three months.
Two people (TK, SC) independently used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to meta‐analyses for prespecified outcomes, and reported the quality of evidence as high, moderate, low, or very low. We used methods and recommendations described in Sections 8.5 and 8.7, and Chapters 11 and 12, of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017; Schünemann 2017a; Schünemann 2017b). We used GRADEpro software to prepare the 'Summary of findings' table (GRADEpro GDT 2015).
We justified all decisions to downgrade the quality of studies by using footnotes and made comments to aid the reader's understanding of the review when necessary. We provided absolute per cent difference and relative per cent change from baseline and, for outcomes with statistically significant differences between intervention groups, the number needed to treat for an additional beneficial or harmful outcome (NNTB or NNTH) in the 'Comments' column of the 'Summary of findings' table, as described in the Measures of treatment effect section above.
Interpreting results and reaching conclusions
We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 12) for interpreting results (Schünemann 2017b), and we were aware of distinguishing lack of evidence of effect from lack of effect. We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice; our implications for research suggest priorities for future research and outline remaining uncertainties in the area.
Results
Description of studies
Results of the search
The search, which was conducted up to 18 September 2020, yielded 350 records across databases (MEDLINE = 68; Embase = 111; CENTRAL = 117; Clinicaltrials.gov = 16; WHO ICTRP = 38). One additional record was identified by screening the reference lists of previously published systematic reviews. After duplicates were removed, 210 unique records remained. Of these, we retrieved 75 for full‐text screening on the basis of title and abstract. We deemed 32 trials eligible for inclusion (Arik 2014; Behera 2015; Branson 2016; Creaney 2011; Dojode 2012; Gautam 2015; Gedik 2016; Gosens 2011; Gupta 2019; Jindal 2013; Kazemi 2010; Krogh 2013; Lebiedziński 2015; Lim 2017; Linnanmäki 2020; Martin 2019; Martínez‐Montiel 2015; Merolla 2017; Mishra 2014; Montalvan 2015; Omar 2012; Ozturan 2010; Palacio 2016; Raeissadat 2014; Schoffl 2017; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Watts 2020; Wolf 2011; Yadav 2015; Yerlikaya 2018). Three trials are awaiting classification (see Characteristics of studies awaiting classification table). We identified 24 ongoing trials in clinical trials registries (see Characteristics of ongoing studies table). We excluded 16 studies, 15 of which were not randomised controlled trials and 1 that used the wrong intervention. A flow diagram of the study selection process is presented in Figure 1.
1.
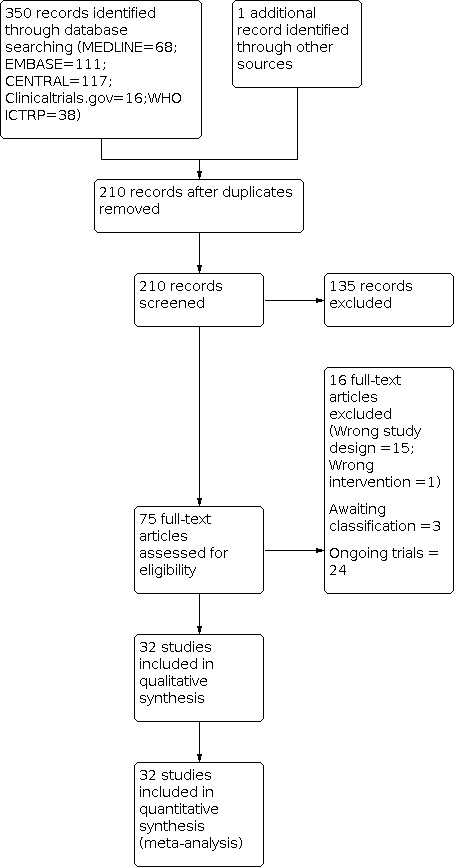
Study flow diagram.
Included studies
We have provided a full description of all included trials in the Characteristics of included studies table. We contacted the authors of five trials to request information about missing data for unreported or partially reported outcomes and received replies from three of them (Creaney 2011; Martin 2019; Martínez‐Montiel 2015).
Study design and setting
Thirty studies were randomised controlled trials (RCTs) and two were quasi‐randomised trials (Jindal 2013; Tetschke 2015). Twenty‐five studies had two intervention arms, and seven had three intervention arms (Branson 2016; Krogh 2013; Linnanmäki 2020; Ozturan 2010; Palacio 2016; Wolf 2011; Yerlikaya 2018).
The included trials were conducted in 19 different countries: Turkey (Arik 2014; Gedik 2016; Ozturan 2010; Yerlikaya 2018), India (Behera 2015; Dojode 2012; Gautam 2015; Gupta 2019; Jindal 2013; Yadav 2015), Australia (Branson 2016), UK (Creaney 2011; Stenhouse 2013; Watts 2020), The Netherlands (Gosens 2011), Iran (Kazemi 2010; Raeissadat 2014), Denmark (Krogh 2013), Finland (Linnanmäki 2020), Poland (Lebiedziński 2015), South Korea (Lim 2017), Spain (Martin 2019), Mexico (Martínez‐Montiel 2015), Italy (Merolla 2017), USA (Mishra 2014; Wolf 2011), France (Montalvan 2015), Egypt (Omar 2012), Brazil (Palacio 2016), Germany (Schoffl 2017; Tetschke 2015), and Greece (Thanasas 2011). The total duration of trials varied between four months and five years. Three studies were funded by manufacturers of the PRP centrifugation system (Gosens 2011; Mishra 2014; Montalvan 2015), two studies were provided with PRP kits (Krogh 2013; Schoffl 2017), one study received funding from PRP kit manufacturers (Watts 2020), and four studies were funded by research grants (Linnanmäki 2020; Martin 2019; Raeissadat 2014; Wolf 2011). The remaining 22 studies did not report a funding source.
Participant characteristics
The 32 trials had randomised 2337 participants to receive autologous blood, PRP, or the control intervention, with numbers ranging between 25 and 230 per trial. The mean age of participants ranged from 36 years to 53 years, and the mean duration of symptoms before study enrolment for the 13 studies that reported it ranged from 1 month to 22 months. Among the 22 studies that reported gender, 56% of participants were female.
Inclusion criteria varied between trials. Seven studies specified a clinical diagnosis of lateral epicondylitis (Arik 2014; Lebiedziński 2015; Linnanmäki 2020; Merolla 2017; Watts 2020; Wolf 2011; Yadav 2015), and 11 studies specified pain on resisted wrist extension as a specific inclusion criterion (Branson 2016; Gosens 2011; Kazemi 2010; Krogh 2013; Martin 2019; Mishra 2014; Omar 2012; Raeissadat 2014; Schoffl 2017; Tetschke 2015; Thanasas 2011). Three studies specified a positive Cozen's test, Maudsley test, and Mill’s manoeuvre (Dojode 2012; Palacio 2016; Yerlikaya 2018), and one study specified a positive Thomsen test (Ozturan 2010). Four studies confirmed the diagnosis of lateral epicondylitis based on ultrasound or MRI (Branson 2016; Krogh 2013; Lim 2017; Stenhouse 2013), and one study excluded other causes of elbow pain using X‐rays (Jindal 2013). Five studies specified an inclusion criterion of recalcitrant lateral epicondylitis, defined as failed conservative treatment (oral medication and physical therapy) for three to six months (Behera 2015; Creaney 2011; Gautam 2015; Gedik 2016; Martínez‐Montiel 2015).
Interventions
A detailed description of the interventions delivered in each trial is summarised in the Characteristics of included studies table. Of the 32 included trials, seven had three intervention arms (Branson 2016; Krogh 2013; Linnanmäki 2020; Ozturan 2010; Palacio 2016; Wolf 2011; Yerlikaya 2018).
Nine trials compared PRP injection to placebo injection (Behera 2015; Krogh 2013; Linnanmäki 2020; Martin 2019; Mishra 2014; Montalvan 2015; Palacio 2016; Schoffl 2017; Yerlikaya 2018), and two trials (out of which one ‐ Linnanmäki 2020 ‐ had three arms comparing autologous blood to PRP to saline) compared autologous blood injection to placebo injection (Linnanmäki 2020; Wolf 2011). Fifteen trials compared PRP to glucocorticoid injection (Arik 2014; Branson 2016; Dojode 2012; Gautam 2015; Gosens 2011; Gupta 2019; Kazemi 2010; Krogh 2013; Lebiedziński 2015; Martínez‐Montiel 2015; Omar 2012; Ozturan 2010; Palacio 2016; Wolf 2011; Yadav 2015); six trials compared autologous blood to glucocorticoid injection (Arik 2014; Branson 2016; Dojode 2012; Kazemi 2010; Ozturan 2010; Wolf 2011); and nine trials compared PRP to glucocorticoid injection (Gautam 2015; Gosens 2011; Gupta 2019; Krogh 2013; Lebiedziński 2015; Martínez‐Montiel 2015; Omar 2012; Palacio 2016; Yadav 2015). Four trials compared PRP to autologous blood injection (Creaney 2011; Linnanmäki 2020; Raeissadat 2014; Thanasas 2011), and one trial compared PRP injection and dry needling to dry needling alone (Stenhouse 2013). Two trials compared autologous blood or PRP injection plus tennis elbow strap and exercise versus tennis elbow strap and exercise alone (Gedik 2016; Lim 2017). One trial compared PRP injection to extracorporeal shock wave therapy (ESWT) (Ozturan 2010); two trials compared PRP injection to surgery (Merolla 2017; Watts 2020). One trial compared PRP injection to laser application (Tetschke 2015), and one trial compared autologous blood injection to polidocanol injection (Branson 2016).
Sixteen studies used a peppering technique (multiple passes to the tendon) to incite fresh tendon injury during injection (Behera 2015; Branson 2016; Gautam 2015; Gosens 2011; Gupta 2019; Krogh 2013; Martin 2019; Mishra 2014; Montalvan 2015; Raeissadat 2014; Schoffl 2017; Stenhouse 2013; Thanasas 2011; Watts 2020; Wolf 2011; Yerlikaya 2018). Omar 2012 did not describe the injection, and remaining trialists described injection without mentioning multiple passes of the needle.
Most participants received one injection. In five studies (Branson 2016; Martin 2019; Montalvan 2015; Stenhouse 2013; Tetschke 2015), participants were given two injections, and in one study (Ozturan 2010), those who did not improve with one injection were given a second injection.
Outcomes
An ORBIT matrix that shows outcomes measured and level of reporting for each outcome in each trial (rated as fully reported, partially reported, measured but not reported, unclear if measured, or not measured) is presented in Table 2.
1. Outcome reporting bias In trials (ORBIT) matrix.
| Study ID | Participant‐reported pain relief ≥ 30% | Pain | Function or disability | Treatment success | Health‐related quality of life | Withdrawal due to adverse events | Adverse events |
| Arik 2014 | ? | Full | Full | Full | ? | ? | Full |
| Behera 2015 | ? | Full | Full | ? | ? | Full | Full |
| Branson 2016 | Not measured | Not measured | Full | Full | Not measured | ? | Full |
| Creaney 2011 | ? | ? | Full | Full | ? | Full | ? |
| Dojode 2012 | ? | Full | ? | Full | ? | Full | Full |
| Gautam 2015 | ? | Partial | Partial | ? | ? | Full | ? |
| Gedik 2016 | ? | ? | Full | Full | ? | Full | ? |
| Gosens 2011 | Not measured | Full | Full | Full | Not measured | Full | Full |
| Gupta 2019 | ? | Full | Full | Full | ? | ? | Full |
| Jindal 2013 | ? | Full | ? | Full | ? | Full | ? |
| Kazemi 2010 | Not measured | Full | Full | Not measured | Not measured | Full | Full |
| Krogh 2013 | Not measured | Full | Full | Not measured | Not measured | Full | Full |
| Lebiedziński 2015 | ? | ? | Full | Full | ? | Full | Full |
| Lim 2017 | ? | Partial | Partial | Full | ? | Full | Full |
| Linnanmäki 2020 | Not measured | Full | Full | Not measured | Not measured | Not measured | Full |
| Martin 2019 | Not measured | Full | Full | Full | Not measured | Full | Full |
| Martínez‐Montiel 2015 | ? | Full | Full | ? | ? | Full | ? |
| Merolla 2017 | ? | Partial | Partial | Measured | ? | Full | ? |
| Mishra 2014 | Full | Partial | Partial | Full | ? | Full | Full |
| Montalvan 2015 | Not measured | Full | Full | Not measured | Not measured | Full | Full |
| Omar 2012 | ? | Full | Full | ? | ? | Full | ? |
| Ozturan 2010 | Full | Full | Full | ? | ? | Full | Full |
| Palacio 2016 | ? | ? | Full | Full | ? | ? | ? |
| Raeissadat 2014 | ? | Full | Full | Full | ? | Full | ? |
| Schoffl 2017 | ? | ? | Full | ? | ? | ? | ? |
| Stenhouse 2013 | ? | Full | Full | Full | ? | Full | Full |
| Tetschke 2015 | ? | Full | Full | Full | ? | Full | ? |
| Thanasas 2011 | ? | Full | Full | ? | ? | Full | Full |
| Wolf 2011 | ? | Full | Full | ? | ? | Full | ? |
| Watts 2020 | not measured | Full | Full | Not measured | Not measured | Not measured | Full |
| Yadav 2015 | ? | Partial | Partial | ? | ? | Full | ? |
| Yerlikaya 2018 | ? | Full | Measured | ? | ? | Full | Measured |
'Full': sufficient data for inclusion in a meta‐analysis were reported (e.g. mean, standard deviation, sample size per group for continuous outcomes). 'Partial': insufficient data for inclusion in a meta‐analysis were reported (e.g. means only, with no measures of variance). 'Measured': outcome was measured but no outcome data were reported. 'Not measured': outcome was not measured by trialists. '?': unclear whether the outcome was measured or not (as a trial protocol was unavailable).
Major outcomes
Participant‐reported pain relief 30% or greater, or 50% or greater
None of the trials reported pain relief > 30% (pre‐planned cutoff), but two trials reported pain relief > 50% (Mishra 2014; Ozturan 2010).
Pain
Twenty‐four trials measured overall pain (mean or mean change) using a 0 to 10‐point visual analogue scale (VAS), with 10 indicating worst pain, and two trials measured pain with the PRTEE pain subscale (Krogh 2013; Watts 2020). Six trials did not report measures of variance or did not clearly report them (Gautam 2015; Gupta 2019; Lim 2017; Merolla 2017; Mishra 2014; Yadav 2015). One trial did not measure pain (Branson 2016). It is unclear whether the five remaining trials that did not report pain measured pain or not, as these trials were not registered and no study protocols were found (Creaney 2011; Gedik 2016; Lebiedziński 2015; Palacio 2016; Schoffl 2017).
Function
Twenty‐nine trials measured function, six of which did not clearly report measures of variance (Gautam 2015; Gupta 2019; Lim 2017; Merolla 2017; Mishra 2014; Yadav 2015). One trial measured function but did not report the results (Yerlikaya 2018). Two trials did not measure function (Dojode 2012; Jindal 2013). Most trials used either the PRTEE questionnaire ‐ Arik 2014; Branson 2016; Creaney 2011; Gedik 2016; Krogh 2013; Merolla 2017; Mishra 2014; Palacio 2016; Watts 2020 ‐ or the DASH questionnaire ‐ Gautam 2015; Gosens 2011; Gupta 2019; Kazemi 2010; Lebiedziński 2015; Linnanmäki 2020; Martin 2019; Omar 2012; Schoffl 2017; Tetschke 2015; Wolf 2011; Yadav 2015. Three trials measured function using the Modified Mayo Clinic Performance Index for Elbow (MMCPIE) (Behera 2015; Lim 2017; Raeissadat 2014); one used the quick DASH (Martínez‐Montiel 2015); one used the Roles‐Maudsley score (Montalvan 2015); and one used upper extremity functional score to measure elbow function (Ozturan 2010). In one trial, elbow function was measured by the Nirschl staging system (Stenhouse 2013), and another trial used the Liverpool elbow score to measure elbow function (Thanasas 2011).
Treatment success
Eighteen trials reported some kind of assessment of treatment success, most of which measured proportion with 25% pain or disability reduction; one trial measured treatment success on the Global Rating of Change (GROC) (Branson 2016), and another trial included patient satisfaction with treatment results along with pain reduction (Gedik 2016). Three trials did not measure treatment success (Kazemi 2010; Krogh 2013; Montalvan 2015); it is unclear whether 11 trials measured treatment success or not, as there was no study protocol (Behera 2015; Gautam 2015; Martínez‐Montiel 2015; Omar 2012; Ozturan 2010; Schoffl 2017; Thanasas 2011; Watts 2020; Wolf 2011; Yadav 2015; Yerlikaya 2018).
Health‐related quality of life
None of the included studies measured or reported this outcome.
Withdrawal due to adverse events
Only two trials reported withdrawal due to adverse events (Martin 2019; Stenhouse 2013).
Adverse events
Eighteen trials reported adverse events, one trial measured but did not report adverse events (Yerlikaya 2018), and in 13 trials it is unclear whether or not adverse events were measured (Creaney 2011; Gautam 2015; Gedik 2016; Jindal 2013; Martínez‐Montiel 2015; Merolla 2017; Omar 2012; Palacio 2016; Raeissadat 2014; Schoffl 2017; Tetschke 2015; Wolf 2011; Yadav 2015).
Minor outcomes
None of the studies reported other pain measures or serious adverse events.
Nine trials reported mean grip strength (Arik 2014; Gautam 2015; Gedik 2016; Gupta 2019; Kazemi 2010; Linnanmäki 2020; Ozturan 2010; Merolla 2017; Yadav 2015).
Excluded studies
We excluded 16 full‐text articles; 15 were not RCTs, and one had only one participant who received different treatments in both arms. Full details can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
A summary of the risk of bias of included studies can be seen in Figure 2, and details are provided in the Characteristics of included studies table. All included trials were susceptible to bias. Overall, 21 (66%) trials were susceptible to selection bias, 20 (62%) were at risk of performance bias, 20 (62%) were at risk of detection bias, seven (22%) were at risk of attrition bias, 25 (78%) were at risk of selective reporting bias, and five (16%) were at risk of other potential bias (Figure 3).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only 11 (34%) trials used appropriate methods to both generate and conceal their allocation sequence, and so we rated these at low risk of selection bias (Branson 2016; Gosens 2011; Gupta 2019; Krogh 2013; Lebiedziński 2015; Linnanmäki 2020; Martin 2019; Martínez‐Montiel 2015; Schoffl 2017; Watts 2020; Wolf 2011).
Eight (25%) trials did not clearly report their method of sequence generation (Arik 2014; Behera 2015; Creaney 2011; Gautam 2015; Omar 2012; Ozturan 2010; Palacio 2016; Yadav 2015), and 17 (53%) trials did not adequately report their method of allocation concealment (Arik 2014; Behera 2015; Creaney 2011; Dojode 2012; Gautam 2015; Gedik 2016; Lim 2017; Merolla 2017; Mishra 2014; Montalvan 2015; Omar 2012; Ozturan 2010; Raeissadat 2014; Stenhouse 2013; Thanasas 2011; Yadav 2015; Yerlikaya 2018). Therefore, the risk of selection bias in these trials was unclear. We judged three trials as having high risk of bias (Jindal 2013; Kazemi 2010; Tetschke 2015), as two were quasi‐randomised (Jindal 2013; Tetschke 2015), and one used a coin‐toss method of randomisation for only the first participant and sequential allocation for the rest of the sample (Kazemi 2010).
Blinding
We judged 11 (34%) trials to be at low risk of performance and detection bias because both participants and study personnel were successfully blinded (Creaney 2011; Gosens 2011; Krogh 2013; Linnanmäki 2020; Martin 2019; Martínez‐Montiel 2015; Mishra 2014; Montalvan 2015; Schoffl 2017; Wolf 2011; Yerlikaya 2018). Of these, seven were placebo‐controlled trials (Krogh 2013; Linnanmäki 2020; Martin 2019; Mishra 2014; Montalvan 2015; Wolf 2011; Yerlikaya 2018), one trial compared autologous blood to PRP (Creaney 2011), two trials compared PRP to glucocorticoid injection (Gosens 2011; Martínez‐Montiel 2015), and one trial compared PRP to dry needling (Schoffl 2017).
We judged 14 (43%) trials to be at risk of high performance and detection bias (Arik 2014; Gautam 2015; Gupta 2019; Jindal 2013; Kazemi 2010; Lebiedziński 2015; Lim 2017; Merolla 2017; Ozturan 2010; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Watts 2020; Yadav 2015). Four trials did not blind participants and study personnel, leading to high risk of bias in the assessment of both subjective and objective outcomes (Arik 2014; Gupta 2019; Ozturan 2010; Watts 2020). Seven trials had high risk of performance and detection bias for subjective outcomes only and measured no objective outcomes (Dojode 2012; Jindal 2013; Kazemi 2010; Lebiedziński 2015; Stenhouse 2013; Tetschke 2015; Thanasas 2011). One trial had high risk of performance bias and detection bias for subjective outcomes and low risk of detection bias for objective outcomes, as assessors were blinded (Lim 2017). Three trials had high risk of performance bias and detection bias for subjective outcomes and unclear risk of detection bias for objective outcomes, as it is unclear whether or not assessors were blinded (Gautam 2015; Merolla 2017; Yadav 2015).
We judged one trial to be at unclear risk of both performance and detection bias for subjective and objective outcomes (Omar 2012). Two trials had unclear risk of performance and detection bias for subjective outcomes and low risk of bias for objective outcomes, as no assessor‐reported outcomes were measured in this study (Behera 2015; Palacio 2016). In Omar 2012, study authors did not report whether participants and study personnel were blinded to treatment allocation, so we judged risk of performance and detection bias as unclear. We judged one trial to be at unclear risk of performance and detection bias for objective outcomes and at high risk of detection bias for subjective outcomes, as participants were unable to be blinded due to the nature of the intervention (injections compared to bandage and exercise) (Gedik 2016). Branson 2016 had low risk of performance bias and unclear risk of detection bias for both subjective and objective outcomes, as study personnel and participants were blinded for the first injection; however investigators do not report whether they were blinded for the second injection.
Incomplete outcome data
We judged 25 (78%) trials to be at low risk of attrition bias (Arik 2014; Behera 2015; Branson 2016; Creaney 2011; Dojode 2012; Gautam 2015; Gedik 2016; Gosens 2011; Gupta 2019; Jindal 2013; Kazemi 2010; Krogh 2013; Lebiedziński 2015; Lim 2017; Martínez‐Montiel 2015; Merolla 2017; Montalvan 2015; Omar 2012; Ozturan 2010; Palacio 2016; Raeissadat 2014; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Yerlikaya 2018). In 12 trials, there were no withdrawals (Arik 2014; Dojode 2012; Gautam 2015; Gupta 2019; Jindal 2013; Kazemi 2010; Krogh 2013; Martínez‐Montiel 2015; Merolla 2017; Omar 2012; Palacio 2016; Yerlikaya 2018). One trial reported only one withdrawal from the control group (Behera 2015); another trial reported one withdrawal from the autologous blood group (Thanasas 2011). In one trial, although there were more withdrawals in the control group, an ITT was performed and data from all withdrawals were used in the final analysis (Branson 2016). In seven trials, withdrawal numbers and reasons were similar across groups (Creaney 2011; Gedik 2016; Gosens 2011; Lebiedziński 2015; Montalvan 2015; Ozturan 2010; Stenhouse 2013). In one trial, almost similar numbers withdrew from both treatment arms and left the study to receive other treatments, hence data from those participants were not sought (Lim 2017). In one trial, two participants left the control group to undergo surgery and were excluded from the final analysis (Tetschke 2015).
We judged three (9%) trials to be at high risk (Mishra 2014; Schoffl 2017; Watts 2020). In Mishra 2014, withdrawal rates in the control group (19%) were twice as high as those in the intervention group (9.8%), reasons for withdrawal were not given, and study authors did not provide withdrawal numbers for each group for final follow‐up. Study authors for Schoffl 2017 reported that they excluded from the study those not achieving satisfactory results, and withdrawal rates were high (28%) for both groups. We judged four (12%) trials to be at unclear risk of attrition bias (Linnanmäki 2020; Martin 2019; Wolf 2011; Yadav 2015). In one trial, although reasons for withdrawal were similar across groups, attrition rates were unbalanced across groups, at 22.5% in the PRP group, 5% in the autologous blood group, and 18% in the placebo group (Linnanmäki 2020). Another trial had high attrition rates (> 30%) that were balanced between groups, but study authors did not provide reasons for withdrawal (Martin 2019). For two trials, authors provided overall withdrawal rates but did not provide group‐wise withdrawal numbers (Wolf 2011; Yadav 2015).
Selective reporting
Risk of selective reporting bias was low in five (16%) trials (Gosens 2011; Kazemi 2010; Linnanmäki 2020; Martin 2019; Montalvan 2015), high in five (16%) trials (Lim 2017; Martínez‐Montiel 2015; Mishra 2014; Palacio 2016; Yerlikaya 2018), and unclear in 22 (68%) trials (Arik 2014; Behera 2015; Branson 2016; Creaney 2011; Dojode 2012; Gautam 2015; Gedik 2016; Gupta 2019; Jindal 2013; Krogh 2013; Lebiedziński 2015; Merolla 2017; Omar 2012; Ozturan 2010; Raeissadat 2014; Schoffl 2017; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Watts 2020; Wolf 2011; Yadav 2015).
We judged Lim 2017 to be at high risk of selective reporting bias, as there was no protocol or trial registration, some outcomes were measured but were not reported, and measures of variance were not reported for any outcome data. No protocol or trial registration is available for Martínez‐Montiel 2015, and study authors did not give a clear description of measurement tools or the intervention used. In Mishra 2014, study authors did not provide measures of variance for self‐reported data, and due to lack of US FDA clearance on the PRP centrifuge, although the trial was registered, no details were provided at clincialtrials.gov. Yerlikaya 2018 did not report any numerical results for subjective and objective outcomes; this trial was not registered, and no study protocol is available.
We judged 19 trials (60%) at unclear risk of selective reporting bias due to lack of study protocol and trial registration (Arik 2014; Behera 2015; Creaney 2011; Dojode 2012; Gautam 2015; Gedik 2016; Gupta 2019; Jindal 2013; Lebiedziński 2015; Merolla 2017; Omar 2012; Ozturan 2010; Raeissadat 2014; Schoffl 2017; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Wolf 2011; Yadav 2015). Branson 2016 stated a secondary outcome (Stratford Pain‐Free Function Questionnaire) at trial registration but did not measure or report it in published results of the trial. Krogh 2013 failed to report secondary outcomes at all time points.
Other potential sources of bias
We judged 27 trials (84%) at low risk of other identified potential sources of bias (Arik 2014; Behera 2015; Branson 2016; Creaney 2011; Dojode 2012; Gautam 2015; Gupta 2019; Jindal 2013; Kazemi 2010; Krogh 2013; Lebiedziński 2015; Lim 2017; Linnanmäki 2020; Martínez‐Montiel 2015; Merolla 2017; Montalvan 2015; Omar 2012; Palacio 2016; Raeissadat 2014; Schoffl 2017; Stenhouse 2013; Tetschke 2015; Thanasas 2011; Watts 2020; Wolf 2011; Yadav 2015; Yerlikaya 2018). We judged two trials at high risk of other sources of bias (Gedik 2016; Mishra 2014). Gedik 2016 administered the intervention to 62% of control group participants during the study (4 weeks), and we judged this trial at high risk of bias due to contamination of results at three months and six months. In Mishra 2014, the study sponsor added a post‐hoc six‐month follow‐up for a subset of participants (n = 119; 52% of the planned sample), and results from this subset may be biased.
We judged three trials at unclear risk of other potential bias (Gosens 2011; Martin 2019; Ozturan 2010). In Gosens 2011, there was risk of contamination of results due to several re‐interventions, which were unplanned and unbalanced across the two groups. In Martin 2019, the number of participants with medial elbow pain was higher in the control group (19%) than in the intervention group (11%), leading to potential contamination in interpretation of results. In Ozturan 2010, administration of re‐interventions across intervention (70%) and control groups (10%) was not balanced, leading to possible contamination in interpretation of results.
Effects of interventions
See: Table 1
See Table 1 for the main comparison autologous blood or PRP injection versus placebo.
Autologous blood or PRP injection versus placebo
Two trials compared autologous blood injection to placebo (saline) injection (Linnanmäki 2020; Wolf 2011), and nine trials compared PRP injection to placebo (saline or local anaesthetic) injection (Behera 2015; Krogh 2013; Linnanmäki 2020; Martin 2019; Mishra 2014; Montalvan 2015; Palacio 2016; Schoffl 2017; Yerlikaya 2018). We judged the ten placebo‐controlled trials to be clinically similar with respect to inclusion criteria and baseline participant characteristics of mean pain, function, and treatment success, facilitating pooling of data in a meta‐analysis. Statistical heterogeneity was unimportant for these outcomes until six weeks, and thereafter Behera 2015 caused substantial heterogeneity in pain and function. The certainty of evidence was moderate for pain and function, low for adverse events, and very low for treatment success, participant‐reported pain relief of 30% or greater or 50% or greater, and withdrawal due to adverse events. The major outcomes are reported in Table 1.
Benefits
Participant‐reported pain relief (≥ 30% or ≥ 50%)
No studies measured participant‐reported pain relief of 30% or greater, and no studies measured this outcome at 3 months. Mishra 2014 measured participant‐reported pain relief (≥ 50%) but reported the outcome selectively at 6 months for a subgroup of 119 participants who were followed up longer than the originally planned 3 months. At 6 months, very low‐certainty evidence (downgraded twice for bias and for small numbers of events) indicates that the proportion of participants with pain relief of 50% or greater may be higher with PRP injection compared with placebo; 46 out of 56 (82%) who received PRP injection reported pain relief of 50% or greater compared with 38 out of 63 (60%) who received placebo injection (risk ratio (RR) 1.36, 95% confidence interval (CI) 1.08 to 1.72) at 6 months. Results show absolute improvement of 22% (5% better to 43% better) and relative improvement of 36% (8% better to 72% better; Analysis 1.1).
1.1. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 1: Pain relief ≥ 30% or ≥ 50%
Mean pain
Based on data from eight trials, we found no clinically important improvement in pain (minimal clinically important difference (MCID) 1.5 points on a 0 to 10 scale; higher is worse pain) at 3 months for autologous blood or PRP injection versus placebo (moderate‐certainty evidence; downgraded once for bias). Statistical heterogeneity was unimportant up to 3 months (I² = 7% to 33%) and was substantial (I² = 76% to 78%) at later follow‐up points, largely driven by one study (Behera 2015).
At 3 weeks, mean pain (0 to 10; higher is worse) was 2.8 points with placebo and 2 points worse (higher scores) (95% CI 0.65 better to 4.65 worse; 1 study, 19 participants) with autologous blood or PRP injection. At 6 weeks, mean pain was 4.8 points with placebo and 0.26 points worse (95% CI 0.14 better to 0.65 worse; 7 studies, 570 participants) with autologous blood or PRP injection. At 3 months (primary time point), mean pain was 3.7 points with placebo and 0.16 points better (95% CI 0.60 better to 0.29 worse; 8 studies, 523 participants; I² = 13%) with autologous blood or PRP injection. This corresponds with absolute improvement of 1.6% (6% better to 3% worse) and relative improvement of 2.3% (9% better to 4% worse). At 6 months, mean pain was 1.64 points with placebo and 0.45 points better (95% CI 1.5 better to 0.59 worse; 7 studies, 387 participants) with autologous blood or PRP injection. At 12 months, mean pain was 2.3 points with placebo and 0.69 points better (95% CI 1.78 better to 0.39 worse; 5 studies, 241 participants) with autologous blood or PRP injection (Analysis 1.2).
1.2. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 2: Mean pain (VAS 0 to 10, PRTEE)
Function
Based on data from seven trials, we found no clinically important improvement in function (MCID 10 points on a 100‐point scale; higher is worse function) for autologous blood or PRP injection versus placebo (moderate‐certainty evidence; downgraded once for bias). Statistical heterogeneity was unimportant (I² = 0 to 9%) up to 3 months and substantial (78% to 82%) at later time points, driven by one study (Behera 2015). Removing Behera 2015 decreased I² to 0 to 3%.
At 3 weeks, function (0 to 100 scale; lower is better) was 24 points with placebo and 12.0 points worse (95% CI 5.33 better to 29.33 worse; 1 study, 19 participants) with autologous blood or PRP injection. At 6 weeks, function was 36.2 points with placebo and 1.3 points worse (95% CI 1.64 better to 4.25 worse; 7 studies, 473 participants) with autologous blood or PRP injection. At 3 months, function was 27.5 points with placebo and 1.86 points better (95% CI 4.97 better to 1.25 worse; 8 studies, 502 participants; I² = 0%) with autologous blood or PRP injection. This corresponds with absolute benefit of 1.9% (5% better to 1% worse) and relative benefit of 4% (11% better to 3% worse). At 6 months, function was 19.2 points with placebo and 1.15 points better (95% CI 8.6 better to 6.3 worse; 7 studies, 379 participants) with autologous blood or PRP injection. At 12 months, function was 20.4 points with placebo and 5.81 points better (95% CI 16.7 better to 5.05 worse; 4 studies, 203 participants) with autologous blood or PRP injection (Analysis 1.3).
1.3. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 3: Function (DASH, MMCPIE, Roles‐Maudsley)
Treatment success
Based upon very low‐certainty evidence (downgraded for bias, indirectness, and imprecision), we are uncertain whether autologous blood or PRP injection improves treatment success compared with placebo injection. Data from four trials show that 121 out of 185 (65%) rated their treatment as successful with placebo versus 125 out of 187 (67%) with autologous blood or PRP injection (RR 1.00, 95% CI 0.83 to 1.19; I² = 38%) for absolute improvement of 0% higher (11.1% lower to 12.4% higher) and relative change 0% higher (17% lower to 19% higher) (95% confidence intervals include both clinically important and unimportant change in treatment success with use of autologous blood or PRP injection (Analysis 1.4) (Martin 2019; Mishra 2014; Montalvan 2015; Palacio 2016).
1.4. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 4: Treatment success (> 25% improvement in pain or function)
Health‐related quality of life
No studies measured this outcome for this comparison.
Minor outcomes
None of the studies reported other pain measures, grip strength, or serious adverse events.
Harms
Withdrawal due to adverse events
Very low‐certainty evidence (downgraded once for bias and twice for very serious imprecision) suggests that we are uncertain whether autologous blood or PRP injection increased the risk of withdrawal due to adverse events.
Martin 2019 reported withdrawal due to adverse events. Six studies reported reasons for withdrawal, and reasons did not include adverse events (judged as zero events) (Behera 2015; Krogh 2013; Mishra 2014; Montalvan 2015; Wolf 2011; Yerlikaya 2018). Thus, the data from these six trials are not included in the pooled estimate (Analysis 1.5).
1.5. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 5: Withdrawal due to AEs
Data from Martin 2019 show withdrawal due to adverse events in 3 out of 39 (8%) with placebo versus 1 out of 41 (2%) with autologous blood or PRP injection (RR 0.32, 95% CI 0.03 to 2.92; 1 study), an absolute change of 5.2% fewer events (7.5% fewer to 14.8% more), and a relative change of 68% fewer events (97% fewer to 192% more) (95% confidence intervals show that autologous blood or PRP injection can cause both greater and lesser harm compared with placebo).
Adverse events
Low‐certainty evidence (downgraded for bias and imprecision) suggests that autologous blood or PRP injection may not increase risk for adverse events compared with placebo. Data from five studies show adverse event rates of 35 out of 208 (17%) with placebo versus 41 out of 217 (19%) with autologous blood or PRP injection (RR 1.14, 95% CI 0.76 to 1.72; 5 studies, 425 participants; I² = 0%), an absolute change of 2.4% more events (4% fewer to 12% more), and a relative change of 14% more events (24% fewer to 72% more) (Analysis 1.6) (Behera 2015; Krogh 2013; Martin 2019; Mishra 2014; Montalvan 2015).
1.6. Analysis.

Comparison 1: Autologous blood or PRP injection versus placebo injection, Outcome 6: Adverse events
Autologous blood or PRP injection versus glucocorticoid injection
Benefits
Participant‐reported pain relief (≥ 30% or ≥ 50%)
Ozturan 2010 reported proportion of participants with 50% or greater improvement in pain. We graded the evidence as low certainty (downgraded for bias and small numbers of events).
Pain relief favoured glucocorticoid injection at 6 weeks but not at 1 year. Pain relief rates were 18 out of 20 (90%) with glucocorticoid injection versus 3 out of 18 (17%) with autologous blood or PRP injection (RR 0.19, 95% CI 0.07 to 0.53) at 6 weeks. At 1 year, pain relief was 10 out of 20 (50%) with glucocorticoid injection versus 15 out of 18 (83%) with autologous blood or PRP injection (RR 1.67, 95% CI 1.03 to 2.71; number needed to treat for additional benefit (NNTB) 3, 95% CI 1.6 to 20) (Analysis 2.1)
2.1. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 1: Pain relief ≥ 50%
Mean pain
We identified 13 trials reporting this outcome and noted considerable statistical heterogeneity up to 3 months (Arik 2014; Dojode 2012; Gautam 2015; Gosens 2011; Gupta 2019; Jindal 2013; Kazemi 2010; Krogh 2013; Martínez‐Montiel 2015; Omar 2012; Ozturan 2010; Wolf 2011; Yadav 2015). At 3 weeks, heterogeneity (I² = 91%) seemed to be driven largely by one study (Arik 2014), but at 6 weeks (I² = 90%) and at 3 months (I² = 71%), heterogeneity could not be explained by study or participant characteristics. After 6 months, statistical heterogeneity was moderate (I² = 61% at 6 months and 62% at 1 year), and with removal of Gupta 2019, heterogeneity dropped to 0% at 1 year. Standard deviation (SD) values reported by Gupta 2019 were unusually small, yielding large weight to this study at 1‐year analysis (Analysis 2.2). Study authors did not respond to queries; thus we report estimates with and without this study.
2.2. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 2: Mean pain
We downgraded this outcome to low‐certainty evidence for bias and inconsistency (effect sizes varied from no effect to clinically meaningful effect). Up to 3 months, PRP may not provide clinically important pain reduction when compared with glucocorticoid injection. At 3 weeks, mean pain (on a 0 to 10 scale; higher is worse) was 3.43 points with glucocorticoid injection and 2.06 points worse (95% CI 0.67 worse to 3.45 worse; 5 studies, 280 participants) with autologous blood or PRP injection. At 6 weeks, mean pain was 2.5 points with glucocorticoid injection and 0.99 points worse (95% CI 0.21 worse to 1.77 worse; 13 studies, 707 participants) with autologous blood or PRP injection.
At 6 months, 12 months, and greater than 1 year follow‐up, mean pain was better with autologous blood or PRP injection. At 3 months, mean pain was 3.1 points with glucocorticoid injection and 1.15 points better (95% CI 1.71 better to 0.59 better; 11 studies, 627 participants) with autologous blood or PRP injection. At 6 months, mean pain was 3.89 points with glucocorticoid injection and 1.55 points better (95% CI 2.21 better to 0.9 better; 8 studies, 427 participants) with autologous blood or PRP injection. At 12 months, mean pain was 4.6 points with glucocorticoid injection and 1.59 points better (95% CI 2.22 better to 0.97 better; 4 studies, 258 participants) with autologous blood or PRP injection. At greater than 1 year, mean pain was 4.24 points with glucocorticoid injection and 2.11 points better (95% CI 3.19 better to 1.03 better; 1 study, 100 participants) with autologous blood or PRP injection (Analysis 2.2).
Excluding Gupta 2019 (which reported unusually small SD values) did not change the results considerably. At 6 weeks, the mean difference was 0.99 points (95% CI 0.21 to 1.77) including this study and 0.80 points (95% CI 0.05 to 1.56) without the study. At 3 months, the mean difference was ‐1.15 points (95% CI ‐1.71 to ‐0.59) including this study and ‐1.04 points (95% CI ‐1.66 to‐0.42) without the study. At 12 months, the mean difference was ‐1.59 points (95% CI ‐2.22 to ‐0.97) with Gupta 2019 and ‐1.95 points (95% CI ‐2.54 to ‐1.35) without it.
Function
Fourteen studies measured function using various measures (Patient‐Rated Tennis Elbow Evaluation (PRTEE) questionnaire, Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire, Quick Dash) (Arik 2014; Branson 2016; Gautam 2015; Gosens 2011; Gupta 2019; Kazemi 2010; Krogh 2013; Lebiedziński 2015; Martínez‐Montiel 2015; Omar 2012; Ozturan 2010; Palacio 2016; Wolf 2011; Yadav 2015). We observed considerable statistical heterogeneity up to 6 months (I² = 93% at 3 weeks; I² = 86% at 6 weeks; I² = 78% at 3 months; I² = 87% at 6 months). At 3 weeks, heterogeneity seemed to be driven by one study (Arik 2014), but at later time points, heterogeneity could not be explained by study or participant characteristics. At 1 year, statistical heterogeneity was unimportant (I² = 22%), and at greater than 1 year follow‐up, there was only one study (Gosens 2011). Similar to mean pain, Gupta 2019 reported unusually small SD values; thus we report these values with and without these results.
We found low‐certainty evidence (downgraded for bias and imprecision) to suggest that PRP may not improve function compared with glucocorticoid injection up to 1 year. At 2 years, PRP may improve function compared to glucocorticoid injection. We considered downgrading for inconsistency, but further downgrading to very low seemed inappropriate, as in all studies, the direction of effect was the same.
At 3 weeks, function (0 to 100; lower is better) was 43.5 points with glucocorticoid injection and 16.5 points worse (95% CI 4.23 better to 37.15 worse; 3 studies, 170 participants) with autologous blood or PRP injection. At 6 weeks, function was 31.2 points with glucocorticoid injection and 6.1 points worse (95% CI 1.79 worse to 10.44 worse; 13 studies, 724 participants) with autologous blood or PRP injection. At 3 months, function was 33.4 points with glucocorticoid injection and 10.2 better (95% CI 6.21 better to 14.1 better; 12 studies, 635 participants) with autologous blood or PRP injection. At 6 months, function was 33.22 points with glucocorticoid injection and 5.07 points better (95% CI 12.66 better to 2.52 worse; 7 studies, 374 participants) with autologous blood or PRP injection. At 1 year, function was 32.2 points with glucocorticoid injection and 8.94 points better (95% CI 5.78 better to 12.1 better; 4 studies, 317 participants) with autologous blood or PRP injection. At greater than1 year, function was 36.5 points with glucocorticoid injection and 18.9 points better (95% CI 28.27 better to 9.53 better; 1 study, 100 participants) with autologous blood or PRP injection (Analysis 2.3).
2.3. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 3: Function (various scales)
Excluding Gupta 2019 did not change the results considerably. At 6 weeks, the mean difference was 6.11 points (95% CI 1.79 to 10.44) with, and 5.39 points (95% CI 0.00 to 10.78) without, the study. At 3 months, the mean difference was ‐10.19 (95% CI ‐14.16 to ‐6.21) with, and ‐9.80 (95% CI ‐15.03 to ‐4.57) without, Gupta 2019. At 1 year, the mean difference was ‐8.94 (95% CI ‐12.09 to ‐5.78) with, and ‐9.73 (95% CI ‐15.89 to ‐3.58) without, Gupta 2019.
Treatment success
Six trials reported some measure of treatment success (Arik 2014; Branson 2016; Dojode 2012; Gupta 2019; Jindal 2013; Lebiedziński 2015). We downgraded the evidence to very low for bias, imprecision, and indirectness. Only one study used patient‐reported global improvement (Branson 2016). Data from five trials suggest that PRP may not improve treatment success compared with glucocorticoid injection.
Treatment success rates were as follows: 76 out of 155 (49%) with glucocorticoid injection versus 27 out of 162 (17%) with autologous blood or PRP injection (RR 0.26, 95% CI 0.07 to 0.95; 5 studies, 317 participants) up to 6 weeks. This corresponds with number needed to treat for additional harm (NNTH) of 2.8 (95% CI 1.6 to 11). At 3 months, 43 out of 94 (46%) with glucocorticoid injection versus 69 out of 94 (73%) with autologous blood or PRP injection (RR 1.56, 95% CI 1.08 to 2.26; 3 studies, 188 participants) were reported. This corresponds with NNTB of 3.8 (95% CI 2.2 to 16.6). At 6 months, 46 out of 90 (51%) with glucocorticoid injection versus 44 out of 97 (45%) with autologous blood or PRP injection (RR 1.02, 95% CI 0.23 to 4.44; 3 studies) were reported. At 12 months, 57 out of 145 (39%) with glucocorticoid injection versus 90 out of 155 (58%) with autologous blood or PRP injection (RR 1.00, 95% CI 0.31 to 3.16; 2 studies, 199 participants) were reported. At greater than 1 year, 51 out of 95 (54%) with glucocorticoid injection versus 58 out of 104 (56%) with autologous blood or PRP injection (RR 1.0, 95% CI 0.31 to 3.16; 2 studies) were reported (Analysis 2.4).
2.4. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 4: Treatment success
Health‐related quality of life
Health‐related quality of life was not measured by any studies.
Minor outcomes
Six trials reported mean grip strength, but measured units were reported only by Arik 2014; thus we used standardised mean difference (SMD) to summarise the data (Arik 2014; Gautam 2015;Gupta 2019; Ozturan 2010; Kazemi 2010; Yadav 2015).
At 2 weeks, the SMD was ‐0.52 (95% CI ‐0.87 to ‐0.16; 3 studies, 170 participants), which back‐transforms to a mean reduction of 8.1 kg (95% CI 2.5 kg worse to 13.57 kg worse) with autologous blood or PRP injection. At 6 weeks, the SMD was ‐0.26 (95% CI ‐0.68 to 0.16; 6 studies, 348 participants), which back‐transforms to a mean reduction of 4,1 kg (95% CI 10.6 kg worse to 2.5 kg better) with autologous blood or PRP injection. At 3 months, the SMD was 0.56 (95% CI 0.19 to 0.93; 348 participants, 6 studies), which back‐transforms to a mean increase of 8.7 kg (95% CI 3 kg better to 14.5 kg better) with autologous blood or PRP injection. At 6 months, the SMD was 0.35 (95% CI ‐0.13 to 0.83; 2 studies, 68 participants), which back‐transforms to a mean increase of 5.5 kg (95% CI 2.03 kg worse to 12.95 kg better) with autologous blood or PRP injection. At 1 year, the SMD was 0.66 (95% CI 0.29 to 1.03; 2 studies, 118 participants), which back‐transforms to a mean increase of 10.3 kg (95% CI 4.5 kg better to 16.1 kg better) with autologous blood or PRP injection (Analysis 2.6).
2.6. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 6: Grip strength
Harms
Withdrawal due to adverse events
None of the studies measured this outcome.
Adverse events
Eight studies reported adverse events, but three had zero events; thus estimates were calculated based on five studies (Arik 2014; Dojode 2012; Krogh 2013; Lebiedziński 2015; Ozturan 2010). Very low‐certainty evidence (downgraded once for bias and twice for very serious imprecision) suggests that we are uncertain whether autologous blood or PRP injection increases adverse events compared with glucocorticoid injection. Statistical heterogeneity was unimportant (I² = 0%).
Adverse event rates were 24 out of 195 (12%) with glucocorticoid injection versus 46 out of 201 (23%) with autologous blood or PRP injection (RR 1.64, 95% CI 0.65 to 4.12; 317 participants, 5 studies) (Analysis 2.5).
2.5. Analysis.

Comparison 2: Autologous blood or PRP injection versus glucocorticoid injection, Outcome 5: Adverse events
PRP and dry needling versus dry needling alone
Benefits
Pain relief
The only study in this comparison did not measure this outcome (Stenhouse 2013).
Mean pain
Low‐certainty evidence (downgraded for bias and imprecision) suggests that PRP and dry needling may not improve pain compared with dry needling alone. The 95% confidence intervals include both clinically meaningful harm and benefit (1.5 points).
At 3 months, mean pain (0 to 10; higher is worse) was 6.02 points with dry needling alone and 0.14 points better (95% CI 2.13 better to 1.85 worse; 1 study, 28 participants) with PRP and dry needling. At 6 months, mean pain was 4.5 points with dry needling alone and 0.35 points better (95% CI 2.88 better to 2.18 worse; 1 study, 28 participants) with PRP and dry needling (Analysis 3.1).
3.1. Analysis.

Comparison 3: PRP and dry needling versus dry needling alone, Outcome 1: Pain
Function
Low‐certainty evidence (downgraded for bias and imprecision) suggests that PRP may not improve function compared with dry needling alone. The 95% confidence intervals include both clinically meaningful harm and benefit (10 points).
At 3 months, function (0 to 100 scale, lower is better) was 28.7 points with dry needling alone and 2.8 points worse (95% CI 16.88 better to 22.48 worse; 1 study, 28 participants) with PRP and dry needling. At 6 months, function was 45.4 points with dry needling alone and 5.7 points worse (95% CI 14.36 better to 25.76 worse; 1 study, 28 participants) with PRP and dry needling (Analysis 3.2).
3.2. Analysis.

Comparison 3: PRP and dry needling versus dry needling alone, Outcome 2: Function
Treatment success
Stenhouse 2013 did not report this outcome.
Health‐related quality of life
Stenhouse 2013 did not report this outcome.
Minor outcomes
None of the studies measured minor outcomes for this comparison.
Harms
Withdrawal due to adverse events
Withdrawal rates were as follows: 1 out of 13 (8%) with dry needling alone versus 2 out of 15 (13%) with PRP and dry needling (RR 1.73, 95% CI 0.18 to 16.99; 1 study) (Analysis 3.3).
3.3. Analysis.

Comparison 3: PRP and dry needling versus dry needling alone, Outcome 3: Withdrawal due to adverse events
Adverse events
Very low‐certainty evidence (downgraded once for bias and twice for very serious imprecision) from Stenhouse 2013 suggests that we are uncertain whether PRP and dry needling increases adverse events compared with dry needling alone.
Adverse event rates were as follows: 1 out of 13 (8%) with dry needling alone versus 2 out of 15 (13%) with PRP and dry needling (RR 1.73, 95% CI 0.18 to 16.99; 1 study) (Analysis 3.4).
3.4. Analysis.

Comparison 3: PRP and dry needling versus dry needling alone, Outcome 4: Adverse events
PRP injection versus autologous blood injection
Benefits
Pain relief
None of the studies measured this outcome.
Mean pain
Three studies measured pain for this comparison (Linnanmäki 2020; Raeissadat 2014; Thanasas 2011). Moderate‐certainty evidence (downgraded for bias) shows that PRP injection probably does not improve pain compared with autologous blood injection. The confidence intervals exclude clinically important benefit at all time points.
At 6 weeks, mean pain (0 to 10; higher is worse) was 1.9 points with autologous blood injection and 0.24 points better (95% CI 1.21 better to 0.73 worse; 3 studies, 169 participants) with PRP injection. At 3 months, mean pain was 2.1 points with autologous blood injection and 0.4 points better (95% CI 1.1 better to 0.3 better; 3 studies, 169 participants) with PRP injection. At 6 months, mean pain was 2.1 points with autologous blood injection and 0.28 points better (95% CI 1.04 better to 0.48 worse; 3 studies, 169 participants) with PRP injection. At 12 months, mean pain was 2.3 points with autologous blood injection and 0.05 points better (95% CI 1.12 better to 1.22 worse; 2 studies, 141 participants) with PRP injection (Analysis 4.1).
4.1. Analysis.

Comparison 4: PRP versus autologous blood, Outcome 1: Mean pain
Function
Four studies measured function for this comparison (Creaney 2011; Linnanmäki 2020; Raeissadat 2014; Thanasas 2011). Moderate‐certainty evidence (downgraded for bias) shows that PRP injection probably does not provide clinically important benefit for function compared with autologous blood injection.
At 6 weeks, function (0 to 100; lower is better) was 31.2 points with autologous blood injection and 3.44 points better (95% CI 6.6 better to 0.28 better; 4 studies, 276 participants) with PRP injection. At 3 months, function was 21.4 points with autologous blood and 3.25 points better (95% CI 6.33 better to 0.17 better; 4 studies, 292 participants) with PRP. At 6 months, function was 18 points with autologous blood and 2.83 points better (95% CI 6.02 better to 0.37 better; 4 studies, 297 participants) with PRP injection. At 12 months, function was 17 points with autologous blood injection and 0.71 points better (95% CI 8.53 better to 7.11 worse; 2 studies, 140 participants) with PRP injection (Analysis 4.2).
4.2. Analysis.

Comparison 4: PRP versus autologous blood, Outcome 2: Function (various scales)
Treatment success
Two studies measured treatment success for this comparison (Creaney 2011; Raeissadat 2014). Low‐certainty evidence (downgraded for bias and imprecision) suggests that PRP injection may not improve rate of treatment success compared with autologous blood injection.
Treatment success rates were as follows: 61 out of 90 (68%) with autologous blood injection versus 69 out of 101 (68%) with PRP injection (RR 1.03, 95% CI 0.77 to 1.37) (Analysis 4.3).
4.3. Analysis.

Comparison 4: PRP versus autologous blood, Outcome 3: Treatment success
Health‐related quality of life
None of the studies measured this outcome.
Minor outcomes
None of the studies measured any minor outcomes for this comparison.
Harms
Withdrawal due to adverse events
None of the studies reported this outcome.
Adverse events
Very low‐certainty evidence (downgraded for bias and very serious imprecision) suggests that we are uncertain whether rate for adverse events differs between PRP and autologous blood injections.
Adverse events rates were as follows: 9 out of 77 (12%) with autologous blood injection versus 4 out of 62 (6%) with PRP injection (RR 2.25, 95% CI 0.9 to 5.62; 2 studies) (Analysis 4.4).
4.4. Analysis.

Comparison 4: PRP versus autologous blood, Outcome 4: Adverse events
Autologous blood injection versus extracorporeal shock wave therapy (ESWT)
Benefits
Pain relief
The only study in this comparison reported proportion with 50% or greater pain improvement (Ozturan 2010). Low‐certainty evidence (downgraded for bias and imprecision) suggests that autologous blood injection may not provide better chance of pain relief compared with ESWT.
Pain relief rates were as follows: 8 out of 20 (40%) with ESWT versus 3 out of 20 (15%) with autologous blood injection (RR 0.38, 95% CI 0.12 to 1.21) at 6 weeks; 18 out of 20 (90%) with ESWT versus 16 out of 20 (80%) with autologous blood injection (RR 0.89, 95% CI 0.68 to 1.16) at 1 year (Analysis 5.1).
5.1. Analysis.

Comparison 5: Autologous blood versus ESWT, Outcome 1: Pain relief > 50%
Mean pain
Low‐quality evidence (downgraded for bias and imprecision; only 1 study) suggests that autologous blood injection may not provide important benefit for pain compared with ESWT. The 95% confidence intervals exclude clinically important benefit.
At 6 weeks, mean pain (0 to 10 scale; higher indicates worse) was 4.42 points with ESWT and 0.63 points worse (95% CI 0.28 better to 1.54 worse; 1 study, 37 participants) with autologous blood injection. At 3 months, mean pain was 2.26 points with ESWT and 0.29 points worse (95% CI 0.75 better to 1.33 worse; 1 study, 37 participants) with autologous blood injection. At 6 months, mean pain was 2.21 points with ESWT and 0.23 points worse (95% CI 0.78 better to 1.24 worse; 1 study, 37 participants) with autologous blood injection. At 1 year, mean pain was 2.10 points with ESWT and 0.23 points worse (95% CI 0.61 better to 1.07 worse; 1 study, 37 participants) with autologous blood injection (Analysis 5.2).
5.2. Analysis.

Comparison 5: Autologous blood versus ESWT, Outcome 2: Mean pain
Function
Low‐certainty evidence (downgraded for bias and imprecision; only 1 study) suggests that autologous blood injection may not provide important benefit for function compared with ESWT. The confidence intervals do not overlap with clinically important benefit at any time point.
At 6 weeks, function (0 to 100 scale; lower is better) was 30.0 points with ESWT and 3.8 points worse (95% CI 1.56 better to 9.16 worse; 1 study, 37 participants) with autologous blood injection. At 3 months, function was 18.1 points with ESWT and 1.4 points worse (95% CI 5.82 better to 8.62 worse; 1 study, 37 participants) with autologous blood injection. At 6 months, function was 19.2 points with ESWT and 1.5 points worse (95% CI 4.17 better to 7.17 worse; 1 study, 37 participants) with autologous blood injection. At 1 year, function was 19.5 points with ESWT and 0.9 points better (95% CI 5.98 better to 4.18 worse; 1 study, 37 participants) with autologous blood injection (Analysis 5.3).
5.3. Analysis.

Comparison 5: Autologous blood versus ESWT, Outcome 3: Function (various scales)
Treatment success
Ozturan 2010 did not measure this outcome.
Health‐related quality of life
Ozturan 2010 did not report this outcome
Minor outcomes
At 6 weeks, grip strength was 33.2 kg with ESWT and 0.4 kg less (95% CI 4.9 more to 5.7 less; 37 participants) with autologous blood injection. At 3 months, grip strength was 36.9 kg with ESWT and 1.1 kg less (95% CI 2.84 more to 5.04 less; 37 participants) with autologous blood injection. At 6 months, grip strength was 37.2 kg with ESWT and 0.3 kg worse (95% CI 3.37 more to 3.97 less; 37 participants) with autologous blood injection. At 1 year, grip strength was 39.6 kg with ESWT and 2.3 kg better (95% CI 5.73 more to 1.13 less; 37 participants) with autologous blood injection (Analysis 5.4).
5.4. Analysis.

Comparison 5: Autologous blood versus ESWT, Outcome 4: Grip strength
Ozturan 2010 did not report other minor outcomes.
Harms
Withdrawal due to adverse events
The only study in this comparison did not measure this outcome (Ozturan 2010).
Adverse events
Very low‐certainty evidence (downgraded once for bias and twice for very serious imprecision) suggests that we are uncertain whether autologous blood injection increases risk for adverse events compared with ESWT.
Adverse event rates were as follows: 12 out of 20 (60%) with ESWT versus 3 out of 20 (15%) with autologous blood injection (RR 0.25, 95% CI 0.08 to 0.75; 1 study) (Analysis 5.5).
5.5. Analysis.

Comparison 5: Autologous blood versus ESWT, Outcome 5: Adverse events
PRP injection versus surgery
Benefits
Pain relief
Studies in this comparison did not measure this outcome.
Mean pain
Moderate‐certainty evidence from two studies shows that PRP injection probably does not improve pain compared with surgery (Merolla 2017; Watts 2020). At 24 months, we found low‐certainty evidence to suggest that PRP injection may increase pain compared with surgery. We downgraded the evidence for risk of bias to moderate certainty at 3 months and 6 months. At 6 weeks and 24 months, evidence was of low certainty (once for bias and once for imprecision). Statistical heterogeneity was 57% at 3 months, 51% at 6 months, and 88% at 12 months.
At 6 weeks, mean pain (0 to 10; higher is worse) was 5.4 with surgery and 0.8 points better with PRP injection (95% CI 0.38 better to 1.98 worse). At 3 months, mean pain was 3.8 points with surgery and 0.14 points better (95% CI ‐1.40 better to 1.12 worse; 2 studies, 153 participants) with PRP injection. At 6 months, mean pain was 2.6 points with surgery and 0.14 points better (95% CI 0.91 better to 1.20 worse; 159 participants, 2 studies) with PRP injection. At 12 months, mean pain was 1.8 points with surgery and 0.39 points better (95% CI 1.86 better to 2.64 worse; 153 participants; 2 studies) with PRP injection. At 24 months, mean pain was 2.1 points with surgery and 5.0 points worse (95% CI 4.02 worse to 5.98 worse; 1 study, 101 participants) with PRP injection (Analysis 6.1).
6.1. Analysis.

Comparison 6: PRP versus surgery, Outcome 1: Mean pain
Function
Low‐certainty evidence from two studies suggests that PRP injection may not improve function compared with surgery (Merolla 2017; Watts 2020). At 24 months, PRP may result in deteriorated function. We downgraded the evidence to low for bias and imprecision.
At 6 weeks, function (0 to 100 scale; lower is better) was 49 with PRP and 7.00 points worse (95% CI 5.94 better to 19.94 worse; 56 participants) with surgery. At 3 months, mean function was 29.9 points with surgery and ‐0.59 points better (95% CI 19.63 better to 18.45 worse; 2 studies, 153 participants) with PRP injection. At 6 months, function was 22.7 points with surgery and 1.36 worse (95% CI 15.92 better to 18.63 worse;159 participants, 2 studies) with PRP injection. At 12 months, function was 17.5 points with surgery and 1.53 points worse (95% CI 13.27 better to 16.33 worse; 153 participants) with PRP injection. At 24 months, function was 21.2 points with surgery and 48.0 points worse (95% CI 40.2 worse to 55.8 worse; 1 study, 101 participants) with PRP injection (Analysis 6.2).
6.2. Analysis.

Comparison 6: PRP versus surgery, Outcome 2: Function
Treatment success
Neither study reported this outcome.
Health‐related quality of life
Neither study reported this outcome.
Minor outcomes
PRP probably does not improve grip strength compared with surgery (moderate‐quality evidence; downgraded for bias). After 6 months, PRP injection may decrease grip strength compared with arthroscopic surgery. The mean difference favoured surgery at every time point, and 95% confidence intervals did not overlap null effect at any time point. Grip strength is a measure of capacity rather than function/disability, and the clinically important difference is unclear in this condition.
At 3 months, grip strength was 48.4 kg with arthroscopic surgery and 1.0 kg worse (95% CI 0.99 better to 2.99 worse; 1 study, 101 participants) with PRP injection. At 6 months, grip strength was 50.2 kg with arthroscopic surgery and 26.8 kg worse (95% CI 29.03 worse to 24.57 worse; 101 participants) with PRP injection. At 12 months, grip strength was 47.3 kg with arthroscopic surgery and 23.7 kg worse (95% CI 25.59 worse to 21.81 worse; 101 participants) with PRP. At 24 months, grip strength was 48.4 kg with arthroscopic surgery and 25.6 kg worse (95% CI 27.31 worse to 23.89 worse; 101 participants) with PRP (Analysis 6.3).
6.3. Analysis.

Comparison 6: PRP versus surgery, Outcome 3: Grip strength
Harms
Withdrawal due to adverse events
Studies in this comparison did not measure this outcome (Merolla 2017).
Adverse events
Watts 2020 reported one adverse event in the surgery group (wound debridement) and zero events in the PRP group; thus we could not estimate the relative risk (very low‐certainty evidence). Merolla 2017 did not report this outcome.
Autologous blood or PRP injection with tennis elbow strap and exercise versus tennis elbow strap and exercise alone
Two studies with 171 participants studied whether autologous blood or PRP injection improves clinical outcomes when added to tennis elbow strap and exercise (Gedik 2016; Lim 2017).
Benefits
Pain relief
Both studies did not measure this outcome.
Mean pain
Only Lim 2017 measured this outcome.
Low‐certainty evidence (downgraded for bias and imprecision) suggests that PRP injection plus tennis elbow strap and exercise may not provide clinically important improvement in pain compared with tennis elbow strap and exercise alone. At 4 weeks, mean pain (0 to 10 scale; higher is worse) had improved by 2.92 points with tennis elbow strap and exercise and by 1.14 additional points (95% CI 1.86 more to 0.42 more; 1 study, 120 participants) with PRP injection plus tennis elbow strap and exercise (Analysis 7.1).
7.1. Analysis.

Comparison 7: Autologous blood plus tennis elbow strap and exercise versus tennis elbow strap and exercise, Outcome 1: Mean pain
Function
Although both studies provided data for this outcome, measurements were obtained at different time points and hence were not pooled. Low‐certainty evidence (downgraded for bias and imprecision) from Lim 2017 suggests that PRP injection plus tennis elbow strap and exercise may not provide clinically important improvement in function compared with tennis elbow strap and exercise alone. At 4 weeks, function (0 to 100 scale; lower is better) had improved by 8.42 points with tennis elbow strap and exercise and by 7.81 additional points (95% CI 12.71 more to 2.91 more; 1 study, 105 participants) with PRP injection plus tennis elbow strap and exercise.
Low‐certainty evidence from Gedik 2016 suggests that autologous blood injection may not improve function when added to tennis elbow strap and exercise. At 3 months, function (0 to 100; lower is better) was 8.6 points with tennis elbow strap and exercise and 1.6 points worse (95% CI 2.19 better to 5.39 worse; 1 study, 45 participants) with autologous blood injection plus tennis elbow strap and exercise. At 6 months, function was 3.9 points with tennis elbow strap and exercise and 2.46 points worse (95% CI 0.41 better to 5.33 worse; 1 study, 45 participants) with autologous blood injection plus tennis elbow strap and exercise. We downgraded the evidence for bias and imprecision ‐ only one study with few participants (Analysis 7.2).
7.2. Analysis.

Comparison 7: Autologous blood plus tennis elbow strap and exercise versus tennis elbow strap and exercise, Outcome 2: Mean function
Treatment success
Only Gedik 2016 provided data for this outcome. Very low‐certainty evidence suggests that we are uncertain whether autologous blood injection affects treatment success when added to tennis elbow strap and exercise. We downgraded the evidence for bias, imprecision, and indirectness; the confidence intervals include no effect, and instead of assessing subjective global success, Gedik 2016 researchers used own non‐validated measure. Treatment success rates were as follows: 13 out of 13 (100%) with tennis elbow strap and exercise versus 29 out of 32 (91%) with autologous blood injection plus tennis elbow strap and exercise (RR 0.93, 95% CI 0.79 to 1.08) at 3 months; 13 out of 13 (100%) with tennis elbow strap and exercise versus 31 out of 32 (97%) with autologous blood injection plus tennis elbow strap and exercise (RR 0.99, 95% CI 0.87 to 1.12) at 6 months (Analysis 7.4).
7.4. Analysis.

Comparison 7: Autologous blood plus tennis elbow strap and exercise versus tennis elbow strap and exercise, Outcome 4: Treatment success
Health‐related quality of life
Both studies did not measure this outcome.
Minor outcomes
Low‐certainty evidence (downgraded for bias and imprecision) suggests that autologous blood injection may not improve grip strength when given in conjunction with tennis elbow strap and exercise. Grip strength is a measure of capacity rather than function/disability, and the clinically important difference is unclear in this condition.
At 3 months, grip strength was 29.1 kg with tennis elbow strap and exercise and 2.2 kg better (95% CI 7.1 better to 2.7 worse; 1 study, 45 participants) with autologous blood injection plus tennis elbow strap and exercise. At 6 months, grip strength was 30.9 kg with tennis elbow strap and exercise and 3.0 kg better (95% CI 8.85 better to 2.85 worse; 1 study, 45 participants) with autologous blood injection plus bandage and exercise (Analysis 7.3).
7.3. Analysis.

Comparison 7: Autologous blood plus tennis elbow strap and exercise versus tennis elbow strap and exercise, Outcome 3: Hand grip strength
Harms
Withdrawal due to adverse events
Both studies did not measure this outcome.
Adverse events
Both studies did not measure this outcome.
PRP injection versus laser application
Benefits
Pain relief
None of the studies measured this outcome.
Mean pain
Compared with low‐lever laser application, PRP injection may not provide important pain improvement (low‐certainty evidence; downgraded for bias and imprecision; 95% CI overlaps with clinically important benefit).
At 3 months, mean pain (0 to 10; higher is worse) was 4.7 points with laser application and 1.0 point better (95% CI 2.13 better to 0.13 worse; 1 study, 56 participants) with PRP injection. At 6 months. mean pain was 3.6 points with laser application and 0.9 points better (95% CI 1.9 better to 0.1 worse; 1 study, 56 participants) with PRP injection. At 12 month, mean pain was 2.7 points with laser applications and 0.9 points better (95% CI 2.03 better to 0.23 worse; 1 study, 56 participants) with PRP injection (Analysis 8.1).
8.1. Analysis.

Comparison 8: PRP versus laser applications, Outcome 1: Pain
Function
Compared with low‐lever laser application, PRP injection may not improve function (low‐certainty evidence; downgraded for bias and imprecision; 95% CI overlaps with clinically important benefit).
At 3 months, function (0 to 100; lower is better) was 38.9 points with laser application and 9.1 points better (95% CI 20.03 better to 1.83 worse; 1 study, 56 participants) with PRP injection. At 6 months, function was 29.0 points with laser applications and 2.5 points better (95% CI 13.22 better to 8.22 worse; 1 study, 56 participants) with PRP injection. At 12 months, function was 26.7 points with laser application and 8.5 points better (95% CI 19.32 better to 2.32 worse; 1 study, 56 participants) with PRP injection (Analysis 8.2).
8.2. Analysis.

Comparison 8: PRP versus laser applications, Outcome 2: Function
Treatment success
The only study in this comparison did not report this outcome (Tetschke 2015).
Minor outcomes
The only study in this comparison did not report any minor outcomes (Tetschke 2015).
Harms
Withdrawal due to adverse events
The only study in this comparison did not measure this outcome (Tetschke 2015).
Adverse events
Tetschke 2015 reported no adverse events in either group (estimates could not be calculated).
Autologous blood injection versus polidocanol injection
Benefits
Pain relief
The only study in this comparison did not measure this outcome (Branson 2016).
Mean pain
The only study in this comparison did not measure this outcome (Branson 2016).
Function
Autologous blood injection may not improve function compared with polidocanol injection (low‐certainty evidence; downgraded for bias and imprecision; 95% CI includes both clinically important benefit and harm at all time points).
At 6 weeks, function (0 to 100; lower is better) was 9.2 points with polidocanol injection and 4.4 points worse (95% CI 10.76 better to 19.56 worse; 1 study, 30 participants) with autologous blood injection. At 3 months, function was 19.9 points with polidocanol injection and 2.1 points better (95% CI 16.78 better to 12.58 worse; 1 study, 30 participants) with autologous blood injection. At 6 months, function was 28.9 points with polidocanol injection and 0.5 points worse (95% CI 15.21 better to 16.21 worse; 1 study, 30 participants) with autologous blood injection (Analysis 9.1).
9.1. Analysis.

Comparison 9: Autologous blood versus polidocanol injection, Outcome 1: Function
Treatment success
Autologous blood injection may not improve treatment success rates compared with polidocanol injection (very low‐certainty evidence; downgraded for bias and twice for very serious imprecision; 95% CI overlaps large effect in both directions).
Treatment success (completely recovered or much improved) rates were as follows: 2 out of 16 (13%) with polidocanol injection versus 3 out of 14 (21%) with autologous blood injection (RR 1.71, 95% CI 0.33 to 8.83; 1 study) at 6 weeks; 6 out of 16 (38%) with polidocanol injection versus 5 out of 14 (36%) with autologous blood injection (RR 0.95, 95% CI 0.37 to 2.45; 1 study) at 3 months; and 13 out of 16 (81%) with polidocanol injection versus 9 out of 14 (64%) with autologous blood injection (RR 0.79, 95% CI 0.5 to 1.25; 1 study) at 6 months (Analysis 8.3).
8.3. Analysis.

Comparison 8: PRP versus laser applications, Outcome 3: Treatment success
Minor outcomes
The only study in this comparison did not measure this outcome (Branson 2016).
Harms
Withdrawal due to adverse events
The only study in this comparison did not measure this outcome (Branson 2016).
Adverse events
The only study in this comparison did not measure this outcome (Branson 2016).
Sensitivity analyses
We performed two sensitivity analyses to assess the effect of excluding studies with high or unclear risk for selection and detection bias for pain and function at the primary time point of 3 months. Sensitivity analyses were performed only for the primary comparison (autologous blood or PRP injection versus placebo).
Removing studies with inadequate or unclear allocation concealment ‐ Behera 2015; Mishra 2014; Montalvan 2015; Yerlikaya 2018 ‐ did not have a clinically important effect on pain estimates (0 to 10 scale; higher is worse) at 3 months. Clinically important benefit with autologous blood or PRP injection was unlikely both with (mean difference (MD) ‐0.16, 95% CI ‐0.60 to 0.29) and without (MD 0.40, 95% CI ‐0.27 to 1.08) studies with inadequate or unclear allocation concealment (Analysis 10.1).
10.1. Analysis.

Comparison 10: Sensitivity analysis (mean pain and function at 3 months), Outcome 1: Pain at 3 months (low vs high or unclear risk of selection bias)
Removing studies with inadequate or unclear allocation concealment ‐ Behera 2015; Mishra 2014; Montalvan 2015 ‐ did not have a clinically important effect on function estimates (0 to 100; higher is worse) at 3 months. Clinically important benefit with autologous blood or PRP injection was unlikely both with (MD ‐1.86, 95% CI ‐4.97 to 1.25) and without (MD ‐0.01, 95% CI ‐4.80 to 4.78) inadequate or unclear allocation concealment (Analysis 10.2).
10.2. Analysis.

Comparison 10: Sensitivity analysis (mean pain and function at 3 months), Outcome 2: Function at 3 months (low vs unclear or high selection bias)
Removing one study with inadequate or unclear participant blinding ‐ Behera 2015 ‐ did not have an important effect on pain estimates (0 to 10; higher is worse). Clinically important benefit was unlikely both with (MD ‐0.16, 95% CI ‐0.60 to 0.29) and without (MD 0.00, 95% CI‐0.47 to 0.47) the only study with inadequate or unclear participant blinding (Analysis 10.3).
10.3. Analysis.

Comparison 10: Sensitivity analysis (mean pain and function at 3 months), Outcome 3: Pain at 3 months (adequate vs inadequate participant blinding)
Removing studies with inadequate or unclear participant blinding ‐ Behera 2015; Palacio 2016 ‐ did not have an important effect on function estimates (0 to 100; higher is worse). Clinically important benefit was unlikely both with (MD ‐1.86, 95% CI ‐4.97 to 1.25) and without (MD ‐0.23, 95% CI ‐3.74 to 3.29) studies with inadequate or unclear participant blinding (Analysis 10.4).
10.4. Analysis.

Comparison 10: Sensitivity analysis (mean pain and function at 3 months), Outcome 4: Function at 3 months (adequate vs inadequate participant blinding)
When we compared fixed‐effect estimates to random‐effects estimates (autologous blood or PRP versus placebo), we did not find evidence of small‐sample bias (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; only random‐effects estimates are shown).
Subgroup analysis
PRP versus autologous blood
Data from seven placebo‐controlled trials were available for analysis at the primary time point (3 months) comparing PRP versus placebo; two trials (with 98 participants receiving autologous blood) compared autologous blood to placebo (Linnanmäki 2020; Wolf 2011). We could include only mean pain and function in this analysis (Analysis 12.1; Analysis 12.2).
12.1. Analysis.

Comparison 12: Subgroup PRP versus autologous blood at 3 months, Outcome 1: Mean pain at 3 months
12.2. Analysis.

Comparison 12: Subgroup PRP versus autologous blood at 3 months, Outcome 2: Mean function at 3 months
The type of injected product did not seem to modify the treatment effect (subgroup heterogeneity: I² = 0% in pain, I² = 22.6% in function). Mean difference in pain with PRP versus placebo was ‐0.19 (95% CI ‐0.63 to 0.25) and with autologous blood versus placebo was ‐0.12 (95% CI ‐1.40 to 1.15). Mean difference in function with PRP versus placebo was ‐2.30 (95% CI ‐5.24 to 0.64) and with autologous blood versus placebo was 0.50 (95% CI ‐6.56 to 7.55).
Leukocyte‐rich versus leukocyte‐poor PRP injection versus placebo
Data from up to seven trials were available for this subgroup analysis. We found no important differences between leukocyte‐rich and leukocyte‐poor PRP versus placebo in pain, function, treatment success, or adverse events.
At 3 months, mean difference in pain was ‐0.21 (95% CI ‐0.71 to 0.30; 292 participants, 3 studies) for leukocyte‐rich and ‐0.07 (95% CI ‐0.80 to 0.66; 193 participants, 4 studies) for leukocyte‐poor PRP versus placebo (Analysis 11.1); mean difference in function was ‐2.34 (95% CI ‐6.91 to 2.23; 272 participants, 3 studies) for leukocyte‐rich and ‐0.09 (95% CI ‐8.36 to 8.18; 132 participants, 3 studies) for leukocyte‐poor PRP versus placebo (Analysis 11.2); risk ratio for treatment success was 1.03 (95% CI 0.67 to 1.59; 275 participants, 2 studies) for leukocyte‐rich and 0.75 (95% CI 0.53 to 1.06; 107 participants, 2 studies) for leukocyte‐poor PRP versus placebo (Analysis 11.3); and risk ratio for adverse events was 1.14 (95% CI 0.71 to 1.84; 270 participants, 2 studies) for leukocyte‐rich and 1.15 (95% CI 0.53 to 2.51; 155 participants, 3 studies) for leukocyte‐poor PRP versus placebo (Analysis 11.4).
11.1. Analysis.

Comparison 11: Subgroup leukocyte‐rich vs leukocyte‐poor PRP at 3 months, Outcome 1: Mean pain
11.2. Analysis.

Comparison 11: Subgroup leukocyte‐rich vs leukocyte‐poor PRP at 3 months, Outcome 2: Function
11.3. Analysis.

Comparison 11: Subgroup leukocyte‐rich vs leukocyte‐poor PRP at 3 months, Outcome 3: Treatment success
11.4. Analysis.

Comparison 11: Subgroup leukocyte‐rich vs leukocyte‐poor PRP at 3 months, Outcome 4: Adverse events
Other planned subgroup analyses could not be performed because included trials did not use frozen products.
Discussion
Summary of main results
Autologous blood or PRP injection versus placebo
Moderate‐certainty evidence indicates that autologous blood or platelet‐rich plasma (PRP) injection probably provides little or no improvement in pain or function for people with lateral elbow pain up to 3 months compared with placebo. Mean differences were clearly under minimal clinically important differences (MCIDs) across outcomes at all time points, and 95% confidence intervals suggest that clinically important benefit is unlikely.
The uncertainty of evidence was related to flaws in design or reporting of included studies, subjecting them to high or unclear risk of bias in various domains. We did not identify any studies comparing autologous blood or PRP injection to placebo with low risk of bias in all domains. However, as studies with risk of bias did not show benefit, we consider it unlikely that unbiased studies will show meaningful benefits.
At 12 months, uncertainty was greater regarding pain and function results, as 95% confidence intervals overlapped with clinically important benefit, and statistical heterogeneity was substantial (largely driven by Behera 2015, with unclear allocation concealment and participant blinding). Removing Behera 2015 decreased heterogeneity, and the estimate aligned with findings from the earlier time points showing no benefit with confidence intervals not including MCID values.
Sensitivity analyses suggest that estimates are robust to possible selection or detection bias; removing studies with inadequate or unclear allocation concealment or participant blinding supported findings from the primary analysis.
Very low‐quality evidence suggests that autologous blood or PRP injection may not improve treatment success, and low‐quality evidence suggests that autologous blood or PRP injection may increase the risk for adverse events. None of the studies in the primary comparison assessed health‐related quality of life. Although reported harms were mostly transient (injection site pain), they were not balanced by any clinically meaningful benefit.
One study found a difference in pain relief in a subset of participants who were followed up to 6 months based on a post‐hoc decision to continue follow‐up for a subset of participants (Mishra 2014). At 3 months, study authors did not report this outcome, and they did not respond to our queries. This may be a spurious finding related to alterations in study design and selective reporting; benefit was not corroborated when mean pain (or function) values were compared at any time point in that particular study, or in the meta‐analysis.
Other comparisons
When compared, low‐certainty evidence suggests that autologous blood or PRP injection is inferior to glucocorticoid injection for pain and function in the first 6 weeks of follow‐up. This transient effect vanishes by 3 months' follow‐up. Low‐certainty evidence suggests that autologous blood or PRP injection may result in greater improvement in pain and function after 6 months' follow‐up. However, confidence intervals overlapped with MCID for pain and function, suggesting that we are uncertain whether the difference is clinically important. Furthermore, as glucocorticoid injection exerts short‐term effects measured only in weeks (up to 6 to 8), the biological rationale for long‐term benefit or harm of glucocorticoid injection is unclear.
Based on moderate‐certainty evidence from four trials (Creaney 2011;Linnanmäki 2020; Raeissadat 2014; Thanasas 2011), PRP probably does not improve pain, function, or treatment success compared with autologous blood. Uncertainty is related to risk of bias in the included studies. This casts doubt over the biological rationale of concentrating platelet‐derived growth factors into the area of pathology to stimulate angiogenesis and healing. It also suggests that the extra cost incurred by the centrifugation process probably is not justified.
Subgroup analyses performed at the primary time point of this review (3 months) suggest that the type of PRP (leukocyte‐rich versus leukocyte‐poor) does not modify treatment effects with regard to mean pain or function, or rates of treatment success.
Other comparisons mainly supported our conclusion from the primary analysis. We did not find evidence of clinically important benefit when autologous blood or PRP injection was compared to extracorporeal shock wave therapy (ESWT), laser applications, or surgery, or when it was given in conjunction with dry needling or tennis elbow strap and exercise. Most of these comparisons included only one trial; thus the results were imprecise. Also, the included studies did not blind participants. Lack of blinding may bias outcomes, but as the comparators were also active treatments with potential placebo effects, it is difficult to say whether bias would alter findings to a considerable extent.
Overall completeness and applicability of evidence
The results of this review likely can be applied to a typical lateral elbow pain population. Studies included participants from 18 countries, and mean age was between 36 years and 53 years ‐ a typical age group for tendinopathy. All participants had lateral elbow pain (mean duration 1 month to 22 months) and were clinically diagnosed to have pain and soreness over the lateral humeral epicondyle. One study also included participants with medial elbow pain but they not report outcomes separately (Martin 2019). As only 11 (15%) participants had medial elbow pain at baseline, we included this study in the analyses.
As long as we have no evidence to indicate that autologous blood or PRP injection improves symptoms in this population (placebo comparison), comparisons to other active treatments offer little additional information. One study assessed the efficacy of PRP given in conjunction with dry needling, which may be analogous to comparison with placebo injection. It is unclear to what extent the observed improvements are related to needle injury and subsequent response in the tendinopathy area, and how much can be attributed to the natural course of the condition. All these aspects compromise the external validity of all other comparisons except placebo.
The results from this review are likely applicable to both PRP and autologous blood injections. We found moderate‐certainty evidence to show that treatment effects do not differ between PRP and autologous blood. Regarding the most pertinent comparison ‐ autologous blood or PRP injection versus placebo ‐ there was only one small study (19 participants receiving autologous blood or saline) injecting autologous blood; thus estimates were imprecise with autologous blood (Wolf 2011).
We found no evidence to support the hypothesis that leukocyte‐rich PRP would be beneficial as opposed to leukocyte‐poor PRP for this population (Fitzpatrick 2017). We did not perform other subgroup analyses, as we did not identify any previous evidence of possible interactions, and no studies used fresh versus frozen products.
For the primary comparison, study authors administered one injection of autologous blood or PRP except in one study (Martin 2019), which administered two injections to participants. Evidence that the number of injections may have any impact on outcomes is limited (Glanzmann 2015), and estimates from Martin 2019 were in line with those of other studies.
None of the included studies for the primary comparison measured health‐related quality of life; thus we could not calculate any estimate for this outcome. As the typical symptom is pain, and functional disturbances strongly correlate with pain (Rompe 2007), it is unlikely that generic health‐related quality of life measures would capture effects beyond pain and function measures. Estimates for pain relief and global perceived treatment success were imprecise; future trials may improve the certainty regarding these outcomes.
Quality of the evidence
Regarding the primary comparison autologous blood or PRP injection versus placebo, the certainty of evidence was moderate for mean pain and function, low for adverse events, and very low for pain relief, treatment success, and withdrawal due to adverse events.
We downgraded the evidence for pain and function once because none of the included trials had low risk of bias in all domains. At 12 months' follow‐up, the confidence intervals for mean pain and function overlapped with clinically important benefit; hence we downgraded the certainty of evidence (another step) to low.
Regarding pain relief, we found no data at 3 months (very low‐certainty evidence), but at 6 months, one study reported this outcome. We downgraded this outcome at 6 months due to bias and low numbers of events.
For adverse events, the certainty of evidence was low, as we downgraded the evidence two levels due to bias and imprecision because the confidence intervals overlapped with no effect. Regarding treatment success, we further downgraded the evidence to very low due to indirectness, in addition to bias and imprecision. None of the studies measured perceived global success but rather reported treatment success based on various definitions, usually using a cutoff value for pain or function score. None of the studies for the primary comparison reported health‐related quality of life; thus we could not calculate estimates (very low‐certainty evidence).
For PRP versus glucocorticoid injection, we downgraded the evidence for pain due to risk of bias and inconsistency; the statistical heterogeneity (I² = 72% to 77%) could not be explained by study characteristics or treatment protocols, and estimates from the included studies ranged from no effect to clinically important effect. For function, we downgraded evidence for bias and imprecision; although we considered downgrading for inconsistency, this was not done, as the direction of effect was consistent across studies.
For other comparisons, the certainty of evidence was low to very low due to bias (primarily detection bias due to lack of blinding) and imprecision. Only for PRP versus autologous blood (and PRP versus surgery) for mean pain, the confidence intervals for function were precise enough to exclude clinically important effects; thus the evidence was graded as moderate certainty.
Potential biases in the review process
We searched all relevant databases with no language restrictions, as well as registries for ongoing trials, and to the best of our knowledge, we identified all relevant trials. Two review authors independently assessed trials for potential inclusion, performed data extraction, and conducted risk of bias assessments, with a third review author adjudicating in case of discrepancy.
One review author (TK) is the study investigator for a trial included in this review (Linnanmäki 2020); another review author (RB) is the study investigator for an ongoing trial in this review (ACTRN12613000616774). To avoid any bias, these trials were independently assessed by two other review authors to discern whether they fulfilled inclusion criteria for this review. Neither review author was involved in data extraction nor risk of bias assessments for his or her own trial. We identified ten ongoing trials comparing PRP with placebo (ACTRN12613000616774; ChiCTR1900024425; EUCTR2013‐000478‐32‐ES; EUCTR2013‐004875‐12‐CZ; ISRCTN12951626; NCT01476605; NCT03984955; NCT03987256; NTR4569; NTR5005); once published, data from these trials may increase the precision of estimates. One study awaiting classification is a placebo‐controlled trial (Chiavaras 2014); similar to the ongoing trials mentioned above, results of this trial may affect estimates.
Agreements and disagreements with other studies or reviews
Several systematic reviews have assessed the effects of autologous blood or PRP injection for lateral elbow pain, with conflicting conclusions ranging from positive in Ahmad 2013; Arirachakaran 2016; Dong 2016; Mi 2017; and Murray 2015 to strong evidence against in de Vos 2014.
Some positive early reviews included non‐randomised trials or observational studies (Ahmad 2013; Rabago 2009); these review authors did not perform meta‐analyses. Some of the positive reviews base their conclusions on the comparison against glucocorticoid injection and not against placebo (Arirachakaran 2016; Mi 2017; Murray 2015). Regarding this specific comparison, their conclusions are in line with ours. However, the efficacy of treatment should first be assessed against placebo; only if the treatment is shown to work, comparison against other (effective) treatments is meaningful.
Authors' conclusions
Implications for practice.
Data in this review do not support the use of autologous blood or PRP injection for treatment of lateral elbow pain. These treatments probably provide little or no benefit for pain or function, and it is uncertain whether they improve treatment success or increase withdrawal due to adverse events. Although risk for harm may not be increased compared with placebo injection, there is always a small risk of infection and pain related to injection therapies, and as long as no evidence shows benefit, the costs or potential harms, even if minimal, are not justified.
Most of the participants in the included studies assessed their pain as low (< 3 on a 0 to 10 scale) after placebo injection. This is in line with the known benign natural course of the condition. However, patients with lateral elbow pain could have pain and disability that persist for a long time.
Implications for research.
Future trials should consider comparing PRP injection only to placebo injection and should follow rigorous research standards to minimise the risk of bias. As long as no solid evidence is available on the efficacy of PRP compared to placebo, comparison to other treatment modalities provides little value.
The data in this review do not provide any viable hypotheses about whether some subgroups of people or some variety in treatment regimens (e.g. multiple injections) or in PRP preparations would yield more favourable outcomes.
Regarding pain and function, the included studies followed up more than 500 participants, findings were robust to selection and detection biases, and it is unlikely that new trials would show clinically important benefit in these outcomes for up to 6 months. However, at later time points, new studies may affect the estimates, although a biological rationale is missing for the late onset of possible effects.
Given that results at 12 months show imprecision, future trials could follow up with participants up to 1 year to improve the certainty of estimates for longer follow‐up. Trialists should consider using core outcome sets proposed for tendinopathy trials to facilitate aggregation of data in future meta‐analyses (Vicenzino 2020).
History
Protocol first published: Issue 2, 2014
Acknowledgements
We thank Tamara Rader, Knowledge Translation Specialist, Cochrane Musculoskeletal Group, for designing the search strategy, and Andrew Barnett, for help in verifying and handling the data.
Appendices
Appendix 1. CENTRAL (Ovid) search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <Sep 2020>
Search Strategy:
1 Tennis Elbow/
2 (Lateral adj2 epicondylitis).tw.
3 (tennis adj2 elbow).tw.
4 (lateral adj2 epicondylalgia).tw.
5 (lateral adj2 elbow).tw.
6 (elbow adj2 pain).tw.
7 (elbow adj3 tendinopathy).tw.
8 (elbow adj3 tendinitis).tw.
9 or/1‐8
10 Blood Transfusion, Autologous/
11 platelet‐rich plasma/
12 ("platelet rich plasma" or "thrombocyte rich plasma" or PRP or auto‐transfusion$ or autotransfusion$ or orthokin$ or regenokin$ or (autologous adj3 (blood or serum or plasma))).tw.
13 or/10‐12
14 9 and 13
Appendix 2. MEDLINE (Ovid) Search strategy
Database: Ovid MEDLINE(R) <1946 to September 2020>
Search Strategy:
1 Tennis Elbow/
2 (Lateral adj2 epicondylitis).tw.
3 (tennis adj2 elbow).tw.
4 (lateral adj2 epicondylalgia).tw.
5 (lateral adj2 elbow).tw.
6 (elbow adj2 pain).tw.
7 (elbow adj3 tendinopathy).tw.
8 (elbow adj3 tendinitis).tw.
9 or/1‐8
10 Blood Transfusion, Autologous/
11 exp Platelet‐Rich Plasma/
12 ("platelet rich plasma" or "thrombocyte rich plasma" or PRP or auto‐transfusion$ or autotransfusion$ or orthokin$ or regenokin$ or (autologous adj3 (blood or serum or plasma))).tw.
13 or/10‐12
14 9 and 13
15 randomized controlled trial.pt
16 controlled clinical trial.pt
17 randomized.ab
18 placebo.ab
19 drug therapy.fs
20 randomly.ab
21 trial.ab
22 groups.ab
23 or/15‐22
24 exp animals/ not humans.sh
25 23 not 24
26 14 and 25
Appendix 3. Embase (Ovid) Search Strategy
Database: Embase <1974 to 2020 September>
Search Strategy:
1 exp Tennis Elbow/
2 (Lateral adj2 epicondylitis).tw.
3 (tennis adj2 elbow).tw.
4 (lateral adj2 epicondylalgia).tw.
5 (lateral adj2 elbow).tw.
6 (elbow adj2 pain).tw.
7 (elbow adj3 tendinopathy).tw.
8 (elbow adj3 tendinitis).tw.
9 or/1‐8
10 exp blood autotransfusion/
11 exp thrombocyte rich plasma/
12 ("platelet rich plasma" or "thrombocyte rich plasma" or PRP or auto‐transfusion$ or autotransfusion$ or orthokin$ or regenokin$ or (autologous adj3 (blood or serum or plasma))).tw.
13 or/10‐12
14 random$.tw
15 factorial$.tw
16 crossover$.tw
17 cross over.tw
18 cross‐over.tw
19 placebo$.tw
20 (doubl$ adj blind$).tw
21 (singl$ adj blind$).tw
22 assign$.tw
23 allocat$.tw
24 volunteer$.tw
25 crossover procedure/
26 double blind procedure/
27 randomized controlled trial/
28 single blind procedure/
29 or/14‐28
30 9 and 13 and 29
Appendix 4. Clinicaltrials.gov
lateral epicondylitis or elbow in Condition
blood or platelet in Intervention
Appendix 5. WHO Registry of Trials Search Strategy
lateral epicondylitis or elbow in Condition
blood or platelet in Intervention
Data and analyses
Comparison 1. Autologous blood or PRP injection versus placebo injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain relief ≥ 30% or ≥ 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 6 months | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.08, 1.72] |
| 1.2 Mean pain (VAS 0 to 10, PRTEE) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 to 3 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐0.65, 4.65] |
| 1.2.2 > 3 weeks to 6 weeks | 7 | 570 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.14, 0.65] |
| 1.2.3 > 6 weeks to 3 months | 8 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.60, 0.29] |
| 1.2.4 > 3 months to 6 months | 7 | 387 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.49, 0.59] |
| 1.2.5 > 6 months to 12 months | 5 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.78, 0.39] |
| 1.3 Function (DASH, MMCPIE, Roles‐Maudsley) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Up to 3 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 12.00 [‐5.33, 29.33] |
| 1.3.2 > 3 weeks to 6 weeks | 6 | 473 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐1.64, 4.25] |
| 1.3.3 > 6 weeks to 3 months | 8 | 502 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐4.97, 1.25] |
| 1.3.4 > 3 months to 6 months | 7 | 379 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐8.62, 6.31] |
| 1.3.5 > 6 months to 12 months | 4 | 203 | Mean Difference (IV, Random, 95% CI) | ‐5.81 [‐16.66, 5.05] |
| 1.4 Treatment success (> 25% improvement in pain or function) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 > 6 weeks to 3 months | 4 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 1.5 Withdrawal due to AEs | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 Total | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 2.92] |
| 1.6 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Total | 5 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.72] |
Comparison 2. Autologous blood or PRP injection versus glucocorticoid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain relief ≥ 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 6 weeks | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.53] |
| 2.1.2 1 year | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.03, 2.71] |
| 2.2 Mean pain | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Up to 3 weeks | 5 | 280 | Mean Difference (IV, Random, 95% CI) | 2.06 [0.67, 3.45] |
| 2.2.2 > 3 weeks to 6 weeks | 13 | 707 | Mean Difference (IV, Random, 95% CI) | 0.99 [0.21, 1.77] |
| 2.2.3 > 6 weeks to 3 months | 11 | 627 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.71, ‐0.59] |
| 2.2.4 > 3 months to 6 months | 8 | 427 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐2.21, ‐0.90] |
| 2.2.5 > 6 months to 1 year | 4 | 258 | Mean Difference (IV, Random, 95% CI) | ‐1.59 [‐2.22, ‐0.97] |
| 2.2.6 > 1 year | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.19, ‐1.03] |
| 2.3 Function (various scales) | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 Up to 3 weeks | 3 | 170 | Mean Difference (IV, Random, 95% CI) | 16.46 [‐4.23, 37.15] |
| 2.3.2 > 3 weeks to 6 weeks | 13 | 724 | Mean Difference (IV, Random, 95% CI) | 6.11 [1.79, 10.44] |
| 2.3.3 > 6 weeks to 3 months | 12 | 635 | Mean Difference (IV, Random, 95% CI) | ‐10.19 [‐14.16, ‐6.21] |
| 2.3.4 > 3 months to 6 months | 7 | 374 | Mean Difference (IV, Random, 95% CI) | ‐5.07 [‐12.66, 2.52] |
| 2.3.5 > 6 months to 1 year | 4 | 317 | Mean Difference (IV, Random, 95% CI) | ‐8.94 [‐12.09, ‐5.78] |
| 2.3.6 > 1 year | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐18.90 [‐28.27, ‐9.53] |
| 2.4 Treatment success | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Up to 6 weeks | 5 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 0.95] |
| 2.4.2 > 6 weeks to 3 months | 3 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.08, 2.26] |
| 2.4.3 > 3 months to 6 months | 3 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.23, 4.44] |
| 2.4.4 > 6 months to 1 year | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.55, 4.75] |
| 2.4.5 > 1 year | 2 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.31, 3.16] |
| 2.5 Adverse events | 5 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.65, 4.12] |
| 2.6 Grip strength | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 Up to 3 weeks | 3 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.87, ‐0.16] |
| 2.6.2 > 3 weeks to 6 weeks | 6 | 348 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.68, 0.16] |
| 2.6.3 > 6 weeks to 3 months | 6 | 348 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.19, 0.93] |
| 2.6.4 > 3 months to 6 months | 2 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.13, 0.83] |
| 2.6.5 > 6 months to 1 year | 2 | 118 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.29, 1.03] |
Comparison 3. PRP and dry needling versus dry needling alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 > 6 weeks to 3 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐2.13, 1.85] |
| 3.1.2 > 3 months to 6 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐2.88, 2.18] |
| 3.2 Function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 > 3 weeks to 6 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 9.60 [‐2.49, 21.69] |
| 3.2.2 > 6 weeks to 3 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐16.88, 22.48] |
| 3.2.3 > 3 months to 6 months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 5.70 [‐14.36, 25.76] |
| 3.2.4 > 6 months to 12 months | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐9.70, 18.30] |
| 3.3 Withdrawal due to adverse events | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.18, 16.99] |
| 3.4 Adverse events | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.18, 16.99] |
Comparison 4. PRP versus autologous blood.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean pain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 > 3 weeks to 6 weeks | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.21, 0.73] |
| 4.1.2 > 6 weeks to 3 months | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.11, 0.30] |
| 4.1.3 > 3 months to 6 months | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.04, 0.48] |
| 4.1.4 > 6 months to 12 months | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐1.12, 1.22] |
| 4.2 Function (various scales) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 > 3 weeks to 6 weeks | 4 | 276 | Mean Difference (IV, Random, 95% CI) | ‐3.44 [‐6.60, ‐0.28] |
| 4.2.2 > 6 weeks to 3 months | 4 | 292 | Mean Difference (IV, Random, 95% CI) | ‐3.25 [‐6.33, ‐0.17] |
| 4.2.3 > 3 months to 6 months | 4 | 297 | Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐6.02, 0.37] |
| 4.2.4 > 6 months to 12 months | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐8.53, 7.11] |
| 4.3 Treatment success | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.37] |
| 4.4 Adverse events | 2 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.90, 5.62] |
Comparison 5. Autologous blood versus ESWT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain relief > 50% | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 6 weeks | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.21] |
| 5.1.2 1 year | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 5.2 Mean pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 > 3 weeks to 6 weeks | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.28, 1.54] |
| 5.2.2 > 6 weeks to 3 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.75, 1.33] |
| 5.2.3 > 3 months to 6 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.78, 1.24] |
| 5.2.4 > 6 months to 1 year | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.61, 1.07] |
| 5.3 Function (various scales) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.3.1 > 3 weeks to 6 weeks | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐1.56, 9.16] |
| 5.3.2 > 6 weeks to 3 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐5.82, 8.62] |
| 5.3.3 > 3 months to 6 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐4.17, 7.17] |
| 5.3.4 > 6 months to 1 year | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐5.98, 4.18] |
| 5.4 Grip strength | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.4.1 > 3 weeks to 6 weeks | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐4.90, 5.70] |
| 5.4.2 > 6 weeks to 3 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.84, 5.04] |
| 5.4.3 > 3 months to 6 months | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐3.37, 3.97] |
| 5.4.4 > 6 months to 1 year | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐5.73, 1.13] |
| 5.5 Adverse events | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.75] |
Comparison 6. PRP versus surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean pain | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 6 weeks | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐0.38, 1.98] |
| 6.1.2 3 months | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.40, 1.12] |
| 6.1.3 6 months | 2 | 159 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.91, 1.20] |
| 6.1.4 12 months | 2 | 153 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐1.86, 2.64] |
| 6.1.5 24 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 5.00 [4.02, 5.98] |
| 6.2 Function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.2.1 6 weeks | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 7.00 [‐5.94, 19.94] |
| 6.2.2 3 months | 2 | 153 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐19.63, 18.45] |
| 6.2.3 6 months | 2 | 159 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐15.92, 18.63] |
| 6.2.4 12 months | 2 | 153 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐13.27, 16.33] |
| 6.2.5 24 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 48.00 [40.20, 55.80] |
| 6.3 Grip strength | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.3.1 3 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐0.99, 2.99] |
| 6.3.2 6 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐26.80 [‐29.03, ‐24.57] |
| 6.3.3 12 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐23.70 [‐25.59, ‐21.81] |
| 6.3.4 24 months | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐25.60 [‐27.31, ‐23.89] |
Comparison 7. Autologous blood plus tennis elbow strap and exercise versus tennis elbow strap and exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Mean pain | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.86, ‐0.42] |
| 7.1.1 > 3 weeks to 6 weeks | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.86, ‐0.42] |
| 7.2 Mean function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 > 3 weeks to 6 weeks | 1 | 105 | Mean Difference (IV, Random, 95% CI) | ‐7.81 [‐12.71, ‐2.91] |
| 7.2.2 3 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐2.19, 5.39] |
| 7.2.3 6 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 2.46 [‐0.41, 5.33] |
| 7.3 Hand grip strength | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.3.1 3 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐7.10, 2.70] |
| 7.3.2 6 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐8.85, 2.85] |
| 7.4 Treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.4.1 3 months | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.08] |
| 7.4.2 6 months | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
Comparison 8. PRP versus laser applications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 >6 weeks to 3 months | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐2.13, 0.13] |
| 8.1.2 >3 months to 6 months | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.90, 0.10] |
| 8.1.3 >6 months to 12 months | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.03, 0.23] |
| 8.2 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.2.1 3 month | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐20.03, 1.83] |
| 8.2.2 6 month | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐13.22, 8.22] |
| 8.2.3 12 month | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐8.50 [‐19.32, 2.32] |
| 8.3 Treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.4 Adverse events | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
8.4. Analysis.

Comparison 8: PRP versus laser applications, Outcome 4: Adverse events
Comparison 9. Autologous blood versus polidocanol injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 6 weeks | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.40 [‐10.76, 19.56] |
| 9.1.2 3 months | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐16.78, 12.58] |
| 9.1.3 6 months | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐15.21, 16.21] |
| 9.2 Treatment success | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.2.1 6 weeks | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.33, 8.83] |
| 9.2.2 3 months | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.37, 2.45] |
| 9.2.3 6 months | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.25] |
9.2. Analysis.

Comparison 9: Autologous blood versus polidocanol injection, Outcome 2: Treatment success
Comparison 10. Sensitivity analysis (mean pain and function at 3 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Pain at 3 months (low vs high or unclear risk of selection bias) | 8 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.60, 0.29] |
| 10.1.1 Adequate allocation concealment | 4 | 166 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.27, 1.08] |
| 10.1.2 Unclear or inadequate | 4 | 357 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.08, 0.02] |
| 10.2 Function at 3 months (low vs unclear or high selection bias) | 8 | 502 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐4.97, 1.25] |
| 10.2.1 Adequate | 5 | 235 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐4.80, 4.78] |
| 10.2.2 Unclear or inadequate | 3 | 267 | Mean Difference (IV, Random, 95% CI) | ‐2.93 [‐7.51, 1.65] |
| 10.3 Pain at 3 months (adequate vs inadequate participant blinding) | 8 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.60, 0.29] |
| 10.3.1 Adequate | 7 | 498 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.47, 0.47] |
| 10.3.2 Unclear or inadequate | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.91, 0.11] |
| 10.4 Function at 3 months (adequate vs inadequate participant blinding) | 8 | 502 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐4.97, 1.25] |
| 10.4.1 Adequate | 6 | 437 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐3.74, 3.29] |
| 10.4.2 Unclear or inadequate | 2 | 65 | Mean Difference (IV, Random, 95% CI) | ‐5.84 [‐10.98, ‐0.70] |
Comparison 11. Subgroup leukocyte‐rich vs leukocyte‐poor PRP at 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Mean pain | 6 | 485 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.56, 0.26] |
| 11.1.1 Leukocyte rich | 3 | 292 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.71, 0.30] |
| 11.1.2 Leukocyte poor | 4 | 193 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.80, 0.66] |
| 11.2 Function | 6 | 404 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐5.61, 1.82] |
| 11.2.1 Leukocyte rich | 3 | 272 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐6.91, 2.23] |
| 11.2.2 Leukocyte poor | 3 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐8.36, 8.18] |
| 11.3 Treatment success | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.65, 1.24] |
| 11.3.1 Leukocyte rich | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.59] |
| 11.3.2 Leucocyte poor | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| 11.4 Adverse events | 5 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.72] |
| 11.4.1 Leucocyte rich | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.71, 1.84] |
| 11.4.2 Leucocyte poor | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.53, 2.51] |
Comparison 12. Subgroup PRP versus autologous blood at 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Mean pain at 3 months | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1.1 PRP vs placebo | 7 | 534 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.63, 0.25] |
| 12.1.2 AB vs placebo | 2 | 98 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐1.40, 1.15] |
| 12.2 Mean function at 3 months | 8 | 581 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐4.60, 0.83] |
| 12.2.1 PRP vs placebo | 7 | 483 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐5.30, 0.82] |
| 12.2.2 AB vs placebo | 2 | 98 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐6.56, 7.55] |
| 12.3 Withdrawals due to adverse events | 7 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 2.92] |
| 12.3.1 PRP vs placebo | 6 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 2.92] |
| 12.3.2 AB vs placebo | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
12.3. Analysis.

Comparison 12: Subgroup PRP versus autologous blood at 3 months, Outcome 3: Withdrawals due to adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arik 2014.
| Study characteristics | ||
| Methods |
Design: single‐centre 2‐arm parallel randomised controlled trial Setting: Orthopaedics and Traumatology Department, Antalya Education and Research Hospital, Turkey Timing: May 2012 to May 2013 Interventions: autologous blood injection vs glucocorticoid injection Sample size: not reported Analysis: intention‐to‐treat |
|
| Participants |
Numbers of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 80 (40 to autologous blood group, 40 to glucocorticoid group) Number included in analysis: 80 (40 in autologous blood group, 40 in glucocorticoid group) Inclusion criteria Lateral epicondylitis diagnosed based on the following criteria
Exclusion criteria
Baseline characteristics Autologous blood injection group
Glucocorticoid injection group
Pre‐treatment group differences: no baseline differences were found between the 2 groups |
|
| Interventions |
Autologous blood injection A single injection of 2 mL of autologous venous blood, which was collected from the antecubital fossa of the ipsilateral side, was mixed with 1 mL of 2% prilocaine hydrochloride and administered Glucocorticoid injection 1 mL of 40 mg methylprednisolone acetate mixed with 1 mL of 2% prilocaine hydrochloride. Injections were given by the same physician in both groups Co‐intervention All participants were instructed to abstain from heavy work; non‐steroidal anti‐inflammatory drugs or physiotherapy was not prescribed to participants in both groups |
|
| Outcomes | Outcomes were reported at baseline and at 15 days, 30 days, and 90 days after treatment in both groups Outcomes
Outcomes used in this review
Time points included in this review 1 month, 3 months, and 6 months (only elbow pain on VAS) |
|
| Notes |
Funding: no funding source was reported Trial registration: not done Withdrawal: none Adverse events Autologous blood injection group Serious adverse events No. of events = 0 No. (%) of participants affected = 0 Other adverse events No. of events = 10 No. (%) of participants affected = 10 (25%) Nature of event: increased pain for up to 2 days after autologous blood injection Total adverse events No. of events = 10 No. (%) of participants affected = 10 (25%) Glucocorticoid injection group Serious adverse events No. of events = 0 No. (%) of participants affected = 0 Other adverse events No. of events = 0 No. (%) of participants affected = 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether random allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both participants and study personnel were not blinded to the treatment received |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded, there is high risk of detection bias in the measurement of self‐reported outcomes of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | High risk | As assessors were not blinded, there is high risk of detection bias in the measurement of grip strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from this study were reported for either group |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was found and the trial was not registered |
| Other bias | Low risk | None is apparent |
Behera 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre single‐blinded parallel‐group 2‐arm randomised controlled trial Setting: Department of Orthopaedics, Postgraduate Institute of Medical Education and Research, India Timing: January 2011 to December 2011 Interventions: single leukocyte‐poor (type‐4B) platelet‐rich plasma (PRP) injection vs bupivacaine injection Sample size: not reported Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported. Number excluded: not reported. Number randomised: 25 (15 to type‐4B PRP group, 10 to bupivacaine group) Number included in analyses: 24 (15 in type‐4B PRP group, 9 in bupivacaine group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group
Bupivacaine group
Pre‐treatment group differences: no baseline differences were found between the 2 groups |
|
| Interventions |
Single leukocyte‐poor (type‐4B) PRP injection 100 mL of blood was collected into an anticoagulant blood bag and was centrifuged at 1500 rpm for 15 minutes to separate red cells from remaining components. The supernatant fluid was transferred into another blood bag. Leukocytes were filtered out using a filter (Imuguard III‐PL, Terumo Penpol, Thiruvananthapuram, India) to obtain leukocyte‐poor PRP, with platelet count between 6 and 8 × 10⁵/μL and leukocyte count a 3‐log reduction. Under ultrasonographic guidance, 3 mL of type‐4B PRP and 0.5 mL of calcium chloride was injected into the maximum hypo‐echoic area of the extensor carpi radialis brevis (ECRB) tendon using the peppering technique. Overall, 5 to 6 passes into the tendon were made using a single skin portal Bupivacaine injection 10 mL of blood was collected but was not used, and 3 mL of bupivacaine and 0.5 mL of normal saline was injected in a similar fashion as described above Co‐intervention In both groups, patients sat for 15 minutes with the arm supported in a sling after receiving the injection. They were advised to rest the arm for 2 days. They were allowed to take oral paracetamol (650 mg) for pain. After 2 days, standard wrist extensor stretching was started at home for 4 weeks under the supervision of a physiotherapist. After 4 weeks, wrist extensor muscle strengthening exercises were started under supervision, with advice to avoid strenuous activities for 3 months. Full activity was allowed after 4 months |
|
| Outcomes | Outcomes were measured at 1, 3, 6, and 12 months Study outcomes
Outcomes used in this review
Time points used in this review 1, 3, 6, and 12 months |
|
| Notes |
Funding: not reported Trial registration: not reported Withdrawal: 0/15 in the PRP group, 1/10 in the bupivacaine group Adverse events PRP group Serious adverse events No. of events = 0 No. (%) of participants affected = 0 Total adverse events No. of events = 0 No. (%) of participants affected = 0 (0%) Bupivacaine group Serious adverse events No. of events = 0 No. (%) of participants affected = 0 Total adverse events No. of events = 1 No. (%) of participants affected = 1 (10%) Nature of event: giddiness after bupivacaine injection, which resolved within 30 minutes Data analysis We reversed the direction of MMCPIE scores to read as 0 = no disability, to ensure consistency in interpretation of function scores in the meta‐analysis. We transformed VAS pain data from 0 to 100 to 0 to 10, to ensure consistency with pain data across studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study outcomes were measured by a single assessor, who was blinded to treatment allocation. However it is unclear whether participants and other personnel were blinded to group allocation |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Unclear risk | As it is unclear whether participants were blinded to the treatment they received, there could be risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐reported outcomes were measured in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/15 in the PRP group and 1/10 in the bupivacaine group were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available and the trial was not registered |
| Other bias | Low risk | None is apparent |
Branson 2016.
| Study characteristics | ||
| Methods |
Design: multi‐centre double‐blind 3‐arm parallel randomised controlled trial Setting: 3 private sports medicine clinics in Melbourne, Australia Timing: between January 2012 and September 2013 Intervention: single glucocorticoid injection vs 2 injections of autologous blood vs 2 injections of polidocanol Sample size: sample size calculation was not reported Analysis: intention‐to‐treat |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Glucocorticoid injection
Autologous blood injection
Polidocanol injection
|
|
| Interventions | Before each injection, 3 mL of blood was drawn from a cubital fossa vein of the other elbow in all participants, regardless of the allocated injection. Participants were blindfolded so they could not see syringe contents. The skin was prepared with a chlorhexidine wash, and neovascularity sites were marked on the skin with a texta pen. No local anaesthetic was injected via any injection option. An experienced musculoskeletal radiologist performed 1 of the following ultrasound‐guided injections using a 25‐g needle: Glucocorticoid injection: 1 mL betamethasone (celestone chondrose) was injected into the abnormal tendon and along the superficial tendon surface Autologous blood injection: multiple dry needling punctures of the abnormal tendon were performed to cause local bleeding. A second 25‐g needle was then used to inject 3 mL of autologous blood targeting the abnormal tendon area. At 4 weeks, participants received a second ultrasound‐guided injection Polidocanol injection: 3 mL of Lauromacrogol was injected superficial to the tendon, targeting regions of neovascularity from lateral to medial back to the normal artery. At 4 weeks, participants received a second ultrasound‐guided injection Post intervention: following the injection, all participants were seen by a physiotherapist who was unaware of the injection type. They were given an information sheet advising them regarding general activity modification (e.g. avoidance of lifting objects with a pronated forearm) and were taught an eccentric home exercise programme |
|
| Outcomes | Outcomes were measured at baseline and at 4, 12, and 26 weeks Primary outcome Global Rating of Change (GROC) measured on a 6‐point Likert scale. GROC scores were dichotomised into completely recovered or much improved vs improved, same, worse, or much worse Secondary outcomes
Outcomes used in this review
Time pointsused in this review 4, 12, and 26 weeks |
|
| Notes |
Funding: no funding was provided Trial registration: ACTRN12614000398606 Withdrawal: 5/14 (36%) withdrew from the glucocorticoid group at final follow‐up due to deterioration in their condition; no withdrawals from the autologous blood or polidocanol injection group were reported Adverse events Glucocorticoid injection Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Autologous blood injection Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Polidocanol injection Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Data analysis Study authors reported grip strength as a ratio of affected to unaffected arms; we requested them for grip strength measurements for the affected side but received no response; hence we could not use these data. We compared autologous blood to glucocorticoid and autologous blood to polidocanol. We were unable to use pain‐free grip strength, as it was reported as a ratio of affected to unaffected elbow |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done via a computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Concealed allocation was performed by a trial nurse who drew from opaque envelopes and was not involved in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study staff and participants were blinded to treatment. Blood was drawn from individuals in all 3 groups; participants were blindfolded and were unable to see contents of the syringe |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Unclear risk | Participants were blinded to treatment allocation for up to 4 weeks, then were given a second injection if they were in the polidocanol and autologous blood groups; it is not explicitly reported whether participants were blinded to the second injection |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | Study assessors were blinded to treatment allocation, and there is low risk of bias in the ultrasound assessments; however as participants were not blinded, there remains unclear risk of bias for measurement of pain‐free grip strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/14 (36%) from the glucocorticoid group at 26 weeks with final follow‐up due to deterioration in their condition; however these data were included in the final analysis, at 0/14 in the autologous blood group and 0/16 in the polidocanol group |
| Selective reporting (reporting bias) | Unclear risk | The Stratford Pain‐Free Function Questionnaire was stated as a secondary outcome in the ANZCTR trial registration, but this outcome was not measured nor reported in the paper. It is unclear whether omission of this tool is related to the results |
| Other bias | Low risk | None is apparent |
Creaney 2011.
| Study characteristics | ||
| Methods |
Design: single‐centre 2‐arm parallel‐group single‐blinded randomised controlled trial Setting: outpatient clinic, United Kingdom Timing: not reported Interventions: platelet‐rich plasma (PRP) injection vs autologous blood injection Sample size: 11 participants per group needed to detect a minimum clinically significant improvement of 25 points (SD 20) on 0 to 100‐point PTREE scale from 0 to 3 months, and 44 participants per group (or 52 per group to allow for 20% loss to follow‐up) needed to detect a minimum clinical between‐group difference of 10 points, based on a 2‐sided type 1 error rate of 5% and power of 90% Analysis: per protocol executed (data from participants lost to follow‐up or having surgery not included in analyses) |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported. Number excluded: not reported. Number randomised: 150 (80 to PRP group, 70 to autologous blood group) Number included in analyses: 130 (70 PRP, 60 autologous blood); for function: 137 (74 PRP, 63 blood) at 1 month, 123 (69 PRP, 54 blood) at 3 months, 128 (69 PRP, 59 blood) at 6 months Inclusion criteria
Exclusion criteria Previous glucocorticoid injection, autologous blood injection, or dry needling therapy Baseline characteristics Platelet‐rich plasma group Mean age (years): 53 Proportion (%) of male:female: 57:43 Mean (95% CI) PRTEE (0 to 100; 0 is no disability): 45.8 (41.9 to 49.6) Autologous blood group Mean (SD) age (years): 48 (no SD reported) Proportion (%) of male:female: 56:44 Mean (95% CI) PRTEE (0 to 100; 0 is no disability): 52.5 (48.5 to 56.5) Pretreatment group differences Groups were similar at baseline |
|
| Interventions |
Platelet‐rich plasma group All participants had blood collected from the antecubital fossa into an 8.5‐mL vacutainer tube with citrate anticoagulant using a 21‐gauge needle. Blood was spun at 2000 g for 15 minutes and 1.5 mL was siphoned from the buddy coat layer. Tendons were surface‐bathed with 2 mL of bupivacaine followed by a 2 minute wait before proceeding to the injection. Injections were ultrasound‐guided into hypo‐echogenic clefts within the tendons via a 23‐gauge needle. No dry needling was performed. An experienced musculoskeletal radiologist performed all injections. Two injections were given ‐ 1 at the initial visit and 1 at the next (after 1 month). The platelet concentration in plasma was 652 (581 to 722) × 10⁹/L Autologous blood group Autologous blood was injected via the same procedure as described above. The platelet concentration in blood was 234 (205 to 253) × 10⁹/L Follow‐up care Participants were advised to continue normal activities and to avoid physical activity or heavy lifting in the first 48 hours post intervention. Paracetamol and icing were permitted, but participants were advised to avoid anti‐inflammatory medications |
|
| Outcomes | Outcomes were reported at baseline and at 1 month (at time of second injection), 3 months, and 6 months Outcomes
Outcomes included in this review
Time points included in this review 1 month, 3 months, and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: 10/80 in the PRP group and 10/70 in the blood group were lost to follow‐up and excluded from final analysis Adverse events PRP group Total adverse events: not reported Serious adverse events: not reported Autologous blood group Total adverse events: not reported Serious adverse events: not reported Data analysis The chief investigator supplied PRTEE outcome data for individual participants on request (see Analysis 4.2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned by means of sealed envelopes, although the process is not defined |
| Allocation concealment (selection bias) | Unclear risk | Allocation was assigned via 'sealed envelopes'; insufficient information to assess whether this was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded. All participants had blood drawn, and centrifugation was done only for the PRP group. Both groups had exactly the same method of injection and the same post‐injection instructions. The study interventionist was not blinded but had no further involvement with study participants |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded to treatment, there is low risk of bias in the measurement of function and treatment success |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No objective outcomes were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10/80 in the PRP group and 10/70 in the blood group were lost to follow‐up and were excluded from final analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered and no study protocol was found |
| Other bias | Low risk | None is apparent |
Dojode 2012.
| Study characteristics | ||
| Methods |
Design: single‐centre parallel‐group 2‐arm open‐label randomised controlled trial Setting: outpatient clinic, Belgaum, India Timing: not reported. Interventions: autologous blood injection vs glucocorticoid injection Sample size: a priori sample size calculation not reported Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 60 (30 to autologous blood group, 30 to glucocorticoid group) Number included in analysis: 60 Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous blood group
Glucocorticoid group
Pretreatment group differences Groups were similar at baseline |
|
| Interventions |
Autologous blood injection 2 mL of autologous blood was drawn from the contralateral arm, mixed with 1 mL of 0.5% bupivacaine, and administered to participants. The participant's elbow was flexed to 90 degrees with palm facing down, and the needle was introduced proximal to the lateral epicondyle along the supracondylar ridge and advanced into the undersurface of the extensor carpi radialis brevis tendon Glucocorticoid injection 2 mL of methylprednisolone acetate 80 mg, mixed with 1 mL of 0.5% bupivacaine, was infiltrated at the elbow region via the procedure described above Follow‐up care Participants were advised to rest the injected limb for 3 days, with no restriction following 3 days |
|
| Outcomes | Outcomes were reported at baseline and at 1 week, 4 weeks, 12 weeks, and 6 months Outcomes
Outcomes included in this review
Time points included in this review Mean pain at 4 weeks, 12 weeks, and 6 months; success at 4 weeks and 12 weeks; adverse events at 6 months |
|
| Notes |
Funding: trial author reported receiving 'no specific grant' Trial registration: not done Withdrawal: none Adverse events Autologous blood group Total adverse events No. (%) of events: 18 (60) Nature of event: increase in pain immediately (and during the following few days) after injection Serious adverse events No. of events: 0 Glucocorticoid group Total adverse events No. (%) of events: 8 (26) Nature of event: increase in pain immediately (and during the following few days) after injection No. (%) of events: 2 (6) Nature of event: local skin atrophy Serious adverse events No. of events: 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated sequentially into 2 groups via a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Study authors used computer‐generated sequence and random numbers table but do not report how the sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and study personnel were not blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded, there is risk of bias in the measurement of pain and treatment success |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐reported outcomes were measured in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and the trial was not registered; it is unclear if 2 outcomes (proportion pain‐free and proportion with recurrence of symptoms) were pre‐planned |
| Other bias | Low risk | No other biases were apparent |
Gautam 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre parallel 2‐arm single‐blind randomised controlled trial Setting: Department of Orthopaedic Surgery, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India Timing: May 2011 to October 2012 Interventions: platelet‐rich plasma (PRP) injection vs glucocorticoid injection Sample size: not reported Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number screened: not reported Number excluded: not reported Number randomised: 30 (15 to PRP injection group, 15 to glucocorticoid injection group) Number analysed: 30 (15 in PRP injection group, 15 in glucocorticoid injection group) Inclusion criteria Recalcitrant (> 6 months) lateral epicondylitis not responsive to oral medication or non‐invasive treatment Aged 18 to 60 years Exclusion criteria
Baseline characteristics PRP injection group
Glucocorticoid injection group
Pretreatment group differences: there were no baseline differences between the 2 groups |
|
| Interventions |
Platelet‐rich plasma (PRP) injection 20 mL of blood was collected in an acid citrate dextrose vacutainer and was centrifuged at 1500 rpm for 15 minutes to separate the blood into layers of red blood cells, buffy coat of leukocytes, and plasma. Platelet counts for PRP and unprocessed blood were calculated. 2 mL of PRP was injected at the most tender point over the lateral epicondyle of the humerus via the peppering technique Glucocorticoid injection Methylprednisolone (40 mg/mL) was injected at the most tender point over the lateral epicondyle of the humerus via the peppering technique Post intervention After injection, patients rested for 30 minutes and were advised against massage or hot fomentation. Ice packs or paracetamol was advised for discomfort, rather than non‐steroidal anti‐inflammatory drugs, as the latter may interfere with platelet function |
|
| Outcomes | Outcomes were measured at 2 weeks, 6 weeks, 3 months, and 6 months Study outcomes
Outcomes used in this review
Time pointsused in this review 6 weeks, 3 months, and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: none Adverse events PRP injection group Total adverse events No.of events: not reported Serious adverse events No.of events: not reported Glucocorticoid injection group Total adverse events No.of events: not reported Serious adverse events No.of events: not reported Data analysis: Study authors do not indicate whether the reported variance is SD or SEM. We assumed SEM because of the size of the reported value in the DASH score, and because mean pain score was too low to be plausibly SD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given that 1 group had blood had drawn and 1 did not, and that no information on blinding was given, we assumed that participants were not blinded. The radiologist assessing ultrasound was blinded; however it is unclear whether the assessor evaluating other outcomes was blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As it is not reported whether participants were blinded to treatment allocation, there is risk of bias in the self‐reported outcomes including VAS, DASH, Oxford elbow score, and modified Mayo score |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | The radiologist assessing ultrasound was blinded to treatment allocation; hence there is low risk of bias in the measurement of ultrasound outcomes; however as it is unclear whether participants were blinded, we assume there is risk of bias in the measurement of hand grip strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from this study were reported |
| Selective reporting (reporting bias) | Unclear risk | This trial was not registered and the study protocol was not found; study authors have not reported the measures of variance accurately |
| Other bias | Low risk | None is apparent |
Gedik 2016.
| Study characteristics | ||
| Methods |
Design: single‐blind 2‐arm parallel randomised controlled trial Setting: Physical Medicine Rehabilitation Clinic, Adıyaman University Education and Research Hospital, Turkey Timing: not reported Interventions: autologous blood injection plus epicondyle bandage and home exercise programme vs epicondyle bandage and home exercise programme Analysis: data analysed for those who completed the study |
|
| Participants |
Number of participants Number screened: not reported Number excluded: not reported Number randomised: 70 (35 to autologous blood group, 35 to bandage and exercise group) Number included in analysis: at 4 weeks, 66 (32 in autologous blood group, 34 in bandage and exercise group). At the end of 4 weeks, 21 participants from the exercise group with poor response to treatment were given autologous blood injection, and a third comparison group was formed: 13 participants in the bandage and exercise group; 32 in the autologous blood group; 21 cross‐overs to autologous blood group Inclusion criteria Lateral epicondylitis in the last 3 months not responding to physical therapy or glucocorticoid local injection therapy Exclusion criteria
Baseline characteristics Autologous blood injection plus epicondyle bandage and home exercise programme
Epicondyle bandage and home exercise programme
Pretreatment group differences: no baseline differences were found between the 2 groups |
|
| Interventions |
Autologous blood injectionplus epicondyle bandage and home exercise programme Autologous blood injection was performed by taking 2 mL of venous blood from the other upper extremity of the participant, mixing it with 1 mL of 2% prilocaine hydrochloride, and administering this around the most sensitive point in the affected lateral epicondyle via a 21‐gauge needle. Participants received physiotherapy for lateral epicondylitis through standardised activity modulation training comprising a 4‐home exercise programme for 2 weeks (twice a day, in 3 sets) with 10 repetitive range of motion, static stretching, and strengthening exercises and lateral epicondyle bandage treatments. Oral or local medication for analgesia was permitted Epicondyle bandage and home exercise programme Participants received physiotherapy for lateral epicondylitis via standardised activity modulation training comprising a 4‐home exercise programme for 2 weeks (twice a day, in 3 sets) with 10 repetitive range of motion, static stretching, and strengthening exercises and lateral epicondyle bandage treatments. Oral or local medication for analgesia was permitted |
|
| Outcomes | Outcomes were measured at baseline and at 4 weeks, 3 months, and 6 months Study outcomes
Outcomes used in this review
Time points used in this review 3 months and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: 3/35 in the autologous group and 1/35 in the non‐injection group; no clear reasons given Adverse events Autologous blood injection plus epicondyle bandage and home exercise programme Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Epicondyle bandage and home exercise programme Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Data analysis PRTEE, hand grip strength, and response to treatment scores were entered for the autologous injection group as the experimental group and for 13 participants from the epicondyle bandage and home exercise group who were not given autologous injections at 4 weeks as the control group. As 21 participants from the control group received autologous blood injections at 4 weeks, data were entered only for 3 month and 6 month time points. We dichotomised scores for response to treatment into 2 categories ‐ 'good', 'excellent', and 'intermediate' into one category; and 'poor' into another category. We inputted good/excellent and 'intermediate' category scores for data analysis Translation: the original article was written in Turkish and was translated into English via Google Translator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random Allocation Software 2.0TM programme was used for generation of the random schedule |
| Allocation concealment (selection bias) | Unclear risk | It is unclear how group allocations were concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study personnel were blinded, but due to the nature of the intervention, participants could not be blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants could not be blinded; there could be risk of bias in the measurement of PRTEE scores and response to treatment scores |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | Although assessors measuring pain thresholds were blinded, participants rating their pain threshold and grip strength were not blinded, so agree with unclear/high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/35 (8.6%) in the autologous group and 1/35 (3%) in the non‐injection group withdrew from the study; no clear reasons were given |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered and study protocol was not available; AEs were not reported |
| Other bias | High risk | After 4 weeks' follow‐up, 21/34 (62%) participants in the bandage and exercise group received autologous blood injection due to poor response. There is risk of contamination in the exercise group; this could mask the effect of autologous blood |
Gosens 2011.
| Study characteristics | ||
| Methods |
Design: multi‐centre (2 sites) parallel‐group 2‐arm double‐blind randomised controlled trial Setting: St. Elisabeth Hospital, Tilburg, The Netherlands, and Haga Hospital, The Hague, The Netherlands Timing: May 2006 to January 2008. Interventions: platelet‐rich plasma injection vs glucocorticoid injection Sample size: 42 participants required per group to detect a 28% difference between groups in the proportion with treatment success (25% reduction in VAS pain score), based on 2‐sided type 1 (alpha) error rate of 0.05 and power of 90%. To account for loss to follow‐up, a minimum of 50 participants were randomised per group Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number of participants screened for eligibility: 106 Number excluded: 6 (did not meet inclusion criteria) Number randomised: 100 (51 to platelet‐rich plasma group, 49 to glucocorticoid group) Number included in analysis: 100 (51 in platelet‐rich plasma group, 49 in glucocorticoid group). There were 6 re‐interventions in the PRP group: 3 had an operation, 3 had re‐injection with glucocorticoids. There were 14 re‐interventions in the glucocorticoid group: 6 had an operation, 1 had re‐injection with glucocorticoids, and 7 crossed over to the PRP group Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean (SD) age (years): 46.8 (8.5) No. male:female: 23:28 Mean (SD) DASH (0 to 100; 0 is no disability): 56.3 (17.7) Mean (SD) VAS pain score (0 to 100; 0 is no pain): 70.2 (15.2) No. (%) dominant arm affected: 38 (74.5) Glucocorticoid group Mean (SD) age (years): 47.3 (7.8) No. male:female: 23:26 Mean (SD) DASH (0 to 100; 0 is no disability): 44.1 (16.2) Mean (SD) VAS pain score (0 to 100; 0 is no pain): 67.1 (13.5) No. (%) dominant arm affected: 37 (75.5) Pretreatment group differences: DASH scores were higher in the PRP group than in the glucocorticoid group |
|
| Interventions |
Platelet‐rich plasma injection Whole blood (27 mL) was drawn from the contralateral arm into a 30‐mL syringe containing 3 mL of sodium citrate, and the platelet‐rich fraction was isolated in the Biomet Recover system; 3 mL of PRP was obtained and buffered to physiologic pH; 8.4% sodium bicarbonate and bupivacaine hydrochloride 0.5% with adrenaline (1:200,000) was added. No activating agent was used. Approximately 1 mL of PRP was injected directly into the area of maximum tenderness (from a covered syringe to maintain blinding), and the remaining 2 mL was injected with a 22‐gauge needle and a peppering technique into the common extensor tendon via a single skin portal and 5 penetrations of the tendon. A supervised orthopaedic resident or a consultant performed all injections in both groups Glucocorticoid injection Whole blood (27 mL) was drawn from the contralateral arm and discarded; 1 mL of glucocorticoid (kenacort 40 mg/mL; triamcinolone acetonide) with bupivacaine hydrochloride 0.5% with adrenaline (1:200,000) was injected directly and 2 mL peppered, via the same injection procedure as in the PRP treatment group Post intervention Participants were kept supine without moving the arm for 15 minutes immediately after the injection, then were instructed to rest the arm for 24 hours. Paracetamol was permitted for pain relief, but non‐steroidal anti‐inflammatory medications were prohibited. A standardised stretching protocol was followed for 2 weeks under physiotherapy supervision, followed by eccentric muscle and tendon strengthening. Participants were permitted to return to normal sporting activities after 4 weeks |
|
| Outcomes | Outcomes were reported at baseline and at 4 weeks, 8 weeks, 12 weeks, 26 weeks, 52 weeks, and 104 weeks Outcomes
Outcomes included in this review
Time points included in this review 4 weeks, 12 weeks, 26 weeks, 1 year, and 2 years |
|
| Notes |
Funding: Biomet sponsored the study by supplying the Recover system at a discounted rate; study authors declared that Biomet did not have any influence on collection and analysis of data for this study Trial registration: http://www.clinicaltrials.gov trial identifier 2007‐004947‐31 Withdrawal: 3/49 in the glucocorticoid group and 3/51 in the PRP group were lost to follow‐up Adverse events PRP group Total adverse events No. (%) of events: not reported Nature of the event: "no complications were seen concerning the use of PRP, except for the initial worsening of pain because of the activation of the inflammation cycle, which usually lasted for 1 to 2 weeks" Serious adverse events None Glucocorticoid group Total adverse events: none reported Serious adverse events: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer using block randomisation of 10 participants was used to create a randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Allocations were placed in sequentially numbered opaque envelopes and were assigned by trial managers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blood was taken from both groups to blind participants. Tubes were masked with opaque tape |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | Participants were blinded to treatment assignment; hence there is low risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No objective outcomes were measured in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/49 in the glucocorticoid group and 3/51 in the PRP group were lost to follow‐up, and 'last observation carried forward' was planned for any missing data |
| Selective reporting (reporting bias) | Low risk | Trial was registered and all outcomes planned in the protocol were reported |
| Other bias | Unclear risk | There is risk of bias due to contamination of results caused by re‐interventions (6 re‐interventions in the PRP group ‐ 3 underwent surgery and 3 had re‐injection of glucocorticoids; 5 out of these 6 re‐interventions were performed in the first year of follow‐up; 14 re‐interventions in the glucocorticoid group ‐ 6 underwent surgery and 1 required re‐injection with glucocorticoids every 3 months and declined surgery, 7 crossed over to the PRP group; 13 out of these 14 re‐interventions were performed in the first year of follow‐up) |
Gupta 2019.
| Study characteristics | ||
| Methods |
Design: single‐centre parallel‐group 2‐arm open‐label randomised controlled trial Setting: Department of Sports Medicine, Sir Ganga Ram Hospital, New Delhi, India Timing: July 2016 to June 2017 Interventions: platelet‐rich plasma injection vs glucocorticoid injection Sample size: 80 participants (40 + 40);initially calculated at 66 (power 90%, α = 0.05, s ~ 1.5), assuming a 20% dropout rate; total sample size was finally set at 80 (40 per group) based on 1.2‐point difference in VAS Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number of participants screened for eligibility: 98. Number excluded: 18 (did not meet inclusion criteria = 12; declined to participate = 6) Number randomised: 80 (40 to platelet‐rich plasma group, 40 to glucocorticoid group) Number included in analysis: 80 (40 in platelet‐rich plasma group, 40 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group (n = 40) Mean age (years): 42.4 No. male:female: 22:21 Mean duration of symptoms (weeks): 15.2 No. with vigorous manual occupation: 10 No. with non manual occupation: 30 Mean VAS: 81 Mean DASH: 87.15 Mean Mayo Elbow Performance Scale score (MEPS): 49.5 Mean grip strength: 67.7 Glucocorticoid group (n = 40) Mean age (years): 39.4 No. male:female: 12:25 Mean duration of symptoms (weeks): 16.5 No. with vigorous manual occupation: 8 No. with non manual occupation: 32 Mean VAS: 77.5 Mean DASH: 85.9 Mean Mayo Elbow Performance Scale score (MEPS): 54 Mean grip strength: 58.9 Pretreatment group differences: no baseline differences between groups |
|
| Interventions |
PRP group Patients were kept anti‐inflammatory‐analgesic‐free for 2 weeks (to allow for relative washout of the drugs). Out of 20 mL whole venous blood, 18 mL was transferred into 4 red‐capped plain tubes (labelled 1, 2, 3, and 4) with 4.5 mL each, and 2 mL was used for cell counts. Two‐spin centrifugation was adopted, with the first at 160 g for 12 minutes at room temperature. The sample was segregated into 3 layers ‐ upper plasma, middle buffy coat, and lower red cell layer. Supernatant plasma and buffy coat from each tube (total 10 to 12 mL) were pipetted into another set of red‐capped plain tubes labelled A and B (5 to 6 mL per tube) under laminar flow. A second spin at 460 g for 18 minutes was then provided at room temperature. Platelet pellets were collected at the bottom of each tube. Around 3 mL of platelet‐poor plasma was discarded, and roughly 2 mL of plasma with platelet pellets was thoroughly mixed in each tube. The final PRP thus obtained was around 4 mL and was transferred from both tubes into 1 plain tube labelled “PRP”, was stored for 15 minutes at room temperature, and was subjected to a platelet count. On a 90‐degree flexed elbow and pronated forearm (passively stretched ECRB allowed clearer identification), the common extensor origin was identified, painted, and draped. Bony landmarks (lateral epicondyle, supracondylar ridge, olecranon, and radial head) were palpated; a 22‐gauge needle was introduced along the supracondylar ridge (proximal to lateral epicondyle) and was gently advanced into the undersurface of the ECRB and the common extensor tendon via a peppering technique: single skin penetration and 10 to 20 tendon penetrations (without emerging from the skin) Glucocorticoid group 40 mg of triamcinolone with 2% xylocaine was injected after peppering Co‐intervention Sterile dressings were removed 2 days later. Discharged after a brief 30‐minute rest, all patients followed standardised rehabilitation (limb rest ‐ 3 days, need‐based cold fomentation and oral paracetamol). Additional requirements were noted on subsequent office visits. Gentle range of movement (ROM) and isotonic exercises were prescribed after a week. Resistive training of wrist extensors using TheraBand (THERABAND, Akron, OH) and rotator cuff and periscapular muscle exercises were started at 3 weeks post intervention |
|
| Outcomes | Outcomes were measured at 6 weeks, 3 months, and 1 year Outcomes
Outcomes used in this review
|
|
| Notes |
Funding: not reported Trial registration: none found Withdrawal: 0 withdrawals in both groups Adverse events: Study authors report, "no major adverse events were reported in any patient" Serious adverse events: none reported Analysis: we transformed VAS to 0 to 10 scores. Table 2 reports S values, which are unusually small for SD and large for SE. We used them as SD (Analysis 2.2; Analysis 2.3), and we conducted a sensitivity analysis using imputed SD. We contacted study authors to clarify whether variance measures were SD or SE; however they did not respond |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random sequence was utilised |
| Allocation concealment (selection bias) | Low risk | Quote: "letters “A” (PRP) and “B” (CS) placed in identical, opaque, sealed and stapled envelopes by an independent researcher (not involved with the care of the patients) to minimise selection bias. The allocation sequence was concealed from the surgeon, and the envelopes were only opened at the time of allocation of intervention" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The need for patient identification during blood withdrawal for PRP injection made the study a non‐blinded one |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | Study authors did not attempt blinding |
| Blinding of outcome assessment (detection bias) objective outcomes | High risk | Study authors did not attempt blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or protocol is available. Study authors report data at 6 weeks, 3 months, and 1 year for all outcomes specified in the methods section |
| Other bias | Low risk | None is apparent |
Jindal 2013.
| Study characteristics | ||
| Methods |
Design: single‐centre single‐blind quasi‐randomised controlled trial Setting: Sawai Man Singh Medical College and Hospital, Jaipur, Rajasthan, India Timing: August 2009 to August 2010 Interventions: autologous blood injection vs glucocorticoid injection Sample size: sample size was calculated on the basis of VAS pain intensity measurements to achieve a minimum expected difference of 1.5 on the pain scale and a P value of 0.05 and power of 90%. Twenty‐one participants were required for each group based on SD of 1.5. With factoring of a dropout rate of 10%, 50 participants were enrolled with 25 in each group Analysis: all participants were analysed as per treatment protocol |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 50 (25 to autologous blood group, 25 to glucocorticoid group) Number included in analysis: 50 (25 in autologous blood group, 25 in glucocorticoid group) Inclusion criteria
Exclusion criteria Other identifiable causes for lateral elbow pain Baseline characteristics Autologous blood group Mean (SD) age (years): 39.04 (6.67) Sex: 11 female:14 male Mean (SD) duration of pain (weeks): 4.48 (1.82) Mean (SD) VAS pain score (0 to 10; 0 is no pain): 5.88 (1.83) Mean (SD) Nirschl stage (0 to 7; 0 is no pain): 4.52 (1.23) Glucocorticoid group Mean (SD) age (years): 37.32 (7.52) Sex: 8 female:17 male Mean (SD) duration of pain (weeks): 4.4 (2.38) Mean (SD) VAS pain score (0 to 10; 0 is no pain): 6.2 (1.61) Mean (SD) Nirschl stage (0 to 7; 0 is no pain): 4.84 (0.94) Pretreatment group differences: groups were similar at baseline |
|
| Interventions |
Autologous blood group 2 mL of venous blood was drawn from either upper limb and was mixed with 1 mL of 2% lignocaine. The needle was introduced just proximal to the lateral epicondyle, and the contents were injected deep to the extensor carpi radialis group of muscles. Injections were administered in the outpatient department with all aseptic precautions observed by the same physician for all participants in both groups Glucocorticoid group 40 mg of methylprednisolone acetate was mixed with 1 mL of 2% lignocaine. The solution was injected as described above Follow‐up care Participants were advised to restrain from activities involving repetitive movements of the wrist and elbow for 3 weeks after injection. Gentle passive stretching exercises of the extensor group of muscles were permitted as soon as the pain subsided |
|
| Outcomes | Outcomes were reported at baseline and at 2 weeks and 6 weeks Outcomes
Outcomes included in this review
Time points included in this review 6 weeks |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: none Adverse events Autologous blood group Total adverse events: not reported Serious adverse events: not reported Glucocorticoid group Total adverse events: not reported Serious adverse events: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were quasi‐randomised on an alternate basis |
| Allocation concealment (selection bias) | High risk | As allocation was done on an alternate basis, the allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and injecting doctors were not blinded to treatment |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to treatment allocation, there is risk of bias in self‐reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Assessors were blinded to treatment but no true objective outcomes were reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and the trial was not registered. Study authors did not state whether there were any adverse events |
| Other bias | Low risk | No other bias was found |
Kazemi 2010.
| Study characteristics | ||
| Methods |
Design: single‐centre single‐blind parallel 2‐arm randomised controlled trial Setting: Outpatient Clinic at Imam Reza University Hospital, Tehran, Iran Timing: September 2007 to September 2008 Interventions: autologous blood injection vs glucocorticoid injection Sample size: a priori sample size calculation estimated 30 participants per group to detect a 30% difference in pain intensity on VAS between the 2 groups, with 80% power and a 2‐tailed P value of 0.05 Analysis: type of analysis (completer or intention‐to‐treat) not reported; appears all randomised were included in analysis |
|
| Participants |
Number of participants Number of participants screened for eligibility: 121 Number excluded: 61 (23 refused to participate, 14 did not meet inclusion criteria, 6 had a history of arthritis, 1 had a previous elbow operation, 1 had a history of elbow trauma, 1 was pregnant or breastfeeding, 13 were taking NSAIDs, 2 were wearing a brace) Number randomised: 60 (30 to autologous blood group, 30 to glucocorticoid group) Number included in analysis: 60 (30 in autologous blood group, 30 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous blood group Mean (SD) age (years): 47.2 (10.6) No. male:female: 7:23 Duration of pain: 2 (7%) less than 1 month, 3 (10%) between 1 and 2 months, 25 (83%) longer than 2 months Mean (SD) pain at rest in the last 24 hours on VAS (0 to 9; 0 is no pain): 6.5 (1.7) Mean (SD) Quick DASH (0 to 100; 0 is no disability): 51.6 (15.1) Mean (SD) modified Nirschl stage (0 to 4; 0 is no pain): 2.8 (0.5) Mean (SD) maximum grip strength: 27.1 (13.7) Mean (SD) pressure pain threshold on algometer: 8.8 (5.8) Glucocorticoid group Mean (SD) age (years): 47.0 (10.3) No. male:female: 4:26 Mean duration of pain: 0 (0%) less than 1 month, 4 (13%) between 1 and 2 months, 26 (87%) longer than 2 months Mean (SD) pain at rest in the last 24 hours (0 to 9; 0 is no pain): 6.7 (1.7) Mean (SD) Quick DASH (0 to 100; 0 is no disability): 52.3 (19.3) Mean (SD) modified Nirschl stage (0 to 4; 0 is no pain): 3.1 (0.6) Mean (SD) maximum grip strength: 27.9 (15.6) Mean (SD) pressure pain threshold on algometer: 9.4 (5.2) Pretreatment group differences Groups were similar at baseline. |
|
| Interventions |
Autologous blood group A total of 2 mL of blood was drawn from the ipsilateral upper limb distally and was mixed with 1 mL of 2% lidocaine. A single dose was injected. The chief resident of physical medicine and rehabilitation administered all intervention injections. To stabilise the elbow, participants were instructed to flex and pronate their affected elbows on a firm surface. The needle was introduced proximal to the lateral epicondyle along the supracondylar ridge and was moved forward to the undersurface of the extensor carpi radialis brevis Glucocorticoid group A single dose of 20 mg methylprednisolone was mixed with 1 mL of 2% lidocaine and was injected by the procedure described above Post‐intervention care All participants were advised to return to normal activities gradually and were advised to avoid pain‐provoking physical stresses that irritated their elbow region, especially within the first 48 hours after injection. Participants were instructed not to use braces, physiotherapy, or analgesia including non‐steroidal or steroidal anti‐inflammatory drugs throughout the duration of the study |
|
| Outcomes | Outcomes were reported at baseline and at 4 weeks and 8 weeks Outcomes Primary outcome Mean pain over last 24 hours at rest, measured on a visual analogue scale (scores from 0 (no pain) to 9 (worst pain)) Secondary outcomes
Outcomes included in this review
Time points included in this review 4 weeks and 8 weeks |
|
| Notes |
Funding: not reported Trial registration: Iranian Registry of Clinical Trials (IRCT), at http://www.irct.ir/, ID: IRCT138711191658N1 Withdrawal: none reported Adverse events: none reported. "there were no noticeable or reported side‐effects of the treatments in either group" Autologous blood group Total adverse events: none reported Serious adverse events: none reported Glucocorticoid group Total adverse events: none reported Serious adverse events: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The first participant was allocated by true randomisation (coin), but after this, study authors used sequential 1:1 allocation; thus investigators could foresee the coming allocation |
| Allocation concealment (selection bias) | High risk | This was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and interventionists were not blinded to treatment allocation; however study assessors were blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded, there is risk of bias in the measurement of pan and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No true objective outcomes are reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals are reported |
| Selective reporting (reporting bias) | Low risk | Trial was registered and results for all prespecified outcomes were reported |
| Other bias | Low risk | No other bias was found |
Krogh 2013.
| Study characteristics | ||
| Methods |
Design: single‐centre double‐blind randomised 3‐arm placebo‐controlled trial Setting: Region Hospital Silkeborg, Silkeborg, Denmark Timing: January 2009 to July 2010 Interventions: platelet‐rich plasma injection vs glucocorticoid injection vs normal saline injection Sample size: a priori calculations were originally made on a 12‐month result. Due to minimal remaining participants after 3 months, 3‐month data were chosen post hoc as the primary outcome. Secondary analyses at 6 and 12 months were based on both last observation carried forward and per protocol. Prospectively, this study was not powered with a superiority design to compare the 2 active arms (platelet‐rich plasma and glucocorticoid). For a 2‐sample pooled t test of a normal mean difference with a 2‐sided significance level of 0.05, assuming a common standard deviation of 10 PRTEE score points, a sample size of 17 per group was required to obtain power of at least 80% to detect a mean difference of 10 PRTEE pain score points. Intention‐to‐treat populations of 20 in each group corresponded to statistical power of 86.9% to detect a mean difference in the PRTEE pain score of 10 in any given 2‐group comparison Analysis: intention to treat |
|
| Participants |
Number of participants Number of participants screened for eligibility: 165 Number excluded: 105 (87 did not meet inclusion criteria, 8 did not consent, 10 were excluded for other reasons) Number randomised: 60 (20 to platelet‐rich plasma group, 20 to glucocorticoid group, 20 to normal saline group) Number included in analysis: 60 (20 in platelet‐rich plasma group, 20 in glucocorticoid group, 20 in normal saline group completed up to 3 months). Due to huge dropout rate (44/60 participants 73%) at 12 months, 12‐month data were not reported and 3‐month data were chosen post hoc as the primary outcome Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean (SD) age (years): 47.6 (7.1) No. male:female: 9:11 Mean (SD) duration of pain (months): 18.1 (36). Mean (SD) PRTEE pain score over last week (scale 0 to 50; 0 is no pain): 27.5 (7.5) Mean (SD) PRTEE disability score over last week (scale 0 to 100; 0 is no disability): 51.5 (19.1) Dominant elbow affected: 17 (85%) Glucocorticoid group Mean (SD) age (years): 43.9 (8.7) No. male:female: 11:9 Mean (SD) duration of pain (months): 35.6 (54.1) Mean (SD) PRTEE pain score over last week (scale 0 to 50; 0 is no pain): 28 (8) Mean (SD) PRTEE disability score over last week (scale 0 to 100; 0 is no disability): 51.1 (22.3) Dominant elbow affected: 15 (75%) Normal saline group Mean (SD) age (years): 44.7 (7.9) No. male:female: 9:11 Mean (SD) duration of pain (months): 15.5 (12.8) Mean (SD) PRTEE pain score over last week (scale 0 to 50; 0 is no pain): 25 (7.3) Mean (SD) PRTEE disability score over last week (scale 0 to 100; 0 is no disability): 47.1 (22.3) Dominant elbow affected: 13 (65%) Pretreatment group differences: duration of symptoms was much longer in the glucocorticoid injection group than in the other groups |
|
| Interventions | All participants were blindfolded throughout the interventional procedure. Under ultrasound guidance, 10 to 15 mL of lidocaine 10 mg/mL was injected into the peritendon before intervention. All participants had 27 mL of blood collected in a 30‐mL syringe containing 3 mL of sodium citrate approximately 20 minutes before intervention. Injections were performed with a 21‐gauge needle by inserting the needle at the most proximal part of the lateral epicondyle and advancing it toward the humeroradial joint Platelet‐rich plasma group Autologous blood was collected and placed in a centrifuge for 15 minutes at 3200 rpm. Platelets were collected with the Recover GPS II system (Biomet Biologics Inc, Warsaw, IN). Approximately 3 to 3.5 mL of PRP, with platelet concentration increased on average 8‐fold compared with whole blood. To achieve physiological pH, PRP was buffered with 8.4% sodium bicarbonate. PRP was injected right after preparation. One injection was given at baseline. The needle was inserted as described, and a peppering technique was used, with 1 skin portal and 7 tendon perforations Glucocorticoid group The injection consisted of 1 mL of triamcinolone 40 mg/mL with 2 mL of lidocaine 10 mg/mL. The needle was inserted as described, and the content was injected at the deepest aspects of the common tendon origin to limit risk of skin atrophy Normal saline group Injection consisted of 3 mL of saline 0.9%. The needle was inserted as described and a peppering technique was used, with 1 skin portal and 7 tendon perforations Follow‐up care Patients were asked to not use or to minimally use the arm for 3 to 4 days and thereafter to gradually return to normal activities if the pain level was acceptable. Acetaminophen was recommended as needed. A standardised stretching and training programme was prescribed (found at www.sportnetdoc.com) |
|
| Outcomes | Outcomes were reported at baseline and at 1 day, 1 month, 3 months, 6 months, and 12 months Primary outcome Mean change in pain measured by the pain section of PRTEE (scale from 0 (no pain) to 50 (worst pain); a validated Swedish version of PRTEE translated into Danish was used) Secondary outcomes
Outcomes included in this review
Time points included in this review 1 month and 3 months |
|
| Notes |
Funding: primary investigator received a 6‐month research grant from Danish Rheumatism Associated. Biomet Biologics provided the Recover GPS II Platelet Concentrate Separation Kit and donated an unrestricted grant to the hospital. The Oak Foundation sponsored the Musculoskeletal Statistics Unit at Parker Institute Trial registration: www.clinicaltrials.gov, identifier number NCT01109446 Withdrawal: There was a large dropout after 3 months (12/20 PRP, 17/20 glucocorticoid, 15/20 saline) because of unsatisfactory effects of initial treatment. ITT was used and 3 months became the primary time point Adverse events PRP group Total adverse events: 7/20 No. (%) of events: 4 (20) Nature of event: persistent pain after PRP injection No. (%) of events: 3 (15) Nature of event: limitation of movement in injected elbow Serious adverse events: 0/20 Glucocorticoid group Total adverse events: 7/20 No. (%) of events: 1 (5) Nature of event: rash at injection site, which resolved spontaneously No. (%) of events: 1 (5) Nature of event: persistent pain after injection No. (%) of events: 1 (5) Nature of event: limitation of movement in injected elbow No. (%) of events: 3 (15) Nature of event: skin atrophy No. (%) of events: 1 (5) Nature of event: loss of pigmentation Serious adverse events: 0/20 Saline group Total adverse events: 3/20 No. (%) of events: 3 (15) Nature of event: persistent pain after injection Serious adverse events: 0/20 Data analysis We analysed 3‐month data, and as there were no withdrawals at 3 months, we judged risk of attrition bias to be low at 3 months while grading the evidence. For mean pain analysis (Analysis 1.2; Analysis 2.2), we transformed PRTEE pain (0 to 50 scale) to a 0 to 10 scale |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomly assigned in permuted blocks of 6, via a simple shuffling envelopes procedure |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed with the use of opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blindfolded and all participants had blood taken. The injecting doctor was not blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded to treatment, risk of bias was low in the measurement of self‐reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although there was a large dropout after 3 months (12/20 PRP, 17/20 glucocorticoid, 15/20 saline), because of an unsatisfactory effect of initial treatment, ITT was used and 3 months was chosen post hoc as the primary time point. For outcomes measured at 6 and 12 months, risk of attrition bias was high, although we have not used that data in our meta‐analysis |
| Selective reporting (reporting bias) | Unclear risk | Study authors have not reported all secondary outcomes at secondary time points (only pain was reported at 6 and 12 months) |
| Other bias | Low risk | None is apparent |
Lebiedziński 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre parallel 2‐arm randomised controlled trial Setting: Orthopaedic Clinic, Poland Timing: between 2009 and 2011 Interventions: autologous conditioned plasma (ACP) injection vs glucocorticoid injection Sample size: not reported Analysis: data for participants who completed the study were analysed |
|
| Participants |
Number of participants Number screened: not reported Number excluded:not reported Number randomised: 120 (64 to ACP group, 56 to glucocorticoid group) Number analysed: 99 (53 in ACP group, 46 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics ACP group Mean (range) age (years): 47 (25 to 67) Mean (SD) DASH score: 53.2 (15.5) Glucocorticoid group Mean (range) age (years): 54 (21 to 96) Mean (SD) DASH score: 58.6 (14.8) |
|
| Interventions |
Autologous conditioned plasma (ACP) injection ACP injections were performed using a 0.8‐mm syringe and were prepared according to the manufacturer’s instructions (Double Syringe System, Arthrex) Glucocorticoidinjection A combination of 1 mL of betamethasone injection and 2 mL of 1% lignocaine was used. Steroid injections were performed with the use of a 0.8‐mm syringe. One mL of Diprophos (Schering‐Plough Labo N.V., Belgium) (6.43 mg of betamethasoni dipropionas band 2.63 mg of betamethasoni natrii phosphas) and 2 mL of 1 % lignocaine was injected subcutaneously. Both Diprophos and ACP injections were done by the same physician in the same way in every case |
|
| Outcomes | Outcomes were measured at baseline and at 6 weeks, 6 months, and 12 months Study outcomes
Outcomes included in this review
Time points included in this review 6 weeks, 6 months, and 12 months |
|
| Notes |
Funding: no funding source was reported Trial registration: not done Withdrawal: 11/64 in the ACP group and 10/56 in the steroid group. No reasons for withdrawal were given Adverse events ACP group Total adverse events: 11/64 No. of events = 11 No. (%) of participants = 11 (17%) Nature of the event: pain related to the injection Serious adverse events No. of events = 0 Glucocorticoid group Total adverse events: 2/56 No. of events = 2 No. (%) of participants = 2 (4%) Nature of the event: pain related to the injection Serious adverse events No. of events = 0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via a computer‐generated schedule |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by the computerised schedule |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the study was not blinded to the researchers and participants, so both groups were aware what kind of treatment was applied" |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | Participants were not blinded, and there is high risk of bias in the measurement of DASH scores |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No outcomes were measured by assessors in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/64 (17%) in the ACP group and 10/56 (18%) in the steroid group withdrew and were not included in the final analysis. No reasons for withdrawal were given |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not found and the trial was not registered |
| Other bias | Low risk | None is apparent |
Lim 2017.
| Study characteristics | ||
| Methods |
Design: single‐centre 2‐arm parallel randomised controlled trial Setting: Chosun University Hospital, South Korea Timing: not reported Interventions: autologous platelet‐rich plasma (PRP) injection plus tennis elbow strap and exercise vs tennis elbow strap and exercise Sample size: not calculated Analysis: data for those who completed the study were analysed |
|
| Participants |
Number of participants Number screened: 156 Number excluded: 36 (peripheral nervous system disease, haematological disease, gout, tumour, osteoarthritis, inflammatory disease, operation or injection for lateral epicondylitis) Number randomised: 120 (61 to PRP plus tennis elbow strap and exercise group, 59 to tennis elbow strap and exercise group) Number included in analysis: 105 (55 in PRP plus tennis elbow strap and exercise group, 50 in tennis elbow strap and exercise group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP plus tennis elbow strap and exercisegroup
Tennis elbow strap and exercise group
Pretreatment group differences: VAS pain scores were much higher in the PRP group than in the sham group |
|
| Interventions |
Autologous platelet‐rich plasma (PRP) injection plus tennis elbow strap and exercise Interventions were performed by 2 radiologists with extensive clinical experience in musculoskeletal intervention procedures. An exploratory echography was performed to identify clefts of hypo‐echogenicity and/or changes in vascularity, and baseline sonographic characteristics were recorded. Ultrasonography‐guided percutaneous needle tenotomy with PRP was performed only once at the beginning of the study. PRP was prepared by drawing blood samples from participants’ unaffected arm. Peripheral venous blood sample was collected into three 9‐mL tubes containing 3.8% (wt/vol) sodium citrate. Anticoagulated blood sample was centrifuged at 1200 rpm for 6 minutes, and PRP was collected by a commercial kit (HUONS, Sungnam, Korea), with care taken to avoid contamination with the buffy coat containing the leukocytes. Plasma sample was kept at room temperature until intervention; the delay between blood extraction and plasma administration should not be > 4 hours. Just preceding PRP administration, 10% calcium chloride was added, up to a final concentration of 22.6 mM (50 mL/mL of PRP), and a 5‐mL Luer‐Lok syringe was filled with activated PRP. By using a single skin portal, a local anaesthetic (2 mL of 1% lidocaine HCl 10 mg/mL) was injected into the subcutaneous tissue of the lateral elbow with a 20‐gauge needle. Once the needle was in place, the 5‐mL Luer‐Lok syringe loaded with the treatment was attached. Local anaesthetics were injected only into subcutaneous tissues and PRP was injected into the tendon after the syringe was changed Tennis elbow strap and exercise Control received only physical therapy plus tennis elbow strap without injection Post intervention Both groups used a tennis elbow strap and performed stretching and strengthening exercises during 6‐month follow‐up |
|
| Outcomes | Outcomes were measured at 4 weeks, 3 months, and 6 months Primary outcome
Secondary outcomes
Outcomes used in this review
Time points included in this review 4 weeks |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: 6/61 (9.8%) in PRP plus tennis elbow strap and exercise group and 9/59 (15%) in tennis elbow strap and exercise group were lost to follow‐up Adverse events "No complications were noted in either group at the treatment period" PRP plus tennis elbow strap and exercisegroup Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Tennis elbow strap and exercise group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Data analysis We reversed the direction of the Modified Mayo Clinic performance index scores to read as 0 = no disability, to ensure consistency in interpretation of function scores in the meta‐analysis. We assumed the variance in the figure was SEM (Fig. 2), and we calculated SD based on that value. For pain assessment, we used VAS scores (0, most satisfactory to 100, poor) for recording changes each time before the treatment procedure in 3 states, namely, rest, daily activity, and work situations, from the beginning up to 8 weeks of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via a computerised random schedule |
| Allocation concealment (selection bias) | Unclear risk | It is not reported whether treatment allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | ParticIpants and interventionists were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to group allocation; there is risk of bias in the measurement of self‐reported outcomes of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | The musculoskeletal radiologist assessing MRIs was blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/61 (9.8%) in the PRP group and 9/59 (15%) in the physical therapy group were lost to follow‐up; they left the study to receive other treatments; hence data from these participants were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Study protocol was not available and the trial was not registered. Grip strength was measured but was not reported. 3‐month data were not reported. Measures of variance were not reported for all outcome data |
| Other bias | Low risk | None is apparent |
Linnanmäki 2020.
| Study characteristics | ||
| Methods |
Design: single‐centre double‐blind parallel‐group 3‐arm randomised controlled trial Setting: Hatanpää City Hospital, Tampere, Finland Timing: between March 2011 and January 2017 Interventions: platelet‐rich plasma (PRP) injection vs autologous blood injection vs saline injection Sample size: estimated enrolment of 120 participants Analysis: intention‐to‐treat was planned but was not executed; completers' analysis was done |
|
| Participants |
Number of participants Number screened: 128 Number excluded: 9 Number randomised: 119 participants (40 to PRP, 40 to autologous blood, 39 to saline) Number analysed: 12 weeks (30 in PRP, 36 in autologous blood, 32 in saline); 26 weeks (27 in PRP, 33 in autologous blood, 34 in saline); 52 weeks (31 in PRP, 38 in autologous blood, 32 in saline) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP Mean (SD) age (years): 46 (5) No.(%) female: 22 (55) Median (IQR) duration of symptoms (weeks): 42 (37) Median (IQR) duration of sick leave (weeks): 3 (9) No. (%) non‐operative treatment: NSAIDs 39 (98), glucocorticoids 25 (63), physiotherapy 37 (93), cast 34 (85) No. (%) frequency of NSAID use: daily 8 (20), several times a week 4 (10), weekly 9 (23), rarely 17 (43) Mean (SD) pain on VAS (0 to 10; 0 no pain): 5.7 (1.7) Mean (SD) function on DASH (0 to 100; 0 best function): 35.6 (15.5) Mean (SD) grip strength: 32 (15.2) Autologous blood Mean (SD) age (years): 46 (10) No.(%) female: 20 (50) Median (IQR) duration of symptoms (weeks): 34 (32) Median (IQR) duration of sick leave (weeks): 5 (12) No. (%) non‐operative treatment: NSAIDs 93 (37), glucocorticoids 38 (15), physiotherapy 93 (37), cast 78 (31) No. (%) frequency of NSAID use: daily 7 (18), several times a week 4 (10), weekly 5 (13), rarely 22 (55) Mean (SD) pain on VAS (0 to 10; 0 no pain): 5.7 (1.5) Mean (SD) function on DASH (0 to 100; 0 best function): 39.4 (13) Mean (SD) grip strength: 35.5 (17.6) Saline Mean (SD) age (years): 49 (8) No.(%) female: 22 (56) Median (IQR) duration of symptoms (weeks): 42 (50) Median (IQR) duration of sick leave (weeks): 3 (8) No. (%) non‐operative treatment: NSAIDs 37 (95), glucocorticoids 23 (59), physiotherapy 35 (90), cast 32 (82) No. (%) frequency of NSAID use: daily 2 (5), several times a week 6 (15), weekly 9 (23), rarely 18 (46) Mean (SD) pain on VAS (0 to 10; 0 no pain): 5.9 (1.8) Mean (SD) function on DASH (0 to 100; 0 best function): 37.8 (14.8) Mean (SD) grip strength: 31.4 (13.3) Pretreatment baseline differences between groups: none were found |
|
| Interventions | Study investigators collected approximately 15 mL of venous blood from the cephalic vein of the asymptomatic arm from all participants using the Arthrex ACP® Double Syringe Kit (Arthrex Inc., Naples, FL). Anticoagulants were not used because the injection was administered within 30 minutes of blood withdrawal in all patients. If the treatment arm was randomised as PRP or saline, investigator placed the syringe in a centrifugation container, and a suitable counterweight was placed in the opposite site. If treatment arm was randomised as autologous blood, centrifugation was performed with 2 counterweights. Blood was therefore saved for injection. Thus, all participants received a similar treatment pre‐injection protocol. For centrifugation, investigators used Hettich Rotofix A32 (Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) with a swing‐out rotor (220 V) at 1500 rpm for 5 minutes for all samples. Investigator asked the participant to close his or her eyes and not open them until the injection was finished and the investigator gave permission. After covering the injection site with a bandage, investigator disposed of syringe, needle, and all equipment, and instructed the participant to open his or her eyes Autologous blood group Investigator cleaned the skin and injected 2 mL of autologous blood with a 22‐gauge × 1.5‐gauge (0.70 mm × 40 mm) needle into the insertion of the extensor tendon at the lateral epicondyle Platelet‐rich plasma group After centrifugation, the smaller syringe of the kit was filled with 4 mL to 6 mL of PRP; the mean concentration of platelets in the PRP kit was 361³/µL × 10³/µL, which was 1.99× larger than the volume of venous blood. Investigator cleaned the skin and injected PRP using a 22‐gauge × 1.5‐gauge (0.70 mm × 40 mm) needle into the insertion of extensor tendons at the lateral epicondyle Saline injection group Investigator cleaned the skin and injected 2 mL of saline with a 22‐gauge × 1.5‐gauge (0.70 mm × 40 mm) needle into the insertion of the extensor tendons at the lateral epicondyle Post intervention: no specific post‐injection regimen was given to patients. However, patients were advised to use non‐steroidal anti‐inflammatory drugs (NSAIDs) if pain at the injection site persisted |
|
| Outcomes | Outcomes were measured at 4, 8, 12, 26, and 52 weeks Outcomes
Outcomes included in this review
Time points included in this review 4 weeks, 3 months, 6 months, 12 months |
|
| Notes |
Funding: Tampere University Hospital, Tampere, Finland Trial registration: NCT01851044 ClinicalTrials.gov Withdrawal: 9/40 (22.5%) in PRP group (3 = loss to follow‐up, 4 = discontinued from study without any reason, 1 = unable to attend follow‐up, 1 = discontinued but came to 26‐week follow‐up) and 2/40 (5%) in autologous blood group (2 = discontinued without reason) and 7/39 (18%) in placebo group (5 = loss to follow‐up, 1 = unable to attend follow‐up, 1 = discontinued without reason) withdrew from the study Adverse events There were no adverse effects in any of the groups. One participant in each group underwent surgery and 2 participants in the PRP group received additional injections (glucocorticoid and botulinum toxin A) during the follow‐up period Data analysis We used pain and function data from 4, 12, 26, and 52 weeks. We compared PRP to saline and PRP to autologous blood, and we used data at the subgroup analysis of PRP vs autologous blood |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was prepared with computer software by a person (HML) not otherwise involved in the study, and the sequence was unknown to all study personnel" |
| Allocation concealment (selection bias) | Low risk | Study personnel used sequentially numbered, sealed, opaque envelopes in allocation of randomised participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded throughout the study "The investigator asked the participant to close his or her eyes and not open them until the injection was finished and the investigator gave permission" Interventionists administering injections were not blinded but were not involved in follow‐up examinations of participants during the study |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded to treatment allocation, there is low risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | "All other hospital staff and persons responsible for treating the participants after the injection were blinded to the allocation" Hence there is low risk of bias for assessment of objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/40 (22.5%) in PRP group (3 = loss to follow‐up, 4 = discontinued from study without any reason, 1 = unable to attend follow‐up, 1 = discontinued but came to 26‐week follow‐up), 2/40 (5%) in autologous blood group (2 = discontinued without reason), and 7/39 (18%) in placebo group (5 = loss to follow‐up, 1 = unable to attend follow‐up, 1 = discontinued without reason) withdrew from the study. Attrition rates are unbalanced across groups, although the reasons for withdrawal are quite similar |
| Selective reporting (reporting bias) | Low risk | Trial was registered |
| Other bias | Low risk | None is apparent |
Martin 2019.
| Study characteristics | ||
| Methods |
Design: single‐centre double‐blind parallel‐group 2‐arm randomised controlled trial Setting: Orthopaedic Department at Cruces University Hospital, Spain Timing: between April 2014 and May 2017 Interventions: platelet‐rich plasma (PRP) injection vs lidocaine injection Sample size: 80 participants Analysis: per‐protocol data provided |
|
| Participants |
Number of participants 82 assessed for eligibility 80 randomised (41 to PRP, 39 to lidocaine) Data available for 71 participants at baseline (36/41 in PRP group, 35/39 in lidocaine group); 54 participants at 6 weeks (28 in PRP group, 26 in lidocaine group); 56 participants at 3 months (29 in PRP group, 27 in lidocaine group); 51 participants at 6 months (26 in PRP group, 25 in lidocaine group); and 46 participants at 12 months (22 in PRP group, 24 in lidocaine group) Inclusion criteria
Exclusion criteria
Baseline data PRP group (n = 35) Mean (SD) age (years): 48.3 (7.6) Number (%) female: 16 (46) Number (%) lateral elbow pain: 31 (89) Number (%) medial elbow pain: 4 (11) Mean BMI (SD): 26.2 (3.2) Mean (SD) DASH: 44.1 (14) Mean (SD) pain: 5.9 (1.5) Lidocaine group (n = 36) Mean (SD) age (years): 50.7 (6.7) Number (%) female: 22 (61) Number (%) lateral elbow pain: 29 (81) Number (%) medial elbow pain: 7 (19) Mean BMI (SD): 25.6 (4.2) Mean (SD) DASH: 44.7 (17) Mean (SD) pain: 5.9 (1.8) |
|
| Interventions | Both groups received 2 treatments, 2 weeks apart. A senior radiologist with more than 20 years of experience in musculoskeletal interventional ultrasonography performed all injections PRP preparation Twenty‐four mL of peripheral blood (i.e. three 9‐mL tubes containing 0.9 mL of sodium citrate; Vacuette, Greiner BioOne, Switzerland) were withdrawn from all patients at every intervention. Pure (leukocyte‐free) PRP was prepared by single spinning at 570 G for 6 minutes, and the plasma layer was collected, under laminar flow, avoiding aspiration of the buffy coat, according to our standard operating procedures. In doing so, we obtained approximately 6 to 8 mL of pure PRP (no leukocytes) without detectable leukocytes and moderated enrichment of platelets (2.30 ± 0.68 times above peripheral blood baseline). According to previous classifications (20), this can be described as pure platelet‐rich plasma (P‐PRP) or leukocyte‐poor platelet‐rich plasma (i.e. preparations without leukocytes and with a low‐density fibrin network after activation). At the interventional radiologist office, PRP is activated with CaCl2 (final concentration 22.5 mM) before 5 mL is loaded into a 10‐mL Luer‐Lok syringe Platelet‐rich plasma group Elbow flexed 120 degrees and forearm in pronation (lateral) or supination (medial), guided by a 4 to 13 MHz high‐frequency linear probe (Esaote MyLab 70 XVG, Esaote S.p.A. Genoa, Italy). Subcutaneous tissues overlying the lateral epicondyle were infiltrated tangential to the plane of the lateral epicondyle with 2 mL lidocaine via a 22‐gauge hypodermic needle. Then, the bulb containing the injectable (PRP or lidocaine) was connected to the needle, which was inserted parallel to the tendon long axis, from distal to proximal. The tendon was repeatedly fenestrated (15 to 25 times) by redirecting the needle in different directions, until softening of the tissue. In addition to piercing the tendon, the tip needle was used to abrade the periosteum. At the same time, the injectable was delivered in areas of hypo‐echogenicity and surrounding areas. Study authors injected 4.47 ± 1.11 (range 1 to 5) of PRP in the first intervention and 4.53 ± 0.88 mL (range 2 to 5) of PRP in the second intervention. After each intervention, patients were instructed to rest for the first 48 hours and to avoid weight lifting. Patients did not follow any post‐procedural exercise programme, but they modified their activities and resumed physical work upon demand Lidocaine injection group Injection site was prepared and injection given the same way as in the PRP group. Study authors injected 4.23 ± 1.09 mL (range 1 to 5) of lidocaine in the first intervention and 4.18 ± 1.14 mL (range 1 to 5) of lidocaine in the second intervention Both groups After each intervention, patients were instructed to rest for the first 48 hours and to avoid weight lifting. Patients did not follow any post‐procedural exercise programme, but they modified their activities and resumed physical work upon demand |
|
| Outcomes | Outcomes were reported at baseline and at 3 months, 6 months, and 12 months Outcomes
Outcomes included in this review
Time points included in this review 3 months, 6 months, and 12 months |
|
| Notes |
Funding: this work was supported by “Instituto de Salud Carlos III”, ISCIII (grant number PI13/01707 to I.A.) co‐financed by FEDER funds Trial registration: NCT01945528, EudraCT 2013‐000478‐32 Withdrawal: 1/41 in PRP group and 3/39 in lidocaine group withdrew from the study because of post‐tenotomy pain Adverse events PRP group Total adverse events No. of events = 6/41 Nature of event: pain and swelling in the 6 weeks following tenotomy Serious adverse events No. of events = 0 Lidocaine group Total adverse events No. of events = 6/39 Nature of event: pain and swelling in the 6 weeks following tenotomy Serious adverse events No. of events = 0 Data analysis Study authors provided proportion with at least 25% improvement as well as mean values (without SD or 95% CI) for pain and DASH. Additional data (SD) were requested from study authors on 15 July 2019. For adverse events (Analysis 1.6), we used number randomised as the nominator. We imputed SD for pain using Montalvan 2015 (1.9) for DASH (Schoffl 2017) (18.2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an independent researcher performed randomization in blocks of four, using EPIDAT3.1" Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Quote: "aluminium paper blinded envelopes with the numbered treatment allocation. The numbered envelopes were opened on the treatment day by the researcher who was in charge of the PRP preparation" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "all physicians (including orthopaedists involved in clinical outcome assessments and radiologists involved in ultrasound assessments), except one radiologist who performed the procedures, were unaware of treatment allocation. All participants were blinded to the treatment. Peripheral blood was drawn from all patients, and in each intervention, the syringe containing the treatment was wrapped with gauze hindering treatment visualization" |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | Participants were blinded to the allocation |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Quote: "assessor blinded"; low risk of bias in ultrasound assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In PRP group, 4/41 (10%) did not receive allocated intervention and 1/41 (2%) discontinued. In lidocaine group, 1/39 (3%) did not receive allocated intervention and 3/39 (8%) discontinued Data missing: at 6 weeks, 13/41 (31%) in PRP group and 13/39 (33%) in lidocaine group; at 3 months, 12/41 (29%) in PRP group and 12/39 (31%) in lidocaine group; at 6 months, 15/41 (37%) in PRP group and 14/39 (36%) in lidocaine group; at 12 months, 19/41 (46%) in PRP group and 15/39 (38%) in lidocaine group Reasons for missing data are not reported. Attrition rates are high but balanced. This may cause bias but the direction is unclear |
| Selective reporting (reporting bias) | Low risk | Trial was registered and protocol was published. Predefined outcomes are reported for each time point. The cutoff for treatment success (25% reduction) is defined in the protocol |
| Other bias | Unclear risk | Medial elbow pain was treated in 4/35 (11%) in the PRP group and 7/36 (19%) in the lidocaine group |
Martínez‐Montiel 2015.
| Study characteristics | ||
| Methods |
Design: single‐blind 2‐arm parallel randomised controlled trial Setting: Mexico Timing: not reported Interventions: platelet‐rich plasma injection vs local glucocorticoid injection Sample size: sample size was calculated based on the statistical calculator of Granmo with a P value of 0.05, which deemed that 30 patients were required in each group Analysis: not clear if intention‐to‐treat was used |
|
| Participants |
Number of participants Number screened: not reported Number excluded:not reported Number randomised: 60 (30 to PRP group, 30 to glucocorticoid group) Number included in analysis: 60 (30 in PRP group, 30 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group
Glucocorticoid group
Pretreatment group differences: groups were similar at baseline. |
|
| Interventions |
Platelet‐rich plasma injection A single injection of platelet‐rich plasma was administered to participants Glucocorticoid injection A single dose of 40 mg of methylprednisolone was injected Post intervention: 400 mg of ibuprofen given to participants in both groups |
|
| Outcomes | Outcomes were measured at 1 month, 3 months, and 6 months Study outcomes
Outcomes used in this review
Time points used in this review 1 month, 3 months, and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: none Adverse events PRP group Total adverse events: none reported Serious adverse events: none reported Glucocorticoid group Total adverse events: none reported Serious adverse events: none reported Data analysis Study authors provided 95% confidence intervals for pain and function data for 1 and 3 months (which were missing in the article) upon request. SDs were calculated from 95% confidence intervals using the formula ((upper CI‐lower CI)/3.92)*(SQRT(N)). Study authors also responded that participants were blinded to treatment allocation, as this information was not provided in the article. 1‐month data were entered under the 6‐week time point Translation: the original article was written in Spanish and was translated into English via Google Translator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised with the programme Research Randomizer Form v.4.0 |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed via Research Randomizer Form v.4.0 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and outcome assessors were blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded, there is low risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐measured outcomes were reported in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported |
| Selective reporting (reporting bias) | High risk | Trial was not registered and the study protocol was not available. Study authors did not give a clear description of the measurement tools or the intervention |
| Other bias | Low risk | None is apparent |
Merolla 2017.
| Study characteristics | ||
| Methods |
Design: single‐centre single‐blinded 2‐arm parallel randomised controlled trial Setting: Shoulder and Elbow Unit Outpatient Office, “D. Cervesi” Hospital, Italy Timing: between June 2010 and December 2012 Interventions: autologous platelet‐rich plasma (PRP) injections vs arthroscopic debridement Sample size: power analysis was performed considering a 2‐point difference in VAS pain score between groups, a standard deviation of the pain score of 2.5 points, and a minimal clinically important difference of 1.8 points. Based on these parameters and power of 0.9, a minimum population of 41 participants per group was required Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number screened: 110 Number excluded: 9 (refused treatment = 4, ineligible = 5) Number randomised: 101 (50 to PRP group, 51 to arthroscopic debridement group) Number included in analysis: 101 (50 in PRP group, 51 in arthroscopic debridement group); 2 participants received arthroscopy after 1 year of PRP treatment due to persistent pain but were analysed in the PRP group until 1 year of treatment Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group
Arthroscopic debridement group
Pretreatment group differences: there were no baseline differences between the 2 groups |
|
| Interventions |
Platelet‐rich plasma (PRP) injection PRP was prepared according to 2011 International Cellular Medicine Society (ICMS) guidelines using autologous blood collected at the hematology unit at the time of treatment. Aliquots of autologous blood components were obtained using a commercial kit (PRPS, BiomedDevice, Modena, Italy). A 30‐mL sample of venous blood yields 3 to 5 mL of PRP. First, blood was centrifuged at constant acceleration, to obtain 3 fractions: an upper layer containing mostly platelets and white blood cells, a thin white blood cell‐rich intermediate layer (buffy coat), and a bottom layer consisting mostly of red blood cells. The upper layer and the buffy coat were placed in an empty sterile tube. Further centrifugation led to formation of soft pellets of erythrocytes and platelets at the bottom of the tube and of a lighter fraction of platelet‐poor plasma that was removed. Two periarticular PRP injections were performed by a single operator under ultrasound guidance using a high‐frequency 7.5‐ to 14‐Hz linear transducer (MyLab Five, Esaote, Reggio Emilia, Italy) according to the technical guidelines of the European Society of Musculoskeletal Radiology. Injections were given on the lateral side of the elbow after the skin was disinfected with iodine solution. The PRP preparation was slowly injected into the area showing fibril discontinuity at the origin of the extensor carpi radialis brevis muscle. The injection site was covered with a sticking plaster, and patients were asked to refrain from taking analgesics or anti‐inflammatories (except rescue medication) for 3 weeks. Each patient received 2 injections administered 2 weeks apart Arthroscopic lateral elbow debridement All procedures were carried out by the same operator under regional block of the brachial plexus using a standard arthroscopic technique with 3 portals: proximal anteromedial, anterolateral, and midlateral. Patients were placed in lateral decubitus position with the shoulder in 90 degrees of abduction and the elbow in 90 degrees of flexion. The anterior aspect of the radiocapitellar joint and the joint capsule were visualised through the anteromedial portal. The first step was to assess the relationship among ECRB, extensor carpi radialis longus (ECRL), and capsule. The undersurface of the extensor carpi radialis brevis was examined, and the capsule was rated according to the classification of Baker et al. The articular aspect of the capsule, above the midline of the radiocapitellar joint, was removed through the midlateral portal with a 3.5‐mm full radius resector shaver to expose the extensor carpi radialis brevis origin on the lateral epicondyle. Debridement of the tendon insertion site was continued below the superior capitellum until the extensor carpi radialis longus fibres came into view, avoiding going posterolaterally beyond half the diameter of the radial head and preserving the lateral collateral ligament to prevent postoperative instability. The superior aspect of the capitellum delineated the anterior margin of the tendon resection. The extensor carpi radialis brevis origin was slightly decorticated with a burr. Finally, the posterior compartment was examined through a posterolateral and a direct posterior portal. At the end of the procedure, the elbow was immobilised in a hinged splint with the elbow in 90 degrees of flexion for 15 days. Gentle elbow range of motion exercises were allowed in the third week until complete range of motion recovery. Strength exercises, forceful gripping, and wrist extension were allowed at 4 weeks Post intervention Rescue pain medication (1000 mg oral paracetamol (acetaminophen)) was allowed at a maximum dosage of 4 g/d; the amount taken by each patient was recorded at each follow‐up visit. The protocol mandated withdrawing pain medication 24 hours before each visit; participants were asked to confirm withdrawal of pain medication during the examination |
|
| Outcomes | Outcomes were measured at 2, 4, 8, 12, and 24 weeks, at 1 year and 2 years in the PRP group, and at 8, 12, and 24 weeks, 1 year, and 2 years in the arthroscopy group, as these participants could not be assessed at Weeks 2 and 4 due to immobilisation and rehabilitation Primary outcome
Secondary outcomes
Outcomes used in this review
Time points used in this review 12 weeks, 24 weeks, 1 year, and 2 years |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: none Adverse events PRP group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Arthroscopic debridement group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Data analysis SDs were calculated from confidence intervals using the formula ((upper CI‐lower CI) /3.92)*(SQRT(N)) for up to 52 weeks. At 2 years, reported confidence intervals were asymmetrical; thus we imputed the median SD value from the other time points |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using the Research Randomizer 2007 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised to 2 groups by an orthopaedic surgeon (G.Z.), who was blinded to their characteristics" It is not clear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study staff were blinded to treatment allocation. Study authors did not attempt to blind participants, and as the nature of the interventions (injection vs surgery) was different, participants must have been aware of the nature of the intervention. It is not clear whether assessors or other staff participating in treatment were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to treatment allocation, there is high risk of bias for the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | It is unclear whether outcome assessors were blinded, so there is unclear risk of bias in the measurement of hand grip strength |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported in either group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not found and trial was not registered; per‐group data for participant global assessment were not provided |
| Other bias | Low risk | None is apparent |
Mishra 2014.
| Study characteristics | ||
| Methods |
Design: multi‐centre (12 sites) parallel 2‐arm double‐blind randomised controlled trial Setting: Menlo Medical Clinic, Stanford University Medical Centre, Menlo Park, California, USA. Other sites were not reported Timing: 2006 to 2011 Interventions: platelet‐rich plasma injection vs local anaesthetic injection Sample size: sample size of 115 patients in each treatment group was calculated to have 87.7% power to detect a difference in treatment success percentage between PRP and bupivacaine injections, assuming a rate of 50% for PRP; that the true difference is 20%; and that the type I error rate is a 1‐sided alpha significance level of 0.025 Analysis: intention‐to‐treat analysis was planned; however this could not be performed and data for those who completed the study were analysed |
|
| Participants |
Number of participants Number of participants screened for eligibility: 301 Number excluded: 71 (48 did not meet inclusion criteria, 22 dropped out before intervention,1 failed blood draw) Number randomised: 230 (116 to platelet‐rich plasma group, 114 to local anaesthetic group) Number included in analysis: at 3 months, 192 (101 (87%) in PRP group and 91 (80%) in local anaesthetic group); at 6 months, 119 (56 (48%) in PRP group and 63 (55%) in local anaesthetic group). Study authors do not report how the 6‐month follow‐up sample was chosen Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean age (years): 48.4 Mean (no SD) PRTEE (0 to 100; 0 is no disability): 54.15 Local anaesthetic group Mean age (years): 47.4 Mean (no SD) PRTEE (0 to 100; 0 is no disability): 57.71 Pretreatment group differences: groups were similar |
|
| Interventions |
Platelet‐rich plasma group Approximately 30 mL of whole blood was drawn from a peripheral vein of each patient. The autologous blood was mixed with an anticoagulant (ACD‐A) and was placed into a sterile separator canister using the Biomet GPS system. The canister was centrifuged for 15 minutes at 3200 rpm. This method of preparation produces type 1A PRP (leukocyte‐enriched PRP with platelets 5× baseline used in an inactivated manner). Type 1A PRP was extracted and was buffered to physiological pH using 8.4% sodium bicarbonate. The injection site was first injected with 0.5% bupivacaine with adrenaline. 2 to 3 mL of the prepared PRP was injected into the extensor carpi radialis brevis tendon and the surrounding area via a peppering technique. This technique consisted of 5 penetrations of the tendon, as the PRP was injected via a single skin penetration Local anaesthetic group Extracted blood was discarded. 2 to 3 mL of bupivacaine was injected as described. To maintain blinding in both groups, the entire 10‐mL syringe including the needle hub was wrapped in black tape to obscure its contents. The participant's arm was draped to prevent inadvertent unblinding during the injection Follow‐up care None was reported for both groups |
|
| Outcomes | Outcomes were reported at baseline and at 4 weeks, 8 weeks, 12 weeks, and 24 weeks Primary outcomes
Secondary outcomes
Outcomes included in this review
Time points included in this review 4 weeks, 12 weeks, and 24 weeks |
|
| Notes |
Funding: Biomet Biologics sponsored this study, and the director of research at Biomet Biologics assisted with statistical analysis of the original paper. Study authors reported numerous research support grants in the past from different companies including Biomet. It is unclear whether in this particular study, Biomet could have had an influence on study results Trial registration: registered at www.clinicaltrials.gov, with identifier number NCT00587613 (no details are available, as the centrifuge was not FDA approved; therefore the FDA redacted the data from the website) Withdrawal: 11/112 in the PRP group and 22/113 in the local anaesthetic group at 12 weeks; withdrawal numbers per group for 24‐week follow‐up were not provided Adverse events PRP group Total adverse events: 25/116 (other adverse events + serious adverse events) Other adverse events: 22/116 No. of events: 22 Nature of the event: pain following injection limited to within 48 hours with pain medication Serious adverse events: 3/116 No. of events: 2 Nature of the event: severe pain (2 days for 1 participant, 4 days for 1 participant) No. of events: 1 Nature of event: unstable angina 4 months after treatment Local anaesthetic group Total adverse events: 22/114 (other adverse events + serious adverse events) Other adverse events: 20/114 No. of events: 20 Nature of the event: pain following injection limited to within 48 hours with pain medication Serious adverse events: 2/114 No. of events: 2 Nature of event: 1 participant had radial/ulnar fracture, and another participant underwent shoulder arthroscopic surgery with decompression Data analysis We contacted study authors to request measures of variance data, allocation concealment methodl however there was no response at the time of publication. For pain, we calculated SD using means and reported P values. We imputed the SD for function (PRTEE) from Krogh 2013 (SD 19.2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 231 participants were randomised by a computerised protocol |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study sponsor, physician‐evaluators, and participants were blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | Participants were blinded to treatment assignment; there is low risk of bias for the measurement of pain and disability |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Physician‐evaluators were blinded to treatment assignment and results until the end of the investigation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/112 (9.8%) in the PRP group and 22/113 (19%) in the local anaesthetic group withdrew at 12 weeks; reasons for withdrawal were not given; withdrawal numbers per group for 24‐week follow‐up were not provided |
| Selective reporting (reporting bias) | High risk | No protocol was found. Trial was registered; however due to lack of US FDA clearance on the PRP centrifuge, no details were provided at the clincialtrials.gov website. Study authors did not provide measures of variance for pain and function. They reported only the percentage of improvement in pain and did not report baseline pain values. We did not receive any communication from them despite several attempts to obtain these data |
| Other bias | High risk | Study sponsor added a 24‐week follow‐up, which was a post hoc decision; the sample available for follow‐up consisted of only 119 participants instead of the estimated sample of 230 participants. Reasons for this were specified as ascertaining safety based on FSDA request, but follow‐up was used for efficacy analysis |
Montalvan 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre double‐blind 2‐arm placebo‐controlled randomised trial Setting: Hospital Ambroise Pare´ Service de Rhumatologie, Boulogne‐Billancourt, France Timing: between October 2010 and April 2014 Interventions: 2 ultrasound‐guided platelet‐rich plasma (PRP) injections vs placebo (saline) injection Sample size: sample size calculated was 22 patients per group, so that this study had power of 90% with type I error rate a = 0.05, to demonstrate significantly greater improvement in global pain score of at least 10% in the PRP group over the placebo group, with a standard deviation of 0.10 Analysis: intention‐to‐treat |
|
| Participants |
Number of participants Number screened: 56 Number excluded: 6 (refused to participate = 3, did not meet inclusion criteria = 3) Number randomised: 50 (25 to PRP group, 25 to placebo group) Number included in analysis: 50 (25 in PRP group, 25 in placebo group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group
Placebo group
Pretreatment group differences: there were no baseline differences between the 2 groups |
|
| Interventions |
Platelet‐rich plasma (PRP) injection PRP was prepared by the autologous conditioned plasma device from Arthrex (Naples, FL), following the supplier’s instructions. The sampling procedure and the injection were performed in compliance with strict aseptic technique. The whole procedure lasted 45 minutes. The patient was lying on a table with arm in the supine position. A nurse collected 12 mL of blood by venipuncture using the double syringe device from Arthrex and placed the needle into the centrifuge. The injector physician discarded the supernatant to isolate the platelet concentrate. According to previous reports, the autologous conditioned plasma preparation from Arthrex results in 1.6‐fold enrichment of platelets, compared with whole‐blood content, without detectable red or white blood cells. 2 mL of 1% lidocaine was injected subcutaneously, followed by an ultrasound‐guided PRP injection. The ultrasound probe was placed longitudinally, parallel to the common tendon of the lateral epicondyle, to visualise its fibres. The needle, guided by ultrasound, was advanced into the tendon, parallel to the tendon fibres, until it achieved bone contact; then the solution was injected into 3 or 4 passages, so that treatment was delivered to superficial, medium, and deep tendon sites. If a fissured area was detected on ultrasound, as a linear hypo‐echoic area or a disruption in fibrillar structure, the injector used the needle to dissect this area. The entire procedure was performed twice for each patient: the first time at inclusion, and the second time after an interval of 4 weeks Placebo (saline) injection Two ultrasound‐guided injections of saline were administered in a similar dose and according to exactly the same steps and schedule as outlined above Post intervention Participants were allowed to take 1 g of paracetamol and to perform local ice application in the event of experiencing pain after injection. Rehabilitation, physical therapy, and local glucocorticoid injections were not allowed |
|
| Outcomes | Outcomes were measured at 1, 3, 6, and 12 months Primary outcome Patient’s assessment of global pain score on a visual analogue scale of 0 to 10 Secondary outcomes
Outcomes used in this review
Time points used in this review 1, 3, 6, and 12 months |
|
| Notes |
Funding: this study was funded by Arthrex, France Trial registration: clinicaltrials.gov: NCT02378285; post hoc trial registration Adverse events PRP group Total adverse events No. (%) of events = 6 (24) Serious adverse events No. of events = 0 Other adverse events No. (%) of events: 4 (16) Nature of events: pain during and after injections, which disappeared after 72 hours No. (%) of events: 2 (8) Nature of events: local cutaneous allergic reaction after first injection of PRP Placebo group Total adverse events No. (%) of events = 3 (12) Serious adverse events No.of events = 0 Other adverse events No. (%) of events: 2 (8) Nature of events: pain during and after injection, which disappeared after 3 days No. (%) of events: 1 (4) Nature of events: haematoma after saline injection, which disappeared within 3 days Withdrawal: 3/25 in the PRP group (lost to follow‐up = 1, discontinued treatment = 2), 3/25 in the placebo group (lost to follow‐up = 2, discontinued treatment = 1); all participants in both groups received glucocorticoid injection, which violated the protocol, and were withdrawn from the study Data analysis Intention‐to‐treat analysis was performed and imputations (last observation carried forward) were done for missing follow up data from withdrawn participants. 1‐month data were entered under the 6‐week time point. Roles‐Maudsley score was transformed to a 0 to 100 scale, with higher score indicating worse function |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the physician in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | It is not clear if group allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and outcome assessors were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded to treatment allocation, there is low risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | As outcome assessors and participants were blinded to treatment allocation, there is low risk of bias in the measurement of triggered pain |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/25 in the PRP group (lost to follow‐up = 1, discontinued treatment = 2) and 3/25 in the placebo group (lost to follow‐up = 2, discontinued treatment = 1); intention‐to‐treat analysis was used and imputations were done for missing outcome data |
| Selective reporting (reporting bias) | Low risk | Trial was registered and results for all outcomes stated in the methods section were reported. However, the study was conducted between October 2010 and April 2014 and the protocol was registered in March 2015, after completion of the study |
| Other bias | Low risk | None is apparent |
Omar 2012.
| Study characteristics | ||
| Methods |
Design: single‐centre unblinded 2‐arm randomised controlled trial Setting: Rheumatology and Rehabilitation Outpatient Clinic, Suez Canal University Hospital, Ismailia, Egypt Timing: October 2009 to May 2010 Interventions: platelet‐rich plasma injection vs glucocorticoid injection Sample size calculation: not reported Analysis: type of analysis (completer or intention‐to‐treat) was not reported; appears all randomised were included in analysis |
|
| Participants |
Number of participants Study had 2 participant groups: 1 for plantar fasciitis and 1 for lateral epicondylitis. Only the lateral epicondylitis group is included in this review Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 60 (30 to lateral epicondylitis group, 15 to platelet‐rich plasma group, 15 to glucocorticoid group) Number included in analysis: 30 (15 in platelet‐rich plasma group, 15 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean (SD) age (years): 40.5 (15.5) No. male:female: 6:9 Mean (SD) VAS pain score (0 to 10; 0 is no pain): 8.0 (1.4) Mean (SD) DASH score (0 to 80; 0 is no disability): 58.9 (10.5) Glucocorticoid group Mean (SD) age (years): 37.5 (17.5) No. male:female: 5:10 Mean (SD) VAS pain score (0 to 10; 0 is no pain): 8.6 (1.6) Mean (SD) DASH score (0 to 80; 0 is no disability): 57.3 (10.3) Pretreatment group differences: groups were similar at baseline |
|
| Interventions |
Platelet‐rich plasma group 150 mL of blood was withdrawn from participants and was added into a quadruple paediatric blood bag system containing 63 mL of citrate phosphate dextrose (CPD) as an anticoagulant (JMS, Singapore Ltd., Singapore) after CPD volume in the original pack was adjusted to 21 mL and excess anticoagulant was passed to the distal satellite pack (JMS Hemoscale was used for shacking and adjusting the donated volume). From donated blood in 1 satellite pack, PRP was separated by 2‐step centrifugation at ambient temperature (for 15 minutes at 320 g (soft spin), then at 2000 g for 15 minutes (hard spin)) and kept at +200° C with continuous shaking on a horizontal shaker (Forma Scientific, Marietta, OH). Platelet count was done using Cell Dyne 1700 (Abbott Diagnostics, Chicago, IL) before and after preparation. PRP contained at least a 2 times increase in platelet concentration. Method of injection was not reported Glucocorticoid group The type, concentration, or volume of glucocorticoid was not reported. Method of injection was not reported Follow‐up care All participants were instructed to rest the elbow and wrist for 48 hours following injection. Acetaminophen was permitted as needed, but non‐steroidal anti‐inflammatory medications were not permitted |
|
| Outcomes | Outcomes were reported at baseline and at 6 weeks Outcomes
Outcomes included in this review
Time points included in this review 6 weeks |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawal: none Adverse events: not reported in either of the 2 groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated randomly; no further detail was provided |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not reported whether participants and study personnel were blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Unclear risk | As it is not known whether participants were blinded, there is unclear risk of bias for the measurement of self‐reported outcomes of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | It is unclear whether outcome assessors were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was found and the trial was not registered |
| Other bias | Low risk | No other bias was found |
Ozturan 2010.
| Study characteristics | ||
| Methods |
Design: single‐centre 3‐arm parallel randomised controlled trial Setting: Department of Orthopedics and Traumatology, Abant Izzet Baysal University, Bolu, Turkey Timing: not reported Interventions: autologous blood injection vs glucocorticoid injection vs extracorporeal shock wave therapy Sample size: not reported Analysis: data for those who completed the study were analysed |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 60 (20 to autologous blood group, 20 to glucocorticoid group, 20 to extracorporeal shock wave therapy) Number included in analysis: 57 (18 in autologous blood group, 20 in glucocorticoid group, 19 in shock wave group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous blood group Mean (SD) age (years): 44 (8.5) No. female:male: 11:7 Mean (SD) duration of pain (months): 10 (2.7) Dominant arm affected: 14 (77.7%) Mean (SD) VAS pain score (0 to 100; 0 is no pain): 75 (12.9) Mean (SD) upper extremity function score (8 to 80; 8 is no disability): 47.2 (10.28) Glucocorticoid group Mean (SD) age (years): 45.8 (8.1) No. female:male:: 10:10 Mean (SD) duration of pain (months): 9.5 (3.1) Dominant arm affected: 15 (75%) Mean (SD) VAS pain score (0 to 100; 0 is no pain): 77 (14.1) Mean (SD) upper extremity function score (8 to 80; 8 is no disability): 46.6 (10.87) Extracorporeal shock wave group Mean (SD) age (years): 47 (8.7) No. female:male: 11:8 Mean (SD) duration of pain (months): 9.6 (2.7) Dominant arm affected: 15 (79%) Mean (SD) VAS pain score (0 to 100; 0 is no pain): 77.8 (13.6) Mean (SD) upper extremity function score (8 to 80; 8 is no disability): 49.9 (9.56) Pretreatment group differences: groups were similar |
|
| Interventions |
Autologous blood group Blood was extracted from the contralateral antecubital fossa and was gently shaken to prevent clotting. Prilocaine (1 mL) was used for local anaesthesia of the cutaneous and subcutaneous tissues. 2 mL of autologous blood was injected with a 22‐gauge needle with 5 skin penetrations at the most tender part of the lateral epicondyle. Fourteen participants whose pain did not improve significantly received a second dose at 6 weeks Glucocorticoid group Participants received a local anaesthetic injection (prilocaine 1 mL) to the skin and subcutaneous tissues, followed by methylprednisolone acetate (1 mL) injection with 5 skin penetrations at the tender part of the tendon, by 1 skin portal according to the technique of Mishra and Pavelko. Two participants whose pain did not improve significantly received a second dose at 6 weeks Extracorporeal shock wave group The most tender point at the participant’s elbow was determined by palpation, and prilocaine (1 mL) was applied for local anaesthesia of the cutaneous and subcutaneous tissues. Ultrasound coupling gel was applied to the skin at the point of contact with the shock wave tube (Stonelith V5 lithotriptor; PCK Electronic Industry & Trade Co., Ankara, Turkey). Active treatment consisted of 1 treatment with 2000 impulses at 0.17 mJ/mm² once a week for 3 weeks |
|
| Outcomes | Outcomes were reported at baseline and at 4 weeks, 12 weeks, 26 weeks, and 52 weeks Outcomes
Outcomes included in this review
Time points included in this review 4 weeks, 12 weeks, 26 weeks, and 52 weeks |
|
| Notes |
Funding: none reported Trial registration: not done Withdrawal: 2/20 in the autologous blood group were lost to follow‐up; 1/20 in the shock wave group discontinued following a traffic accident Adverse events Autologous blood group Total adverse events: 3/20 No. of events: 3 Nature of event: after the first injection, pain caused by the injection for 4 to 6 days for 2 participants; after the second injection, for 4 days for 1 participant Serious adverse events: none Glucocorticoid group Total adverse events: 6/20 No. of events: 5 Nature of event: pain caused by injection for > 5 days No. of events: 1 Nature of event: discolouration at injection site Serious adverse events: none Extracorporeal shock wave group Total adverse events: 12/20 No. of events: 4 Nature of event: nausea after shock wave No. of events: 4 Nature of event: erythaema at the elbow No. of events: 3 Nature of event: swelling at the elbow No. of events: 1 Nature of event: tremor in the affected arm after shock wave Serious adverse events: none All participants in all 3 groups had pain after injection, which subsided within 1 to 2 days after treatment with acetaminophen; study authors did not document these episodes as adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that there was a randomisation process, although it provides no further detail |
| Allocation concealment (selection bias) | Unclear risk | There is no information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and injecting doctors were not blinded to treatment |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to treatment allocation, there is risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | High risk | Outcome assessors were not blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/20 in the autologous blood group were lost to follow‐up, and 1/20 in the shock wave group discontinued following a traffic accident. Events were unlikely due to treatment |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and the trial was not registered |
| Other bias | Unclear risk | In the autologous blood group, 14 participants received re‐injection at 6 weeks; in the glucocorticoid group, 2 participants received re‐injection at 6 weeks. Study authors do not report on excluding these participants from the main analysis nor discuss effects of re‐intervention on study findings; possible risk of contamination |
Palacio 2016.
| Study characteristics | ||
| Methods | Design: single‐centre triple‐blind 3‐arm randomised controlled trial Setting: Department of Orthopedics, Traumatology and Exercise Medicine and Sports, School of Medicine of Marilia (Famema), Marilia, SP, Brazil Timing: February 2012 to February 2014 Interventions: PRP injection vs dexamethasone injection vs neocaine injection Sample size: based on power analysis, trialists planned 20 participants per group Analysis: intention‐to‐treat | |
| Participants |
Number of participants
Number of participants screened for eligibility: 72
Number randomised: 60 (to group 1 neocaine: n = 20, to group 2 dexamethasone: n = 20, to group 3 PRP: n = 20)
Number included in analysis: 60 (group 1 neocaine: n = 20, group 2 dexamethasone: n = 20, group 3 PRP: n = 20) Inclusion criteria
Exclusion criteria
Baseline characteristics Neocaine injection group Mean (95% CI) age (years): 47.9 (42.2 to 53.6) Mean (SE) DASH: 49.7 (3.0) Mean (SE) PRTEE: 51.7 (4.4) Dexamethasone injection group Mean (95% CI) age (years): 46.2 (41 to 51.50) Mean (SE) DASH: 44.3 (4.4) Mean (SE) PRTEE: 42.9 (4.3) PRP injection group Mean (95% CI) age (years): 46.6 (41.6 to 51.6) Mean (SE) DASH: 45.7 (3.8) Mean (SE) PRTEE: 47.1 (4.9) |
|
| Interventions |
Neocaine injection group
Local digital pressure was used to identify patients with the region of greatest pain. Aseptic and antiseptic chlorhexidine procedures were performed, then patients underwent local injection of 3 mL of 0.5% neocaine. The syringe was covered with a double layer of aluminium foil before injection by a person who was unconnected to the study
Dexamethasone injection
3 mL of dexamethasone acetate was injected by the procedure described above PRP group 60 mL of blood drawn from the patient was placed in six 10‐mL sodium citrate tubes. The tubes were then subjected to 2 centrifugation cycles under 400 g and 800 g forces for 10 minutes. Two‐thirds of the original volume (platelet‐poor plasma) was discarded in this method; only one‐third of the original blood sample consisted of PRP. Local digital pressure was used to identify the patient with the region of greatest pain. Aseptic and antiseptic chlorhexidine procedures were performed, then patients underwent local injection of 3 mL of PRP. The syringe was covered with a double layer of aluminium foil before injection by non‐study personnel |
|
| Outcomes | Outcomes were reported at baseline and at 90 days. 180 days was planned, but results could not be found in the published paper Outcomes
Outcomes used in this review
Time points included in this review 3 months |
|
| Notes | Funding: no funding source was reported Trial registration: not done Withdrawals: none reported Adverse events: none reported Data analysis: SDs for all outcomes were derived from the standard errors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not clearly described; paper authors have cited 2 references on randomisation but have not clearly reported which method they used to generate the random sequence |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by means of opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “all syringes were covered with a double layer of aluminium foil prior to infiltration by non‐study personnel” Not clear whether blood was drawn from all participants |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Unclear risk | The study is reported as "triple blinded," but it is unclear whether trialists masked the interventions adequately by drawing blood from all participants |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐reported outcomes were reported in this study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported |
| Selective reporting (reporting bias) | High risk | Trial was not registered and no study protocol was found. Although 90 and 180 days' follow‐up were planned, data for only 90 days were reported |
| Other bias | Low risk | None is apparent |
Raeissadat 2014.
| Study characteristics | ||
| Methods |
Design: Single‐centre single‐blind 2‐arm parallel randomised controlled trial Setting: Physical Medicine and Rehabilitation Clinic, Shahid Modarres Hospital, Iran Timing: September 2011 to October 2013 Interventions: platelet‐rich plasma injection vs autologous blood injection Sample size: not reported. Analysis: data for those who completed the study were analysed |
|
| Participants |
Number of participants Number screened: 76 Number excluded: 12 (not eligible = 2, declined participation = 3, NSAID usage = 7) Number randomised: 64 (33 to PRP group, 31 to whole blood group) Number included in analysis: 61 (31 in PRP group, 30 in whole blood group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous platelet‐rich plasma (PRP) injection group Mean (SD) age (years): 43 (6) No. (%) male:female: 8 (26):23(74) Mean (SD) duration of symptoms (months): 14.5 (3) Mean (SD) VAS score: 7.1 (1.2) Mean (SD) Mayo score: 53.9 (16) Mean (SD) PPT score: 17 (5.6) Autologous whole blood injection group Mean (SD) age (years): 44 (7) No. (%) male:female: 6(20):24 (80) Mean (SD) duration of symptoms (months): 14.5 (3) Mean (SD) VAS score: 6.8 (1.5) Mean (SD) Mayo score: 48.8 (18) Mean (SD) PPT score: 16.9 (5.4) Pretreatment group differences: there were no baseline differences between the 2 groups |
|
| Interventions |
PRP preparation PRP processing was done with the Rooyagen kit (made by Arya Mabna Tashkhis Corporation; RN: 312569). For preparing 2 mL of PRP with concentration 4 to 6 times average normal values, 20 mL of blood was first collected from the patient’s upper limb cubital vein with an 18‐G needle. Then 2 mL of ACD‐A was added to the sample as an anticoagulant. One mL of the blood sample was sent for complete blood count. The rest of the sample passed 2 stages of centrifuge (first with 1600 rpm for 15 minutes for separation of erythrocytes, and next with 2800 rpm for 7 minutes to concentrate platelets). The final product was 2 mL of PRP containing leukocytes (leukocyte‐rich PRP). The PRP quantification and qualification procedure was performed with laboratory analyser Sysmex KX 21. Exogenous factor was not used for the process of platelet activation. The mean platelet count of all patients at baseline was 250,000 ± 53,000/μL, which increased to 1,227,000 ± 250,000/μL (4.8 times concentration) in PRP preparation. Leukocyte count was 6740 ± 1396/μL in the PRP group and 6453 ± 1193/μL in AWB groups Autologous platelet‐rich plasma (PRP) injection The participant was placed in an appropriate and comfortable position that allowed for sterility and access to the site of injection. Two mL of 1% lidocaine was injected 8 minutes before the PRP injection. The skin at the injection site was prepped and draped, and the liquid PRP was injected in a sterile condition using an 18‐G needle. A single injection of 2 mL of autologous PRP was administered deep at the origin of wrist extensors, into the maximal tenderness point at the elbow region under aseptic technique via a peppering technique, spreading in a clock‐like manner to achieve a more expansive zone of delivery Autologous whole blood injection A single injection of 2 mL of autologous peripheral whole blood was administered by the same technique as the PRP group. Two mL of 1% lidocaine was injected 8 minutes before the whole blood injection using the same technique as for the PRP group Post intervention No cortisone or non‐steroidal anti‐inflammatories were prescribed during follow‐up. For pain relief, oral paracetamol and ice therapy were used. Participants from both groups were requested to refrain from heavy labour activities for a week. Tennis elbow strap (Oppo trademark) was administered for all participants, and they were instructed to apply the strap 2 cm below the maximal tenderness point on the elbow. Participants from both groups were followed via weekly telephone calls and were instructed how to use the elbow splint and perform exercises. Three days after the injection, each participant was asked to start a simple programme of extensor muscle stretching, and 2 weeks after injection, eccentric loading exercises were prescribed to be performed on an individual basis twice every day for 5 weeks. Participants were allowed to perform full activities of daily living after 4 weeks |
|
| Outcomes | Outcomes were measured at 4 weeks, 8 weeks, 6 months, and 12 months Study outcomes
Outcomes used in this review
Time points used in this review 4 weeks, 6 months, and 12 months |
|
| Notes |
Funding: research grant from Faculty of Medicine, Shahid Beheshti University of Medical Sciences Trial registration: not done Withdrawals: 2/33 in the PRP group (loss to follow‐up = 1, moved to another city = 1), 1/31 in the whole blood group (moved to another city = 1) Adverse events: not reported in both groups Autologous PRP group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Autologous whole blood group Total adverse event No. of events = 0 Serious adverse events No. of events = 0 Data analysis: we used 4‐week data for the 6‐week time point. We reversed the direction of Modified Mayo Clinic Performance Index scores to read as 0 = no disability, to ensure consistency in interpretation of function scores in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked covariate adaptive randomisation was used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. "The assessors filling out the questionnaire and performing PPT, also the statistician were blinded to the group of the patient" |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded, there is risk of bias in the measurement of pain, function, and treatment success |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | As assessments of pain thresholds were not truly objective, there is risk of bias associated with the measurement of PPT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/33 in the PRP group (loss to follow up = 1, moved to another city = 1) and 1/31 in the whole blood group (moved to another city = 1) withdrew from the study |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered, and no study protocol was found |
| Other bias | Low risk | None is apparent |
Schoffl 2017.
| Study characteristics | ||
| Methods |
Design: double‐blind parallel 2‐arm randomised controlled trial Setting: hospital in Germany Timing: not reported Interventions: autologous conditioned plasma and dry needling vs placebo (saline) injection with dry needling Sample size: 50 (25 in PRP and dry needling, 25 in placebo and dry needling) Analysis: not intention‐to‐treat |
|
| Participants |
Number of participants Number screened not reported Number randomised: 50 (72%) participants randomised (25 to PRP and dry needling, 25 to placebo and dry needling) Number included in analysis: 36 (18 in PRP and dry needling, 18 in placebo and dry needling) analysed at 4 weeks and at 6 months Inclusion criteria
Exclusion criteria
Baseline characteristics PRP and dry needling group Mean (SD) age (years): 52.6 (11.5) Mean (SD) duration of symptoms (months): 18.8 (14.6) Number (%) female: 9 (50) Mean (SD) VAS pain: 4.2 (1.8) Mean (SD) DASH 41 (18) Placebo and dry needling group Mean (SD) age (years): 52.6 (11.6) Mean (SD) duration of symptoms (months): 14.8 (13.2) Number (%) female: 9 (50) Mean (SD) VAS pain: 5.4 (0.7) Mean (SD) DASH: 36.4 (17.7) |
|
| Interventions |
PRP preparation: study authors used Arthrex kit (leukocyte‐poor). No further details reported Both groups 1 mL of Scandicain® (equivalent to 10 mg mepivacaine hydrochloride) was infiltrated along the surface of the tendon with a 23‐G needle. Following the standard wait time of several minutes for the anaesthetic to take effect, the needle tip was positioned into the site of maximum tendon injury, specified as maximum point tenderness. The tendon was then dry‐needled to create fenestrations, initiating fibril disruption and internal bleeding. The syringe was covered with opaque tape to blind the physician and the participant PRP injection and dry needling ACP was then slowly injected into the site of tendinosis. The time frame between blood aspiration and ACP/placebo injection was 10 to 15 minutes. Three applications of ACP were performed with a free interval of 7 to 10 days between therapy sessions, with dry needling and anaesthetic procedures preceding only the first ACP injection Placebo injection and dry needling Blood was collected similar to the PRP group, but the nurse filled the syringe with 0.9% saline. The injection was given in a similar manner as the ACP injection Post intervention Patients were instructed to continue their normal daily activities except those that could aggravate their symptoms |
|
| Outcomes | Study authors measured outcomes at baseline and at 4 weeks and 6 months Study outcomes
Outcomes used in this review
Time points used in this review
|
|
| Notes |
Funding: Arthrex provided ACP kits for free Trial registration: no registration Withdrawals: data missing from 7 participants in each group; reasons not reported Adverse events: not reported; unclear if measured Serious adverse events: not reported; unclear if measured. Study author provided information on allocation concealment performed with the use of sealed opaque envelope |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were externally randomized into the treatment control (placebo) group by an independent statistician with the help of Microsoft Excel, randomised number between 0 and 1 were generated and listed" |
| Allocation concealment (selection bias) | Low risk | Study author provided information on allocation concealment performed with the use of sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the syringe was blinded by the study nurse through external coverage with adhesive and opaque tape wrapping" This ensured blinding of both interventionists and participants |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | Participants were blinded; there is low risk of bias in the measurement of self‐reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐reported outcomes were used in this study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals were 7/25 (28%) in both groups. Study authors reported that "those not achieving a satisfactory result were discontinued from the study and hence did not qualify for re‐evaluation at follow up" |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available; trial was not registered; baseline data were provided only for those who achieved satisfactory results; adverse events were not measured |
| Other bias | Low risk | None is apparent |
Stenhouse 2013.
| Study characteristics | ||
| Methods |
Design: single‐centre unblinded parallel 2‐arm randomised controlled trial Setting: Rheumatology or Orthopaedic Outpatient Clinic, West Middlesex University Hospital, Middlesex, United Kingdom Timing: June 2010 to August 2012 Interventions: autologous conditioned plasma injection and dry needling vs dry needling Sample size: not reported Analysis: data for participants who completed the study were analysed |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 28 (15 in autologous blood group, 13 in dry needling group) Number included in analysis: 25 (13 in autologous blood group, 12 in dry needling group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous conditioned plasma and dry needling group Mean (SD) age (years): 53.2 (9.87) No. male:female: 8:7 Mean (SD) duration of pain (months): 18.9 (17.8) Dominant arm affected: 13 (86.6%) Mean (SD) VAS pain score (0 to 10; 0 is no pain): 8.07 (1.18) Mean (SD) Nirschl stage (80 to 0; 80 is no pain): 11.1 (14.3) Dry needling group Mean (SD) age (years): 47.6 (6.12) No. male:female: 5:8 Mean (SD) duration of pain (months): 22.2 (14.5) Dominant arm affected: 11 (84.6) Mean (SD) VAS pain score (0 to 10; 0 is no pain): 6.87 (2.15) Mean (SD) Nirschl stage (0 to 80; 80 is no pain): 22.9 (19.1) Pretreatment group differences: Nirschl scores were higher in the dry needling group compared with the ACP and dry needling group |
|
| Interventions | The abnormal common extensor origin tendon was identified with a 15 L 8‐W transducer on a GE Logiq E9 ultrasound machine (Milwaukee, WI). Before treatment, the skin was cleaned with antiseptic solution and 1 to 2 mL of 1 % lignocaine was injected immediately deep into the deep fascia with a 23‐G needle. Care was taken to avoid local anaesthetic injection into the tendon. Following a short interval (to allow the anaesthetic to act), the patient was treated with either dry needling alone or dry needling followed by ACP injection. The procedure was repeated at 1 month Autologous conditioned plasma and dry needling group Participants had 10 mL of blood taken from their contralateral antecubital vein. The ACP double syringe (manufactured by Arthrex Double Syringe System, Inc., Naples, FL) containing whole blood was then centrifuged at 1500 rpm for 5 minutes. This separated red blood cells from platelet‐containing plasma. The plasma was drawn into the smaller syringe. Dry needling was performed initially for 2 minutes followed by a 2‐mL injection of ACP Dry needling group Dry needling consisted of passing a fine needle (23G) in and out through the long axis of the tendon without exiting the skin approximately 40 to 50 times to pepper the tendon. This lasted a total of 2 minutes Follow‐up care Participants were advised to undertake normal activities but to avoid anything that may aggravate symptoms |
|
| Outcomes | Outcomes were reported at baseline and at 2 months and 6 months Outcomes
Outcomes included in this review
Time points included in this review 2 months and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawals: 2/15 in the autologous blood group and 1/13 in the dry needling group were lost to follow‐up due to increasing elbow pain after the first treatment Adverse events Autologous conditioned plasma and dry needling group Total adverse events: 2/15 No. of events: 2 Nature of event: increased elbow pain after first treatment Serious adverse events: none reported Dry needling group Total adverse events: 1/13 No. of events: 1 Nature of event: increased elbow pain after first treatment Serious adverse events: none reported Although study authors reported no adverse events in either group, 3 participants were lost from the study ‐ 2 from the autologous blood group and 1 from the dry needling group ‐ due to increasing elbow pain after first injection. We regarded these data as withdrawal due to adverse events and documented increased elbow pain following treatment as an adverse event |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using an Internet platform called 'research randomised form v4.0'” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not adequately reported in the article |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to group allocation; study authors report "a separate investigator was enrolled to evaluate the outcome measures" |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to treatment allocation, there is risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | There were no truly objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/15 (13%) in the autologous blood group and 1/13 (7.7%) in the dry needling group were discontinued after first intervention due to increased elbow pain after the first injection |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found and the trial was not registered |
| Other bias | Low risk | No other bias was found |
Tetschke 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre 2‐arm parallel single blind quasi‐randomised controlled trial Setting: Orthopedic Department, University Hospital, Magdeburg (Otto‐von‐Guericke University Magdeburg), Germany Timing: between March 2012 and July 2013 Interventions: autologous conditioned plasma (ACP) injections vs low‐level laser application Sample size: sample size calculation was not done Analysis: data for those who completed outcome assessments were analysed |
|
| Participants |
Number of participants Number screened: 61 Number excluded: 5 (3 not willing to participate, 2 not eligible) Number quasi‐randomised: 56 (27 to ACP group, 29 to laser group) Number included in analysis: 52 (26 in ACP group, 26 in laser group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous conditioned plasma (ACP) injection group Mean (SD) age (years): 51.5 (10.4) No. male:female: 12:14 Mean (SD) VAS score: 5.2 (1.8) Mean (SD) DASH score: 37 (18.3) Laser application group Mean (SD) age (years): 53.1 (12.7) No. male:female: 9:17 Mean (SD) VAS score: 6.7 (2.0) Mean (SD) DASH score: 47 (19.6) Pretreatment group differences: VAS and DASH scores were higher in the laser group than in the ACP group |
|
| Interventions |
Autologous conditioned plasma (ACP) injection For preparation of ACP, the ACP Double Syringe System by Arthrex, Inc. (Naples, FL) was used. 10 mL of whole blood was collected from a vein in the region of the cubital fossa with a 21‐gauge butterfly needle into a double‐lumen syringe. Centrifugation of blood at 1500 rounds per minute for 5 minutes resulted in 3 to 5 mL of supernatant. The extracted growth factor rich plasma (supernatant) was now transferred from the larger outer syringe into the smaller inner syringe. ACP was injected with a 25‐gauge needle at first subfascially in the region of the common head of the extensors. Then a further, intralesional dispersion was followed by a 2 times over fan‐like wheal injection. Three intralesional ACP injections were administered with an interval of 7 days in between Laser application Laser application was conducted with a low‐level laser BTL 5000 (a GaAs‐infrared laser with a wavelength of 830 nm and a dose of 7 J/cm²; BTLMedizintechnik GmbH, Ulm, Germany). During this procedure, special glasses were used to protect the retina. Laser radiation was applied in circular movements to the region of the lateral epicondyle. Treatment was managed by 2 previously instructed physiotherapy practices. Myofascial manipulation was done after laser application for additional benefit of hyperaemia and metabolism activation Post intervention Physiotherapy was given after 8 weeks to all participants in both groups. This was based on 12 sessions with manual therapy techniques for trigger point elimination in the initial phase, stretching and strengthening exercises in the first 2 weeks, and patient‐adapted muscle‐trophic training in the advanced phase. Therapists were instructed by a treatment protocol, which was prepared in collaboration between physicians and physiotherapists. The standardised physiotherapy protocol involved 2 phases ‐ an initial phase to restore the regenerative process, and a final phase with muscle trophic training for optimisation of functionality. In addition, patients were assigned to do daily, self‐contained stretching exercises |
|
| Outcomes | Outcomes were measured at baseline and at 2 months, 6 months, and 12 months Study outcomes
Outcomes used in this review
Time points used in this review 2 months, 6 months, and 12 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawals: 1/27 in the ACP group was lost to follow‐up as were 3/29 in the laser group (1 = loss to follow up, 2 = underwent surgery) Adverse events ACP injection group Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Laser application group Total adverse events No.of events: 0 Serious adverse events No.of events: 0 Data analysis: 2‐month data were entered as 3‐month data. This is a quasi‐randomised study, and risk of bias assessments were done in a similar manner as for randomised studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was not done, as this is a quasi‐randomised design whereby participants were allocated sequentially into 2 treatment groups |
| Allocation concealment (selection bias) | High risk | Allocation was not random and therefore was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and study personnel was not done |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants were not blinded to group allocation, there is risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No outcomes were assessed by study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/27 (3.7%) in the ACP group was lost to follow‐up and 3/29 (10.3%) in the laser group (1 = loss to follow up, 2 = underwent surgery) were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered and study protocol was not available |
| Other bias | Low risk | No other bias is apparent |
Thanasas 2011.
| Study characteristics | ||
| Methods |
Design: single‐centre single‐blinded 2‐arm parallel randomised controlled trial Setting: Red Cross Hospital, Athens, Greece Timing: not reported Interventions: platelet‐rich plasma injection vs autologous blood injection Sample size: sample estimation was performed via Lehr formula; power was fixed at 80%. The minimum sufficient number of participants was 13 for each group, with level of significance as P < 0.05 Analysis: only those who completed follow‐up assessments were included in the final analysis |
|
| Participants |
Number of participants Number of participants screened for eligibility: not reported Number excluded: not reported Number randomised: 28 (14 to platelet‐rich plasma group, 14 to autologous blood group) Number included in analysis at 6 months: 27 (14 in platelet‐rich plasma group, 13 in autologous blood group) Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean age (years): 35.9 Sex: 10 female:5 male Mean duration of pain (months): 4.7 Dominant arm affected: 11 Mean (SD) VAS pain score (0 to 10; 0 is no pain): 6.1 (1.28) Mean (SD) Liverpool Elbow score (10 to 0; 10 is no disability): 6.99 (0.3) Autologous blood group Mean age (years): 36.6 Sex: 11 female:3 male Mean duration of pain (months): 5.1 Dominant arm affected: 13 Mean (SD) VAS pain score (0 to 10; 0 is no pain): 6.0 (1.3) Mean (SD) Liverpool Elbow score (10 to 0; 10 is no disability): 6.97 (0.61) Pretreatment group differences: groups were similar at baseline |
|
| Interventions | All injections were performed under ultrasound guidance Platelet‐rich plasma group 27 to 55 mL of autologous blood was extracted and mixed with 3 to 5 mL of anticoagulant. This was centrifuged at 3200 rpm for 15 minutes via the Biomet GPS III system. To estimate the concentration of the PRP extraction, samples of 2 healthy volunteers were examined (blood test parameters within normal limits). Compared with whole blood, the concentration of platelets was found to be raised from 235,000/mL to 1,292,500/mL (5.5 times on average). White blood cells are included in the concentrate, with an average ratio of 111/1 (platelets/leukocytes). 3 mL of PRP was injected deep to the origin of wrist extensors via a peppering technique using a single skin portal Autologous blood group Single injection of 3 mL of autologous peripheral whole blood, deep at the origin of wrist extensors was administered via a peppering technique using an aseptic technique with the assistance of ultrasound guidance Follow‐up care No cortisone or non‐steroidal anti‐inflammatory medications were prescribed during follow‐up. Oral paracetamol and ice therapy was permitted as needed. Participants were requested to refrain from heavy physical activity for 1 week. A week after the injection, each participant was reassessed and was given a simple programme of stretching and eccentric loading exercises to be performed on an individual basis twice every day for 5 weeks |
|
| Outcomes | Outcomes were reported at baseline and at 6 weeks, 3 months, and 6 months Outcomes
Outcomes included in this review
Time points included in this review 6 weeks, 3 months, and 6 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawals: 1/14 from autologous blood group was lost to follow‐up Adverse events PRP injection group Total adverse events: 9/14 No. (%) of events: 9 (64) Nature of the event: local pain and discomfort from the day of injection, which subsided gradually Serious adverse events No. of events = 0 Autologous blood group Total adverse events: 4/14 No. (%) of events: 4 (28) Nature of the event: local pain and discomfort from the day of injection, which subsided gradually Serious adverse events No. of events = 0 Data analysis: we changed the direction of Liverpool function scores to read as 0 = no disability, to ensure consistency in interpretation of function scores in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using an Internet platform called '‘research randomiser form v4.0'’’ |
| Allocation concealment (selection bias) | Unclear risk | It is unclear how allocation to the 2 groups was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and injecting doctors were not blinded to treatment. Study assessors were blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | Participants were not blinded to treatment, so there is risk of bias in the measurement of pain and function |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Assessors were blinded to treatment, but there were no truly objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/14 in the autologous blood group was lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was found and the trial was not registered |
| Other bias | Low risk | No other bias was found |
Watts 2020.
| Study characteristics | ||
| Methods |
Design: single‐centre unblinded 2‐arm parallel‐group randomised controlled trial Setting: sIngle specialist centre, Wrightington, Wigan, UK Timing: not reported Interventions: platelet‐rich plasma injection vs open surgery Sample size: the power calculation for the primary endpoint was based on mean change in PRTEE pain; study authors powered (power 0.8) the study to detect 15 points difference with 45 participants per group Analysis: study authors claim they used ITT analysis, but in discussion they state, "data were only available for the primary outcome measure in 52 of 81 subjects (64%) in part due to the high cross‐over from L‐PRP to surgery". Thus, it seems that participants who crossed over were excluded from subsequent analyses (i.e. reported data are likely from per‐protocol analysis) |
|
| Participants |
Number of participants Number of participants screened for eligibility: 100 Number excluded: 17 (8 did not meet inclusion criteria, 9 declined) Number randomised: 83 (41 to platelet‐rich plasma group, 42 to surgery) Number included in analysis: 31 for PRP and 25 for surgery at 6 weeks; 27 for PRP and 25 for surgery at 3 months; 28 for PRP and 30 for surgery at 6 months; 24 (59%) in PRP group and 28 (68%) in surgery group: 52 (63%), 24 (59%) in PRP group, 28 (68%) in surgery group Inclusion criteria
Exclusion criteria
Baseline characteristics Platelet‐rich plasma group Mean age (years): 47 Sex: 17 female:23 male Mean duration of pain (months): 23 Mean number of glucocorticoid injections: 3 Mean (SD) PRTEE pain score (0 to 50; 0 is no pain): 32 (7) Mean (SD) PRTEE function score (0 to 50; 0 is no disability): 28 (10) Mean (SD) PRTEE total score (0 to 100; 0 is no disability): 58 (16) Mean (SD) DASH score (0 to 100; 0 is no disability) 47(17) Surgery group Mean age (years): 48 Sex: 17 female:24 male Mean duration of pain (months): 22 Mean number of glucocorticoid injections: 2 Mean (SD) PRTEE pain score (0 to 50; 0 is no pain): 33 (7) Mean (SD) PRTEE function score (0 to 50; 0 is no disability): 29 (10) Mean (SD) PRTEE total score (0 to 100; 0 is no disability): 62 (17) Mean (SD) DASH score (0 to 100; 0 is no disability): 45 (18) |
|
| Interventions |
Platelet‐rich plasma group L‐PRP was prepared using the Zimmer Biomet Recover Platelet Separation Kit (Biomet Biologics Inc., Warsaw, IN). 27 mL of venous whole blood was drawn from the patient into a 30‐mL syringe containing 3 mL of ACD‐A (anticoagulant). The mixture was transferred to a single‐use sterile mini‐separation tube and placed in a desktop centrifuge for 15 minutes at 3200 r/min to separate L‐PRP from plasma and red blood corpuscles. During centrifugation, 2 mL of 2% plain lidocaine was injected into the dermis and subdermal tissues at the site of maximal tenderness determined by manual palpation. L‐PRP was extracted from the GPS III device with a 10‐mL syringe. 1 to 3 mL of L‐PRP was injected via an 18‐gauge hypodermic needle into the ECRB tendon via a ‘peppering’ technique. Ultrasound was not routinely used to guide the injection Surgery group Surgery was undertaken using the technique described by Nirschl, which was common to both surgeons. Under general or regional anaesthesia, a 3– to 5‐cm curvilinear incision was created centred on the lateral epicondyle. The ECRL tendon was retracted anteromedially to expose the ECRB tendon origin. Any area of tendinosis was excised from the ECRB tendon. After haemostasis, the ECRB aponeurosis was repaired with a Vicryl suture and wound closure was performed with a subcuticular suture. A bulky bandage was applied for 48 hours |
|
| Outcomes | Outcomes were reported at 6 weeks, 3 months, 6 months, and 12 months Outcomes
Outcomes included in this review
|
|
| Notes |
Funding: study author(s) disclosed funding for this study from Zimmer Biomet, which manufactures the PRP kit Trial registration: not done Adverse events: 1 adverse event (surgical wound debridement) in the surgery group, zero events in PRP group Withdrawals: not reported Data analysis: PRTEE pain score (0 to 50) was transformed to 0 to 10 scale. PRTEE total score was used for function, similar to the other study in the comparison (Merolla 2017). One patient in the surgery arm had an L‐PRP injection in the same area for persistent symptoms 10.6 months post surgery. Thirteen patients in the L‐PRP arm underwent surgical treatment for persistent symptoms of TE at a mean time of 6.2 months. Patients who crossed over were excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 100 envelopes were shuffled |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised to either L‐PRP injection or surgical intervention using 100 opaque envelopes shuffled and sequentially numbered, 50 stating ‘Surgery’ and 50 stating ‘PRP injection" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the clear difference between the two techniques, blinding of the patient or surgeon was not possible" Not attempted |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | Not attempted (surgery vs injection) |
| Blinding of outcome assessment (detection bias) objective outcomes | High risk | Not attempted. All outcomes were subjective |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were available for 31 for PRP and 25 for surgery at 6 weeks; 27 for PRP and 25 for surgery at 3 months; 28 for PRP and 30 for surgery at 6 months; and 24 (59%) in PRP group and 28 (68%) in surgery group. Large but balanced loss, but study authors state that data for patients crossing over were included up to the time of their second intervention (mean, 6.2 months). Thus attrition may be biased as cross‐over rates increased |
| Selective reporting (reporting bias) | Unclear risk | No protocol or registration is available. Outcomes that were specified in the methods were reported at all time points |
| Other bias | Low risk | Study was funded by the manufacturer of the PRP kit. 14 patients had additional interventions within 12 months of the index intervention. 1 patient in the surgery arm had an L‐PRP injection in the same area for persistent symptoms 10.6 months post surgery (3% cross‐over). 13 patients in the L‐PRP arm underwent surgical treatment for persistent symptoms of TE at a mean time of 6.2 months (3 to 10.6); 30% cross‐over. Data for these patients are included up to the time of their second intervention |
Wolf 2011.
| Study characteristics | ||
| Methods |
Design: multi‐centre (2 sites) single‐blinded 3‐arm parallel randomised controlled trial Setting: Denver Health Medical Centre and Denver Veterans Administration Medical Centre, Denver, Colorado, USA Timing: not reported. Interventions: autologous blood injection vs glucocorticoid injection vs normal saline injection Sample size: based on DASH outcomes scores, and assuming a large effect size (f 0.70), a total of 8 participants per group were required to detect a statistically significant difference with 0.05 Analysis: type of analysis (completer or intention‐to‐treat) not reported; not all randomised were included in analysis. Likely to be completers |
|
| Participants |
Number of participants Number of participants screened for eligibility: 108 Number excluded: 74 (did not consent) Number enrolled: 34 Number randomised: 34 (per group numbers not given). 3 participants dropped out of the study after 2 weeks, and another 3 dropped out after the initial injection Number included in analysis at 6 months: 28 (10 in autologous blood group, 9 in glucocorticoid group, 9 in saline group) Inclusion criteria
Exclusion criteria
Baseline characteristics Autologous blood group Mean (SD) VAS pain score (0 to 10; 0 is no pain): 5 (3.1) Mean (SD) DASH score (0 to 80; 0 is no disability): 38 (14.1) Glucocorticoid group Mean (SD) VAS pain score (0 to 10; 0 is no pain): 5 (1.4) Mean (SD) DASH score (0 to 80; 0 is no disability): 37 (14.1) Normal saline group Mean (SD) VAS pain score (0 to 10; 0 is no pain): 5 (3) Mean (SD) DASH score (0 to 80; 0 is no disability): 38 (10.0) Pretreatment group differences: baseline scores were similar. Demographic information was not reported |
|
| Interventions | All participants had 3 mL of blood drawn before injection. Injections were prepared behind a curtain and were covered by foil to conceal contents. After sterile preparation of the lateral epicondyle, the needle was advanced under the extensor origin and all injections were performed with multiple passes of the needle in a fan‐like fashion Autologous blood group 2 mL of autologous blood was mixed with 1 mL of lidocaine and injected as described Glucocorticoid group 2 mL of a glucocorticoid (type not reported) was mixed with 1 mL of lidocaine and injected as described Normal saline group 2 mL of normal saline was mixed with 1 mL of lidocaine and injected as described Follow‐up care Participants were given a standardised sheet of stretching exercises. No other therapy was permitted |
|
| Outcomes | Outcomes were reported at baseline and at 2 weeks, 2 months, and 6 months Outcomes
Outcomes included in this review
Time points included in this review 2 months and 6 months |
|
| Notes |
Funding: Clinical Research Grant from the American Society for Surgery of the Hand. Trial authors report no financial conflict of interest Trial registration: not done Adverse events: not reported Withdrawals: 3 participants dropped out of the study after 2 weeks, and another 3 did not follow up after initial injection. Numbers per group were not reported Data analysis: 2‐month data were used for 6‐week time point. DASH scores were used to report function, and VAS scores for pain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to 3 treatment groups generated centrally by a random numbers table |
| Allocation concealment (selection bias) | Low risk | Concealment was achieved through sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants from all groups had 3 mL of blood drawn before the injection was prepared. Each injection was mixed by the physician, who stayed behind a curtain or screen and then covered the syringe with aluminium foil. Physicians performing the injections were not blinded to the type of injection given; however they were not involved in after‐injection care |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | Participants were unaware of treatment groups; thus low risk of bias for self‐reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | No assessor‐reported outcomes were used in this study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors reported that 6/34 (18%) participants did not complete the study. Numbers and reasons per group were not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found; trial was not registered. Participant recruitment is not clearly reported; study authors state 34 were enrolled but do not provide numbers per group; only per‐group data at analysis are provided |
| Other bias | Low risk | None is apparent |
Yadav 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre 2‐arm parallel randomised controlled trial Setting: Safdarjang Hospital, New Delhi, India Timing: between October 2012 and April 2014 Interventions: platelet‐rich plasma (PRP) injection vs glucocorticoid injection Sample size: sample size calculation not done Analysis: data for those who completed the study were analysed |
|
| Participants |
Number of participants Number screened: not reported Number excluded: not reported Number randomised: 65 (numbers to PRP group and glucocorticoid group not reported) Numbers included in analysis at 3 months: 60 (30 in PRP group, 30 in glucocorticoid group) Inclusion criteria
Exclusion criteria
Baseline characteristics PRP group
Glucocorticoid group
Pre‐treatment group differences: no baseline differences were found between the 2 groups |
|
| Interventions |
Platelet‐rich plasma (PRP) injection Participants received a single injection of PRP (1 mL), with absolute platelet count of 1 million platelets/mm³ as confirmed by manual counting. PRP was prepared under aseptic conditions as per the procedure standardised in the departmental laboratory. A 9001:2000 ISO certified R‐23 centrifuge was used for the purpose of platelet concentration. PRP was injected into the common extensor origin at the lateral epicondyle of the humerus under aseptic conditions Glucocorticoid injection Participants received a single injection of glucocorticoid (methylprednisolone, 40 mg in 1 mL). The site of injection and the technique used were the same in both groups Post intervention Only paracetamol (500 mg) tablets were allowed as rescue medication for a maximum period of 1 week in both groups |
|
| Outcomes | Outcomes were measured at baseline and at 15 days, 1 month, and 3 months Study outcomes
Outcomes used in this review
Time pointsused in this review 1 month and 3 months |
|
| Notes |
Funding: not reported Trial registration: not done Withdrawals: 5/65 were lost to follow‐up; however group‐wise numbers were not reported Adverse events PRP injection group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Glucocorticoid injection group Total adverse events No. of events = 0 Serious adverse events No. of events = 0 Data analysis: 1‐month data were entered for the 6‐week time point analysis. Study authors did not report standard deviation for the groups. We calculated SD for pain, function, and grip strength using reported P values. At 3 months, P values were reported as P < 0.0001; thus we could not calculate SD, therefore we imputed it using SD from 4‐week analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors do not describe any method of blinding, and blood was likely not drawn in the glucocorticoid group. Thus, participants must have been aware of the intervention they received. There was no report of interventionists being blinded |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | High risk | As participants most likely were not blinded, there is high risk of bias in the measurement of self‐reported outcomes |
| Blinding of outcome assessment (detection bias) objective outcomes | Unclear risk | It is unclear whether assessors measuring grip strength were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/65 (7.6%) participants were lost to follow‐up, but study authors did not report group‐wise missing data. Data from these 5 participants were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available and the trial was not registered; measures of variance for study outcomes were not reported |
| Other bias | Low risk | None is apparent |
Yerlikaya 2018.
| Study characteristics | ||
| Methods |
Design: single‐centre double‐blinded three‐arm parallel randomised controlled trial Setting: Erciyes University, Turkey Timing: between 15 February 2015 and 15 June 2015 Interventions: Leukocyte‐poor‐PRP injection versus Leukocyte‐rich‐PRP injection versus saline injection Sample size: sample size calculation not done Analysis: data analysed from participants who completed the study |
|
| Participants |
Number of participants: Number screened: not reported Number excluded:not reported Number randomised: 90 (30 to the Leukocyte‐poor‐PRP group 30 to the Leukocyte‐rich‐PRP group and 30 to the saline group) Number included in analysis: 90 (30 to the Leukocyte‐poor‐PRP group 30 to the Leukocyte‐rich‐PRP group and 30 to the saline group) Inclusion criteria:
Exclusion criteria:
Baseline characteristics: LP‐PRP group: Mean (SD) age in years: 45 (8.6) No. (%) male/female: 4/26 (13.3/86.7) Mean (SD) VAS scores for nocturnal pain: 7.4 (2.1) Mean (SD) VAS scores for pain during activity: 8.7 (1.7) LR‐PRP group: Mean (SD) age in years: 46.5 (8.7) No. (%) male/female: 11/19 (36.7/63.3) Mean (SD) VAS scores for nocturnal pain: 6.5 (2.4) Mean (SD) VAS scores for pain during activity: 7.9 (1.8) Saline group: Mean (SD) age in years: 47.6 (9.1) No. (%) male/female: 11/19 (36.7/63.3) Mean (SD) VAS scores for nocturnal pain: 6.8 (1.8) Mean (SD) VAS scores for pain during activity: 8.2 (1.7) Pre‐treatment group differences: There were no baseline differences found between any of the groups. |
|
| Interventions |
PRP preparation: Blood (16 mL) was drawn from cubital vein; then citrate (4 mL) was added to prevent premature clotting. Following placement of blood samples in 10 mL tubes, the LP‐PRP was centrifuged at 1075 rpm for 15 minutes in the first step. Then, it was re‐centrifuged at 1495 rpm for 15 minutes under layer 1 and layer 2 of laminar flow. Following centrifugation, the platelets were initially collected by a poor plasma syringe and these platelets were not used for injection. Thereafter, the remaining platelets were collected from the tube and two aliquots containing approximately 1.5 mL of PRP were obtained. Of these, one was injected to the patient while the other was used to determine cell count. After receiving the same amount of blood for LR‐PRP, the blood was centrifuged at 1190 rpm for 20 minutes in the first step. Then, it was re‐centrifuged for 15 minutes at 1890 rpm in the second step. The platelet concentration was intended to be 2‐ or 8‐fold higher than the basal value while the leukocyte concentration was intended to be three‐fold lower and three‐fold higher than the basal value in the LP‐PRP and LR‐PRP groups, respectively. Leukocyte‐poor‐PRP (LP‐PRP) injection: LP‐PRP (1.5 mL) was injected to the participants via peppering technique at the most sensitive point. Peppering technique involves insertion of a needle into the tendon to inject some of the blood; followed by retraction of the needle to subcutaneous tissue and redirection and reinsertion of the needle. The injections were performed without ultrasound guidance. Leukocyte‐rich‐PRP (LR‐PRP) injection: LR‐PRP (1.5 mL) was injected to the participants following the same process as described above. Saline injection: Normal saline (1.5 mL) was injected to the participants following the same process as described above. Post‐intervention: In all groups, an exercise program was prescribed including three sets per day consisting of 10 repetitive range of motion, stretching, strengthening, and gripping exercises for over four weeks. The exercise program was initiated on the second day after injection. The participants were given brochures containing a written statement. Participant compliance of the exercise program was recorded. Non‐steroidal antiinflammatory drug use was prohibited for 1 week before and after PRP application. The use of paracetamol up to 4 g/day for pain before and after injection was allowed. |
|
| Outcomes | Outcomes were measured at 4 weeks and 8 weeks Study outcomes:
Outcomes used in this review:
Timepointsused in this review: 4 weeks and 8 weeks |
|
| Notes |
Funding: none reported Trial registration: not done Withdrawals: none reported Adverse events: LP‐PRP group: Total adverse event: No. of events = 0 Serious adverse events: No. of events = 0 LR‐PRP group: Total adverse event: No. of events = 0 Serious adverse events: No. of events = 0 Saline group: Total adverse event: No. of events = 0 Serious adverse events: No. of events = 0 Data analysis: We pooled the leucocyte‐poor and leucocyte rich groups together in the pain analysis (Analysis 1.2). Function and adverse events measured but not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned into three groups (n=30 in each) by using the block randomisation method." |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded to the received treatment. |
| Blinding of outcome assessment (detection bias) for self reported subjective outcomes (pain, function, treatment success, quality of life) | Low risk | As participants were blinded to group allocation there is low risk of bias in the measurement of self‐reported outcomes of pain and function. |
| Blinding of outcome assessment (detection bias) objective outcomes | Low risk | Outcome assessors were blinded to group allocation, hence there is low risk of bias in the measurement of grip strength and tendon thickness. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported in any group. |
| Selective reporting (reporting bias) | High risk | Numerical results for patient‐based forearm questionnaire, daily life questionnaire, grip strength and tendon thickness were not reported in the article.The method of participant blinding was not clearly reported. The trial was not registered and study protocol was not available. |
| Other bias | Low risk | none apparent. |
ACP: autologous conditioned plasma.
AE: adverse event.
BMI: body mass index.
DASH: Disabilities of the Arm, Shoulder and Hand questionnaire.
ECRB: extensor carpi radialis brevis tendon.
IQR: interquartile ratio.
ITT: intention‐to‐treat.
LE: lateral epicondylitis.
MCID: minimum clinically important difference
MMCPIE: Modified Mayo Clinic Performance Index for Elbow.
NSAID: non‐steroidal anti‐inflammatory drug.
PREE: Patient‐Reported Elbow Evaluation.
PRP: platelet‐rich plasma.
PRTEE: Patient‐Rated Tennis Elbow Evaluation.
SD: standard deviation.
SEM: standard error of the mean.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernstein 2013 | Not an RCT: case study and review |
| Dong 2018 | Not an RCT |
| Jiang 2020 | Not an RCT |
| Johnson 2007 | Not an RCT: review article |
| Kapoor 2012 | Not an RCT. Letter to the Editor |
| Kaux 2012 | Not an RCT. Lecture |
| Li 2019 | Not an RCT. Systematic review |
| Mishra 2006 | Not an RCT. Cohort study |
| Mishra 2009 | Not an RCT. Case discussion |
| Mishra 2010 | Not an RCT. Review article |
| Naam 2020 | Not an RCT. Commentary |
| Nichols 2012 | Not an RCT. Commentary |
| Orchard 2011 | Not an RCT. Commentary |
| Scudeller 2011 | Not autologous blood or PRP injection: platelet lysate. N = 1 study |
| Shiple 2013 | Not an RCT. Commentary |
| Szabo 2009 | Not an RCT. Review article |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12613000616774.
| Methods | Multi‐site 3‐arm triple‐blinded parallel randomised controlled trial |
| Participants |
Inclusion criteria • Lateral elbow pain > or = 6 weeks' duration • Reproducibility of pain by ≥ 2 of the following tests: palpation of the lateral epicondyle and/or the common extensor origin of the elbow, gripping, resisted wrist, or second or third finger extension (dorsiflexion) • Ultrasound‐confirmed lesion • Ability to read and write in English • Aged 18 to 65 years Exclusion criteria • Bilateral symptoms of lateral elbow pain • Any other elbow pathology • Generalised inflammatory arthritis such as rheumatoid arthritis • Concurrent shoulder and/or neck pain and/or pain proximal to the elbow on affected side • Any wound or skin lesion on the lateral side of affected elbow • Neurological symptoms or signs in affected arm • Severe infection • Known malignancy • Bleeding disorder • Previous surgery to the elbow • Local glucocorticoid injection in previous 6 months • Oral glucocorticoids in previous 3 months • Large tear > or = 15 mm in common extensor origin or torn lateral collateral ligament • Lack of informed consent • Any other reason thought likely to result in inability to complete the trial |
| Interventions | • Single ultrasound‐guided injection of autologous platelet‐rich plasma (2 mL) into the site of maximal abnormality in the elbow. To obtain autologous platelet‐rich plasma, 5 mL of venesected blood will be centrifuged for 7 minutes at 1500 revs/s, then the buffy coating and 2 mL of the supernatant will be aspirated via a 22‐gauge needle into a 3‐mL syringe. This 2 mL will then be injected into the elbow • Single ultrasound‐guided injection of glucocorticoid (Celestone Chronodose 1 mL + 1 mL normal saline) into the site of maximal abnormality in the elbow • Placebo ‐ single ultrasound‐guided injection of saline (2 mL) into the site of maximal abnormality in the elbow |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | Status at 20 December 2020: study is completed but is not yet published. Recruitment ended 24 January 2019, and follow‐up was completed 31 October 2019 (151 recruited participants) |
Chiavaras 2014.
| Methods |
Design: multi‐centre single‐blinded parallel‐group randomised controlled trial Setting: Hamilton General Hospital, Ontario, Canada, and University of Michigan, USA Interventions: platelet‐rich plasma (PRP) injection vs autologous blood injection vs dry needle fenestration vs sham injection Sample size: 25 participants per group needed to ensure over 80% power to detect a 3‐point difference in pain scores at 52 weeks |
| Participants |
Inclusion criteria Minimum 18 years of age Pain at the common extensor tendon origin and pain elicited with active extension of the wrist in pronation and elbow extension Sonographic evidence of common extensor tendinosis based on tendon thickening, areas of hypo‐echogenicity, and loss of normal echo texture Minimum symptom duration of 3 months Minimum pain score of 5 on a visual analogue scale (VAS) Exclusion criteria Elbow trauma within 1 week Rheumatoid arthritis Malignancy Pregnancy Participants requiring anti‐platelet medication for treatment of heart attack, stroke, or other medical condition Previous surgery for lateral epicondylitis Previous local injection, including steroid, within the past 6 months Signs of other causes for lateral elbow pain (posterior interosseous nerve entrapment, osteochondral lesion) |
| Interventions | All participants will have their lateral elbow injected with 1% lidocaine for local anaesthetic before intervention Autologous blood group A 22‐gauge needle will be inserted into the tendon, followed by dry needling in multiple sites A total of 6 mL of autologous blood will be drawn from the contralateral arm via a butterfly needle The tendon will be injected with 3 mL of whole blood via a fenestration technique Platelet‐rich plasma group A 22‐gauge needle will be inserted into the tendon, followed by dry needling in multiple sites A total of 16 mL of autologous blood will be drawn from the contralateral arm via a butterfly needle. Blood is centrifuged at 1500 rpm for 5 minutes through the Arthex ACP system The tendon will be injected with 3 mL of PRP via a fenestration technique Dry needling group A total of 3 mL of autologous blood will be drawn from the contralateral arm and discarded A 22‐gauge needle will be inserted into the tendon, followed by dry needling in multiple sites Sham injection group A total of 3 mL of autologous blood will be drawn from the contralateral arm and discarded A 22‐gauge needle will be inserted into the superficial subcutaneous soft tissues, with no injection or tendon penetration Follow‐up care Participants were given standardised physical therapy exercises. No other therapy was permitted |
| Outcomes | Outcomes will be reported at baseline and at 6 weeks, 12 weeks, 24 weeks, and 52 weeks Outcomes Mean pain measured on a visual analogue scale (scale from 0 (no pain) to 10) Mean disability measured by the Liverpool Elbow Score (scale from 10 (no disability) to 0) Health‐related quality of life measured by the 12‐Item Short Form Health Survey Psychological impairment measured on the Hospital Anxiety and Depression Scale Outcomes included in this review Mean pain measured on a visual analogue scale Mean disability measured by the Liverpool Elbow Score Health‐related quality of life measured by the 12‐Item Short Form Health Survey Time points included in this review 6 weeks, 12 weeks, 24 weeks, and 52 weeks |
| Notes | Trial registration: NCT01668953 Currently recruiting participants Sponsored by Arthrex, Inc. Expected completion: January 2017 |
Im 2012.
| Methods |
Study design: double‐blind randomised controlled trial Setting: SOL Hospital, Seoul, Korea Timing: not reported Intervention: platelet‐rich plasma (PRP) injection and extracorporeal shock wave therapy (ESWT) Sample size: 136 participants |
| Participants | 136 participants with chronic lateral epicondylitis randomly assigned to PRP (n = 73) or ESWT (n = 54) groups |
| Interventions |
Intervention: PRP 3 PRP injections through a peppering technique at weekly intervals in the PRP group Control: ESWT 5 ESWT sessions at weekly intervals in the ESWT group with a sonographic guide |
| Outcomes | Visual analogue scale (VAS) for pain Quick DASH Outcome Measure scores (DASH: Disabilities of the Arm, Shoulder, and Hand) for function before and at 6 months |
| Notes | Conference poster. No full text available |
Kulkarni 2020.
| Methods |
Study design: 2‐arm prospective comparative trial Setting: India Timing: not reported Intervention: platelet‐rich plasma (PRP) injection and methylprednisolone acetate injection Sample size: 50 |
| Participants | 50 participants with lateral epicondylitis |
| Interventions |
Intervention: PRP Local injection of platelet‐rich plasma via BD Vacutainer® blood collection needle and adapter Control: methylprednisolone acetate injection Local injection of methylprednisolone acetate |
| Outcomes | VAS score and maximum grip strength measured at 6 weeks, 12 weeks, 24 weeks, and 52 weeks |
| Notes | Full text available; however, the paper does not clearly state whether this is an RCT or not. Study authors have been contacted, but we received no response |
AQoL: Assessment of Quality of Life.
DASH: Disabilities of the Arm, Shoulder and Hand questionnaire.
ESWT: extracorporeal shock wave therapy.
PRP: platelet‐rich plasma.
PRTEE: Patient‐Rated Tennis Elbow Evaluation.
VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900024425.
| Study name | Ultrasound‐guided leukocyte‐poor platelet‐rich plasma injection for lateral epicondylitis: a randomized, double‐blinded, controlled trial |
| Methods | Single‐centre 2‐arm parallel placebo‐controlled randomised trial |
| Participants |
Inclusion criteria Diagnosis of lateral epicondylitis over 3 months, with pain on VAS (visual analogue scale) ≥ 5, aged 18 to 65 years, ultrasonographic confirmation of lateral epicondylitis Exclusion criteria Immune system disease or neurological disorder, such as cervical radiculopathy or carpal tunnel syndrome, peripheral nerve injury; affected upper arm with elbow or wrist fracture; history of elbow surgery; taking oral NSAIDs within last 2 weeks or local steroid injection within last 6 weeks; history of allergic reaction, cardiopulmonary insufficiency, thrombocytopenia, or other coagulation disorder; pregnancy; tumour; or those who cannot complete this trial. |
| Interventions | Leukocyte‐poor platelet‐rich plasma vs normal saline |
| Outcomes | VAS ASES Quick DASH |
| Starting date | 01/09/2019 |
| Contact information | Yu Xi 363 Furong Avenue, Wenjing District, Chengdu, Sichuan +86 15680670929 912606137@qq.com |
| Notes | Recruitment pending |
ChiCTR2000032210.
| Study name | A randomized, double‐blind, controlled clinical trial of platelet‐rich plasma combined with arthroscopy in the treatment of lateral epicondylitis |
| Methods | 3‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria • Diagnosis of LE by MRI or ultrasound without obvious tear of tendon • Symptoms longer than 6 months • After conservative treatment is ineffective or has poor effect • Repeated symptoms (≥ 2 times) Exclusion criteria • Without any treatment • Underwent surgery or PRP treatment that was ineffective • Haematological disease, infection, coagulation dysfunction, etc.; not suitable for surgery or PRP treatment • Patient does not accept enrolment or follow‐up plan • Mental symptoms and cannot complete the test and follow‐up evaluation |
| Interventions | Group 1: PRP Group 2: arthroscopic surgery Group 3: arthroscopic surgery with PRP |
| Outcomes | VAS |
| Starting date | 01/05/2020 |
| Contact information | Liu Yan 363 Furong Avenue, Wenjing District, Chengdu, Sichuan +86 18190030357 123315971@qq.com |
| Notes | Recruiting |
CTRI/2017/05/008554.
| Study name | Comparison of physiotherapy and two modalities of local injection in long‐standing tennis elbow |
| Methods | Single‐centre parallel 3‐arm randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Steroid injection 1 mL of methylprednisolone mixed with 3 mL of xylocaine 2%, making 4 mL, infiltrated into the maximal area of tenderness PRP injection 4 mL of platelet‐rich plasma infiltrated into the maximal area of tenderness Local ultrasonic therapy Ultrasound given in sitting position at intensity of 1.5 W/cm² by 1 MHz ultrasound sound therapy machine for 8 minutes for 12 treatment sessions over 2 weeks |
| Outcomes | Outcomes are collected at 3 weeks, 6 weeks, 3 months, 6 months, and 1 year Primary outcome Improvement in pain on visual analogue scale (VAS) Secondary outcome None specified |
| Starting date | 01/06/2017 |
| Contact information | Koushik Narayan Subramanyam, Sri Sathya Sai Institute of Higher Medical Sciences ‐ Prasanthigram, Andhra Pradesh, India |
| Notes | Not yet recruiting |
EUCTR2006‐002198‐32‐NL.
| Study name | Prospective randomized study of the effect of autologous concentrated thrombocytes versus glucocorticoid injections in lateral epicondylitis ‐ autologous thrombocytes injections in lateral epicondylitis |
| Methods | 2‐arm single‐blind parallel randomised controlled trial |
| Participants |
Inclusion criteria • Men and women with lateral epicondylitis > 3 months • Baseline elbow pain score > 5/10, > 100/200 during resisted wrist extension • Informed consent • Pain by palpation of the lateral epicondyle • Pain that is unresponsive to physical therapy or a brace Exclusion criteria • < 18 years of age • History of any blood disorder • History of bleeding disorder • History of carpal tunnel syndrome • Cervical radiculopathy • Systemic disorder such as diabetes, rheumatoid arthritis, or hepatitis • Uncooperative patient or patient with neurological disorder who is incapable of following directions or is predictably unwilling to return for follow‐up examinations • Previous surgery or glucocorticoid injection for lateral epicondylitis • Active bilateral elbow tendinosis • Hypothyroidism • Haemoglobin above or below the following range: 14.0 to 17.7 g/dL • Haematocrit outside the following range: 40.0% to 52.0% • Platelet count outside the following range: 150 to 400 × 103/dL • Arthrosis or fracture of the elbow, as confirmed by X‐ray diagnosis (AP, lateral views) • Does not speak Dutch |
| Interventions |
PRP injection Single local injection Glucocorticoid injection Injection of lidocaine and glucocorticoid |
| Outcomes |
Primary outcome Decrease in pain and duration of the condition Secondary outcome Not specified |
| Starting date | 14/01/2008 |
| Contact information | The Netherlands; no other details given |
| Notes | Authorised ‐ recruitment may be ongoing or finished This trial is also registered as EUCTR2007‐004947‐31‐NL |
EUCTR2013‐000478‐32‐ES.
| Study name | Study to prove the safety and efficacy of platelet rich plasma tenotomy for the treatment of chronic epicondylitis |
| Methods | Single‐centre single‐blind 2‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | PRP injection Lidocaine injection |
| Outcomes | Outcomes are measured at 6 weeks and at 3, 6, and 12 months Primary outcome DASH scores Secondary outcome
|
| Starting date | 12/07/2013 |
| Contact information | Isabel Andi Ortiz, Plaza de Cruces, 48003, Barakaldo, Spain |
| Notes | Authorised ‐ recruitment may be ongoing or finished |
EUCTR2013‐004875‐12‐CZ.
| Study name | A clinical trial to assess the efficacy and safety of platelet concentrates for the treatment of tennis elbow |
| Methods | Single‐centre 4‐arm placebo‐controlled parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria • Acute or chronic infection or acute inflammatory complications (general or local); treated with antibiotic therapy for suspected infectious disease during 6 weeks before inclusion in the study or in the event of Infectious complications in observed elbow at least 6 months before the study • Pregnant and nursing women and women of childbearing potential not using reliable contraceptive method • Presumption of survival for < 2 years • Severe disorder of blood coagulation and platelet disorder • Bone marrow disease in history • Kidney disease in the last stage with dependence on dialysis • Use of immunosuppressive drugs • Known abuse of alcohol or drugs, or other factor that might affect the participant's behaviour during the study or during interpretation of results • Patient whose participation is considered by the investigator to be inappropriate • Unwillingness or inability of the patient to give informed consent for inclusion in the study • Application of glucocorticoid in the elbow < 6 weeks before enrolment • Rehabilitation measures < 6 weeks before enrolment • Surgical procedure < 3 months before enrolment • X‐ray signs of osteoarthritis of the elbow ‐ over II degree by Kellgren • Consequences of severe injury such as bone fracture, resulting in non‐anatomical position |
| Interventions | Autologous platelet‐rich plasma injection Mesocain 1% injection Diprophos injection Saline injection |
| Outcomes | Outcomes are measured at 12 months Primary outcome Adverse events Secondary outcomes VAS for pain DASH |
| Starting date | 24/09/2014 |
| Contact information | Martina Robenkkove Ethical committee Ul.17.Listopadu 708 52 Ostrava Czech Republic |
| Notes | Not recruiting |
EUCTR2018‐002650‐67‐ES.
| Study name | Efficacy of plasma injections and extracorporeal shock wave therapy in the treatment of work–related lateral epicondylitis |
| Methods | Single‐centre 3‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Outcomes are measured at 10 days, 1 month, and 3 months Primary outcomes VAS pain score DASH score Biomechanical assessment using ultrasound Secondary outcomes Cost‐effectiveness of the 3 treatment care protocol modalities in controlling the work‐related sick leave period |
| Starting date | 15/11/2018 |
| Contact information | Departamento Proyectos Sanitarios, Calle Ramirez de Arellano 27, 28043, Madrid, Spain |
| Notes | Authorised ‐ recruitment may be ongoing or finished |
IRCT20180422039382N2.
| Study name | Comparison of the efficacy of local autologous blood and glucocorticoid injection on pain and function in patients with tennis elbow |
| Methods | 3‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria All patients with tennis elbow who came to the hospital Exclusion criteria None specified |
| Interventions | Intervention 1: patients who have been injected with glucocorticoid (methylprednisolone acetate 40 mg) at the site of the lateral epicondyle Intervention 2: patients who have been injected with autologous blood (2 mL of venous blood) at the site of the lateral epicondyle Intervention 3: 1 group used a brace for 3 weeks |
| Outcomes | Pain and function Time points: Day 0; Day 15; Day 30; Day 90 Method of measurement: evaluation with patient‐rated tennis elbow evaluation |
| Starting date | March 2019 |
| Contact information | Dr. Raheleh Javer Babol University of Medical Sciences Ganjafrooz Street; Babol; Iran +98 11 3225 6285 Bluesky9344@gmail.com |
| Notes | Recruitment complete |
IRCT20191105045335N1.
| Study name | Comparing the effects of platelet‐rich plasma (PRP) with glucocorticoid injection in resistant tennis elbow patients to non‐surgical treatment |
| Methods | Single‐centre parallel 2‐arm randomised controlled trial |
| Participants |
Inclusion criteria
Diagnosed with lateral epicondylitis and related functional impairment after 6 weeks of *non‐surgical care such as resting, avoiding severe activities, behaviour reform, physical therapy, non‐steroidal anti‐inflammatory drug (NSAID)
In physical examination, resisted wrist extension test in forearm pronation and elbow extension; VAS score = 5 or more; symptoms lasting at least 3 months Exclusion criteria 70 years of age or older History of infectious disease or fever in recent days History of any malignancy Diagnosed carpal tunnel syndrome or any other peripheral nerve lesion or cervical radiculopathy Systemic disease such as ischaemic heart disease, diabetes mellitus, rheumatoid arthritis, or hepatitis Any bone malformation or bone injury in humerus or elbow joint History of autoimmune disease History of platelet dysfunction Use of antiplatelet medication during last 10 days or use of NSAIDs during last 48 hours before injection Use of steroids during last 3 weeks Hb ≤ 10 Platelet count < 150,000 History of vasovagal reaction Pregnancy or breastfeeding |
| Interventions | Intervention 1: intervention group: injection of 2 mL platelet‐rich plasma, obtained from patient's blood centrifuge, at maximum tenderness point, by peppering method Intervention 2: control group: injection of 40 mg of prednisolone acetate at maximum tenderness point by peppering method |
| Outcomes | Function time point: before injection, 2 weeks, 1 and 3 months after injection Method of measurement: Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire Adverse events |
| Starting date | 15/02/2020 |
| Contact information | Fatemeh Ochi Ardebili No,74‐Shahid Ali Hatami Ave‐Golshahr 3198688381 Karaj Iran +98 26 3350 9825 lamy@chmail.ir |
| Notes | Recruiting |
ISRCTN12951626.
| Study name | A pilot study of platelet‐rich plasma (PRP) versus autologous whole blood versus saline in the treatment of resistant tennis elbow |
| Methods | Multi‐centre 3‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Injection of whole blood, platelet‐rich plasma or saline via a technique called needle barbotage, which disrupts tendon fibres and promotes the healing process |
| Outcomes | Outcomes are measured at 6 weeks, 3 months, 6 months, and 12 months Primary outcome Patient‐rated tennis elbow evaluation (PRTEE) Secondary outcomes
|
| Starting date | 15/07/2015 |
| Contact information | Dr. Johanna Wales, Robert Jones & Agnes Hunt Orthopaedic & District Hospital, Oswestry (UK) |
| Notes | Trial completed; no longer recruiting |
NCT01476605.
| Study name | Efficacy of injection therapy for lateral epicondylosis |
| Methods | Single‐centre unblinded parallel‐group randomised controlled trial |
| Participants |
Inclusion criteria Age between 18 and 65 years Lateral epicondylitis defined by pain over the lateral epicondyle and pain on 2 extensor muscle provocation tests Minimum symptom duration of 6 months Failure of ≥ 2 non‐steroidal anti‐inflammatory medications, physical therapy, or glucocorticoid injection Exclusion criteria Bilateral disease. Steroid injection within past 3 months Previous PrT or PRP injection Concurrent carpal tunnel syndrome, other elbow pathology, or acute trauma of the affected limb History of bleeding disorder, other haematological condition, inflammatory arthritis, systemic nervous system disease, upper extremity surgery, or neuropathy Current use of opioid medication Anticoagulation or immunosuppressive therapy within the past 1 month Intention to use NSAID or oral/injected steroid Allergy to dextrose, acetaminophen, or lidocaine Contraindication to having MRI scan Unresolved litigation Pregnancy |
| Interventions |
Platelet‐rich plasma group No details regarding PRP are specified PrT‐DMS group A solution of 1 mL of 5% morrhuate sodium, 1.5 mL of 50% dextrose, 1 mL of 2% lidocaine, and 3.5 mL of normal saline will be injected into the tendon PrT‐D group A solution of 4 mL of 50% dextrose, 2 mL of 2% lidocaine, and 3 mL of normal saline will be injected into the tendon No intervention group Participants will receive no intervention |
| Outcomes | Outcomes will be reported at baseline, 16 weeks, and 32 weeks Outcomes Mean change in disability measured by Patient‐Rated Tennis Elbow Evaluation score (scores ranging from 0 (no disability) to 50) Grip strength measured by a dynamometer Ultrasonographic and magnetic resonance imaging changes in the common extensor origin Outcomes included in this review Mean change in disability measured by the Patient‐Rated Tennis Elbow Evaluation score Grip strength measured by a dynamometer Time points included in this review 16 weeks and 32 weeks |
| Starting date | June 2009 |
| Contact information | David P Rabago, MD Department of Family Medicine University of Wisconsin |
| Notes | Expected completion: December 2015. No results published |
NCT01668862.
| Study name | A study to determine safety & efficacy of autologous human platelet lysate in lateral epicondylitis (tennis elbow) |
| Methods | Multi‐centre open‐label parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Autologous human platelet lysate group
Participants will receive 1 injection of autologous human platelet lysate in the lateral epicondyle space Glucocorticoid group Participants will receive 1 injection of glucocorticoid in the lateral epicondyle space |
| Outcomes | Outcomes will be measured at 1 month, 2 months, and 3 months Primary outcome
Secondary outcomes
|
| Starting date | 20 August 2012 |
| Contact information | Kaushal Shah, PhD; +91‐22‐41173472 kaushal.shah@kasiakresearch.com |
| Notes | Status unknown; ClinicalTrials.gov; accessed 25 July |
NCT02052089.
| Study name | Comparative study for the optimal treatment method of lateral epicondylosis |
| Methods | 4‐arm double‐blind parallel‐group randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Physiotherapy group
Not specified Quote: "physiotherapy is the treatment of a wide range of conditions and injuries to the body through the use of various forms of passive mobilisation, massage, electrotherapy and exercises" Extracorporeal shock wave therapy group Not specified Quote: "extracorporeal shockwave therapy (ESWT) is noninvasive procedure, and has been shown to be effective in the treatment of chronic tendon pathology in the elbow, shoulder and plantar fascia. Shock wave therapy is traditionally categorized as either low energy (<0.2 mJ/mm²) or high energy (>0.2 mJ/mm²). Rompe et al have hypothesized that there is an overstimulation of nerve fibers, resulting in an immediate analgesic effect (hyperstimulation analgesia). Physical effects on cell permeability and induction of diffusible radicals have also been postulated to cause disruption of the tendon tissue, resulting in induction of a healing process" Prolotherapy group Not specified Quote: "prolotherapy has been defined as the iatrogenic stimulation of wound healing and tissue repair through the injection of an irritant solution into damaged ligaments and tendons. Prolotherapy solutions are purported to initiate an inflammatory cascade at the site of injection, which induces fibroblast proliferation and subsequent collagen synthesis, resulting in a tighter and stronger ligament or tendon. The primary mechanism of action of prolotherapy is to induce a small inflammatory response to promote adequate healing or more viable scar tissue formation that results in stronger fibrous tissue at the lateral epicondyle, which leads to improved function and reduced pain" Platelet‐rich plasma Not specified Quote: "platelet‐rich plasma (PRP) is defined as an autologous concentration of human platelets in a small volume of plasma which is mechanically treated to increase the concentration of platelets compared to whole blood. The supraphysiological concentration of platelets will provide a locally increased concentration of growth factors and cytokines that are contained within the platelets themselves.Based on these concepts, it is believed that PRP can augment or stimulate healing with the same biologic healing process that normally occurs in the human body after injury" |
| Outcomes | Outcomes are collected at 3‐month follow‐up, 6‐month follow‐up, 18‐month follow‐up, and 24‐month follow‐up Primary outcome measure Disabilities of Arm Shoulder and Hand (DASH) questionnaire (0 to 100; higher is worse) Secondary outcome measure
SSS includes the following question: "how do you feel now compared to the condition before treatment in terms of satisfaction?" (scale 0 to 5; higher is better) 0 ‐ It is worse. Not satisfied at all 1 ‐ There are no changes and still it is as uncomfortable as before. Not satisfied at all 2 ‐ Slightly improved but < 50% of the pain has subsided. Not so satisfied 3 ‐ About 50% of the pain or discomfort is gone. Slightly satisfied, but not so much 4 ‐ About 75% of the pain or discomfort is gone. Definitely better. Satisfied 5 ‐ More than 75% of the pain or discomfort is gone. Much better and very satisfied
Study authors measure changes in the hypo‐echogenicity of the tendon and via colour doppler to determine the amount of vascularity noted in the tendon |
| Starting date | March 2009 |
| Contact information | Sang‐Hoon Lhee, Director of CM Chungmu Hospital, CM Chungmu Hospital |
| Notes | Status completed. Did not find a publication. ClinicalTrial.gov (accessed 26 July 2019) |
NCT02325063.
| Study name | Leukocyte and platelet rich plasma versus type A botulinum toxin versus glucocorticoids for the treatment of lateral epicondylalgia |
| Methods | Multi‐centre double‐blind parallel 3‐arm randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
Non‐inclusion criteria for investigational and auxiliary medicinal products
Non‐inclusion criteria for the medical device used for PRP samples (SmartPReP2 sampling system)
Non‐inclusion criteria for interfering disease or condition
|
| Interventions |
PRP‐L injection Patients randomised to this group will be treated with an injection of leukocyte and platelet‐rich plasma (PRP‐L) Botox injection Patients randomised to this group will be treated with an injection of type A botulinum toxin (Xeomin®, MERZ) Corticoid injection Patients randomised to this group will be treated with an injection of corticoid |
| Outcomes | Outcomes are measured at 3 weeks, 6 weeks, 3 months, and 6 months Primary outcome Visual analogue scale (VAS) score for pain over the last 24 hours at 6 months Secondary outcomes
|
| Starting date | 17/12/2015 |
| Contact information | Matthieu Vaucher, MD, Centre Hospitalier Universitaire de Nîmes, France |
| Notes | Recruitment has been terminated |
NCT02755727.
| Study name | Platelet‐rich plasma vs open surgery in the treatment of chronic lateral epicondylar tendinopathy (tennis elbow): a pilot randomized control trial |
| Methods | 2‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Platelet‐rich plasma group
In the outpatient setting, a sample of venous whole blood will be taken from the patient (40 mL) from the antecubital fossa via standard phlebotomy techniques. The Angel™ system will be available also in the outpatient clinic and will be used to separate the blood to yield a PRP sample (approximately 15 minutes' preparation time). The PRP sample will then be injected into the common extensor origin. 2 injections will be used per patient over 2 weeks Open surgical release group Standardised surgical technique based on the Nirschl technique will be used. The patient will then have a small scar centred over the lateral epicondyle and the plane opened between ECRL (extensor carpi radialis longus) and EDC (extensor digitorum communis) to expose the damaged ECRB (extensor carpi radialis brevis) tendon. The amount of abnormal tendon will be documented and excised. EDC will also be inspected, and any abnormal tissue documented, then excised. The footprint of the excised ECRB ± EDC is cleared of soft tissue and the bone scored with an osteotome to promote bleeding. The interval is closed with suture material and the skin wound closed |
| Outcomes | Outcomes will be assessed at 6 weeks, 3 months, 6 months, and 12 months Primary outcome measure
Secondary outcome measures
|
| Starting date | 29 April 2016 |
| Contact information | C Gardener Research Development Directorate ‐ Royal Devon and Exeter Hospital |
| Notes | ClinicalTrials accessed 24 July 2019; recruitment completed |
NCT03072381.
| Study name | PEAK platelet rich plasma injection treatment for chronic lateral epicondylosis |
| Methods | Parallel 2‐arm double‐blind randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Platelet‐rich plasma group
Participants will receive a single ultrasound‐guided intratendinous (common extensor tendon origin) injection of up to 3 mL of autologous PEAK platelet‐rich plasma Glucocorticoid control group Participants will receive a single ultrasound‐guided intratendinous injection of approximately (may be limited by tendon/soft tissue limitations of the subject) 2 mL of 1% lidocaine + 1 mL of 40 mg Kenalog (triamcinolone hexacetonide, 40 mg/mL) at Week 0 |
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
| Starting date | March 2017 |
| Contact information | John J. Wilson, MD; MS608‐440‐6543; wilson@ortho.wisc.edu Kenneth S. Lee, MD; 608‐263‐8183; klee2@uwhealth.org |
| Notes | Status: recruiting ClinicalTrials.gov (accessed 26 July 2019) |
NCT03300531.
| Study name | Impact of autologous pure platelet‐rich plasma in the treatment of tendon disease |
| Methods | Parallel‐group randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Ultrasound‐guided pure platelet‐rich plasma (P‐PRP) injection group
Blood will be drawn, and tendon will be penetrated with dry needle under ultrasound guidance. Pure platelet‐rich plasma will be injected into the tendon once a week 3 times Ultrasound‐guided platelet‐rich plasma (PRP) injection group Blood will be drawn, and tendon will be penetrated with dry needle under ultrasound guidance. Platelet‐rich plasma will be injected into the tendon once a week 3 times Ultrasound‐guided compound betamethasone injection Tendon will be penetrated with dry needle under ultrasound guidance. Compound betamethasone will be injected into the tendon once a week 3 times |
| Outcomes |
Primary outcome measure
Secondary outcome measures (depend on the condition)
|
| Starting date | October 2017 |
| Contact information | Weiliang Shen, Doctor; +86‐13757101563 Email: wlshen@zju.edu.cn |
| Notes | Status: not yet recruiting ClinicalTrials.gov (accessed 26 July 2019) |
NCT03504111.
| Study name | PRINT trial (platelet rich injection vs needle tenotomy) (PRINT) |
| Methods | Parallel‐group 2‐arm participant‐blinded randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Percutaneous needle tenotomy group A sham phlebotomy sample will be drawn on all study participants. Participants in this treatment group will be given local anaesthesia with 1% lidocaine and then will be blinded to the intervention via a blindfold or shielding. Ultrasound‐guided needle tenotomy with be performed at the common extensor tendon at the area of tendinosis. There will be approximately 25 passes through the tendon with an 18‐gauge needle. Investigators will keep track of the number of passes through the tendon. Investigators will keep track of the amount and type of anaesthetic used to provide adequate and effective anaesthesia PRP group Participants in this treatment group will be given local anaesthesia with 1% lidocaine and then will be blinded to the intervention via a blindfold or shielding. Ultrasound‐guided injection of PRP will be performed at the common extensor tendon at the area of tendinosis. Investigators will keep track of the amount and type of anaesthetic used to provide adequate and effective anaesthesia to local skin |
| Outcomes | Outcomes will be collected at 1 year Primary outcome Change in DASH symptoms score (scale 0 to 100; higher is worse) |
| Starting date | 20 April 2018 |
| Contact information | Marissa S. Vasquez, Kaiser Permanente, USA |
| Notes | Status: active, not recruiting ClinicalTrials.gov (accessed 25 July 2019) |
NCT03984955.
| Study name | Comparing injection treatments for tennis elbow (CITTE) |
| Methods | Prospective controlled randomised double‐blind single‐centre trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Platelet‐rich plasma injection group
Platelet‐rich plasma (PRP) describes an autologous blood plasma fraction enriched with platelets, which is injected at the site of tendon injury Sodium hyaluronate with mannitol (ostenil tendon) group 1 mL of isotonic solution contains 20.0 mg of sodium hyaluronate and sodium chloride, disodium phosphate, sodium dihydrogen phosphate, mannitol, and water for injection Subcutaneous sham injection group Sham injection will penetrate the skin, but no therapeutic substance will be injected |
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
| Starting date | June 2009 |
| Contact information | Contact: Adam C Watts; +441257256365; Adam.C.Watts@wwl.nhs.uk |
| Notes | Status: recruiting ClinicaTrials (accessed 26 July 2019) |
NCT03987256.
| Study name | Tennis elbow, randomized study: needling with and without platelet‐rich plasma after failure of up‐to‐date rehabilitation |
| Methods | Single‐centre 2‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Needling with PRP vs needling with saline solution |
| Outcomes |
Primary outcome measure Pain during isometric contraction of the ECRB [Time Frame: 3 months] Pain is evaluated on a 0 to 10 scale (0 = no pain) during isometric contraction manoeuvre of the ECRB Secondary outcome measures Pain during isometric contraction of the ECRB [Time Frame: ‐3, 0, 6, & 12 months] Pain is evaluated on a 0 to 10 scale (0 = no pain) during isometric contraction manoeuvre of the ECRB Overall pain evaluation (mean of last 3 days) [Time Frame: ‐3, 0, 3, 6, & 12 months] Pain is evaluated on a 0 to 10 scale (0 = no pain) SANE score (Single Assessment Numeric Evaluation) [Time Frame: ‐3, 0, 3, 6, & 12 months] Function is evaluated on a 0 to 100% scale (100 = good function) PRTEE score (Patient‐Rated Tennis Elbow Evaluation) [Time Frame: ‐3, 0, 3, 6, & 12 months] Score going from 0 to 100 (0 = good outcome) Strength on Jamar test (hand grip strength) [Time Frame: ‐3, 0, 3, 6, & 12 months] Grip strength measured in kg (higher strength = better outcome) Proportion of patients cured with re‐education protocol [Time Frame: 0 months] Descriptive statistics: evaluation of the proportion of patients for which tendon needling is not necessary Volume of PRP prepared [Time Frame: 0 months] Descriptive statistics: quantity of PRP prepared (in mL) Volume of PRP (or saline solution) injected [Time Frame: 0 months] Descriptive statistics: quantity of PRP (or saline solution) injected (in mL) Ultrasonographic aspect of the epicondylar tendon: hypo‐echogenic lesion [Time Frame: ‐3, 0, 3, & 6 months] The tri‐dimensional volume of the lesion is measured in mm³ Ultrasonographic aspect of the epicondylar tendon: Doppler [Time Frame: ‐3, 0, 3, & 6 months] The Doppler reaction will be evaluated on a subjective scale (none, mild, average, high, huge) Ultrasonographic aspect of the epicondylar tendon: solution of continuity [Time Frame: ‐3, 0, 3, & 6 months] During active contraction of the ECRB, an eventual solution of continuity will be measured in mm Ultrasonographic aspect of the epicondylar tendon: thickness [Time Frame: ‐3, 0, 3, & 6 months] The thickness of the common epitrochlear will be measured in mm Ultrasonographic aspect of the epicondylar tendon: compressibility [Time Frame: ‐3, 0, 3, & 6 months] The presence or absence of compressibility of the common epitrochlear tendon (binary outcome) Ultrasonography of the epicondylar tendon: sonopalpation [Time Frame: ‐3, 0, 3, & 6 months] Patient pain on sonopalpation will be evaluated on a 0 to 10 scale (0 = no pain) |
| Starting date | 1 January 2020 |
| Contact information | Adrien Schwitzguébel, Hôpital La Providence, Sports Medicine Neuchâtel, Switzerland +4179 762 05 62 adrien.schwitzguebel@gmail.com |
| Notes | Recruiting |
NCT04384809.
| Study name | Comparison of leukocyte‐rich platelet rich plasma injection to percutaneous tenotomy in the treatment of chronic common extensor tendinopathy |
| Methods | 2‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Leukocyte‐rich platelet‐rich plasma injection • Patient will be injected with leukocyte‐rich platelet‐rich plasma into the common extensor tendon Percutaneous tenotomy • Patient will undergo percutaneous tenotomy of the common extensor tendon via the Tenex tenotomy device |
| Outcomes |
Primary outcome measures
|
| Starting date | 15/05/2020 |
| Contact information | Robert Flannery University Hospitals Cleveland Medical Center 216‐844‐3078 Robert.Flannery@UHhospitals.org |
| Notes | Not yet recruiting |
NTR4569.
| Study name | Standardised needle therapy in LE |
| Methods | 3‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria • Patients referred by their GP to the orthopaedic surgeon with diagnosed unilateral lateral epicondylitis lasting longer than 6 weeks • Age between 18 and 65 years • Unsuccessful conservative treatment • Able to read and write in Dutch • Provision of informed consent by patient. Exclusion criteria • Prior injection therapy (during this episode of LE), surgery, or trauma at the affected elbow • Inflammatory disease (i.e. rheumatoid arthritis, psoriatic arthritis, or reactive arthritis) • Any other elbow pathology • Neck pain or shoulder pain correlated with elbow pain such as C6 radiculopathy or disability of the arm or other chronic widespread pain syndrome • Traumatic onset of LE • Bilateral LE (mild case of LE on the contralateral elbow without functional limitations allowed) • Abnormality on X‐ray • Additional pain at the medial epicondyle • Allergy to lidocaine |
| Interventions | All treatments will be performed ultrasound‐guided and in a standardised and automated way Autologous blood injection Perforation with infiltration of 0.4 cc autologous blood; blood is taken by venipuncture and is directly injected into the affected tendon Dextrose injection Perforation with infiltration of 0.4 cc dextrose: solution with 4 mL of 50% dextrose + 4 mL of 90% saline + 2 mL of 1% lidocaine Sham injection Perforation without infiltration |
| Outcomes | Outcomes are measured at 5 months Primary outcome Pain measured on VAS (0 to 100). After provocation test, pain during resisted dorsiflexion of the wrist during full elbow extension Secondary outcomes Pain on VAS Quality of life Adverse events |
| Starting date | 01/11/2015 |
| Contact information | R. Keijsers; reneekeijsers@gmail.com; The Netherlands |
| Notes | Recruitment pending |
NTR5005.
| Study name | Effect of a platelet rich plasma (PRP) injection on the outcome of chronic lateral epicondylitis: a double blinded randomised controlled clinical trial |
| Methods | 2‐arm parallel randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Platelet‐rich plasma (PRP) 3‐mL injection Saline 3‐mL injection |
| Outcomes | Outcomes are measured at 6 months Primary outcome Patient Rated Tennis Elbow Evaluation (PRTEE) questionnaire Secondary outcomes
|
| Starting date | 01/01/2015 |
| Contact information | Reinoud Meijer; MeijerR@zgv.nl; The Netherlands |
| Notes | Recruitment pending |
ASES: American Shoulder and Elbow Surgeons score.
BMI: body mass index.
CLE: chronic lateral epicondylosis.
DASH: Disabilities of the Arm, Shoulder and Hand questionnaire.
ECRB: extensor carpi radialis brevis tendon.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on 5 dimensions
EVA: Electronic visual analog
LE: lateral epicondylitis.
NSAID: non‐steroidal anti‐inflammatory drug.
PRP: platelet‐rich plasma.
PrT‐D: Prolotherapy Dextrose
PrT‐DMS: Prolotherapy Dextrose and Morrhuate sodium
TC: Total count
VAS: visual analogue scale.
Differences between protocol and review
Post‐hoc specification of main comparison: autologous blood or PRP injection versus placebo.
Post‐hoc time point was not specified in the protocol; we used 3 months.
Background was updated to reflect advances in different types of autologous blood products that have become available since the protocol was published.
Contributions of authors
TK performed data extraction and analyses.
RJ performed risk of bias and grade assessments and drafted and commented on review.
MS selected studies for inclusion, performed data extraction and drafted sections of the review.
EO selected studies for inclusion, performed data extraction and drafted sections of the review.
SC performed data extraction and analyses.
RB conceived the review and contributed to drafting the review.
All review authors contributed to the final manuscript.
Sources of support
Internal sources
-
Cabrini Institute, Cabrini Hospital, Australia
In‐kind support
-
Monash University, Australia
In‐kind support
External sources
-
National Health and Medical Research Council (NHMRC), Australia
Rachelle Buchbinder is supported by an NHMRC Senior Principal Research Fellowship
Declarations of interest
TK: none known. TK was co‐author in one of the included trials Linnanmäki 2020, and he did not perform data extraction or risk of bias assessments for this trial.
MS: none known
EO: none known
RJ: Renea Johnston is the Managing Editor of Cochrane Musculoskeletal but is not involved in editorial decisions regarding this review. She is the recipient of an NHMRC (Australia) Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funding source did not participate in the conduct of this review.
SC: none known
RB: Rachelle Buchbinder is the Co‐ordinating Editor of Cochrane Musculoskeletal but is not involved in editorial decisions regarding this review. She is the recipient of a National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal ‐ Australia and Cochrane Australia, but the funding source did not participate in the conduct of this review. (RB) is the study investigator for an ongoing trial in this review (ACTRN12613000616774) and did not perform data extraction for this trial.
New
References
References to studies included in this review
Arik 2014 {published data only}
- Arik HO, Kose O, Guler F, Deniz G, Egerci OF, Ucar M. Injection of autologous blood versus corticosteroid for lateral epicondylitis: a randomised controlled study. Journal of Orthopaedic Surgery 2014;22(3):333-7. [DOI] [PubMed] [Google Scholar]
Behera 2015 {published and unpublished data}
- Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. Journal of Orthopaedic Surgery 2015;23(1):6-10. [DOI] [PubMed] [Google Scholar]
Branson 2016 {published data only}
- Branson R, Naidu K, du Toit C, Rotstein AH, Kiss R, McMillan D, et al. Comparison of corticosteroid, autologous blood or sclerosant injections for chronic tennis elbow. Journal of Science and Medicine in Sport 2016;20(6):528-33. [DOI] [PubMed] [Google Scholar]
Creaney 2011 {published data only}
- Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. British Journal of Sports Medicine 2011;45(12):966-71. [DOI] [PubMed] [Google Scholar]
Dojode 2012 {published data only}
- Dojode CM. A randomised control trial to evaluate the efficacy of autologous blood injection versus local corticosteroid injection for treatment of lateral epicondylitis. Bone & Joint Research 2012;1(8):192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautam 2015 {published data only}
- Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. Journal of Orthopaedic Surgery 2015;23(1):1-5. [DOI] [PubMed] [Google Scholar]
Gedik 2016 {published data only}
- Gedik DE, Sürücü GD, Yildirim A, Karabiber M. Effectiveness of autologous blood injection in the treatment of lateral epicondylitis: randomized clinical study. Duzce Medical Journal 2016 2016;18(1):1-7. [Google Scholar]
Gosens 2011 {published data only}
- Gosens T, Peerbooms JC, Laar W, Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. American Journal of Sports Medicine 2011;39(6):1200-8. [DOI] [PubMed] [Google Scholar]
- Peerbooms JC, et al. The cost effectiveness of platelet rich plasma versus corticosteroids in the treatment of lateral epicondylitis. Value in Health 2012;15(7):A357. [Google Scholar]
- Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. American Journal of Sports Medicine 2010;38(2):255-62. [DOI] [PubMed] [Google Scholar]
Gupta 2019 {published data only}
- Gupta PK, Acharya A, Khanna V, Roy S, Khillan K, Sambandam SN. PRP versus steroids in a deadlock for efficacy: long-term stability versus short-term intensity-results from a randomised trial. Musculoskeletal Surgery 2019;https://doi.org/:10.1007/s12306-019-00619-w. [DOI] [PubMed] [Google Scholar]
Jindal 2013 {published data only}
- Jindal N, Gaury Y, Banshiwal RC, Lamoria R, Bachhal V. Comparison of short term results of single injection of autologous blood and steroid injection in tennis elbow: a prospective study. Journal of Orthopaedic Surgery and Research 2013;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazemi 2010 {published data only}
- Kazemi M, Azma K, Tavana B, Rezaiee Moghaddam F, Panahi A. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. American Journal of Physical Medicine & Rehabilitation 2010;89(8):660-7. [DOI] [PubMed] [Google Scholar]
Krogh 2013 {published data only}
- Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. American Journal of Sports Medicine 17/1/2013;41(3):625-35. [DOI] [PubMed] [Google Scholar]
Lebiedziński 2015 {published data only}
- Lebiedziński R, Synder M, Buchcic P, Polguj M, Grzegorzewski A, Sibiński M. A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. International Orthopaedics (SICOT) 2015;39:2199–203. [DOI] [PubMed] [Google Scholar]
Lim 2017 {published data only}
- Lim W, Park SH, Kim B, Kang SW, Lee JW, Moon YL. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. Journal of Orthopaedic Research 2018;36(3):913-20. [DOI] [PubMed] [Google Scholar]
Linnanmäki 2020 {unpublished data only}
- Lehtinen JT, Leppanen O, Linnamaki L. The treatment of lateral epicondylitis: the effect of platelet rich plasma on healing - a randomized controlled double-blinded trial. Journal of Shoulder and Elbow Surgery 2019;3(4):237. [Google Scholar]
- Linnanmäki L, Kanto K, Karjalainen T, Leppänen OV, Lehtinen J. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clinical Orthopaedics and Related Research 2020;478(8):1892-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2019 {published data only}
- Martin JI, Atilano L, Merino J, Gonzalez I, Iglesias G, Areizaga L, et al. Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: a randomized controlled trial. Journal of Orthopaedic Surgery and Research 2019;14(1):109. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez‐Montiel 2015 {published data only}
- Martínez-Montiel O, Valencia-Martínez G, Blanco-Bucio P, Villalobos-Campuzano C. Treatment of elbow epicondylitis with platelet-rich plasma versus local corticosteroid. Acta Ortopédica Mexicana 2015;29(3):155-8. [PubMed] [Google Scholar]
Merolla 2017 {published data only}
- Merolla G, Dellabiancia F, Ricci A, Mussoni MP, Nucci S, Zanoli G, et al. Arthroscopic debridement versus platelet-rich plasma injection: a prospective, randomized, comparative study of chronic lateral epicondylitis with a nearly 2-year follow-up. Journal of Arthroscopic and Related Surgery 2017;33(7):1320-9. [DOI] [PubMed] [Google Scholar]
Mishra 2014 {published data only}
- Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, Vermillion DA, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. American Journal of Sports Medicine 2014;42(2):463-71. [DOI] [PubMed] [Google Scholar]
Montalvan 2015 {published data only}
- Montalvan B, Goux PL, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology 2016;55:279285. doi:10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
Omar 2012 {published data only}
- Omar AS, Ibrahim ME, Ahmed AS, Said M. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: randomized clinical trial. Egyptian Rheumatologist 2012;34(2):43-9. [Google Scholar]
Ozturan 2010 {published data only}
- Ozturan KE, Yucel I, Cakici H, Guven M, Sungur I. Autologous blood and corticosteroid injection and extracoporeal shock wave therapy in the treatment of lateral epicondylitis. Orthopedics 2010;33(2):84-91. [DOI] [PubMed] [Google Scholar]
Palacio 2016 {published data only}
- Palacio EP, Schiavetti RR, Kanematsu M, Ikeda TM, Mizobuchi RR, Galbiatti JA. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Revista Brasileira de Ortopedia 2016;51(1):90-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raeissadat 2014 {published data only}
- Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Science, Medicine, and Rehabilitation 2014;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeissadat SA, Sedighipour L, Rayegani SM, Bahrami MH, Bayat M, Rahimi R. Effect of platelet-rich plasma (PRP) versus autologous whole blood on pain and function improvement in tennis elbow: a randomized clinical trial. Pain Research and Treatment 2014;2014:191525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoffl 2017 {published data only}
- Schoffl V, Willauschus W, Sauer F, Kupper T, Schoffl I, Lutter C, et al. Autologous conditioned plasma versus placebo injection therapy in lateral epicondylitis of the elbow: a double blind, randomized study [Autologes konditioniertes Plasma versus Placebo in der Therapie der lateralen Epikondylitis - eine randomisierte Doppelblindstudie.]. Sportverletzung Sportschaden 2017;31(1):31-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stenhouse 2013 {published data only}
- Stenhouse G, Sookur P, Watson M. Do blood growth factors offer additional benefit in refractory lateral epicondylitis? A prospective, randomized pilot trial of dry needling as a stand-alone procedure versus dry needling and autologous conditioned plasma. Skeletal Radiology 2013;42(11):1515-20. [DOI] [PubMed] [Google Scholar]
Tetschke 2015 {published data only}
- Tetschke E, Rudolf M, Lohmann CH, Starke C. Autologous proliferative therapies in recalcitrant lateral epicondylitis. American Journal of Physical Medicine & Rehabilitation 2015;94:696Y706. [DOI] [PubMed] [Google Scholar]
Thanasas 2011 {published data only}
- Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. American Journal of Sports Medicine 2/8/2011;39(10):2130-4. [DOI] [PubMed] [Google Scholar]
Watts 2020 {published data only}
- Watts AC, Morgan BW, Birch A, Nuttall D, Trail IA. Comparing leukocyte-rich platelet-rich plasma injection with surgical intervention for the management of refractory tennis elbow. A prospective randomised trial. Shoulder & Elbow 2020;12(1):46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2011 {published data only}
- Wolf JM, Ozer K, Scott F, Gordon MJ, Williams AE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. Journal of Hand Surgery 2011;36(8):1269-72. [DOI] [PubMed] [Google Scholar]
Yadav 2015 {published data only}
- Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. Journal of Clinical and Diagnostic Research 2015;9(7):RC05-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yerlikaya 2018 {published data only}
- Yerlikaya M, Çalis TH, Sutbeyaz ST, Sayan H, Ibis N, Koc A, Karakukcu C. Comparison of effects of leukocyte-rich and leukocyte-poor platelet-rich plasma on pain and functionality in patients with lateral epicondylitis. Archives of Rheumatology 2018;33(1):73-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bernstein 2013 {published data only}
- Bernstein J, Wolf JM. Autologous blood and platelet-rich plasma injections for enthesopathy of the extensor carpi radialis brevis origin. Journal of Hand Surgery - American Volume 2013;38(5):992-4. [DOI] [PubMed] [Google Scholar]
Dong 2018 {published data only}
- Dong G, Jun L, Chenhui D, Yinshuan D, Yanfeng Z, Xinle Y, et al. Efficacy of platelet-rich plasma versus glucocorticoid for lateral humeral epicondylitis: a meta-analysis. Chinese Journal of Tissue Engineering Research 2018;22(35):5735-40. [Google Scholar]
Jiang 2020 {published data only}
- Jiang W, Zhuang J, Zhang Y, Gao M. The effect of platelet-rich plasma in the treatment of external humeral epicondylitis and an analysis of the influencing factors. International Journal of Clinical and Experimental Medicine 2020;13(6):3866-74. [Google Scholar]
Johnson 2007 {published data only}
- Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. American Family Physician 2007;76(6):843-50+53. [PubMed] [Google Scholar]
Kapoor 2012 {published data only}
- Kapoor S. Pain management of chronic lateral epicondylitis: emerging new therapeutic options. Pain Medicine (United States) 2012;13(6):848. [DOI] [PubMed] [Google Scholar]
Kaux 2012 {published data only}
- Kaux JF, Crielaard JM. Current evidence and indications for prolotherapy with platelet rich plasma in chronic musculoskeletal conditions. Regional Anesthesia and Pain Medicine 2012;1:104-6. [Google Scholar]
Li 2019 {published data only}
- Li A, Wang H, Yu Z, Zhang G, Feng S, Liu L, et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: a systematic review and meta-analysis. Medicine 2019;98(51):e18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2006 {published data only}
- Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. American Journal of Sports Medicine 2006;34(11):1774-8. [DOI] [PubMed] [Google Scholar]
Mishra 2009 {published data only}
- Mishra A, Collado H, Fredericson M. Platelet-rich plasma compared with corticosteroid injection for chronic lateral elbow tendinosis. Physical Medicine & Rehabilitation 2009;1(4):366-70. [DOI] [PubMed] [Google Scholar]
Mishra 2010 {published data only}
- Mishra A. Platelet-rich plasma. Orthopedics 2010;33:486-7. [DOI] [PubMed] [Google Scholar]
Naam 2020 {published data only}
- Naam. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clinical Orthopaedics and Related Research 2020;478(8):1901-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 2012 {published data only}
- Nichols AW. Two-year follow-up of injection with platelet-rich plasma versus corticosteroid for lateral epicondylitis. Clinical Journal of Sport Medicine [Internet] 2012;22(5):451-2. [DOI] [PubMed] [Google Scholar]
Orchard 2011 {published data only}
- Orchard J. Corticosteroid injection for lateral epicondylalgia is helpful in the short term, but harmful in the longer term; data for non-corticosteroid injections and other tendinopathies are limited. Evidence-Based Medicine 2011;16(4):116-7. [DOI] [PubMed] [Google Scholar]
Scudeller 2011 {published data only}
- Scudeller L, Fante C, Perotti C, et al. N of 1, two contemporary arm, randomised controlled clinical trial for bilateral epicondylitis: a new study design. BMJ 2011;343:7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiple 2013 {published data only}
- Shiple BJ. How effective are injection treatments for lateral epicondylitis? Clinical Journal of Sport Medicine 2013;23(6):502-3. [DOI] [PubMed] [Google Scholar]
Szabo 2009 {published data only}
- Szabo RM. Steroid injection for lateral epicondylitis. Journal of Hand Surgery - American Volume 2009;34(2):326-30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12613000616774 {published data only}
Chiavaras 2014 {unpublished data only}
- Chiavaras MM, Jacobson JA, Carlos R, Maida E, Bentley T, Simunovic N, . Impact of platelet rich plasma over alternative therapies in patients with lateral epicondylitis (IMPROVE): protocol for a multicenter randomized controlled study: a multicenter, randomized trial comparing autologous platelet-rich plasma, autologous whole blood, dry needle tendon fenestration, and physical therapy exercises alone on pain and quality of life in patients with lateral epicondylitis. Academic Radiology 2014;21(9):1144-55. [DOI] [PubMed] [Google Scholar]
Im 2012 {published data only}
- Im SH. Effects of an autologous platelet-rich plasma (PRP) and electrical shock wave therapy (ESWT) in lateral epicondylitis. Double-blind randomized controlled trial. Physical Medicine & Rehabilitation 2012;1:271-2. [Google Scholar]
Kulkarni 2020 {published data only}
- Kumar S, Kulkarni PN, Kshirsagar AY. A single blind randomised clinical study of outcome of local injection of platelet rich plasma vs methyl prednisolone acetate In management of lateral epicondylitis. International Journal of Research in Pharmaceutical Sciences 2020;11(3):4949-55. [Google Scholar]
References to ongoing studies
ChiCTR1900024425 {published data only}
- Ultrasound-guided leukocyte-poor platelet-rich plasma injection for lateral epicondylitis: a randomized, double-blinded, controlled trial. Ongoing study. 01/09/2019. Contact author for more information.
ChiCTR2000032210 {published data only}
- A randomized, double-blind, controlled clinical trial of platelet-rich plasma combined with arthroscopy in the treatment of lateral epicondylitis. Ongoing study. 01/05/2020. Contact author for more information.
CTRI/2017/05/008554 {published data only}
- Comparison of physiotherapy and two modalities of local injection in long-standing tennis elbow. Ongoing study. 01/06/2017. Contact author for more information.
EUCTR2006‐002198‐32‐NL {published data only}
- Prospective randomized study of the effect of autologous concentrated thrombocytes versus glucocorticoid injections in lateral epicondylitis - autologous thrombocytes injections in lateral epicondylitis. Ongoing study. 14/01/2008. Contact author for more information.
EUCTR2013‐000478‐32‐ES {published data only}
- Study to prove the safety and efficacy of platelet rich plasma tenotomy for the treatment of chronic epicondylitis. Ongoing study. 12/07/2013. Contact author for more information.
EUCTR2013‐004875‐12‐CZ {published data only}
- A clinical trial to assess the efficacy and safety of platelet concentrates for the treatment of tennis elbow. Ongoing study. 24/09/2014. Contact author for more information.
EUCTR2018‐002650‐67‐ES {published data only}
- Efficacy of plasma injections and extracorporeal shock wave therapy in the treatment of work–related lateral epicondylitis. Ongoing study. 15/11/2018. Contact author for more information.
IRCT20180422039382N2 {published data only}
- Comparison of the efficacy of local autologous blood and glucocorticoid injection on pain and function in patients with tennis elbow. Ongoing study. March 2019. Contact author for more information.
IRCT20191105045335N1 {published data only}
- Comparing the effects of platelet-rich plasma (PRP) with glucocorticoid injection in resistant tennis elbow patients to non-surgical treatment. Ongoing study. 15/02/2020. Contact author for more information.
ISRCTN12951626 {published data only}
- A pilot study of platelet-rich plasma (PRP) versus autologous whole blood versus saline in the treatment of resistant tennis elbow. Ongoing study. 15/07/2015. Contact author for more information.
NCT01476605 {published data only}
- Efficacy of injection therapy for lateral epicondylosis. Ongoing study. June 2009. Contact author for more information.
NCT01668862 {published data only}
- A study to determine safety & efficacy of autologous human platelet lysate in lateral epicondylitis (tennis elbow). Ongoing study. 20 August 2012. Contact author for more information.
NCT02052089 {published data only}
- Lhee. Comparative study for the optimal treatment method of lateral epicondylosis. https://clinicaltrials.gov/ct2/show/NCT02052089.
NCT02325063 {published data only}
- Leukocyte and platelet rich plasma versus type A botulinum toxin versus glucocorticoids for the treatment of lateral epicondylalgia. Ongoing study. 17/12/2015. Contact author for more information.
NCT02755727 {published data only}
- Gardener C. Platelet rich plasma vs open surgery in the treatment of chronic lateral epicondylar tendinopathy (tennis elbow): a pilot randomized control trial. ClinicalTrial.gov.
NCT03072381 {published data only}
- Wilson J. PEAK: platelet rich plasma injection treatment for chronic lateral epicondylosis. https://clinicaltrials.gov/ct2/show/NCT03072381.
NCT03300531 {published data only}
- Shen W. Impact of autologous pure platelet-rich plasma in the treatment of tendon disease. https://clinicaltrials.gov/ct2/show/NCT03300531.
NCT03504111 {published data only}
- Vasquez M. PRINT trial (platelet rich injection vs needle tenotomy) (PRINT). https://clinicaltrials.gov/ct2/show/NCT03504111.
NCT03984955 {published data only}
- Watts A. Comparing injection treatments for tennis elbow (CITTE). https://clinicaltrials.gov/ct2/show/NCT03984955.
NCT03987256 {published data only}
- ECRB tendinopathy: needling ± PRP after failure of rehabilitation. https://clinicaltrials.gov/ct2/show/NCT03987256.
NCT04384809 {published data only}
- Platelet rich plasma injection vs percutaneous tenotomy for common extensor tendinopathy. https://clinicaltrials.gov/ct2/show/NCT04384809.
NTR4569 {published data only}
- Keijsers R, Kuijer P, Koenraadt K, den Bekerom M, Gerritsma-Bleeker C, Beumer A, et al. Effectiveness of standardized ultrasound guided percutaneous treatment of lateral epicondylitis with application of autologous blood, dextrose or perforation only on pain: a study protocol for a multi-center, blinded, randomized controlled trial with a 1 year follow up. BMC Musculoskeletal Disorders 2019;20(1):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR5005 {published data only}
- Effect of a platelet rich plasma (PRP) injection on the outcome of chronic lateral epicondylitis: a double blinded randomised controlled clinical trial. Ongoing study. 01/01/2015. Contact author for more information.
Additional references
Ahmad 2013
- Ahmad Z, Brooks R, Kang SN, Weaver H, Nunney I, Tytherleigh-Strong G, et al. The effect of platelet-rich plasma on clinical outcomes in lateral epicondylitis. Arthroscopy 2013;29(11):1851-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Alizadehkhaiyat 2007
- Alizadehkhaiyat O, Fisher AC, Kemp GJ, Frostick SP. Pain, functional disability, and psychologic status in tennis elbow. Clinical Journal of Pain 2007;23:482-9. [DOI] [PubMed] [Google Scholar]
Arirachakaran 2016
- Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. Journal of Orthopaedics and Traumatology 2016;17(2):101-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Assendelft 1996
- Assendelft WJJ, Hay EM, Adshead R, Bouter LM. Cortiosteroid injections for lateral epicondylitis: a systematic overview. British Journal of General Practice 1996;46:209-16. [PMC free article] [PubMed] [Google Scholar]
Bell 2013
- Bell KJ, Fulcher ML, Rowlands DS, Kerse N. Impact of autologous blood injections in treatment of mid-portion Achilles tendinopathy: double blind randomised controlled trial. BMJ 2013;346:f2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennell 2017
- Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Current Rheumatology Reports 2017;19(24:):https://doi.org/10.1007/s11926-017-0652-x. [DOI: 10.1007/s11926-017-0652-x] [DOI] [PubMed] [Google Scholar]
Bisset 2005
- Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. British Journal of Sports Medicine 2005;39(7):411-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisset 2006
- Bisset L, Beller E, Jull G, Brooks P, Darnell R, Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ 2006;333(7575):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjordal 2008
- Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, Stergioulas A, et al. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskeletal Disorders 2008;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borkholder 2004
- Borkholder CD, Hill VA, Fess EE. The efficacy of splinting for lateral epicondylitis: a systematic review. Journal of Hand Therapy 2004;17(2):181-99. [DOI] [PubMed] [Google Scholar]
Bot 2005
- Bot SD, Waal JM, Terwee CB, Windt DA, Schellevis FG, Bouter LM, et al. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Annals of the Rheumatic Diseases 2005;64(1):118-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2005
- Buchbinder R, Green SE, Youd JM, Assendelft WJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD003524. [DOI: 10.1002/14651858.CD003524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2011
- Buchbinder R, Johnston RV, Barnsley L, Assendelft WJJ, Bell SN, Smidt N. Surgery for lateral elbow pain. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD003525. [DOI: 10.1002/14651858.CD003525.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carless 2011
- Carless PA, Rubens FD, Anthony DM, O'Connell D, Henry DA. Platelet-rich-plasmapheresis for minimising peri-operative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD004172. [DOI: 10.1002/14651858.CD004172.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2008
- Visual Rx [Computer program]. Version 3. Dr. Christopher Cates. EBM website. URL: http://www.nntonline.net,2008.
Clarke 2010
- Clarke AW, Ahmad M, Curtis M, Connell DA. Lateral elbow tendinopathy: correlation of ultrasound findings with pain and functional disability. American Journal of Sports Medicine 2010;38:1209-14. [DOI] [PubMed] [Google Scholar]
Coombes 2010
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010;376(9754):1751-67. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DeLong 2012
- DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy 2012 Jul;28(7):998-1009. [DOI] [PubMed] [Google Scholar]
De Vos 2010
- De Vos RJ, Weir A, Van Schie HTM, Bierma-Zeinstra SMA, Verhaar JAN, Weinans H, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 2010;303(2):144-9. [DOI] [PubMed] [Google Scholar]
de Vos 2014
- Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. British Journal of Sports Medicine 2014;48(12):952-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dong 2016
- Dong W, Goost H, Lin XB, Burger C, Paul C, Wang ZL, et al. Injection therapies for lateral epicondylalgia: a systematic review and Bayesian network meta-analysis. British Journal of Sports Medicine 2016;50(15):900-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI] [PubMed] [Google Scholar]
Edwards 2003
- Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. Journal of Hand Surgery 2003;28(2):272-8. [DOI] [PubMed] [Google Scholar]
Engebretsen 2010
- Engebretsen L, Steffen K, Alsousou J, Anitua E, Bachl N, Devilee R, et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. British Journal of Sports Medicine 2010;44:1072-81. [DOI] [PubMed] [Google Scholar]
Eppley 2004
- Eppley B, Woodell J, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and Reconstructive Surgery 2004;114:1502-8. [DOI] [PubMed] [Google Scholar]
Evans 2016
- Evans CH, Chevalier X, Wehling P. Autologous conditioned serum. Physical Medicine and Rehabilitation Clinics 2016;27(4):893-908. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2017
- Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. American Journal of Sports Medicine 2017;45(1):226-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glanzmann 2015
- Glanzmann MC, Audige L. Platelet-rich plasma for chronic lateral epicondylitis: is one injection sufficient? Archives of Orthopaedic and Trauma Surgery 2015;135(12):1637-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015
- GRADEpro Guideline Development Tool [Computer program]. McMaster University (developed by EvidencePrime, Inc.). Available from www.gradepro.org, 2015.
Green 2002
- Green S, Buchbinder R, Barnsley L, Hall S, White M, Smidt N, et al. Acupuncture for lateral elbow pain. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD003527. [DOI: 10.1002/14651858.CD003527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2012
- Griffin XL, Wallace D, Parsons N, Costa ML. Platelet rich therapies for long bone healing in adults. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD009496. [DOI: 10.1002/14651858.CD009496.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gummesson 2003
- Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskeletal Disorders 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herd 2008
- Herd CR, Meserve BB. A systematic review of the effectiveness of manipulative therapy in treating lateral epicondylalgia. Journal of Manual and Manipulative Therapy 2008;16(4):225-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2017a
- Higgins JT, Altman DG, Sterne JAC (editors). Chapter 7. Selecting studies and collecting data. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2017b
- Higgins JT, Altman DG, Sterne JAC (editors). Chapter 16. Special topics in statistics. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2017c
- Higgins JT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hume 2006
- Hume PA, Reid D, Edwards T. Epicondylar injury in sport: epidemiology, type, mechanisms, assessment, management and prevention. Sports Medicine 2006;36:151-70. [DOI] [PubMed] [Google Scholar]
Kampa 2010
- Kampa R, Connell D. Treatment of tendinopathy: is there a role for autologous whole blood and platelet rich plasma injection? International Journal of Clinical Practice 2010;64(13):1813-23. [DOI] [PubMed] [Google Scholar]
Kohia 2008
- Kohia M, Brackle J, Byrd K, Jennings A, Murray W, Wilfong E. Effectiveness of physical therapy treatments on lateral epicondylitis. Journal of Sport Rehabilitation 2008;17(2):119-36. [DOI] [PubMed] [Google Scholar]
Lana 2017
- Lana JFSD, Purita J, Paulus C, Huber SC, Rodrigues BL, Rodrigues AA, et al. Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regenerative Medicine 2017;12(5):565-74. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Archives of Plastic Surgery 2013;40(5):530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2007
- Lo MY, Safran MR. Surgical treatment of lateral epicondylitis: a systematic review. Clinical Orthopaedics and Related Research 2007;463:98-106. [DOI] [PubMed] [Google Scholar]
Mallen 2009
- Mallen CD, Chesterton LS, Hay EM. Tennis elbow. BMJ 2009;339:b3180. [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2012
- Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD006899. [DOI: 10.1002/14651858.CD006899.pub2] [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2013
- Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Bellmunt-Montoya S, Cid J, Urrútia G. Autologous platelet-rich plasma for treating surgical wounds. Cochrane Database of Systematic Reviews 2013, Issue 9. Art. No: CD010739. [DOI: 10.1002/14651858.CD010739] [DOI] [Google Scholar]
Mautner 2015
- Mautner K, Malanga GA, Smith J, Shiple B, Ibrahim V, Sampson S, et al. A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. Physical Medicine & Rehabilitation 2015;7(4 Suppl):S53-9. [DOI] [PubMed] [Google Scholar]
Mi 2017
- Mi B, Liu G, Zhou W, Lv H, Liu Y, Wu Q, et al. Platelet rich plasma versus steroid on lateral epicondylitis: meta-analysis of randomized clinical trials. The Physician and Sportsmedicine 2017;45(2):97-104. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 2015
- Murray DJ, Javed S, Jain N, Kemp S, Watts AC. Platelet-rich plasma injections in treating lateral epicondylosis: a review of the recent evidence. Journal of Hand and Microsurgery 2015;7(2):320-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Clinical Excellence. Autologous blood injection for tendinopathy (interventional procedure guidance IPG 438). January 2013. http://guidance.nice.org.uk/IPG438 (accessed 29 January 2014).
Nirschl 1979
- Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. Journal of Bone and Joint Surgery 1979;61(6A):832-9. [PubMed] [Google Scholar]
Pattanittum 2013
- Pattanittum P, Turner T, Green S, Buchbinder R. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD003686. [DOI: 10.1002/14651858.CD003686.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabago 2009
- Rabago D, Best TM, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. British Journal of Sports Medicine 2009;43(7):471-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranney 1995
- Ranney D, Wells R, Moore A. Upper limb musculoskeletal disorders in highly repetitive industries: precise anatomical physical findings. Ergonomics 1995;38:1408-23. [DOI] [PubMed] [Google Scholar]
RevMan 2014
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rompe 2007
- Rompe JD, Overend TJ, MacDermid JC. Validation of the Patient-rated Tennis Elbow Evaluation Questionnaire. Journal of Hand Therapy 2007;20:3-10. [DOI] [PubMed] [Google Scholar]
Samson 2008
- Samson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Current Reviews in Musculoskeletal Medicine 2008;1:165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017a
- Schünemann HJ, Oxman AD, Higgins JT, Vist GE, Glasziou P, Akl E, et al (editors). Chapter 11. Completing Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT,Deeks JJ, Glasziou P, Akl E, Guyatt GH (editors). Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS(editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017).Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Silverstein 2006
- Silverstein BA, Adam D. Work-related disorders of the back and upper extremity in Washington State, 1989-1996. Olympia, Washington: SHARP Program, Washington State Department of Labor and Industries, 2006.
Smidt 2002b
- Smidt N, Assendelft WJJ, Windt DAWM, Hay EM, Buchbinder R, Bouter LM. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain 2002;96:23-40. [DOI] [PubMed] [Google Scholar]
Smidt 2003
- Smidt N, Assendelft WJ, Arola H, Malmivaara A, Green S, Buchbinder R, et al. Effectiveness of physiotherapy for lateral epicondylitis: a systematic review. Annals of Medicine 2003;35(1):51-62. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10. Addressing reporting biases. In: Higgins JT,Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Struijs 2002
- Struijs PAA, Smidt N, Arola H, Dijk CN van, Buchbinder R, Assendelft WJJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD001821. [DOI: 10.1002/14651858.CD001821] [DOI] [PubMed] [Google Scholar]
Suresh 2006
- Suresh SP, Ali KE, Jones H, Connell DA. Medial epicondylitis: is ultrasound guided autologous blood injection an effective treatment? British Journal of Sports Medicine 2006;40:935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2002
- Taylor MA, Norman TL, Clovis NB, Blaha JD, Taylor MA, Norman TL, et al. The response of rabbit patellar tendons after autologous blood injection. Medicine and Science in Sports and Exercise 2002;34:70-3. [DOI] [PubMed] [Google Scholar]
Vicenzino 2020
- Vicenzino B, Vos RJ, Alfredson H, Bahr R, Cook JL, Coombes BK, et al. ICON 2019-International Scientific Tendinopathy Symposium Consensus: there are nine core health-related domains for tendinopathy (CORE DOMAINS): Delphi study of healthcare professionals and patients. British Journal of Sports Medicine 2020;54:444-51. [DOI] [PubMed] [Google Scholar]
Walker‐Bone 2004
- Walker-Bone K, Palmer K, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis & Rheumatism 2004;51(4):642-51. [DOI] [PubMed] [Google Scholar]
Wehling 2007
- Wehling P, Moser C, Frisbie D, McIlwraith CW, Kawcak CE, Krauspe R, et al. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs 2007;21:323-32. [DOI] [PubMed] [Google Scholar]


