Abstract
Background
The efficacy of biological therapies used for the treatment of chronic plaque psoriasis can be influenced by numerous variables including body mass index (BMI).
Objective
This study aimed to evaluate the impact of BMI on the short-term and long-term efficacy of biological therapies in clinical practice and to identify the best therapeutic options in obese patients (BMI ≥ 30 kg/m2).
Methods
A multicentric retrospective study was conducted in patients who initiated a biological therapy during the period January 2006–December 2019. The proportion of patients achieving a 90% improvement of baseline Psoriasis Area and Severity Index at weeks 12 and 24 was calculated also recording the 12- and 24-month drug survival as a measure of long-term efficacy, performing multivariate analyses to assess the impact of different variables.
Results
Five hundred and four patients with psoriasis were included. After 12 and 24 weeks, the proportion of patients achieving a 90% improvement of baseline Psoriasis Area and Severity Index response was higher in patients with a BMI < 30 kg/m2 compared with those with a BMI ≥ 30 kg/m2 [54.90% vs 43.45% (p = 0.014) at week 12 and 66.84% vs 56.55% (p = 0.021) at week 24]. The Kaplan–Meier survival curves showed how obese patients had a higher probability of discontinuation due to a lack or loss of efficacy (p = 0.0192) compared with non-obese patients. The drug survival analysis also showed that BMI negatively affected the drug survival of secukinumab (odds ratio 1.27, p < 0.001) and ustekinumab (odds ratio 1.06, p = 0.050), while the long-term efficacy of adalimumab, etanercept, and ixekizumab was not influenced by BMI.
Conclusions
Obesity (BMI ≥ 30 kg/m2) negatively affects the clinical response of biological drugs in psoriatic patients, with anti-interleukin drugs being more affected by BMI than anti-tumor necrosis factor drugs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-021-01080-z.
Key Points
| Our results highlight the importance of considering patient-related and disease-related variables when choosing the most appropriate treatment for each subject affected by psoriasis. |
| Obesity can negatively affect the short-term and long-term efficacy of biological therapies. |
| In our analysis, anti-interleukin drugs seem to be more affected by a patient’s weight than anti-tumor necrosis factor drugs. |
Introduction
Psoriasis is a chronic inflammatory skin disease affecting 3.0% of the Italian population [1]. Despite psoriasis being originally regarded as primarily driven by Th1 cell-derived cytokines (namely tumor necrosis factor [TNF]-α and interferon-γ), several studies have supported the central role of the interleukin (IL)-23/Th17 axis. Interleukin-17 and IL-23, together with TNF-α are now considered key mediators in psoriasis pathogenesis as demonstrated by the high efficacy rates of biological agents inhibiting these cytokines [2, 3]. As a result of the better understanding of psoriasis immune-mediated pathways, several biological therapies with different targets and mechanisms of action are now available for the treatment of moderate-to-severe plaque psoriasis. In the context of the many therapeutic options, a tailored approach that takes into consideration patient-related and disease-related factors, such as comorbidities, is recommended to provide a personalized therapy and avoid multiple, potentially unnecessary, therapeutic switches [4]. Several studies demonstrated that the efficacy of biological therapies can be influenced by numerous variables, potentially leading to a lack or loss of response to the treatment. Among these factors, obesity, defined as a body mass index (BMI) ≥ 30 kg/m2 [5], might affect the response to biological therapies [6–8]. Obesity was also suggested as a negative predictor of response to systemic therapies being associated with an inferior likelihood of achieving a 75% improvement of the baseline Psoriasis Area and Severity Index (PASI) score in patients with a high BMI compared with those with a normal BMI (< 25 kg/m2) [9]. However, several sub-analyses of randomized clinical trials evaluating the impact of BMI on the efficacy of biological therapies [10–12] showed conflicting results as obesity was found to negatively affect the efficacy of certain drugs while showing scarce or no impact on the efficacy of other biological treatments. Real-life studies have mainly considered patients treated with anti-TNF or anti-IL-12/23 drugs, while few studies include subjects treated with the novel anti-IL drugs. Moreover, data about the impact of BMI on long-term efficacy and drug survival of biological therapies are scarce, and to date, it is unclear whether drugs with a particular mechanism of action are preferred over others in obese patients. The aim of the present multicentric study was to evaluate the impact of BMI on the short-term and long-term efficacy of biological therapies in psoriatic patients and to assess whether the potential influence of BMI differs among the biological therapies included in the analysis, trying to identify the best therapeutic options in obese patients.
Patients and Methods
A retrospective analysis was performed in a cohort of patients with chronic plaque psoriasis, with or without psoriatic arthritis, who initiated treatment with a biological therapy during the period January 2006–December 2019. The study population consisted of patients attending the outpatient clinics of two dermatologic centers (Università Cattolica del Sacro Cuore, Rome, Italy and Policlinico San Martino-IRCCS, Genova, Italy). All enrolled patients were > 18 years old and affected by chronic plaque psoriasis. Subjects with palmoplantar, erythrodermic, or pustular forms were excluded, as well as patients concurrently treated with other systemic therapies. Biological therapies were administered at European Medicines Agency-approved dosages, and no dose or frequency variations were permitted. For each patient, demographic and clinical data (age, sex, BMI, age of onset and duration of disease, comorbidities, and previous use of systemic conventional or biological therapies) were collected at the time of enrolment. The severity of the psoriasis was measured by the PASI [13] at baseline and after 12 and 24 weeks of treatment. Moreover, treatment duration was recorded, and the reasons for any drug withdrawal were categorized into adverse events and a lack or loss of efficacy, while interruptions up to 90 days were accepted and not considered as a withdrawal. Patients who stopped treatment for reasons related to psoriatic arthritis were excluded from the analysis. Only cases with complete data were included. Patients treated with infliximab were not included in the analysis because of the small number of subjects in the two participating centers. Short-term clinical efficacy was assessed as the proportion of patients who achieved a PASI reduction ≥ 90% (PASI90) at weeks 12 and 24, compared with the PASI score measured at baseline. Long-term efficacy was assessed with a drug survival analysis, as it can be considered a global indicator of effectiveness and adherence, reflecting information on drug efficacy, safety, and patient satisfaction [14–17]. The examination of drug survival patterns was carried out using Kaplan–Meier survival curves for overall discontinuation, a lack or loss of efficacy, and adverse events assessed at 12 and 24 months of observation. The entire study was conducted according to the principles of the Helsinki Declaration.
Statistical Analysis
Demographic and clinical characteristics of the study population were described through absolute and relative frequencies (%), means and standard deviations, or medians and interquartile range where appropriate. The Kruskal–Wallis test and Chi-squared test were used to compare the quantitative and qualitative characteristics of the populations treated with the different drugs. Univariate and multivariate logistic regression analyses were used to evaluate potential differences in efficacy (PASI) among the various treatments, choosing adalimumab as the reference drug and adjusting for potential confounders (age, sex, BMI, previous biologic drugs, baseline PASI, and the presence of psoriatic arthritis). The “non-responder imputation” method, an approach that imputes individuals with missing data as non-responders, was used to handle missing data [18, 19]. Differences in drug survival between the various drugs were examined using the Kaplan–Meier survival analysis and the log-rank test. “Event” was defined as treatment discontinuation or switching of a biologic therapy and the “event date” was considered as the date of treatment discontinuation. Patients who had not discontinued treatment at the time they were lost to follow-up were censored. Cox regression analyses with adjustment for covariates collected at the entry in the study or before the start of a new therapy were used, whenever possible [20], to compare treatment discontinuation times. Adjusted hazard ratios, 95% confidence intervals, and corresponding p-values were calculated for each clinical characteristic to compare different treatments and the reference drug. A p-value < 0.05 was considered statistically significant. The statistical analysis was performed using the software STATA 13 (StataCorp LP, College Station, TX, USA).
Results
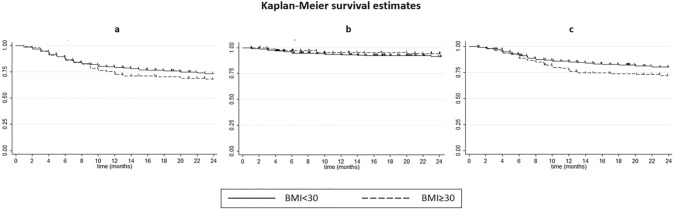
The study population included 504 subjects, whose clinical and demographic characteristics are reported in Table 1. The median BMI value was 26.0 kg/m2 (interquartile range 23.66–29.32) with a BMI ≥ 30 kg/m2 in 102/504 (20.2%) patients. Psoriatic arthritis was found in 178/504 (35.3%) patients with a mean duration of disease of 12 years. A total of 706 cycles of therapy with biological drugs were registered, with 563 cycles in patients with a BMI < 30 kg/m2 and 143 cycles in patients with a BMI ≥ 30 kg/m2. Among the cycles considered, 359 (50.8%), 278 (39.4%), and 94 (13.3%) cycles were referred to patients previously treated with cyclosporine, methotrexate, and acitretin, respectively, while 70 cycles of therapy were started after two conventional systemic therapies. Last, in the study population, 397 cycles of therapy (56.4%) were carried out in patients naïve to biological drugs. The PASI values at baseline were higher in patients with a BMI ≥ 30 kg/m2 compared with those registered at baseline in patients with a BMI < 30 kg/m2 (17.2 vs 15.1). After 12 and 24 weeks of therapy, the proportion of patients achieving PASI90 response after a cycle of biological therapy was higher in patients with a BMI < 30 kg/m2 compared with those with a BMI ≥ 30 kg/m2: 54.90% vs 43.45% (p = 0.014) and 66.84% vs 56.55% (p = 0.021), respectively (Fig. 1). A logistic regression analysis (adjusted for the presence of psoriatic arthritis, sex, age, previous biological treatments, and baseline PASI) showed that BMI was the only parameter influencing the odds of achieving a PASI90 response at week 12 (p = 0.014) and week 24 (p = 0.011), with high BMI values being associated with a less likely clinical response (Table 2). The same logistic regression analysis was carried out for each biological drug considered in the study, showing a negative impact of BMI on clinical response only for secukinumab at weeks 12 and 24 (odds ratio [OR] 0.81, p = 0.004 and OR 0.78, p = 0.001 respectively) and for ixekizumab at week 24 (OR 0.86, p = 0.009). The history of previous biological treatment(s) negatively affected the clinical response at week 12 for adalimumab (OR 0.51, p = 0.05) and at week 24 for ustekinumab (OR 0.30, p = 0.004). The presence of psoriatic arthritis had a positive impact on clinical response to etanercept at weeks 12 and 24 (OR 2.35, p = 0.02 and OR 2.93, p = 0.003, respectively) and on the clinical response to adalimumab at week 24. Male sex was associated with a significant higher probability of achieving PASI90 at week 12 and 24 in patients treated with ixekizumab (OR 4.61, p < 0.001 and OR 4.30, p = 0.003, respectively), while male sex negatively affected the clinical response to secukinumab at weeks 12 and 24 (OR 0.19, p = 0.003 and OR 0.24, p = 0.014, respectively). In addition, a logistic regression analysis taking adalimumab as the reference drug and adjusted for potential confounders (presence of psoriatic arthritis, sex, age, previous biological treatment(s), and baseline PASI) showed a significant superiority of anti-IL drugs vs anti-TNF drugs in achieving PASI90 at week 12 and 24 in patients with a BMI < 30 kg/m2. In particular, ixekizumab, secukinumab, and ustekinumab showed a superiority compared with adalimumab at week 12 (2.33, p = 0.02; 5.99, p < 0.001; 3.02, p = 0.003, respectively) and week 24 (4.47, p < 0.001; 5.75, p < 0.001; 3.55, p < 0.001, respectively). The same analysis was carried out in patients with a BMI ≥ 30 kg/m2 showing no differences among the drugs considered at weeks 12 and 24 (Table 3). Long-term efficacy analysis carried out using Kaplan–Meier survival curves showed no differences between patients with a BMI ≥ 30 or < 30 kg/m2 when evaluating both overall survival and discontinuations related to adverse events at 12 and 24 months of observation (Fig. 2a, b), while obese patients showed a higher probability of discontinuation due to a lack or loss of efficacy at 24 months of observation (p = 0.0192) (Fig. 2c). A regression analysis, adjusted for the above-mentioned potential confounders, showed that BMI was the only variable influencing the drug’s survival, with high BMI values adversely influencing the treatment continuation (hazard ratio 1.03, p = 0.029) at 24 months of observation, while no correlations were found at 12 months of observation. Sex, psoriatic arthritis, previous biological therapies, age, and baseline PASI showed no significant impact on the risk of discontinuation in both time-points (data not shown). The same regression analysis performed for each biological drug and considering 24 months of observation showed a similar negative impact of BMI on the drug survival of secukinumab (OR 1.274, p < 0.001) and ustekinumab (OR 1.06, p = 0.050), while the long-term efficacy of adalimumab, etanercept, and ixekizumab was not influenced by BMI values. The presence of psoriatic arthritis positively influenced the treatment retention in etanercept (OR 0.43, p < 0.001), while male sex and a history of previous biological therapies had a negative impact on the survival of secukinumab (OR 5.04, p = 0.002 and OR 12.39, p = 0.001, respectively). As for the short-term efficacy, a regression analysis taking adalimumab as the reference drug and adjusted for the presence of arthritis, sex, age, previous biological therapies, and baseline PASI was performed considering a 24-month period of observation, showing better survival curves for anti-IL drugs vs anti-TNF drugs in patients with a BMI < 30 kg/m2. The presence of psoriatic arthritis and the history of previous biological therapies were the only statistically significant variables in patients with a BMI < 30 kg/m2 (both p = 0.001). However, no differences among the various drugs were found in patients with a BMI ≥ 30 kg/m2 (Table 4). Further data for each drug included in the analysis can be found in Table 1 of the Electronic Supplementary Material.
Table 1.
Clinical and demographic characteristics of the study population
| Characteristics | Population with a BMI < 30 kg/m2 (563 cycles of biological therapy) | Population with a BMI ≥ 30 kg/m2 (143 cycles of biological therapy) | P-value |
|---|---|---|---|
| Male sex | 319 (56.6%) | 100 (69.9%) | 0.003 |
| Age (years) | 53.56 ± 14.55 | 55.06 ± 12.71 | NS |
| Weight (kg) | 72.43 ± 13.31 | 100.14 ± 12.80 | 0.001 |
| Psoriasis duration (months) | 23.11 ± 14.46 | 25.46 ± 13.21 | NS |
| Psoriatic arthritis | 191 (34.0%) | 59 (41.2%) | NS |
| Previous therapies | |||
| Cyclosporine | 311 (55.2%) | 48 (33.6%) | 0.001 |
| Acitretin | 82 (14.6%) | 12 (8.4%) | 0.001 |
| Methotrexate | 219 (38.9%) | 59 (41.3%) | NS |
| Biologics | 249 (44.2%) | 59 (41.3%) | 0.02 |
| Baseline PASI | 15.1 | 17.2 | 0.05 |
| Biological therapy | |||
| Adalimumab | 129 (22.9%) | 45 (31.5%) | NS |
| Etanercept | 121 (21.5%) | 25 (17.5%) | NS |
| Ixekizumab | 105 (18.7%) | 18 (12.6%) | NS |
| Secukinumab | 108 (19.2%) | 14 (9.8%) | NS |
| Ustekinumab | 100 (17.8%) | 41 (28.7%) | NS |
Values are expressed as mean ± SD or n (%)
BMI body mass index, NS not significant, PASI Psoriasis Area and Severity Index
Fig. 1.
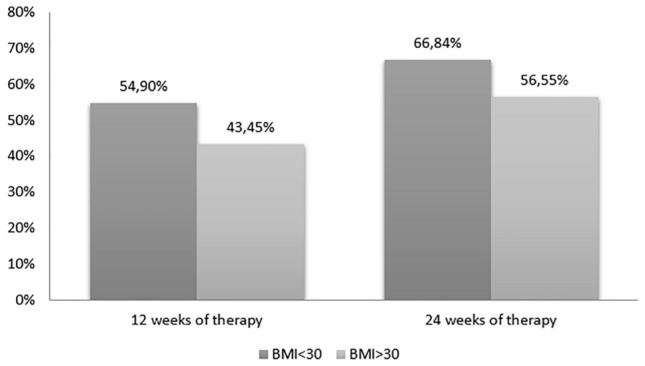
Proportions of patients achieving a 90% improvement of the baseline Psoriasis Area and Severity Index (PASI90) response
Table 2.
Logistic regression analysis of variables influencing PASI90 response at week 12 and 24
| Variable | PASI90 at week 12 | PASI90 at week 24 | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P-value | 95% CI | Odds ratio | P-value | 95% CI | |
| BMI | 0.96 | 0.014 | 0.93–0.99 | 0.96 | 0.011 | 0.93–0.99 |
| Psoriatic arthritis | 1.03 | 0.829 | 0.75–1.42 | 1.24 | 0.217 | 0.88–1.73 |
| Sex | 1.24 | 0.178 | 0.91–1.69 | 1.26 | 0.159 | 0.91–1.75 |
| Age | 0.99 | 0.384 | 0.98–1.00 | 1.00 | 0.8323 | 0.99–1.01 |
| Previous biologics | 0.95 | 0.769 | 0.71–1.29 | 0.81 | 0.194 | 0.59–1.11 |
| Baseline PASI | 1.00 | 0.977 | 0.98–1.02 | 1.00 | 0.813 | 0.98–1.02 |
BMI body mass index, CI confidence interval, PASI Psoriasis Area and Severity Index
Table 3.
Multivariate regression analysis showing PASI90 achievement rates at week 12 and week 24 in patients with a BMI < 30 and a BMI ≥ 30 kg/m2
| Variable | PASI90 at week 12 | PASI90 at week 24 | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P-value | 95% CI | Odds ratio | P-value | 95% CI | |
| BMI < 30 kg/m2 | ||||||
| Etanercept | 0.64 | 0.100 | 0.37–1.09 | 0.87 | 0.603 | 0.51–1.48 |
| Ixekizumab | 1.94 | 0.020 | 1.11–3.38 | 4.07 | 0.000 | 2.20–7.53 |
| Secukinumab | 7.16 | 0.000 | 3.76–13.6 | 7.53 | 0.000 | 3.78–14.9 |
| Ustekinumab | 2.40 | 0.003 | 1.36–4.24 | 2.95 | 0.000 | 1.62–5.37 |
| Psoriatic arthritis | 1.75 | 0.009 | 1.14–2.67 | 2.21 | 0.000 | 1.42–3.46 |
| Sex | 1.31 | 0.142 | 0.91–1.88 | 1.26 | 0.236 | 0.86–1.85 |
| Age | 0.99 | 0.125 | 0.98–1.00 | 0.99 | 0.565 | 0.98–1.01 |
| Previous biological therapies | 0.61 | 0.012 | 0.41–0.89 | 0.46 | 0.000 | 0.30–0.69 |
| Baseline PASI | 0.99 | 0.319 | 0.96–1.01 | 0.99 | 0.458 | 0.96–1.02 |
| BMI ≥ 30 kg/m2 | ||||||
| Etanercept | 0.46 | 0.166 | 0.16–1.38 | 0.72 | 0.534 | 0.26–2.00 |
| Ixekizumab | 0.86 | 0.800 | 0.26–2.81 | 0.79 | 0.693 | 0.25–2.54 |
| Secukinumab | 1.79 | 0.347 | 0.53–6.07 | 1.17 | 0.797 | 0.35–3.94 |
| Ustekinumab | 1.05 | 0.914 | 0.42–2.61 | 1.36 | 0.518 | 0.54–3.42 |
| Psoriatic arthritis | 1.22 | 0.594 | 0.59–2.53 | 1.50 | 0.275 | 0.72–3.11 |
| Sex | 1.11 | 0.788 | 0.51–2.45 | 1.48 | 0.327 | 0.68–3.21 |
| Age | 1.01 | 0.719 | 0.98–1.04 | 1.01 | 0.527 | 0.98–1.04 |
| Previous biological therapies | 0.75 | 0.447 | 0.36–1.57 | 1.05 | 0.904 | 0.50–2.18 |
| Baseline PASI | 1.05 | 0.064 | 0.99–1.10 | 1.04 | 0.146 | 0.99–1.09 |
BMI body mass index, CI confidence interval, PASI Psoriasis Area and Severity Index
Fig. 2.
Kaplan–Meier curves of drug survival. a Overall survival (p > 0.05), b discontinuation because of adverse events (p > 0.05), and c discontinuation because of a lack or loss of efficacy (p = 0.0192)
Table 4.
Multivariate regression analysis showing drug withdrawal at 24 months in patients with a BMI < 30 and a BMI ≥ 30 kg/m2
| Variable | Odds ratio | P-value | 95% CI |
|---|---|---|---|
| BMI < 30 kg/m2 | |||
| Etanercept | 1.05 | 0.785 | 0.73–1.51 |
| Ixekizumab | 0.15 | < 0.001 | 0.08–0.29 |
| Secukinumab | 0.24 | < 0.001 | 0.14–0.42 |
| Ustekinumab | 0.25 | < 0.001 | 0.15–0.42 |
| Psoriatic arthritis | 0.65 | 0.010 | 0.47–0.90 |
| Sex | 0.94 | 0.679 | 0.69–1.27 |
| Age | 1.00 | 0.534 | 0.99–1.01 |
| Previous biological therapies | 1.67 | 0.001 | 1.22–2.29 |
| Baseline PASI | 1.01 | 0.354 | 0.99–1.03 |
| BMI ≥ 30 kg/m2 | |||
| Etanercept | 1.19 | 0.642 | 0.58–2.44 |
| Ixekizumab | 0.51 | 0.227 | 0.17–1.53 |
| Secukinumab | 1.02 | 0.966 | 0.39–2.63 |
| Ustekinumab | 0.60 | 0.177 | 0.29–1.25 |
| Psoriatic arthritis | 0.91 | 0.745 | 0.52–1.59 |
| Sex | 1.29 | 0.411 | 0.70–2.39 |
| Age | 0.99 | 0.845 | 0.97–1.02 |
| Previous biological therapies | 1.22 | 0.490 | 0.69–2.14 |
| Baseline PASI | 0.98 | 0.469 | 0.95–1.02 |
BMI body mass index, CI confidence interval, PASI Psoriasis Area and Severity Index
Discussion
Our study confirmed the well-known association between psoriasis and obesity, with 20.2% of the analyzed patients having a BMI ≥ 30 kg/m2. In a study conducted on a cohort of 44,164 patients, Kaye et al. found obesity to be more prevalent in patients affected by psoriasis when compared with the general population (6.3% vs 5.5%) [21], similarly to other studies [22, 23]. Several hypotheses have been made to explain this association. Social isolation, depression, and scarce physical activity in psoriatic patients can lead to the development of obesity. However, the inflammatory nature of obesity and the several cytokines shared in both psoriasis and obesity pathogenesis suggest a common inflammatory background. In particular, obese patients carry a higher risk of developing inflammatory diseases such as psoriasis as a consequence of the high levels of pro-inflammatory cytokines and adipokines released from adipose tissue [24]. Moreover, psoriasis severity seems to be linked to the likelihood of developing obesity, with a higher risk for those patients affected by severe forms of psoriasis [25].
Our results also highlighted the potential negative impact of obesity on the odds of obtaining a clinical response from a biological therapy in patients affected by psoriasis, similarly to other studies [26, 27]. In both short-term and long-term efficacy analysis, our results found worse response rates in obese patients compared with those with lower BMI values. A significant difference in the likelihood of obtaining a PASI90 response was detected between patients with a BMI < 30 kg/m2 and those with a BMI ≥ 30 kg/m2 at both week 12 [54.90% vs 43.45% (p = 0.014)] and week 24 [66.84% vs 56.55% (p = 0.021)]. A similar result emerged from the drug survival analyses, with higher rates of drug withdrawal due to a lack or loss of efficacy in obese patients (p = 0.0192) at 24 months. Moreover, among the clinical and demographic variables considered in the multivariate analysis for short-term and long-term efficacy, BMI was the only factor negatively affecting clinical response. These results may find an explanation again in the connection between psoriasis and obesity. Obese patients are at greater risk of developing more severe forms of psoriasis, with high BMI values being related to a more severe skin disease [28]. The inflammatory cross-talk between obesity and psoriasis can represent the underlying mechanism of this association. As mentioned above, both diseases share an immunopathogenic pathway involving pro-inflammatory cytokines such as TNFα. In the adipose tissue of obese patients, activated macrophages are responsible for the release of pro-inflammatory mediators (TNFα, IL-6, macrophage-colony stimulating factor, monocyte chemoattractant protein-1), also stimulating the production of the same mediators from adipocytes [29], which are also involved in the release of adipokines, stimulating insulin resistance, inflammation, and endothelial damage. In psoriatic patients, high levels of inflammatory adipokines (such as leptin and resistin) have been detected in peripheral blood, together with a down-regulation of regulatory adipokines such as adiponectin [30–32]. In this context, TNFα is also able to increase the levels of circulating leptin, leading to a stimulation of keratinocyte proliferation and correlating with both psoriasis severity and duration, stressing the connection between these two inflammatory diseases and the potential influence of BMI on psoriasis clinical presentation and course [33–35]. A reduced efficacy of biological therapies in psoriatic patients can also be explained by the different dosages among the various drugs [36], mostly not adapted to the patient’s weight except for infliximab and ustekinumab, although the latter can only be administered in two different dosages (45 mg vs 90 mg) if a patient’s weight is below or above 100 kg, respectively. Pharmacokinetic studies showed that excessive body weight could negatively affect the drug clearance and volume of distribution, depending on the different lipophilicity of the molecules. On the contrary, weight loss in psoriatic patients, as achieved with diet and lifestyle changes, has been demonstrated to have a positive impact on disease severity and response to treatments [37, 38]. Thus, the impact of BMI on the pharmacokinetic properties of the different drugs together with the presence of more severe forms of psoriasis in obese patients could provide an explanation to our results.
Analysing the impact of BMI on each drug considered, in both short-term and long-term efficacy analyses, high BMI values negatively affected the efficacy of anti-IL drugs (ixekizumab, secukinumab, ustekinumab), while in the multivariate analysis carried out in patients with a BMI < 30 kg/m2, anti-IL drugs showed the best efficacy rates at weeks 12 and 24 and the best drug survival rates at 24 months of therapy; no statistically significant differences were found in obese patients. Thus, in the study population, anti-TNF drugs were less influenced by high BMI values when compared with anti-IL drugs. Of note, several randomized clinical trials reported a general superiority of anti-IL drugs vs anti-TNF drugs in terms of efficacy [39, 40], but few data are available on the comparison of anti-IL drugs and anti-TNF drugs in obese patients. Real-life studies also confirm the superiority of anti-IL drugs vs anti-TNF drugs. In a recent study, Blauvelt et al. compared the overall survival of ixekizumab and adalimumab with results similar to our drug survival analysis, although the impact of BMI on these results was not analyzed [41]. Therefore, our findings highlight an unexpected result, showing a greater influence of high BMI values on the efficacy of anti-IL drugs when compared with anti-TNF drugs, with worse performances in obese patients, in line with the negative impact of BMI on the efficacy and drug survival of anti-IL drugs reported in recent studies [42–44]. Our results might be explained by the inflammatory background of obese patients, in which TNFα is the most represented inflammatory mediator, owing to its release from adipose tissue under the influence of resistin. Moreover, literature data regarding the impact of BMI on the response of anti-TNF drugs are conflicting. Several studies showed that elevated BMI is correlated to a reduced therapeutic response to anti-TNF [45, 46], while other authors reported that the efficacy and drug survival of anti-TNF drugs and in particular adalimumab is not affected by a patient’s BMI [47], as also confirmed by our study. In this context, a recent meta-analysis carried out in psoriatic patients treated with etanercept, adalimumab, infliximab, and ustekinumab found that obesity could negatively affect the drug survival of these therapies, except for the patients treated with adalimumab when singularly analyzed [48].
In contrast, it is well known that anti-TNF drugs could lead to an increase in body weight [49], as TNFα plays an important metabolic role, favoring lipolysis and proteolysis, and suppressing the release of anabolic hormones such as insulin and insulin growth factor-1. Moreover, TNFα is involved in the release of leptin from adipose tissue, a crucial mediator in inducing the sense of satiety. Anti-TNF drugs could therefore lead to an increased appetite in patients treated with these molecules [50], while ixekizumab, secukinumab, and ustekinumab do not seem to induce weight gain or increased appetite [51–53]. The use of drugs causing weight gain in patients who are already predisposed to a high BMI may raise concerns for physicians, stressing the need to consider positive and negative aspects of each therapy in the single patient.
The main limitations of the present study are mostly represented by the retrospective nature and the time frame considered, starting when anti-IL-17 and anti-IL-12/23 drugs were not yet licensed. Therefore, in the case of no response or a sub-optimal response to anti-TNF therapies, patients could not be switched to another drug class: this aspect may have affected overall survival. Moreover, the overall number of treatments given with anti-IL drugs in obese patients was lower than for other types of treatment, and no data regarding potential changes in weight during the observation period were available.
Conclusions
Obese patients with psoriasis still represent a challenge for the dermatologist. Our results showed that high BMI values can negatively affect the clinical response of these patients. Of note, our study highlighted that anti-IL drugs are more affected by body weight than anti-TNF drugs and this finding should be considered in choosing the most appropriate treatment in obese patients. Prospective studies would be useful to confirm the data from our analysis aiming to perform a personalized therapy, with positive effects in terms of appropriateness of therapy, a patient’s quality of life, and pharmacoeconomics.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Conflict of interest/Competing interest
Giacomo Caldarola reports consulting fees or honorarium and payment for lectures from Lilly and Novartis, outside the submitted work. Andrea Chiricozzi reports consulting fees or honorarium from Abbvie, Novartis, Lilly, Leo Pharma, Janssen, Sanofi, UCB Pharma, and Pfizer, outside the submitted work. Clara De Simone reports consulting fees or honorarium from Abbvie, Amgen, Novartis, Celgene, Sanofi, UCB Pharma, Janssen, and Lilly and payment for lectures from Abbvie, Lilly, Novartis, UCB Pharma, and Celgene, outside the submitted work. Ketty Peris reports consulting fees or honorarium from Almirall, AbbVie, Biogen, Lilly, Celgene, Galderma, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, Sun Pharma, and Janssen, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Availability of data and material
Data were derived from the internal database and medical charts.
Code availability
Not applicable.
Authors’ contributions
F. Pirro, G. Caldarola, K. Peris, and C. De Simone conceptualized this study, analyzed the data, and wrote the manuscript. M. Mariani performed and verified the statistical analyses. F. Pirro, G. Caldarola, A. Chiricozzi, M. Burlando, M. Mariani, A. Parodi, K. Peris, and C. De Simone contributed to both the data collection and the interpretation of the results. All authors revised the manuscript and approved the submitted version.
Ethics approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments). No ethics committee approval was requested because of the retrospective and observational nature of the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Federico Pirro and Giacomo Caldarola contributed equally to this manuscript.
References
- 1.Prignano F, Rogai V, Cavallucci E, et al. Epidemiology of psoriasis and psoriatic arthritis in Italy-a systematic review. Curr Rheumatol Rep. 2018;20(7):43. doi: 10.1007/s11926-018-0753-1. [DOI] [PubMed] [Google Scholar]
- 2.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Investig Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 3.Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol. 2018;19(1):1–13. doi: 10.1007/s40257-017-0328-3. [DOI] [PubMed] [Google Scholar]
- 5.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl.):s176–s185. [PubMed] [Google Scholar]
- 6.Shan J, Zhang J. Impact of obesity on the efficacy of different biologic agents in inflammatory diseases: a systematic review and meta-analysis. Jt Bone Spine. 2019;86(2):173–183. doi: 10.1016/j.jbspin.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443–446. doi: 10.1016/j.jaad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007–1011. doi: 10.1111/j.1468-3083.2011.04065.x. [DOI] [PubMed] [Google Scholar]
- 9.Naldi L, Addis A, Chimenti S, Giannetti A, Picardo M, Tomino C, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Dermatology. 2008;217:365–373. doi: 10.1159/000156599. [DOI] [PubMed] [Google Scholar]
- 10.Strober B, Gottlieb A, Leonardi C, Papp K. Levels of response of psoriasis patients with different baseline characteristics treated with etanercept. J Am Acad Dermatol. 2006;54:AB220. [Google Scholar]
- 11.Menter A, Gordon K, Goldblum O, Gu Y. Efficacy and safety of adalimumab are consistent across weight quartiles in patients with moderate to severe psoriasis: subanalysis of REVEAL. J Am Acad Dermatol. 2009;60:AB173. doi: 10.1016/j.jaad.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Notario J, Deza G, Vilarrasa E, et al. Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain. J Dermatol Treat. 2019;30(5):424–429. doi: 10.1080/09546634.2018.1528000. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 14.Costanzo A, Malara G, Pelucchi C, et al. Effectiveness end points in real-world studies on biological therapies in psoriasis: systematic review with focus on drug survival. Dermatology. 2018;234(1–2):1–12. doi: 10.1159/000488586. [DOI] [PubMed] [Google Scholar]
- 15.Lin PT, Wang SH, Chi CC. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068. doi: 10.1038/s41598-018-34293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Investig Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 17.Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Br J Dermatol. 2020;183(2):294–302. doi: 10.1111/bjd.18981. [DOI] [PubMed] [Google Scholar]
- 18.Moore RA, Straube S, Eccleston C, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain. 2012;153(2):265–268. doi: 10.1016/j.pain.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Misumi T, Maruo K. A comparison of multiple imputation methods for incomplete longitudinal binary data. J Biopharm Stat. 2018;28(4):645–667. doi: 10.1080/10543406.2017.1372772. [DOI] [PubMed] [Google Scholar]
- 20.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 21.Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902. doi: 10.1111/j.1365-2133.2008.08707.x. [DOI] [PubMed] [Google Scholar]
- 22.Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141(12):1527–1534. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 23.Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Investig Dermatol. 2005;125(1):61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 24.de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debbaneh M, Millsop JW, Bhatia BK, Koo J, Liao W. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71(1):133–140. doi: 10.1016/j.jaad.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gisondi P, Del Giglio M, Di Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88(5):1242–1247. doi: 10.3945/ajcn.2008.26427. [DOI] [PubMed] [Google Scholar]
- 28.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 31.Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30(1):13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- 32.Johnston A, Arnadottir S, Gudjonsson JE, et al. Obesity in psoriasis: leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol. 2008;159(2):342–350. doi: 10.1111/j.1365-2133.2008.08655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Investig. 1997;100(11):2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83(10):1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 35.Cerman AA, Bozkurt S, Sav A, Tulunay A, Elbaşi MO, Ergun T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br J Dermatol. 2008;159(4):820–826. doi: 10.1111/j.1365-2133.2008.08742.x. [DOI] [PubMed] [Google Scholar]
- 36.Naldi L, Addis A, Chimenti S, et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis: evidence from the Psocare project. Dermatology. 2008;217(4):365–373. doi: 10.1159/000156599. [DOI] [PubMed] [Google Scholar]
- 37.Minno MND, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 2014;73(6):1157–1162. doi: 10.1136/annrheumdis-2012-202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39(8):1197–1202. doi: 10.1038/ijo.2015.64. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi C, Reich K, Foley P, Torii H, Gerdes S, Guenther L, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther (Heidelb) 2020;10(3):431–447. doi: 10.1007/s13555-020-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al., ERASURE Study Group, FIXTURE Study Group. Secukinumab in plaque psoriasis: results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. [DOI] [PubMed]
- 41.Blauvelt A, Shi N, Burge R, et al. Comparison of real-world treatment patterns among psoriasis patients treated with ixekizumab or adalimumab. Patient Prefer Adherence. 2020;14:517–527. doi: 10.2147/PPA.S233993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiricozzi A, Balato A, Conrad C, et al. Secukinumab demonstrates improvements in absolute and relative psoriasis area severity indices in moderate-to-severe plaque psoriasis: results from a European, multicentric, retrospective, real-world study. J Dermatol Treat. 2020;31(5):476–483. doi: 10.1080/09546634.2019.1671577. [DOI] [PubMed] [Google Scholar]
- 43.Torres T, Balato A, Conrad C, et al. Secukinumab drug survival in patients with psoriasis: a multicenter, real-world, retrospective study. J Am Acad Dermatol. 2019;81(1):273–275. doi: 10.1016/j.jaad.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–579. doi: 10.1007/s40257-021-00598-4. [DOI] [PubMed] [Google Scholar]
- 45.Di Lernia V, Ricci C, Lallas A, Ficarelli E. Clinical predictors of non-response to any tumor necrosis factor (TNF) blockers: a retrospective study. J Dermatol Treat. 2014;25(1):73–74. doi: 10.3109/09546634.2013.800184. [DOI] [PubMed] [Google Scholar]
- 46.Hojgaard P, Glintborg B, Kristenses LE, Gudbjornsson B, Love TJ, Dreyer L. The influence of obesity on response to tumor necrosis factor-a inhibitors in psoriatic arthritis: results from the DANBIO and ICEBO registries. Rheumatology (Oxf) 2016;55:2191–2199. doi: 10.1093/rheumatology/kew326. [DOI] [PubMed] [Google Scholar]
- 47.Chiricozzi A, Zangrilli A, Bavetta M, et al. Real-life 9-year experience with adalimumab in psoriasis and psoriatic arthritis: results of a single-centre, retrospective study. J Eur Acad Dermatol Venereol. 2017;31(2):304–311. doi: 10.1111/jdv.13771. [DOI] [PubMed] [Google Scholar]
- 48.Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2019;181(3):450–458. doi: 10.1111/bjd.17738. [DOI] [PubMed] [Google Scholar]
- 49.Saraceno R, Schipani C, Mazzotta A, et al. Effect of anti-tumor necrosis factor-alpha therapies on body mass index in patients with psoriasis. Pharmacol Res. 2008;57(4):290–295. doi: 10.1016/j.phrs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Carrascosa JM, Rocamora V, Fernandez-Torres RM, et al. Obesity and psoriasis: inflammatory nature of obesity, relationship between psoriasis and obesity, and therapeutic implications. Actas Dermosifiliogr. 2014;105(1):31–44. doi: 10.1016/j.ad.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Gisondi P, Conti A, Galdo G, Piaserico S, De Simone C, Girolomoni G. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol. 2013;168(5):1124–1127. doi: 10.1111/bjd.12235. [DOI] [PubMed] [Google Scholar]
- 52.Takamura S, Takahashi A, Inoue Y, Teraki Y. Effects of tumor necrosis factor-α, interleukin-23 and interleukin-17A inhibitors on bodyweight and body mass index in patients with psoriasis. J Dermatol. 2018;45(9):1130–1134. doi: 10.1111/1346-8138.14526. [DOI] [PubMed] [Google Scholar]
- 53.Egeberg A, Wu J, Korman N, et al. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J Am Acad Dermatol. 2018;79:104–9.e8. doi: 10.1016/j.jaad.2018.02.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.