Abstract
We define the minimal important change (MIC) as a threshold for a minimal within-person change over time above which patients perceive themselves importantly changed. There is a lot of confusion about the concept of MIC, particularly about the concepts of minimal important change and minimal detectable change, which questions the validity of published MIC values. The aims of this study were: (1) to clarify the concept of MIC and how to use it; (2) to provide practical guidance for estimating methodologically sound MIC values; and (3) to improve the applicability of PROMIS by summarizing the available evidence on plausible PROMIS MIC values. We discuss the concept of MIC and how to use it and provide practical guidance for estimating MIC values. In addition, we performed a systematic review in PubMed on MIC values of any PROMIS measure from studies using recommended approaches. A total of 50 studies estimated the MIC of a PROMIS measure, of which 19 studies used less appropriate methods. MIC values of the remaining 31 studies ranged from 0.1 to 12.7 T-score points. We recommend to use the predictive modeling method, possibly supplemented with the vignette-based method, in future MIC studies. We consider a MIC value of 2–6 T-score points for PROMIS measures reasonable to assume at this point. For surgical interventions a higher MIC value might be appropriate. We recommend more high-quality studies estimating MIC values for PROMIS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11136-021-02925-y.
Keywords: Minimal important change, Interpretation, Patient-reported outcomes, PROMIS, Methodology
Introduction
There are several ways to interpret change scores arising from patient-reported outcome measures (PROMs). One possible threshold is the minimal important change (MIC) estimate, which refers to the smallest change in score that patients consider important. The MIC is the lower bound of a distribution of thresholds for important change. There is a lot of confusion about the concept of MIC, which questions the validity of published MIC values [1, 2]. First, there is inconsistency in terminology used (e.g., minimal important change, minimal important difference, minimal clinically important difference, meaningful change threshold, to name a few). Similar terms may refer to different concepts and vice versa. Second, there is particular confusion about the concepts of minimal important change and minimal detectable change, which refer to different concepts [3, 4]. Third, there are differences in methods used for estimating the MIC, some more and some less methodologically sound [5]. This confusion hampers and may even bias the interpretation of PROM change scores in research and clinical practice.
An increasingly used, innovative set of PROMs is the Patient-Reported Outcomes Measurement Information System (PROMIS®). It covers domains of health-related quality of life (HRQOL), such as pain, fatigue, physical function, anxiety, depression, and the ability to participate in social roles and activities, that are commonly important for adults and children with and without (chronic) medical conditions [6, 7]. Most PROMIS measures are rooted in item response theory (IRT)-based item banks (i.e., large sets of calibrated questions measuring the same domain (construct)), which enables efficient measurement through fixed-length short forms and/or computerized adaptive testing (CAT) [8–10]. A number of studies have estimated MIC values for PROMIS measures. However, in light of its increasing use across the world [11–19], and the aforementioned confusion in the interpretation literature, additional guidance is needed on interpreting PROMIS change scores.
The aims of this study were: (1) to clarify the concept of MIC and how to use it; (2) to provide practical guidance for estimating methodologically sound MIC values; and (3) to improve the applicability of PROMIS by summarizing the available evidence on plausible PROMIS MIC values.
Part 1: the concept of MIC and how to use it
We define the MIC as a threshold for a minimal within-person change over time above which patients perceive themselves importantly changed. Assuming that all patients have their individual threshold of what they consider a minimal important change, the MIC can be conceptualized as the mean of these individual thresholds [20, 21]. This definition of MIC is made up of three important elements: first, it refers to a threshold for a minimal change above which patients perceive themselves as changed (improved or deteriorated). Second, it refers to a change that is considered important to patients. And third, it refers to a within-patient change over time.
These three elements do not only define what the MIC is but also clarifies what the MIC is not. The MIC does not refer to thresholds for changes that are considered more than minimal (e.g., a mean change in patients who reported to be “much better” is not a MIC). There are other relevant concepts that reflect meaningful change thresholds that are larger than minimal, such as Clinically Significant Change [22], Sufficiently Important Difference [23] or Smallest Worthwhile Effect [24]. These concepts are outside the scope of this paper.
Next, the MIC is not a minimal detectable change (MDC, also referred to as smallest detectable change (SDC)). The MDC is the smallest change in score than can be detected statistically with some degree of certainty (e.g., 95 or 90%), based on the standard error of measurement (SEM) or limits of agreement from a test–retest reliability design. The MDC does not relate to the importance of change to the patients under investigation [4, 25–27]. The MDC is also an important benchmark for interpreting PROM change scores, but it is also outside the scope of this paper.
Finally, the MIC is not a difference between (groups of) patients. For example, a difference between patients who reported to be “a little better” and those who reported to be “about the same” refers to a minimal important difference (MID), not a minimal important within-person change (MIC). The MID is another relevant benchmark for interpreting PROM scores but is also outside the scope of this paper.
The MIC, as defined above, can be used for different purposes. In research, some use the MIC value as a threshold to determine the number of responders in clinical trials or other studies (i.e., patients who have a change at least as large as the MIC value) [28, 29]. This responder definition adds a meaningful interpretation to study results from the patients’ perspective. In clinical practice, the MIC value can also be used to determine the number of responders in groups of patients who receive certain treatments to inform future patients about the expected effects of treatments. For example, a patient can be told that about 70% of patients experience a minimal important change after a given treatment. This may facilitate shared-decision making. However, it is necessary to acknowledge that the estimated MIC value is derived from a wider sample of patients, and the threshold may not apply to the individual patient in the clinical trial or in the consultation room. If a responder is defined as an individual whose PROM change score exceeds the MIC, then on a group level the percentage of responders will probably be correct. However, this doesn’t mean that all patients have been classified correctly, based on their individual PROM change score being smaller or greater than their individual MIC. This is because all patients have their own individual threshold of what they consider a minimal important change [20]. Furthermore, measurement error in the PROM change score further contributes to misclassification of individuals.
In addition to being used as a threshold for responder definitions, the MIC value can be used as a probabilistic value, rather than a deterministic cut-point, by clinicians to interpret change scores in light of the probability that an individual patient has experienced a meaningful change. For example, if the estimated MIC value of a PROM is 10 points and an individual patient has changed more than 10 points, it is more likely that the patient has importantly improved than that the patient has not importantly improved. This might help the clinician start a conversation with the patient.
Part 2: guidance for estimating MIC values
A variety of methods have been used in the literature to estimate MIC values [1, 30, 31]. Many methods, however, do not refer to the concept of MIC as described above. MIC methods are often categorized into distribution-based and anchor-based methods. Distribution-based methods use statistical parameters, such as a standard deviation (SD) or standard error of measurement (SEM) for estimating the MIC value. These parameters refer to measurement error (minimal detectable change) but do not relate to the importance of the change to the patients under investigation and, while they add useful context to interpreting MIC values, they do not capture the spirit of the MIC [3, 4, 27].
Anchor-based methods are generally more appropriate because they relate change scores on the instrument of interest to an external criterion of important change. Often, a single question at follow-up is being used as the external criterion (the anchor), asking patients how much they have changed, for example on a global 5- or 7-point rating scale ranging from “much worse” to “much better”. The most simple and prevalent method used to estimate the MIC value is the mean change method, where the MIC value (further referred to as MICmean) is defined as the change score on the measure of interest in the subgroup of patients that reported to be “a little better” (minimal important improvement) or “a little worse” (minimal important deterioration) on the anchor question [32]. Studies have shown that a MIC for improvement may not be the same as a MIC for deterioration [33–35]. The mean change method has some important drawbacks. First, the subgroup of patients who reported to be “a little better” is often small, which results in imprecise MICmean estimates. More importantly, the MICmean value does not reflect a threshold for minimal improvement because it is defined as the mean of the entire group of patients who reported to be “a little better”. As all patients in this group reported to be minimal importantly changed on the anchor, the mean change in score on the PROMs of interest in this group of patients is higher than the threshold for minimal important change. Finally, it has been shown that if the anchor is not completely accurate, MICmean estimates are more severely biased than other anchor-based methods and will always be biased downwards [36].
Two additional, more appropriate, anchor-based MIC methods are the ROC method and the MIC predictive modeling method, which are described in more detail below and in Online supplement 2. In addition, a relatively new qualitative method, based on comparing vignettes (descriptions of health status of hypothetical patients), is also described below.
ROC method
The Receiver Operating Characteristic (ROC) curve method is based on the ability of a measure to distinguish patients who reported to be improved from patients who reported to be not improved (i.e., stayed the same or worsened) on the anchor. The MIC value (further referred to as MICROC) is most often defined as the value for which the sum of the proportions of misclassifications ([1-sensitivity] + [1-specificity]) is smallest [32]. An advantage of this method is that it uses the entire study sample, leading to more reliable estimates than the MICmean. Moreover, it estimates the threshold between ‘not changed’ and ‘a little better’ (minimal important improvement) or ‘a little worse’ (minimal important deterioration). A disadvantage is that the MICROC will be biased if the percentage of improved patients is not 50% [20].
Predictive modeling method
The predictive modeling approach is based on the predicted probability that a patient belongs to the improved group (based on the anchor) given the observed change score [21]. This method uses logistic regression analysis with the group variable (improved versus not improved [stayed the same and worsen] on an anchor) as the dependent variable and the change score on the instrument of interest as the independent variable. The MIC value (further referred to as MICpredict) is defined as the change score associated with a likelihood ratio of 1, which is the change score where the posttest probability of belonging to the improved group (i.e., after knowing the patient’s PROM change score) equals the pretest probability of belonging to the improved group (before knowing the patient’s PROM change score, the pretest probability is the percentage of improved patients in the sample) [20, 21]. The MICpredict is more precise than the MICROC and a formula has been published to correct the MICpredict for bias if the percentage of improved patients is not 50% [20]. It is therefore considered as a better option than the MICROC. In Online supplement 2 we provide additional details and SPSS and R codes (See also [37]) for how MICROC and MICpredict can be calculated.
Vignette-based method
The anchor-based MIC methods described above depend on the reliability and validity of the anchor question, which has been criticized [30, 38, 39]. An alternative method for instruments with IRT-based scores is a vignette-based method, often referred to as bookmarking or standard setting. With this method, patients are asked to compare vignettes (descriptions of health status of hypothetical patients) in focus groups or in a survey [40–42]. Each vignette represents a health status with an associated score on the underlying IRT metric. Patients are asked to indicate whether a hypothetical change in health status from one vignette to another would be considered an important change. The MIC (further referred to as MICvignette) has been defined as the mean difference in scores between pairs of vignettes that represent a minimal important change. If the mean difference is used to estimate the MICvignette, this method may suffer a similar issue to MICmean in that it represents a value higher than the minimal threshold. Alternatively, it would also be possible to ask patients to rate the change between two (or more) vignettes on an anchor question and then use the predictive modeling method to estimate the MICpredict.
In Box 1 we provide a summary of general recommendations for the design and analysis of MIC studies.
Box 1: Recommendations for conducting and reporting MIC studies.
The predictive modeling or ROC method should be used over the mean change method because MICpredict and MICROC provide a threshold between improved and not improved patients [21], while the MICmean does not reflect a threshold for minimal improvement, but rather a mean in a subgroup of patients who considered themselves as minimally improved. The MICpredict is more precise than the MICROC and can be corrected for bias if the percentage of improved patients is not 50%, and is therefore recommended as best option. Vignette-based methods can also be considered or used in addition to the MICpredict or MICROC because they do not require a longitudinal study. The MICvignette is typically determined in a qualitative or survey study [40, 97].
The MICpredict or MICROC, should be determined in a longitudinal study, where patients complete the instrument of interest at baseline and again after a relevant time period (e.g., after an intervention). The most efficient design is one in which about half of the patients are expected to change (at least to a minimal important degree) on the domain of interest (e.g., physical function) and about half of the patients are expected not to change. If an intervention is applied between baseline and follow-up measurement, this intervention should be clearly described.
An anchor question should be completed by the patients at follow-up. The anchor question should measure the same construct as the instrument of interest. For example, for estimating the MIC of a fatigue instrument, the anchor question should state “how much has your fatigue changed since …”. The anchor question should refer to a change since the previous measurement (e.g., since before treatment). The anchor question can have 3–7 response options, ranging from “much worse” to “much better”. Patients who report to be “a little better” or more will be included in the improved group, while the rest of the patients will be included in the not improved group.
The sample size of the MIC study should be at least 100 patients [2]. Ideally, the percentage of patients in the improved group should be 50%. The percentage of improved patients should be reported. If the percentage of improved patients is not about 50%, the adjusted MICpredict should be used (see Online supplement 2) [20].
We recommend to plot the distributions of change scores in the improved group and in the not improved group (see Online supplement 2) to visualize how well the instrument of interest can distinguish between the improved and not improved patients [32].
The correlation between the change score on the instrument of interest and the anchor question should be at least 0.30 to assume validity of the anchor [2]. This correlation should be reported. If the correlation is too low, the data are not suitable for estimating MIC value.
A 95% confidence interval around the MIC value should also be calculated and reported (see Online supplement 2) [98].
Part 3: evidence on plausible MIC values of PROMIS measures
To summarize the available evidence on plausible MIC values of PROMIS measures we performed a search in PubMed from inception up to May 31, 2021 to identify all studies that estimated the MIC of one or more PROMIS measures.
Methods
We extracted relevant search terms from the COSMIN PubMed filter for finding studies on measurement properties [43]. The full search strategy is presented in Online supplement 3. One author (CBT) screened the abstracts.
We included studies that determined a MIC value for any PROMIS measure (adults and pediatric, any domain, any language, any version (e.g., v1.0, v2.0), full bank, short form or CAT) in any population. We extracted the following information: PROMIS measure(s) used (including domain, version number, administration type, language, age version) and country in which data were collected, study population, intervention(s), length of follow-up, sample size on which the MIC values(s) was/were based, MIC methods used, correlation between PROMIS change scores and the anchor (Spearman correlation if presented, otherwise Pearson correlation), percentage of patients improved based on the anchor (only for studies estimating MICROC or MICpredict), and MIC values.
We only extracted MIC values based on anchor-based methods or vignette-based methods. We did not extract distribution-based MIC values. We only extracted MIC values based on longitudinal anchors, referring to within-person change over time. We did not extract values based on cross-sectional anchors, referring to minimal important differences between groups of patients (e.g., difference between patients who reported to be “slightly improved” and patients who reported to be “not changed” [44] or differences between patients with different levels of disease [45]) because these values refer to a minimal important difference (MID) rather than a minimal important change (MIC). When MIC values of other instruments were used as an anchor, we checked whether these MIC values were based on anchor-based methods. Furthermore, we did not extract MIC values that referred to more than a minimal important change (for example, MICmean values based on mean changes in patients who reported to be “much better” were not included). We extracted MIC values for minimal important improvement and for minimal important deterioration separately. MIC values determined in groups of less than 10 patients were not extracted. Data extraction was initially performed by one author (either JDP, RC, PG, or CBT) for each paper, and extracted data were checked by another author (CBT or LBM). Missing information (for example, regarding the version numbers of PROMIS measures used) was requested by email (by CBT) to the primary authors of the papers.
All PROMIS measures are scored on a T-score metric, in which 50 is the mean of a relevant reference population (often a general population) with a standard deviation (SD) of 10. Higher scores mean more of the concept being measured (e.g., worse fatigue, better physical function).
Results
The search yielded 911 abstracts, including 50 studies that estimated a MIC value of a PROMIS measure [41, 44–92]. All studies used self-reported PROMIS data, no studies on proxy-reported data were found. Of these 50 studies, 10 studies used only distribution-based methods [49, 50, 52, 55, 58, 66, 68, 74, 75, 77]; five studies estimated a minimal important difference (MID) rather than minimal important change (MIC) [44, 62, 63, 72, 73]; one study averaged estimates based on cross-sectional and longitudinal anchors as well as distribution-based estimates [84]; one study estimated a MIC value that referred to more than a minimal important change [92]; and two studies intended to calculate an anchor-based MIC but reported only a distribution-based MIC because the area under the ROC curve was considered too low [82, 83]. Data from these 19 studies were not extracted.
MIC values from the remaining 31 studies were extracted and presented in Tables 1, 2, 3 (See also Tables S1 through S11 in Online supplement 1) [41, 44–48, 51, 53, 54, 56, 57, 59–61, 64, 65, 67, 69–71, 76, 78–81, 85–91]. Twenty-eight of these 31 studies used anchor-based methods. Anchor-based MIC values from these studies were extracted. Distribution-based MIC values that were also presented in 17 of these studies were not extracted [45–48, 53, 54, 59, 64, 69–71, 78, 81, 85–87, 91], three MIC values based on cross-sectional anchors were not extracted [45, 46, 48], and one MIC value based on patients who experienced a “meaningful change” (more than minimal) was also not extracted [53]. Out of the 28 anchor-based studies, 24 used (a variation of) a mean change method [45–48, 51, 53, 54, 55, 59, 60, 64, 65, 69–71, 76, 78, 79, 85–89, 91], five studies used an ROC method [53, 54, 56, 67, 81], of which two studies used both methods [53, 54], and one study used the predictive modeling method [90]. In addition to the 28 studies that used anchor-based methods, the MIC values of three studies that used a vignette method to estimate MIC values were also extracted [41, 61, 80].
Table 1.
Minimal important change values for adult PROMIS pain interference
| References | PROMIS measure | Language | Population | Intervention | Method used | Follow-up | Na | Correlation of PROMIS change score with anchor | MIC valueb | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Amtmann et al. [46] | V1.0 pain interference CAT | English (US) | Low back pain | Epidural steroid injection or antidepressants, psychotherapy, or both | Mean change in patients that reached an MIC on other PROMs (2–3 point positive or negative change on NRS average and worse pain intensity, 5–10 points on RMDQ, 0.5–1.0 × SD on BPI) | 1 Month | Subgroups of 414 | Not reported | 3.5–5.5 |
Sample size on which MIC is based not reported Anchor for BPI distribution-based Improved and deteriorated patients combined |
| Bartlett et al. [78] |
V1.0 pain interference 8a V1.0 pain interference 4a |
English (US) | Rheumatoid arthritis | Medication | Mean change of individuals responding a little better or a little worse on an anchor question on change in pain interference | 4.6 (2.4) Months | 51 | Not reported | Improvement: 1.1 | |
| 60 | Deterioration: 1.8 | |||||||||
| 50 | Improvement: 1.1 | |||||||||
| 59 | Deterioration: 1.7 | |||||||||
| Beaumont et al. [79] | V1.1 pain interference 6b | English (US) | Rheumatoid arthritis | Not reported | Mean change of individuals responding a little better or a little worse on an anchor question on response to treatment | 6 Months | 60 | 0.24 | Improvement: 1.9 | |
| 126 | Deterioration: 0.6 | |||||||||
| 12 Months | 53 | 0.29 | Improvement: 1.8 | |||||||
| 122 | Deterioration: 1.5 | |||||||||
| Bernstein et al. [47] | V1.0 pain interference CAT | English (US) | Carpal tunnel release | Surgery | Mean change in subgroup that reached an MIC on MHQ Pain (13 points) or BCTQ (0.74 points) | 6 Weeks or 3 months | 52 (MHQ) | Not reported | 8.9 | MIC of MHQ pain was based on MHQ satisfaction, where ‘satisfied’ was defined based on effect size (distribution-based) |
| 40 (BCTQ) | 9.7 | |||||||||
| Bingham et al. [80] | V1.1 pain interference | English (US) | Rheumatoid arthritis | NA | Qualitative bookmarking method | NA | 11 | NA | Improvement: 10 | |
| Deterioration: 10 | ||||||||||
| Chen et al. [48] | V1.0 pain interference 4a, 6a, 6b, 8a | English (US) | Chronic low back pain | Pharmacological or behavioral approaches | Mean change scores corresponding to a 1-point change in prospective change in global rating of pain (range − 1 to +4) | 6 Months | Subgroups of 261 | 0.23–0.49 | Improvement: 2.3 | Sample size on which MIC is based not reported |
| Deterioration: 3.8 | ||||||||||
| Mean change scores corresponding to one category shift on an anchor question on change in pain; | Improvement: 2.4 | |||||||||
| Deterioration: 1.9 | ||||||||||
| Chen et al. [48] | V1.0 pain interference 4a, 6a, 6b, 8a | English (US) | Chronic back pain or hip or knee osteoarthritis pain | Opioid therapy or non-opioid medication | Mean change scores corresponding to a 1-point change in prospective change in global rating of pain (range − 1 to +4) | 3 Months | Subgroups of 240 | 0.23–0.49 | Improvement: 3.8 | Sample size on which MIC is based not reported |
| Deterioration: 3.8 | ||||||||||
| Mean change scores corresponding to one category shift on an anchor question on change in pain | Improvement: 2.4 | |||||||||
| Deterioration: 2.4 | ||||||||||
| Chen et al. [48] | V1.0 pain interference 4a, 6a, 6b, 8a | English (US) | Stroke survivors | Stroke self-management | Mean change scores corresponding to a 1-point change in prospective change in global rating of pain (range − 1 to +4) | 3 Months | Subgroups of 258 | 0.23–0.49 | Improvement: 3.1 | Sample size on which MIC is based not reported |
| Deterioration: 5.6 | ||||||||||
| Mean change scores corresponding to one category shift on an anchor question on change in pain | Improvement: 2.0 | |||||||||
| Deterioration: 3.1 | ||||||||||
| Forlenza et al. [81] | Pain interference CAT | English (US) | Biceps tenodesis | Surgery | Optimal ROC cut-off point to distinguish patients who reported improvement from patients who reported no improvement on an anchor question on overall function of the shoulder | 7.6 (6.0–9.3) months | 112 | Not reported | 5.6 | |
| Hung et al. [53] | V1.1 pain interference CAT | English (US) | Foot and ankle disorders | Surgery | Optimal ROC cut-off point to distinguish patients who reported much worse, worse, improved and much improved from patients who reported slightly worse, no change, slightly improved | 3 Months |
64 vs 41 (61% changed) |
Not reported | 8.0 | |
| > 3 Months |
321 vs 187 (63% changed) |
12.4 | ||||||||
| > 6 Months |
170 vs 100 (63% changed) |
5.5 | ||||||||
| Katz et al. [69] | V1.1 pain interference 4a | English (US) | Rheumatic diseases (> 93% RA) | Not reported | Mean change of individuals responding somewhat better or somewhat worse on an anchor question on change in pain interference. MIC also calculated per baseline category of NRS pain (low, moderate, high) | Average of three periods of 6 months | ± 400 | Not reported |
Improvement: Total: 1.7 Low baseline pain: 0.4 Moderate baseline pain: 2.2 High baseline pain: 4.1 |
Each patient was included three times in the analyses |
| > 550 |
Deterioration: Total: 1.8 Low baseline pain: 5.0 Moderate baseline pain: 1.7 High baseline pain: 0.4 |
|||||||||
| Katz et al. [70] | V1.1 pain interference 4a | English (US) | SLE | Not reported | Mean change of individuals responding somewhat better or somewhat worse on an anchor question on change in function | 6 Months | 29 | Not reported | Improvement: 2.0 | |
| 37 | Deterioration: 1.9 | |||||||||
| Kenney et al. [56] | Pain interference CAT | English (US) | Patients undergoing knee arthroscopy | knee arthroscopy | Optimal ROC cut-off point to distinguish patients who reached a MIC (11.5 points on 0–100 scale) on the IKDC | 2 Weeks to 12 months |
51 vs 25 (67% improved) |
− 0.67 | 3.2 | MIC (slightly) overestimated because percentage of improved patients > 50% |
| Khutok et al. [87] | Pain interference 4a (part of PROMIS-29 v2.1) | Thai | Chronic low back pain | Many received standard physical therapy | Mean change of individuals reporting little improvement on an anchor question on change in pain interference | 4 Weeks | 49 | 0.19 | 1.6 | |
| Kuhns et al. [88] | Pain interference CAT | English (US) | Patients undergoing hip arthroscopy, (including 61 athletes) | Hip arthroscopy | Optimal ROC cut-off point to distinguish patients who reported their level of function after surgery excellent or good versus patients who reported their level of function after surgery average, fair or poor | 21.3 (± 4.4) months | 113 | Not reported | 10.9 | Anchor question about current (postoperative) level of functioning instead of change |
| Lee et al. [60] | V1.0 pain interference 6b | English (US) | Adults (40 +) with Knee OA | Tai Chi or physical therapy | Mean change in patients that reached 1–2 MICs on SF-36 subscale | 12 Weeks | 20–30 | 0.53 | 2.4 |
Unclear which MIC values for SF-36 were used and whether they were anchor-based Lower bound of MICs was increased to > SEM |
| Schwartz et al. [65] | V1.0 pain interference 6b | English (US/Canada) | Disc herniation or spinal stenosis | Surgery | Mean change at each follow-up time point for patients who reported being somewhat better | Baseline-6 weeks | 32 | Not reported | 6.5 | |
| 6 Weeks-3 months | 32 | 0.7 | ||||||||
| 3–6 Months | 23 | 1.8 | ||||||||
| 6–12 Months | 16 | 0.9 | ||||||||
| Stephan et al. [99] | V1.0 pain interference 4a | German (Switzerland) | Foot and ankle disorders | Orthopedic foot and ankle surgery | Optimal ROC cut-off point to distinguish patients who reported operation did help or operation helped a lot from patients who reported operation helped only a little, did not help or made things worse | 6 Months | 166 vs 36 (82% improved) | 0.45 | 5.0 |
Anchor does not refer to change in pain interference MIC overestimated due to high percentage of patients improved |
| Yost et al. [45] | V1.1 Cancer Pain Interference− 10 | English (US) | Advanced-stage cancer |
Chemotherapy only (74.3%) Chemo- and radiation therapy (9.9%) Other mixed modalities (13.8%) Missing 2.0% |
Mean change in patients who changed 2–3 points on an anchor with range – 10 to 10 Mean change in patients who changed 1–2 MICs on BPI [0.5 × SD (1.4 points)] Mean change in patients who reported a little better or moderately better or a little worse or moderately worse |
6–12 Weeks | Subgroups of 88 | > 0.30 | Median 3.5 (range 2.2–4.8) |
PROMIS Cancer scales are on the same metric as the PROMIS generic item banks Estimates for improvement and deterioration were lumped together MIC on BPI distribution-based |
BCTQ boston carpal tunnel questionnaire, BPI brief pain inventory, CAT computerized adaptive testing, IKDC international knee documentation committee; MHQ Michigan hand questionnaire, MIC minimal important change, NA not applicable, NRS numerical rating scale, OA osteoarthritis, PROMs patient-reported outcome measures, PROMIS patient-reported outcomes measurement information system, RA rheumatoid arthritis, RMDQ roland morris disability questionnaire, ROC receiver operating characteristics, SF-36 short form 36
aN reflects the number of patients on which the presented MIC values are based (often a subset of the study population)
bMIC values for minimal important improvement, unless otherwise specified. For all values, higher MIC values indicate more improvement or more deterioration for the construct being measured
Table 2.
Minimal important change values for adult PROMIS physical function
| References | PROMIS measure | Language | Population | Intervention | Method used | Follow-up | Na | Correlation of PROMIS change score with anchor | MIC valueb | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartlett et al. [78] | V1.0 Physical Function 20a | English (US) | Rheumatoid arthritis | Medication | Mean change of individuals responding a little better or a little worse on an anchor question on change in physical function | 4.6 (2.4) Months | 33 | Not reported | Improvement: 2.8 | |
| 46 | Deterioration: 1.4 | |||||||||
| 33 | Improvement: 2.5 | |||||||||
| V1.0 physical function 4a | 45 | Deterioration: 1.8 | ||||||||
| Bernstein et al. [47] | V1.0 physical function | English (US) | Carpal tunnel release | Surgery | Mean change in subgroup who reached a MIC on MHQ Function (23 point) or BCTQ (0.74 points) | 6 Weeks or 3 months | 52 (MHQ) | Not reported | 1.8 | MIC of MHQ Function was based on MHQ satisfaction, where ‘satisfied’ was defined based on effect size (distribution-based) |
| 40 (BCTQ) | 2.8 | |||||||||
| Hays et al. [51] | V1.0 physical function 19 (out of 20a) | English (US) | Rheumatoid arthritis | Any treatment | Mean change in patients who reported “a little better” or “a little worse” on an anchor question on how you are feeling now compared to 6 months ago |
0–6 Months 6–12 months |
35 | 0.33 |
Improvement: 1.6 2.0 |
Anchor question does not refer to change in physical function |
|
0–6 Months 6–12 months |
113 |
Deterioration: 0.8 1.7 |
||||||||
| Hung et al. [53] | V1.2 physical function CAT | English (US) | Foot and ankle disorders | Surgery | Optimal ROC cut-off point to distinguish patients who reported much worse, worse, improved and much improved from patients who reported slightly worse, no change, slightly improved on an anchor question on change in physical function | 3 Months |
32 vs 42 (43% changed) |
Not reported | 12.0 | Patients who were improved and patients who were deteriorated were lumped together in the analyses |
| > 3 Months |
219 vs 337 (39% changed) |
7.9 | ||||||||
| > 6 Months |
128 vs 178 (42% changed) |
10.5 | ||||||||
| Hung et al. [54] | V1.2 physical function CAT | English (US) | Pathology of the hip or knee | Surgical and non-surgical interventions | Optimal ROC cut-off point to distinguish patients who reported much worse, worse, improved and much improved from patients who reported slightly worse, no change, slightly improved on an anchor question on change in physical function | 3 Months |
54 vs 88 (38% improved) |
Not reported | 2.0 | |
| > 3 Months |
366 vs 577 (39% improved) |
3.4 | ||||||||
| 6 Months |
21 vs 34 (38% improved) |
3.5 | ||||||||
| > 6 Months |
192 vs 421 (31% improved) |
8.2 | ||||||||
| Katz et al. [70] | Physical Function 4a | English (US) | SLE | Not reported | Mean change of patients who reported somewhat better on an anchor question on change in functioning | 4 Periods of 6 months | 141 | Not reported | Improvement: 0.8 | Each patient was included up to 4 times in the analysis |
| 169 | Deterioration: 1.1 | |||||||||
| Kazmers et al. [86] | V2.0 physical function CAT | English (US) | Non-shoulder hand and upper extremity pathology | Recovering from surgery, undergoing surgery, corticosteroid injection, other | Mean difference between patients reporting no change and patients reporting (slightly) improved on an anchor question (2 anchor questions) | 6 (± 4) Weeks | 381 | Not reported | 2.1, 3.6 | |
| Kenney et al. [56] | Physical function CAT | English (US) | patients undergoing knee arthroscopy | knee arthroscopy | Optimal ROC cut-off point to distinguish patients who reached a MIC (11.5 points on 0–100 scale) on the IKDC | 2 Weeks to 12 months |
51 vs 25 (67% improved) |
0.76 | 3.3 | Version not reported |
| Khutok et al. [87] | Physical function 4a (part of PROMIS-29 v2.1) | Thai | Chronic low back pain | Many received standard physical therapy | Mean change of individuals reporting little improvement on an anchor question on change in pain interference | 4 Weeks | 54 | 0.26 | 0.1 | |
| Kuhns et al. [88] | Physical function CAT | English (US) | Patients undergoing hip arthroscopy, (including 61 athletes) | Hip arthroscopy | Optimal ROC cut-off point to distinguish patients who reported their level of function after surgery excellent or good versus patients who reported their level of function after surgery average, fair or poor | 21.3 (± 4.4) Months | 113 | Not reported | 5.1 | Anchor question about current (postoperative) level of functioning instead of change |
| Lapin et al. [59] | V1.0 physical function CAT | English (US) | Ischemic and hemorrhagic stroke patients | Routine care | Mean change in patients who indicated minimally or much improved/worse on an anchor on change in function | 5–6 Months | 167 | 0.28 | Improvement: 4.0 (± 7.4) | Minimally and much improved patients were combined, MIC likely overestimated |
| 34 | Deterioration: 0.3 (± 7.0) | |||||||||
| Lee et al. [60] | V1.0 physical function 10a | English (US) | Adults (40+) with knee OA | Tai Chi or physical therapy | Mean change in patients that reached 1–2 MICs on SF-36 PF | 12 Weeks | 13–28 | 0.59 | 1.9 to 2.2 |
Unclear which MIC values for SF-PF were used and whether they were anchor-based Lower bound of MICs was increased to > SEM |
| Lee et al. [76] | V1.2/V2.0 physical function CAT | English (US) | Thumb carpometacarpal arthritis | Operative or non-operative treatment | Mean change score in the mild improvement group according to an anchor question on change in your condition since last visit | 63 days (IQR 42–125) | 70 | Not reported | 3.9 (95% CI 3.3–4.7) | Anchor question does not refer to change in physical function |
| Sandvall et al. [64] | V1.2/V2.0 physical function CAT | English (US) | Unilateral distal radius fracture | Nonsurgical care | Mean change in patients who reported mildly better on an anchor question on change in your condition | Median 35 days (IQR, 25 − 45) | 138 | Not reported | 3.6 | Anchor question does not refer to change in physical function |
| Smit et al. [90] | 24-item PROMIS physical function geriatric rehabilitation (PROMISPF-GR) short form | Dutch (NL) | patients admitted for geriatric inpatient rehabilitation | Usual care in nursing homes | predictive modeling to distinguish patients who reported to be improved versus patients who reported to be unchanged on an anchor question on change in physical function | 41 Days | 167 | 0.32 | 8.0 (95% CI 4.1–12.5) | |
| Speck et al. [91] | V1.0 physical function 13-item custom short form | English (US, Canada, EU, and Australia) | Tenosynovial giant cell tumors | pexidartinib or placebo | Mean change in patients who changed 1 point on a 5-point global rating of physical function | 25 Weeks | 27 | Not reported | 4.0 | Design and sample size not clearly reported |
| Stephan et al. [99] | V2.0 physical function 4a | German (Switzerland) | Foot and ankle disorders | Orthopedic foot and ankle surgery | Optimal ROC cut-off point to distinguish patients who reported operation did help or operation helped a lot from patients who reported operation helped only a little, did not help or made things worse | 6 Months |
166 vs 36 (82% improved) |
0.45 | 4.6 |
Anchor does not refer to change in physical function MIC overestimated due to high percentage of patients improved |
| Yost 2011 [45] | V1.1 cancer physical function-10 | English (US) | Advanced-stage cancer |
Chemotherapy only (74.3%) Chemo- and radiation therapy (9.9%) Other mixed modalities (13.8%) Missing 2.0% |
Mean change in patients who changed 1–2 MICs on SF-36 PF (8–16 points) Mean change in patients who reported a little better or moderately better or a little worse or moderately worse |
6–12 Weeks | Subgroups of 88 | > 0.30 |
Median 3.0 (range 3.0–3.0) |
PROMIS Cancer scales are on the same metric as the PROMIS generic item banks Estimates for improvement and deterioration were lumped together MIC of SF-36 partly based on distribution-based methods |
BCTQ boston carpal tunnel questionnaire, CAT computerized adaptive testing, IKDC international knee documentation committee, IQR inter quartile range, MIC minimal important change, MHQ Michigan hand questionnaire, NA not applicable, NRS numerical rating scale, OA osteoarthritis, PROMIS patient-reported outcomes measurement information system, ROC receiver operating characteristics, SEM standard error of measurement, SF-36 PF short form 36, physical function subscale
aN reflects the number of patients on which the presented MIC values are based (often a subset of the study population)
bMIC values for minimal important improvement, unless otherwise specified. For all values, higher MIC values indicate more improvement or more deterioration for the construct being measured
Table 3.
Minimal important change values for adult PROMIS fatigue
| References | PROMIS measure | Language | Population | Intervention | Method used | Follow-up | Na | Correlation of PROMIS change score with anchor | MIC valueb | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartlett et al. [78] | V1.0 fatigue 8a | English (US) | Rheumatoid arthritis | Medication | Mean change of individuals responding a little better or a little worse on an anchor question on change in fatigue | 4.6 (2.4) months | 37 | Not reported | Improvement: 2.7 | |
| 62 | Deterioration: 1.3 | |||||||||
| V1.0 fatigue 7a | 37 | Improvement: 2.6 | ||||||||
| 62 | Deterioration: − 0.3 | |||||||||
| V1.0 fatigue 4a | 37 | Improvement: 3.3 | ||||||||
| 60 | Deterioration: 1.2 | |||||||||
| Beaumont et al. [79] | V1.0 fatigue 7a | English (US) | Rheumatoid arthritis | Not reported | Mean change of individuals responding a little better or a little worse on an anchor question on change in fatigue | 6 months | 41 | 0.29 | Improvement: 2.6 | Correlation with anchor very low |
| 119 | Deterioration: 1.7 | |||||||||
| 12 months | 31 | 0.13 | Improvement: 1.3 | |||||||
| 133 | Deterioration: 0.9 | |||||||||
| Bingham et al. [80] | V1.0 fatigue | English (US) | Rheumatoid arthritis | NA | Qualitative bookmarking method | NA | 11 | NA | Improvement: 5 | |
| Deterioration: 10–15 | ||||||||||
| Katz et al. [70] | V1.0 fatigue 4a | English (US) | SLE | Not reported | Mean change of individuals responding somewhat better or somewhat worse on an anchor question on change in function | 6 months | 26 | Not reported | Improvement: 2.3 | |
| 57 | Deterioration: 1.9 | |||||||||
| Khutok et al. [87] | Fatigue 4a (part of PROMIS-29 v2.1) | Thai | Chronic low back pain | Many received standard physical therapy | Mean change of individuals reporting little improvement on an anchor question on change in pain intensity | 4 weeks | 45 | 0.13 | 1.9 | |
| Lapin et al. [59] | V1.0 fatigue CAT | English (US) | Ischemic and hemorrhagic stroke patients | Routine care | Mean change in patients who indicated minimally or much improved/worse | 5–6 months | 140 | 0.27 | Improvement: 2.4 (± 8.3) | Minimally and much improved patients were combined, MIC likely overestimated |
| 29 | Deterioration: 3.0 (± 7.7) | |||||||||
| Yost et al. [45] | V1.0 cancer fatigue-17 | English (US) | Advanced-stage cancer |
Chemotherapy only (74.3%) Chemo- and radiation therapy (9.9%) Other mixed modalities (13.8%) Missing 2.0% |
Mean change in patients who had a one point change on an anchor with range − 4 to 4 | 6–12 weeks | Subgroups of 88 | > 0.30 | Median 2.6 (range 1.9–3.6) | Cancer scales are on the same metric as the generic item banks |
| Mean change in patients who changed 1–2 MICs on FACIT-Fatigue (3–6 points) | ||||||||||
| V1.0 cancer fatigue-7 | Mean change in patients who reported a little better or moderately better or a little worse or moderately worse | Median 2.4 (range 1.9–4.2) | Estimates for improvement and deterioration were lumped together |
CAT computerized adaptive testing, FACIT functional assessment of chronic illness therapy, MIC minimal important change, PROMIS patient-reported outcomes measurement information system
aN reflects the number of patients on which the presented MIC values are based (often a subset of the study population)
bMIC values for minimal important improvement, unless otherwise specified. For all values, higher MIC values indicate more improvement or more deterioration for the construct being measured
Out of the 28 studies that used anchor-based methods 12 studies reported the correlation between the PROMIS change scores and the anchor. These correlations ranged from 0.02 to 0.76.
In several studies MIC values were presented for more than one PROMIS item bank. Regarding the adult PROMIS item banks, most MIC estimates were found for Pain Interference [17 studies, including 19 patient samples, MIC values for improvement ranged from 0.7 to 12.4 (Table 1)] and Physical Function [18 studies, MIC values for improvement ranged from 0.1 to 12.0 (Table 2)]. Multiple studies were found for Fatigue [7 studies, MIC values for improvement ranged from 1.3 to 5 (Table 3)], Anxiety [5 studies, MIC values for improvement ranged from 2.3 to 3.5 (Table S1 is found in Online Supplement 1)], Depression [4 studies, MIC values for improvement ranged from 1.5 to 3.7 (Table S2 is found in Online Supplement 1)], Upper Extremity [4 studies, MIC values for improvement ranged from 3.0 to 10.3 (Table S3 is found in Online Supplement 1)], Sleep Disturbance [3 studies, MIC values for improvement ranged from 0.9 to 2.4 (Table S4 is found in Online Supplement 1)], Ability to Participate in Social Roles and Activities [3 studies, MIC values for improvement ranged from 0.4 to 2.2 (Table S5 is found in Online Supplement 1)], and Pain Intensity [2 studies, MIC values for improvement ranged from 1.2 to 4.0 (Table S7 is found in Online Supplement 1)]. For the domains Satisfaction with Social Roles and Activities, Gastrointestinal Symptoms, Itch, and Global Health, only one study was found (Tables S6, S8, S9, S10, S11 is found in Online Supplement 1).
Only two studies estimated MIC values for five different PROMIS pediatric item banks (Mobility, Upper Extremity, Pain Interference, Fatigue, and Depressive Symptoms, Table S11), with MIC values ranging from 0.1 to 12.7 [41, 61].
Discussion
We defined the minimal important change (MIC) as a threshold for a minimal within-person change over time above which patients perceive themselves importantly changed. Assuming that all patients have their individual threshold of what they consider a minimal important change, the MIC can be conceptualized as the mean of these individual thresholds. The MIC can be used to determine the number of responders in a group of patients to interpret study results or to inform patients about expected treatment results, or to help clinicians to estimate the probability that an individual patient has experienced a meaningful change, facilitating a conversation with the patient.
There is no perfect MIC method. Distribution-based methods are not appropriate because they do not relate to the importance of the change to patients. We consider the predictive modeling method the most appropriate anchor-based method, because, unlike the mean change method, it refers to a threshold for minimal important change. Moreover, the MICpredict is more precise than the MICROC and a formula has been published to correct the MICpredict for bias if the percentage of improved patients is not 50% [20]. A disadvantage of all anchor-based MIC methods is the concern about the reliability and validity of the anchor question. The relatively new vignette-based method does not depend upon an anchor question, but the MICvignette may represent a value higher than a minimal threshold if based on mean differences between vignettes. We recommend the predictive modeling method, possibly supplemented with the vignette-based method if time and knowledge to design vignettes and recruit patients for that kind of study is available.
Our systematic review showed that published MIC estimates for PROMIS measures vary widely (larger than the range of MIC estimates currently published on the HealthMeasures website [93]) and were often generated by less appropriate methods. The lower end of the observed range of MIC values (0.1 T-score points) is, in our opinion, implausible as a MIC threshold. The highest MIC values (7 T scores points of higher) were almost all found in adult patients undergoing surgery. It has been suggested before that an invasive procedure like surgery might require a higher change to be considered an important improvement, but results in the literature have been inconsistent [94, 95]. For non-surgical interventions, we consider a MIC value of 2–6 points (covering about two thirds of the published MIC values) reasonable to assume at this point. There is not enough evidence yet to make more specific domain-specific or population-specific recommendations. Further studies are needed to examine whether MIC values differ across domains or between adults and children.
We particularly noticed several methodological concerns which might result in such a wide range of MIC estimates. First, most of these studies used the mean change method, which may represent a value higher than a minimal threshold. We did not exclude these results because this method is currently the most widely used method in the field (despite the critiques raised here) and only five studies used the ROC method, one study used the predictive modeling method [90], and three studies used a vignette-based method. In theory, it is likely that MICmean values represent an overestimation of the MIC (Fig. 1); however, many reported MIC values were rather low. Second, sample sizes on which the MIC estimates were based were often small. Third, some studies used the MIC of another instrument as an anchor. These MIC values were sometimes untraceable, based on the MIC value of yet another instrument, based on instruments that may not measure a sufficiently-similar construct or that lack evidence for responsiveness, or based on distribution-based methods. Fourth, only 12 out of 28 anchor-based studies presented the correlation between the PROMIS change score and the anchor question and about one third of the correlations were lower than 0.30 (excluding these values would not change our conclusions). Fifth, in some studies it was not clear whether the MIC estimate was based on patients who improved minimally. Sixth, in some studies the lower bound of recommended MIC values was increased to the SEM. However, the SEM represents the amount of measurement error and does not reflect changes that patients consider important. For this reason, setting a MIC lower bound to be in the detectable range may eliminate changes that patients find important. More broadly, researchers should be mindful of instruments with large measurement error and attempt to reduce the measurement error (e.g., using CAT), instead of adjusting the MIC value [3]. Finally, in some studies improved and deteriorated patients were combined together, while the MIC for improvement might be different than the MIC for deterioration, making inferences about the estimated MIC difficult [31, 33–35].
Fig. 1.
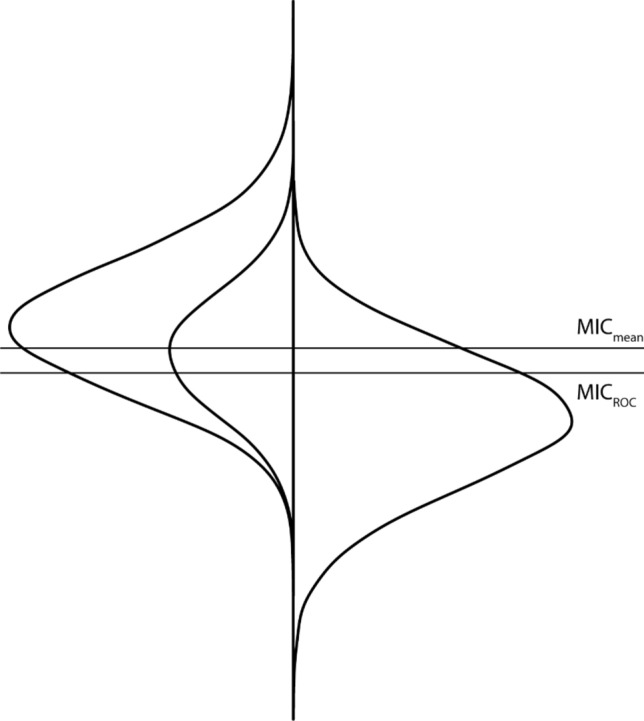
MICmean and MICROC. On the left, the distribution of change scores in all patients who are ‘improved’ (larger distribution) and in patients who are ‘a little better’ (smaller distribution), on the right the distribution of change scores in patients who are ‘not improved’. The upper line represents the MICmean (based on the smaller distribution on the left side), the lower line represents the MICROC (based on the larger left-sided distribution and the right-sided distribution)
Another problem is that important details of the MIC studies were often not reported, such as version numbers (while different versions of PROMIS measures may have a different metric), percentage of patients improved, correlation between the PROMIS change score and the anchor, and samples size on which MIC value was based. Recently, a reporting guideline for all publications using PROMIS and other HealthMeasures instruments was published [96]. We strongly recommend PROMIS users to use these reporting recommendations. A reporting guideline for MIC studies is being developed by an international group led by researchers from McMaster University, Canada (personal communication).
To gain more insight in the meaning of PROMIS change scores, more high-quality MIC studies are needed. To increase the understanding of the concept of MIC and improve the field, we need to agree on a clear definition of the MIC and report MIC values that are based on this definition. We recommend not to publish MIC values based on data where the correlation between the change score and the anchor is too low. We recommend to report the anchor correlations and state that the low correlation prevents MIC estimation, rather than publish MIC values based on distribution-based methods. We offer recommendations for conducting MIC studies (Box 1) that may help preventing the situation where the correlation between the change score and the anchor is too low. Alternatively, we recommend to use vignette-based methods. The recommendations in Box 1 can also be used to re-analyze existing data. More data are also needed to examine whether the MIC value differs across the PROMIS metric and across settings (e.g., duration of disease, kind of intervention, length of follow-up) [26]. In case researchers need to analyze a study (e.g., responders in a clinical trial) and no credible anchor-based MIC value is available, researchers could decide to use a distribution-based value, such as 0.5 × SD, or use a range of different values in a sensitivity analysis, but we argue that these values should not be called MIC values because distribution-based values refer to the concept of measurement error and are not based on the concept of MIC. However, as stated in part 1, researchers should keep in mind that the estimated MIC value is derived from a wider sample of patients, and the MIC threshold or responder classification may not apply to the individual patient in the clinical trial or in the consultation room.
This study has some limitations. First, we only searched PubMed and the abstracts were screened by one author only, so we may have missed some MIC studies. Second, we based our review on one definition of minimal important change and excluded studies and MIC estimates that were not in line with this definition. Others may have different opinions, and the excluded studies and estimates may nevertheless provide relevant information about the interpretation of PROMIS (change) scores. Strong points of the study were that data extraction was checked by a second author and missing information was requested by email from the corresponding authors of the papers.
In conclusion, 50 studies estimated the MIC of a PROMIS measure, of which 19 studies used less appropriate methods. MIC values of the remaining 31 studies ranged from 0.1 to 12.7 T scores points. We consider a MIC value of 2–6 T-score points for PROMIS measures reasonable to assume at this point. For surgical interventions a higher MIC value might be appropriate. We recommend more high-quality studies estimating MIC values for PROMIS. This paper provides recommendations for designing and analyzing future MIC studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No funding was received for conducting this study.
Declarations
Conflict of interest
D. Cella was co-author on one of the included PROMIS MIC papers [44] and CB. Terwee was co-authors of another included PROMIS MIC paper [89], but both were not involved in the data extraction of these papers. CB. Terwee and D. Cella are board members of the PROMIS Health Organization. The other authors have no conflicts of interest to declare that are relevant to the content of this article.
Research involving human participants and/or animals
This study does not include human participants or animals.
Informed consent
Because the study does not include human participants, informed consent is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.King MT. A point of minimal important difference (MID): A critique of terminology and methods. Expert Review of Pharmacoeconomics & Outcomes Research. 2011;11(2):171–184. doi: 10.1586/erp.11.9. [DOI] [PubMed] [Google Scholar]
- 2.Devji T, Carrasco-Labra A, Qasim A, Phillips M, Johnston BC, Devasenapathy N, Zeraatkar D, Bhatt M, Jin X, Brignardello-Petersen R, Urquhart O, Foroutan F, Schandelmaier S, Pardo-Hernandez H, Vernooij RW, Huang H, Rizwan Y, Siemieniuk R, Lytvyn L, Patrick DL, Ebrahim S, Furukawa T, Nesrallah G, Schünemann HJ, Bhandari M, Thabane L, Guyatt GH. Evaluating the credibility of anchor based estimates of minimal important differences for patient reported outcomes: instrument development and reliability study. BMJ. 2020;369:m1714. doi: 10.1136/bmj.m1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vet HC, Terwee CB. The minimal detectable change should not replace the minimal important difference. Journal of Clinical Epidemiology. 2010;63(7):804–805. doi: 10.1016/j.jclinepi.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 4.de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: Distinction between minimally detectable change and minimally important change. Health and Quality of Life Outcomes. 2006;4:54. doi: 10.1186/1477-7525-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terwee CB. Estimating minimal clinically important differences and minimal detectable change. Journal of Hand Surgery. 2019;44(12):e1. doi: 10.1016/j.jhsa.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The patient-reported outcomes measurement information system (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorner JB, Chang CH, Thissen D, Reeve BB. Developing tailored instruments: Item banking and computerized adaptive assessment. Quality of Life Research. 2007;16(Suppl 1):95–108. doi: 10.1007/s11136-007-9168-6. [DOI] [PubMed] [Google Scholar]
- 9.Cook KF, O'Malley KJ, Roddey TS. Dynamic assessment of health outcomes: Time to let the CAT out of the bag? Health Services Research. 2005;40(5 Pt 2):1694–1711. doi: 10.1111/j.1475-6773.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Embretsen SE, Reise SP. Item response theory for psychologists. New York: Psychology Press; 2000. [Google Scholar]
- 11.Alonso J, Bartlett SJ, Rose M, Aaronson NK, Chaplin JE, Efficace F, Leplege A, Lu A, Tulsky DS, Raat H, Ravens-Sieberer U, Revicki D, Terwee CB, Valderas JM, Cella D, Forrest CB. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health and Quality of Life Outcomes. 2013;11:210. doi: 10.1186/1477-7525-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liegl G, Rose M, Correia H, Fischer HF, Kanlidere S, Mierke A, Obbarius A, Nolte S. An Initial psychometric evaluation of the German PROMIS v1.2 physical function item bank in patients with a wide range of health conditions. Clinical Rehabilitation. 2018;32(1):84–93. doi: 10.1177/0269215517714297. [DOI] [PubMed] [Google Scholar]
- 13.Terwee CB, Roorda LD, de Vet HC, Dekker J, Westhovens R, Cella D, Correia H, Arnold B, Perez B, Boers M. Dutch-Flemish translation of 17 item banks from the patient-reported outcomes measurement information system (PROMIS) Quality of Life Research. 2014;23(6):1733–1741. doi: 10.1007/s11136-013-0611-6. [DOI] [PubMed] [Google Scholar]
- 14.Evans JP, Smith A, Gibbons C, Alonso J, Valderas JM. The national institutes of health patient-reported outcomes measurement information system (PROMIS): A view from the UK. Patient Relat Outcome Meas. 2018;9:345–352. doi: 10.2147/PROM.S141378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilagut G, Forero CG, Adroher ND, Olariu E, Cella D, Alonso J. Testing the PROMIS(R) Depression measures for monitoring depression in a clinical sample outside the US. Journal of Psychiatric Research. 2015;68:140–150. doi: 10.1016/j.jpsychires.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett SJ, Witter J, Cella D, Ahmed S. Montreal accord on patient-reported outcomes (PROs) use series—paper 6: Creating national initiatives to support development and use-the PROMIS example. Journal of Clinical Epidemiology. 2017;89:148–153. doi: 10.1016/j.jclinepi.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Katarzyna K, Glinkowski WM. Patient-reported outcomes of carpal tunnel syndrome surgery in a non-industrial area. Annals of Agricultural and Environmental Medicine. 2019;26(2):350–354. doi: 10.26444/aaem/99004. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Hinds PS, Wang J, Correia H, Du S, Ding J, Gao WJ, Yuan C. Translation and linguistic validation of the pediatric patient-reported outcomes measurement information system measures into simplified Chinese using cognitive interviewing methodology. Cancer Nursing. 2013;36(5):368–376. doi: 10.1097/NCC.0b013e3182962701. [DOI] [PubMed] [Google Scholar]
- 19.Schnohr CW, Rasmussen CL, Langberg H, Bjorner JB. Danish translation of a physical function item bank from the patient-reported outcome measurement information system (PROMIS) Pilot Feasibility Stud. 2017;3:29. doi: 10.1186/s40814-017-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terluin B, Eekhout I, Terwee CB. The anchor-based minimal important change, based on receiver operating characteristic analysis or predictive modeling, may need to be adjusted for the proportion of improved patients. Journal of Clinical Epidemiology. 2017;83:90–100. doi: 10.1016/j.jclinepi.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Terluin B, Eekhout I, Terwee CB, de Vet HC. Minimal important change (MIC) based on a predictive modeling approach was more precise than MIC based on ROC analysis. Journal of Clinical Epidemiology. 2015;68(12):1388–1396. doi: 10.1016/j.jclinepi.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- 23.Barrett B, Brown D, Mundt M, Brown R. Sufficiently important difference: Expanding the framework of clinical significance. Medical Decision Making. 2005;25(3):250–261. doi: 10.1177/0272989X05276863. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira ML, Herbert RD, Ferreira PH, Latimer J, Ostelo RW, Nascimento DP, Smeets RJ. A critical review of methods used to determine the smallest worthwhile effect of interventions for low back pain. Journal of Clinical Epidemiology. 2012;65(3):253–261. doi: 10.1016/j.jclinepi.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2005;2(1):63–67. doi: 10.1081/COPD-200050663. [DOI] [PubMed] [Google Scholar]
- 26.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Turner D, Schünemann HJ, Griffith LE, Beaton DE, Griffiths AM, Critch JN, Guyatt GH. The minimal detectable change cannot reliably replace the minimal important difference. Journal of Clinical Epidemiology. 2010;63(1):28–36. doi: 10.1016/j.jclinepi.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: The clinician’s perspective. Health and Quality of Life Outcomes. 2006;4:62. doi: 10.1186/1477-7525-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brozek JL, Guyatt GH, Schünemann HJ. How a well-grounded minimal important difference can enhance transparency of labelling claims and improve interpretation of a patient reported outcome measure. Health and Quality of Life Outcomes. 2006;4:69. doi: 10.1186/1477-7525-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coon CD, Cook KF. Moving from significance to real-world meaning: Methods for interpreting change in clinical outcome assessment scores. Quality of Life Research. 2018;27(1):33–40. doi: 10.1007/s11136-017-1616-3. [DOI] [PubMed] [Google Scholar]
- 31.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. Journal of Clinical Epidemiology. 2003;56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 32.de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in medicine. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 33.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Quality of Life Research. 2002;11(3):207–221. doi: 10.1023/A:1015276414526. [DOI] [PubMed] [Google Scholar]
- 34.Conijn AP, Jonkers W, Rouwet EV, Vahl AC, Reekers JA, Koelemay MJ. Introducing the concept of the minimally important difference to determine a clinically relevant change on patient-reported outcome measures in patients with intermittent claudication. Cardiovascular and Interventional Radiology. 2015;38(5):1112–1118. doi: 10.1007/s00270-015-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrikx J, Fransen J, Kievit W, van Riel PL. Individual patient monitoring in daily clinical practice: A critical evaluation of minimal important change. Quality of Life Research. 2015;24(3):607–616. doi: 10.1007/s11136-014-0809-2. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths P, Williams A, Brohan E, Cocks K. Understanding the role of anchor correlations in the calculation of meaningful change thresholds for health-related quality of life research. Value Health. 2019;22:S826. doi: 10.1016/j.jval.2019.09.2266. [DOI] [Google Scholar]
- 37.Terluin, B., Eekhout, I., Terwee, C. B., & De Vet, H. C. W. (2015). from https://www.jclinepi.com/article/S0895-4356(15)00160-2/fulltext
- 38.Guyatt GH, Norman GR, Juniper EF, Griffith LE. A critical look at transition ratings. Journal of Clinical Epidemiology. 2002;55(9):900–908. doi: 10.1016/S0895-4356(02)00435-3. [DOI] [PubMed] [Google Scholar]
- 39.Carragee EJ. The rise and fall of the minimum clinically important difference. Spine. 2010;10(4):283–284. doi: 10.1016/j.spinee.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Cook KF, Kallen MA, Coon CD, Victorson D, Miller DM. Idio Scale Judgment: Evaluation of a new method for estimating responder thresholds. Quality of Life Research. 2017;26(11):2961–2971. doi: 10.1007/s11136-017-1625-2. [DOI] [PubMed] [Google Scholar]
- 41.Thissen D, Liu Y, Magnus B, Quinn H, Gipson DS, Dampier C, Huang IC, Hinds PS, Selewski DT, Reeve BB, Gross HE, DeWalt DA. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Quality of Life Research. 2016;25(1):13–23. doi: 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staunton H, Willgoss T, Nelsen L, Burbridge C, Sully K, Rofail D, Arbuckle R. An overview of using qualitative techniques to explore and define estimates of clinically important change on clinical outcome assessments. Journal of Patient Reported Outcomes. 2019;3(1):16. doi: 10.1186/s41687-019-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terwee CB, Jansma EP, Riphagen II, de Vet HC. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Quality of Life Research. 2009;18(8):1115–1123. doi: 10.1007/s11136-009-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazmers NH, Qiu Y, Yoo M, Stephens AR, Tyser AR, Zhang Y. The minimal clinically important difference of the PROMIS and QuickDASH instruments in a nonshoulder hand and upper extremity patient population. Journal of Hand Surgery. 2020;45(5):399–407.e396. doi: 10.1016/j.jhsa.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. Journal of Clinical Epidemiology. 2011;64(5):507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for patient reported outcomes measurement information system pain interference for individuals with back pain. Journal of Pain Research. 2016;9:251–255. doi: 10.2147/JPR.S93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernstein DN, Houck JR, Mahmood B, Hammert WC. Minimal clinically important differences for PROMIS physical function, upper extremity, and pain interference in carpal tunnel release using region- and condition-specific PROM tools. Journal of Hand Surgery. 2019;44(8):635–640. doi: 10.1016/j.jhsa.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, Bair MJ, Damush TM, Monahan PO. Estimating minimally important differences for the PROMIS pain interference scales: Results from 3 randomized clinical trials. Pain. 2018;159(4):775–782. doi: 10.1097/j.pain.0000000000001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gausden EB, Levack A, Nwachukwu BU, Sin D, Wellman DS, Lorich DG. Computerized adaptive testing for patient reported outcomes in ankle fracture surgery. Foot and Ankle International. 2018;39(10):1192–1198. doi: 10.1177/1071100718782487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halperin DM, Huynh L, Beaumont JL, Cai B, Bhak RH, Narkhede S, Totev T, Duh MS, Neary MP, Cella D. Assessment of change in quality of life, carcinoid syndrome symptoms and healthcare resource utilization in patients with carcinoid syndrome. BMC Cancer. 2019;19(1):274. doi: 10.1186/s12885-019-5459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2015;74(1):104–107. doi: 10.1136/annrheumdis-2013-204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hays RD, Spritzer KL, Sherbourne CD, Ryan GW, Coulter ID. Group and individual-level change on health-related quality of life in chiropractic patients with chronic low back or neck pain. Spine. 2019;44(9):647–651. doi: 10.1097/BRS.0000000000002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung M, Baumhauer JF, Licari FW, Voss MW, Bounsanga J, Saltzman CL. PROMIS and FAAM minimal clinically important differences in foot and ankle orthopedics. Foot and Ankle International. 2019;40(1):65–73. doi: 10.1177/1071100718800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hung M, Bounsanga J, Voss MW, Saltzman CL. Establishing minimum clinically important difference values for the patient-reported outcomes measurement information system physical function, hip disability and osteoarthritis outcome score for joint reconstruction, and knee injury and osteoarthritis outcome score for joint reconstruction in orthopaedics. World Journal of Orthopedics. 2018;9(3):41–49. doi: 10.5312/wjo.v9.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazmers NH, Hung M, Bounsanga J, Voss MW, Howenstein A, Tyser AR. Minimal clinically important difference after carpal tunnel release using the PROMIS platform. Journal of Hand Surgery. 2019;44(11):947–953. doi: 10.1016/j.jhsa.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenney RJ, Houck J, Giordano BD, Baumhauer JF, Herbert M, Maloney MD. Do patient reported outcome measurement information system (PROMIS) scales demonstrate responsiveness as well as disease-specific scales in patients undergoing knee arthroscopy? American Journal of Sports Medicine. 2019;47(6):1396–1403. doi: 10.1177/0363546519832546. [DOI] [PubMed] [Google Scholar]
- 57.Khanna D, Hays RD, Shreiner AB, Melmed GY, Chang L, Khanna PP, Bolus R, Whitman C, Paz SH, Hays T, Reise SP, Spiegel B. Responsiveness to change and minimally important differences of the patient-reported outcomes measurement information system gastrointestinal symptoms scales. Digestive Diseases and Sciences. 2017;62(5):1186–1192. doi: 10.1007/s10620-017-4499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroenke K, Yu Z, Wu J, Kean J, Monahan PO. Operating characteristics of PROMIS four-item depression and anxiety scales in primary care patients with chronic pain. Pain Medicine. 2014;15(11):1892–1901. doi: 10.1111/pme.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lapin B, Thompson NR, Schuster A, Katzan IL. Clinical utility of patient-reported outcome measurement information system domain scales. Circulation Cardiovascular Quality and Outcomes. 2019;12(1):e004753. doi: 10.1161/CIRCOUTCOMES.118.004753. [DOI] [PubMed] [Google Scholar]
- 60.Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 patient-reported outcomes measurement information system short forms: Physical function, pain interference, depression, and anxiety in knee osteoarthritis. The Journal of Pain. 2017;18(9):1096–1110. doi: 10.1016/j.jpain.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan EM, Mara CA, Huang B, Barnett K, Carle AC, Farrell JE, Cook KF. Establishing clinical meaning and defining important differences for patient-reported outcomes measurement information system (PROMIS((R))) measures in juvenile idiopathic arthritis using standard setting with patients, parents, and providers. Quality of Life Research. 2017;26(3):565–586. doi: 10.1007/s11136-016-1468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purvis TE, Andreou E, Neuman BJ, Riley LH, 3rd, Skolasky RL. Concurrent validity and responsiveness of PROMIS health domains among patients presenting for anterior cervical spine surgery. Spine. 2017;42(23):E1357–E1365. doi: 10.1097/BRS.0000000000002347. [DOI] [PubMed] [Google Scholar]
- 63.Purvis TE, Neuman BJ, Riley LH, 3rd, Skolasky RL. Discriminant ability, concurrent validity, and responsiveness of PROMIS health domains among patients with lumbar degenerative disease undergoing decompression with or without arthrodesis. Spine. 2018;43(21):1512–1520. doi: 10.1097/BRS.0000000000002661. [DOI] [PubMed] [Google Scholar]
- 64.Sandvall B, Okoroafor UC, Gerull W, Guattery J, Calfee RP. Minimal clinically important difference for PROMIS physical function in patients with distal radius fractures. Journal of Hand Surgery. 2019;44(6):454–459.e451. doi: 10.1016/j.jhsa.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz CE, Zhang J, Rapkin BD, Finkelstein JA. Reconsidering the minimally important difference: Evidence of instability over time and across groups. Spine. 2019;19(4):726–734. doi: 10.1016/j.spinee.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Shahgholi L, Yost KJ, Kallmes DF. Correlation of the National Institutes of Health patient reported outcomes measurement information system scales and standard pain and functional outcomes in spine augmentation. AJNR American Journal of Neuroradiology. 2012;33(11):2186–2190. doi: 10.3174/ajnr.A3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stephan A, Mainzer J, Kümmel D, Impellizzeri FM. Measurement properties of PROMIS short forms for pain and function in orthopedic foot and ankle surgery patients. Quality of Life Research. 2019;28(10):2821–2829. doi: 10.1007/s11136-019-02221-w. [DOI] [PubMed] [Google Scholar]
- 68.Donovan LM, Yu L, Bertisch SM, Buysse DJ, Rueschman M, Patel SR. Responsiveness of patient-reported outcomes to treatment among patients with type 2 diabetes mellitus and OSA. Chest. 2020;157(3):665–672. doi: 10.1016/j.chest.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katz P, Kannowski CL, Sun L, Michaud K. Estimation of Minimally important differences and patient acceptable symptom state scores for the patient-reported outcomes measurement information system pain interference short form in rheumatoid arthritis. ACR Open Rheumatology. 2020;2(6):320–329. doi: 10.1002/acr2.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katz P, Pedro S, Alemao E, Yazdany J, Dall'Era M, Trupin L, Rush S, Michaud K. Estimates of responsiveness, minimally important differences, and patient acceptable symptom state in five patient-reported outcomes measurement information system short forms in systemic lupus erythematosus. ACR Open Rheumatology. 2020;2(1):53–60. doi: 10.1002/acr2.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khalil LS, Darrith B, Franovic S, Davis JJ, Weir RM, Banka TR. Patient-reported outcomes measurement information system (PROMIS) global health short forms demonstrate responsiveness in patients undergoing knee arthroplasty. Journal of Arthroplasty. 2020;35(6):1540–1544. doi: 10.1016/j.arth.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 72.Kroenke K, Baye F, Lourens SG. Comparative responsiveness and minimally important difference of common anxiety measures. Medical Care. 2019;57(11):890–897. doi: 10.1097/MLR.0000000000001185. [DOI] [PubMed] [Google Scholar]
- 73.Kroenke K, Stump TE, Chen CX, Kean J, Bair MJ, Damush TM, Krebs EE, Monahan PO. Minimally important differences and severity thresholds are estimated for the PROMIS depression scales from three randomized clinical trials. Journal of Affective Disorders. 2020;266:100–108. doi: 10.1016/j.jad.2020.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kroenke K, Stump TE, Kean J, Talib TL, Haggstrom DA, Monahan PO. PROMIS 4-item measures and numeric rating scales efficiently assess SPADE symptoms compared with legacy measures. Journal of Clinical Epidemiology. 2019;115:116–124. doi: 10.1016/j.jclinepi.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 75.Lawrie CM, Abu-Amer W, Barrack RL, Clohisy JC. Is the patient-reported outcome measurement information system feasible in bundled payment for care improvement in total hip arthroplasty patients? Journal of Arthroplasty. 2020;35(5):1179–1185. doi: 10.1016/j.arth.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 76.Lee, D. J., & Calfee, R. P. (2019). The minimal clinically important difference for promis physical function in patients with thumb carpometacarpal arthritis. Hand (N Y), Oct 18 [Online, ahead of print]. [DOI] [PMC free article] [PubMed]
- 77.Steinhaus ME, Iyer S, Lovecchio F, Khechen B, Stein D, Ross T, Yang J, Singh K, Albert TJ, Lebl D, Huang R, Sandhu H, Rawlins B, Schwab F, Lafage V, Kim HJ. Minimal clinically important difference and substantial clinical benefit using PROMIS CAT in cervical spine surgery. Clinical Spine Surgery. 2019;32(9):392–397. doi: 10.1097/BSD.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 78.Bartlett SJ, Gutierrez AK, Andersen KM, Bykerk VP, Curtis JR, Haque UJ, Orbai AM, Jones MR, Bingham CO., 3rd identifying minimal and meaningful change in PROMIS(®) for rheumatoid arthritis: Use of multiple methods and perspectives. Arthritis Care and Research. 2020 doi: 10.1002/acr.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beaumont JL, Davis ES, Fries JF, Curtis JR, Cella D, Yun H. Meaningful change thresholds for patient-reported outcomes measurement information system (PROMIS) fatigue and pain interference scores in patients with rheumatoid arthritis. Journal of Rheumatology. 2021 doi: 10.3899/jrheum.200990. [DOI] [PubMed] [Google Scholar]
- 80.Bingham CO, Butanis AL, Orbai AM, Jones M, Ruffing V, Lyddiatt A, Schrandt MS, Bykerk VP, Cook KF, Bartlett SJ. Patients and clinicians define symptom levels and meaningful change for PROMIS pain interference and fatigue in RA using bookmarking. Rheumatology. 2021 doi: 10.1093/rheumatology/keab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forlenza EM, Lu Y, Cohn MR, Baker J, Lavoie-Gagne O, Yanke AB, Cole BJ, Verma NN, Forsythe B. Establishing clinically significant outcomes for patient-reported outcomes measurement information system after biceps tenodesis. Arthroscopy. 2021;37(6):1731–1739. doi: 10.1016/j.arthro.2020.12.236. [DOI] [PubMed] [Google Scholar]
- 82.Haunschild ED, Condron NB, Gilat R, Fu MC, Wolfson T, Garrigues GE, Nicholson G, Forsythe B, Verma N, Cole BJ. Establishing clinically significant outcomes of the PROMIS upper extremity questionnaire after primary reverse total shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2021;S1058–2746(21):00355–364. doi: 10.1016/j.jse.2021.03.147. [DOI] [PubMed] [Google Scholar]
- 83.Haunschild ED, Gilat R, Fu MC, Tauro T, Huddleston HP, Yanke AB, Forsythe B, Verma NN, Cole BJ. Establishing the minimal clinically important difference, patient acceptable symptomatic state, and substantial clinical benefit of the PROMIS upper extremity questionnaire after rotator cuff repair. American Journal of Sports Medicine. 2020;48(14):3439–3446. doi: 10.1177/0363546520964957. [DOI] [PubMed] [Google Scholar]
- 84.Ibaseta A, Rahman R, Andrade NS, Skolasky RL, Kebaish KM, Sciubba DM, Neuman BJ. Determining validity, discriminant ability, responsiveness, and minimal clinically important differences for PROMIS in adult spinal deformity. Journal of Neurosurgery Spine. 2021 doi: 10.3171/2020.8.SPINE191551. [DOI] [PubMed] [Google Scholar]
- 85.Kazmers NH, Qiu Y, Ou Z, Presson AP, Tyser AR, Zhang Y. Minimal clinically important difference of the PROMIS upper-extremity computer adaptive test and QuickDASH for ligament reconstruction tendon interposition patients. Journal of Hand Surgery. 2021;46(6):516–516. doi: 10.1016/j.jhsa.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Kazmers NH, Qiu Y, Yoo M, Stephens AR, Zeidan M, Zhang Y. Establishing the minimal clinically important difference for the promis upper extremity computer adaptive test version 2.0 in a nonshoulder Hand and upper extremity population. Journal of Hand Surgery. 2021;S0363–5023(21):00093. doi: 10.1016/j.jhsa.2021.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khutok K, Janwantanakul P, Jensen MP, Kanlayanaphotporn R. Responsiveness of the PROMIS-29 scales in individuals with chronic low back pain. Spine. 2021;46(2):107–113. doi: 10.1097/BRS.0000000000003724. [DOI] [PubMed] [Google Scholar]
- 88.Kuhns BD, Reuter J, Lawton D, Kenney RJ, Baumhauer JF, Giordano BD. Threshold values for success after hip arthroscopy using the patient-reported outcomes measurement information system assessment: Determining the minimum clinically important difference and patient acceptable symptomatic state. American Journal of Sports Medicine. 2020;48(13):3280–3287. doi: 10.1177/0363546520960461. [DOI] [PubMed] [Google Scholar]
- 89.Silverberg JI, Lai JS, Cella D. Reliability and meaningful change of the patient-reported outcomes measurement information system(®) itch questionnaire (PIQ) item banks in adults with atopic dermatitis. British Journal of Dermatology. 2021 doi: 10.1111/bjd.20066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smit EB, Bouwstra H, Roorda LD, van der Wouden JHC, Wattel ELM, Hertogh C, Terwee CB. A patient-reported outcomes measurement information system short form for measuring physical function during geriatric rehabilitation: Test-retest reliability, construct validity, responsiveness, and interpretability. Journal of the American Medical Directors Association. 2021;S1525–8610(21):00141–149. doi: 10.1016/j.jamda.2021.01.079. [DOI] [PubMed] [Google Scholar]
- 91.Speck RM, Ye X, Bernthal NM, Gelhorn HL. Psychometric properties of a custom patient-reported outcomes measurement information system (PROMIS) physical function short form and worst stiffness numeric rating scale in tenosynovial giant cell tumors. Journal of Patient Reported Outcomes. 2020;4(1):61. doi: 10.1186/s41687-020-00217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bongers MER, Groot OQ, Thio Q, Bramer JAM, Verlaan JJ, Newman ET, Raskin KA, Lozano-Calderon SA, Schwab JH. Prospective study for establishing minimal clinically important differences in patients with surgery for lower extremity metastases. Acta Oncologica. 2021 doi: 10.1080/0284186X.2021.1890333. [DOI] [PubMed] [Google Scholar]
- 93.http://www.healthmeasures.net/score-and-interpret/interpret-scores/promis
- 94.Terwee CB, Roorda LD, Dekker J, Bierma-Zeinstra SM, Peat G, Jordan KP, Croft P, de Vet HC. Mind the MIC: Large variation among populations and methods. Journal of Clinical Epidemiology. 2010;63(5):524–534. doi: 10.1016/j.jclinepi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 95.Hao Q, Devji T, Zeraatkar D, Wang Y, Qasim A, Siemieniuk RAC, Vandvik PO, Lähdeoja T, Carrasco-Labra A, Agoritsas T, Guyatt G. Minimal important differences for improvement in shoulder condition patient-reported outcomes: A systematic review to inform a BMJ rapid recommendation. British Medical Journal Open. 2019;9(2):028777. doi: 10.1136/bmjopen-2018-028777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanmer J, Jensen RE, Rothrock N. A reporting checklist for HealthMeasures’ patient-reported outcomes: ASCQ-Me, Neuro-QoL, NIH Toolbox, and PROMIS. Journal of Patient Reported Outcomes. 2020;4(1):21. doi: 10.1186/s41687-020-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cook KF, Cella D, Reeve BB. PRO-bookmarking to estimate clinical thresholds for patient-reported symptoms and function. Medical Care. 2019;57(Suppl 5):S13–S17. doi: 10.1097/MLR.0000000000001087. [DOI] [PubMed] [Google Scholar]
- 98.de Vet HC, Terluin B, Knol DL, Roorda LD, Mokkink LB, Ostelo RW, Hendriks EJ, Bouter LM, Terwee CB. Three ways to quantify uncertainty in individually applied minimally important change values. Journal of Clinical Epidemiology. 2010;63(1):37–45. doi: 10.1016/j.jclinepi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 99.Stephan A, Mainzer J, Kummel D, Impellizzeri FM. Measurement properties of PROMIS short forms for pain and function in orthopedic foot and ankle surgery patients. Quality of Life Research. 2019;28(10):2821–2829. doi: 10.1007/s11136-019-02221-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


