Abstract
Objectives
To identify predictors of poor prognosis in previously healthy young individuals admitted to hospital with coronavirus disease 2019 (COVID-19).
Methods
We studied a cohort of patients hospitalized with COVID-19. All patients without co-morbidities, without usual treatments and ≤65 years old were selected from an international registry (HOPE-COVID-19, NCT04334291). We focused on baseline variables—symptoms and signs at admission—to analyse risk factors for poor prognosis. The primary end point was a composite of major adverse clinical events during hospitalization including mortality, mechanical ventilation, high-flow nasal oxygen therapy, prone, sepsis, systemic inflammatory response syndrome and embolic events.
Results
Overall, 773 healthy young patients were included. The primary composite end point was observed in 29% (225/773) and the overall mortality rate was 3.6% (28/773). In the combined event group, 75% (168/225) of patients were men and the mean age was 49 (±11) years, whereas in the non-combined event group, the prevalence of male gender was 43% (238/548) and the mean age was 42 (±13) years (p < 0.001 for both). On admission, respiratory insufficiency and cough were described in 51.4% (114/222) and 76% (170/223) of patients, respectively, in the combined event group, versus 7.9% (42/533) and 56% (302/543) of patients in the other group (p < 0.001 for both). The strongest independent predictor for the combined end point was desaturation (Spo2 <92%) (OR 5.40; 95% CI 3.34–8.75; p < 0.001), followed by tachypnoea (OR 3.17; 95% CI 1.93–5.21; p < 0.001), male gender (OR 3.01; 95% CI 1.96–4.61; p < 0.001) and pulmonary infiltrates on chest X-ray at admission (OR 2.21; 95% CI 1.18–4.16; p 0.014).
Conclusions
Major adverse clinical events were unexpectedly high considering the baseline characteristics of the cohort. Signs of respiratory compromise at admission and male gender, were predictive for poor prognosis among young healthy patients hospitalized with COVID-19.
Keywords: COVID-19, Mortality, Lung disease, Prognosis, Healthy, Young
Introduction
In early 2021, the coronavirus disease-2019 (COVID-19) pandemic had already been responsible for more than 2 million deaths worldwide [1]. It has been observed that severe forms of COVID-19 mainly affect older individuals with multiple co-morbidities [[2], [3], [4], [5], [6], [7]]. According to previous series, the mortality rate in symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections has been observed to range from 4.3% to 30.4%. Most of the previous studies comprised a mixed population including healthy young individuals as well as older people with several chronic diseases. Co-morbidities such as hypertension and cardiovascular, respiratory or kidney diseases, along with older age, have been identified as the main risk factors for poor prognosis and death in COVID-19. Both factors are related with a chronic systemic inflammation state as a consequence of an over-secretion of cytokines and chemokines, which attenuates the immune response [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Nevertheless, some series have also described unexpectedly high rates of complications in healthy young patients. For instance, Liu et al. studied a young cohort with a median age of 47 years, and found complication rates greater than expected: secondary infection (13.2%), shock (5.3%) or requirement of invasive mechanical ventilation (7.9%) [12]. Likewise, Zhang et al. studied a group of adults younger than 50 years, describing complications such as severe pneumonia, encephalitis and Kawasaki-like disease [14].
Some genetic immunological disorders have been suggested as factors playing a role in adverse outcomes in young people [15,16].
However, there is a paucity of data regarding clinical outcomes in younger individuals without co-morbidities.
Our objective was to assess risk factors at presentation for complications in a previously healthy cohort of individuals admitted to hospital with COVID-19, without any apparent risk factor linked to serious outcomes in this condition.
Materials and methods
Study design and population
We conducted a cohort study including consecutive patients hospitalized with confirmed or highly suspected COVID-19. From the entire HOPE-cohort, all patients ≤65 years old and without co-morbidities were selected. We fixed 65 years old as the age cut-off point because in a previous HOPE publication, we found that this age was the best cut-off value for predicting in-hospital mortality (through a Youden index calculation) [17].
Patients receiving chronic therapies or suffering from chronic diseases such as any cardiovascular risk factor (hypertension, dyslipidaemia, diabetes, obesity) or any heart, lung, or neurological disease, were excluded from the analysis.
The patients were included in the HOPE-COVID-19 registry (Health Outcome Predictive Evaluation for COVID-19, NCT04334291), a multicentre international registry with no conflicts of interest, designed as an ambispective cohort [18]. In short, the selected HOPE cohort assessed here came from 31 centres from seven countries: Spain (16 centres), Italy (seven centres), Ecuador (three centres), Germany (two centres), Cuba (one centre), Canada (one centre) and China (one centre). From 1 January 2020 to 31 May 2020, patients discharged from hospital, alive or deceased, with a diagnosis of COVID-19 were included. An on-line anonymized database was provided to be filled in by each participating centre (www.HopeProjectMD.com) [18].
Definitions and study end points
The primary end point was a composite of major adverse clinical events including in-hospital death, any kind of mechanical ventilation (invasive and non-invasive with Bi-level Positive Airways Pressure), high-flow nasal oxygen therapy, the need for a prone position, sepsis, systemic inflammatory response syndrome and embolic events. As all patients were free from any chronic disease or condition, we analysed risk factors for the primary combined end point focusing on symptoms and signs reported at admission. Laboratory results were collected as categorical variables (normal or abnormal values). Further details on definitions can be found in the Supplementary material (online expanded protocol, Appendix S1).
Ethical considerations
This study has been conducted in accordance with the Declaration of Helsinki principles and Good Clinical Practice Guidelines. It has been approved by the Ethics Research Committee from the Hospital Clinico San Carlos (Madrid, Spain) (20/241-E) and the Spanish Agency for Medicines and Health Products classification (EPA-0D).
Statistical analysis
Data are presented as mean (standard deviation) for continuous variables with a normal distribution, median (interquartile range) for continuous variables with a non-normal distribution, and as frequency (%) for categorical variables. Student's t test was used to compare continuous variables and the χ2 test was used to compare categorical variables. Univariate analysis was performed for qualitative variables and reported as OR with 95% CI. Two-sided p values < 0.05 were accepted as statistically significant. Based on the univariate analysis, those significant variables considered clinically relevant, and with a plausible and potential role as confounding factors were selected and entered into the multivariate analysis. A logistic regression model using the enter method was performed.
Statistical analysis was performed using SPSS v. 22.0 (IBM, Armonk, NY, USA) and STATA v. 16.0 (Stata Corp., College Station, TX, USA).
Results
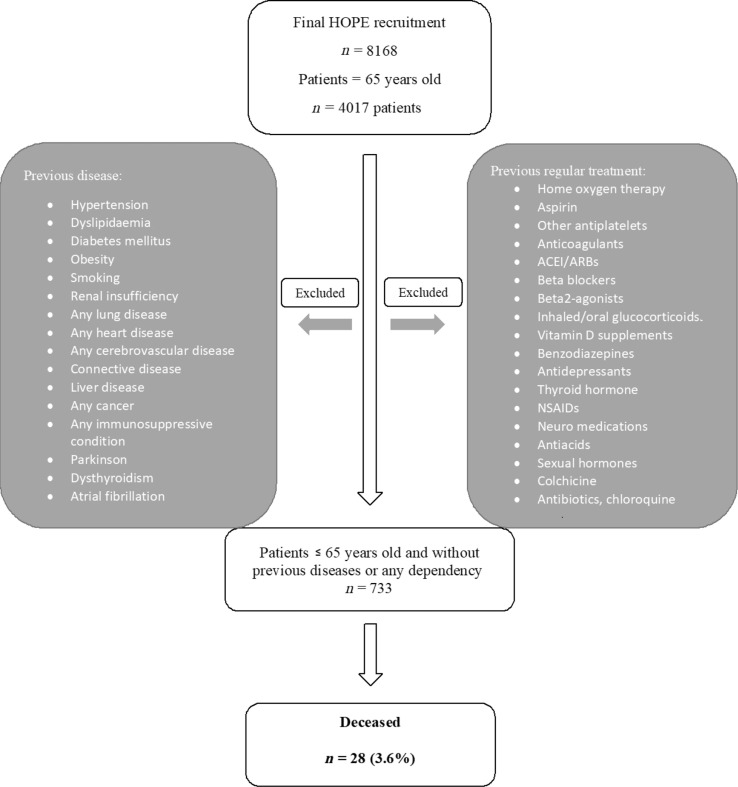
From the entire HOPE-cohort of 8168 patients, 773 healthy young patients were obtained. Fig. 1 shows the flow chart with co-morbidities and therapies excluded.
Fig. 1.
Flowchart of patients included/excluded from the analysis.
The primary composite end point of major adverse events was observed in 29% (225/773) of patients. The mortality rate was 3.6% (28/773) in the overall cohort. The most frequent cause of death was respiratory (19/28).
Patients in the combined event group were older than individuals without the combined end point, with a mean age of 49 years (SD ± 11 years) versus a mean age of 42 years (SD: 13 years old), (p < 0.001).
On admission, the group that presented with the primary combined end point had higher rates of dyspnoea (159/225; 71%) compared with the group without the primary outcome (230/543; 42%), (p < 0.001). In the combined event group, 90% of patients (202/225) presented fever, and cough was described in 76% (170/223) of them, while in the group without the combined outcome fever was present in 68% (371/544) of patients and cough in 56% (302/543) of individuals (p < 0.001 for both symptoms). Twenty-five per cent (56/222) of patients complained of sore throat in the combined event group whereas 16% (86/532) of them described this symptom in the other group, (p 0.004).
Likewise, signs such as tachypnoea or desaturation (oxygen saturation levels <92%) at admission, were significantly more frequent among individuals in the combined event group compared with the non-combined event group (Table 1 ).
Table 1.
Clinical presentation and laboratory tests at admissiona
| Non-combined event group (n = 548) | Combined event group (n = 225) | p value | Or (95% CI) | |
|---|---|---|---|---|
| Male gender | 238/548 (43.4%) | 168/225 (74.7%) | <0.001 | 3.84 (2.72–5.42) |
| Age (y), mean ± SD | 42 ± 13 | 49 ± 11 | <0.001 | |
| A/oligosymptomatic | 110/546 (20.1%) | 9/225 (4%) | <0.001 | 0.17 (0.08–0.33) |
| Dyspnoea | 230/543 (42.4%) | 159/225 (70.7%) | <0.001 | 3.28 (2.35–4.58) |
| Tachypnoea | 44/541 (8.1%) | 94/221 (42.5%) | <0.001 | 8.36 (5.56–12.57) |
| Fatigue | 165/542 (30.4%) | 117/223 (52.5%) | <0.001 | 2.52 (1.83–3.47) |
| Anosmia | 59/532 (11.1%) | 28/221 (12.7%) | 0.537 | 1.16 (0.72–1.88) |
| Dysgeusia | 60/532 (11.3%) | 29/219 (13.2%) | 0.449 | 1.20 (0.75–1.92) |
| Sore throat | 86/532 (16.2%) | 56/222 (25.2%) | 0.004 | 1.75 (1.20–2.56) |
| Fever | 371/544 (68.2%) | 202/225 (89.8%) | <0.001 | 4.10 (2.57–6.54) |
| Cough | 302/543 (55.6%) | 170/223 (76.2%) | <0.001 | 2.56 (1.80–3.64) |
| Vomiting | 38/535 (7.1%) | 19/222 (8.6%) | 0.490 | 1.22 (0.69–2.17) |
| Diarrhoea | 73/536 (13.6%) | 41/222 (18.5%) | 0.089 | 1.44 (0.95–2.19) |
| Myalgia or arthralgia | 157/540 (29.1%) | 95/223 (42.6%) | <0.001 | 1.81 (1.31–2.50) |
| Spo2 <92% | 42/533 (7.9%) | 114/222 (51.4%) | <0.001 | 12.34 (8.19–18.60) |
| Chest X-ray abnormality | 349/515 (67.8%) | 199/214 (93.0%) | <0.001 | 6.31 (3.62–11.00) |
| Hypotension (SBP <90 mmHg) | 15/524 (2.9%) | 20/218 (9.2%) | <0.001 | 3.43 (1.72–6.83) |
| Glasgow coma score <15 | 2/514 (0.4%) | 6/200 (3.0%) | 0.003 | 7.92 (1.58–39.56) |
| Elevated D-dimer | 166/492 (33.7%) | 132/190 (69.5%) | <0.001 | 4.47 (3.12–6.41) |
| Elevated procalcitonin | 25/418 (6.0%) | 49/158 (31.0%) | <0.001 | 7.07 (4.18–11.96) |
| Elevated CRP | 312/529 (59.0%) | 195/216 (90.3%) | <0.001 | 6.46 (3.99–10.46) |
| Elevated transaminases | 120/519 (23.1%) | 111/198 (56.1%) | <0.001 | 4.24 (3.00–6.00) |
| Elevated ferritin | 92/294 (31.3%) | 115/133 (86.5%) | <0.001 | 14.03 (8.06–24.43) |
| Elevated LDH | 211/508 (41.5%) | 154/191 (80.6%) | <0.001 | 5.86 (3.93–8.74) |
| Creatinine >1.5 mg/dL | 40/529 (7.6%) | 22/219 (10%) | 0.262 | 1.37 (0.79–2.36) |
| Lymphocytes <1500 μL | 288/529 (54.4%) | 171/217 (78.8%) | <0.001 | 3.11 (2.15–4.50) |
Abbreviations: CRP, C-reactive protein; LDH, lactate dehydrogenase; OR, odds ratio; SBP, systolic blood pressure; SD, standard deviation; Spo2, oxygen saturation.
Univariate analysis. Risk estimations for the composite end point.
Concerning laboratory findings at admission, C-reactive protein was elevated in 90% (195/216) of patients who presented the combined outcome versus in 59% (312/529) of patients in the non-combined event group (p < 0.001). Other laboratory markers significantly increased in the combined event group were lactate dehydrogenase and D dimer (p < 0.001 for both). Laboratory test results at admission are depicted in Table 1.
At admission, chest X-ray results were available in 729 of 773 patients. Some chest X-ray abnormality, including unilateral or bilateral pulmonary infiltrates, was found in 93% (199/214) of patients in the primary end-point group, versus 68% (349/515) of patients in the other group (p < 0.001).
In contrast with previous findings, the prevalence of neurological and gastrointestinal symptoms at admission such as dysgeusia or anosmia, as well as diarrhoea or vomiting, was not significantly different between the two groups (Table 1).
On multivariable analysis, the following variables were included: age, gender, tachypnoea, sore throat, cough, Spo 2 <92% and any chest radiography abnormality on admission (Table 2 ). We selected these variables according to their clinical relevance, beyond the statistical significance, excluding correlated variables. Laboratory findings were excluded from the multivariate analysis because of the high percentage of missing data (>3% of the study population). Likewise, most of the laboratory results were inflammatory markers and were related between them. Chest X ray abnormalities were included in the analysis because of their specificity and clinical significance, despite the percentage of missing data (5.7%).
Table 2.
Multivariate analysis for the composite end point
| Multivariate analysis for the composite end pointa,b |
|||
|---|---|---|---|
| OR | 95% CI | p value | |
| Age (per year) | 1.025 | 1.005–1.044 | 0.011 |
| Male gender | 3.005 | 1.960–4.605 | <0.001 |
| Tachypnoea | 3.169 | 1.929–5.209 | <0.001 |
| Sore throat | 1.869 | 1.129–3.096 | 0.015 |
| Cough | 1.761 | 1.106–2.804 | 0.017 |
| Oxygen saturation (Spo2) <92% | 5.404 | 3.339–8.746 | <0.001 |
| Any chest X-ray abnormality | 2.214 | 1.178–4.161 | 0.014 |
Composite end point of death, mechanical ventilation (including invasive and non-invasive mechanical ventilation with Bi-level Positive Airways Pressure), high flow nasal oxygen therapy, prone position, sepsis, systemic inflammatory response syndrome and embolic events.
Binary logistic regression model. Goodness-of-fit (Hosmer and Lemeshow), p 0.795.
The strongest independent predictor for the combined end point was desaturation (Spo 2 <92%) at admission with a five-fold increased risk for the combined end point (OR 5.4; 95% CI 3.4–8.7, p < 0.001). Other important predictors were male gender and tachypnoea (with a three-fold increased risk for both).
Patients presenting the combined event received more corticosteroids (106/225; 47%) than individuals in the group without the primary combined end point (54/548; 9%; p < 0.001). Anticoagulant drugs, including both, therapeutic and prophylactic doses, were more commonly used in the combined event group (158/204; 77%), than in the non-combined event group (198/520; 38%) (p < 0.001). The mortality rate was 12% (28/225) in the combined event group.
Discussion
We present here a unique analysis on young patients admitted with COVID-19 but previously healthy without relevant co-morbidities or use of previous medications. The mortality rate in this cohort was 3.6% (28/773). The primary end point focused on major clinical events, and we analysed a composite end point of severe events including in-hospital death. Twenty-nine per cent of patients (225/773) developed the primary end point despite their good baseline health status. We sought to identify those risk features at presentation related with serious outcomes developed during hospital admission. The main independent predictor for the combined end point was desaturation at admission (Spo 2 <92%) followed by male gender and tachypnoea. Surprisingly, complications and mortality rates were greater than those that we would have expected in healthy patients under 65 years old.
Previous studies included older patients with multiple co-morbidities in their analysis, reporting in-hospital mortality rates for COVID-19 between 4.3% and 30.4% [[2], [3], [4], [5], [6], [7], [8], [9]]. It has been observed that individuals aged 65 years and over represent 80% of admitted patients for COVID-19 disease and present a 20-fold higher risk of death than younger people [11]. In our series, despite excluding all relevant co-morbidities in a group up to 65 years old, the age linear independent relationship with prognosis was maintained.
There are different theories explaining poor outcomes in healthy individuals. Environmental or immunological factors have been related with worse outcomes in young patients with COVID-19. More virulent SARS-CoV-2 or higher inoculum levels, as well as pollution or climate have been described as potential risk factors. Moreover, it has been suggested that a previous infection with another coronavirus might play a protective role by immunological memory via the T and B lymphocytes [14]. Interestingly, some authors have proposed unknown inborn immunological disorders as a mechanistic explanation to this phenomenon [14]. A number of monogenic disorders have been identified in other infectious diseases such as Epstein–Barr virus infection or influenza A virus pneumonia [14].
Specifically regarding COVID-19, it has been observed that individuals with severe COVID-19 have a dysfunctional type I interferon response leading to deficient viral clearance [19,20]. In line with this, van der Made et al. studied genetic variations in healthy young men with severe SARS-CoV-2 infections. A genetic variation in the Toll-like receptor 7 (TLR7) pathway, implying a loss of function of X-chromosomal TLR7, was observed to be related to deficient type I and II interferon responses [15]. TLR7 had previously been identified as related in the recognition of ssRNA of the Middle East respiratory syndrome coronavirus and SARS-CoV infections in mice [21]. Furthermore, because of its localization on the X chromosome, TLR7 genetic variation might explain the male sex bias in COVID-19 [15].
Ellinghaus et al. studied multigenic disorders in 1980 patients from Italy and Spain with severe COVID-19. In this study, a 3p21.31 gene cluster was found to be a genetic susceptibility locus associated with respiratory failure in COVID-19 [16].
Limitations
This cohort only represents 9.4% of patients in the entire HOPE-cohort because most healthy and young individuals could be managed on an outpatient basis. Although we intended to include patients requiring hospitalization, we found that some individuals were asymptomatic or oligosymptomatic at admission. The decision to admit a patient was made by the local attending physician. Admission criteria have changed over the course of the pandemic and were influenced by the point on the pandemic curve together with the local specific protocols and resource availability.
In this study, the associations between clinical management and therapies and the outcome were not specifically evaluated. We found an inverse relationship between the use of steroids and anticoagulants drugs and the primary outcome because these drugs were related to worse outcomes in our cohort. This finding is the opposite to what was observed in previous randomized controlled trials. Our results may reflect that those patients with more severe COVID-19 received more aggressive therapies, such as corticoids or anticoagulants drugs, with the subsequent bias. The facts that we did not specifically evaluate the relationship between therapies and results and did not adjust by confounding factors might explain these results.
Conclusions
To sum up, healthy and young individuals may develop severe SARS-CoV-2 infections. The combined end point was unexpectedly high (29%). Signs at admission such as desaturation or tachypnoea have been identified as worse outcomes predictors. Among baseline characteristics, male gender is related to increased risk of severe events. It is important to highlight that everybody is at risk to develop severe forms of COVID-19.
Author contributions
CE and IN wrote the original draft; all authors review and edited the manuscript; Conceptualization was by CE and IN; all authors contributed to the investigations; methodology was by IN and so was the formal analysis.
Transparency declaration
The authors have no conflicts of interest to declare.
Funding
There is no financial interest to report.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.021.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.WHO coronavirus disease (COVID-19) dashboard [Internet]. Available at: https://covid19.who.int, accessed 24 October 2020.
- 2.Du R.-H., Liang L.-R., Yang C.-Q., Wang W., Cao T.-Z., Li M., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uribarri A., Núñez-Gil I.J., Aparisi A., Becerra-Muñoz V.M., Feltes G., Trabattoni D., et al. Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. J Nephrol. 2020;33:737–745. doi: 10.1007/s40620-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Núñez-Gil I.J., Fernández-Pérez C., Estrada V., Becerra-Muñoz V.M., El-Battrawy I., Uribarri A., et al. Mortality risk assessment in Spain and Italy, insights of the HOPE COVID-19 registry. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., et al. COVID-19 is an emergent disease of aging. medRxiv. 2020 doi: 10.1111/acel.13230. vol. 2020.04.15.20060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S.-Y., Zhang Q., Casanova J.-L., Su H.C., COVID Team Severe COVID-19 in the young and healthy: monogenic inborn errors of immunity? Nat Rev Immunol. 2020;20:455–456. doi: 10.1038/s41577-020-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Severe Covid-19 GWAS Group Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepe M., Maroun-Eid C., Romero R., Arroyo-Espliguero R., Fernàndez-Rozas I., Aparisi A., et al. Clinical presentation, therapeutic approach, and outcome of young patients admitted for COVID-19, with respect to the elderly counterpart. Clin Exp Med. 2021;21:249–268. doi: 10.1007/s10238-021-00684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Núñez-Gil I.J., Estrada V., Fernández-Pérez C., Feltes G., Vedia O., Vergara-Uzcategui C.E., et al. Health Outcome Predictive Evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemp Clin Trial. Commun. 2020;20:100654. doi: 10.1016/j.conctc.2020.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1