Abstract
Ferroptosis, a cell death modality characterized by iron-dependent lipid peroxidation, is involved in the development of multiple pathological conditions, including ischemic tissue damage, infection, neurodegeneration, and cancer. The cellular machinery responsible for the execution of ferroptosis integrates multiple pro-survival or pro-death signals from subcellular organelles and then ‘decides’ whether to engage the lethal process or not. Here, we outline the evidence implicating different organelles (including mitochondria, lysosomes, endoplasmic reticulum, lipid droplets, peroxisomes, Golgi apparatus, and nucleus) in the ignition or avoidance of ferroptosis, while emphasizing their potential relevance for human disease and their targetability for pharmacological interventions.
Subject terms: Cancer, Cell biology
Facts
Ferroptosis is a type of regulated cell death caused by the imbalance between the levels of oxidants and antioxidants.
Lipid peroxidation is the central biochemical and metabolic event leading to plasma membrane damage during ferroptosis.
The regulation of ferroptosis involves a network involving multiple subcellular organelles to generate signals for iron accumulation, lipid synthesis, and lipid peroxidation.
The direct effector of ferroptosis remains unclear.
Open questions
Are the ferroptosis-relevant damage or repair mechanisms affecting the plasma membrane and internal, organelle-specific membranes different?
What are the key molecules that maintain or disrupt the communication between subcellular organelles in ferroptosis?
How can we develop molecular probes to dynamically monitor ferroptosis-associated changes in organellar morphology and function?
Do the biogenesis and turnover of specific organelles affect the susceptibility of cells to ferroptosis?
Introduction
Ferroptosis was originally described as a cell death pathway occurring in cancer cells expressing mutant RAS [1]. Today, this type of iron-dependent regulated cell death (RCD) is known to occur in a variety of transformed or non-transformed cell lines and in tissues [2]. Ferroptosis is morphologically and biochemically different from apoptosis, the most studied form of RCD [3]. For instance, ferroptosis is accompanied by cell swelling and plasma membrane rupture, while apoptotic cells usually exhibit cell shrinkage and plasma membrane blebbing [4]. Ferroptosis is driven by unrestricted lipid peroxidation, which does not require the activation of caspase (key executors of apoptosis) [5]. The autophagic degradation pathway usually protects cells from apoptosis, but selective autophagy (e.g., ferritinophagy [6, 7] and lipophagy [8]) can favor ferroptosis. Altogether, ferroptosis has unique cellular and molecular mechanism that shift the balance between oxidants and antioxidants in favor of the oxidative damage of plasma membrane and subcellular organelles [9]. Of note, ferroptosis may cause inflammation due to the release of endogenous damage-associated molecular pattern molecules (DAMPs), resulting in the recruitment and activation of immune cells [10–13].
Ferroptosis plays a dual role in health and disease [14–18]. On the one hand, physiological ferroptosis might contribute to eliminating harmful cells to maintain tissue homeostasis and development. On the other hand, pathological ferroptosis is increasingly recognized as a significant factor that contributes to the pathogenesis of diseases, including, but not limited to, cancer, neurodegenerative disorders, ischemia-reperfusion injury, and infectious states. Although the implementation of ferroptosis in translational medicine faces many obstacles, certain investigational small molecule compounds (e.g., erastin, ferrostatin-1, liproxstatin-1, and RSL3) [5, 19] or Food and Drug Administration (FDA)/European Medicines Agency (EMA)-approved drugs (e.g., sulfasalazine, sorafenib, zalcitabine, and doxorubicin) [20–22] have been used in preclinical models to induce or inhibit ferroptosis. However, a recent study suggests that sorafenib may not be a strong activator of ferroptotic cancer cell death and erastin only triggers ferroptosis in certain cancer cells [23].
All cellular organelles may sense, attenuate or amplify stress signals [24] and thus contribute to the regulation or execution of different types of RCD, including apoptosis and necroptosis [25]. Likely, this concept can be extended to ferroptosis as well. In this review, we summarize the key processes of ferroptosis and discuss how signals from different organelles modulate the ignition and execution of ferroptotic cell death.
Central events of ferroptosis
The generation of reactive oxygen species (ROS) and subsequent hydroxyl radical (·OH)-mediated lipid peroxidation culminating with plasma membrane damage are the core events leading to ferroptosis. These processes are inhibited by integrated antioxidant or membrane repair systems.
Oxidative damage
ROS for ferroptosis can be generated from multiple sources, such as the iron-mediated Fenton reaction, mitochondrial electron transport chain (ETC), nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX), and myeloperoxidase (MPO) [12, 26–29]. The accumulation of iron in cells is one of the hallmarks of ferroptosis [30]. Many proteins mediate iron uptake (e.g., transferrin [TF] [31], transferrin receptor [TFRC] [32], and lactotransferrin [LTF] [33]), storage (e.g., ferritin [6, 7]), utilization (e.g., iron-sulfur proteins [34]), distribution (e.g., CDGSH iron–sulfur domain 1 [CISD1] [35]), and export (e.g., solute carrier family 40 member 1 [SLC40A1] [36, 37], prominin 2 [PROM2] [38], and lipocalin 2 [LCN2] [39]), meaning that they affect the sensitivity of cells to ferroptosis (Fig. 1a). However, the dynamic relationship of iron metabolism and different ROS resources in promoting ferroptosis remains poorly investigated [40].
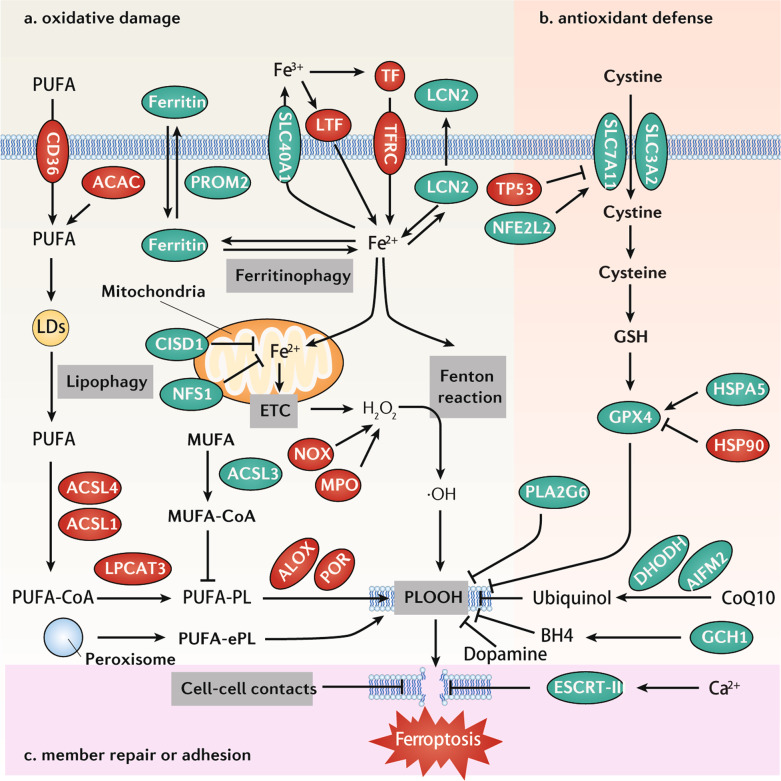
Fig. 1. Core mechanisms of ferroptosis.
a Oxidative damage. Ferroptosis is caused by lipid peroxidation with the involvement of various enzymes (ACSL, LPCAT4, ALOX, and POR). This is further regulated by fatty acid metabolism, including CD36-mediated PUFA uptake, ACAC-dependent PUFA synthesis, or lipophagy-induced PUFA production. In addition, ferroptosis is activated by the iron-mediated Fenton reaction. Therefore, ferroptosis sensitivity is highly related to iron metabolism, including iron uptake (e.g., TF, TFRC, and LTF), storage (e.g., ferritin), utilization (e.g., NFS1), distribution (e.g., CISD1), and export (e.g., SLC40A1, PROM2, and LCN2). b Antioxidant defense. The SLC7A11-GSH-GPX4 pathway and GSH-independent pathway (CoQ10, BH4, dopamine, and PLA2G6) are the main antioxidant systems for ferroptotic cell death by inhibiting lipid peroxidation. c ESCRT-III–mediated membrane repair or cell adhesion also inhibits ferroptosis through blocking membrane damage induced by lipid peroxidation. Abbreviations: ACAC acetyl-CoA carboxylase, ACSL1 acyl-CoA synthetase long-chain family member 1, ACSL3 acyl-CoA synthetase long-chain family member 3, ACSL4 acyl-CoA synthetase long-chain family member 4, AIFM2/FSP1 apoptosis-inducing factor mitochondria-associated 2, ALOX lipoxygenase, CISD1 CDGSH iron sulfur domain 1, DHODH dihydroorotate dehydrogenase (quinone), ESCRT-III endosomal sorting complex required for transport-III, GCH1 GTP cyclohydrolase 1, GPX4 glutathione peroxidase 4, GSH glutathione, HSP90 heat shock protein 90, HSPA5/GRP78/BIP heat shock protein family A (Hsp70) member 5, LCN2 lipocalin 2, LTF lactotransferrin, MPO myeloperoxidase, MUFA monounsaturated fatty acid, NFE2L2/NRF2 nuclear factor erythroid 2-like 2, NOX NADPH oxidase, PLA2G6/iPLA2β phospholipase A2 group VI, PLOOH phospholipid hydroperoxides, POR cytochrome P450 oxidoreductase, PROM2 prominin 2, PUFA polyunsaturated fatty acid, PUFA-ePL polyunsaturated ether phospholipid, PUFA-PL polyunsaturated phospholipid, SLC3A2 solute carrier family 3 member 2, SLC7A11 solute carrier family 7 member 11, SLC40A1 solute carrier family 40 member 1, TF transferrin, TFRC transferrin receptor, TP53 tumor protein p53.
Although oxidative DNA damage is also conducive to ferroptosis, lipid peroxidation of polyunsaturated fatty acids (PUFAs) plays a major role in driving lytic cell death. Accordingly, CD36-mediated PUFA uptake [41, 42], acetyl-CoA carboxylase (ACAC)-dependent PUFA synthesis [43], or lipophagy-induced PUFA production [8] might facilitate ferroptosis. The production of oxidative metabolites of PUFA requires additional enzymes. Long-chain acyl-CoA synthetases (ACSLs) activate fatty acids by the addition of a coenzyme A (CoA) group and provide substrates for specific metabolic pathways. ACSL4 [44–46] and ACSL1 [47] are essential for arachidonic acid/adrenic acid-mediated and linolenic acid-mediated ferroptosis, respectively. ACSL3 is responsible for the activation of monounsaturated fatty acids (MUFAs), which competitively inhibit PUFA-induced ferroptosis [48]. Later, lysophosphatidylcholine acyltransferase 3 (LPCAT3) is involved in phospholipid remodeling for ferroptosis [45]. Finally, different members of the lipoxygenase (ALOX) family mediate ferroptosis through the oxygenation of PUFAs in a cell type-dependent manner [21, 49–51]. Alternatively, cytochrome P450 oxidoreductase (POR) transfers electrons to oxygen and then mediates lipid peroxidation during ferroptosis in an ALOX-independent manner [52, 53]. In addition to PUFAs, the production of plasmalogens in peroxisomes can provide substrates for lipid peroxidation during ferroptosis [54]. This complex complementary pathway of lipid metabolism affects the susceptibility to ferroptosis [55]. It is possible, yet remains to be demonstrated, that sophisticated biochemical methods allowing for the identification of distinct lipid peroxidation products will facilitate a sort of “molecular diagnosis” of the etiology of ferroptotic cell death.
Antioxidant defense
Tremendous progress has been made in deconvoluting enzymatic and non-enzymatic antioxidant defense systems in ferroptosis [56]. The most characteristic system is the system xc−-glutathione (GSH)–glutathione peroxidase 4 (GPX4) axis (Fig. 1b). Many classical ferroptosis inducers (e.g., erastin and RSL3) are inhibitors of this axis. System xc−, a glutamate/cystine transporter, consists of solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2). System xc− can maintain intracellular GSH content by mediating the uptake of cystine into cells. GSH acts as a cofactor of many antioxidant enzymes, including GPX4. GPX4 requires GSH to reduce phospholipid hydroperoxides (PLOOHs) to nontoxic phospholipid alcohols (PLOHs) [57]. During ferroptosis, the activity or expression of SLC7A11 and GPX4 are regulated on multiple levels. For example, SLC7A11-mediated cystine uptake also promotes GPX4 protein synthesis through the mechanistic target of rapamycin kinase (MTOR) pathway [58]. GPX4 protein can be stabilized by heat shock protein family A (Hsp70) member 5 (HSPA5) [59], but destabilized by heat shock protein 90 (HSP90) in a context-dependent manner [60]. The transcription of SLC7A11 is upregulated or downregulated by nuclear factor erythroid 2-like 2 (NFE2L2, best known as NRF2) [61] or tumor protein p53 (TP53) [62], respectively. The identification of a large number of GPX4 or SLC7A11 binding proteins further exacerbates the complexity of the regulation of this pathway in ferroptosis [63–66].
In addition to GSH, several intracellular antioxidants, such as coenzyme Q10 (CoQ10), tetrahydrobiopterin (BH4), and dopamine, prevent lipid peroxidation during ferroptosis. Mechanically, apoptosis-inducing factor mitochondria-associated 2 (AIFM2/FSP1) [67, 68] and dihydroorotate dehydrogenase (DHODH) [69] inhibit ferroptosis through reducing cytosolic and mitochondrial ubiquinone (CoQ10) to generate ubiquinol, respectively. GTP cyclohydrolase 1 (GCH1) is the rate-limiting enzyme for the synthesis of BH4, which acts as a radical trapping antioxidant in inhibiting ferroptosis [70, 71]. Dopamine enhances GPX4 protein stability, which in turn limits lipid peroxidation in ferroptosis [72]. Distinct from GPX4, phospholipase A2 group VI (PLA2G6, also known as iPLA2β) averts ferroptosis by hydrolyzing oxidized phosphatidylethanolamine [73, 74]. These findings underscore the notion that an integrated antioxidant system limits ferroptosis caused by excessive oxidative stress.
Membrane repair or adhesion
Cell membrane disruption induces not only a rapid and massive influx of Ca2+ into the cytosol but also an efflux or release of various endogenous proteins, such as high-mobility group box 1 (HMGB1), which is considered as a major pro-inflammatory DAMP [75]. Although the key effectors responsible for the formation of plasma membrane pores have not yet been determined, the activation of Ca2+-dependent endosomal sorting complex required for transport-III (ESCRT-III) machinery can promote plasma membrane repair, thereby limiting the occurrence of ferroptosis and the release of pro-inflammatory DAMPs (Fig. 1c) [76–78]. In addition to ferroptosis, ESCRT-III has a conserved function to repair plasma membrane damage in pyroptosis and necroptosis [79, 80]. Additional work is needed to identify whether the membrane repair mechanisms of specific organelles (e.g., mitochondria or lysosomes) are involved in the defense against ferroptosis [81]. Mounting evidence shows that cell-cell contacts confer cell resistance to ferroptosis [28, 82, 83]. In adjacent cells, ferroptosis may propagate in a rapid wave-like propagation [84]. It can be speculated that specific cytoskeleton-related dynamic changes transmit or limit the oxidative damage of plasma membranes at the contact sites between interacting cells. The elucidation of such hypothetical mechanisms will require the development and standardization of spatially resolved assays for the detection of ferroptosis-associated membrane damage.
Role of different organelles in ferroptosis
Ferroptosis is a strictly regulated process that requires multiple modulators involved in a series of complex signals in different organelles, including mitochondria, lysosomes, endoplasmic reticulum (ER), lipid droplets (LDs), peroxisomes, Golgi apparatus, and nucleus (Table 1).
Table 1.
Role of subcellular organelles in ferroptosis.
| Organelle | Stimulus | Morphological and functional changes | Organelle-specific regulators |
|---|---|---|---|
| Mitochondria |
Erastin Glutamate Doxorubicin Zalcitabine |
Swollen mitochondria Decrease in cristae Mitochondrial membrane potential ↓ Mitochondrial membrane permeability ↑ Mitochondrial ROS ↑ Mitochondrial lipid peroxidation ↑ Mitochondrial iron ↑ Mitochondrial DNA stress ↑ |
AIFM1, CISD1, CISD2, DHODH, ETC complex, FH, FXN, GLS2, GPX4, IDH2, ISCU, LONP1 MFN1, MFN2, MGST1, MPC1, NFS1, PDK4, POLG, SOD2, TFAM, VDAC |
| Lysosome |
Erastin Glutamate |
Lysosomal cathepsins ↑ Lysosomal lipid peroxidation ↑ Lysosomal iron ↑ Lysosomal nitric oxide ↑ |
ATG, CTSB, PSAP, SMPD1 |
| ER |
Erastin RSL3 |
Viscosity of ER ↑ Lipid peroxidation ↑ ER stress ↑ MUFA synthesis ↓ Zinc transport from ER to cytosol |
AGPAT3, EIF2AK3, SCD, SLC39A7/ZIP7, STING1 |
| LD |
RSL3 Orlistat |
Formation of LDs ↑ Lipophagy ↑ |
RAB7A, TPD52, FAF2 |
| Peroxisome |
Erastin RSL3 ML210 |
Plasmalogen synthesis ↑ | AGPS, FAR1, PEDS1, PEX |
| Golgi complex |
Brefeldin A AMF-26 Golgicide A |
Golgi dispersal | Unknown |
| Nucleus |
Erastin RSL3 |
DNA oxidative damage ↑ DNA damage and repair ↑ |
AIFM1, CTSB, FANCD2, HMGB1, PIR, TP53 |
AGPAT3 1-acylglycerol-3-phosphate O-acyltransferase 3, AGPS Alkylglycerone phosphate synthase, AIFM1/AIF Apoptosis-inducing factor mitochondria-associated 1, ATG Autophagy-related gene, CISD1 CDGSH iron sulfur domain 1, CISD2 CDGSH iron sulfur domain 2, CTSB cathepsin B, DHODH Dihydroorotate dehydrogenase (quinone), EIF2AK3/PERK Eukaryotic translation initiation factor 2 alpha kinase 3, ER Endoplasmic reticulum, ETC Electron transport chain, FANCD2 FA complementation group D2, FAR1 Fatty acyl-CoA reductase 1, FH Fumarate hydratase, FXN Frataxin, GLS2 Glutaminase 2, GPX4 Glutathione peroxidase 4, HMGB1 High-mobility group box 1, IDH2 Isocitrate dehydrogenase (NADP[+]) 2, ISCU Iron-sulfur cluster assembly enzyme, LDs Lipid droplets, LONP1 Lon peptidase 1, mitochondrial, MFN1 Mitofusin 1, MFN2 Mitofusin 2, MGST1 Microsomal glutathione S-transferase 1, MPC1 Mitochondrial pyruvate carrier 1, PDK4 Pyruvate dehydrogenase kinase 4, PEDS1/TMEM189 Plasmanylethanolamine desaturase 1, PEX Peroxisomal biogenesis factor, PIR Pirin, POLG DNA polymerase gamma, catalytic subunit, PSAP Prosaposin, RAB7A Member RAS oncogene family, SCD/SCD1 Stearoyl-CoA desaturase, SLC39A7/ZIP7 Solute carrier family 39 member 7, SMPD1/ASM Sphingomyelin phosphodiesterase 1, SOD2 Superoxide dismutase 2, STING1/TMEM173 Stimulator of interferon response cGAMP interactor 1, TFAM Transcription factor A, mitochondrial, TP53 Tumor protein p53, TPD52 Tumor protein D52, VDAC Voltage-dependent anion channel.
Mitochondria
Ferroptotic cells usually exhibit swollen mitochondria, accompanied by a decrease in cristae, dissipation of the mitochondrial membrane potential, as well as an increase in mitochondrial membrane permeability [5], indicating that mitochondrial dysfunction has occurred. However, the role of mitochondria in ferroptosis is controversial. Early study suggests that mitochondria are not required for ferroptosis because when human osteosarcoma 143B cells are depleted of mitochondrial DNA (mtDNA), which are known as ρ° cells, it has no effect on the pro-ferroptotic effects of SLC7A11 inhibitor erastin [5]. It is important to note that cells lacking mtDNA do have mitochondria. Thus, these results are reminiscent of the initially fallacious interpretation of results involving ρ° cells that were fully susceptible to apoptosis induction (“no need for mitochondria in apoptosis”) [85] that were later reinterpreted to mean that the close-to-obligatory contribution of mitochondrial membrane permeabilization to apoptosis does not require mtDNA [86, 87]. Cells that eliminate mitochondria through parkin RBR E3 ubiquitin protein ligase (PRKN)-mediated mitophagy are less sensitive to ferroptosis triggered by cystine starvation or erastin, but are more sensitive to ferroptosis induced by GPX4 inhibitors [26, 88, 89]. Increasing evidence indicates that mitochondria play a significant role in promoting ferroptosis through context-dependent metabolic effects. Altogether, it appears plausible that mitochondrial biogenesis, dynamics, and turnover affect the number and quality of mitochondria, thereby fine-tuning the activity of ferroptosis inducers.
Mitochondrial ROS
In the process of oxidative phosphorylation, mitochondria are an important source of ROS in most mammalian cells. Local ROS generation does not only lead to mitochondrial damage, but also affects the redox status of the rest of the cell. Because mitochondrial ROS mainly induce apoptosis, they were initially thought not to be involved in ferroptosis [5, 19]. However, later studies suggest that increased mitochondrial ROS promote ferroptosis, a process that can be inhibited by mitochondrial-targeted antioxidants or enzymes, as shown in several complementary studies (Fig. 2a). First, C11-BODIPY 581/591 (a fluorescent radio probe for detecting lipid peroxidation) staining and quantitative analysis of malondialdehyde (MDA, an end product of lipid peroxidation) show that lipid ROS are increased in mitochondria during erastin- or doxorubicin-induced ferroptosis in human fibrosarcoma HT1080 cells, mouse embryonic fibroblasts (MEFs), or cardiomyocytes [26, 90]. The accumulation of mitochondrial lipid ROS may be partly explained by depletion of mitochondrial GSH during ferroptosis [91]. Thus, mitochondria-targeted ROS scavengers, such as MitoTEMPO and mitoquinone, can inhibit ferroptosis in various cell types, including cancer cells, cardiomyocytes, hippocampal neuronal cells, and MEFs [22, 92]. Second, several mitochondrial antioxidant enzymes play a significant role in inhibiting ferroptosis. GPX4 can be localized in the cytosol and mitochondrial intermembrane space [93–95], and its mitochondrial form plays a role in mitigating mitochondria oxidative damage during cell death, including ferroptosis [90]. Superoxide dismutase 2 (SOD2/MnSOD), a member of the iron/manganese superoxide dismutase family located in the mitochondrial matrix of eukaryotes as well as in various prokaryotes, also has the ability to prevent mitochondrial ROS-induced ferroptosis in non-small cell lung cancer cells [96]. In addition, microsomal glutathione S-transferase 1 (MGST1), an antioxidant enzyme located predominantly in mitochondria and ER, limits lipid peroxidation and ferroptosis by binding to ALOX5 [97]. Third, similar to its role in extramitochondrial membranes, the mitochondrial CoQ10 effectively prevents ferroptosis [69]. Mitochondrial DHODH mediates the oxidation of dihydroorotate to orotate, a process coupled to the reduction of CoQ10 to ubiquinol, and limits mitochondrial lipid peroxidation and ferroptosis caused by GPX4 downregulation [69]. Fourth, mitochondrial oxidative damage induces the release of certain mitochondrial apoptosis regulators, such as apoptosis-inducing factor mitochondria-associated 1 (AIFM1, a factor initially involved in caspase-independent apoptosis [98]), which promotes ferroptosis through its translocation to the nucleus in mouse hippocampal HT22 cells or MEFs [99, 100], highlighting a molecular link between apoptosis and ferroptosis. However, CRISPR-mediated knockout of AIFM1 cannot rescue ferroptosis induced by GPX4 deletion in MEFs [67]. Unlike mitochondrial fission that promotes apoptosis [101], mitochondrial fusion favors mitochondrial oxidative damage and subsequent ferroptosis through the stimulator of interferon response cGAMP interactor 1 (STING1)-mitofusin 1/2 (MFN1/2) pathway [102]. Since mitochondrial ROS induce apoptosis by releasing mitochondrial proteins (such as AIFM1 and the caspase activators cytochrome c [CYCS] and SMAC/DIABLO), it may be expected that mitochondrial ROS-mediated ferroptosis is coupled to the release of cytotoxic mitochondrial proteins as well. Nevertheless, deficient oxidative phosphorylation or consumption of adenosine triphosphate (ATP) by uncoupled mitochondria might contribute to ferroptosis as well. Hence, further studies of mitochondrial derangements accompanying ferroptosis are warranted.
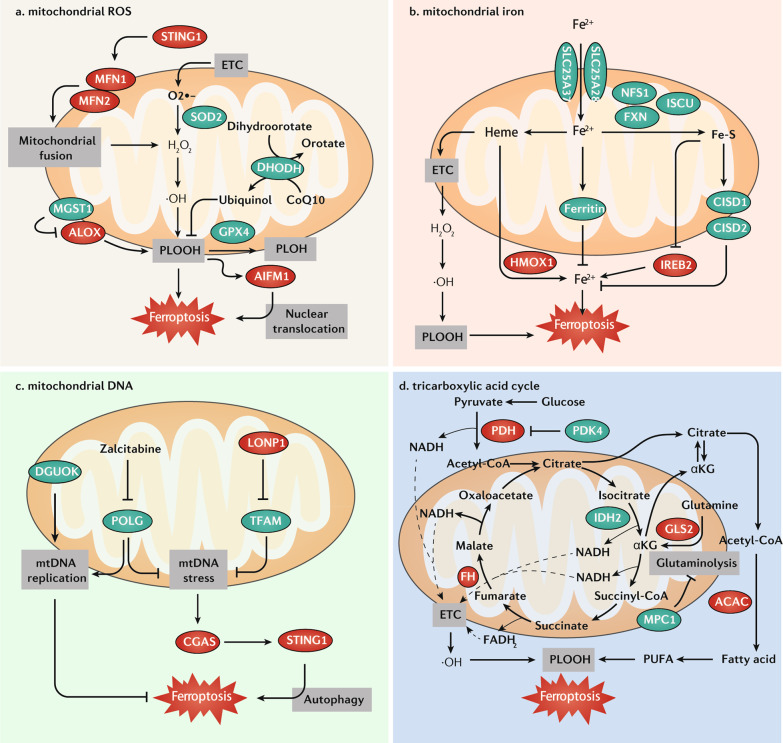
Fig. 2. Role of mitochondria in ferroptosis.
a Mitochondrial ROS. GPX4, SOD2, and MGST1 are mitochondria-associated antioxidant proteins, which play a major role in protecting mitochondria from oxidative damage during ferroptosis. In addition, mitochondrial DHODH limits ferroptosis through the reduction of CoQ10 to ubiquinol. Mitochondrial oxidative damage induces the release of AIFM1, which promotes ferroptosis through its translocation to the nucleus. The STING1-MFN1/2 pathway triggers ferroptosis by inducing mitochondrial fusion and subsequent ROS production. b Mitochondrial iron. Iron can be transported to the mitochondria via SLC25A37 and SLC25A28 and used to synthesize heme/Fe-S, or it can be stored in ferritin in mitochondria. Heme is catalyzed by HMOX1 and decomposes into Fe2+, and acts as a key cofactor of ETC by which heme promotes ferroptosis. The inhibition of iron-sulfur cluster assembly proteins, including NFS1, FXN, and ISCU, can enhance ferroptosis by the activation of an IREB2-mediated iron-starvation response. Fe-S proteins CISD1 and CISD2 inhibit ferroptosis by decreasing intracellular iron levels. c Mitochondrial DNA (mtDNA). POLG (DNA polymerase) and DGUOK (deoxynucleoside synthesis enzyme) are required for mtDNA replication. The inhibition of POLG by zalcitabine and TFAM degradation induce mtDNA stress, which activates GAS-STING1 pathway-dependent autophagy to mediate ferroptosis. d The TCA cycle transfers electrons to the ETC, which releases ROS to induce ferroptosis. The TCA cycle also enhances ferroptosis by promoting ACAC-mediated fatty acid synthesis. This effect is inhibited by PDK4-mediated glucose metabolism or enhanced by GLS2-mediated glutaminolysis. In addition, the suppression of MPC1 also enhances ferroptosis partly through increasing glutaminolysis. Abbreviations: ACAC acetyl-CoA carboxylase, AIFM1/AIF apoptosis-inducing factor mitochondria-associated 1, ALOX lipoxygenase, CGAS cyclic GMP-AMP synthase, CISD1 CDGSH iron sulfur domain 1, CISD2 CDGSH iron sulfur domain 2, DGUOK deoxyguanosine kinase, DHODH dihydroorotate dehydrogenase (quinone), ETC electron transport chain, FH fumarate hydratase, FXN frataxin, GLS2 glutaminase 2, GPX4 glutathione peroxidase 4, HMOX1/HO1 heme oxygenase 1, IDH2 isocitrate dehydrogenase (NADP[+]) 2, IREB2/IRP2 iron-responsive element binding protein 2, ISCU iron–sulfur cluster assembly enzyme, LONP1 Lon peptidase 1, mitochondrial, MFN1 mitofusin 1, MFN2 mitofusin 2, MGST1 microsomal glutathione S-transferase 1, MPC1 mitochondrial pyruvate carrier 1, NADH dihydronicotinamide adenine dinucleotide, PDH pyruvate dehydrogenase, PDK4 pyruvate dehydrogenase kinase 4, PLOOH phospholipid hydroperoxide, POLG DNA polymerase gamma, catalytic subunit, PUFA polyunsaturated fatty acid, ROS reactive oxygen species, SLC25A28/mitoferrin-2 solute carrier family 25 member 28, SLC25A37/mitoferrin-1 solute carrier family 25 member 37, SOD2 superoxide dismutase 2, STING1/TMEM173 stimulator of interferon response cGAMP interactor 1, TFAM transcription factor A, mitochondrial.
Mitochondrial iron
Extracellular iron is taken up by cells and can be imported into mitochondria via the mitochondrial iron importer solute carrier family 25 member 37 (SLC25A37, also known as mitoferrin-1) and solute carrier family 25 member 28 (SLC25A28, also known as mitoferrin-2). Mitochondrial Fe2 + can be used to synthesize heme and Fe-S clusters, or stored in mitochondrial ferritin. In contrast, excessive mitochondrial iron can mediate the production of ROS or cause abnormal enzyme activity. Impaired mitochondrial iron metabolism leads to ferroptosis (Fig. 2b). First, heme directly induces ferroptosis in primary neurons or in human monocytic cells [103, 104], and this process can be further dually regulated by cytosolic or mitochondrial heme oxygenase 1 (HMOX1), likely in a cell type-dependent manner [105, 106]. Second, the component of iron-sulfur cluster assembly machinery, such as NFS1 cysteine desulfurase, frataxin (FXN), and iron-sulfur cluster assembly enzyme (ISCU), generally play an anti-ferroptotic role in various conditions. For example, the suppression of NFS1 activates the iron-responsive element binding protein 2 (IREB2, also known as IRP2)-mediated iron-starvation response and sensitizes lung cancer cells to ferroptosis [34, 107]. The suppression of FXN also induces the accumulation of free iron, thereby enhancing erastin- or alcohol-induced ferroptosis in cancer or live cells [108, 109]. FXN deficiency is related to Friedreich’s ataxia and can be relieved by ferroptosis inhibitors [110]. In addition, the overexpression of ISCU attenuates dihydroartemisinin-induced ferroptosis by regulating iron metabolism and mitochondrial function [111]. Third, mitochondrial iron exporters, such as CISD1 and CDGSH iron sulfur domain 2 (CISD2), inhibit ferroptosis by protecting mitochondria against lipid peroxidation in cancer cells [35, 112, 113]. Fourth, similar to cytoplasmic ferritin, mitochondrial ferritin increases iron storage and protects against ferroptosis in human neuroblastoma SH-SY5Y cells or primary human macrophages caused by erastin or hypoxia [114, 115]. Together, these findings help identify new proteins to clarify the pathways involved in mitochondrial iron homeostasis during ferroptosis.
mtDNA
mtDNA is a circular double-stranded DNA condensed into nucleoids due to the interaction with mitochondrial transcription factor A (TFAM). In mammals, the DNA polymerase gamma, catalytic subunit (POLG) is required for mtDNA replication. Various mitochondrial stresses, including bioenergetic and environmental factors, can lead to mtDNA release into the cytoplasm. The released mtDNA activates a plethora of innate immune responses, especially the cyclic GMP-AMP synthase (CGAS)-STING1–dependent DNA sensing pathway, which can initiate a type I interferon response, autophagy, or cell death [116, 117]. It is widely accepted that mtDNA damage is an initial signal of cell death. Zalcitabine, an antiviral drug that targets POLG to induce Lon peptidase 1, mitochondrial (LONP1)-dependent TFAM degradation, has been shown to induce ferroptosis in human pancreatic cancer cells through the induction of mtDNA release and subsequent STING1-related autophagic cell death (Fig. 2c) [21]. Deoxyguanosine kinase (DGUOK) is a rate-limiting enzyme for mitochondrial deoxynucleoside salvage pathway enzymes involved in precursor synthesis for mtDNA replication. A loss-of-function mutation of DGUOK can cause hepatic mtDNA depletion syndrome with enhanced ferroptosis sensitivity (Fig. 2c) [118]. However, mtDNA-depleted human osteosarcoma 143B cells (ρ° cells) display sensitivity to erastin-induced ferroptosis that is equivalent to that of parental cells [5], contrasting with the observation that they contain higher levels of ALOX for lipid peroxidation to induce apoptosis [119]. Thus, unknown defense mechanisms might limit ALOX activity in ρ° cells in response to ferroptosis activators, but not other cell death inducers.
The tricarboxylic acid cycle
The tricarboxylic acid (TCA) cycle is a mitochondrial matrix-located enzymatic pathway that interfaces with various metabolic pathways in the cytosol. It uses acetyl-CoA produced from glucose as a starting material, and transfers electrons to the ETC through a series of redox reactions, thus allowing ATP production by oxidative phosphorylation. The energy sensor AMP-activated protein kinase (AMPK) regulated by the cellular ADP:ATP ratio plays a dual role in ferroptosis according to its phosphorylated substrate [43, 120]. The TCA cycle enzyme fumarate hydratase (FH) catalyzes the reversible hydration of fumarate to malate. FH-mutant renal cancer cells are less sensitive to cystine starvation-induced ferroptosis [26]. However, FH knockout sensitizes renal cancer cells to erastin-induced cell death [121]. Inhibitors of the mitochondrial ETC complexes I/II/III/IV selectively inhibit ferroptosis caused by cysteine starvation or erastin, rather than the GPX4 inhibitor RSL3 [26, 122]. These findings raise questions about the subcellular localization of erastin and RSL3, and erastin has indeed been shown to target voltage-dependent anion channels (VDACs) in mitochondria [123]. Nevertheless, ROS from mitochondrial ETC affords considerable flexibility in the regulation of ferroptotic cell death (Fig. 2d).
Glutaminolysis can fuel the TCA cycle by producing glutamate from glutamine via glutaminase (GLS). Mitochondrial GLS2, but not cytosolic GLS1, is responsible for glutaminolysis-associated ferroptosis [31, 124]. The suppression of mitochondrial pyruvate carrier 1 (MPC1) also increases vulnerability to ferroptosis partly by increasing glutaminolysis in erlotinib-resistant cancer cells [125]. Whether GLS2 and MPC1 have direct antagonistic effects on the induction of ferroptosis remains to be investigated.
Mitochondrial isocitrate dehydrogenase (NADP[ +]) 2 (IDH2) catalyzes the conversion of isocitrate to α-ketoglutarate (αKG), which is the first oxidative decarboxylation of the TCA cycle. The downregulation of IDH2 sensitizes cancer cells to erastin-induced ferroptosis through decreasing the mitochondrial NADPH pool [126]. Acetyl-CoA produced from the TCA cycle or glucose-mediated pyruvate oxidation in mitochondria can be used for fatty acid synthesis and elongation in the cytosol [127]. In contrast, pyruvate dehydrogenase kinase 4 (PDK4) inhibits glucose-mediated susceptibility to ferroptosis by limiting pyruvate oxidation and subsequent fatty acid synthesis in pancreatic cancer cells [128]. The anaplerotic conversion of glutamate to αKG provides an additional way for fatty acids to be synthesized for amplifying ferroptosis in cancer and non-malignant cells [26]. Thus, the TCA cycle provides an interconnected redox hub for the integration of metabolic signals from glycolysis and amino acid catabolism to generate ferroptosis-favoring PUFAs (Fig. 2d). Future metabolic flux analyses might unveil new mechanisms and feedback loops that participate to the mitochondrial regulation of ferroptosis.
Lysosomes
Lysosomes are acidic membrane-bound organelles that contribute to ferroptosis through three mechanisms: (i) the activation of autophagy, (ii) the release of lysosomal cathepsins, and (iii) the accumulation of lysosomal iron or nitric oxide.
Macroautophagy (to which we refer as ‘autophagy’) is a lysosome-dependent degradation pathway characterized by the formation of double-membrane bound vesicles called autophagosomes, which are hierarchically executed by the sequential contribution of autophagy-related (ATG) proteins [129]. The knockdown of ATG genes, such as ATG3, ATG5, ATG7, ATG13, BECN1 (also known as ATG6), and microtubule-associated protein 1 light chain 3 B (MAP1LC3B, also known as ATG8), inhibits ferroptosis in many cancer cells [6, 7, 130]. However, the knockdown of ATG2A promotes ferroptosis in human cervical cancer Hela cells by increasing Fe2+ uptake [131]. Several selective autophagy pathways promote ferroptosis by removing different cargoes (Fig. 3a). First, nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy [6, 7] and the sequestosome 1 (SQSTM1/p62)-mediated autophagic degradation of SLC40A1 [37] promote ferroptosis by increasing intracellular Fe2+ levels. Second, the lipophagy-dependent degradation of LDs increases free fatty acid supplies for subsequent lipid peroxidation during ferroptosis [8]. Third, the SQSTM1-mediated autophagic degradation of aryl hydrocarbon receptor nuclear translocator-like (ARNTL/BMAL1), a process known as clockophagy, facilitates ferroptosis induction through increasing intracellular levels of PUFA [132]. Fourth, chaperone-mediated autophagy (CMA) facilitates GPX4 degradation, resulting in an increase in lipid peroxidation that favors ferroptosis [60]. This process is further enhanced by the activation of sphingomyelin phosphodiesterase 1 (SMPD1, also known as ASM), a lysosomal enzyme that plays a major role in sphingolipid metabolism [133]. Although these data support the notion that ferroptosis is an autophagy-dependent form of cell death, the specific pathways used only for this process remain to be characterized [134, 135]. In particular, the question arises whether there would be some kind of specificity in the mechanism of selective autophagy and lipid metabolism that favor ferroptosis [136, 137].
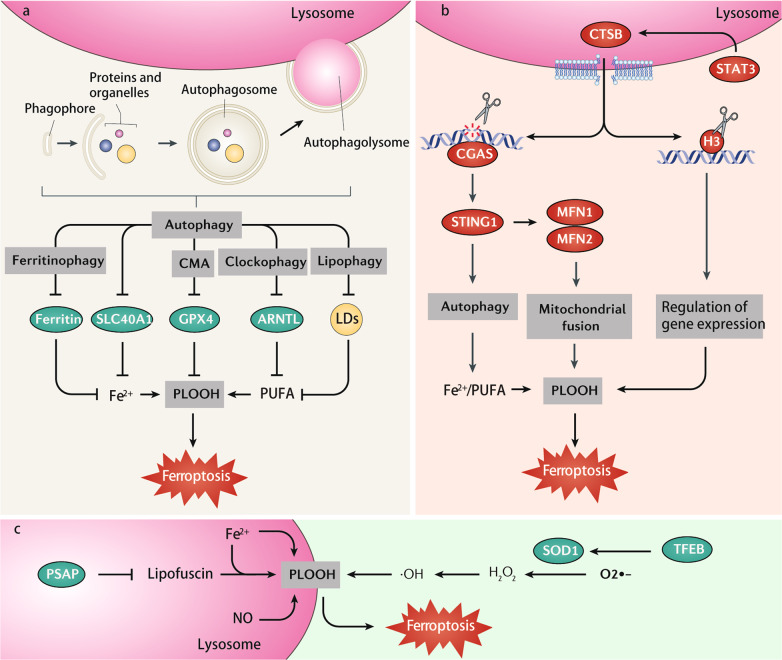
Fig. 3. Role of lysosomes in ferroptosis.
a Several selective autophagy pathways (ferritinophagy, chaperone-mediated autophagy [CMA], clockophagy, and lipophagy) promote ferroptosis by removing different cargoes, including ferritin, SLC40A1, GPX4, ARNTL, and lipid droplets (LDs). b Lysosomal CTSB is considered as an executioner of ferroptosis. CTSB is upregulated by the activation of transcription factor STAT3. CTSB translocates from lysosomes to the nucleus, where it cleaves DNA or histone H3. CTSB-mediated DNA damage activates STING1-dependent autophagy, while H3 cleavage might change ferroptosis-related gene expression. c The accumulation of lysosomal iron or nitric oxide (NO) can promote lysosome-dependent ferroptosis through induction of lipid peroxidation. The inhibition of PSAP triggers an accumulation of lipofuscin, which traps iron to induce ferroptosis. TFEB-mediated SOD2 expression attenuates ferroptosis by inhibiting ROS production. Abbreviations: ARNTL/BMAL1 aryl hydrocarbon receptor nuclear translocator-like, CGAS cyclic GMP-AMP synthase, CTSB cathepsin B, GPX4 glutathione peroxidase 4, H3 histone H3, MFN1 mitofusin 1, MFN2 mitofusin 2, PLOOH phospholipid hydroperoxides, PSAP prosaposin, PUFA polyunsaturated fatty acid, SLC40A1 solute carrier family 40 member 1, SOD1 superoxide dismutase 1, STAT3 signal transducer and activator of transcription 3, STING1/TMEM173 stimulator of interferon response cGAMP interactor 1, TFEB transcription factor EB.
An increased lysosomal membrane potential is the initial signal of lysosome-dependent cell death driven by various cell death stimulations. Recently, the release of lysosomal cathepsins, especially cathepsin B (CTSB), has been considered to be contributing to ferroptosis (Fig. 3b). The activation of signal transducer and activator of transcription 3 (STAT3) is required for the upregulation and subsequent lysosomal release of CTSB [138]. CTSB mediates ferroptosis through at least two potential mechanisms. First, CTSB translocates from lysosomes to the nucleus, causing DNA damage and subsequent STING1-dependent ferroptosis [139]. CTSB can also act as a specific histone H3 protease and cleave H3 for ferroptosis [140]. In addition to inhibitors of lysosomal function (e.g., bafilomycin A1, ammonium chloride, pepstatin A, and CA-074Me), genetic blockade of cathepsin limits erastin-induced ferroptosis in cancer cells and MEFs [139, 140].
Other mechanisms of lysosomal-dependent ferroptosis involve the accumulation of lysosomal iron or nitric oxide (Fig. 3c) [141, 142]. This process is responsible for the dichloroacetate-induced inhibition of stemness in colorectal cancer cells [143], the loss of lysosomal protein prosaposin (PSAP)-mediated neuronal death [144], or nonthermal plasma-activated Ringer’s lactate-triggered ferroptosis in malignant mesothelioma cells [145]. As a defense mechanism, the activation of nuclear transcription factor EB (TFEB) can inhibit lysosomal-dependent ferroptosis by inducing antioxidant superoxide dismutase 1 (SOD1) gene expression [146]. CD44-mediated iron uptake in endocytic vesicles replenishes lysosomal iron, leading to increased sensitivity to ferroptosis [147]. Together, the crosstalk between the lysosome and the nucleus can establish a feedback mechanism for the modulation of ferroptosis. It is not clear whether lysosomal exocytosis results in membrane remodeling and repair during ferroptosis.
Endoplasmic reticulum
Under normal conditions, the endoplasmic reticulum (ER) is the central organelle for the synthesis and processing of proteins as well as lipid secretion [148]. ER stress triggers an unfolded protein response to restore protein homeostasis, but can also trigger cell death when cells fail to restore homeostasis [149]. ER stress plays a dual role in ferroptosis (Fig. 4a). For example, erastin can induce a significant ER stress response by activating the eukaryotic translation initiation factor 2A (EIF2A)/activating transcription factor 4 (ATF4) pathway, which determines cell fate [20]. On one hand, ATF4-mediated HSPA5 expression prevents the degradation of GPX4, thereby increasing the resistance of pancreatic cancer cells or glioma cells to ferroptosis caused by gemcitabine or dihydroartemisinin [59, 150]. ATF4-mediated SLC7A11 upregulation is also implicated in ferroptosis resistance in human glioma cells [151]. On the other hand, the ATF4-mediated transcriptional expression of GSH-degrading enzyme ChaC glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1) enhances artesunate- or cystine starvation-induced ferroptosis in breast cancer cells [152, 153]. Thus, the diversity of ATF4 target genes confer ATF4 multiple biological functions in ferroptosis. The ER stress response also contributes to artesunate- or erastin-induced ferroptosis via the activation of autophagic degradation [154]. In stark contrast, ER stress-associated Ca2+ influx triggers ESCRT-III accumulation in plasma membranes to prevent membrane damage during ferroptosis [76, 77]. Notably, ferrostatin-1 might exert its anti-ferroptotic effect through its accumulation in the ER, rather than in lysosomes and mitochondria [88]. Quantitative measurements by two-photon phosphorescent lifetime imaging revealed that the viscosity of the ER increases during erastin-induced ferroptosis [155]. These two studies further support the involvement of the ER in regulating ferroptosis.
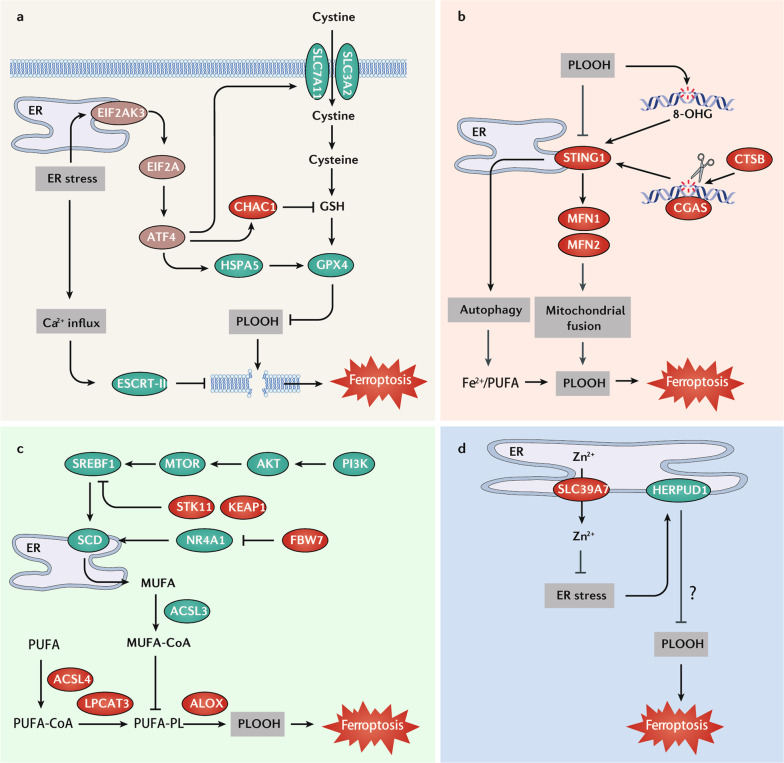
Fig. 4. Role of endoplasmic reticulum in ferroptosis.
a Endoplasmic reticulum (ER) stress, in particular from the EIF2AK3-EIF2A-ATF3 pathway, plays a dual role in ferroptosis. ATF4 inhibits ferroptosis by promoting HSPA5-mediated GPX4 protein stabilization or SLC7A11 expression, whereas it induces ferroptosis by upregulation of CHAC1 to degrade GSH. In addition, ER stress induces Ca2+ influx to trigger ESCRT-III–mediated membrane repair, which prevents membrane damage during ferroptosis. b STING1, a transmembrane protein on the ER, is activated by oxidized 8-OHG or CTSB-mediated DNA damage. STING1 then promotes ferroptosis by autophagy or an MFN1/MFN2 mediated-mitochondrial fusion pathway. c The ER enzyme SCD, a target gene of SREBP1, is essential for the biosynthesis of MUFA. ACSL3 is responsible for the activation of MUFA, which competitively inhibits PUFA-induced ferroptosis. SREBP1-SCD can be activated by the PI3K-AKT-MTOR pathway, a double mutation of STK11 and KEAP1, or the FBW7-NR4A1 pathway. d Zinc ions (Zn2+) can induce ferroptosis. The inhibition of zinc transporter SLC39A7 on the ER can trigger the expression of the ER stress-associated gene HERPUD1, which drives ferroptosis resistance. Abbreviations: ACSL3 acyl-CoA synthetase long-chain family member 3, ACSL4 acyl-CoA synthetase long-chain family member 4, ALOX lipoxygenase, ATF4 activating transcription factor 4, CGAS cyclic GMP-AMP synthase, CHAC1 ChaC glutathione-specific gamma-glutamylcyclotransferase 1, CTSB cathepsin B, EIF2A eukaryotic translation initiation factor 2A, EIF2AK3/PERK eukaryotic translation initiation factor 2 alpha kinase 3, ESCRT-III endosomal sorting complex required for transport-III, GPX4 glutathione peroxidase 4, GSH glutathione, HERPUD1 homocysteine inducible ER protein with ubiquitin-like domain 1, HSPA5/GRP78/BIP heat shock protein family A (Hsp70) member 5, LPCAT3 lysophosphatidylcholine acyltransferase 3, MFN1 mitofusin 1, MFN2 mitofusin 2, MTOR mechanistic target of rapamycin kinase, MUFA monounsaturated fatty acid, PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase, PLOOH phospholipid hydroperoxide, PUFA polyunsaturated fatty acid, SCD/SCD1 stearoyl-CoA desaturase, SLC39A7/ZIP7 solute carrier family 39 member 7, SLC3A2 solute carrier family 3 member 2, SLC7A11 solute carrier family 7 member 11, SREBF1/SREBP1 sterol regulatory element-binding transcription factor 1, STING1/TMEM173 stimulator of interferon response cGAMP interactor 1.
Several ER proteins play a broad role in regulating ferroptosis sensitivity. For example, STING1, a transmembrane protein on the ER, is reported to translate the oxidative response of nuclear or mitochondrial structures into a ferroptotic response (Fig. 4b). STING1 depletion attenuates acute pancreatitis and KRAS-driven pancreatic tumor formation in mice that are prone to ferroptosis due to a high-iron diet or genetic GPX4 deletion in pancreatic acinar cells [156]. The oxidized nucleobase 8-hydroxyguanine (8-OHG) released by ferroptotic cells has been identified as a ligand for active STING1-dependent innate immunity in macrophages [156]. Mitochondrial damage caused by zalcitabine or erastin can trigger STING1-dependent ferroptosis in pancreatic cancer cells through convoluted pathways involving autophagy or mitochondrial fusion [21, 102]. As a negative feedback mechanism, unrestricted lipid peroxidation might reduce the transport of STING1 from the ER to the Golgi complex and the subsequent immune response by its carbonylation [157]. These findings underscore the multifunctional role of STING1 in mediating ferroptosis.
ER proteins can block ferroptosis, as exemplified by stearoyl-CoA desaturase (SCD). The biosynthesis of MUFA requires SCD, which competitively inhibits PUFA-mediated ferroptosis [48]. The expression of SCD is regulated by multiple factors, including transcription factors, kinases, hypoxia, and nutrition signals (Fig. 4c) [158]. For example, sterol regulatory element-binding transcription factor 1 (SREBF1, also known as SREBP1), a nuclear transcription factor regulating lipid metabolism, acts as a ferroptosis repressor by the induction of SCD expression [159, 160]. Ferroptosis inhibition by the SREBP1-SCD pathway can also result from the activation of pro-survival phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-AKT-MTOR signaling in MDA-MB-453 and BT474 human breast cancer cells [159]. Lactate formed during anaerobic glycolysis inhibits the phosphorylation of AMPK, thereby activating the SREBP1-SCD pathway to inhibit ferroptosis in liver cancer cells [160]. The co-mutation of serine/threonine kinase 11 (STK11) and Kelch-like ECH-associated protein 1 (KEAP1) results in ferroptosis resistance in lung cancer cells partly through the upregulation of SCD [161]. Additionally, the nuclear protein F-box and WD repeat domain containing 7 (FBW7) can inhibit SCD expression in pancreatic cancer cells through blockade of nuclear receptor subfamily 4 group A member 1 (NR4A1) [162]. Thus, the combination of SCD inhibitors and ferroptosis inducers might be a potential strategy for anticancer treatment that warrants to be explored.
In addition to iron, zinc ions have the ability to induce ferroptosis [163]. Solute carrier family 39 member 7 (SLC39A7, also known as ZIP7), a resident ER protein that mediates zinc transport from the ER to cytosol, is a promoter of ferroptosis (Fig. 4d) [163]. The inhibition of SLC39A7 triggers the expression of ER stress-associated genes, such as homocysteine-inducible ER protein with ubiquitin-like domain 1 (HERPUD1), which drives ferroptosis resistance [163]. These findings not only uncover an unexpected role for ER stress in mediating zinc-induced ferroptosis, but also challenge the current notion that ferroptosis is exclusively dominated by iron-dependent redox reactions. Since mitochondria can form contacts with the ER to regulate vital cellular homoeostatic functions [164], researchers should investigate these connections in ferroptosis.
Lipid droplets
Lipid droplets (LDs) serve as storage organelles for neutral lipids, such as triacylglycerol and sterol esters. LDs are also in dynamic contact with other organelles (such as mitochondria, the ER, peroxisomes, and lysosomes) to facilitate the exchange of lipids, metabolites, and ions [165]. It is widely accepted that increasing the formation of LDs protects cells from PUFA-induced lipotoxicity and ER stress [166, 167]. The number of LDs increases in the early stages, but decreases in the final stages, of ferroptosis. The balance between the degradation and storage of LDs affects the sensitivity to ferroptosis. For example, RAB7A-mediated lipophagy increases intracellular PUFA production, thereby enhancing RSL3-induced ferroptosis in liver cancer cells. In contrast, tumor protein D52 (TPD52)-mediated lipid storage might limit ferroptosis by sequestering toxic oxidized lipids [8]. Exogenous PUFAs induces LD formation and accumulates in LDs, resulting in enhanced lipid ROS and ferroptosis in cervical (SiHa), colorectal (HCT-116), and hypopharyngeal (FaDu) cancer cells [168]. Moreover, Fas-associated factor family member 2 (FAF2), a molecule regulating LD formation and homeostasis, is downregulated in orlistat-induced ferroptosis in A549 and H1299 lung cancer cells, supporting the anti-ferroptotic role of LDs [169]. These observations highlight an urgent need to uncover the mechanisms of the LD dynamics in ferroptosis. In addition, lipolysis (the hydrolysis of triacylglycerol) occurs on the surface of LDs, releasing fatty acids for bioenergetic or anabolic reactions. Several enzymes, such as patatin-like phospholipase domain containing 2 (PNPLA2, also known as ATGL) and lipase E, hormone-sensitive type (LIPE, also known as HSL), play crucial roles in lipolysis [170], but their precise roles in ferroptosis remain to be uncovered.
Peroxisomes
Peroxisomes are organelles that generate ROS and reactive nitrogen species (RNS) through pro-oxidant enzymes, such as xanthine dehydrogenase (XDH) and nitric oxide synthase 2 (NOS2) [171]. Conversely, peroxisomes also contain antioxidant enzymes, such as catalase (CAT), SOD1, peroxiredoxin 5 (PRDX5), and glutathione S-transferase kappa 1 (GSTK1) [172]. Nonetheless, a recent study showed that peroxisome-mediated lipid synthesis rather than ROS or RNS generation promotes ferroptosis [54]. In particular, ether lipids are synthesized through a well-characterized process that begins in peroxisomes and finishes in the ER [54]. Within peroxisomes, the enzymes fatty acyl-CoA reductase 1 (FAR1) and alkylglycerone phosphate synthase (AGPS) catalyze the biosynthesis of the ether lipid precursor 1-O-alkyl-glycerol-3-phosphate (AGP). The is then delivered to the ER where it is acylated and dehydrogenated to form plasmalogens. The knockdown of peroxisome resident enzymes FAR1 and AGPS, or ER resident enzyme 1-acylglycerol-3-phosphate O-acyltransferase 3 (AGPAT3), diminishes the sensitivity of the cells to ferroptosis induced by GPX4 inhibition [54]. Consistently, the knockdown of peroxisomal biogenesis factors (PEXs), including PEX3, PEX10, PEX16, and PEX19, limits the production of polyunsaturated ether phospholipids (PUFA-ePLs), especially plasmalogens [54, 173]. Moreover, plasmanylethanolamine desaturase 1 (PEDS1, also known as TMEM189), which introduces the characteristic vinyl ether double bond into plasmalogens [174], limits ferroptosis through downregulating FAR1 protein levels [173]. These findings support the key role of peroxisome-driven PUFA-ePLs in modulating susceptibility to ferroptosis [175]. However, neurons from plasmalogen-deficient (PEX7 knockout) mice are more susceptible to ROS-mediated damage [176], indicating that ether phospholipids might act as endogenous antioxidants as well. In addition to lipid synthesis and redox balance, peroxisomes are also involved in the biosynthesis and signaling of steroid and peptide hormones [177], which in turn might indirectly impinge on the regulation of ferroptosis.
Golgi apparatus
The Golgi apparatus, a membranous organelle, has important functions in processing and sorting lipids and proteins for secretion or cellular use. Pharmacological Golgi stress inducers (e.g., AMF-26/M-COPA, brefeldin A, and golgicide A) trigger ferroptosis in HeLa cells, and this can be avoided by overexpression of SLC7A11 or GPX4, as well as the depletion of ACSL4 [178], indicating that Golgi-dependent ferroptosis requires classical ferroptotic regulators. The induction of ferroptosis by brefeldin A is also influenced by the availability of extracellular cystine [178]. The transsulfuration pathway (a source of cysteine for GSH in cells) can protect cells against brefeldin A-induced ferroptosis [178]. Although the exact mechanism of Golgi stress-induced ferroptosis is still poorly understood, it is suspected that Golgi dispersal might induce the loss of antioxidant molecules (e.g., CoQ10) [179]. Regardless, the sorting and transportation of cellular cargos in the entire cell by the Golgi apparatus might be impaired during ferroptosis, hence exemplifying yet another pathway in which disrupted protein homeostasis contributes to cell death.
Nucleus
In a previous review, we discussed the contribution of various transcription factors to the regulation of ferroptosis [180]. Here, we will focus on the discussion of non-transcriptional aspects of the nuclear implication in ferroptosis. Although early studies did not detect any obvious morphological changes in the nucleus, oxidative damage of nuclear DNA is a biochemical correlate of ferroptosis, which is associated with nuclear DAMP (e.g., HMGB1) release [10]. Several DNA damage response pathways, such as TP53, ataxia telangiectasia mutated (ATM), and FA complementation group D2 (FANCD2), play a context-dependent role in inhibiting or promoting ferroptosis. For example, TP53 activation can promote ferroptosis by the downregulation of SLC7A11 in breast cancer cells [62], whereas TP53 loss can trigger ferroptosis by activating the dipeptidyl peptidase 4 (DPP4)-dependent NOX pathway in colon cancer cells [27]. Radiotherapy-activated ATM transcriptionally represses SLC7A11 expression to promote ferroptosis in HT1080 cells [181]. FANCD2-mediated DNA repair inhibits erastin-induced ferroptosis in bone marrow cells [182]. The iron-binding protein pirin (PIR) is a nuclear redox-sensor, which limits autophagy-dependent ferroptosis by retaining HMGB1 in the nucleus [183]. In contrast, the translocation of lysosomal CTSB [139, 140] or mitochondrial AIFM1 [99, 100] to the nucleus can cause local damage and induce ferroptotic cell death. Thus, the translocation of different proteins between nuclear and extranuclear compartments profoundly affects the susceptibility of cells to ferroptosis. Future proteomic studies should provide a systematic and refined analysis of such ferroptosis-relevant translocation events.
Conclusions and perspectives
Selected metabolic changes, such as iron accumulation and lipid peroxidation, are considered as the biochemical hallmarks of ferroptosis [30]. Different organelles are involved in this metabolic cascade, which eventually leads to the rupture of the plasma membrane. Unlike other types of RCD, the specific effector of ferroptosis is still unknown. One hypothesis is that toxic lipids might directly mediate ferroptosis without the involvement of pore-forming proteins [2]. The production of toxic lipids involves a dynamic pathway, which connects lipid synthesis, degradation, storage, transformation, utilization, and peroxidation [55]. This process is further regulated by organelle-specific signals and pathways. Thus, multiple antioxidant systems and membrane repair pathways can synergistically antagonize organelle damage and ferroptosis induced by oxidative stress [19, 67–70, 103, 184, 185]. However, the contribution of exogenous (e.g., ferrostatin-1 and liproxstatin-1) or endogenous antioxidants (e.g., GSH, CoQ10, BH4, and dopamine) to specific organelles has largely not been verified. Apparently, all major organelles of the cell may modulate the ‘decision’ phase during which the threshold for lethal membrane peroxidation is reached or avoided. Moreover, several major organelles, in particular mitochondria and lysosomes, may contribute to the lethal process as a result of their membrane permeabilization, hence liberating hydrolytic enzymes and increasing the entropy of the cellular system.
Although significant advances have been made in our understanding of the machinery of ferroptosis [186], several basic questions must be answered before the development of specific ferroptosis-related therapies may be envisaged. Are the ferroptosis-relevant damage or repair mechanisms affecting the plasma membrane and internal, organelle-specific membranes different? What are the key molecules that maintain or disrupt the communication between subcellular organelles in ferroptosis? How can we develop molecular probes to dynamically monitor ferroptosis-associated changes in organellar morphology and function? Do the biogenesis and turnover of specific organelles affect the susceptibility of cells to ferroptosis? And finally, which strategies may guide the identification of subtle modulators of ferroptosis that act on peculiar, ideally cell type or organ-specific, receptors and hence can be used for the therapeutic avoidance of excessive ferroptosis or, on the contrary, for its selective induction in cancer cells? Elucidating the role of organelle-specific membranes in ferroptosis would be an attractive research area in the future.
Acknowledgements
We thank Dave Primm (Department of Surgery, University of Texas Southwestern Medical Center) for his critical reading of the manuscript. We thank the numerous colleagues in the field of ferroptosis. We also apologize to the researchers who were not referenced due to space limitations.
Author contributions
X.C. and D.T. wrote the manuscript. G.K. and R.K. edited the manuscript. All authors approved the submitted version.
Funding
Not applicable
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent for publication
All authors agree to publish.
Footnotes
Edited by M. Piacentini
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Kang, Email: rui.kang@utsouthwstern.edu.
Guido Kroemer, Email: kroemer@orange.fr.
Daolin Tang, Email: daolin.tang@utsouthwestern.edu.
References
- 1.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang D, Kroemer G. Ferroptosis. Curr Biol. 2020;30:R1–R6. doi: 10.1016/j.cub.2019.12.061. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 11.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–83. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11:5424. doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–90. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218:e20210518. doi: 10.1084/jem.20210518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology, and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhang Y, Liu J, Kang R, Klionsky DJ, Tang D, et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2020;17:948–60. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–80. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021;12:698. doi: 10.1038/s41419-021-03998-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Otin C, Kroemer G. Hallmarks of Health. Cell. 2021;184:33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014;16:728–36. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 26.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63 e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 28.Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, et al. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28:2501–8 e2504. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Cancer Res. 2020;18:79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Comish PB, Tang D, Kang R. Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol. 2021;9:637162. doi: 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–23 e3417. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Liu Y, Liu J, Kang R, Tang D. NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531:581–7. doi: 10.1016/j.bbrc.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–43. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharm Sci. 2018;22:3826–36. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Liu J, Xu Y, Wu R, Chen X, Song X, et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy. 2021 doi: 10.1080/15548627.2021.1872241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–86 e574. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Song X, Kuang F, Zhang Q, Xie Y, Kang R, et al. NUPR1 is a critical repressor of ferroptosis. Nat Commun. 2021;12:647. doi: 10.1038/s41467-021-20904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Yu C, Kang R, Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol. 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–12 e1005. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu S, Chaudhary O, Rodriguez-Morales P, Sun X, Chen D, Zappasodi R, et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity. 2021;54:1561–77. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 47.Beatty A, Singh T, Tyurina YY, Tyurin VA, Samovich S, Nicolas E, et al. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat Commun. 2021;12:2244. doi: 10.1038/s41467-021-22471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol. 2019;26:420–32 e429. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966–4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–41 e626. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–91. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–9. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 2020;81:355–69. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–8. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Z, Liu J, Kang R, Yang M, Tang D. Lipid metabolism in ferroptosis. Adv Biol (Weinh) 2021;10:e2100396. doi: 10.1002/adbi.202100396. [DOI] [PubMed] [Google Scholar]
- 56.Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol. 2020;8:586578. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiorino M, Thomas JP, Girotti AW, Ursini F. Reactivity of phospholipid hydroperoxide glutathione peroxidase with membrane and lipoprotein lipid hydroperoxides. Free Radic Res Commun. 1991;12-13(Pt 1):131–5. doi: 10.3109/10715769109145777. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12:1589. doi: 10.1038/s41467-021-21841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu S, Zhang Q, Sun X, Zeh HJ, 3rd, Lotze MT, Kang R, et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017;77:2064–77. doi: 10.1158/0008-5472.CAN-16-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, et al. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–71. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 62.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system X(c)(-) activity. Curr Biol. 2018;28:2388–99.e2385. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vuckovic AM, Bosello Travain V, Bordin L, Cozza G, Miotto G, Rossetto M, et al. Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14-3-3epsilon. FEBS Lett. 2020;594:611–24. doi: 10.1002/1873-3468.13631. [DOI] [PubMed] [Google Scholar]
- 65.Han L, Bai L, Fang X, Liu J, Kang R, Zhou D, et al. SMG9 drives ferroptosis by directly inhibiting GPX4 degradation. Biochem Biophys Res Commun. 2021;567:92–98. doi: 10.1016/j.bbrc.2021.06.038. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Wang Y, Liu J, Kang R, Tang D. Interplay between MTOR and GPX4 signaling modulates autophagy-dependent ferroptotic cancer cell death. Cancer Gene Ther. 2021;28:55–63. doi: 10.1038/s41417-020-0182-y. [DOI] [PubMed] [Google Scholar]
- 67.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 68.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16:1351–60. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D, Peng Y, Xie Y, Zhou B, Sun X, Kang R, et al. Antiferroptotic activity of non-oxidative dopamine. Biochem Biophys Res Commun. 2016;480:602–7. doi: 10.1016/j.bbrc.2016.10.099. [DOI] [PubMed] [Google Scholar]
- 73.Sun WY, Tyurin VA, Mikulska-Ruminska K, Shrivastava IH, Anthonymuthu TS, Zhai YJ, et al. Phospholipase iPLA2beta averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol. 2021;17:465–76. doi: 10.1038/s41589-020-00734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen D, Chu B, Yang X, Liu Z, Jin Y, Kon N, et al. iPLA2beta-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nat Commun. 2021;12:3644. doi: 10.1038/s41467-021-23902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. HMGB1 in health and disease. Mol Asp Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pedrera L, Espiritu RA, Ros U, Weber J, Schmitt A, Stroh J, et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2020;28:1644–57. doi: 10.1038/s41418-020-00691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai E, Meng L, Kang R, Wang X, Tang D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun. 2020;522:415–21. doi: 10.1016/j.bbrc.2019.11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 Blocks Ferroptosis Independent of Ubiquinol Metabolism. Biochem Biophys Res Commun. 2020;523:966–71. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 79.Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300 e216. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–60. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 81.Liu J, Kang R, Tang D. ESCRT-III-mediated membrane repair in cell death and tumor resistance. Cancer Gene Ther. 2021;28:1–4. doi: 10.1038/s41417-020-0200-0. [DOI] [PubMed] [Google Scholar]
- 82.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–6. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wenz C, Faust D, Linz B, Turmann C, Nikolova T, Dietrich C. Cell-cell contacts protect against t-BuOOH-induced cellular damage and ferroptosis in vitro. Arch Toxicol. 2019;93:1265–79. doi: 10.1007/s00204-019-02413-w. [DOI] [PubMed] [Google Scholar]
- 84.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–8. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993;361:365–9. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- 86.Marchetti P, Susin SA, Decaudin D, Gamen S, Castedo M, Hirsch T, et al. Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res. 1996;56:2033–8. [PubMed] [Google Scholar]
- 87.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 88.Gaschler MM, Hu F, Feng H, Linkermann A, Min W, Stockwell BR. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol. 2018;13:1013–20. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basit F, van Oppen LM, Schockel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747. doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang S, Chapa-Dubocq XR, Tyurina YY, St Croix CM, Kapralov AA, Tyurin VA, et al. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021;45:102021. doi: 10.1016/j.redox.2021.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R, et al. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med. 2018;117:45–57. doi: 10.1016/j.freeradbiomed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 93.Liang H, Yoo SE, Na R, Walter CA, Richardson A, Ran Q. Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. J Biol Chem. 2009;284:30836–44. doi: 10.1074/jbc.M109.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang H, Van Remmen H, Frohlich V, Lechleiter J, Richardson A, Ran Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem Biophys Res Commun. 2007;356:893–8. doi: 10.1016/j.bbrc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 95.Schneider M, Forster H, Boersma A, Seiler A, Wehnes H, Sinowatz F, et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009;23:3233–42. doi: 10.1096/fj.09-132795. [DOI] [PubMed] [Google Scholar]
- 96.Ma CS, Lv QM, Zhang KR, Tang YB, Zhang YF, Shen Y, et al. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharm Sin. 2021;42:613–23. doi: 10.1038/s41401-020-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuang F, Liu J, Xie Y, Tang D, Kang R. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem Biol. 2021;28:765–75. doi: 10.1016/j.chembiol.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 99.Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R, et al. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 2017;12:558–70. doi: 10.1016/j.redox.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Perfettini JL, Roumier T, Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–83. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Li C, Liu J, Hou W, Kang R, Tang D. STING1 promotes ferroptosis through MFN1/2-dependent mitochondrial fusion. Front in Cell and Dev Biol. 2021;9:1564. doi: 10.3389/fcell.2021.698679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–79 e1225. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 104.Imoto S, Kono M, Suzuki T, Shibuya Y, Sawamura T, Mizokoshi Y, et al. Haemin-induced cell death in human monocytic cells is consistent with ferroptosis. Transfus Apher Sci. 2018;57:524–31. doi: 10.1016/j.transci.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 105.Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393–403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF, et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Ren Physiol. 2018;314:F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terzi EM, Sviderskiy VO, Alvarez SW, Whiten GC, Possemato R. Iron-sulfur cluster deficiency can be sensed by IRP2 and regulates iron homeostasis and sensitivity to ferroptosis independent of IRP1 and FBXL5. Sci Adv. 2021;7:eabg4302. doi: 10.1126/sciadv.abg4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Du J, Zhou Y, Li Y, Xia J, Chen Y, Chen S, et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020;32:101483. doi: 10.1016/j.redox.2020.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, He H, Wang J, Guo X, Lin H, Chen H, et al. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology. 2020;445:152584. doi: 10.1016/j.tox.2020.152584. [DOI] [PubMed] [Google Scholar]
- 110.Cotticelli MG, Xia S, Lin D, Lee T, Terrab L, Wipf P, et al. Ferroptosis as a novel therapeutic target for Friedreich’s Ataxia. J Pharm Exp Ther. 2019;369:47–54. doi: 10.1124/jpet.118.252759. [DOI] [PubMed] [Google Scholar]
- 111.Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–69. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 112.Kim EH, Shin D, Lee J, Jung AR, Roh JL. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180–90. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 113.Homma T, Kobayashi S, Fujii J. Cysteine preservation confers resistance to glutathione-depleted cells against ferroptosis via CDGSH iron sulphur domain-containing proteins (CISDs) Free Radic Res. 2020;54:397–407. doi: 10.1080/10715762.2020.1780229. [DOI] [PubMed] [Google Scholar]
- 114.Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci. 2016;8:308. doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuhrmann DC, Mondorf A, Beifuss J, Jung M, Brune B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. doi: 10.1016/j.redox.2020.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang R, Kang R, Tang D. The STING1 network regulates autophagy and cell death. Signal Transduct Target Ther. 2021;6:208. doi: 10.1038/s41392-021-00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murthy AMV, Robinson N, Kumar S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differ. 2020;27:2989–3003. doi: 10.1038/s41418-020-00624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo J, Duan L, He X, Li S, Wu Y, Xiang G, et al. A combined model of human iPSC-derived liver organoids and hepatocytes reveals ferroptosis in DGUOK mutant mtDNA depletion syndrome. Advanced. Science. 2021;8:2004680. doi: 10.1002/advs.202004680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomita K, Takashi Y, Ouchi Y, Kuwahara Y, Igarashi K, Nagasawa T, et al. Lipid peroxidation increases hydrogen peroxide permeability leading to cell death in cancer cell lines that lack mtDNA. Cancer Sci. 2019;110:2856–66. doi: 10.1111/cas.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc(-) activity. Curr Biol. 2018;28:2388–99 e2385. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kerins MJ, Milligan J, Wohlschlegel JA, Ooi A. Fumarate hydratase inactivation in hereditary leiomyomatosis and renal cell cancer is synthetic lethal with ferroptosis induction. Cancer Sci. 2018;109:2757–66. doi: 10.1111/cas.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Homma T, Kobayashi S, Sato H, Fujii J. Superoxide produced by mitochondrial complex III plays a pivotal role in the execution of ferroptosis induced by cysteine starvation. Arch Biochem Biophys. 2021;700:108775. doi: 10.1016/j.abb.2021.108775. [DOI] [PubMed] [Google Scholar]
- 123.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niu Y, Zhang J, Tong Y, Li J, Liu B. Physcion 8-O-beta-glucopyranoside induced ferroptosis via regulating miR-103a-3p/GLS2 axis in gastric cancer. Life Sci. 2019;237:116893. doi: 10.1016/j.lfs.2019.116893. [DOI] [PubMed] [Google Scholar]
- 125.You JH, Lee J, Roh JL. Mitochondrial pyruvate carrier 1 regulates ferroptosis in drug-tolerant persister head and neck cancer cells via epithelial-mesenchymal transition. Cancer Lett. 2021;507:40–54. doi: 10.1016/j.canlet.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 126.Kim H, Lee JH, Park JW. Down-regulation of IDH2 sensitizes cancer cells to erastin-induced ferroptosis. Biochem Biophys Res Commun. 2020;525:366–71. doi: 10.1016/j.bbrc.2020.02.093. [DOI] [PubMed] [Google Scholar]
- 127.Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21:805–21. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 128.Song X, Liu J, Kuang F, Chen X, Zeh HJ, 3rd, Kang R, et al. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021;34:108767. doi: 10.1016/j.celrep.2021.108767. [DOI] [PubMed] [Google Scholar]
- 129.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiong Q, Li X, Li W, Chen G, Xiao H, Li P, et al. WDR45 mutation impairs the autophagic degradation of transferrin receptor and promotes ferroptosis. Front Mol Biosci. 2021;8:645831. doi: 10.3389/fmolb.2021.645831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thayyullathil F, Cheratta AR, Alakkal A, Subburayan K, Pallichankandy S, Hannun YA, et al. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis. 2021;12:26. doi: 10.1038/s41419-020-03297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 136.Xie Y, Li J, Kang R, Tang D. Interplay between lipid metabolism and autophagy. Front Cell Dev Biol. 2020;8:431. doi: 10.3389/fcell.2020.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X, Yu C, Kang R, Kroemer G, Tang D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021;28:1135–48. doi: 10.1038/s41418-020-00728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D, et al. Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun. 2018;503:1550–6. doi: 10.1016/j.bbrc.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 139.Kuang F, Liu J, Li C, Kang R, Tang D. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem Biophys Res Commun. 2020;533:1464–9. doi: 10.1016/j.bbrc.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 140.Nagakannan P, Islam MI, Conrad M, Eftekharpour E. Cathepsin B is an executioner of ferroptosis. Biochim Biophys Acta Mol Cell Res. 2021;1868:118928. doi: 10.1016/j.bbamcr.2020.118928. [DOI] [PubMed] [Google Scholar]
- 141.Hirayama T, Miki A, Nagasawa H. Organelle-specific analysis of labile Fe(ii) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics. 2019;11:111–7. doi: 10.1039/C8MT00212F. [DOI] [PubMed] [Google Scholar]
- 142.Mai TT, Hamai A, Hienzsch A, Caneque T, Muller S, Wicinski J, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9:1025–33. doi: 10.1038/nchem.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun J, Cheng X, Pan S, Wang L, Dou W, Liu J, et al. Dichloroacetate attenuates the stemness of colorectal cancer cells via trigerring ferroptosis through sequestering iron in lysosomes. Environ Toxicol. 2021;36:520–9. doi: 10.1002/tox.23057. [DOI] [PubMed] [Google Scholar]
- 144.Tian R, Abarientos A, Hong J, Hashemi SH, Yan R, Drager N, et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci. 2021;24:1020–34. doi: 10.1038/s41593-021-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang L, Zheng H, Lyu Q, Hayashi S, Sato K, Sekido Y, et al. Lysosomal nitric oxide determines transition from autophagy to ferroptosis after exposure to plasma-activated Ringer’s lactate. Redox Biol. 2021;43:101989. doi: 10.1016/j.redox.2021.101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li L, Sun S, Tan L, Wang Y, Wang L, Zhang Z, et al. Polystyrene nanoparticles reduced ROS and inhibited ferroptosis by triggering lysosome stress and TFEB nucleus translocation in a size-dependent manner. Nano Lett. 2019;19:7781–92. doi: 10.1021/acs.nanolett.9b02795. [DOI] [PubMed] [Google Scholar]
- 147.Muller S, Sindikubwabo F, Caneque T, Lafon A, Versini A, Lombard B, et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12:929–38. doi: 10.1038/s41557-020-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 149.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402. doi: 10.1186/s13046-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang N, Zeng GZ, Yin JL, Bian ZX. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s Lymphoma. Biochem Biophys Res Commun. 2019;519:533–9. doi: 10.1016/j.bbrc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 153.Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS, Lee HC, et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8:114588–602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee YS, Kalimuthu K, Seok Park Y, Makala H, Watkins SC, Choudry MHA, et al. Ferroptotic agent-induced endoplasmic reticulum stress response plays a pivotal role in the autophagic process outcome. J Cell Physiol. 2020;235:6767–78. doi: 10.1002/jcp.29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hao L, Zhong YM, Tan CP, Mao ZW. Quantitative tracking of endoplasmic reticulum viscosity during ferroptosis by an iridium complex via TPPLM. Chem Commun (Camb) 2021;57:5040–2. doi: 10.1039/D1CC01062J. [DOI] [PubMed] [Google Scholar]
- 156.Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R, et al. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. 2020;11:6339. doi: 10.1038/s41467-020-20154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jia M, Qin D, Zhao C, Chai L, Yu Z, Wang W, et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat Immunol. 2020;21:727–35. doi: 10.1038/s41590-020-0699-0. [DOI] [PubMed] [Google Scholar]
- 158.Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79:5355–66. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–97. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 161.Wohlhieter CA, Richards AL, Uddin F, Hulton CH, Quintanal-Villalonga A, Martin A, et al. Concurrent mutations in STK11 and KEAP1 promote ferroptosis protection and SCD1 dependence in lung cancer. Cell Rep. 2020;33:108444. doi: 10.1016/j.celrep.2020.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye Z, Zhuo Q, Hu Q, Xu X, Mengqi L, Zhang Z, et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021;38:101807. doi: 10.1016/j.redox.2020.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen PH, Wu J, Xu Y, Ding CC, Mestre AA, Lin CC, et al. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 2021;12:198. doi: 10.1038/s41419-021-03482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee S, Min KT. The interface between ER and mitochondria: molecular compositions and functions. Mol Cells. 2018;41:1000–7. doi: 10.14348/molcells.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137–55. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jarc E, Kump A, Malavasic P, Eichmann TO, Zimmermann R, Petan T. Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:247–65. doi: 10.1016/j.bbalip.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 167.Petan T, Jarc E, Jusovic M. Lipid droplets in cancer: guardians of fat in a stressful world. Molecules. 2018;23:1941. doi: 10.3390/molecules23081941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dierge E, Debock E, Guilbaud C, Corbet C, Mignolet E, Mignard L, et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 2021;33:1701–15. doi: 10.1016/j.cmet.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 169.Zhou W, Zhang J, Yan M, Wu J, Lian S, Sun K, et al. Orlistat induces ferroptosis-like cell death of lung cancer cells. Front of Med. 2021. 10.1007/s11684-020-0804-7. [DOI] [PubMed]
- 170.Miyoshi H, Perfield JW, 2nd, Obin MS, Greenberg AS. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J Cell Biochem. 2008;105:1430–6. doi: 10.1002/jcb.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith JJ, Aitchison JD. Peroxisomes take shape. Nat Rev Mol Cell Biol. 2013;14:803–17. doi: 10.1038/nrm3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fransen M, Nordgren M, Wang B, Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta. 2012;1822:1363–73. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 173.Cui W, Liu D, Gu W, Chu B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021;28:2536–51. doi: 10.1038/s41418-021-00769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Werner ER, Keller MA, Sailer S, Lackner K, Koch J, Hermann M, et al. The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens. Proc Natl Acad Sci USA. 2020;117:7792–8. doi: 10.1073/pnas.1917461117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tang D, Kroemer G. Peroxisome: the new player in ferroptosis. Signal Transduct Target Ther. 2020;5:273. doi: 10.1038/s41392-020-00404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luoma AM, Kuo F, Cakici O, Crowther MN, Denninger AR, Avila RL, et al. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic Biol Med. 2015;84:296–310. doi: 10.1016/j.freeradbiomed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 177.Weinhofer I, Kunze M, Forss-Petter S, Berger J. Involvement of human peroxisomes in biosynthesis and signaling of steroid and peptide hormones. Subcell Biochem. 2013;69:101–10. doi: 10.1007/978-94-007-6889-5_6. [DOI] [PubMed] [Google Scholar]
- 178.Alborzinia H, Ignashkova TI, Dejure FR, Gendarme M, Theobald J, Wolfl S, et al. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun Biol. 2018;1:210. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang Z, Hu Z, Zeng L, Lu W, Zhang H, Li T, et al. The role of the Golgi apparatus in oxidative stress: is this organelle less significant than mitochondria? Free Radic Biol Med. 2011;50:907–17. doi: 10.1016/j.freeradbiomed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 180.Dai C, Chen X, Li J, Comish P, Kang R, Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;27:645–56. doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 181.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Disco. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480:443–9. doi: 10.1016/j.bbrc.2016.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hu N, Bai L, Dai E, Han L, Kang R, Li H, et al. Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis. Biochem Biophys Res Commun. 2021;536:100–6. doi: 10.1016/j.bbrc.2020.12.066. [DOI] [PubMed] [Google Scholar]
- 184.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–22 e421. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 186.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2020. 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed]