Abstract
Aglaia, with about 120 species, is one of the largest genera of the plant family Meliaceae (the mahogany plants), and is native to the tropical rainforests of the Indo-Australian region, ranging from India and Sri Lanka eastwards to Polynesia and Micronesia. Various Aglaia species have been investigated since the 1960s for their phytochemical constituents and biological properties, with the cyclopenta[b]benzofurans (rocaglates or flavaglines) being of particular interest. Phytochemists, medicinal chemists and biologists have conducted extensive work in establishing these secondary metabolites as potential lead compounds with antineoplastic and antiviral effects, among others. The varied biological properties of rocaglates can be attributed to both their unusual structures and their ability to act as inhibitors of the eukaryotic translation initiation factor 4A (eIF4A), hence affecting protein translation. The present review provides an update on the recently reported phytochemical constituents of Aglaia species, focusing on rocaglate derivatives. Furthermore, laboratory work performed on investigating the biological activities of these chemical constituents is also covered.
Keywords: Aglaia, Meliaceae, natural products, rocaglates, flavaglines, rocaglamide, silvestrol, biological effects, antineoplastic activity, antiviral activity, protein translation inhibition
Graphical Abstract
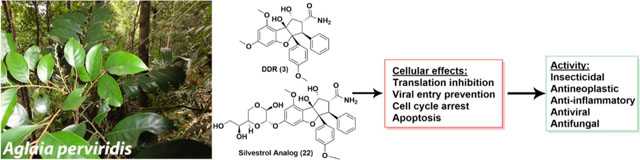
Introduction
Since the first phytochemical report of the tetracyclic triterpene, aglaiol, from the leaves of the oriental plant Aglaia odorata in 1965 [1], many studies have appeared describing the chemical constituents of Aglaia species in terms of elucidating the structurally diverse natural products present. Examples of secondary metabolite compound classes isolated from Aglaia species include bisamides [2–4], flavonoids [2, 5], lignans [6], and triterpenoids, particularly of the baccharane, cycloartane, and dammarane types [7–9]. In addition, more than 100 biogenetically related oxygen-containing heterocyclic secondary metabolites have been isolated to date, and are characteristic of many Aglaia species, and are known collectively as rocaglamide (1) derivatives. Such compounds have been divided into three sub-classes: (i) cyclopenta[b]benzofurans (CPBFs; “flavaglines” or “rocaglates”); (ii) cyclopenta[b]benzopyrans (“thapsakins” or “aglain” derivatives) and (iii) benzo[b]oxepines (“thapoxepines” or “forbaglines”), which are considered to be formed by the cycloaddition of a cinnamic acid amide and a flavonoid unit [10, 11]. Of these three groups, rocaglates are the most potently bioactive and well-investigated chemical constituents of Aglaia species and thus the main focus of the present article.
(−)-Rocaglamide (1), a CPBF, was the first compound obtained in its class and was isolated in 1982 from the dried roots and stems of Aglaia elliptifolia as an in vivo-active antileukemic agent [12]. In subsequent years, more than 60 rocaglate derivatives have been isolated and structurally identified from several Aglaia species [13, 14]. With most substitutions occurring at the C-1 and C-2 positions of their phenyl rings (Fig. 1), some examples of rocaglate derivatives are methyl rocaglate (2) [15, 16], didesmethylrocaglamide (DDR, 3) [13, 15], rocaglaol (4) [17], aglaroxin C (5) [18], cyclorocaglamide (6) [19], and isothapsakon A (7) [20]. Another rocaglate derivative that has garnered much scientific attention is silvestrol (8), which was isolated and structurally characterized along with its 5‴-epimer, episilvestrol (9) [21]. These two rocaglate congeners were purified from the stem bark of Aglaia foveolata (originally misidentified taxonomically as Aglaia silvestris) collected in Indonesia, and contain an unprecedented dioxanyl ring connected to the CPBF core at the C-6 position of the phenyl ring A (Fig. 1). The presence of a dioxanyl ring was demonstrated to enhance the cytotoxic potency of rocaglates [2, 22, 23] and has led to extensive work on the synthesis and structure-activity requirement exploration of rocaglamide, silvestrol, and related analogues [22–28].
Fig. 1.
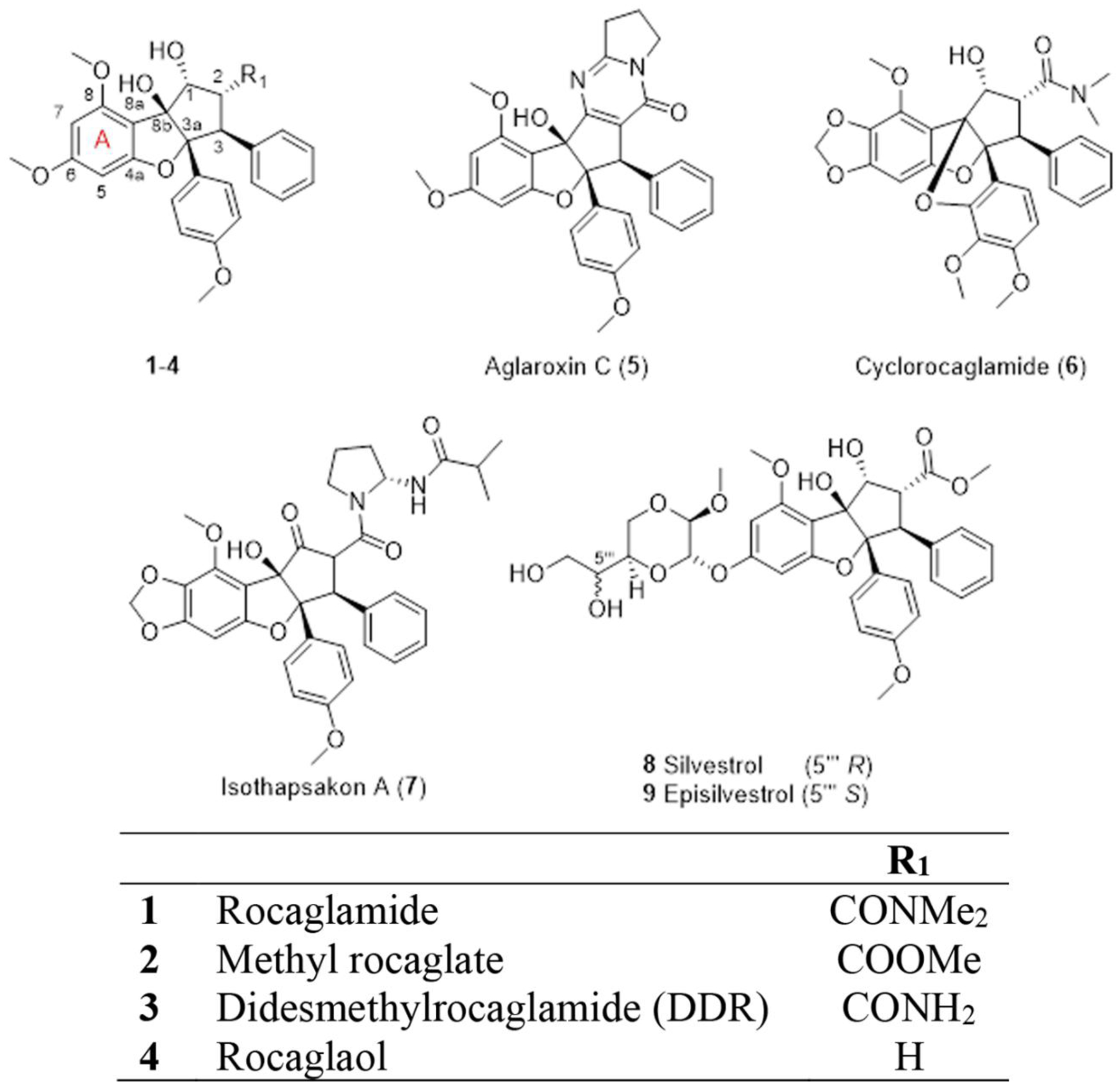
Examples of selected flavaglines (1-9) isolated from various Aglaia species
A pivotal paper exploring the cellular mechanism of activity of the rocaglates was published in 2008 [29], in which Pelletier and his associates reported silvestrol as an inhibitor of protein translation by modulating the activity of the eukaryotic initiation factor (eIF) 4A, an RNA helicase subunit of the eIF4F complex. This work has been complemented by further mechanistic reports from the same group [30, 31], with selected rocaglates also being documented to act at the cellular level to modulate the Raf-MEK-ERK pathway via targeting prohibitins (PHBs) 1/2 [32], MAPK [33], and FMS-like receptor tyrosine kinase 3 (FLT3) and the microRNA-155 (miR-155) gene [34]. Such biological studies have laid the foundation for developing rocaglamide and silvestrol as potential drug leads against different disease states including, in particular, cancer, and more recently, certain viruses.
This review describes the work on rocaglates from Aglaia species, primarily in terms of their phytochemical isolation, structural characterization, and biological activities, as reported during the period 2014–2020. It is intended as an update of the two previous review articles we wrote on this same topic in 2006 [22] and 2014 [23], and includes experimental contributions from the respective laboratories of the three current senior authors. Additionally, a summary is included of the collection of several Aglaia species from three Southeast Asian countries (Indonesia, Laos and Vietnam) under various formal Memoranda of Agreement (MOA) with the University of Illinois at Chicago, as part of two multi-institutional collaborative research projects funded by the U.S. National Cancer Institute (NCI) [35–37]. To assist with the writing of this review, the SciFinder literature database (Chemical Abstracts Service, Columbus, OH, USA) was searched using keywords such as Aglaia, rocaglamide and silvestrol and then categorized and refined for relevant publications and patents from 2014 onwards.
Taxonomy and Collection
The genus Aglaia is a large group of plants, mostly comprised of trees, belonging to the family Meliaceae. These species have a natural distribution spanning the tropics from Sri Lanka and India, east to the Pacific through Burma, southern mainland China, Taiwan, Vietnam, Malaysia, Indonesia, the Philippines, New Guinea, Northern Australia, and the Western Pacific. In the volume “A Taxonomic Monograph of The Genus Aglaia Lour. (Meliaceae)”, Dr. Caroline Pannell (University of Oxford, Oxford, UK), a leading taxonomic specialist of this genus, included 104 species as belonging to Aglaia [38]. However, today, approximately 120 Aglaia species are recognized [39]. The largest concentration of this genus is found in Indonesia, followed by Malaysia, the Philippines, and the Indochina region (including Thailand and southern mainland China). In the taxonomic system, Aglaia belongs to the Tribe Aglaieae, within the Subfamily Melioideae and is made up of two taxonomic sections, namely, the Section Aglaia and the Section Amoora [38]. In 2005, based on a phylogenetic study, Muellner and coworkers [40] recognized three taxonomic sections within the genus, viz., the Sections Amoora, Neoaglaia, and Aglaia, which are defined morphologically primarily by their fruit characteristics and by the numbers of flower parts.
All species of Aglaia are woody, ranging in size from a few meters high to large trees up to 40 m tall. The bark of these trees and their branches tend to exude a sticky white latex when incised. Most species have imparipinnate leaves, although these are occasionally simple or trifoliolate. Characteristically, branchlets and leaves are covered by an indumentum of peltate scales or stellate hairs, which may be used to identify certain species. The flowers of the members of the genus are small (1–10 mm long) and subglobose or ellipsoid and unisexual, normally set in a terminal and axillary paniculate inflorescence. In classifying the species, the staminal tube in the female flower provides the most taxonomic information. The fruits are either ellipsoid, obovoid or pear-shaped, dehiscent or indehiscent and covered by a stellate type of scales or hairs, with the pericarp thick and pliable. The outer layer of the seed coat (referred to as the aril) is usually fleshy [38].
Members of the genus Aglaia are found growing in both evergreen and monsoon primary and secondary forests, from sea level to an altitude of 1,500 m, or rarely higher, and occur mostly in evergreen forests and less commonly in monsoon forests. Aglaia species populations are normally scattered, not in a dense and dominant cluster, and many species have become rare due to forest clearance and may be threatened. Examples include A. foveolata, A. spectabilis and A. perviridis [41]. Most species yield good hardwood timber that can be used in construction, such as for buildings, bridges, houses, and furniture. Several species have found traditional medicinal uses, especially the leaves, for the treatment of bodily afflictions, such as wounds, fever, headache, asthma, jaundice, and as a tonic, e.g., after childbirth [42, 43]. One species, A. odorata, often found in cultivation, has a history of wide use in systems of traditional medicine to treat various diseases [44, 45].
During the time of operation of the two NCI supported multi-institutional research projects [35–37], a total of 17 identified species of Aglaia were collected for investigation for potential anticancer activity (Table 1). The greatest number of collections came from Indonesia (11 species), while a small number came from each of Laos (three species), Vietnam (two species), and Thailand (one species). Voucher specimens of these collections are in deposit at the Herbarium of the Field Museum of Natural History, Chicago, IL, USA, as well as at herbarium institutions in the county where each collection was made.
Table 1:
Aglaia species collected in Southeast Asia under two NCI-funded research projects
| COUNTRY | SPECIES | VOUCHER SPECIMEN |
|---|---|---|
| Thailand | Aglaia elliptica Bl. | Nantasan s.n. |
| Laos | Aglaia cf. macrocarpa King | Soejarto et al. 15399 |
| Laos | Aglaia cf. oligophylla | Soejarto et al. 15396 |
| Laos | Aglaia spectabilis (Miq.) Jain & Bennet | Soejarto et al.15410 |
| Vietnam | Aglaia cf. aquatica (Pierre) Harms | Soejarto et al. 15176 |
| Vietnam | Aglaia perviridis Hiern | Soejarto et al. 14863 |
| Indonesia | Aglaia cf. argentea Bl. | Riswan ML-039 |
| Indonesia | Aglaia edulis (Roxb.) Wall. | Riswan SR-022 |
| Indonesia | Aglaia elliptica Bl. | Riswan ML-033 |
| Indonesia | Aglaia foveolata Pannell | Riswan KP-034 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS02 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS17 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS18 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS23 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS24 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS24 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS25 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS26 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS26 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS27 |
| Indonesia | Aglaia foveolata Pannell | Riswan SR-IS28 |
| Indonesia | Aglaia foveolata Pannell | Riswan Z-34 |
| Indonesia | Aglaia korthalsii (Miq.) Pellegr. | Kardono SC87 |
| Indonesia | Aglaia leptantha Miq. | Riswan SR-IS01 |
| Indonesia | Aglaia odorata Lour. | Riswan A-12 |
| Indonesia | Aglaia odoratissima Bl. | Riswan SR-072 |
| Indonesia | Aglaia rubiginosa (Hiern) Pannell | Riswan Z-55 |
| Indonesia | Aglaia silvestris Merr. | Riswan SR-J17 |
| Indonesia | Aglaia silvestris Merr. | Riswan SR-068 |
| Indonesia | Aglaia silvestris Merr. | Riswan SR-CJR068 |
| Indonesia | Aglaia teysmanniana (Miq.) Miq. | Riswan SR-J20 |
| Indonesia | Aglaia tomentosa Teijsm. & Binn. | Riswan B-037 |
Phytochemical Reports of Rocaglate (Cyclopenta[b]benzofurans) Constituents of Aglaia Species (2014–2020)
In 2014, our group at The Ohio State University (OSU) published a comprehensive review focusing on rocaglamide (1), silvestrol (8), and their structurally related bioactive compounds [23], inclusive of isolation of several new rocaglates from four Aglaia species (Aglaia cucullata, A. edulis, A. odorata, and A. perviridis). Recent reports published largely during the period 2014–2020 have described the isolation of new rocaglate derivatives from only a relatively few Aglaia spp., namely, A. odorata, A. oligophylla, A. perviridis, and A. stellatopilosa. Many of the research reports on the Aglaia CPBFs published over the last few years have focused on their syntheses, analogue development and biological activity evaluation, with the last-mentioned topic, in particular, to be covered in subsequent sections of this review.
In 2015, An et al. reported the structures of eight new benzo[b]oxepine derivatives, aglaodoratins A-H [46], and eight new cyclopenta[b]benzopyrans, biosynthetic precursors to rocaglates, aglapervirisins B-J [47], from the leaves of Aglaia odorata and Aglaia perviridis, respectively. Of these 16 secondary metabolites, aglaodoratins C (10), D (11) and E (12) (Fig. 2) were observed to exhibit moderate cytotoxicity, with their IC50 values in the range from 0.097 to 6.25 μM, against three human cancer cell lines (HT-29 colon cancer, SMMC-7721 hepatocellular cancer, and MG-63 osteosarcoma). Additionally, aglaodoratin C (10) inhibited cellular proliferation by arresting cells at the G2/M cell-cycle phase and thereby inducing apoptosis in HepG2 liver cancer cells in vitro [46]. In contrast, aglapervirisins B-J were either only weakly active or non-cytotoxic for a panel of four human cancer cell lines [47]. Interestingly, Kong and colleagues recently published a further phytochemical investigation on the leaves of A. perviridis, where they reported four new aglain glycosides (aglapervirisins J-M) with weak anti-inflammatory activity, as determined by an in vitro nitric oxide inhibition assay using the RAW264.7 mouse macrophage tumor cell line [48].
Fig. 2.
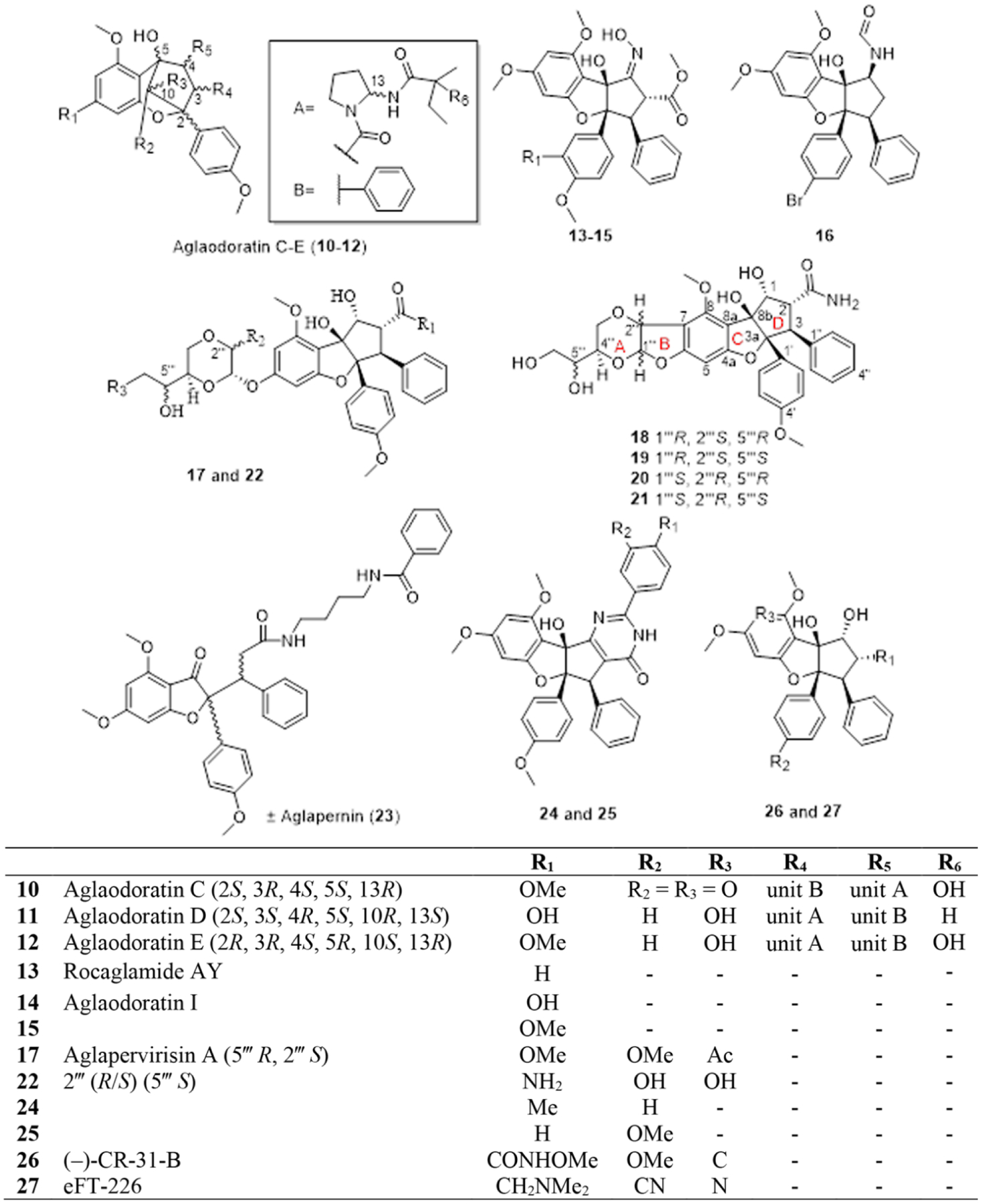
Structures of cytotoxic rocaglates isolated from various Aglaia species and of several related synthetic congeners (10-27)
Two separate groups reported the similar compounds rocaglamide AY (13) [49] and aglaodoratin I (14) [46], isolated from the leaves of A. oligophylla and A. odorata, respectively. Both these rocaglates have an oxime group at the C-1 position but aglaodoratin I (14) possesses a hydroxy group at the C-3′ position instead of a hydrogen atom, as seen in rocaglamide AY (13) (Fig. 2). The limited quantity of 14 obtained prevented it from being evaluated for cytotoxicity [46]. Although no biological test results were reported for rocaglamide AY (13) either, it was mentioned that many rocaglamide congeners possess insecticidal properties against the insect agricultural pest, Spodoptera littoralis [49]. Another paper published in 1999 reported a similar compound 15, also from the twigs and leaves of Aglaia odorata, which exhibited moderate insecticidal activity (LC50 of 1.3 ppm) toward Spodoptera litttoralis larvae [10]. Additionally, a rocaglaol derivative 16, with a formamide group at the C-1 (S) position and a bromine at C-4′ instead of a methoxy group, was found to have cytotoxic activity ranging from 0.5–2.3 nM against an array of human cancer cell lines [50], suggesting an amide at C-1 may lead to a more potent cytotoxic effect when compared to rocaglate derivatives with a more typical hydroxy group found at this position.
In 2016, Othman and colleagues published the isolation of silvestrol (8) and its epimer 5‴-episilvestrol (9), in addition to several new 2,3-seco-dammarane triterpenoids, from the stems of Aglaia stellatopilosa, collected in Sarawak, Malaysia [51]. Earlier, silvestrol (8) was reported in a patent application as an antineoplastic constituent of the Malaysian plant Aglaia leptantha, which was later re-identified as A. stellatopilosa [52]. However, the structures for the isolated compound described in this patent did not show full configurational details, and did not distinguish between compounds 8 and 9. Silvestrol (8) and episilvestrol (9) were also purified from the leaves of Aglaia periviridis in 2016, collected in Yunnan Province of the People’s Republic of China [47]. Moreover, our group confirmed the presence of compounds 8 and 9 in a root sample of A. perviridis collected in Vietnam [53]. Therefore, to date, cyclopenta[b]benzopyrans containing a dioxanyl ring as in silvestrol (8) have been found in only three of the approximately 120 Aglaia species (i.e., A. foveolata, A. perviridis, and A. stellatopilosa), making them rare constituents of this genus.
In addition to episilvestrol (5′′′-episilvestrol) (9), a small number of additional structural modified variants of silvestrol (8) have been reported from Aglaia species. In 2010, as a result of a large-scale recollection of the stem bark of A. foveolata from Kalimantan, Indonesia, the compounds 2′′′-episilvestrol and 2′′′,5′′′-diepisilvestrol were obtained as trace constituents, with both having reduced cytotoxic potencies when compared to silvestrol [8]. As a result of this work, which utilized specialized large-scale isolation facilities available at NCI, a sufficient amount of silvestrol (ca. 100 mg) was purified from the recollected plant material in order to conduct extensive biological testing at OSU and elsewhere [34, 54, 55]. An et al. in 2016 reported aglapervirisin A (17) as a new silvestrol analogue with an acetate group at the C-6‴ position of the dioxanyl ring side chain (Fig. 2) [47]. Cytotoxic profiling of aglapervirisin A (17) in four human cell lines (HT-29 colon cancer, HepG2 hepatocarcinoma, HL-60 leukemia, MCF-7 breast cancer) showed that it had comparable potencies to silvestrol (8) and episilvestrol (9), with IC50 values ranging from 8–14 nM. Further mechanistic evaluation of 17 against HepG2 cells, uncovered the ability of this mono-acetylated molecule to lower the expression levels of tyrosine phosphatases, Cdc2, and Cdc25, thereby causing apoptosis by arresting cells at the G2/M phase [47].
In 2019, our group at OSU reported five new cyclopenta[b]benzofuran analogues (18–22) from the leaves of Aglaia perviridis collected in the Nui Chua National Park in Vietnam [53]. Of these compounds, 18-21 were observed to have a fused dihydrofuran ring to both the dioxanyl and aryl rings of the rocaglate core (Fig. 2) and an amide moiety at C-2. Compound 22, elucidated as a 2‴-hydroxy derivative of episilvestrol (9) with an amide moiety at C-2, was isolated as an enantiomeric mixture (Fig. 2). Of these isolates, only compound 22 exhibited low micromolar cytotoxic potency against the human colon (HT-29) and prostate cancer (PC-3) cell lines. However, this study provided some structure-cytotoxic activity information, in that a hydroxy group at C-2‴ and the rigidity in structure between the dioxanyl and CPBF core might be detrimental to the cytotoxic activity of these flavaglines [53].
In the most recent report on Aglaia perviridis by Kong and colleagues [48], a novel rocaglate derivative was described, (±) aglapernin (23), which did not show cytotoxicity against cancer cell lines, but exhibited weak antibacterial activity (125 μM) against Porphyromonas gingivalis.
Another recent development worthy of mention, is the silvestrol-based antibody drug conjugates (ADCs), developed in 2017 by Genentech, Inc. [56]. ADCs are target-specific prodrugs, with a warhead (the cytotoxic drug) connected to a specific antibody via a linker [57–59]. The warhead bioactive moiety of the ADC provides the biological activity to the macromolecule, in antibody-targeted cells. In their patent, the Genentech team synthesized various silvestrol-ADC analogues, incorporating a dioxanyl ring system, with different antibodies. These ADCs were then evaluated both in vitro and in vivo, in breast cancer cells and B-cell malignancies. Silvestrol-ADCs connected to cysteine modified CD22 antibodies demonstrated promising results against a CD22-expressing xenograft mouse model [56].
Therapeutic Potential of Rocaglates (Update 2014–2020)
Since the initial report of rocaglamide (2) from Aglaia elliptifolia as an antileukemic agent in the early 1980s [12], the therapeutic potential of CPBFs has been evaluated and reported by a number of research groups. The diverse range of biological activities evaluated for these compounds have included antineoplastic, insecticidal, anti-inflammatory, neuroprotective, and more recently, antiviral properties [2, 22, 23, 60]. Fig. 3 gives a graphical representation of the published work on these compounds over the period 2014–2020. Continued modifications of the functional groups at specific positions have contributed to a better understanding of the structure-activity relationships of rocaglates as potential therapeutic agents. Substantial work has been conducted in establishing their biological targets, of which two have been explored to the greatest extent, namely, eIF4A [29, 30, 61] and PHB1/2 [32, 62, 63].
Fig. 3:
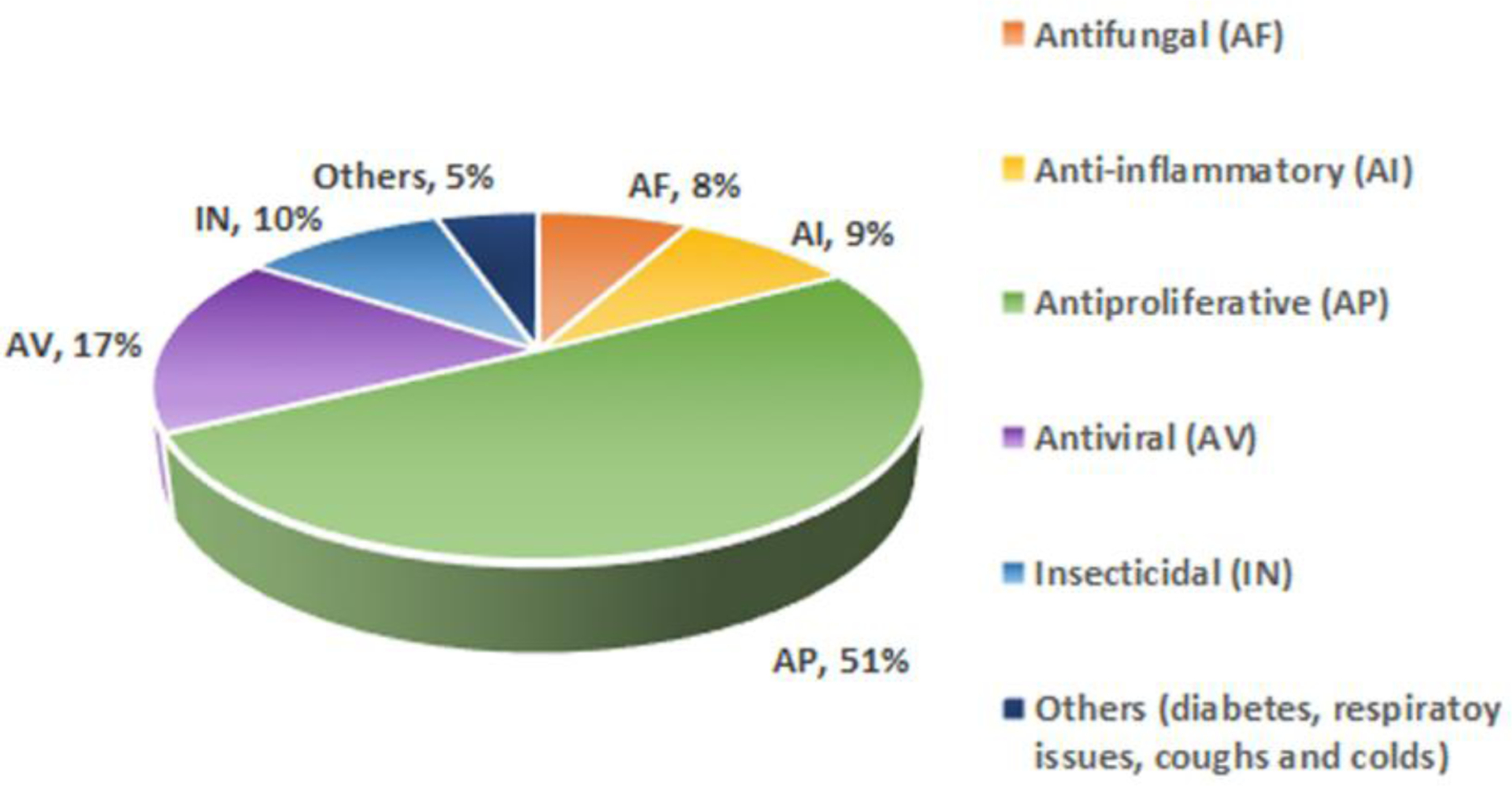
Graphical representation of publications on Aglaia spp. and rocaglate derivatives, with respect to different disease states, 2014–2020 (n = 77, primary research articles).
The initial studies exploiting the cellular mechanistic action of silvestrol (8) were published just a few years after its isolation, where it was observed to influence the interaction between eIF4A and RNA [29, 30]. This work was followed by the demonstration of synthesized biotinylated 5‴-episilvestrol to selectively inhibit eIF4AI/II [26].
Over the last few years, several studies have been published from our collaborative work on the effects of various rocaglate derivatives against a number of neurofibromatosis-associated tumors and pediatric sarcomas, with the biological testing experimental work performed at the Nationwide Children’s Hospital, Columbus, Ohio, USA. In an initial study using silvestrol (8), it was shown that the eIF4F components, including eIF4A, are potential therapeutic targets in malignant peripheral nerve sheath tumors (MPNSTs) and vestibular schwannomas [64]. Genetic depletion of eIF4A using short-hairpin RNAs and pharmacological inhibition using the natural eIF4A inhibitor silvestrol potently suppresses the growth of MPNSTs and schwannomas by decreasing the levels of multiple mitogenic signaling molecules including AKT, ERKs, Aurora A, and cyclins, important for tumor growth. The decrease of tumor growth was correlated with elevated levels of phospho-histone H3 and with G2/M arrest and apoptosis observed in the tumor cells treated with silvestrol [64].
The inhibition of the overexpressed eIF4F components in meningiomas was also investigated using a panel of 23 natural products, inclusive of representatives of the cucurbitacin, diarylheptanoid, rocaglate, simaroubolide, stilbenoid, sesquiterpene lactone, and xanthone structural classes [65]. Of the compounds examined, silvestrol (8) and episilvestrol (9) were the two most growth-inhibitory compounds, with silvestrol being more potent (IC50 10 nM) than episilvestrol (IC50 32 nM) against NF2-deficient meningioma Ben-Men-1 cells. Like in MPNSTs and schwannoma cells, silvestrol (8) treatment induced G2/M arrest in the meningioma cells. Taken together, it was suggested that inhibiting protein translation is a potential treatment approach for for MPNSTs, schwannomas, and meningiomas, including those associated with neurofibromatosis [64, 65].
However, silvestrol (8) has suboptimal drug-like properties, including a bulky structure, poor oral bioavailability [66], and sensitivity to multi-drug resistance 1 (MDR1) efflux [67]. Moreover, a toxicity study in larger animals conducted by our colleagues and collaborators at OSU and the NCI Developmental Therapeutic Program (DTP) revealed toxic effects of silvestrol in the lungs of dogs [68]. Consequently, further development of silvestrol as a cancer therapy was suspended. To search for compounds with better drug-like properties, alternative rocaglate congeners to silvestrol (8) were sought as potential growth inhibitors of MPNST, schwannoma and meningioma cells [68]. Upon side-by-side comparison of ten rocaglates lacking the dioxanyl ring with silvestrol (8), rocaglamide (1) and DDR (3) were found to exhibit growth-inhibitory activity comparable to silvestrol (8). Like silvestrol, both rocaglamide (1) and DDR (3) arrested tumor cells at G2/M, and induced apoptosis and a DNA-damage response, while decreasing the expression of multiple mitogenic kinases, consistent with translation inhibition. In collaboration with colleagues at the NCI DTP, rocaglamide (1) was observed to be 50% orally bioavailable and did not show any discernible pulmonary toxicity in dogs. In addition, both rocaglamide (1) and DDR (3) were not sensitive to MDR1 inhibition, possibly due to the lack of a dioxanyl ring. Most importantly, when administered either intraperitoneally or orally, rocaglamide (1, NSC326408) potently inhibited tumor growth in an orthotopic MPNST model. In addition, rocaglamide (1) exhibited broad antitumor activity in patient-derived xenograft (PDX) models for a Ewing sarcoma, an osteosarcoma, and an alveolar rhabdomyosarcoma. In a comparative in vitro study of 11 rocaglate congeners, including rocaglamide (1), methyl rocaglate (2), DDR (3), rocaglaol (4), and silvestrol (8), DDR (3, IC50 between 5 and 15 nM) was found to be the most potent compound, when tested against a panel of MPNST, a schwannoma, and a meningioma cell lines [68]. (‒)-DDR (3) was obtained earlier in our work as a trace constituent from the combined leaves, twigs and fruits of Aglaia perviridis when collected in Vietnam, and differs from rocaglamide (1) in possessing an amide unit instead of a dimethylamide functionality at the C-2 position of the CPBF core (Fig. 1) [13]. In a follow-up investigation, chemically synthesized DDR (3) was also found to effectively block tumor growth in orthotopic cell line-derived xenograft (CDX) and PDX models of osteosarcoma [69]. It was suggested that both rocaglamide (1) and DDR (3) are worthy of further evaluation as potential treatments for pediatric bone and soft-tissue sarcomas.
Additional studies have been performed on the cellular mechanism of action of rocaglate family members as eIF4A inhibitors. Chu and colleagues used CRISPR-Cas9 as a tool for drug-target validation in vivo. They validated the rocaglate-eIF4A relationship by introducing an eIF4A1 mutant allele (F163L) into cells and showed that eIF4A1(F163L) retains helicase activity but was unresponsive to rocaglates, such as silvestrol (8) [70]. Iwasaki et al. reported that rocaglamide (1) does not repress translation of specific messenger RNAs by reducing cellular eIF4A availability, but rather by clamping eIF4A onto the polypurine sequences in an ATP-independent manner [71]. This same group later elucidated the crystal structure of a complex of human eIF4A-rocaglamide-polypurine RNA, and showed rocaglamide to target a bimolecular cavity between eIF4A and polypurine RNA [72]. Recently, Sidraharan et al. treated breast cancer stem cells (BCSCs) with rocaglamide (1), determining eIF4A as a valid molecular target for both BCSCs and bulk tumor cells. They further suggested that eIF4A inhibitors may be combined synergistically with existing chemo-, radio- and/or immunotherapies [73].
Extensive follow-up work has been done more recently since an initial documentation of rocaglates and their inhibitory effect on PHB1/2 [32]. This includes not only the investigation of their antineoplastic activity but also their potential antiviral effects. Liu and colleagues evaluated rocaglamide (1) and aglaroxin C (5) in hepatitis C virus (HCV)-infected human hepatoma cells [63]. HCV, responsible for hepatitis C and liver cancer in humans, enters human hepatocyte cells utilizing different membrane proteins, particularly based on the interaction between its glycoprotein E2 and PHB1/2 [74]. Rocaglamide (1) inhibits HCV entry into human hepatoma cells by targeting PHB1/2, which in turn inhibits the CRaf/RAS pathway, an integral component in cell proliferation and signaling [75]. While synthesized aglaroxin C (5, Fig. 1) was found to exhibit a weak effect on HCV replication or entry into cells, several further analogues of 5 were shown to be more effective HCV entry inhibitors, including 24 and 25, in which the C-aryl group of the pyramidinone is differentially substituted [76, 77]. These two compounds exhibited low cytotoxicity (EC50 = 0.5 μM), 3-fold greater in comparison to aglaroxin C (5), against human hepatoma Huh-7.5.1 cells infected with HCV and were suggested to inhibit viral entry rather than replication as their mechanism of action [76]. Another similar study with enterovirus 71 (EV71), responsible for hand, foot, and mouth disease in humans, demonstrated a dependence on PHB for cell entry, with rocaglamide (1) used to investigate EV71 translation and entry inhibition [78]. An in vivo study of EV71-infected mice showed that mice survived longer with lower viral loads in the brain and spinal cord, on treatment with rocaglamide (1, 0.25 mg/kg), as compared to those treated with vehicle (0.25% DMSO in olive oil). These data were supported by an in vitro study of EV71-infected motor-neuron NSC-34 cells, where a dose-dependent reduction in viral load was observed in cells treated with rocaglamide (1, 10–100 nM) [78].
In 2017, silvestrol (8) was evaluated in vitro for its antiviral activity against the Ebola virus (EBOV) [79]. This study by Biedenkof et al. demonstrated the ability of silvestrol (8) to inhibit EBOV infection at a low non-cytotoxic concentration (10 nM). Additionally, they demonstrated that reduction of EBOV propagation correlated with the disappearance of viral nucleoprotein (NP), which is comparable to translational inhibition of PIM1, a cellular kinase known to be affected by silvestrol (8) [79]. In another antiviral study, Slaine et al. examined the role of silvestrol (8) in blocking the replication of influenza A virus (IAV) [80]. They showed that silvestrol treatment during early IAV infection induced stress granule formation, inhibited viral protein synthesis, and blocked viral replication. Interestingly, the viral protein synthesis was “recovered” upon silvestrol (8) withdrawal, suggesting a reversible translation inhibition mode of action [80].
Several further evaluations of rocaglamide (1) and silvestrol (8) as potential antiviral agents have been performed against hepatitis E virus (HEV) [81, 82], corona- and picornaviruses [83], chikungunya virus [84], Ebola virus and Marburg virus [85] and zika virus [86]. All these studies were based on the assumption that efficient translation of the mRNAs of these viruses, which contain highly structured 5′-untranslated regions (5-UTRs), requires the DEAD-box RNA helicase eIF4A. (‒)-CR-31-B (26), a synthesized rocaglate hydroxamate, was evaluated by Müller and colleagues for its antiviral activity against HEV, corona-, zika-, Lassa- and Crimean Congo hemorrhagic fever viruses, in comparison with silvestrol [87]. It was found that (‒)-CR-31-B (26) exhibited slightly more potent viral inhibition than silvestrol (8), with EC50 values in the low nanomolar range for most of the viruses examined. However, the inhibitory activity of (‒)-CR-31-B (26) against HEV replication was somewhat weaker in comparison to silvestrol (8), suggesting a potential difference in the antiviral mode of action between these two rocaglates [87]. Recently, the synthetic rocaglate (‒)-CR-1–31-B (26) was employed to show that eIF4A is a therapeutic target in pancreatic duct adenocarcinoma (PDA), and it suppressed tumor growth and extended the survival time in a genetically engineered mouse PDA model [88].
Two additional mechanistic targets of rocaglates have been suggested, namely, KRAS [89], a member of the RAS family of a small GTPases, and DDX3, a DEAD-box RNA helicase [90]. RAS proteins are imperative for triggering multiple signaling pathways required for cell proliferation and survival [91]. Mutations in KRAS, have been frequently found in several types of cancer, including pancreatic, lung, and colon cancers, and non-small-cell lung carcinoma [92]. According to Yurugi et al., flavaglines, particularly rocaglamide (1), potently inactivate RAS by inhibiting its GTP loading, and deterring its nanocluster formation at the phospholipid-enriched sites on the plasma membrane [89]. In turn, Chen et al. [90] focused on DDX3, a highly conserved DEAD-box helicase involved in cell-cycle regulation, differentiation, survival, and apoptosis [93]. Rocaglamide (1) was discerned to clamp DDX3 on its polypurine sequences in an ATP-independent manner, and the glutamine at position 360 was found to be a critical residue for DDX3 binding by this rocaglate [90].
Other Biological Properties of Rocaglate Derivatives
In 1985, Chiu published an initial report of the antifeedant activities against three agricultural pests of an acetone extract of Aglaia odorata [94]. This was followed up in 1993 by the work of Ishibashi and colleagues showing that two CPBF constituents, rocaglamide (1) and methyl rocaglate (2), from this plant demonstrated potent insecticidal activities against the larvae of the variegated cutworm (Peridroma saucia) [16]. Subsequently, several studies have evaluated the potential insecticidal effects of rocaglamide and its analogues. Although the exact mechanism of action for the insecticidal property of CPBFs is as yet unknown, phytochemists and medicinal chemists have obtained several congeners to evaluate their structure-activity relationships. For instance, the free hydroxy groups at both C-1 (R) and C-8b were essential for mediation of the insecticidal activity of rocaglamide (1) (Fig. 1), when evaluated against the pest insect Spodoptera littoralis [95, 96]. Moreover, favorable modifications by chemical synthesis at C-2 or C-4′ for insecticidal activity are a hydroxamide and halogen (Br or Cl) functional group, respectively. Such derivatives were well-tolerated in comparison to rocaglamide (1), and exhibited LC50 values ranging from 3 to 12.5 mg/L against an array of pests and beetles inclusive of Diabrotia balteata, Heliothis vierescens, Plutella xylostella, and Spodoptera littoralis [97].
Treatment of cerebral malaria, caused in particular by infection of Plasmodium falciparum, has proven to be a continued challenge. In spite of the widely available synthetic analogs of the plant-derived sesquiterpene lactone, artemisinin, resistance to this compound class by the causative organisms has been observed [98, 99]. Langlias and associates recently suggested the possibility of using rocaglates as potential therapeutic intervention agents for malaria. They showed the synthetic rocaglate derivative (‒)-CR-31-B (26) to exhibit antiplasmodial activity. According to their report, owing to its potential to inhibit eIF4A, (‒)-CR-31-B (26) not only inhibited Plasmodium protein synthesis at low nanomolar levels (ranging between 0.9 and 2.8 nM) in vitro but also showed similar effects in a dose-dependent manner in mice infected with Plasmodium berghei [100]. Additionally, their study highlighted the anti-inflammatory activity of (‒)-CR-31-B (26) by suppressing the production of interferon regulatory factor 1 (IRF1), a pro-inflammatory transcription factor important for the expression of critical inflammatory factors, like GBP2 and CXCL10 [100]. Complemented with a previous study that established good pharmacokinetic properties of this synthetic rocaglate [101], Langlais et al. proposed that (‒)-CR-31-B (26), warrants further evaluation as a potential therapy for cerebral malaria, either as a single agent or in combination with artemisinin [100].
In a recent publication, Wang et al. evaluated the potential neuroprotective effects of a 95% ethanol extract of A. odorata leaves [102]. This plant extract was found to exhibit a neuroprotective effect in a middle cerebral artery occlusion (MCAO) rat model. Treatment with this extract reduced the number of apoptotic cells and increased mitochondrial membrane potential in oxygen-glucose deprivation/reperfusion (OGC/R)-induced PC12 cells. It was hypothesized that the extract exerts a neuroprotective effect against cerebral ischemia by suppression of the p53/Puma mediated signaling pathway [102]. While these biological results look interesting, the investigation was not supported by any detailed phytochemical work, but only with a preliminary chromatographic profiling method indicating the presence of triterpenoids in the extract [102]. It is possible that the actual active constituents may include rocaglate derivatives, as already reported from A. odorata [16, 46].
Conclusions
Unlike many other structural classes of specialized metabolites from higher plants that have long been known, the novel rocaglate (flavagline) derivative, (−)-rocaglamide (1), was first reported in 1982 from the leaves of Aglaia elliptifolia [12]. At the time of its isolation, the structure and absolute configuration of this CPBF were determined by single-crystal X-ray crystallographic analysis, and it was shown to exhibit antileukemic activity (T/C value of 156%) in a P388 murine leukemia in vivo assay at a non-toxic dose (1.0 mg/kg) [12]. Likewise, the antileukemic activity of the dioxanyl-ring containing CPBF (−)-silvestrol (8) was reported in 2004 from Aglaia foveolata, and its full structure and stereochemistry were determined also by X-ray diffraction analysis [21]. Dioxanyl ring-containing CPBFs are rare constituents in Aglaia species, and, at the present time they have been found in only three of the approximately 120 members of this genus [8, 21, 47, 51–53]. Subsequently, and particularly over the last decade, the key cellular mechanism inhibition of eIF4A [29, 30] and PHB1/2 [32] has made rocaglamide (1) and silvestrol (8) of wide interest to the biomedical community as standard protein translation inhibitors. Both these compounds are now available commercially from fine-chemical scientific suppliers for research use. As a potential means of increasing their supply, rocaglamide [103–106] and silvestrol [107, 108] have been subjected to total chemical synthesis. In addition, methods have been developed for synthesizing rocaglate analogues to establish further structure-activity relationship information [109, 110]. However, it should be reiterated that while silvestrol has proven to be a useful pharmacological tool, it has sub-optimal drug-like properties and can cause pulmonary toxicity [68]. Therefore, this dioxanyl ring-containing natural product needs to be modified structurally for further development as a bioactive molecule drug lead.
Several review articles have appeared recently by various groups on the pharmacological activities of rocaglates, and have dealt, in particular, with their antineoplastic [14, 25, 111], antiviral [111, 112], and miscellaneous biological effects [14]. In terms of drug development, work on a synthetic derivative of rocaglamide (1), eFT-226 (27, Zotatifin), which was elucidated to have good pharmacokinetic properties and potent antitumor effects, like rocaglamide (1) and DDR (3), seems promising. The potent eIF4A inhibitor, Zotatifin, is the first compound with this mechanism of action to have entered into a clinical trial, as a potential treatment for patients with advanced solid-tumor malignancies [113].
Acknowledgements
The plant collection and laboratory studies by our group mentioned in this article were supported by grants U19 CA52956 and P01 CA125066 from NCI to ADK, W81XWH-18-1-0547 from the U.S. Department of Defense to LSC, and P30CA16058 to The OSU Comprehensive Cancer Center. We are very grateful to many faculty colleagues, research staff, postdoctoral fellows, and graduate students who have contributed to this work.
ABBREVIATIONS
- 5′-UTRs 5′
untranslated regions
- ADC
antibody-drug conjugate
- AMPPNP
adenylyl-imidodiphosphate
- BCSCs
breast cancer stem cells
- CDX
cell line-derived xenograft
- CPBF
cyclopenta[b]benzofuran ring system
- DDR
didesmethylrocaglamide
- DDX3
DEAD-box RNA helicase 3
- EBOV
Ebola virus
- eIF4A
eukaryotic initiation factor 4A
- EV71
enterovirus 71
- FLT3
FMS-like receptor tyrosine kinase 3
- HCV
hepatitis C virus
- HepG2 cells
human hepatoblastoma cell line
- HEV
hepatitis E virus
- IGF-1
insulin-like growth factor-1
- IRF1
interferon regulatory factor 1
- MDR1
multi-drug-resistance protein 1
- MPNST
malignant peripheral nerve sheath tumor
- NSCLC
non-small-cell lung carcinoma
- p53/Puma
p53 upregulated modulator of apoptosis
- PC-3
prostate cancer cell line
- PDA
pancreatic ductal adenocarcinoma
- PDX
patient-derived xenograft
- PHB1/2
prohibitins 1 and 2
- PIM1
proto-oncogene serine/threonine-protein kinase
- Raf-MEK-ERK
mitogen-activated protein kinases (MAPKs) involved in cell proliferation and survival
Footnotes
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- [1].Shiengthong D, Verasarn A, NaNonggai-Suwanrath P. Constituents of Thai medicinal plants-I. Aglaiol. Tetrahedron 1965; 21: 914–924 [Google Scholar]
- [2].Ebada SS, Lajkiewicz N, Porco JA Jr, Li-Weber M, Proksch P. Chemistry and biology of rocaglamides (= flavaglines) and related derivatives from Aglaia species (Meliaceae). In: Kinghorn AD, Falk H, Kobayashi J, eds, Progress in the Chemistry of Organic Natural Products. Vienna: Springer-Verlag; 2011; 94: 1–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang L, Wang LH, Yang YF, Yang SM, Zhang JH, Tan CH. Aglaianine, a new bisamide from Aglaia abbreviata. Nat Prod Res 2011; 25: 1676–1679 [DOI] [PubMed] [Google Scholar]
- [4].Sianturi J, Purnamasari M, Darwati, Harneti D, Mayanti T, Supratman U, Awang K, Hayashi H. New bisamide compounds from the bark of Aglaia eximia (Meliaceae). Phytochem Lett 2015; 13: 297–301 [Google Scholar]
- [5].Zhang H, Song Z-J, Chen W-Q, Wu X-Z, Xu H-H. Chemical constituents from Aglaia odorata Lour. Biochem Syst Ecol 2012; 41: 35–40 [Google Scholar]
- [6].Peng L, Fu WX, Zeng CX, Zhou L, Bao MF, Cai XH. Two new lignans from twigs of Aglaia odorata. J Asian Nat Prod Res 2016; 18: 147–152 [DOI] [PubMed] [Google Scholar]
- [7].Joycharat N, Plodpai P, Panthong K, Yingyongnarongkul B-E, Voravuthikunchai SP. Terpenoid constituents and antifungal activity of Aglaia forbesii seed against phytopathogens. Can J Chem 2010; 88: 937–944 [Google Scholar]
- [8].Pan L, Kardono LBS, Riswan S, Chai H-B, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale re-collection of Aglaia foveolata. J Nat Prod 2010; 73: 1873–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harneti D, Supriadin A, Maharani R, Nurlelasari, Mayanti T, Hidayat AT, Anwar R, Supratman U, Awang K, Shiono Y. Triterpenoids from the bark of Aglaia glabrata and their in vitro effects on P-388 murine leukemia cells. Orient J Chem 2019; 35: 134–139 [Google Scholar]
- [10].Nugroho BW, Edrada RA, Wray V, Witte L, Bringmann G, Gehling M, Proksch P. An insecticidal rocaglamide derivative and related compounds from Aglaia odorata (Meliaceae). Phytochemistry 1999; 51: 367–376 [Google Scholar]
- [11].Bacher M, Hofer O, Brader G, Vajrodaya S, Greger H. Thapsakins: possible biogenetic intermediates towards insecticidal cyclopenta[b]benzofurans from Aglaia edulis. Phytochemistry 1999; 52: 253–263 [Google Scholar]
- [12].King ML, Chiang C-C, Ling H-C, Fujita E, Ochiai M, McPhail AT. X-Ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J Chem Soc, Chem Commun 1982; 1982: 1150–1151 [Google Scholar]
- [13].Pan L, Muñoz-Acuña U, Li J, Jena N, Ninh TN, Pannell CM, Chai H-B, Fuchs JR, Carcache de Blanco EJ, Soejarto DD, Kinghorn AD. Bioactive flavaglines and other constituents isolated from Aglaia perviridis. J Nat Prod 2013; 76: 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harneti D, Supratman U. Phytochemistry and biological activities of Aglaia species. Phytochemistry 2021; 181: 112540. [DOI] [PubMed] [Google Scholar]
- [15].Dumontet V, Thoison O, Omobuwajo OR, Martin M-T, Perromat G, Chiaroni A, Riche C, Pais M, Sevenet T. New nitrogenous and aromatic derivatives from Aglaia argentea and A. forbesii. Tetrahedron 1996; 52: 6931–6942 [Google Scholar]
- [16].Ishibashi F, Satasook C, Ismant MB, Towers GHN. Insecticidal 1H-cyclopentatetrahydro[b]benzofurans from Aglaia odorata. Phytochemistry 1993; 32: 307–310 [Google Scholar]
- [17].Su B-N, Chai H-B, Mi Q, Riswan S, Kardono LBS, Afriastini JJ, Santarsiero BD, Mesecar AD, Farnsworth NR, Cordell GA, Swanson SM, Kinghorn AD. Activity-guided isolation of cytotoxic constituents from the bark of Aglaia crassinervia collected in Indonesia. Bioorg Med Chem 2006; 14: 960–972 [DOI] [PubMed] [Google Scholar]
- [18].Kokpol U, Venaskulchai B, Simpson J, Weavers RT. Isolation and X-ray structure determination of a novel pyrimidinone from Aglaia odorata. J Chem Soc, Chem Commun 1994; 1994: 773–774 [Google Scholar]
- [19].Bringmann G, Mühlbacher J, Messer K, Dreyer M, Ebel R, Nugroho W, Wray V, Proksch P Cyclorocaglamide, the first bridged cyclopentatetrahydrofuran, and a related “open chain” rocaglamide derivative from Aglaia oligophylla. J Nat Prod 2003, 66:80–85 [DOI] [PubMed] [Google Scholar]; Wang S-K, Cheng Y-J, Duh C-Y. Cytotoxic constituents from leaves of Aglaia elliptifolia. J Nat Prod 2001; 64: 92–94 [DOI] [PubMed] [Google Scholar]
- [20].Dreyer M, Nugroho BW, Bohnenstengel FI, Ebel R, Victor V, Witte L, Bringmann G, Mühlbacher J, Herold M, Hung P, Kiet LC, Proksch P New insecticidal rocaglamide derivatives and related compounds from Aglaia oligophylla. J Nat Prod 2001, 64:415–420 [DOI] [PubMed] [Google Scholar]
- [21].Hwang BY, Su B-N, Chai H-B, Mi Q, Kardono LBS, Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, Fairchild CR, Vite GD, Rose WC, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem 2004; 69: 3350–3358; ibid. 2004; 69: 6156 [DOI] [PubMed] [Google Scholar]
- [22].Kim S, Salim AA, Swanson SM, Kinghorn AD. Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anticancer Agents Med Chem 2006; 6: 319–345 [DOI] [PubMed] [Google Scholar]
- [23].Pan L, Woodard JL, Lucas DM, Fuchs JR, Kinghorn AD. Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species. Nat Prod Rep 2014; 31: 924–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ribeiro N, Thuaud F, Bernard Y, Gaiddon C, Cresteil T, Hild A, Hirsch EC, Michel PP, Nebigil CG, Désaubry L. Flavaglines as potent anticancer and cytoprotective agents. J Med Chem 2012; 55: 10064–10073 [DOI] [PubMed] [Google Scholar]
- [25].Ribeiro N, Thuaud F, Nebigil C, Désaubry L. Recent advances in the biology and chemistry of the flavaglines. Bioorg Med Chem 2012; 20: 1857–1864 [DOI] [PubMed] [Google Scholar]
- [26].Chambers JM, Lindqvist L,M, Webb A, Huang DCS, Savage GP, Rizzacasa MA. Synthesis of biotinylated episilvestrol: highly selective targeting of the translation factor eIF4AI/II. Org Lett 2013; 15: 1406–1409 [DOI] [PubMed] [Google Scholar]
- [27].Basmadjian C, Zhao Q, de Gramont A, Serova M, Falvre S, Raymond E, Vagner S, Robert C, Nebigil CG, Désaubry L. Bioactive Flavaglines: Synthesis and Pharmacology. In: Brahmachari G ed, Bioactive Natural Products. Weinheim: Wiley-VCH; 2015: 171–199 [Google Scholar]
- [28].Zhao Q, Abou-Hamdan H, Désaubry L. Recent advances in the synthesis of flavaglines, a family of potent bioactive natural compounds originating from traditional Chinese medicine. Eur J Org Chem 2016; 2016: 5908–5916 [Google Scholar]
- [29].Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SM, Wendel HG, Brem B, Greger H, Lowe SW, Porco JA Jr., Pelletier J Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J Clin Invest 2008; 118: 2651–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cencic R, Carrier M, Galicia-Vázquez G, Bordeleau ME, Sukarieh R, Bourdeau A, Brem B, Teodoro JG, Greger H, Tremblay ML, Porco JA Jr., Pelletier J Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS One 2009; 4: e5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cencic R, Carrier M, Trnkus A, Porco JA Jr., Minden M, Pelletier J Synergistic effect of inhibiting translation initiation in combination with cytotoxic agents in acute myelogenous leukemia cells. Leuk Res 2010; 34: 535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Polier G, Neumann J, Thuaud F, Ribeiro N, Gelhaus C, Schmidt H, Giaisi M, Kohler R, Muller WW, Proksch P, Leippe M, Janssen O, Désaubry L, Krammer PH, Li-Weber M. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem Biol 2012; 19: 1093–1104 [DOI] [PubMed] [Google Scholar]
- [33].Zhu JY, Lavrik IN, Mahlknecht U, Giaisi M, Proksch P, Krammer PH, Li-Weber M. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int J Cancer 2007; 121: 1839–1846 [DOI] [PubMed] [Google Scholar]
- [34].Alachkar H, Santhanam R, Harb JG, Lucas DM, Oaks JJ, Hickey CJ, Pan L, Kinghorn AD, Caligiuri MA, Perrotti D, Byrd JC, Garzon R, Grever MR, Marcucci G. Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia. J Hematol Oncol 2013; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bueno Pérez L, Still PC, Naman CB, Ren Y, Pan L, Chai H-B, Carcache de Blanco EJ, Ninh TN, Van Thanh B, Swanson SM, Soejarto DD, Kinghorn AD. Investigation of Vietnamese plants for potential anticancer agents. Phytochem Rev 2014; 13: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Henkin JM, Sydara K, Xayvue M, Souliya O, Kinghorn AD, Burdette JE, Chen W-L, Elkington BG, Soejarto DD. Revisiting the linkage between ethnomedical use and development of new medicines: A novel plant collection strategy towards the discovery of anticancer agents. J Med Plant Res 2017; 11: 621–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Henkin JM, Ren Y, Soejarto DD, Kinghorn AD. The search for anticancer agents from tropical plants. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, Asakawa Y, Liu J-K eds, Progress in the Chemistry of Organic Natural Products. Cham, Switzerland: Springer International; 2018; 107: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pannell CM. A Taxonomic monograph of the genus Aglaia Lour. (Meliaceae). Richmond, Surrey, UK: HMSO: Kew; 1992 [Google Scholar]
- [39].Online Brahms. Department of Plant Sciences, University of Oxford. Copyright© 1985–2020. Accessed October 6, 2020. Available at: https://herbaria.plants.ox.ac.uk/bol/aglaia;
- [40].Müellner AN, Samuel R, Chase MW, Pannell CM, Greger H. Aglaia (Meliaceae): An evaluation of taxonomic concepts based on DNA data and secondary metabolites. Am J Bot 2005; 92: 534–543 [DOI] [PubMed] [Google Scholar]
- [41].IUCN. The IUCN Red List of Threatened Species. Version 2020. Accessed November 7, 2020.Available at: https://www.iucnredlist.org/;
- [42].Widodo SH. Aglaia (PROSEA Medicinal Plants). Plant Resources of Southeast Asia. Accessed October 8, 2020. Available at: https://uses.plantnet-project.org/en/Aglaia_(PROSEA_Medicinal_plants)#:~:text=Several%20Aglaia%20species%20are%20used,diseases%2C%20and%20bark%20against%20tumours; [Google Scholar]
- [43].Priya R, Sowmiya P, Muthuraman MS. An overview on the biological perspectives of Aglaia species. Asian J Pharm Clin Res 2018; 11: 42 [Google Scholar]
- [44].Stuart Jr GU. Aglaia odorata. Philippine Medicinal Plants. Accessed October 6, 2020. Available at: http://www.stuartxchange.com/Sinamomong-sungsong.html; [Google Scholar]
- [45].Hong Kong Baptist University. Aglaia odorata. Medicinal Plant Images Database. School of Chinese Medicine, Hong Kong Baptist Church. Accessed October 6, 2020. Available at: https://web.archive.org/web/20150510023028/http://libproject.hkbu.edu.hk/was40/detail?channelid=1288&lang=en&searchword=herb_id%3DD00922; [Google Scholar]
- [46].An F-L, Wang J-S, Wang H, Wang X-B, Yang M-H, Guo Q-L, Dai Y, Luo J, Kong L-Y. Cytotoxic flavonol-diamide [3+2] adducts from the leaves of Aglaia odorata. Tetrahedron 2015; 71: 2450–2457 [Google Scholar]
- [47].An FL, Wang XB, Wang H, Li ZR, Yang MH, Luo J, Kong LY. Cytotoxic rocaglate derivatives from leaves of Aglaia perviridis. Sci Rep 2016; 6: 20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].An F-L, Xu W-J, Yang M-H, Luo J, Kong L-Y. Anti-inflammatory flavagline glycosides and putrescine bisamides from Aglaia perviridis leaves. Tetrahedron 2020; 76: 131257 [Google Scholar]
- [49].Duong NT, Edrada-Ebel R, Ebel R, Lin W, Duong AT, Dang XQ, Nguyen NH, Proksch P. New rocaglamide derivatives from Vietnamese Aglaia species. Nat Prod Commun 2014; 9: 833–834 [PubMed] [Google Scholar]
- [50].Thuaud F, Ribeiro N, Gaiddon C, Cresteil T, Désaubry L. Novel flavaglines displaying improved cytotoxicity. J Med Chem 2011; 54: 411–415 [DOI] [PubMed] [Google Scholar]
- [51].Othman N, Pan L, Mejin M, Voong JC, Chai H-B, Pannell CM, Kinghorn AD, Yeo TC. Cyclopenta[b]benzofuran and secodammarane derivatives from the stems of Aglaia stellatopilosa. J Nat Prod 2016; 79: 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Meurer-Grimes BM, Yu J, Vairo GL. Therapeutic compounds and methods. U.S. Patent 6710075 B2, 2004
- [53].Agarwal G, Wilson JR, Kurina SJ, Anaya-Eugenio GD, Ninh TN, Burdette JE, Soejarto DD, Cheng X, Carcache de Blanco EJ, Rakotondraibe LH, Kinghorn AD. Structurally modified cyclopenta[b]benzofuran analogues isolated from Aglaia perviridis. J Nat Prod 2019; 82: 2870–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, Davis ME, Rozewski DM, Johnson AJ, Su BN, Goettl VM, Heerema NA, Lin TS, Lehman A, Zhang X, Jarjoura D, Newman DJ, Byrd JC, Kinghorn AD, Grever MR. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood 2009; 113: 4656–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Callahan KP, Minhajuddin M, Corbett C, Lagadinou ED, Rossi RM, Grose V, Balys MM, Pan L, Jacob S, Frontier A, Grever MR, Lucas DM, Kinghorn AD, Liesveld JL, Becker MW, Jordan CT. Flavaglines target primitive leukemia cells and enhance anti-leukemia drug activity. Leukemia 2014; 28: 1960–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pillow T, Polson AG, Zheng B. Silvestrol antibody-drug conjugates and methods of use. US Pat Pub Appl 20170348422, December 7, 2017 [Google Scholar]
- [57].Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev 1998; 31: 89–104 [DOI] [PubMed] [Google Scholar]
- [58].Rautio J, Meanwell NA, Di L, Hageman MJ. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov 2018; 17: 559–587 [DOI] [PubMed] [Google Scholar]
- [59].Agarwal G, Blanco Carcache PJ, Addo EM, Kinghorn AD. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol Adv 2020; 38: 107337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grünweller A, Hartmann RK. Silvestrol: A potential future drug for acute Ebola and other viral infections. Future Virol 2017; 11: 243–245 [Google Scholar]
- [61].Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol 2011; 12: 235–245 [DOI] [PubMed] [Google Scholar]
- [62].Thuaud F, Ribeiro N, Nebigil CG, Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem Biol 2013; 20: 316–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Liu S, Wang W, Brown LE, Qiu C, Lajkiewicz N, Zhao T, Zhou J, Porco JA Jr., Wang TT. A novel class of small molecule compounds that inhibit hepatitis C virus infection by targeting the prohibitin-CRaf pathway. EBioMedicine 2015; 2: 1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Oblinger JL, Burns SS, Akhmametyeva EM, Huang J, Pan L, Ren Y, Shen R, Miles-Markley B, Moberly AC, Kinghorn AD, Welling DB, Chang LS. Components of the eIF4F complex are potential therapeutic targets for malignant peripheral nerve sheath tumors and vestibular schwannomas. Neuro Oncol 2016; 18: 1265–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Oblinger JL, Burns SS, Huang J, Pan L, Ren Y, Shen R, Kinghorn AD, Welling DB, Chang LS. Overexpression of eIF4F components in meningiomas and suppression of meningioma cell growth by inhibiting translation initiation. Exp Neurol 2018; 299: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sadlish H, Galicia-Vazquez G, Paris CG, Aust T, Bhullar B, Chang L, Helliwell SB, Hoepfner D, Knapp B, Riedl R, Roggo S, Schuierer S, Studer C, Porco JA Jr., Pelletier J, Movva NR. Evidence for a functionally relevant rocaglamide binding site on the eIF4A-RNA complex. ACS Chem Biol 2013; 8: 1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gupta SV, Sass EJ, Davis ME, Edwards RB, Lozanski G, Heerema NA, Lehman A, Zhang X, Jarjoura D, Byrd JC, Pan L, Chan KK, Kinghorn AD, Phelps MA, Grever MR, Lucas DM. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J 2011; 13: 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chang LS, Oblinger JL, Burns SS, Huang J, Anderson LW, Hollingshead MG, Shen R, Pan L, Agarwal G, Ren Y, Roberts RD, O’Keefe BR, Kinghorn AD, Collins JM. Targeting protein translation by rocaglamide and didesmethylrocaglamide to treat MPNST and other sarcomas. Mol Cancer Ther 2020; 19: 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chang LS, Oblinger JL, Agarwal G, Wilson TA, Roberts R, Fuchs J, O’Keefe BR, Kinghorn AD, Collins JM. The eIF4A inhibitors didesmethylrocaglamide and rocaglamide as effective treatments for pediatric bone and soft-tissue sarcomas. Cancer Res; 2020; 80 (Suppl 16): abs. no. 1950 [Google Scholar]
- [70].Chu J, Galicia-Vazquez G, Cencic R, Mills JR, Katigbak A, Porco JA Jr., Pelletier J. CRISPR-mediated drug-target validation reveals selective pharmacological inhibition of the RNA helicase, eIF4A. Cell Rep 2016; 15: 2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Iwasaki S, Floor SN, Ingolia NT. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 2016; 534: 558–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Iwasaki S, Iwasaki W, Takahashi M, Sakamoto A, Watanabe C, Shichino Y, Floor SN, Fujiwara K, Mito M, Dodo K, Sodeoka M, Imataka H, Honma T, Fukuzawa K, Ito T, Ingolia NT. The translation inhibitor rocaglamide targets a bimolecular cavity between eIF4A and polypurine RNA. Mol Cell 2019; 73: 738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sridharan S, Robeson M, Bastihalli-Tukaramrao D, Howard CM, Subramaniyan B, Tilley AMC, Tiwari AK, Raman D. Targeting of the eukaryotic translation initiation factor 4A against breast cancer stemness. Front Oncol 2019; 9: 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sainz B Jr., Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med 2012; 18: 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chiu CF, Ho MY, Peng JM, Hung SW, Lee WH, Liang CM, Liang SM. Raf activation by Ras and promotion of cellular metastasis require phosphorylation of prohibitin in the raft domain of the plasma membrane. Oncogene 2013; 32: 777–787 [DOI] [PubMed] [Google Scholar]
- [76].Zhang W, Liu S, Maiga RI, Pelletier J, Brown LE, Wang TT, Porco JA Jr. Chemical synthesis enables structural reengineering of aglaroxin C leading to inhibition bias for hepatitis C viral infection. J Am Chem Soc 2019; 141: 1312–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Porco JA Jr., Zhang W, Wang TT, Liu S. Preparation of rocaglates for inhibiting viral infection. US Pat Pub Appl 20200123170, April 23, 2020 [Google Scholar]
- [78].Too IHK, Bonne I, Tan EL, Chu JJH, Alonso S. Prohibitin plays a critical role in Enterovirus 71 neuropathogenesis. PLoS Pathog 2018; 14: e1006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Biedenkopf N, Lange-Grünweller K, Schulte FW, Weisser A, Müller C, Becker D, Becker S, Hartmann RK, Grünweller A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antiviral Res 2017; 137: 76–81 [DOI] [PubMed] [Google Scholar]
- [80].Slaine PD, Kleer M, Smith NK, Khaperskyy DA, McCormick C. Stress granule-inducing eukaryotic translation initiation factor 4A inhibitors block influenza A virus replication. Viruses 2017; 9: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Todt D, Moeller N, Praditya D, Kinast V, Friesland M, Engelmann M, Verhoye L, Sayed IM, Behrendt P, Dao Thi VL, Meuleman P, Steinmann E. The natural compound silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo. Antiviral Res 2018; 157: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Glitscher M, Himmelsbach K, Woytinek K, Johne R, Reuter A, Spiric J, Schwaben L, Grünweller A, Hildt E. Inhibition of hepatitis E virus spread by the natural compound silvestrol. Viruses 2018; 10: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Müller C, Schulte FW, Lange-Grünweller K, Obermann W, Madhugiri R, Pleschka S, Ziebuhr J, Hartmann RK, Grünweller A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antiviral Res 2018; 150: 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Henss L, Scholz T, Grünweller A, Schnierle BS. Silvestrol inhibits chikungunya virus replication. Viruses 2018; 10: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Grünweller A, Hartmann RK, Lange-Grünweller K, Schulte FW, Becker S, Biedenkopf N, Ziebuhr J, Müller C, Schlitzer M. Usage of silvestrol, episilvestrol analogs for the treatment of viral infections caused by viruses with cap-dependent translation. EP 33052889 A, 2018 [Google Scholar]
- [86].Elgner F, Sabino C, Basic M, Ploen D, Grünweller A, Hildt E. Inhibition of zika virus replication by silvestrol. Viruses 2018; 10: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Müller C, Obermann W, Schulte FW, Lange-Grünweller K, Oestereich L, Elgner F, Glitscher M, Hildt E, Singh K, Wendel HG, Hartmann RK, Ziebuhr J, Grünweller A. Comparison of broad-spectrum antiviral activities of the synthetic rocaglate CR-31-B (−) and the eIF4A-inhibitor silvestrol. Antiviral Res 2020; 175: 104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chan K, Robert F, Oertlin C, Kapeller-Libermann D, Avizonis D, Gutierrez J, Handly-Santana A, Doubrovin M, Park J, Schoepfer C, Da Silva B, Yao M, Gorton F, Shi J, Thomas CJ, Brown LE, Porco JA Jr., Pollak M, Larsson O, Pelletier J, Chio IIC. eIF4A supports an oncogenic translation program in pancreatic ductal adenocarcinoma. Nat Commun 2019; 10: 5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yurugi H, Zhuang Y, Siddiqui FA, Liang H, Rosigkeit S, Zeng Y, Abou-Hamdan H, Bockamp E, Zhou Y, Abankwa D, Zhao W, Désaubry L, Rajalingam K. A subset of flavaglines inhibits KRAS nanoclustering and activation. J Cell Sci 2020; 133: jcs.244111. [DOI] [PubMed] [Google Scholar]
- [90].Chen M, Asanuma M, Takahashi M, Shichino Y, Mito M, Fujiwara K, Saito H, Floor SN, Ingolia NT, Sodeoka M, Dodo K, Ito T, Iwasaki S. Dual targeting of DDX3 and eIF4A by the translation inhibitor rocaglamide A. Cell Chem Biol 2021, 28: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res 2012; 72: 2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Castagnola P, Giaretti W. Mutant KRAS, chromosomal instability and prognosis in colorectal cancer. Biochim Biophys Acta 2005; 1756: 115–125 [DOI] [PubMed] [Google Scholar]
- [93].Bol GM, Xie M, Raman V. DDX3, a potential target for cancer treatment. Mol Cancer 2015; 14: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chiu S-F. Recent research findings on Meliaceae and other promising botanical insecticides in China. J Plant Dis Prot 1985; 92: 310–319 [Google Scholar]
- [95].Chaidir, Hiort J, Nugroho BW, Bohnenstengel FI, Wray V, Witte L, Hung PD, Kiet LC, Sumaryono W, Proksch P. New insecticidal rocaglamide derivatives from flowers of Aglaia duperreana. Phytochemistry 1999; 52: 837–842 [Google Scholar]
- [96].Dreyer M, Nugroho BW, Bohnenstengel FI, Ebel R, Wray V, Witte L, Brigmann G, Muhlbacher J, Herold M, Hung PD, Kiet LC, Proksch P. New insecticidal rocaglamide derivatives and related compounds from Aglaia oligophylla. J Nat Prod 2001; 64: 415–420 [DOI] [PubMed] [Google Scholar]
- [97].Hall RG, Bruce I, Cooke NG, Diorazio LJ, Cederbaum F, Dobler MR, Irving E. Investigating the structure-activity relationship of the insecticidal natural product rocaglamide. Chimia 2017; 71: 845–850 [DOI] [PubMed] [Google Scholar]
- [98].Miller LH, Su X. Artemisinin: discovery from the Chinese herbal garden. Cell 2011; 146: 855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Duru V, Witkowski B, Menard D. Plasmodium falciparum resistance to artemisinin derivatives and piperaquine: A major challenge for malaria elimination in Cambodia. Am J Trop Med Hyg 2016; 95: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Langlais D, Cencic R, Moradin N, Kennedy JM, Ayi K, Brown LE, Crandall I, Tarry MJ, Schmeing M, Kain KC, Porco JA Jr., Pelletier J, Gros P. Rocaglates as dual-targeting agents for experimental cerebral malaria. Proc Natl Acad Sci USA 2018; 115: E2366–E2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rodrigo CM, Cencic R, Roche SP, Pelletier J, Porco JA. Synthesis of rocaglamide hydroxamates and related compounds as eukaryotic translation inhibitors: synthetic and biological studies. J Med Chem 2012; 55: 558–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang JK, Guo Q, Zhang XW, Wang LC, Liu Q, Tu PF, Jiang Y, Zeng KW. Aglaia odorata Lour. extract inhibit ischemic neuronal injury potentially via suppressing p53/Puma-mediated mitochondrial apoptosis pathway. J Ethnopharmacol 2020; 248: 112336. [DOI] [PubMed] [Google Scholar]
- [103].Davey AE, Schaeffer MJ, Taylor RJK. Synthesis of the novel anti-leukaemic tetrahydrocyclopenta[b]benzofuran, rocaglamide. J Chem Soc, Chem Commun 1991; 16: 1137–1139 [Google Scholar]
- [104].Davey AE, Schaeffer MJ, Taylor RJK. Synthesis of the novel anti-leukemic tetrahydrocyclopenta[b]benzofuran, rocaglamide and related synthetic studies. J Chem Soc, Perkin Trans 1 1992; 20: 2657–2666 [Google Scholar]
- [105].Gerard B, Jones G, Porco JA. A biomimetic approach to the rocaglamides employing photogeneration of oxidopyryliums derived from 3-hydroxyflavones. J Am Chem Soc 2004; 126: 13620–13621 [DOI] [PubMed] [Google Scholar]
- [106].Zhou Z, Dixon DD, Jolit A, Tius MA. The evolution of the total synthesis of rocaglamide. Chem Eur J 2016; 22: 15929–15936 [DOI] [PubMed] [Google Scholar]
- [107].Gerard B, Cencic R, Pelletier J, Porco JA Jr. Enantioselective synthesis of the complex rocaglate (‒)-silvestrol. Angew Chem Int Ed 2007; 46: 7831–7834 [DOI] [PubMed] [Google Scholar]
- [108].El Sous M, Khoo ML, Holloway G, Owen D, Scammells PJ, Rizzacasa MA. Total synthesis of (‒)-episilvestrol and (‒)-silvestrol. Angew Chem Int Ed 2007; 46: 7835–7838 [DOI] [PubMed] [Google Scholar]
- [109].Hawkins BC, Lindqvist LM, Nhu D, Sharp PP, Segal D, Powell AK, Campbell M, Ryan E, Chambers JM, White JM, Rizzacasa MA, Lessene G, Huang DC, Burns CJ. Simplified silvestrol analogues with potent cytotoxic activity. ChemMedChem 2014; 9: 1556–1566 [DOI] [PubMed] [Google Scholar]
- [110].Arai MA, Kofuji Y, Tanaka Y, Yanase N, Yamaku K, Fuentes RG, Karmakar UK, Ishibashi M. Synthesis of rocaglamide derivatives and evaluation of their Wnt signal inhibitory activities. Org Biomol Chem 2016; 14: 3061–3068 [DOI] [PubMed] [Google Scholar]
- [111].Schulz G, Victoria C, Kirschning A, Steinmann E. Rocaglamide and silvestrol: a long story from anti-tumor to anti-coronavirus compounds. Nat Prod Rep 2021; 38: 18–23 [DOI] [PubMed] [Google Scholar]
- [112].Nebigil CG, Moog C, Vagner S, Benkirane-Jessel N, Smith DR, Désaubry L. Flavaglines as natural products targeting eIF4A and prohibitins: From traditional Chinese medicine to antiviral activity against coronaviruses. Eur J Med Chem 2020; 203: 112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ernst JT, Thompson PA, Nilewski C, Sprengeler PA, Sperry S, Packard G, Michels T, Xiang A, Tran C, Wegerski CJ, Eam B, Young NP, Fish S, Chen J, Howard H, Staunton J, Molter J, Clarine J, Nevarez A, Chiang GG, Appleman JR, Webster KR, Reich SH. Design of development candidate eFT226, a first in class inhibitor of eukaryotic initiation factor 4A RNA helicase. J Med Chem 2020; 63: 5879–5955 [DOI] [PubMed] [Google Scholar]


