Bullous pemphigoid (BP) is the most common autoimmune bullous disease, commonly encountered in the elderly and characterized by autoantibodies toward basement membrane zone antigens BP180 and BP230. Many trigger factors for BP have been identified such as ultraviolet (UV), radiation, drugs, trauma and burns. Post-vaccination bullous pemphigoid is rare. A few cases have been reported following diphtheria, tetanus, whooping cough, poliomyelitis, influenza, pneumococcal, meningococcal, hepatitis B, BCG and rabies vaccinations [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. We report the first case of BP triggered by a COVID-19 vaccine.
A 77-year-old patient, with no significant pathological history, consulted for a diffuse itchy bullous eruption that appeared 24 hours after the first injection of AstraZeneca COVID-19 vaccine. The clinical examination found tense bullous lesions, with clear or hemorrhagic content, over erythematous skin, on the trunk, scalp and limbs (Fig. 1 ). No mucosal bullous lesion was noticed. Considering the age and the clinical characteristics, BP was suspected. Skin biopsy showed a sub-epidermal cleavage and linear deposit of Ig G at the membrane zone using direct and indirect immunofluorescence assays, confirming the diagnosis. The occurrence of the eruption one day after the AstraZeneca COVID-19 vaccine was in favor of a post-vaccine BP that was reported to the pharmacovigilance department. The patient was treated by high potency topical steroids (propionate of clobetasol 0.05% cream) once a day and doxycycline (100 mg/day) with a favorable outcome. The vaccine booster was contraindicated.
Fig. 1.
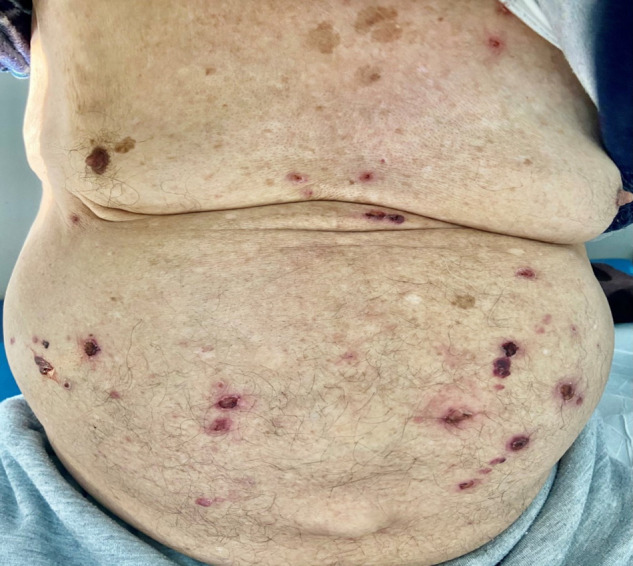
Diffuse bullous eruption with hemorrhagic or tense blisters on the trunk.
BP is an autoimmune bullous disease, the main antigenic target of which is the trans membrane protein BP180 [16]. Various triggering factors have been reported in the literature, mostly radiotherapy, but also different drugs [17]. When a genetically predisposed individual is exposed to a trigger, it may lead to the revelation of the BP180 antigen, generating an immune response mediated by B-cells, leading to the production of auto-antibodies against BP180 antigen. These antibodies lead to the activation of the complement pathway causing protease release resulting in degradation of the basement membrane zone [18].
The skin side effects of vaccines are numerous. In addition to hypersensitivity reactions at the injection sites and drug eruptions, the onset or exacerbation of some skin diseases is possible. The occurrence of autoimmune bullous diseases after vaccination has already been reported in the literature. A few cases of pemphigus vulgaris have been reported after rabies and hepatitis B vaccines [19], [20]. Post-vaccination BP is rare; only ten cases have been reported in the literature in adults and in children [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The period between the vaccine injection and the occurrence of BP varies between 1 day and 1 month [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. The mechanism for induction of BP in response to vaccine is not very well understood. It has been proposed that BP can be triggered in some genetically predisposed individuals after attenuated life exposure vaccine that strengthens the immune response. The main mechanism by which specially inactivated vaccines provide immunity is the humoral pathway, that is, by stimulation of B-lymphocytes, leading to the production of antibodies. Post-vaccine BP has the same clinical, histological and immunological characteristics as other pemphigoids. Scalp involvement as in our observation is rare and could be a characteristic of post-vaccination BP. However, this has been never reported in published cases of post-vaccination BP. A causal relationship between vaccination and BP is difficult to establish, as vaccinations are extremely frequent. Thus, we cannot eliminate a coincidence between the occurrence of this bullous autoimmune skin disease in our patient and the AstraZeneca COVID-19 vaccine but a short delay of one day between the vaccination and BP may be in favor of a causal link.
Recurrence cases after another vaccine booster injection confirms the hypothesis of the role of vaccine and require more vigilance before proposing a vaccine booster to these patients [4]. However, in children it remains difficult to prohibit a vaccine booster, especially for certain serious diseases.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Cunha P.R., Thomazaski P.V., Hipolito, Michalany N.S., Bystryn J.C. Bullous pemphigoid in a 2-month-old infant. Int J Dermatol. 1998;37:935–938. doi: 10.1046/j.1365-4362.1998.00615.x. [DOI] [PubMed] [Google Scholar]
- 2.Baykal C., Okan G., Sarica R. Childhood bullous pemphigoid developed after the first vaccination. J Am Acad Dermatol. 2001;44:348–350. doi: 10.1067/mjd.2001.103034. [DOI] [PubMed] [Google Scholar]
- 3.Erbagci Z. Childhood bullous pemphigoid following hepatitis B immunization. J Dermatol. 2002;29:781–785. doi: 10.1111/j.1346-8138.2002.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 4.Merida C., Martinez-Escribano J.A., Frias J.F., Sánchez-Pedreno P., Corbalán R. Bullous pemphigoid in an infant after vaccination. Actas Dermosifiliogr. 2005;96:255–257. doi: 10.1016/s0001-7310(05)73081-7. [DOI] [PubMed] [Google Scholar]
- 5.Toyama T., Nakamura K., Kuramochi, Ohyama B., Hashimoto T., Tsuchida T. Two cases of childhood bullous pemphigoid. Eur J Dermatol. 2009;19:368–371. doi: 10.1684/ejd.2009.0694. [DOI] [PubMed] [Google Scholar]
- 6.Hafiji J., Bhogal B., Rytina E., Burrows N.P. Bullous pemphigoid in infancy developing after the first vaccination. Clin Exp Dermatol. 2010;35:940–941. doi: 10.1111/j.1365-2230.2010.03839.x. [DOI] [PubMed] [Google Scholar]
- 7.Valdivielso-Ramos M., Velázquez D., Tortoledo A., Hernanz J.M. Infantile bullous pemphigoid developing after hexavalent, meningococcal and pneumococcal vaccinations. Ann Pediatr. 2011;75:199–202. doi: 10.1016/j.anpedi.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Jindal A., Nayak S.U., Shenoi S.D., Rao R., Monappa V. Bullous pemphigoid triggered by rabies vaccine. Indian J Dermatol Venereol Leprol. 2020;86:66–68. doi: 10.4103/ijdvl.IJDVL_666_18. [DOI] [PubMed] [Google Scholar]
- 9.Walmsley N., Hampton P. Bullous pemphigoid triggered by swine flu vaccination: case report and review of vaccine triggered pemphigoid. J Dermatol Case Rep. 2011;5:74–76. doi: 10.3315/jdcr.2011.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lear J.T., Tan B.B., English J.S. Bullous pemphigoid following influenza vaccination. Clin Exp Dermatol. 1996;21:392. doi: 10.1111/j.1365-2230.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 11.Fournier B., Descamps V., Bouscarat F., Crickx B., Belaich S. Bullous pemphigoid induced by vaccination. Br J Dermatol. 1996;135:153–154. doi: 10.1046/j.1365-2133.1996.d01-963.x. [DOI] [PubMed] [Google Scholar]
- 12.Bisherwal K., Pandhi D., Singal A., Sharma S. Infantile bullous pemphigoid following vaccination. Indian Pediatr. 2016;53:425–426. doi: 10.1007/s13312-016-0867-3. [DOI] [PubMed] [Google Scholar]
- 13.Neri I., Greco A., Bassi A., Orgaz-Molina J., Balestri R., Oranges T., et al. Bullous pemphigoid in infant post vaccination: myth or reality? Int J Immunopathol Pharmacol. 2016;29:295–299. doi: 10.1177/0394632015603796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baroero L., Coppo P., Bertolino L., Maccario S., Savino F. Three case reports of post immunization and post viral bullous pemphigoid: looking for the right trigger. BMC Pediatr. 2017;17:60. doi: 10.1186/s12887-017-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs A.M., Lear J.T., Bower C.P., Kennedy C.T. Does influenza vaccination induce bullous pemphigoid? A report of four cases. Br J Dermatol. 1998;138:363. doi: 10.1046/j.1365-2133.1998.02097.x. [DOI] [PubMed] [Google Scholar]
- 16.Bernard P., Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18:513–528. doi: 10.1007/s40257-017-0264-2. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt E., Zillikens D. The diagnosis and treatment of autoimmune blistering skin diseases. Dtsch Arztebl Int. 2011;108:399–405. doi: 10.3238/arztebl.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata H., Kitajima Y. Bullous pemphigoid: role of complement and mechanisms for blister formation within the lamina lucida. Exp Dermatol. 2013;22:381–385. doi: 10.1111/exd.12146. [DOI] [PubMed] [Google Scholar]
- 19.Yalçin B., Alli N. Pemphigus vulgaris following antirabies vaccination. J Dermatol. 2007;34:734–735. doi: 10.1111/j.1346-8138.2007.00373.x. [DOI] [PubMed] [Google Scholar]
- 20.Berkun Y., Mimouni D., Shoenfeld Y. Pemphigus following hepatitis B vaccination-coincidence or causality? Autoimmunity. 2005;38:117–119. doi: 10.1080/08916930400027078. [DOI] [PubMed] [Google Scholar]


