Abstract
We describe a diagnostic PCR assay (D-PCR) and a quantitative PCR assay (Q-PCR) for the detection of human cytomegalovirus (CMV) in plasma and serum. In the D-PCR, DNA was purified from plasma or serum together with internal control (IC) DNA, which monitored both DNA extraction efficiency and PCR efficiency. DNA was subjected to PCR with a single primer pair, and the amount of PCR products was determined by electrochemiluminescence (ECL) in the QPCR System 5000 (Perkin-Elmer) after hybridization with Tris (2,2′-bipyridine) ruthenium (II) chelate-labeled probes. The lower limit of sensitivity of the D-PCR was reached at about 25 CMV particles/ml. Even with extremely low DNA inputs (four molecules of IC DNA/200 μl of plasma), very high yields (near 100%) were reached. DNA extracted from specimens that were CMV positive by the D-PCR was subsequently used in the Q-PCR, which was similar to the D-PCR. The viral load was calculated directly from the ratio of CMV and IC signals obtained by ECL. The Q-PCR assay is quantitative in the range of 100 to 150,000 copies of CMV/ml, independent of the anticoagulant. Interassay variation, intra-assay variation, and interspecimen variation were about 25%, suggesting that the Q-PCR will reliably detect fourfold differences in viral load. Comparison of paired serum and plasma specimens from CMV-infected individuals showed that serum CMV loads were frequently more than 10-fold lower than plasma CMV loads.
Infection with cytomegalovirus (CMV) is an important cause of morbidity and mortality in immunocompromised individuals, like transplant recipients, AIDS patients, and newborns. For the monitoring of patients at risk for the development of CMV disease and for the monitoring of treatment, rapid, specific, and sensitive tests are needed. One such test is PCR (32), which can detect CMV DNA present in the blood compartment as cell-associated virus in the leukocytes and as free virus in serum and plasma.
Since the first detection of CMV in serum and plasma (7, 23, 36), many reports on the qualitative (diagnostic) detection of CMV DNA in plasma or serum have appeared. In bone marrow transplant recipients (3, 21, 23, 28, 39), renal transplant recipients (7, 11, 16, 39), and liver transplant recipients (16, 29, 30, 33), the presence of CMV DNA in plasma or serum has been shown to be an early marker for CMV infection and CMV disease. In congenitally infected newborns (26) and in children with AIDS (27), CMV can readily be detected in serum. In human immunodeficiency virus-seropositive patients, the presence of CMV DNA in plasma or serum has been shown to precede CMV disease by a median of 46 days (14) to 3 months (20). Although the negative predictive power for disease in human immunodeficiency virus-seropositive patients is high, the positive predictive power is still a matter of debate (14, 25). The levels of CMV DNA in plasma or serum were significantly lower than those in leukocytes (15, 43); therefore, very sensitive assays are needed for CMV detection.
Most of the diagnostic procedures mentioned above are not readily implemented in the routine setting of a clinical virology laboratory, and nested PCR was frequently used to reach the high sensitivity needed. However, nested PCR precludes the use of N-uracil-glycosylase to avoid false-positive results due to amplimer carryover. Another problem in the detection of CMV is the preparation of CMV DNA from serum and plasma. Although simple pretreatments (e.g., proteinase digestion and heat treatment or treatment with alkali) will detect high viral loads, low loads may remain undetected (3, 23). In previous studies in which CMV DNA was purified before PCR, controls that monitored DNA extraction efficiency were not included and controls that monitored PCR inhibition were usually absent. Therefore, the sensitivities of many of these diagnostic assays were unknown.
Quantitation of CMV in serum or plasma may be important for the detection of patients at risk for disease, monitoring of the efficacy of therapy, development of resistance, and prognosis. Several procedures for the quantitative assessment of CMV in serum and plasma have been developed. Some of these procedures are based on limiting dilution and nested PCR or detection with radioactive probes (36, 38). Others are based on coamplification of internal control DNA (competitive PCR) which shares the primer sequences with the target of interest but which generates an altered PCR product (8, 17, 19, 34, 35, 41, 43). Internal DNA corrects for variations in PCR efficiency due to inhibitory substances and well-to-well variations in the thermocycler (10, 13). To derive absolute quantitative information by competitive PCR, the target and internal control DNAs must be amplified with equal efficiencies with identical primer pairs. Under these conditions the initial ratio of target to competitor remains constant throughout amplification (including the plateau phase), and this can be used to calculate viral load, provided that the amplification products are accurately measured (10). Measurement of amplification products has been done by high-pressure liquid chromatography (8) and by polyacrylamide gel electrophoresis (34, 35), procedures that are not readily implemented in a routine clinical virology laboratory. Amplification products have also been measured after slot blotting and hybridization with digoxigenin-labeled probes followed by enzymatic conversion of a substrate into a colored reaction product which is subsequently measured by light absorbance (19). Recently, a plate assay has been described (17, 31, 41, 43). In that assay amplification products were hybridized to a specific detector probe bound to the wells of a plate. The amount of hybrids was subsequently quantified spectrophotometrically after enzymatic conversion of substrate. Since the ratios of CMV signals over IC signals are not a direct reflection of the amount of CMV, an external reference curve must be prepared for each assay.
We describe a highly sensitive diagnostic PCR assay (D-PCR) for the detection of CMV in serum and plasma in which 35 molecules of IC DNA, mimicking the CMV target, are included in the DNA extraction, and we evalute the performance of a quantitative PCR assay (Q-PCR). The viral load (expressed as the number of copies of CMV per milliliter) was calculated by a straightforward algorithm from the ratio of CMV over IC signals obtained after solution hybridization with Tris (2,2′-bipyridine) ruthenium (II) chelate (TBR)-labeled probes and measurement by electrochemiluminescence (ECL). In clinical specimens, the loads observed in serum were often significantly lower (10-fold or more) than those observed in the corresponding plasma specimens.
MATERIALS AND METHODS
Chemicals and enzymes.
Taq DNA polymerase (Amplitaq), uracil-N-glycosylase (Amperase), and streptavidin-coated magnetic beads were from Perkin-Elmer. Bovine serum albumin was from Boehringer Mannheim. Human placental DNA was from Sigma Chemical Company. Pools of human citrate plasma (ESDEP; Octapharma Pharmazeutica Produktions-Gesellschaft m.b.H., Vienna, Austria) were obtained from the Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands; this plasma is referred to as the reference plasma. Plasma and serum specimens were obtained in Vacutainer tubes (containing tripotassium EDTA, sodium citrate, and lithium heparin; Becton Dickinson Systems, Meylan, France).
Human CMV.
Sucrose density gradient-purified human CMV AD 169 (lot no. 80-165-1; 5.38 × 109 viral particles/ml of virus dilution buffer [10 mM Tris · HCl, 150 mM NaCl, 1 mM EDTA [pH 7.5]), as determined electron microscopically by direct particle count determination, which discriminated between full and empty particles) was obtained from Advanced Biotechnologies Inc. (Columbia, Md.). According to the manufacturer, the error in viral particle count was estimated to be ±0.5 log. We performed limiting-dilution experiments for the detection of CMV DNA purified from virus preparations by the silica-guanidinium thiocyanate (GuSCN) procedure (5) followed by PCR, which suggested that the actual amount of viral DNA was about three times higher than that expected from the viral particle count, assuming a 100% recovery of viral DNA. The CMV concentrations provided in this paper were based on this correction factor.
Laboratory parameters for CMV infection.
Viral culture was done by cocultivation of blood buffy coat cells and human diploid fibroblasts and microscopic examination for the appearance of CMV-specific cytopathologic effects. CMV immunoglobulin G (IgG) in serum was determined by the IMx CMV IgG assay (Abbott Laboratories); CMV IgM in serum was determined by the VIDAS IgM assay (Biomérieux, Lyon, France).
PCR.
Primers (purified by high-pressure liquid chromatography) were from Perkin-Elmer and were diluted in TE buffer (10 mM Tris · HCl, 1 mM EDTA [pH 8.0]) to 100 ng/μl. The primer pair used for amplification consisted of CMV-531 (5′-ACA AGG TGC TCA CGC ACA TTG ATC-3′; nucleotide positions [nt] 2034 to 2057) and Bio-CMV-1107 (5′-CAC TGG CTC AGA CTT GAC AGA CAC-3′, 5′ biotinylated; nt 2588 to 2611); nucleotide numbering was according to Akrigg et al. (1). This primer pair amplifies a 578-bp DNA fragment from exon 4 of the major immediate-early gene of human CMV or a fragment of identical size and GC content from internal control (IC) DNA. The primer pair was chosen in perfectly conserved areas (1, 9, 37) of exon 4 of the major immediate-early gene. In the D-PCR 25 μl of DNA eluate (corresponding to 50 μl of plasma or serum) was used as input for the PCR. For Q-PCR, 20 μl of DNA eluate (corresponding to 40 μl of plasma or serum) and 5 μl of reference IC DNA (see below) was subjected to PCR. The final reaction mixture (50 μl) contained 28 pmol of each primer (CMV-531 and Bio-CMV-1107), 2.5 U of Amplitaq DNA polymerase, 0.5 U of Amperase (uracil-N-glycosylase; Perkin-Elmer), 5 μg of bovine serum albumin (Boehringer Mannheim), 10 mM Tris · HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, dATP, dGTP, and dCTP at a concentration of 200 μM each, and 400 μM dUTP (Perkin-Elmer). The PCRs were done in a Perkin-Elmer 9600 thermocycler: 2 min at 50°C, 5 min at 95°C, followed by 35 cycles each consisting of 20 s at 95°C, 20 s at 63°C, and 1 min at 72°C, followed by 5 min at 72°C. No PCR products were obtained when the DNAs of other herpesviruses (Advanced Biotechnologies Inc.) were used in the PCRs. These included herpes simplex virus type 1 MacIntyre, herpes simplex virus type 2 G, Epstein-Barr virus B95-8, human herpesvirus 6 Z-29, and varicella-zoster virus Rod.
IC DNA.
The construction of IC DNA has been described previously (6). Relative to the CMV immediate-early DNA sequence, the AocI site (5′-CCTGAGG-3′; nt 2246 to 2252) was replaced by the sequence 5′-CCTGACC-3′; similarly, the HhaI site (5′-GCGC-3′; nt 2269 to 2272) was replaced by the sequence 5′-CCGC-3′. In addition, the CMV DNA sequence at nt 2292 to 2316 was replaced by the sequence 5′-CCC TTT ACA TCT TTC TGA AGT AGG G-3′ to serve as a probe area. These modifications allowed discrimination between CMV and IC DNA amplimers (which have the same sizes and GC contents and which are amplified by the same primer pair) resulting from the PCR described above by the restriction enzymes HhaI and AocI. These restriction enzymes cleaved uracil-containing CMV amplimers but not IC amplimers; HhaI and AocI sites were present in the amplimers obtained by PCR from all (n = 80) clinical CMV isolates tested (data not shown). The presence of AocI and HhaI sites in CMV DNA could therefore be used to discriminate between CMV and IC DNA amplimers by gel electrophoresis. The modified DNA fragment (578 bp) was cloned into a plasmid vector (PCRII; 3,932 bp; Promega) resulting in plasmid pCMV-marker. pCMV-marker was purified from bacterial cultures as described previously (22), linearized by HindIII digestion, and purified by the silica-GuSCN procedure (5); and the DNA was quantitated by measuring the UV absorption at 260 nm and was stored in Tris-EDTA (TE) buffer (at 100 μg of plasmid/ml) at −20°C. Dilutions of linearized plasmid were made in TE buffer containing 20 ng of human placental DNA per μl; this DNA served to stabilize very dilute DNA preparations upon storage. Reference IC DNA contained 7 molecules of linearized plasmid (as determined by limiting dilution followed by PCR) and 20 ng of human placental DNA per μl.
Preparation of lysis buffer L7A.
Lysis buffer L7A was prepared by the addition of alpha-casein (C 6780; Sigma) to lysis buffer L6 (prepared as described previously [5]) to a final concentration of 1 mg of alpha-casein per ml of L6. The performance of this lysis buffer has recently been evaluated (6).
DNA purification.
DNA was purified from 200 μl of serum, plasma, or cerebrospinal fluid or from 50 μl of whole blood as described previously (5), with the following modifications: 20 μl of size-fractionated silica particles was used in combination with 900 μl of lysis buffer L7A, and DNA was eluted in 100 μl of TE buffer. In the D-PCR and Q-PCR, 5 μl of reference IC DNA was added to the lysis buffer-silica mixture, and then the clinical specimen was added. The presence of 35 molecules of IC DNA during extraction served as a control for a lower limit of detection of 175 molecules per ml in the diagnostic procedure.
Removal of excess primers.
Initial experiments revealed that the amount of biotin-labeled primers used in the PCR exceeded the biotin binding capacity of the streptavidin-coated magnetic beads. A protocol (Protocol Delta Y-A) was developed to remove excess primers. By that protocol, 40 μl of PCR product was added to a mixture of 1 ml of lysis buffer L6 and 20 μl of size-fractionated silica particles; after vortexing, the tubes were left at ambient temperature for 10 min and were subsequently centrifuged (1 min at approximately 12,000 × g). The supernatant was removed and the tube was again centrifuged (1 min at approximately 12,000 × g), after which the supernatant was carefully removed. The silica pellet was washed once with 1 ml of acetone, and after centrifugation (1 min at 12,000 × g) the supernatant was carefully removed. These steps removed most of the GuSCN present in the lysis buffer because it would otherwise interfere with hybridization. The silica pellets were dried (10 min at 56°C; open lids) and the DNA was eluted (10 min at 56°C) in 100 μl of 1× PCRII buffer (10 mM Tris · HCl [pH 8.3], 50 mM KCl; Perkin-Elmer), followed by vortexing and centrifugation at approximately 12,000 × g for 2 min. The supernatant containing the purified PCR product was subsequently used for hybridization. About 24 samples could be handled in an hour; the yields were high (nearly 100% for both IC and CMV DNA amplimers, as judged from ethidium bromide-stained gels), and the primers were efficiently removed. This protocol was chosen because it gave the lowest variation (coefficient of variation [CV], 5%) when purified amplimers were used in hybridization and ECL measurements.
Hybridization and measurement by ECL.
For D-PCR, the purified PCR product was used directly for hybridization. For Q-PCR, the purified PCR product was diluted five times in 1× PCRII buffer. Twenty microliters of TBR-labeled probe (1 ng/μl; 1.3 pmol; either CMV or IC DNA specific) was added to 30 μl of purified PCR product, and hybridization was done in a Perkin-Elmer 9600 thermocycler (2 min at 95°C, 5 min at 56°C). Next, 10 μl of streptavidin-coated magnetic beads (Perkin-Elmer) was added, and the mixture was incubated for 15 min at 56°C. Forty microliters of the bead-hybrid suspension was added to 400 μl of the QPCR buffer (Perkin-Elmer), and the ECL signal, expressed in luminosity units (LU), was measured by the QPCR System 5000 (Perkin-Elmer). In this device, excess TBR-labeled probe is automatically removed by washing, and the amount of labeled hybrids is determined after excitation by applying an electric field. TBR-labeled probes were diluted in 1× PCRII buffer (10 mM Tris · HCl [pH 8.3], 50 mM KCl; Perkin-Elmer) to 1 ng/μl and were stored at −20°C. The probes were TBR-CMV-1 (CMV-specific probe; 5′-TGA AGG TCT TTG CCC AGT ACA TTC T-3′; nt 2292 to 2316; 5′ labeled with TBR) and TBR-CMV-2 (IC-specific probe; 5′-CCC TTT ACA TCT TTC TGA AGT AGG G-3′; 5′ labeled with TBR). No cross-hybridization was observed for IC DNA-specific and CMV DNA-specific probes. TBR-CMV-1 was chosen in a highly conserved area (1, 9, 37).
Criteria for D-PCR.
In the D-PCR a signal of >80 LU (which is equal to four times the mean background signal for either probe) was considered to be positive. In the D-PCR a serum or plasma specimen was considered to be positive for CMV DNA if >80 LU was measured with the CMV probe, regardless of the results for IC DNA (with loads of greater than 50,000 copies of CMV DNA/ml, IC DNA levels were near the background levels in the D-PCR). A specimen was considered negative for CMV DNA if <80 LU was obtained with the CMV probe and, additionally, IC DNA was detected at >80 LU. If neither CMV DNA nor IC DNA was detected (<80 LU), the test result was considered false negative and the result was rejected. At present we have tested over 500 plasma (and whole-blood) specimens, and no false-negative results have been obtained.
In the D-PCR three controls were included in the DNA extraction-one positive control (a reference plasma specimen containing 400 copies of CMV DNA/ml) and two negative controls. The first negative control contained human chromosomal DNA (100 ng) and 35 molecules of IC DNA and served as a control for the entire Q-PCR procedure and as a control for the sensitivity of the procedure. The second negative control contained human chromosomal DNA (100 ng) only and should give negative results with both probes.
Algorithm for quantitation in Q-PCR.
The algorithm assumes ideal circumstances for each of the steps of the procedure and would be valid if (i) recovery of CMV DNA and IC DNA from plasma were 100%, (ii) CMV DNA and IC DNA were amplified with equal efficiencies, (iii) CMV DNA and IC DNA amplimers were purified with equal efficiencies, (iv) hybridization with either probe was quantitative, and (v) bead capture and measurement by ECL of the amounts of hybrids were quantitative. In the Q-PCR, DNA was purified from 200 μl of plasma or serum together with 35 molecules of IC DNA, and DNA was eluted in 100 μl of TE buffer. Twenty microliters of DNA was subjected to Q-PCR in the presence of an additional 35 molecules of IC DNA which was present in the PCR master mixture, and the ECL signals obtained after hybridization with TBR-labeled probes were determined as described above. Background signals (mean, 20 LU; CV, 40%) for CMV DNA-specific and IC DNA-specific probes were determined for 23 plasma specimens previously shown to be CMV negative. After correction for the background, the ratio CMV DNA-specific signal/IC DNA-specific signal (R) was calculated, and the number of copies of CMV DNA per milliliter of plasma was calculated by amplifying R by a factor 1,050. This factor was reached from two separate sets of factors, (7 + 35) × 25. The factor (7 + 35) represents the number of IC DNA molecules present during the PCR (sum of the numbers of coextracted and added IC DNA molecules, respectively) and the factor 25 is required to reach the copy number per milliliter of plasma. Thus, a clear-cut algorithm, number of copies CMV per milliliter = R × 1,050, is used throughout this paper for all Q-PCR experiments.
RESULTS
Amplimer detection by TBR-labeled probes.
Comparison of ethidium bromide staining and ECL detection of the amplimers showed a lower level of detection of about 5 × 109 molecules (3,000 pg) and 3 × 108 molecules (200 pg), respectively. Thus, for an amplimer of 578 bp, ECL detection was about 15-fold more sensitive than ethidium bromide staining. When a constant amount of bead-hybrid complex was used as input for ECL measurement, the CV for the ECL signals was 2%. When a constant amount of PCR product was used as input for primer removal followed by hybridization, bead binding, and ECL measurement, the CV for the ECL signals was 5%.
Recovery of DNA from plasma and lower limit of detection.
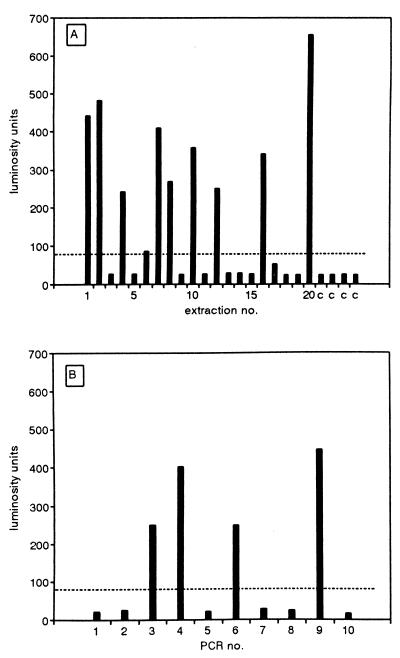
The silica-GuSCN procedure (5) has been widely used for the isolation of DNA from clinical specimens, but as yet, no information about DNA yields with very low inputs is available. To study the recovery of DNA from plasma with very low input DNA concentrations, 200-μl aliquots of reference plasma were supplemented with 4 molecules of IC DNA, DNA was extracted from 20 of such aliquots, and one-quarter of each of the extracted DNAs was used for the PCR. With a 100% extraction efficiency, this would result in the presence of a single molecule of IC DNA per PCR. In such cases Poisson statistics predict that 63% of the reactions will be positive (12). In Fig. 1A it is shown that 50% of the PCRs were positive, suggesting that the rate of recovery of DNA with this extremely low input was near 100%. Control extractions were all negative (Fig. 1A). As a control for the concentration of the IC DNA preparation used to prepare the IC DNA and plasma mixtures, the same IC DNA preparation was used directly in 10 PCRs at an input expected to contain a mean of 1 molecule of IC DNA per PCR. In this case the PCR was positive for 40% of the reactions, confirming the IC DNA concentration (Fig. 1B). These data indicate that even with extremely low DNA inputs, DNA was recovered at very high (near 100%) yields.
FIG. 1.
Recovery of IC DNA from plasma at the single-molecule level. (A) Reference plasma was supplemented with IC DNA to a concentration of 4 molecules per 200 μl of plasma. DNA was extracted from 20 200-μl aliquots, and one-quarter of the extracted DNA was used in the PCR (bars 1 to 20). DNA was also extracted from reference plasma only, which served as a negative control (bars c). The amount of amplimers was determined and expressed in LU. The cutoff level is indicated with a broken line. (B) In the same experiment IC DNA was used directly in 10 separate PCRs at an input expected to contain a mean of 1 molecule of IC DNA per PCR (bars 1 to 10). The amount of amplimers was determined by ECL and expressed in LU. The cutoff level is indicated with a broken line.
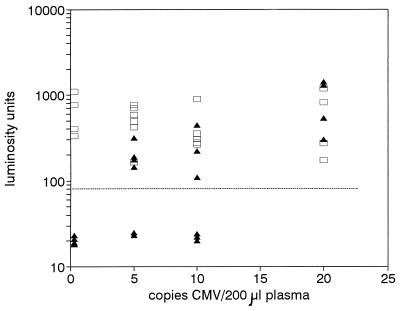
To study the lower limit of detection of CMV DNA in plasma, DNA was extracted from reference plasma samples supplemented with CMV particles to a concentration of 100 CMV particles/ml of plasma and serial twofold dilutions thereof. Figure 2 shows that with extraction inputs as low as 5 viruses per 200 μl of plasma (resulting in a mean of 1 copy of CMV DNA in the PCR with 100% extraction efficiency), the assay was still positive for CMV DNA in four of six reactions, which is in accord with the number of expected positive reactions with 100% extraction efficiency according to the Poisson distribution (12). The negative results were truly negative since the ECL signals for coextracted IC DNA were all positive. Together these data suggest that CMV DNA was recovered at high yields even at viral loads as low as 25 copies of CMV/ml of plasma.
FIG. 2.
Lower limit of detection of CMV DNA in plasma. DNA was purified from 200-μl reference plasma samples containing 20, 10, 5, or 0 CMV particles; 35 molecules of IC DNA were coextracted. Extractions were done four times for the specimens containing 20 and 0 particles and six times for specimens containing 10 and 5 particles. One-fifth of the extracted DNA was subjected to PCR, and the amount of CMV amplimers (filled triangles) and IC amplimers (open squares) was determined by ECL and expressed in LU. The cutoff level is indicated with a broken line.
Diagnostic assay.
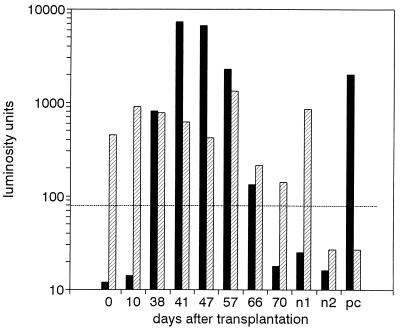
In the D-PCR, coextraction of IC DNA (35 molecules) with serum or plasma DNA served as a control for the entire diagnostic procedure, including a control for sensitivity. Figure 3 shows an example of the results of the diagnostic assay in which eight consecutive serum specimens from a renal transplant patient (CMV seronegative with a seropositive kidney donor) were tested for the presence of CMV DNA. Initial serum specimens (obtained on days 0 and 10 after transplantation) were CMV DNA negative, followed by a period in which CMV DNA was detected (days 38 to 66 after transplantation). During this period, seroconversion to CMV positivity was observed, and the patient presented with hepatitis and fever and was treated with ganciclovir. In the last specimen, obtained 70 days after transplantation, CMV DNA was no longer detected.
FIG. 3.
Diagnostic PCR. Sequential serum specimens from a renal transplant patient were subjected to D-PCR. Two negative controls (nl, human DNA + 35 molecules IC DNA; n2, human DNA only) and one positive control (pc, reference plasma seeded with CMV to 400 particles per milliliter) were included. Filled bars, CMV probe; hatched bars, IC probe. The amount of amplimers was expressed in LU. The cutoff level is indicated with a broken line.
Quantitative assay.
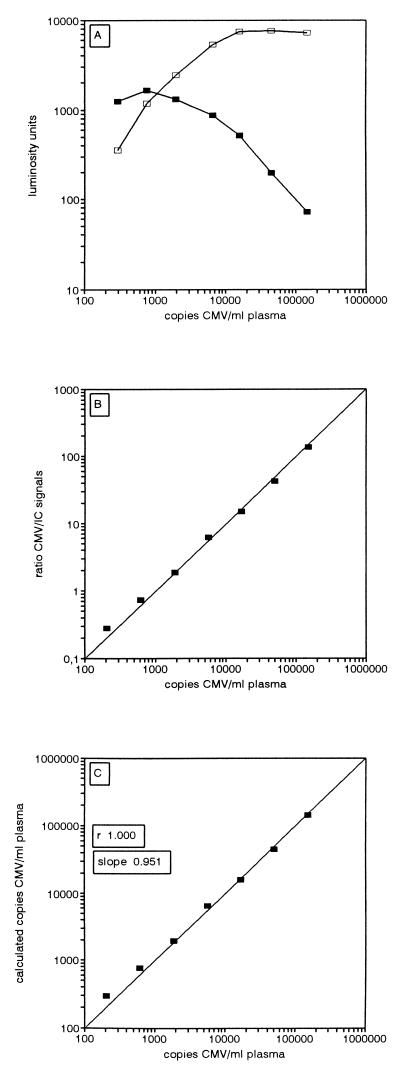
Reference plasma was seeded with CMV to 150,000 copies/ml, and serial threefold dilutions were subjected to Q-PCR. Figure 4A shows the ECL signals obtained by Q-PCR after hybridization. With increasing virus load the amount of CMV amplimers increased until a plateau was reached. This plateau was a reflection of the amount present at the plateau phase of the PCR (data not shown). With low CMV concentrations, IC DNA signals were unaffected by increasing viral loads; with higher viral loads the IC DNA signals decreased. This decrease was due to the fact that the PCR reached the plateau earlier with higher viral loads. When this plateau is reached during PCR, IC DNA amplification also enters a plateau phase at a level determined by the viral load. Thus, the initial ratio (CMV DNA/IC DNA) present at the start of the PCR will be maintained throughout the PCR and the plateau phase. If the CMV DNA and IC DNA amplimers were accurately quantitated in the next steps of the procedure, the CMV DNA/IC DNA ratio after background correction (R) would be a straight line with a slope corresponding to the result for a serial threefold dilution. Figure 4B shows that the ratios obtained for this plasma dilution series were in accordance with the expected ratios. R was amplified by a constant (see Materials and Methods for the algorithm) to calculate the viral load, expressed as number of CMV copies per milliliter (Fig. 4C). The expected and calculated values were in good accordance for viral loads of from 206 to 150,000 copies of CMV DNA/ml.
FIG. 4.
Quantitative PCR. Reference plasma was supplemented with CMV particles to a concentration of 150,000 CMV copies/ml, serial threefold dilutions were made in the same plasma, and Q-PCR was done for this dilution series. (A) ECL signals (LU) obtained with the CMV DNA probe (open squares) and the IC DNA probe (filled squares). (B) Values of the CMV DNA/IC DNA ratios after background correction (R). (C) CMV loads (number of copies of CMV per milliliter of plasma) were calculated by amplifying R by a constant factor (1,050). Negative controls (reference plasma) were included; the calculated loads for these controls varied between 0 and −2 copies/ml. The correlation coefficient (r) and slope were obtained by least-squares linear regression analysis.
Variation.
Interassay variation of the Q-PCR was determined by repeating the experiment described above another six times. To determine the intra-assay variation, each plasma dilution was tested eight times by the Q-PCR. Negative controls (reference plasma) were included in each run. The data, summarized in Table 1, indicate that the interassay variation and the intra-assay variation were similar (mean CV, 25%).
TABLE 1.
Inter- and intra-assay variationsa
| No. of CMV particles/ml of plasma | Variation (mean no. of copies of CMV DNA/ml [% CV])
|
|
|---|---|---|
| Interassay | Intra-assay | |
| 150,000 | 151,908 (18) | 124,791 (36) |
| 50,000 | 51,346 (29) | 48,258 (39) |
| 16,665 | 18,213 (28) | 15,423 (27) |
| 5,556 | 6,720 (14) | 8,128 (8) |
| 1,851 | 3,231 (38) | 2,790 (24) |
| 617 | 940 (16) | 884 (19) |
| 206 | 336 (29) | 308 (22) |
Human reference plasma was supplemented with CMV to 150,000 copies/ml, and serial threefold dilutions were made in the same plasma. Q-PCR was done for these specimens to determine interassay (n = 7) and intra-assay (n = 8) variations.
To study interspecimen variation, 10 serum specimens from different CMV-seronegative subjects (including one renal transplant patient and nine chronic fatigue syndrome patients) were seeded with CMV to 15,000 and 1,667 CMV particles/ml or were left unseeded. The unseeded specimens were tested by D-PCR and were found to be negative for CMV DNA (data not shown). For the 10 serum specimens containing 15,000 CMV particles/ml, a mean value of 15,065 copies of CMV DNA/ml was calculated, with a CV of 25%. A similar CV (23%) was obtained for the 10 serum specimens containing 1,667 CMV particles/ml, with a calculated mean of 2,363 copies of CMV DNA/ml. Since this variation was the same as that obtained for the inter- and intra-assay variations, it was concluded that the Q-PCR reliably measures viral loads, independent of the serum donor. Results similar to those described above were obtained when plasma specimens rather than serum specimens were tested by Q-PCR.
To study the contribution of variations in DNA extraction efficiency to the overall variation of the Q-PCR, the DNA extracts from the 10 serum specimens supplemented with CMV to 1,667 particles/ml mentioned above were pooled and the viral load was determined 10 times. The mean calculated load was 2,527 copies of CMV DNA/ml, with a CV of 16%. These data suggest that variation in DNA extraction efficiency contributed only 7% to the total observed variation of 23%, as calculated for the separate extractions followed by Q-PCR.
Quantitation of CMV in clinical specimens by Q-PCR.
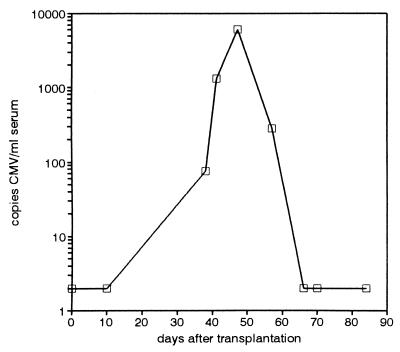
The serum specimens from the renal transplant patient analyzed by D-PCR (Fig. 3) as described above were also tested by Q-PCR. Figure 5 shows that after an initial CMV-negative phase, a viral load of 75 copies/ml was observed at day 38, and this load increased rapidly to a level of 1,275 copies/ml at day 41 and further increased to 6,025 copies/ml at day 47 after transplantation. At this time, the patient presented with fever and elevated liver enzyme levels and seroconverted (for both CMV IgG and CMV IgM). Ganciclovir therapy was started at day 47. The serum CMV DNA level decreased, and CMV was undetectable at day 66. CMV culture was first positive for buffy coat cells obtained at day 38 (after 24 days of culture) and remained negative thereafter.
FIG. 5.
Quantitation of CMV load by Q-PCR. The sequential serum specimens from the renal transplant patient described in Fig. 3 were subjected to Q-PCR. Points for specimens found to be negative were arbitrarily plotted as 2 copies of CMV/ml for graphical representation.
Q-PCR reliably quantifies CMV loads in serum and plasma irrespective of anticoagulant.
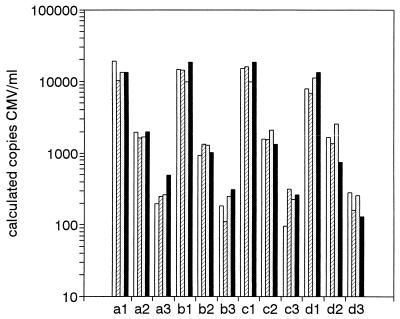
To establish whether plasma specimens with different anticoagulants (heparin, EDTA, or citrate) and serum were equivalent substrates for Q-PCR, serum and plasma specimens from four healthy subjects were seeded with constant amounts of CMV (to 15,000, 1,000, and 185 CMV particles/ml) and were subjected to Q-PCR. Unseeded specimens were negative for CMV DNA. The data presented in Fig. 6 indicate that the viral loads determined by Q-PCR were not significantly different for serum and plasma (repeated measures analysis of variance, P = 0.65, P = 0.21, and P = 0.45 for 15,000, 1,000, and 185 CMV particles/ml, respectively). These data indicate that CMV loads in serum and plasma specimens can be reliably determined by Q-PCR, irrespective of the anticoagulant that is used.
FIG. 6.
Serum and plasma specimens are equivalent substrates for Q-PCR. Serum, plasma containing heparin, plasma containing EDTA, and plasma containing citrate were tested. The plasma was obtained from each of four healthy volunteers (subjects a, b, c, and d; subject a, CMV seronegative; subjects b to d, CMV seropositive) and was seeded with CMV to 15,000 particles/ml (a1, b1, c1, and d1), 1,000 particles/ml (a2, b2, c2, and d2), and 185 particles/ml (a3, b3, c3, and d3), and the CMV loads were determined by Q-PCR. Open bars, plasma containing heparin; hatched bars, plasma containing citrate; stippled bars, plasma containing EDTA; filled bars, serum.
In clinical specimens serum CMV loads are frequently significantly lower than plasma CMV loads.
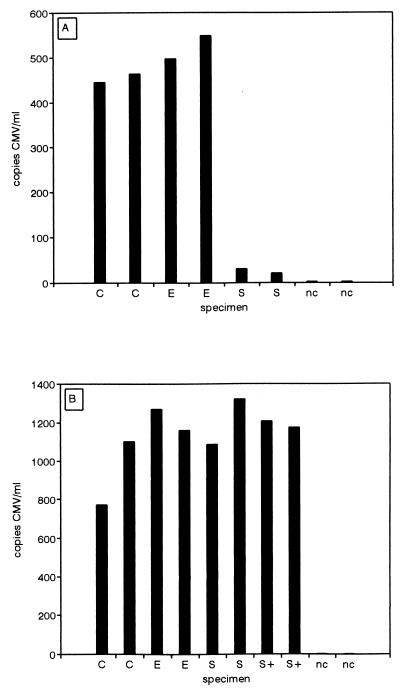
When paired serum and plasma specimens from CMV-infected patients were tested by Q-PCR, it was observed that serum DNA levels were often significantly lower than plasma DNA levels (Table 2). An example is shown in Fig. 7A in which CMV loads in plasma containing citrate, plasma containing EDTA, and serum were determined in duplicate by Q-PCR. The mean serum CMV load was 25 copies/ml, whereas the mean plasma CMV load was 489 copies/ml. To rule out the possibility that this particular patient serum specimen was not a proper substrate for Q-PCR, CMV was added to 750 particles/ml. As a control, plasma and serum were obtained from a healthy, CMV DNA-negative volunteer and plasma containing citrate, plasma containing EDTA, and serum were also seeded with CMV to the same level. Q-PCR showed that similar loads were obtained (Fig. 7B), suggesting that the observed differences between loads in patient serum and plasma specimens were not an artifact of the Q-PCR procedure.
TABLE 2.
Comparison of CMV DNA loads in patient serum and plasma specimensa
| Subject | No. of copies of CMV DNA/ml
|
|||
|---|---|---|---|---|
| Plasma with heparin | Plasma with citrate | Plasma with EDTA | Serum | |
| A (RTX) | NAb | 446 | 497 | 31 |
| NA | 463 | 549 | 20 | |
| B (RTX) | 57 | 160 | 159 | Negc |
| 59 | 146 | 137 | Neg | |
| C (RTX) | NA | 351 | 689 | Neg |
| NA | 802 | 668 | 58 | |
| D (ABMT) | ||||
| Day 49 | NA | NA | 287 | 166 |
| Day 52 | NA | NA | 631 | 387 |
| Day 54 | NA | NA | 3,872 | 1,121 |
| E (AIDS) | 5,518 | 2,313 | 10,649 | 809 |
| 7,095 | 6,774 | 9,644 | 580 | |
| F (RTX) | NA | NA | 300 | Neg |
| G (RTX) | ||||
| Day 32 | NA | NA | 1,715 | 75 |
| Day 41 | NA | NA | 1,636 | 83 |
Q-PCR CMV loads in paired serum and plasma specimens from five renal transplant patients (RTX), one AIDS patient, and one allogeneic bone marrow transplantation (ABMT) patient; days after transplantation are given where applicable. Data for subjects A, B, C, and E were obtained from duplicate Q-PCR assays.
NA, not available.
Neg, negative.
FIG. 7.
CMV loads observed in patient serum may be significantly lower than those observed in plasma. (A) Plasma containing citrate (C), plasma containing EDTA (E), and serum (S) obtained in parallel from the same patient (subject A in Table 2) were subjected to Q-PCR (in duplicate). (B) Plasma containing citrate (C), plasma containing EDTA (E), and serum (S) obtained from a CMV-negative healthy volunteer and the serum from the patient (S+) were seeded to 750 CMV particles/ml, and the CMV loads were determined by Q-PCR. nc, negative controls (reference plasma).
DISCUSSION
In the study described in this paper we evaluated a highly sensitive diagnostic assay (D-PCR) and quantitative assay (Q-PCR) for the detection of CMV in plasma and serum. A total of 20 clinical specimens and four controls could be examined (either by D-PCR or by Q-PCR) within an 8-h workday. Costs per specimen for materials were about $US10, and the hands-on time was about 5 h.
We have described a highly sensitive diagnostic assay (D-PCR) for the detection of CMV in serum and plasma in which 35 molecules of IC DNA mimicking the CMV target were included in the DNA extraction and all subsequent steps of the procedure and in which both the DNA extraction efficiency and PCR efficiency were monitored, thus excluding false-negative reactions. The PCR was performed in a nonnested format and a single primer pair was used for amplification of CMV and IC DNA. The uracil-N-glycosylase system (Perkin-Elmer) was used to prevent false-positive results due to amplimer carryover. High sensitivity and specificity were reached by solution hybridization with nonradioactive TBR-labeled probes. The amount of hybrids was subsequently determined by ECL in the Perkin-Elmer System 5000. For a CMV-positive specimen, this gave rise to a mixture of IC DNA and CMV DNA amplimers with identical lengths (587 bp) and GC contents. CMV DNA and IC DNA amplimers were discriminated by hybridization with TBR-labeled probes followed by ECL. The use of TBR-labeled probes and detection by ECL was about 15-fold more sensitive than ethidium bromide staining of agarose gels, with a lower limit of detection of about 3 × 108 molecules (200 pg) of amplimer, in accord with the results of previous investigations (24, 40). The lower limit of sensitivity of the D-PCR assay was about 25 CMV DNA copies/ml of plasma or serum. The presence of 35 molecules of IC DNA during extraction from 200 μl of plasma or serum allowed us to draw the conclusion that a specimen found to be negative for CMV DNA but positive for IC DNA would contain less than 175 copies of CMV DNA/ml of plasma or serum.
We have previously reported on a procedure for the purification of nucleic acids from clinical specimens (Silica-GuSCN procedure [5]). This procedure has been used for the extraction of CMV DNA from clinical specimens (2, 18, 19, 25, 42), but at present no published information concerning DNA extraction efficiency with very low DNA concentrations is available. The experiments described here, in which DNA extractions were done with a recently described lysis buffer containing alpha-casein (6), suggest that even with extremely low DNA inputs (4 molecules of IC DNA present in 200 μl of plasma), DNA yields were near 100%. Similar high yields were obtained with a linearized 10-kb plasmid (pES [4]) containing the CMV immediate-early region (data not shown).
The virus concentrations mentioned in this paper were established on the basis of the assumption that the CMV DNA extraction efficiency was also 100% (see Materials and Methods). Since this high extraction efficiency could be validated only with standards with known amounts of relatively small (linearized) plasmids, it would still be possible that CMV DNA (which is large [about 250 kb]) was isolated at a lower efficiency. Therefore, the Q-PCR assay was also used to determine the number of copies of the CMV immediate-early genes which are stably integrated into the chromosomal DNA of Rat-9G cells (4). Copy numbers previously determined by Southern blot analysis (4) and the values determined by Q-PCR (about 15 copies of the immediate-early gene/cell) were in good accordance, suggesting that high-molecular-weight DNA was also isolated at a very high efficiency (data not shown). The high DNA extraction efficiency in combination with a sensitive PCR and sensitive detection of amplimers by ECL resulted in a highly sensitive diagnostic assay which was still positive for CMV in four of six reactions with viral loads as low as 25 copies of CMV DNA/ml of plasma.
DNA extracted from clinical specimens that were CMV positive by the D-PCR was used for quantitation by Q-PCR. In the Q-PCR an additional constant amount of IC DNA (35 molecules) was present during PCR. This additional IC DNA served to dampen the variation due to the Poisson distribution and to extend the dynamic range of the Q-PCR. After PCR the viral load was calculated from the ratio of the CMV DNA over the IC DNA ECL signals by the most straightforward algorithm possible. That algorithm assumed ideal circumstances for each of the steps of the Q-PCR. Under these conditions this ratio would be a direct reflection of the amount of CMV present in the clinical specimen. The data indicate that the expected and calculated CMV loads were in good accordance. The mean interassay, intra-assay, and interspecimen variations of the Q-PCR were about 25%, suggesting that the Q-PCR will reliably detect fourfold differences in viral load in serum and plasma specimens in the range of 100 to 150,000 copies of CMV DNA/ml.
The contribution of the variation in DNA yield to the total observed variation in the Q-PCR was shown to be relatively small (7% of a total variation of 23%) for serum specimens obtained from different patients. When the contribution of the variation in DNA yield to the total observed variation in the Q-PCR was determined with a single serum specimen, only 2% of a total variation of 18% could be ascribed to a variation in extraction efficiency.
Since the D-PCR is internally controlled by a very small number of IC DNA molecules, quantitative information could also be obtained from D-PCR data by an algorithm, number of copies of CMV per milliliter = R × 175, which was based on the same criteria used for Q-PCR. Although quantitative data obtained by either D-PCR or Q-PCR were similar in the lower range (100 to 50,000 copies of CMV DNA/ml), the observed variation did not allow for reliable measurement of fourfold differences in viral load. Preliminary experiments have suggested that the Q-PCR also performed well when whole blood (50 μl) and cerebrospinal fluid (200 μl) were used for DNA extraction.
In reconstruction experiments in which a known amount of virus was added to the specimen, it was found that the Q-PCR procedure performed equally well for serum and plasma specimens, regardless of the anticoagulant used (citrate, heparin, or EDTA). In clinical specimens, serum CMV loads were often found to be significantly lower (10-fold or more) than plasma CMV loads. These data are in contrast to those of Patel et al. (29), who described similar levels of CMV DNA in serum and EDTA-anticoagulated plasma in liver transplant recipients. At present we have no explanation for this discrepancy, which may be related to different procedures for the handling of clinical specimens, differences in patient populations, and differences in quantitation procedures. The clinical significance of this observation, if any, remains to be determined.
ADDENDUM
During the review process it appeared that Perkin-Elmer no longer supplied the QPCR System 5000. In the United States, assay buffer (to which sodium azide should be added to 0.05%), cell cleaner, and equipment are now available from the original manufacturer (IGEN International, Inc., 16020 Industrial Dr., Gaithersburg, MD 20877; in Europe, they are now available from Biozym Nederland B.V., Landgraaf, The Netherlands). TBR-labeled probes and streptavidin-coated magnetic beads (4.5-μm streptavidin Dynabeads suspended in assay buffer) can still be obtained from Perkin-Elmer.
ACKNOWLEDGMENTS
We thank Ans van Strien, Fokla Zorgdrager, John Dekker, Wim van Est, Alex van Breda, Joke Spaargaren, Pien Defoer, Spencer Valli, and the members of the Clinical Virology section for their contributions to this study.
REFERENCES
- 1.Akrigg A, Wilkinson G W G, Oram J D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985;2:107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- 2.Allen R D, Pellett P E, Stewart J A, Koopmans M. Nonradioactive PCR–enzyme-linked immunosorbent assay method for detection of human cytomegalovirus DNA. J Clin Microbiol. 1995;33:725–728. doi: 10.1128/jcm.33.3.725-728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspin M A, Gallez-Hawkins G M, Giugni T D, Tegtmeier B, Lang D J, Schmidt G M, Forman S J, Zaia J. Comparison of plasma PCR and bronchoalveolar lavage fluid culture for detection of cytomegalovirus infection in adult bone marrow transplant recipients. J Clin Microbiol. 1994;32:2266–2269. doi: 10.1128/jcm.32.9.2266-2269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom R, Geelen J L, Sol C J, Raap A K, Minnaar R P, Klaver B P, van der Noordaa J. Establishment of a rat cell line inducible for the expression of human cytomegalovirus immediate-early gene products by protein synthesis inhibition. J Virol. 1986;58:851–859. doi: 10.1128/jvi.58.3.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom R, Sol C, Beld M, Weel J, Goudsmit J, Wertheim-van Dillen P. Improved silica-guanidiniumthiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J Clin Microbiol. 1999;37:615–619. doi: 10.1128/jcm.37.3.615-619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brytting M, Xu W, Wahren B, Sundqvist V. Cytomegalovirus DNA detection in sera from patients with active cytomegalovirus infections. J Clin Microbiol. 1992;30:1937–1941. doi: 10.1128/jcm.30.8.1937-1941.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan A, Zhao J, Krajden M. Polymerase chain reaction kinetics when using a positive internal control target to quantitatively detect cytomegalovirus target sequences. J Virol Methods. 1994;48:223–236. doi: 10.1016/0166-0934(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 9.Chou S. Effect of interstrain variation on diagnostic DNA amplification of the cytomegalovirus major immediate-early region. J Clin Microbiol. 1992;30:2307–2310. doi: 10.1128/jcm.30.9.2307-2310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross N C P. Quantitative PCR techniques and applications. Br J Haematol. 1995;89:693–697. [PubMed] [Google Scholar]
- 11.Cunningham R, Harris A, Frankton A, Irving W. Detection of cytomegalovirus using PCR in serum from renal transplant recipients. Clin Pathol. 1995;48:575–577. doi: 10.1136/jcp.48.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaco R. Practical considerations for the design of quantitative PCR assays. In: Innis M A, Gelfand D H, Sninsky J J, editors. PCR strategies. New York, N.Y: Academic Press, Inc.; 1995. pp. 84–108. [Google Scholar]
- 13.Dickover R E, Donovan R M, Goldstein E, Dandekar S, Bush C E, Carlson J R. Quantitation of human immunodeficiency virus DNA by using the polymerase chain reaction. J Clin Microbiol. 1990;28:2130–2133. doi: 10.1128/jcm.28.9.2130-2133.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodt K K, Jacobsen P H, Hofmann B, Meyer C, Kolmos H J, Skinhoj P, Norrild B, Mathiesen L. Development of cytomegalovirus (CMV) disease may be predicted in HIV-infected patients by CMV polymerase chain reaction and the antigenemia test. AIDS. 1997;11:F21–F28. doi: 10.1097/00002030-199703110-00001. [DOI] [PubMed] [Google Scholar]
- 15.Drouet E, Michelson S, Denoyel G, Colimon R. Polymerase chain reaction detection of human cytomegalovirus in over 2000 blood specimens correlated with virus isolation and related to urinary virus excretion. J Vir Methods. 1993;45:259–276. doi: 10.1016/0166-0934(93)90112-5. [DOI] [PubMed] [Google Scholar]
- 16.Freymuth F, Gennetay E, Petitjean J, Eugene G, Hurault de Ligny B, Ryckelynck J-P, Legoff C, Hazera P, Bazin C. Comparison of nested PCR for detection of DNA in plasma with pp65 leukocytic antigenemia procedure for diagnosis of human cytomegalovirus infection. J Clin Microbiol. 1994;32:1614–1618. doi: 10.1128/jcm.32.6.1614-1618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallez-Hawkins G M, Tegtmeier B R, ter Veer A, Niland J C, Forman S J, Zaia J C. Evaluation of a quantitative plasma PCR plate assay for detecting cytomegalovirus infections in marrow transplant recipients. J Clin Microbiol. 1997;35:788–790. doi: 10.1128/jcm.35.3.788-790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerna G, Baldanti F, Sarasini A, Furione M, Percivalle E, Revello M G, Zipeto D, Zella D the Italian Foscarnet Study Group. Effect of foscarnet induction treatment on quantitation of human cytomegalovirus (HCMV) DNA in peripheral blood polymorphonuclear leukocytes and aqueous humor of AIDS patients with HCMV retinitis. Antimicrob Agents Chemother. 1994;38:38–44. doi: 10.1128/aac.38.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerna G, Furione M, Baldanti F, Sarasini A. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J Clin Microbiol. 1994;32:2709–2717. doi: 10.1128/jcm.32.11.2709-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen K K, Ricksten A, Hofmann B, Norrild B, Olofsson S, Mathiesen L. Detection of cytomegalovirus DNA in serum correlates with clinical cytomegalovirus retinitis in AIDS. J Infect Dis. 1994;170:1271–1274. doi: 10.1093/infdis/170.5.1271. [DOI] [PubMed] [Google Scholar]
- 21.Hebart H, Muller C, Loffler J, Jahn G, Einsele H. Monitoring of CMV infection: a comparison of PCR from whole blood, plasma-PCR, pp65-antigenemia and virus culture in patients after bone marrow transplantation. Bone Marrow Transplant. 1996;17:861–868. [PubMed] [Google Scholar]
- 22.Ish-Horowicz D, Burke J F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981;9:2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishigaki S, Takeda M, Kura T, Ban N, Satoi T, Sakamaki S, Watanabe N, Kogho Y, Nitsu Y. Cytomegalovirus DNA in the sera of patients with cytomegalovirus pneumonia. Br J Haematol. 1991;79:198–204. doi: 10.1111/j.1365-2141.1991.tb04522.x. [DOI] [PubMed] [Google Scholar]
- 24.Kenten J H, Gudibande S, Link J, Willey J J, Curfman B, Major E O, Massey R J. Improved electrochemiluminescent label for DNA probe assays: rapid quantitative assays of HIV-1 polymerase chain reaction products. Clin Chem. 1992;38:873–879. [PubMed] [Google Scholar]
- 25.Laue T, Mertenskötter T, Grewing T, Degen O, van Lunzen J, Dietrich M, Schmitz H. Clinical significance of qualitative human cytomegalovirus (HCMV) detection in cell-free serum samples in HIV-infected patients at risk for HCMV disease. AIDS. 1997;11:1195–1196. doi: 10.1097/00002030-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Nelson C T, Istas A S, Wilkerson M K, Demmler G J. PCR detection of cytomegalovirus DNA in serum as a diagnostic test for congenital cytomegalovirus infection. J Clin Microbiol. 1995;33:3317–3318. doi: 10.1128/jcm.33.12.3317-3318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigro G, Krzysztofiak A, Gattinara G C, Mango T, Mazzocco M, Porcaro M A, Provvedi S, Booth J C. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS. 1996;10:1127–1133. [PubMed] [Google Scholar]
- 28.Nolte F S, Emmens R K, Thurmond C, Mitchell P S, Pascuzzi C, Devine S M, Saral R, Wingard J R. Early detection of human cytomegalovirus viremia in bone marrow transplant recipients by DNA amplification. J Clin Microbiol. 1995;33:1263–1266. doi: 10.1128/jcm.33.5.1263-1266.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel R, Smith T F, Espy M, Wiesner R H, Krom R A F, Portela D, Paya C V. Detection of cytomegalovirus DNA in sera of liver transplant recipients. J Clin Microbiol. 1994;32:1431–1434. doi: 10.1128/jcm.32.6.1431-1434.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel R, Smith T F, Espy M, Portela D, Wiesner R H, Krom R A F, Paya C V. A prospective comparison of molecular diagnostic techniques for the early detection of cytomegalovirus in liver transplant recipients. J Infect Dis. 1995;171:1010–1014. doi: 10.1093/infdis/171.4.1010. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen L, Morris S, Zipeto D, Fessel J, Wolitz R, Dowling A, Merigan T C. Quantitation of human cytomegalovirus DNA from peripheral blood cells of human immunodeficiency virus-infected patients could predict cytomegalovirus retinitis. J Infect Dis. 1995;171:177–182. doi: 10.1093/infdis/171.1.177. [DOI] [PubMed] [Google Scholar]
- 32.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C A, Oettle H, Peng R, Neuhaus P, Blumhardt G, Lohmann R, Wilborn F, Osthoff K, Oertel J, Timm H, Siegert W. Comparison of polymerase chain reaction from plasma and buffy coat with antigen detection and occurrence of immunoglobulin M for the demonstration of cytomegalovirus infection after liver transplantation. Transplantation. 1995;59:1133–1138. [PubMed] [Google Scholar]
- 34.Shinkai M, Bozette S A, Powderly W, Frame P, Spector S A. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of AIDS patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997;175:302–308. doi: 10.1093/infdis/175.2.302. [DOI] [PubMed] [Google Scholar]
- 35.Smith I L, Shinkai M, Freeman W R, Spector S A. Polyradiculopathy associated with ganciclovir-resistant cytomegalovirus in an AIDS patient: phenotypic and genotypic characterization of sequential virus isolates. J Infect Dis. 1996;173:1481–1484. doi: 10.1093/infdis/173.6.1481. [DOI] [PubMed] [Google Scholar]
- 36.Spector S A, Merrill R, Wolf D, Dankner W M. Detection of human cytomegalovirus in plasma of AIDS patients during acute visceral disease by DNA amplification. J Clin Microbiol. 1992;30:2359–2365. doi: 10.1128/jcm.30.9.2359-2365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenberg R M, Thomsen D R, Stinski M F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984;49:190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokimatsu I, Tashiro T, Nasu M. Early diagnosis and monitoring of human cytomegalovirus pneumonia in patients with adult T-cell leukemia by DNA amplification in serum. Chest. 1995;107:1024–1027. doi: 10.1378/chest.107.4.1024. [DOI] [PubMed] [Google Scholar]
- 39.Wolf D G, Spector S A. Early diagnosis of human cytomegalovirus disease in transplant recipients by DNA amplification in plasma. Transplantation. 1993;56:330–334. doi: 10.1097/00007890-199308000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Bruno J G, Cheng T, Calomiris J J, Goode M T, Gatto-Menking D L. A comparative study of PCR product detection and quantitation by electrochemiluminescence and fluorescence. J Biolumin Chemilumin. 1995;10:239–245. doi: 10.1002/bio.1170100407. [DOI] [PubMed] [Google Scholar]
- 41.Zaia J A, Gallez-Hawkins G M, Tegtmeier B R, ter Veer A, Li X, Niland J C, Forman S J. Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J Infect Dis. 1997;176:782–785. doi: 10.1086/517301. [DOI] [PubMed] [Google Scholar]
- 42.Zipeto D, Baldanti F, Zella D, Furione M, Cavicchini A, Milanesi G, Gerna G. Quantification of human cytomegalovirus DNA in peripheral blood polymorphonuclear leukocytes of immunocompromised patients by the polymerase chain reaction. J Virol Methods. 1993;44:45–56. doi: 10.1016/0166-0934(93)90006-d. [DOI] [PubMed] [Google Scholar]
- 43.Zipeto D, Morris S, Hong C, Downing A, Wolitz R, Merigan T C, Rasmussen L. Human cytomegalovirus (CMV) DNA in plasma reflects quantity of CMV DNA present in leukocytes. J Clin Microbiol. 1995;33:2607–2611. doi: 10.1128/jcm.33.10.2607-2611.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]