Abstract
The synthetic copper-containing compound, CuATSM, has emerged as one of the most promising drug candidates developed for the treatment of amyotrophic lateral sclerosis (ALS). Multiple studies have reported CuATSM treatment provides therapeutic efficacy in various mouse models of ALS without any observable adverse effects. Moreover, recent results from an open label clinical study suggested that daily oral dosing with CuATSM slows disease progression in patients with both sporadic and familial ALS, providing encouraging support for CuATSM in the treatment of ALS. Here, we assessed CuATSM in high copy SOD1G93A mice on the congenic C57BL/6 background, treating at 100 mg/kg/day by gavage, starting at 70 days of age. This dose in this specific model has not been assessed previously. Unexpectedly, we report a subset of mice initially administered CuATSM exhibited signs of clinical toxicity, that necessitated euthanasia in extremis after 3–51 days of treatment. Following a 1-week washout period, the remaining mice resumed treatment at the reduced dose of 60 mg/kg/day. At this revised dose, treatment with CuATSM slowed disease progression and increased survival relative to vehicle-treated littermates. This work provides the first evidence that CuATSM produces positive disease-modifying outcomes in high copy SOD1G93A mice on a congenic C57BL/6 background. Furthermore, results from the 100 mg/kg/day phase of the study support dose escalation determination of tolerability as a prudent step when assessing treatments in previously unassessed models or genetic backgrounds.
Subject terms: Amyotrophic lateral sclerosis, Drug safety
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease involving deterioration of both upper and lower motor neurons. Disease progression is rapid, with patients developing progressive muscle weakness, subsequently leading to respiratory failure and death typically within 2–3 years1. Currently, there are two FDA approved therapeutics for ALS; riluzole and edaravone. Both of these drugs provide minimal therapeutic benefit2–5 highlighting a need for more effective treatment options.
A large majority of ALS cases have no hereditary origin and are classified as sporadic ALS (sALS); whereas, approximately 10% of cases are inherited and classified as familial ALS (fALS). The first gene to be associated with ALS was SOD1, which now has > 160 identified mutations linked with ALS6. SOD1 encodes for the Cu/Zn superoxide dismutase (SOD1) enzyme that functions as a free radical scavenger by converting superoxide (O2·−) to hydrogen peroxide and molecular oxygen7. Many fALS SOD1 mutations do not alter the activity of the enzyme, which remains similar to wild-type SOD18. In addition, SOD1 knock-out models do not exhibit ALS pathology, indicating loss-of-function is not sufficient to cause ALS pathology9. Conversely, mice overexpressing wild-type SOD1 reportedly display neuromuscular deficits, further indicating a gain-of-function mechanism as the mode of SOD1 toxicity10,11. Moreover, transgenic mice overexpressing mutant SOD1 (i.e. D90A, G85R, G37R, G93A) display ALS-like symptoms and pathology to varying degrees and severity (for review see12). However, all fALS-associated SOD1 mutations share the unifying feature of promoting SOD1 to misfold, leading to the formation of insoluble protein aggregates/inclusions in motor neurons and proximal cells13,14. The folding pathway of SOD1 involves several post-translational modifications to achieve its final, homodimeric, functional form. In its native conformation, SOD1 is thermodynamically stable and remains catalytically active even in high concentrations of denaturant15,16. A critical step in the proper maturation of SOD1 structure is the addition of a redox active copper ion. In addition to SOD1 stability, copper is an essential element with its bioavailability and in vivo homeostasis tightly controlled. Furthermore, SOD1 rodent models exhibit copper dyshomeostasis17–20. Copper bioavailability was recently reported to be altered in sALS post-mortem tissue, and associated accumulation of iron in the motor cortex21 suggesting that changes to copper metabolism may not be specific to SOD1 fALS22.
CuATSM (diacetylbis(N(4)-methylthiosemicarbazonato)copper(II)) is a stable (Ka = 1018), low molecular weight, charge neutral, lipophilic, compound that is able to cross the blood–brain barrier17,18,23,24. CuATSM has neuroprotective activity in animal models of diverse neurodegenerative disorders, including cerebral ischemia, neuroinflammation, Parkinson's disease and ALS23,25–27. Preclinical investigation of CuATSM treatment (30–200 mg/kg/day) in numerous SOD1 ALS mouse models has demonstrated the ability of CuATSM to significantly delay the onset of ALS symptoms, reduce weight loss and extend lifespan (Table 1), with no reported adverse side effects attributed to CuATSM treatment. In light of these encouraging preclinical results, the tolerability and efficacy of CuATSM in patients with ALS is now being assessed in a multicentre, randomized, double-blind, placebo controlled study in Australia (NCT04082832 and NCT02870634).
Table 1.
Therapeutic outcomes for CuATSM across multiple mouse models of amyotrophic lateral sclerosis.
| Mouse model | Genetic background | Commencement of treatment | Daily dose (mg/kg) | Administration route | Increase in survival | Body weight | Motor function | Neurological score | Study |
|---|---|---|---|---|---|---|---|---|---|
| Low copy SOD1-G93A | Congenic; C57BL/6 | 140 days (presymptomatic) | 30 (5 days per week) | Oral | 14% | Delayed onset | Delayed onset/slowed progression | Delayed onset | 23 |
| Low copy SOD1-G93A | Congenic; C57BL/6 | 200 days (symptomatic) | 30 (5 days per week) | Oral | 10% | NR | Slowed progression | NR | 23 |
| High copy SOD1-G37R | Congenic; C57BL/6 | 40 days (presymptomatic) | 10 (7 days per week) | Oral | 8% | NR | Delayed onset/slowed progression | NR | 24 |
| High copy SOD1-G37R | Congenic; C57BL/6 | 40 days (presymptomatic) | 30 (7 days per week) | Oral | 18% | NR | Delayed onset/slowed progression | NR | 24 |
| High copy SOD1-G37R | Congenic; C57BL/6 | 40 days (presymptomatic) | 60 (7 days per week) | Oral | 26% | NR | Delayed onset/slowed progression | NR | 24 |
| High copy SOD1-G37R | Congenic; C57BL/6 | 149 days (symptomatic) | 60 (7 days per week) | Oral | 12% | NR | Slowed progression | NR | 24 |
| High copy SOD1-G37R | Congenic; C57BL/6 | 40 days (presymptomatic) | 30 (7 days per week) | Oral | 18% | NR | Delayed onset/slowed progression | NR | 17 |
| High copy SOD1-G93A | Mixed; B6SJL | 5 days | 200 (7 days per week) | Transdermal | 25% | Slowed progression | NR | Delayed onset | 18 |
| High copy SOD1-G93A | Mixed; B6SJL | 50 days | 200 (7 days per week) | Transdermal | 19% | Slowed progression | NR | Delayed onset | 18 |
| High copy SOD1-G93A × CCS | Mixed; B6SJL | Prenatal | 60 (7 days per week) | Transdermal | 2800% | Slowed progression | NR | NR | 18 |
| High copy SOD1-G93A | Mixed; B6SJL | 50 days (presymptomatic) | 100 (7 days per week) | Oral | 9% | NR | Delayed onset/slowed progression | Delayed onset | 20 |
| High copy SOD1-G93A | Mixed; B6SJL | 50 days (presymptomatic) | 30 (7 days per week) | Oral | *Improved survival by 5.5 days | Slowed progression | NR | Delayed onset | 45 |
| Neurotoxin; β-sitosterol β-d-glucoside | Outbred;CD-1 | 63 days | 30 (5 days per week) | Transdermal | NR | No significant difference | Slowed progression | Improved leg extension reflex | 47 |
*Trends toward extended lifespan in mice treated with CuATSM compared to vehicle-treated mice, although the effects were not statistically significant. NR not reported.
In the current study, we tested an oral dose of 100 mg/kg/day CuATSM in a high copy number SOD1G93A mouse model on a congenic C57BL/6 background. This dose in this specific model has not been assessed previously. CuATSM was suspended in a standard suspension vehicle (0.9% w/v NaCl, 0.5% w/v Na-carboxymethylcellulose, 0.5% v/v benzyl alcohol, 0.4% v/v Tween-80) and administered by oral gavage twice daily from 70 days old. We observed that at this dose in SOD1G93A mice maintained on a C57BL/6 background, a subset of CuATSM-treated mice exhibited clinical signs of toxicity including hunched posture, piloerection and were hypoactive following 3–51 days of treatment. Subsequently, mice remaining in the cohort were given a 1-week wash-out period and recommenced CuATSM treatment at 60 mg/kg/day, which consequently delayed disease progression and prolonged lifespan.
Results
Oral CuATSM administration (100 mg/kg/day) induces clinical signs of toxicity and weight loss in a subset of SOD1G93A mice on C57BL/6 background
To investigate the therapeutic efficacy of CuATSM on ALS progression, age- and gender-matched SOD1G93A mice (C57BL/6 background) were treated twice daily with vehicle or CuATSM (total dose of 100 mg/kg/day) from 70 days old (presymptomatic with no clinical symptoms or motor impairment).
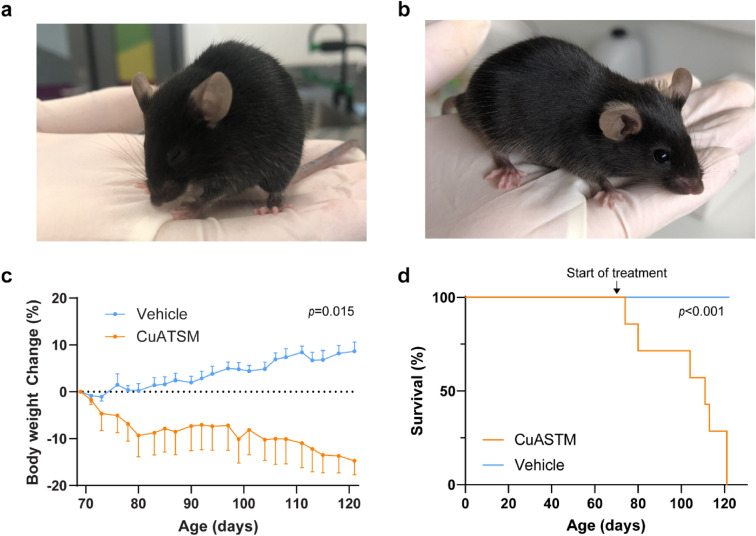
CuATSM (100 mg/kg/day) was seemingly tolerated for the majority of SOD1G93A mice. However, seven CuATSM-treated mice (six male, one female) developed clinical signs of toxicity including; hunched posture, orbital tightening, piloerection, and low activity following CuATSM administration that were not observed in vehicle-treated mice (Fig. 1a,b; Table 2). The onset of observed clinical symptoms of toxicity was varied and ranged from 3–51 days following the commencement of CuATSM treatment. Furthermore, we observed CuATSM-treated mice produced dark brown/black faeces. SOD1G93A mice typically exhibit weight gain until 110–130 days old28,29, when the onset of disease phenotype occurs, and gradual weight loss is observed thereafter. However, clinical symptoms observed in CuATSM-treated mice were accompanied by weight loss before 100 days old (Fig. 1c). A repeated measures one-way ANOVA of percentage body weight (compared to maximum weight prior to treatment) showed the subset of CuATSM-treated SOD1G93A mice that exhibited signs of clinical toxicity progressively declined in percentage body weight from the commencement of treatment, while age-matched vehicle-treated SOD1G93A mice increased over the same period (p = 0.015; Fig. 1c).
Figure 1.
CuATSM (100 mg/kg/day) causes clinical signs of toxicity and weight loss in SOD1G93A mice (maintained on a C57BL/6 background). SOD1G93A mice were administered CuATSM (100 mg/kg/day) or vehicle via oral gavage. A subset of CuATSM-treated SOD1G93A mice (7/20) developed clinical signs of toxicity including (a) hunched posture, orbital tightening, piloerection and low activity/respiration compared to vehicle-treated mice that exhibited, (b) typical posture and activity. (c) Percent body weight change of the subset of CuATSM-treated SOD1G93A mice that developed clinical signs of toxicity compared to age- and sex-matched SOD1G93A vehicle-treated mice and (d) survival of SOD1G93A mice that exhibited toxicity following daily oral gavage administration of CuATSM (100 mg/kg/day) compared to age- and sex-matched SOD1G93A vehicle-treated mice. Data represents mean SEM (n = 7/treatment. Repeated measures ANOVA and Mantel–Cox tests were used to compare body weight change and survival, respectively, to compare relative differences between CuATSM- and matched vehicle-treated mice SOD1G93A mice.
Table 2.
Symptoms of CuATSM–associated toxicity, including weight loss, posture abnormalities and activity.
| Symptom | Vehicle (n = 20) | CuATSM (n = 20) |
|---|---|---|
| Weight loss* | 0 | 7 |
| Hunched posture | 0 | 6 |
| Orbital tightening | 0 | 5 |
| Piloerection | 0 | 5 |
| Hypoactive | 0 | 5 |
*Weight loss was defined as 10% body weight loss since beginning treatment.
To assess if the neurological score or motor coordination were negatively affected in the mice exhibiting clinical signs of toxicity, we analysed the neurological score and motor coordination in these mice compared to age-matched controls. The analysis showed no difference of neurological score, rotarod and pole test performance between CuATSM-treated mice exhibiting clinical symptoms and age-matched vehicle-treated mice (Supplementary Figure 1a–c, all p > 0.05), indicating CuATSM did not exacerbate ALS-associated motor symptoms in this subset of mice.
Once signs of either weight loss, hunched posture or orbital tightening were observed, mice were monitored closely. In all cases, the health of the mouse drastically declined within 96 h of observing any of the aforementioned symptoms and euthanaised by recommendation of the University of Wollongong’s animal welfare officer. A Mantel–Cox analysis of survival of CuATSM-treated mice at the dose of 100 mg/kg/day and subsequently euthanised due to observed clinical toxicity revealed a significantly reduced median survival compared to age-matched vehicle-treated SOD1G93A mice (p < 0.001; Fig. 1d). The median survival for CuATSM-treated mice was 111 days (41 days following commencement of CuATSM (100 mg/kg/day) treatment), whilst all mice within the vehicle treatment group remained alive. Furthermore, to determine if CuATSM-induced toxicity was sex-dependent, a Fisher’s exact test was performed on male and female CuATSM-treated mice. Whilst there was a trend for male mice to be more susceptible to CuATSM-induced toxicity, this did not reach statistical significance (p = 0.057), likely due to the low sample size. Subsequently, CuATSM (100 mg/kg/day) treatment was ceased due to animal welfare concerns.
Oral CuATSM administration (60 mg/kg/day) improved neurological function in SOD1G93A mice
After observing clinical signs of toxicity and the subsequent euthanasia of a subset of CuATSM-treated mice, treatment was ceased in all mice. The remaining mice showed no signs clinical signs of toxicity following CuATSM 100 mg/kg/day treatment and were given a 1 week wash-out period where no treatment was administered in either group. Body weight, neurological score and motor coordination were continually assessed during this period. Following the 1 week wash-out period, mice recommenced CuATSM treatment at a new daily dose (60 mg/kg/day), and their body weight, ALS score and motor coordination were recorded. To avoid an age- or sex-bias, only vehicle-treated mice with age- and sex-matched CuATSM-treated counterparts were included in the remaining data used to determine the therapeutic efficacy of CuATSM treatment at 60 mg/kg/day. Due to the staggered age of the cohort, mice started to receive the lower 60 mg/kg/day CuATSM at various ages. The average age of mice upon recommencement of treatment was 112 days old for both the vehicle- and CuATSM-treatment groups (Supplementary Table 1). Furthermore, analysis of the remaining mice showed no significant difference in total body weight, percentage body weight change from pre-disease maximum and neurological score (p > 0.05; Supplementary Table 1) at the recommencement of CuATSM treatment (60 mg/kg/day).
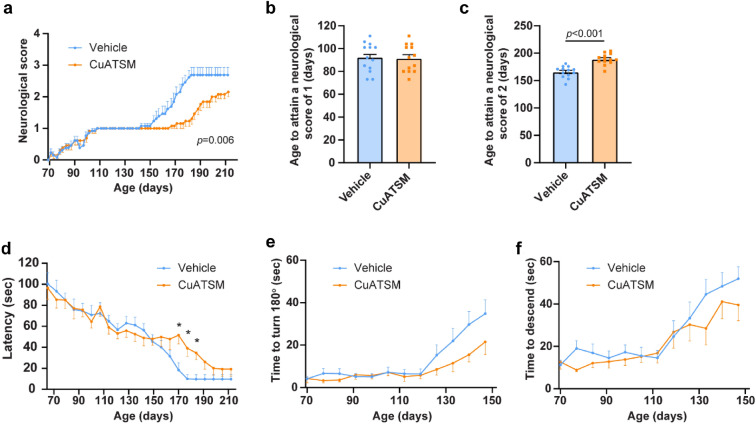
Assessment of neurological function was recorded three times a week by a blinded observer. Whilst onset of disease (neurological score = 1) was the same for both treatment groups (p > 0.05, Fig. 2b), repeated measures one-way ANOVA revealed CuATSM-treated mice exhibited a reduced rate of disease progression (p = 0.006, Fig. 2a), with CuATSM treatment delaying the mean onset of hind-leg paralysis by 23 ± 4 days (p < 0.0001, Fig. 2c). To further assess disease progression, mice performed weekly rotarod and pole test tasks. Mice from both treatment groups displayed a progressive decline in both rotarod and pole test performance (Fig. 2d–f). CuATSM treatment mitigated motor coordination decline on the rotarod, with CuATSM-treated mice having a significantly longer latency to fall from the rotarod when compared to vehicle-treated mice on days 170, 177 and 184 (all p < 0.05). Further assessment of motor coordination using the pole test task revealed whilst CuATSM-treated mice showed a general trend to turn 180° and descend the pole faster than vehicle-treated mice (Fig. 2e,f), no significant differences between treatment groups in the pole test were observed until 150 days old, when mice were unable to securely hold onto the pole and assessment was ceased.
Figure 2.
Oral CuATSM treatment slows disease progression and loss of motor coordination in SOD1G93A mice. SOD1G93A mice were administered CuATSM or vehicle via oral gavage. To assess neurological function, mice completed (a) Neurological scoring three times a week and the age to attain a neurological score of (b) 1 and (c) 2 was measured. In addition (d) rotarod and (e,f) pole test tasks were performed. Data are shown as mean ± SEM (n = 13/treatment). Repeated measures ANOVA were used to compare neurological score, latency, time to turn 180° and time to descend followed by post-hoc with Fisher’s least significance difference corrections. Independent t test were used to compare age to attain a neurological score of 1 and 2 between CuATSM- and vehicle-treated mice SOD1G93A mice. *p < 0.05 compared to age- and sex-matched vehicle treated mice.
Oral CuATSM administration (60 mg/kg/day) reduced body weight loss and improved survival in SOD1G93A mice
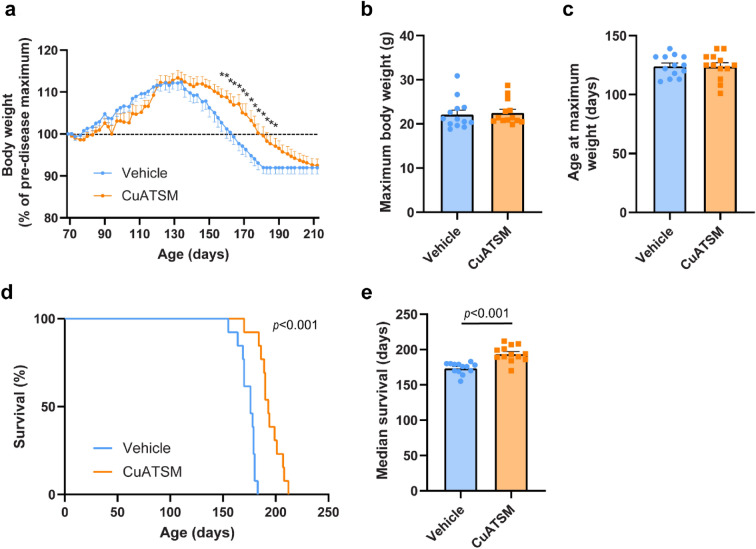
Body weight of CuATSM- and vehicle-treated SOD1G93A mice was recorded three times a week throughout the duration of the study with both treatment groups reaching peak body weight at 124 days (Fig. 3a–c). Furthermore, there were no significant differences in peak body weight or the mean age at which maximum body weight was reached between treatment groups (p > 0.05, Fig. 3b,c). Both CuATSM- and vehicle-treated mice showed a gradual decline in body weight from 124 days onwards (Fig. 3a). Repeated measures one-way repeated ANOVA of body weight (percentage of pre-disease maximum) revealed a significant main effect of time (p < 0.001), but not significant treatment effect (p > 0.05). However, a significant interaction between factors was observed (p < 0.001), with post-hoc analysis showing CuATSM-treated mice slowed body weight loss and displayed a significantly higher percentage of maximum body weight between 155–188 days of age compared to vehicle-treated mice (all p < 0.05).
Figure 3.
Oral CuATSM treatment delays weight loss and extends survival in SOD1G93A mice (n = 13/treatment). SOD1G93A mice were administered CuATSM or vehicle and (a) body weight recorded three times a week. (b) The mean age to reach peak body weight (+ SEM) and (c) maximum mean body weight (+ SEM) were measured. (d) A Kaplan–Meier curve of CuATSM- and vehicle-treated SOD1G93A mice and (e) median survival (+ SEM) (n = 13/treatment). Repeated measures ANOVA were used to compare body weight change followed by post-hoc with Fisher’s least significance difference corrections. Independent t test were used to compare maximum body weight, age at maximum body weight and median survival between CuATSM- and vehicle-treated mice SOD1G93A mice. *p < 0.05 compared to age- and sex-matched vehicle treated mice.
The humane-end stage was determined when mice displayed either a 20% weight loss compared to their maximum weight or an inability to right themselves within 30 s after being placed on their side. CuATSM treatment increased the survival of SOD1G93A mice (p < 0.001), extending median end-stage by 9%, from 176 (vehicle-treated mice) to 193 days (p < 0.001, Fig. 3d,e).
Oral CuATSM administration (60 mg/kg/day) increased SOD1 levels and activity
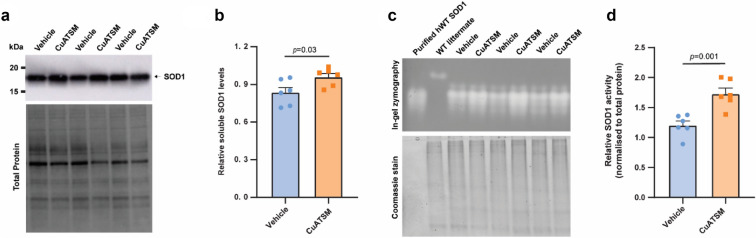
CuATSM treatment has previously shown to increase soluble SOD1 levels and its enzymatic activity in the lumbar spinal cord of SOD1 mice17,18,20. To investigate the effect of CuATSM on SOD1 levels, PBS-soluble and -insoluble fractions were prepared from the lumbar spinal cord of CuATSM- and matched-vehicle-treated mice. Analysis of SOD1 western blots revealed CuATSM-treated mice exhibited a 15% increase in PBS-soluble SOD1 in the spinal cord compared to vehicle-treated mice (p = 0.03, Fig. 4a,b).
Figure 4.
Oral CuATSM treatment increases soluble SOD1 levels and activity in the lumbar spinal cord of SOD1G93A mice. (a) The relative levels of SOD1 protein were determined via western blot in (b). PBS-soluble fractions obtained from the lumbar spinal cord of vehicle- and CuATSM-treated mice. Quantification of relative SOD1 levels were normalised to total protein loading for each sample. (c) PBS-soluble homogenate from the lumbar spinal cord of CuATSM- and vehicle-treated mice were separated on a native 8% gel and SOD1 activity determined by in-gel zymography. Equal total protein amount across samples were determined by Coomassie signal. (d) Quantification of relative SOD1 normalised to total protein. Data shown are means ± SEM (n = 6/treatment). Data are from two independent experiments. Student’s t test was used to compare relative differences between CuATSM- and vehicle-treated mice SOD1G93A mice. Full-length blots and gels are presented in Supplementary Figures 2 and 3, respectively.
To assess if SOD1 activity was increased in the lumbar spinal cord of CuATSM-treated mice, in-gel zymography was performed on the PBS-soluble fraction (Fig. 4c). Indeed, CuATSM-treated mice showed a 44% increase in SOD1 activity compared to vehicle-treated mice (p = 0.001; Fig. 4d).
Discussion
Currently, CuATSM has emerged one of the most promising pharmacotherapeutic agents for the treatment of ALS. CuATSM has shown to delay the onset of motor impairment and prolongs survival in various ALS mouse models (Table 1), with no reported adverse events. Here, we sought to assess the therapeutic efficacy of a high dose of CuATSM (100 mg/kg/day) in high copy SOD1G93A mice maintained on a congenic background (C57BL/6), a combination that had not been tested previously.
Results obtained from Phase I clinical trials observed that 8/14 patients receiving the highest dose of CuATSM (> 72 mg/day) exhibited reversible transaminitis30, consequently leading to the recommended Phase II dose set at 72 mg/day. In our SOD1G93A model, administration of 100 mg/kg/day, across two doses a day by oral gavage led to morbidity and euthanasia in extremis for 7 of 20 treated mice after 3–51 days of treatment. It is unclear why only a subset of mice exhibited signs of toxicity, however we cannot eliminate the possibility that the remaining mice did not exhibit observable levels of pathological toxicity. To our knowledge, this is the first study to report adverse effects following CuATSM treatment. The signs of toxicity we observed, included weight loss, hunched posture and a hypoactive state, are consistent with symptoms reported previously in rodents given 100 or 200 mg/kg/day oral copper sulphate31,32 and intraperitoneal injections of copper lactate33. Histopathologic analyses of rodents given high doses of oral copper sulphate showed toxic effects on liver and kidney31. We were unable to confirm such effects on liver and/or kidney in CuATSM-treated mice that displayed signs of toxicity; histopathologic analyses on five of seven affected mice showed no significant lesions in liver or kidney (Supplementary Table 3). However, the albumin:globulin ratio and glomerular filtration rate, which are the most important markers for kidney disease, were not assessed. Moreover, of the seven CuATSM affected mice, we were able to obtain plasma samples for two, which subsequently showed elevated alanine aminotransferase (ALT) levels (Supplementary Table 4). Although, elevated ALT is excreted from both necrotic muscle and liver tissue. Differentiating between transaminases released from muscle and liver, requires testing for gamma glutamyl transferase (GGT), creatinine phosphokinase (CPK), and bilirubin, in addition to transaminases. Whilst, this information is important to provide a detailed understanding of the nature of the adverse events reported here, they remained beyond the initial scope of this work.
Although the clinical symptoms observed in a subset of mice treated with 100 mg/kg/day CuATSM displayed symptoms similar to previous reports of oral copper sulphate treatment in rodents, it is important to distinguish between mice treated with copper salts such as copper sulphate and CuATSM. The latter, is a charge neutral, stable, lipophilic compound with high membrane permeability and low redox potential (in the same range of redox potential as NADH), which is reduced by hypoxic but not normal mitochondria34. The complex remains stable when in copper(II) oxidation state but upon reduction to copper(I) the metal ion can be exchanged with high affinity copper(I) binding proteins. The reduction potential of CuATSM prevents reduction to copper(I) and subsequent release of copper in normal tissues35, however is selectively released in hypoxic cells or those with damaged mitochondrial electron transport chains36, as is the case in affected neuroglia cells in ALS. Under certain conditions, oxidation of CuATSM can lead to release of the metal ion37,38. Gastrointestinal microbiota secrete a diverse array of enzymes that metabolize orally administered drugs39,40, therefore oxidative metabolism in the gastrointestinal tract may lead to release of copper from CuATSM, but at this stage this is merely speculation and requires more detailed investigations.
There have been two previous studies which have utilised doses equal or higher than the 100 mg/kg/day dose initially used in the present study, without any reported adverse events18,20. However, it should be noted that the aforementioned studies utilised SOD1G93A mice maintained on a B6/SJL background, unlike the present study where mice were maintained on a congenic C57BL/6 background, indicating toxicity may be strain-dependent. Strain-dependent differences in rodents have previously been reported in copper homeostasis and metabolism41,42. Furthermore, whilst there is a well-established strain difference regarding disease pathology of SOD1G93A mice43–46, to our knowledge, there are no reports of pharmacotoxicology strain-dependent differences between SOD1G93A mice maintained on different backgrounds. In addition, to potential strain-dependent tolerance differences, Williams et al.18 delivered CuATSM 200 mg/kg/day transdermally, in contrast to Hilton et al.20 and the present study, whereby CuATSM was administered orally. Bioavailability and pharmacokinetic comparisons between transdermal and oral gavage CuATSM delivery have not yet been performed and therefore it is not yet clear whether tolerability at 200 mg/kg/day via transdermal administration is the result of fundamental differences in pharmacological response or the fact that the 200 mg/kg/day study involved mice on the mixed B6/SJL background. It is also important to highlight that CuATSM is insoluble in aqueous media and the particle size of laboratory preparations may vary greatly from one study to the next due to variations from batch-to-batch within a laboratory or across laboratories. Thus, it is possible that different particle sizes may lead to differing dose-dependent absorption and saturation levels, and it is possible that high doses (100 mg/kg/day) delivered by oral gavage could lead to accumulation of the insoluble compound in the gastrointestinal tract. Although, the particle size of CuATSM used in this study was not assessed, histopathology assessment of affected mice showed no gastrointestinal abnormalities (Supplementary Table 3). Whilst we were unable to determine the underlying cause of toxicity, the present results from mice administered 100 mg/kg/day CuATSM provides important information for future preclinical work investigating CuATSM.
Despite, a subset of mice showing clinical signs of toxicity following 100 mg/kg/day CuATSM treatment, the remaining mice were subsequently given a 1 week wash-out period and administered 60 mg/kg/day CuATSM. These results support previous reports CuATSM has the capacity to delay disease progression and extend survival in SOD1 mice (Table 1), particularly earlier intervention appears to lead to better therapeutic outcomes18,23, justifying our decision to begin treatment at a presymptomatic stage. We observed 60 mg/kg/day CuATSM treatment delayed weight loss, which has been attributed to muscle wastage47 and metabolic alterations48 in SOD1G93A mice. A study performed by ALS Therapy Development Institute reported CuATSM slowed disease progression and provided a trend to extended lifespan in high copy number SOD1G93A mice (maintained on a C57BL/6 background)29. The authors of the ALS Therapy Development Institute study acknowledged that their results reflected their use of a low daily dose (30 mg/kg/day) which had previously only produced statistically significant survival outcomes in low copy number SOD1G93A mice or SOD1G37R mice17,23,24. Dose–response studies have not been performed in high copy number SOD1G93A mice. However, retrospective comparisons of CuATSM efficacy in high copy number SOD1G93A mice suggests that increasing the dose improves outcomes. Williams et al.18 treated SOD1G93A mice (maintained on a B6/SJL background) with 200 mg/kg/day from 50 days old, which resulted in increased mean survival of 19%, whilst Hilton et al.20 only reported a 9% increase when using 100 mg/kg/day. Notably, this apparent relationship between dose and survival in the high copy number SOD1G93A mice came from two different studies which involved different methods for administering CuATSM (i.e., transdermal vs. oral). Collectively, multiple investigations utilising different mouse models of ALS and different methods of drug administration have shown that the therapeutic benefit derived from CuATSM relates to the dose of compound administered. Data presented herein for the dose of 60 mg/kg/day are supportive of this. However, our data presented for the higher dose of 100 mg/kg/day demonstrate that treatment beyond a maximum tolerated dose can be associated with serious adverse events. This highlights the importance of dose finding studies for tolerability, as per the successfully completed Phase 1 assessment of CuATSM in ALS patients are essential30.
Supportive of the growing body of preclinical and clinical evidence for the broad the therapeutic benefits of CuATSM, reports on the compound’s potential therapeutic mechanism of action appear diverse. There is substantial evidence that CuATSM increases copper bioavailability36, to improve physiological metalation and stability of SOD1, potentially benefiting patients with SOD1 fALS17,20,24. Here, we were able to reproduce previous studies demonstrating CuATSM-treated mice display increased soluble SOD1 levels and activity17,20,23. Moreover, previous in vitro work performed by our group suggests CuATSM may provide greater protection against wild-type-like SOD1 mutations and not metal-binding region (MBR) mutations49. These data raises questions about whether a copper bioavailability mechanism of action for CuATSM is suitable for patients carrying SOD1 MBR mutations. The efficacy of CuATSM has yet to be tested in a mouse model carrying SOD1 MBR mutations or a non-SOD1 mouse model of fALS. However, there is ample evidence from numerous lines of investigation to illustrate that the therapeutic activity of CuATSM is not restricted to a mechanism of action that involves copper delivery to SOD1. For example, a recent pilot study reported CuATSM improves motor coordination and survival in a neurotoxin β-sitosterol β-d-glucoside model of sALS50. Whilst it is not known if SOD1 folding or copper homeostasis is altered in this model, evidence indicates that the therapeutic efficacy of CuATSM in this model may be through SOD1-independent mechanisms (reviewed by51). Alternative mechanisms may include scavenging peroxynitrite23,25 and anti-ferroptotic activity52, which are pathways implicated in both fALS and sALS.
Future work revealing pertinence of the mechanism(s) underlying the beneficial effects of CuATSM for both fALS and sALS will help further elucidate the disrupted molecular pathways in ALS. Furthermore, whilst the present study was not specifically designed to assess the toxicity of CuATSM, collectively, these initial results support the notion that high dose CuATSM may cause adverse effects, however further dose tolerance studies specifically addressing this issue are required.
Materials and methods
Animals
All research was approved by the Animal Ethics Committee (AE19/10) of the University of Wollongong (Wollongong, Australia) and complied with the National Health and Medical Research Institute, Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Mice hemizygous for the human SOD1G93A transgene maintained on a C57BL/6 background (B6-Tg (SOD1-G93A)1Gur/j) were bred at the Australian Bioresources Animal Facility (Moss Vale, Australia) and housed in individually ventilated cages (IVC) (Type Mouse Version 1; Airlaw, Smithfield, Australia; air change: 90‐120 times per hour averaged; passive exhaust ventilation system). Mice were genotyped at the age of weaning (~ 3–4 weeks old) and SOD1G93A transgenic mice (~ 4–5 weeks old) were transported and housed at the University of Wollongong in IVC cages (Greenline GM500, Techniplast, Australia) under a 12:12 h light–dark cycle (illuminated from 0700 to 1900 h). Mice were caged with littermates where attainable, with 2–4 females or 1–3 males per cage. IVC cages included a layering of Bed-O’Cobs™ corncob bedding (Techniplast, Australia), tissue, Bed r’Nest™ (Techniplast, Australia), a plastic house and a PVC tunnel. Food and water were available ad libitum. When mice reached 100 days old, water-soaked food pellets were placed on the cage floor and longer sippers placed on water bottles. Mice matched for date of birth and sex were equally divided into two treatment groups (n = 10/treatment/sex).
CuATSM preparation and initial dosing regime
We chose to utilise CuATSM at 100 mg/kg/day in the present study based on a previous dose–response study which demonstrated a dose-proportional increase in life-span of SOD1G37R mice up to 60 mg/kg/day24 and recent evidence demonstrating 100 mg/kg/day is therapeutically effective in SOD1G93A mice (maintained on a B6/SJL background)20. CuATSM was synthesised as previously described53. CuATSM was suspended in a standard suspension vehicle (SSV; 0.9% w/v NaCl, 0.5% w/v Na-carboxymethylcellulose, 0.5% v/v benzyl alcohol, 0.4% v/v Tween-80) and sonicated via probe for five minutes immediately prior to treatment as previously described29. The CuATSM suspension was administered by oral gavage to SOD1G93A mice twice daily (0830–0930 and 1730–1830) at a total dose of 100 mg/kg/day as previously described20. Equivalent volumes of SSV were administered to the vehicle-treated mice. Treatment commenced when mice reached 70 days of age.
Adjusted CuATSM dosing regime
During the initial CuATSM treatment regime (100 mg/kg/day), clinical signs of toxicity were observed in a subset of CuATSM-treated mice following 3–51 days of treatment. Subsequently, treatment was ceased and remaining mice within the cohort (n = 13/treatment) were given a 1-week wash-out period and administered a lower dose of CuATSM (60 mg/kg/day) in a single daily treatment session (0830–0930) as previously described24. The revised dose of CuATSM (60 mg/kg/day) is the highest dose previously investigated and therapeutically effective in transgenic SOD1 mice maintained on a C57BL/6 background. Previous work has also shown CuATSM at 60 mg/kg/day does not produce any toxic effects or weight alterations in wild-type C57BL/6 mice24. However, CuATSM at a 60 mg/kg/day dose has not been previously investigated in SOD1G93A mice on a C57BL/6 background. Due to the staggered age of the cohort, mice started the adjusted treatment regimen (CuATSM; 60 mg/kg/day) at various ages (range = 80–125 days old), however the mean age, body weight and ALS score of mice within each treatment group were not significantly different once treatment recommenced (p < 0.05, Supplementary Table 1). The same CuATSM batch and drug preparation steps were not changed from the initial treatment regimen. Equivalent volumes of SSV were administered to the vehicle-treated mice.
Weight and neurological score
Body weight was recorded three times a week, prior to the first daily treatment. Mice were also scored using the criteria outlined by the ALS Therapy Development Institute (TDI)54 (Supplementary Table 2) three times a week to assess neurological deficit. Scoring commenced at the beginning of treatment (70 days old) and was performed by observers blinded to treatment.
Rotarod
The locomotor function of mice was assessed weekly, beginning at the first week of treatment (70 days old), using a five-lane accelerating rotarod (RotaRod Advanced, TSE Systems, Hesse, Germany). Mice were habituated to the rotarod assay 2 weeks prior to recording. Habituation sessions consisted of three acclimatisation sessions, with the first session run at a continuous 4 rotations per minute (rpm) for 180 s. The second and third habituation sessions were performed at an inclining speed of 4–20 rpm over a 180 s period. During the recording period (testing) the rotation speed of the rotarod was accelerated from 4 to 20 rpm over a 180 s period with the time taken to fail the task (latency to fall) recorded for each mouse. Mice were given three independent runs with a 30–60 s rest between runs. The maximum time each mouse was able to remain on the rod was recorded and included in the data analysis. To control for odour cues, the apparatus was cleaned with 70% ethanol after each trial.
Pole test
Motor coordination, including grip strength, was also assessed through the use of the pole test with minor modifications as previously described55. Mice were placed facing upwards on a wooden vertical pole (diameter: 1 cm; length: 55 cm). Mice were given 60 s to descend the pole and complete the task. The time to turn 180° and subsequently reach the bottom of the pole was recorded. Mice who either did not turn around or slid down the pole with no active climbing were given a maximum score of 60 s. Each mouse was tested twice, with a 30–60 s rest between runs. The minimum time to descend the pole was recorded and included in the analysis. To control for odour cues, each apparatus was cleaned with 70% ethanol between each trial. All mice were assessed until 150 days old or until they were unable to be securely placed on the pole.
Survival and end-stage
Mice treated with CuATSM (100 mg/kg/day) displaying signs of toxicity through weight loss, hunching, orbital tightening, and piloerection were indicators of a humane end-point and euthanasia determined by the University of Wollongong animal welfare officer. For mice treated with CuATSM (60 mg/kg/day) and matched vehicle-treated mice, disease end-stage was defined when the mice displayed either a 20% loss in maximum body weight or reached a clinical score of 4. Once end-stage was identified, mice were euthanised via asphyxiation using a slow-fill carbon dioxide technique, transcardially perfused with phosphate buffered saline solution (PBS) and lumbar spinal cords removed, snap frozen in liquid nitrogen and stored at − 80 °C.
Tissue homogenisation and fractionation
Lumbar spinal cord (~ 30 mg) collected from PBS-perfused mice was homogenised in buffer containing PBS + 0.1% Triton-X100 supplemented with Halt™ protease and phosphatase inhibitors (ThermoFisher, Australia) with polypropylene pestles. Homogenates were centrifuged (20,000×g for 30 min at 4 °C) and the supernatant collected (PBS-soluble fraction). Protein concentration was determined via a DC assay as per the manufacturer’s instructions (Bio-Rad, Australia), and tissue homogenate stored at − 80 °C.
Immunoblot
PBS-soluble fractions from the lumbar spinal cord (10 µg) were separated in stain-free TGX Any-kDa SDS-PAGE precast gels (Bio-Rad, Australia) under reducing conditions. Proteins were transferred onto 0.2 µm polyvinylidene difluoride membranes (GE Healthcare, Australia). Membranes were blocked in blocking solution containing 5% skim milk powder/Tris-buffered saline with 0.02% Tween 20 (w/v) for 1 h at room temperature (RT). Subsequently, membranes were incubated with anti-SOD1 antibody (Abcam, ab13498; 1:10,000) in blocking solution overnight at 4 °C. Following primary antibody incubation, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for anti-rabbit IgG (Dako, P0448; 1:5000) in blocking solution for 1 h at RT. To visualise bands, membranes were incubated with ECL (GE Healthcare, Australia) and imaged with an Amersham 600RB Imager (GE Healthcare). SOD1 band quantification was achieved through the use of ImageJ software (Version 1.48, https://imagej.nih.gov/ij/)56 and intensity normalised to total protein and a pooled sample (containing equal amounts of each sample) to account for equal protein loading between samples and gels, respectively. Each sample was run in duplicate.
In-gel zymography
SOD1 enzymatic activity was determined using in-gel zymography as previously described57. Briefly, lumbar spinal cord PBS-soluble fractions (2.5 μg) and 100 ng of purified recombinant human SOD1WT protein was separated in 8% native PAGE gels. Subsequently, gels were incubated in 5 mM nitrotetrazolium blue chloride (Sigma-Aldrich) for 20 min with gentle agitation, before incubation in developer solution (10 mM tetramethylethylenediamine and 30 µM riboflavin) for 15 min. Gels were developed by exposure to fluorescent light until sufficient contrast between the achromatic zones and background was achieved and images captured using a calibrated densitometer (GS-900; Bio-Rad, Australia). SOD1 activity was quantified as absorbance of the clear bands using ImageJ software (version 1.48, https://imagej.nih.gov/ij/). Each sample was run in duplicate. To account for equal protein between samples, SOD1 activity was normalised to InstantBlue Coomassie stain (Sigma-Aldrich) from samples run on an adjacent gel.
Data presentation and statistical analyses
For each data point, the mean ± standard error of the mean (SEM) is reported, unless otherwise indicated. Body weight, neurological score, rotarod- and pole test-activity were determined through repeated measures one-way ANOVA with Fisher's least significant difference test for comparisons between CuATSM- and vehicle-treated mice. Survival of CuATSM- and vehicle-treated mice was compared using a Mantel–Cox test. Student's t test was used to assess any other comparisons between CuATSM and vehicle treatment groups. To mitigate potential age- or sex-bias due to the attrition that resulted from treating with CuATSM at 100 mg/kg/day, only vehicle-treated mice with age- and sex-matched CuATSM-treated counterparts were included in the remaining data used to determine the therapeutic efficacy of CuATSM treatment at 60 mg/kg/day.
Ethics declarations
All research was approved by the Animal Ethics Committee (AE19/10) of the University of Wollongong (Wollongong, Australia) and complied with the National Health and Medical Research Institute, Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The study was carried out in compliance with the ARRIVE guidelines.
Supplementary Information
Acknowledgements
We thank Dr Sarah Toole, our attending veterinarian and animal welfare officer for her support in monitoring the health of mice, in addition to her assistance identifying and managing emerging health issues.
Author contributions
Conceptualization: J.S.L., L.M., J.J.Y.; Methodology: J.S.L., L.M., D.L., K.L.V., T.K., F.K., S.N., P.J.C., J.J.Y.; Validation: J.S.L., J.J.Y.; Formal analysis: J.S.L., M.L.B., J.J.Y.; Investigation: J.S.L., M.L.B., N.E.F., D.L., C.G.C., L.M., J.S.; Resources: P.S.D., L.E.M., J.J.Y.; Data curation: J.S.L., M.L.B., N.E.F., J.J.Y.; Writing—original draft: J.S.L., M.L.B., P.S.D., J.J.Y.; Writing—review and editing: J.S.L., M.L.B., N.E.F., L.M., D.L., C.G.C., J.S., K.L.V., T.K., F.K., L.E.M., S.N., P.J.C., P.S.D., J.J.Y.; Visualization: J.S.L., M.L.B., N.E.F., J.J.Y.; Supervision: J.S.L., J.J.Y.; Project administration: J.S.L., J.J.Y.; Funding acquisition: J.J.Y.
Funding
JJY was supported by a University of Wollongong Professorship in Neurodegenerative Diseases, and JJY, and NEF were supported by a National Health and Medical Research Council, Australia Dementia Teams Grant (1095215). LM was supported by a Motor Neuron Disease Research Institute of Australia Bill Gole Postdoctoral Fellowship. CGC was supported by a University of Wollongong-Yerbury Family Scholarship. TK is supported by a National Health and Medical Research Council (NHMRC) Dementia Research Team Initiative (#1095215) and two NHMRC Project Grants (#1102012 and #1141789) as well as the Ainsworth Medical Research Innovation Fund. Funding sources had no role in the study, design or interpretation of data.
Competing interests
Collaborative Medicinal Development LLC has licensed intellectual property related to this subject from the University of Melbourne where the inventors include PSD. PJC is an unpaid consultant for Collaborative Medicinal Development LLC. JSL, MLB, NEF, LM, DL, CGC, JS, KLV, TK, FK, LEM, SN, JJY declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98317-w.
References
- 1.Kiernan MC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 3.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/S0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 4.Miller RG, Appel SH. Introduction to supplement: The current status of treatment for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 2017;18:1–4. doi: 10.1080/21678421.2017.1361447. [DOI] [PubMed] [Google Scholar]
- 5.Al-Chalabi A, et al. July 2017 ENCALS statement on edaravone. Amyotroph. Lateral Scler. Frontotemporal Degener. 2017;18:471–474. doi: 10.1080/21678421.2017.1369125. [DOI] [PubMed] [Google Scholar]
- 6.Abel O, et al. Development of a smartphone app for a genetics website: The Amyotrophic Lateral Sclerosis Online Genetics Database (ALSoD) JMIR mHealth uHealth. 2013;1:e18. doi: 10.2196/mhealth.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathis S, Goizet C, Soulages A, Vallat J-M, Masson GL. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019;399:217–226. doi: 10.1016/j.jns.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Borchelt DR, et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaume AG, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 10.Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: A model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-V. [DOI] [PubMed] [Google Scholar]
- 11.Jaarsma D, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol. Dis. 2000;7:623–643. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 12.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAlary L, Yerbury JJ. Strategies to promote the maturation of ALS-associated SOD1 mutants: Small molecules return to the fold. Neural Regen. Res. 2019;14:1511–1512. doi: 10.4103/1673-5374.255962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei G, et al. Denaturation of human copper-zinc superoxide dismutase by guanidine hydrochloride: A dynamic fluorescence study. Biochemistry. 1992;31:7224–7230. doi: 10.1021/bi00147a003. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez JA, et al. Familial amyotrophic lateral sclerosis-associated mutations decrease the thermal stability of distinctly metallated species of human copper/zinc superoxide dismutase. J. Biol. Chem. 2002;277:15932–15937. doi: 10.1074/jbc.M112088200. [DOI] [PubMed] [Google Scholar]
- 17.Roberts BR, et al. Oral treatment with Cu(II)(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34:8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JR, et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SODG93A mice co-expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton JB, White AR, Crouch PJ. Endogenous Cu in the central nervous system fails to satiate the elevated requirement for Cu in a mutant SOD1 mouse model of ALS. Metallomics. 2016;8:1002–1011. doi: 10.1039/C6MT00099A. [DOI] [PubMed] [Google Scholar]
- 20.Hilton JB, et al. CuII(atsm) improves the neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord. Sci. Rep. 2017;7:42292. doi: 10.1038/srep42292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan JY, et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: Correlating 7 Tesla MRI and pathology. PLoS One. 2012;7:e35241. doi: 10.1371/journal.pone.0035241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilton, J. B. Disrupted copper availability in sporadic ALS: Implications for CuII (atsm) as a treatment option. Preprint at https://www.biorxiv.org/content/10.1101/2020.04.17.047704v1 (2020). [DOI] [PMC free article] [PubMed]
- 23.Soon CPW, et al. Diacetylbis(N(4)-methylthiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J. Biol. Chem. 2011;286:44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllum EJ, et al. Therapeutic effects of CuII(atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14:586–590. doi: 10.3109/21678421.2013.824000. [DOI] [PubMed] [Google Scholar]
- 25.Hung LW, et al. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson’s disease. J. Exp. Med. 2012;209:837–854. doi: 10.1084/jem.20112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huuskonen MT, et al. The copper bis(thiosemicarbazone) complex CuII(atsm) is protective against cerebral ischemia through modulation of the inflammatory milieu. Neurotherapeutics. 2017;14:519–532. doi: 10.1007/s13311-016-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choo XY, et al. CuII(atsm) Attenuates Neuroinflammation. Front. Neurosci. 2018;12:668. doi: 10.3389/fnins.2018.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly D, et al. The P2X7 receptor antagonist JNJ-47965567 administered thrice weekly from disease onset does not alter progression of amyotrophic lateral sclerosis in SOD1G93A mice. Purinergic Signal. 2020;16:109–122. doi: 10.1007/s11302-020-09692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira FG, et al. CuATSM efficacy is independently replicated in a SOD1 mouse model of ALS while unmetallated ATSM therapy fails to reveal benefits. IBRO Rep. 2017;2:47–53. doi: 10.1016/j.ibror.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe, D., Mathers, S. & Smith, G. Modification of ALS disease progression in a phase 1 trial of CuATSM. 29th International Symposium on ALS/MND Glasgow (2018).
- 31.Hebert, C. NTP technical report on the toxicity studies of cupric sulfate (CAS No. 7758-99-8) administered in drinking water and feed to F344/N rats and B6C3F1 mice. Toxicity Report Series29, 1-D3 (1993). [PubMed]
- 32.Kumar V, Kalita J, Misra UK, Bora HK. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015;29:269–274. doi: 10.1016/j.jtemb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Pal A, et al. Biochemical, histological, and memory impairment effects of chronic copper toxicity: A model for non-Wilsonian brain copper toxicosis in Wistar rat. Biol. Trace Elem. Res. 2013;153:257–268. doi: 10.1007/s12011-013-9665-0. [DOI] [PubMed] [Google Scholar]
- 34.Fujibayashi Y, et al. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 35.Dearling J, Packard A. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010 doi: 10.1016/j.nucmedbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly PS, et al. An impaired mitochondrial electron transport chain increases retention of the hypoxia imaging agent diacetylbis(4-methylthiosemicarbazonato)copperII. Proc. Natl. Acad. Sci. U.S.A. 2012;109:47–52. doi: 10.1073/pnas.1116227108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padgitt-Cobb L. 234-Investigation into the oxidation of CuATSM as a mechanism for copper release. Free Radic. Biol. Med. 2016;100:S107. doi: 10.1016/j.freeradbiomed.2016.10.275. [DOI] [Google Scholar]
- 38.Sirois JJ, et al. Oxidative release of copper from pharmacologic copper bis(thiosemicarbazonato) compounds. Inorg. Chem. 2018;57:8923–8932. doi: 10.1021/acs.inorgchem.8b00853. [DOI] [PubMed] [Google Scholar]
- 39.Sousa T, et al. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570:462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farr C, Hunt DM. Genetic differences in zinc and copper induction of liver metallothionein in inbred strains of the mouse. Biochem. Genet. 1989;27:199–217. doi: 10.1007/BF02401801. [DOI] [PubMed] [Google Scholar]
- 42.Huster D, et al. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168:423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heiman-Patterson TD, et al. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J. Neurol. Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Heiman-Patterson TD, et al. Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: A window of opportunity in the search for genetic modifiers. Amyotroph. Lateral Scler. 2011;12:79–86. doi: 10.3109/17482968.2010.550626. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso R, et al. Effect of genetic background on onset and disease progression in the SOD1-G93A model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2012;13:302–310. doi: 10.3109/17482968.2012.662688. [DOI] [PubMed] [Google Scholar]
- 46.Pfohl SR, Halicek MT, Mitchell CS. Characterization of the contribution of genetic background and gender to disease progression in the SOD1 G93A mouse model of amyotrophic lateral sclerosis: A meta-analysis. J. Neuromusc. Dis. 2015;2:137–150. doi: 10.3233/JND-140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharp PS, Dick JRT, Greensmith L. The effect of peripheral nerve injury on disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuroscience. 2005;130:897–910. doi: 10.1016/j.neuroscience.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 48.Dupuis L, Oudart H, René F, Gonzalez de Aguilar J-L, Loeffler J-P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrawell NE, Yerbury MR, Plotkin SS, McAlary L, Yerbury JJ. CuATSM protects against the in vitro cytotoxicity of wild-type-like copper-zinc superoxide dismutase mutants but not mutants that disrupt metal binding. ACS Chem. Neurosci. 2019;10:1555–1564. doi: 10.1021/acschemneuro.8b00527. [DOI] [PubMed] [Google Scholar]
- 50.Kuo MTH, Beckman JS, Shaw CA. Neuroprotective effect of CuATSM on neurotoxin-induced motor neuron loss in an ALS mouse model. Neurobiol. Dis. 2019;130:104495. doi: 10.1016/j.nbd.2019.104495. [DOI] [PubMed] [Google Scholar]
- 51.Nikseresht S, Hilton JBW, Kysenius K, Liddell JR, Crouch PJ. Copper-ATSM as a treatment for ALS: Support from mutant SOD1 models and beyond. Life. 2020;10:271. doi: 10.3390/life10110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Southon A, et al. CuII(atsm) inhibits ferroptosis: Implications for treatment of neurodegenerative disease. Br. J. Pharmacol. 2020;177:656–667. doi: 10.1111/bph.14881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blower, P. J. et al. Structural trends in copper(II) bis(thiosemicarbazone) radiopharmaceuticals. Dalton Trans. 4416–4425 (2003). 10.1039/B307499D.
- 54.Hatzipetros T, et al. A quick phenotypic neurological scoring system for evaluating disease progression in the SOD1-G93A mouse model of ALS. J. Vis. Exp. 2015 doi: 10.3791/53257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karl T, Pabst R, von Hörsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 56.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.