Abstract
We report a case of a heart transplant recipient who presented with a rapidly growing Epstein-Barr virus (EBV)–positive, diffuse large B-cell lymphoma 7 days after receiving the first dose of the ChAdOx1 nCoV-19 vaccine. Because of the atypical radiologic presentation, the initial tentative diagnosis was a mediastinal abscess. This observation indicates a potential risk of EBV reactivation after coronavirus disease 2019 (COVID-19) vaccination, which might lead to or aggravate the presentation of posttransplant lymphoproliferative disorder in transplantation patients. Transplant surgeons should be aware of the potential immunomodulatory effects of the COVID-19 vaccination.
During the coronavirus disease 2019 (COVID-19) pandemic, vaccination is recommended for immunocompromised patients, including those who have received solid organ transplantation [1]. The most common adverse reactions include pain at the injection site, headache, fatigue, myalgia, fever, and joint pain [2]. Less common side effects include lymphadenopathy or lymphadenitis in the ipsilateral axillary region [3]. Additionally, reactivation of the Gammaherpesvirinae subfamily, such as Epstein-Barr virus (EBV) and herpesvirus, have been reported in case studies [4], [5], [6]. However, information on COVID-19 vaccines among immunocompromised patients (eg, solid organ transplant recipients, patients with blood and solid organ cancers) is limited, and further investigation is required regarding efficacy and safety [7].
This study was approved by the institutional review board of the National Cheng Kung University Hospital (IRB No. B-EC-110-024, 08/01/2021). Written informed consent for the publication of details and images was obtained from the patient.
Case Presentation
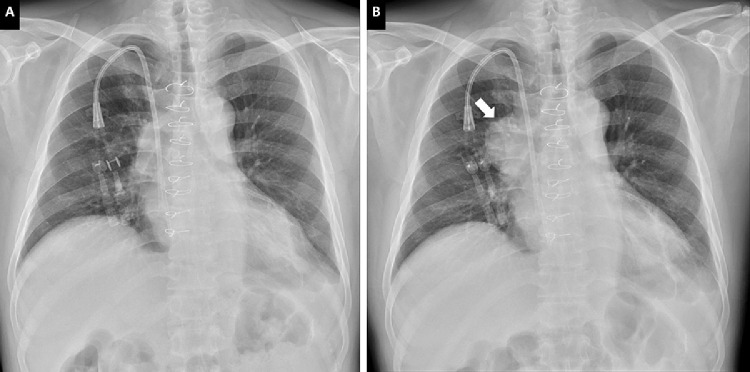
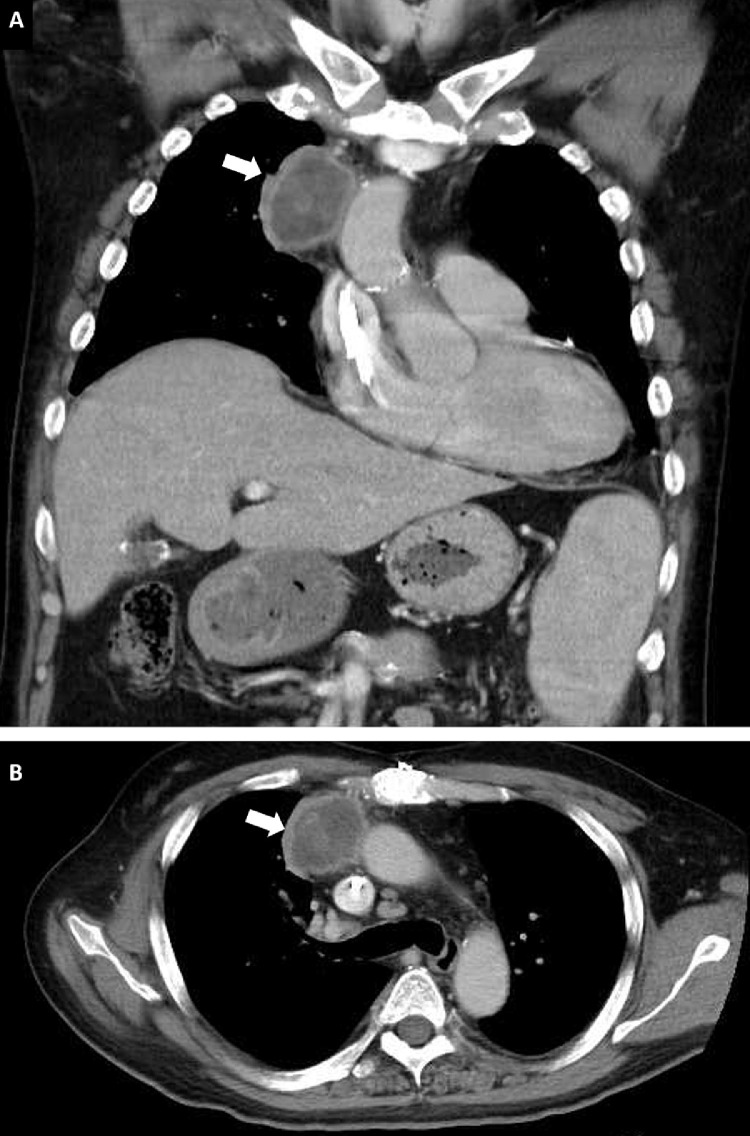
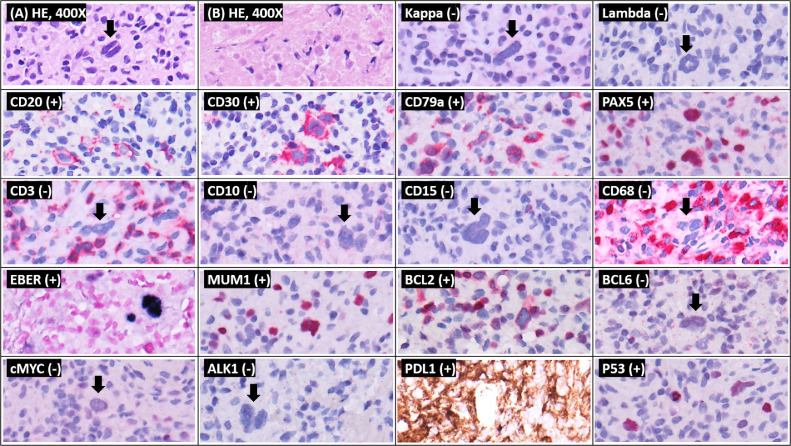
A 51-year-old man was admitted with a chief complaint of fever up to 38°C and generalized soreness at 1 week after the first dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca) in the right deltoid muscle. The symptoms lasted for 3 days. He had been diagnosed with idiopathic dilated cardiomyopathy and received an orthotopic heart transplantation on February 6, 2014. He developed end-stage renal disease and required hemodialysis in December 2018. His recent immunosuppressant regimen included tacrolimus (1 mg twice a day) and mycophenolate mofetil (1.25 g/d). Chest radiography showed a new mass in the right upper mediastinum, which had not been seen on the chest film 1 month before admission (Fig 1 ). Physical examination was unremarkable, and there was no palpable axillary or supraclavicular lymphadenopathy. Testing with nasopharyngeal swab severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription–polymerase chain reaction (PCR) (Cobas 6800 system) was negative. Laboratory tests revealed normal white blood cell count (4.0 × 103/uL), platelet count (2.76 × 105/uL), and C-reactive protein level (9.4 mg/L). The whole blood trough concentration of tacrolimus was 6.5 ng/mL. Contrast-enhanced computed tomography of the chest revealed a ring-enhancing mass of approximately 52 × 50 × 42 mm in the right upper mediastinum (Fig 2 ). Computed tomography–guided drainage was performed under the impression of a mediastinal abscess, which failed to drain any contents. Therefore, he underwent debulking surgery for the mass lesion using video-assisted thoracoscopy. Pathology examination revealed EBV-positive diffuse large B-cell lymphoma (Fig 3 ), which was consistent with a diagnosis of monomorphic posttransplant lymphoproliferative disorder (PTLD). Blood, sputum, and excisional specimens were sent for bacterial, fungal, and mycobacterial cultures and real-time PCR (Xpert MTB/RIF assay [Cepheid, Sunnyvale, Calif, United States]) analyses. Abbott real-time PCR was used to detect viremia, and data on cytomegalovirus, EBV, and SARS-CoV-2 as well as herpes simplex virus type 1, herpes simplex virus type 2, hepatitis B virus, and hepatitis C virus serology were obtained. In addition, deoxyribonucleic acid (DNA) microarray technology was used for mold, fungus, and mycobacterium detection, and 16S ribosomal ribonucleic acid (RNA) gene PCR was used to detect bacterial pathogens. All studies evaluating the infectious etiology were negative. Even Abbott real-time EBV PCR of serum was negative 2 months before and 1 month after admission. The patient was treated with a 50% reduction in immunosuppressant dosages in conjunction with rituximab once a week for 4 weeks as an inpatient.
Fig 1.
Chest radiography shows a mediastinal mass that bulges the right upper mediastinal contour (B), which was not seen 1 month previously (A).
Fig 2.
Computed tomography of the chest reveals a ring-enhancing mass in the right upper mediastinum and splenomegaly (A, coronal view; B, axial view)
Fig 3.
Sections show (HE 400×) (A) large, atypical lymphocytes with bi-lobation within a lymphohistiocytic background. There was extensive necrosis in the other parts (HE 400×). (B) Immunohistochemically, the atypical lymphocytes were positive for CD20 (>50%), CD30, CD79a, PAX5, MUM1, and BCL2, and negative for κ, lambda, CD3, CD10, CD15, CD68, BCL6, and c-MYC. ALK1 staining was negative. Atypical large lymphocytes were positive for EBER in situ hybridization. P53 staining was heterogeneously positive. PDL1 was also positive. ALK1, activin A receptor like type 1; BCL, B-cell lymphoma; CD, cluster of differentiate; c-MYC, cellular myelocytomatosis oncogene; EBER, Epstein-Barr encoding region; HE, hematoxylin and eosin; MUM, multiple myeloma oncogen; PAX5, paired box 5; PDL, programmed death-ligand.
Discussion
PTLD refers to a group of lymphoid disorders with heterogeneous presentations ranging from indolent to aggressive disease [8,9]. The highest incidence occurs in cases of multivisceral transplants, followed by lung transplants and heart transplants [8]. The risk contribution of specific immunosuppressive agents varies in reports, but greater impact has been reported of higher intensity and longer duration immunosuppression (ie, total immunosuppressive burden) [8,9]. The pathogenesis of PTLD is closely associated with infection or reactivation of EBV in the context of chronic immunosuppression as the main predisposing factor [8], [9], [10]. In the classic model, most EBV-positive PTLD results from EBV-infected B cells with a latency III pattern that proliferates only under conditions of reduced cytotoxic T cells [8,10]. Additionally, different EBV life phases are associated with different immune conditions [8]. Therefore, cell-mediated immunity is crucial for quiescent coexistence with EBV, especially for organ recipients who have made great efforts to strike an optimal risk balance between infection and immunosuppression. Nonetheless, EBV-positive PTLD only develops in a minority of solid organ transplant recipients receiving immunosuppression [8].
This suggests that the key step toward lymphomagenesis is multifactorial and extends beyond the scope of chronic immunosuppression. In a population-based study conducted in Nebraska, the risk of non-Hodgkin lymphoma was positively associated with receiving influenza vaccination (odds ratio [OR] = 1.53; confidence interval [CI] = 1.14-2.06) [11]. In particular, follicular lymphoma (OR = 1.23; CI = 1.23-3.18) and diffuse large B-cell lymphoma (OR = 1.88; CI = 1.13-3.12) were among the most common forms of lymphoma. Alteration of T helper 1 and Th2 cell balance after vaccination is inferred to be the cause of this increased risk. Accordingly, vaccination for SARS-CoV-2 may be an external stimulus and may temporarily alter this fragile balance [6], based on the experience gained from various vaccines [[4], [5], [6],11]. As a result, tissue EBV reactivation may occur after COVID-19 vaccination [3].
Although it is far-fetched to establish a straightforward relationship between the COVID-19 vaccine and PTLD, 2 in vitro studies have implicated it in oncogenesis. Chen et al [12] reported that transfection of SARS-CoV-2 spike protein S vectors led to a significant increase in representative lytic gene expression in the iSLK.219 cell line harboring recombinant Kaposi sarcoma-associated herpesvirus.219 (rKSHV.219). This indicates that protein S, one of SARS-CoV-2 major structural proteins and the target antigen encoded by the COVID-19 vaccine [2], has the potential to induce lytic reactivation of KSHV in chronically infected cells. EBV and KSHV are members of the Gammaherpesvirinae subfamily, which has a biphasic life cycle comprising lytic and latent phases and can cause a variety of cancers in humans [13]. In recent years, the role of the lytic cycle in tumorigenesis and tumor progression of both viruses have been elucidated and are well recognized as the main contributors to genomic instability and the necessary antiapoptotic and immunomodulatory signals [10,12,13]. Chiang et al [14] demonstrated the ability of SARS-CoV-1 spike protein to transform B cells in vitro, and proposed that devastating viral ligands may accelerate abnormal conversion of lymphocytes under a dampened immune system. Although SARS-CoV-2 and SARS-CoV-1 have different S sequences and bind angiotensin-converting enzyme 2 via distinct regions in the S protein receptor-binding motif [15], the B-cell conversion ability of coronavirus S protein is indirectly implied.
Conclusion
In summary, we hypothesize that the vaccine may contribute to B lymphocyte tumorigenesis via the reactivation of latent EBV. However, it has been acknowledged that chronically EBV-infected B cells accumulate pro-oncogenic aberrations (eg, A20, BCL2, BCL6, MYC, PAX5, and TP53) under impaired immune surveillance and can transform into lymphoma by themselves at some point in time [8,9]. In this reported case, PTLD occurred 7 years after transplantation, which falls within the range of the second peak [9]. The patient received a heart transplant and was maintained on tacrolimus, both of which are associated with a higher risk of PTLD [9]. Nevertheless, the blood concentration of tacrolimus was kept within low limits of the therapeutic range, and EBV viral load in the serum was undetectable for 5 years, even 4 weeks after diagnosis of EBV-positive PTLD. Additionally, although serum EBV DNA kinetics have been demonstrated to be important in the highest-risk hematopoietic stem cell transplantation group, with a median of 7 days from EBV DNAemia onset to EBV-related lymphoproliferative disorders, evidence in solid organ transplant is less compelling because of the lack of sensitivity to localized EBV-positive PTLD and EBV-negative PTLD [8].
We report a case of PTLD after COVID-19 vaccination in a heart transplant recipient, which might show a possible pathogenic link. Although we are not able to exclude the coincidence of COVID-19 vaccination and the development of PTLD in this case, transplant surgeons should be aware of the immunomodulatory effect after COVID-19 vaccination and always think the worst until proven otherwise.
Acknowledgment
We thank Wiley Editing Services for enhancing our writing.
Footnotes
Supported by grants from the Ministry of Science and Technology, Executive Yuan, Taiwan (grant nos. MOST 109-2314-B-006-079 and 110-2314-B-006-104 to J.-N.R.).
References
- 1.International Society of Heart and Lung Transplantation . International Society of Heart and Lung Transplantation; 2021. Guidance from the International Society of Heart and Lung Transplantation regarding the SARS CoV-2 pandemic 2021.https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf <. >. [Google Scholar]
- 2.European Medicines Agency . European Medicines Agency; 2021. Vaxzevria (previously COVID-19 vaccine AstraZeneca): EPAR product information.www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca#product-information-section <. >. [Google Scholar]
- 3.Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA vaccine with ipsilateral axillary lymph node reactivity on imaging. JAMA Oncol. 2021;7:1241–1242. doi: 10.1001/jamaoncol.2021.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series [e-pub ahead of print]. Rheumatology (Oxford) doi:10.1093/rheumatology/keab345, accessed August 9, 2021. [DOI] [PMC free article] [PubMed]
- 5.Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021;93:5231–5232. doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel) 2021;9:572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam S, Goldstein DR, Vos R, Gelman AE, Kittleson MM, Wolfe C, et al. COVID-19 vaccination in our transplant recipients: the time is now. J Heart Lung Transplant. 2021;40:169–171. doi: 10.1016/j.healun.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. doi: 10.1038/nrdp.2015.88. [DOI] [PubMed] [Google Scholar]
- 9.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]
- 10.Münz C. Latency and lytic replication in Epstein-Barr virus–associated oncogenesis. Nat Rev Microbiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- 11.Lankes HA, Fought AJ, Evens AM, Weisenburger DD, Chiu BCH. Vaccination history and risk of non-Hodgkin lymphoma: a population-based, case-control study. Cancer Causes Control. 2009;20:517–523. doi: 10.1007/s10552-008-9259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Dai L, Barrett L, James J, Plaisance-Bonstaff K, Post SR, et al. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun Biol. 2021;4:682. doi: 10.1038/s42003-021-02220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manners O, Murphy JC, Coleman A, Hughes DJ, Whitehouse A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr Opin Virol. 2018;32:60–70. doi: 10.1016/j.coviro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang SF, Lin TY, Chow KC, Chiou SH. SARS spike protein induces phenotypic conversion of human B cells to macrophage-like cells. Mol Immunol. 2010;47:2575–2586. doi: 10.1016/j.molimm.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatmal MM, Alshaer W, Al-Hatamleh MAI, Hatmal M, Smadi O, Taha MO, et al. Comprehensive structural and molecular comparison of spike proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and their interactions with ACE2. Cells. 2020;9:2638. doi: 10.3390/cells9122638. [DOI] [PMC free article] [PubMed] [Google Scholar]