Abstract
Aims
Autoimmune systemic inflammatory diseases (SIDs) are associated with an increased risk of cardiovascular (CV) disease, particularly myocardial infarction (MI). However, there are limited data on the prevalence and effects of SID among adults who experience an MI at a young age. We sought to determine the prevalence and prognostic implications of SID among adults who experienced an MI at a young age.
Methods and results
The YOUNG-MI registry is a retrospective cohort study from two large academic centres, which includes patients who experienced a first MI at 50 years of age or younger. SID was ascertained through physician review of the electronic medical record (EMR). Incidence of death was ascertained through the EMR and national databases. The cohort consisted of 2097 individuals, with 53 (2.5%) possessing a diagnosis of SID. Patients with SID were more likely to be female (36% vs. 19%, P = 0.004) and have hypertension (62% vs. 46%, P = 0.025). Over a median follow-up of 11.2 years, patients with SID experienced an higher risk of all-cause mortality compared with either the full cohort of non-SID patients [hazard ratio (HR) = 1.95, 95% confidence interval (CI) (1.07–3.57), P = 0.030], or a matched cohort based on age, gender, and CV risk factors [HR = 2.68, 95% CI (1.18–6.07), P = 0.018].
Conclusions
Among patients who experienced a first MI at a young age, 2.5% had evidence of SID, and these individuals had higher rates of long-term all-cause mortality. Our findings suggest that the presence of SID is associated with worse long-term survival after premature MI.
Keywords: Inflammation, Myocardial infarction, Young, Autoimmunity
Introduction
Patients with systemic inflammatory conditions have a higher risk of myocardial infarction (MI) and cardiovascular (CV) mortality compared with the general population. These patients have also been noted to have a higher prevalence of CV risk factors; however, these typical risk factors do not fully account for this elevated CV risk.1–5 Instead, systemic inflammation has been implicated as the key driver of excess risk.6–10 Common inflammatory conditions associated with increased CV risk include psoriasis, systemic lupus erythematosus (SLE), and rheumatoid arthritis (RA). Although each disease has unique pathogenesis and immunopathobiology, systemic inflammation is a common theme among these disorders. In fact, the 2019 American College of Cardiology/American Heart Association (ACC/AHA) prevention guidelines have categorized each of these conditions as a ‘risk enhancer’ that can be used to further stratify patients determined to be at intermediate risk and for whom primary prevention statin therapy is being considered.11
While CV mortality has declined substantially in the USA over the last 50 years, progress has stalled among young adults (age < 55 years), especially among young women. Similarly, the incidence of MI has declined substantially across the USA over the past 10 years but not in young adults.12 The onset of systemic autoimmune inflammatory disorders often occurs in young adulthood, with women being more frequently affected than men.13–16 Thus, we hypothesized that systemic inflammatory disorders in this age group might be associated with a higher risk of long-term CV events. The objective of this study was to examine the overall prevalence of systemic inflammatory diseases (SIDs), as well as their association with CV risk factor profiles and long-term mortality among patients admitted with a first MI at or before the age of 50.
Methods
Study population
The design of the YOUNG-MI registry has been previously described.17 In brief, this is a retrospective cohort study from two large academic medical centres (Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, MA, USA), which included all consecutive patients who experienced an MI at or before 50 years of age between 2000 and 2016. All records were adjudicated by a team of study physicians, as previously described,17 using the Third Universal Definition of MI.18 For the present analysis, only patients with type 1 MI were included. Individuals with known coronary artery disease (CAD) (defined as prior MI or revascularization) were excluded. A waiver of consent for the YOUNG-MI registry was granted by the Institutional Review Board at Partners HealthCare.
Risk factors
The presence of CV risk factors was ascertained by means of a detailed review of electronic medical records (EMRs) from the period during and prior to the index admission. For each risk factor, we also determined whether it was known prior to admission or diagnosed during the index hospitalization. Diabetes was defined as fasting plasma glucose >126 mg/dL or haemoglobin A1c ≥6.5% or diagnosis/treatment for diabetes. Hypertension was defined as having a documented diagnosis and/or treatment of hypertension. Dyslipidaemia was defined as having a documented diagnosis and/or treatment of dyslipidaemia. Obesity was defined as having a body mass index ≥30 kg/m2 or a diagnosis of obesity. Smoking was defined as current (tobacco products used within a month prior to the index admission), former, or never. Family history of premature CAD, defined as a fatal MI, nonfatal MI, or coronary revascularization occurring before 55 years of age for first-degree male family members and before 65 years of age for first-degree female family members, was captured by a thorough review of the EMRs, which included all clinic notes prior to admission, admission history and physical, discharge summaries, and follow-up visit notes. The atherosclerotic cardiovascular disease (ASCVD) risk score was calculated based on data available prior to MI or at time of presentation using the pooled cohort equation. Risk factors that were diagnosed after the index hospitalization for MI were not used for calculating the risk scores, as the intent of our study was to evaluate the known risk factor profile prior to presentation. Given the systemic nature of inflammatory conditions, we also calculated the Charlson Comorbidity Index (CCI) score, a composite score that combines underlying medical comorbidities, using International Classification of Diseases, 9th and 10th Revision (ICD-9, ICD-10) diagnosis and billing codes associated with the index hospitalization.19
Cardiac biomarkers
Laboratory values were obtained during the index admission and extracted from the EMR. The maximum troponin value during in-patient admission was used. Because different assays were used to measure troponin during the study period, the troponin value was standardized by dividing it by the 99th percentile (i.e. the upper limit of normal) for the particular assay. Further details are provided in ref.20
System inflammatory disease
The existence of SIDs was initially assessed ICD-9 or ICD-10 diagnosis codes within the EMR or natural language processing21 for key search word/terms for autoimmune systemic inflammatory disorders. A physician blinded to all outcomes data then reviewed each chart to confirm the clinical criteria. Only patients who had the inflammatory disease diagnosis listed in the medical record at or before the time of the index MI were included.
Outcomes
The primary outcome of interest was all-cause mortality. Vital status was assessed by means of the Social Security Administration Death Master File, the Massachusetts Department of Vital Statistics, and the National Death Index. Cause of death was adjudicated independently by two physicians, with all instances of disagreement reviewed by an adjudication committee and decisions reached by consensus.
Statistical analysis
All analyses were performed using Stata Version 15.1 (StataCorp, College Station, TX, USA). Categorical variables are reported as frequencies and proportions and were compared with χ2 or Fisher’s exact tests, as appropriate. Continuous variables are reported as means or medians and compared with t-tests or Mann–Whitney tests, as appropriate. The proportional hazards assumption was assessed by analysing the Schoenfeld residuals. Survival curves were compared using the log-rank test. A two-tailed P-value less than 0.05 was considered statistically significant.
Cox proportional hazards modelling were used to assess the association with SID and obtain corresponding hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality. Patients were censored on the date of querying their source of vital statistics. Cox proportional hazard modelling was initially performed on a univariate basis using all patients in the cohort. Following this, given the small cohort size and number of events, an analysis was performed after generating a sub-sample based on Mahalanobis Distance matching on age, sex, and key CV risk factors that included diabetes, tobacco use, and hypertension. Subsequently, multivariable modelling was performed to further adjust for estimated glomerular filtration rate (eGFR) and length of stay (LOS). This additional adjustment was performed based on selecting variables which had a significant univariate association with all-cause mortality in the matched cohort and for which there is an established association with all-cause mortality.22
Results
Prevalence of systemic inflammatory disorders among patients with type 1 MI age ≤50
The cohort consisted of 2097 patients who experienced a Type 1 MI at or before age 50 years. The median age was 45 years, of whom 19% were female and 53% presented with an ST-elevation MI. Among the total cohort, 53 (2.5%) possessed a diagnosis of SID at or before their index MI. The distribution is shown in Figure 1 among which 64% of patients had a diagnosis of psoriatic disease, 23% SLE, 9% RA, and 4% other SID.
Figure 1.
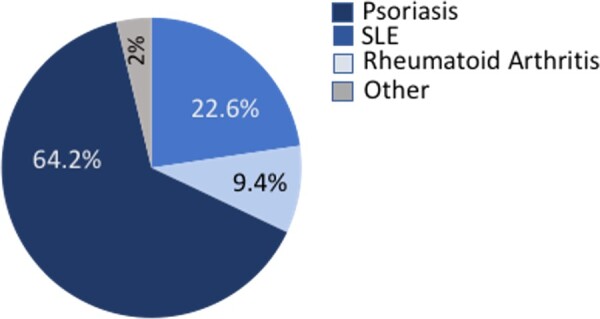
Distribution of systemic inflammatory disease among young adults with type 1 MI. A pie chart is shown subdividing the type of systemic inflammatory condition that characterized the study cohort population. The distribution was as follows: 23% systemic lupus erythematosus, 9% rheumatoid arthritis, 64% psoriasis, and 4% other inflammatory arthritis.
The demographics of patients with SID compared with the overall cohort is shown in Table 2. Patients with SID were more likely to be female (36% vs. 19%, P = 0.004) and be diagnosed with hypertension (62% vs. 46%, P = 0.025). There were no significant differences in the prevalence of other CV risk factors: diabetes, smoking, dyslipidaemia, or a family history of premature CAD. The mean CCI was also similar between patients with SID and the rest of the cohort [1.7 (0.8) vs. 1.5 (1.0), P = 0.3].
Table 2.
Baseline characteristics of a matched sub-sample and systemic inflammatory disease patients
| Matched sub-sample (n = 138) | Systemic inflammatory disease cohort (n = 53) | P-value | |
|---|---|---|---|
| Age at event, median (IQR) | 46 (41–49) | 46 (42–48) | 0.54 |
| Female, n (%) | 48 (34.8%) | 19 (35.8%) | 0.89 |
| Caucasian, n (%) | 95 (68.8%) | 40 (75.5%) | 0.37 |
| Hypertension, n (%) | 84 (60.9%) | 33 (62.3%) | 0.86 |
| Hyperlipidaemia, n (%) | 124 (89.9%) | 46 (86.8%) | 0.54 |
| Diabetes, n (%) | 30 (21.7%) | 11 (20.8%) | 0.88 |
| Obesity, n (%) | 55 (39.9%) | 24 (46.2%) | 0.43 |
| Current tobacco use, n (%) | 57 (41.6%) | 23 (44.2%) | 0.74 |
| Charlson index, mean (SD) | 1.6 (1.0) | 1.7 (0.8) | 0.36 |
IQR, interquartile range; SD, standard deviation.
Table 1.
Baseline demographics of study cohort
| Individuals without systemic inflammatory disease (n = 2044) | Individuals with systemic inflammatory disease (n = 53) | P-value | |
|---|---|---|---|
| Age at event, median (IQR) | 45 (41–48) | 46 (42–48) | 0.54 |
| Female, n (%) | 385 (18.8%) | 19 (35.8%) | 0.002a |
| Caucasian, n (%) | 1497 (73.2%) | 40 (75.5%) | 0.72 |
| Hypertension, n (%) | 947 (46.3%) | 33 (62.3%) | 0.02a |
| Hyperlipidaemia, n (%) | 1868 (91.4%) | 46 (86.8%) | 0.24 |
| Diabetes, n (%) | 405 (19.8%) | 11 (20.8%) | 0.87 |
| Obesity, n (%) | 756 (38.3%) | 24 (46.2%) | 0.25 |
| Family history of premature CAD, n (%) | 565 (27.6%) | 17 (32.1%) | 0.48 |
| Charlson index, mean (SD) | 1.5 (1.0) | 1.7 (0.8) | 0.3 |
CAD, coronary artery disease; IQR, interquartile range; SD, standard deviation.
Patients with SID were less likely to present with ST-elevation MI (39.6% vs. 53.8%, respectively P = 0.04), but had similar rates of revascularization during their index hospitalization, including percutaneous coronary intervention and coronary artery bypass graft surgery (Table 3). We observed that patients with inflammatory disease were less likely to be prescribed aspirin (88% vs. 95%, P = 0.049) or a statin (76% vs. 89%, P = 0.008) upon discharge when compared with the rest of the cohort. There was no significant difference in the prescription rates of angiotensin-converting enzyme inhibitors, beta-blockers, or P2Y12 inhibitors on discharge (Table 3).
Table 3.
Cardiovascular biomarkers and outcomes during index type 1 MI admission between patients with systemic inflammatory disease and controls
| Individuals without systemic inflammatory disease (n = 2044) | Individuals with systemic inflammatory disease (n = 53) | P-value | |
|---|---|---|---|
| Laboratory values, median (IQR) | |||
| Total cholesterol (mg/dL) | 187 (158–218) | 185 (148–229) | 0.71 |
| Triglycerides (mg/dL) | 149 (102–223) | 164 (130–274) | 0.04 |
| HDL-C (mg/dL) | 36 (30–42) | 35.0 (28–39) | 0.072 |
| LDL-C (mg/dL) | 116 (91–142) | 110 (79–149) | 0.5 |
| Max troponin | 42.2 (10.7–152) | 14.2 (3.8–57) | 0.003 |
| Creatinine (mg/dL) | 1.0 (0.9–1.1) | 1.0 (0.9–1.2) | 0.71 |
| Discharge medications,an (%) | |||
| Aspirin | 1898 (94.7%) | 43 (87.8%) | 0.04 |
| Statin therapy | 1793 (89.4%) | 37 (75.5%) | 0.002 |
| P2Y12 inhibitor | 1640 (81.8%) | 36 (73.5%) | 0.14 |
| Beta-blocker | 1834 (91.5%) | 41 (83.7%) | 0.06 |
| ACE inhibitor/ARB | 1240 (61.8%) | 27 (55.1%) | 0.34 |
| Outcomes, n (%) | |||
| Cardiac cath | 1923 (94.1%) | 48 (90.6%) | 0.29 |
| Coronary revascularization | 1690 (82.7%) | 45 (84.9%) | 0.67 |
| CABG | 199 (9.7%) | 7 (13.2%) | 0.4 |
ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL, low-density lipoprotein cholesterol.
Shown are admission labs with median values with IQR except for troponin. Troponin was standardized by the assay upper limit of normal and then a maximum median value is shown.20 Lipid values reflect >85% of individuals without SID and n = 49 with SID.
Discharge medications reflect 2005 patient’s without SID that were discharged and 49 patients with SID that were discharged.
Cardiovascular outcomes and all-cause mortality
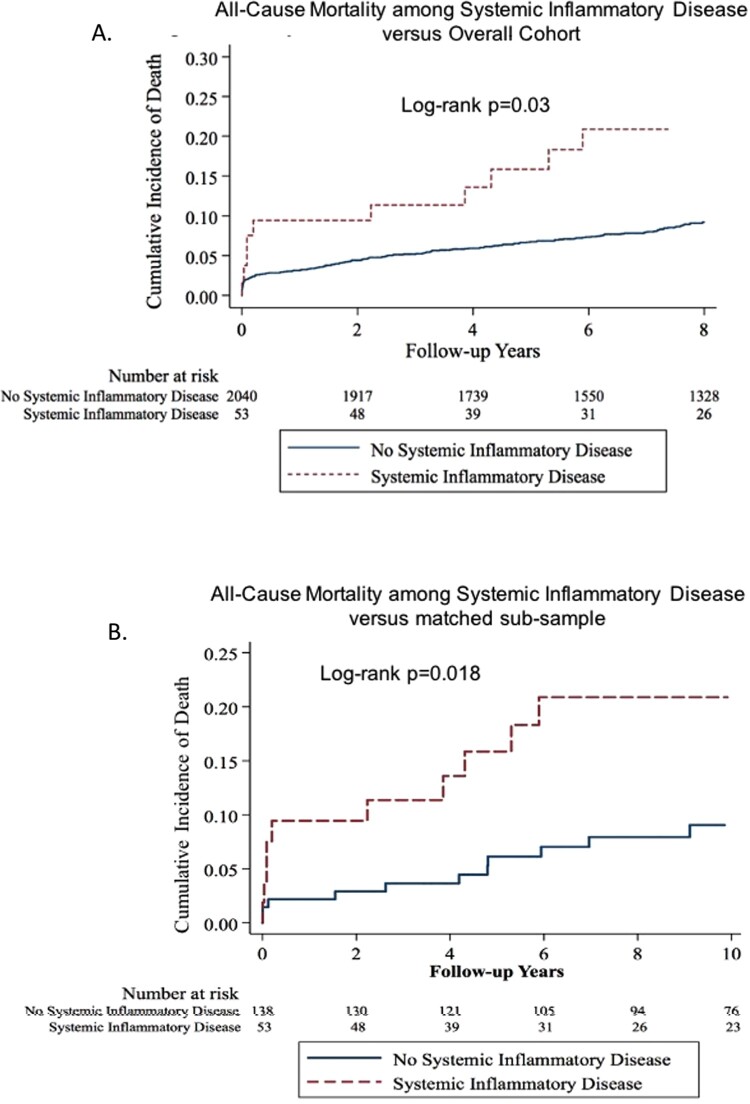
Over a median follow-up 11.2 years, 11 (20.8%) of the 53 with SID died as compared with 243 (11.9%) of the 2044 individuals without a diagnosis of SID at or before their index MI (P = 0.083). Within the full cohort, the unadjusted HR for death with a diagnosis of SID at or before the index MI was 1.95 (95% CI 1.07–3.57, P = 0.030) ) (Figure 2A), which remained significant after adjusting for eGFR and LOS with an adjusted HR 1.86 [adjusted HR of 1.86 (95% CI 1.02–3.42, P = 0.044].
Figure 2.
(A) Kaplan–Meier curves of all-cause mortality in patients with systemic inflammatory disease compared with the full-cohort (log-rank P = 0.03). (B) Kaplan–Meier curves of all-cause mortality in patients with systemic inflammatory disease compared with a matched cohort (n = 138) (log-rank P = 0.018).
Similar findings were observed when the SID cohort was compared with the matched subsample of 138 individuals matched on the basis of age, sex, and key CV risk factors: 11 (20.8%) of the 53 with SID died compared with 12 (8.7%) of the 138 individuals without SID (P = 0.027). The corresponding HR of a diagnosis of SID on all-cause mortality was 2.68 (95% CI 1.18–6.07, P = 0.018) ) (Figure 2B). These results remained similarly robust after adjusting for eGFR and LOS, with an adjusted HR for the matched sub-sample of 2.41 (95% CI 1.04–5.61, P = 0.041), as shown in Table 4.
Table 4.
Univariate and multivariate model for all-cause mortality comparing patients with systemic inflammatory diseases to matched controls
| Model | HR (95% CI) | P-value |
|---|---|---|
| Univariate: matched sub-sample (n = 138) vs. SID (n = 53) | 2.67 (1.18–6.07) | 0.018 |
| Length of stay | 2.74 (1.19–6.27) | 0.017 |
| eGFR | 2.35 (1.03–5.40) | 0.043 |
| Final model: length of stay and eGFR | 2.41 (1.04–5.61) | 0.041 |
Shown is the Cox proportional model among SID compared to the sub-group matched on age, gender, HTN, DM, and tobacco use.
eGFR, estimated glomerular filtration rate; SID, systemic inflammatory disease.
Assessment of relevant biomarkers
Systemic inflammation can alter lipid levels, including the well-described paradoxical increase in LDL-C levels with a reduction in inflammation in RA patients.23,24 We examined the lipid levels obtained during the index hospitalization. Patients with systemic inflammatory conditions had similar median levels of total cholesterol, LDL cholesterol, and triglycerides, and a trend towards higher triglyceride values [164 mg/dL (130–274) vs. 149 mg/dL (102–223), P = 0.04] compared to the whole cohort without SID. Cardiac injury was assessed based on maximum troponin value first standardized by the assay upper limit of normal obtained during in-patient admission which was lower among patients with systemic inflammatory conditions compared with the remainder of the cohort [14.2 (3.8–57) vs. 42.2 (10.6–152), P = 0.003]. Importantly, given the known renal manifestations of many systemic inflammatory conditions, baseline renal function (eGFR) at the time of index MI was similar between the groups (Table 3).
Discussion
Autoimmune SIDs are associated with excess CV risk although limited data exist on adults who experience an MI at a young age. Our findings extend prior observations as we provide the first study, to our knowledge, to determine the prevalence and prognostic value of SID among adults who experience an MI at young age. Among a large cohort of patients with premature MI, approximately 2.5% had evidence of a SID, and these patients experienced higher all-cause mortality, even when compared with patients who had a similar CV risk factor profile. One possible mechanism that links SID with higher rates of mortality after an MI is ongoing systemic inflammation and a dysregulated immune response, including both adaptative and innate immunity, which impairs the normal healing response.25–28 Indeed, recent data in the general population from the Colchicine Cardiovascular Outcomes Trial (COLCOT) suggests that reducing inflammation with colchicine in the post-MI period reduces rates of major CV events.29
Despite the enhanced CV risk associated with systemic inflammatory disorders, it is known that the 10-year ASCVD risk score underestimates the true risk.10,30,31 Inflammatory disease-specific CV risk calculators have been proposed; however, these risk scores do not perform better than the current ACC/AHA ASCVD risk calculator and are not routinely used in general practice.32,33 Our data demonstrate a similar distribution of ASCVD risk scores when comparing the patients with SID with the remainder of the cohort, despite higher overall mortality. A better understanding of the risk factors and outcomes among young adults with systemic inflammatory conditions is needed, since age remains the dominant risk factor for 10-year ASCVD risk prediction, and as a result, most patients with SID who experienced MI at a young age would not have been eligible for primary prevention statin therapy based on currently guidelines, as is also the case in the large population of patients who experienced an MI at a young age.34
Prescription of secondary prevention CV medications at discharge after an acute coronary syndrome has been shown to reduce morbidity and mortality. Our finding that patients with systemic inflammatory conditions were less likely to be prescribed aspirin and statin on discharge was surprising. The reason for this discrepancy is not known and should be explored in future studies. Patients with systemic inflammatory conditions are often on baseline immunosuppression, and whether the difference in discharge prescription rate or secondary preventative CV therapies is a result of concern for drug-drug interactions or medical complexity of the patient is not known.
Lipid levels may be falsely lowered at the time of acute MI, and it is possible that these values could be a less accurate representation of steady state; however, this has been examined in several studies, and a large study did not find a clinical meaningful change between baseline lipid profile and that during an acute coronary syndrome.35,36 In addition, patients with RA have been noted to exhibit the ‘lipid paradox’, where LDL is paradoxically lower during periods of high inflammation, which is likely similarly true in other SID conditions. Psoriasis is the most common systemic inflammatory skin disorder and it is not surprising that this contributed the highest portion of the cohort. Despite being a known biomarker of elevated CV risk even in patients without SIDs,37 high-sensitivity C-reactive protein (hsCRP) was measured in only a small minority of the cohort as it was not routinely checked as part of care. The absence of information on disease severity, including hsCRP or disease-specific severity index scores, is a limitation of the study. Further work that incudes larger scale, disease-specific prospective studies will be needed to determine the specific features of disease severity and treatment that are relevant to prediction of CV events at a young age.
The primary limitations of the study include a small sample size that is retrospective in nature. However, this retrospective cohort design is ideal to examine less frequent conditions, such as systemic inflammatory conditions and MI in young individuals. Given the retrospective nature of this study and the small sample size of young adults with SID, we were not able to control for all baseline characteristics or discharge medications and thus focused on the most significant and clinically relevant characteristics. Nonetheless, we believe these findings are of importance and to our knowledge, is the first study to specifically examine a cohort of patients with underlying systemic inflammatory conditions who experience an MI at a young age. In addition, instead of relying on billing or other coded information, the small sample size allowed our team to perform a manual review of all notes within the EMR prior to, during, and at discharge from the index admission to adjudicate the presence of a systemic inflammatory disorder and the presence of other CV risk factors. While we do not have follow-up for recurrent CV events in this cohort, the finding of increased all-cause death is a highly robust and meaningful outcome in this young population. Because our cohort was limited to individuals who experienced an MI, we were not able to determine the prevalence of systemic inflammatory conditions in the at-risk population and, therefore, were unable to provide data on the relative risk of systemic inflammatory conditions for causing a first MI.
In conclusion, systemic inflammatory conditions were present in 2.5% of patients with an MI at age <50 years and were associated with worse long-term all-cause mortality over a median follow-up of 11.2 years, a disparity not fully explained by higher rates of typical CV risk factors. Furthermore, these patients were less likely to be prescribed guideline-based secondary prevention aspirin and statin therapy after acute MI. These findings highlight the need for focused attention to SIDs in CV risk assessment and for implementation of more aggressive preventive therapies to reduce the burden of adverse CV events in young patients with underlying SID.
Funding
ThThis work was supported by the NHLBI T32 HL094301 (to B.W., A.N.B., S.D.), NHLBI T32 HL007604 (J.M.B.).is work was supported by the NHLBI T32 HL094301 (to B.W., A.N.B., S.D.), T32 HL007604 (J.M.B.).
Conflict of interest: Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, MyoKardia, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr. Di Carli reports grants from Gilead Sciences and Spectrum Dynamics, and personal consulting fees from Janssen and Bayer, outside the submitted work. Dr. Blankstein reports research support from Amgen Inc. and Astellas Inc.
Data availability
Data may be available upon request, subject to institutional policies
References
- 1. Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM.. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol 2012;148:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Pratt JE, Tracy RP, Kuller LH, Manzi S.. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum 2004;50:151–159. [DOI] [PubMed] [Google Scholar]
- 3. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM.. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829–835. [DOI] [PubMed] [Google Scholar]
- 4. Prey S, Paul C, Bronsard V, Puzenat E, Gourraud P-A, Aractingi S, Aubin F, Bagot M, Cribier B, Joly P, Jullien D, Le Maitre M, Richard-Lallemand M-A, Ortonne J-P.. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol 2010;24:23–30. [DOI] [PubMed] [Google Scholar]
- 5. Bartoloni E, Alunno A, Gerli R.. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat Rev Cardiol 2018;15:33–44. [DOI] [PubMed] [Google Scholar]
- 6. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB.. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735. [DOI] [PubMed] [Google Scholar]
- 7. Egeberg A, Skov L, Joshi AA, Mallbris L, Gislason GH, Wu JJ, Rodante J, Lerman JB, Ahlman MA, Gelfand JM, Mehta NN.. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol 2017;77:650–656.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D.. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–1529. [DOI] [PubMed] [Google Scholar]
- 9. Meune C, Touzé E, Trinquart L, Allanore Y.. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–1313. [DOI] [PubMed] [Google Scholar]
- 10. Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, Berger RD, Côte R, Grover SA, Fortin PR, Clarke AE, Senécal J-L.. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–2337. [DOI] [PubMed] [Google Scholar]
- 11.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. et al. et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM.. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol 2014;64:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper GS, Stroehla BC.. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119–125. [DOI] [PubMed] [Google Scholar]
- 14. Henseler T, Christophers E.. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985;13:450–456. [DOI] [PubMed] [Google Scholar]
- 15. Ballou SP, Khan MA, Kushner I.. Clinical features of systemic lupus erythematosus: differences related to race and age of onset. Arthritis Rheum 1982;25:55–60. [DOI] [PubMed] [Google Scholar]
- 16. Amador-Patarroyo MJ, Rodriguez-Rodriguez A, Montoya-Ortiz G.. How does age at onset influence the outcome of autoimmune diseases? Autoimmune Dis 2012;2012:1–7. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh A, Collins B, Qamar A, Gupta A, Fatima A, Divakaran S, Klein J, Hainer J, Jarolim P, Shah RV, Nasir K, Di Carli MF, Bhatt DL, Blankstein R.. Study of young patients with myocardial infarction: Design and rationale of the YOUNG-MI Registry. Clin Cardiol 2017;40:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand J-P, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon J-L, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S.. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 20. Singh A, Gupta A, DeFilippis EM, Qamar A, Biery DW, Almarzooq Z, Collins B, Fatima A, Jackson C, Galazka P, Ramsis M, Pipilas DC, Divakaran S, Cawley M, Hainer J, Klein J, Jarolim P, Nasir K, Januzzi JL, Di Carli MF, Bhatt DL, Blankstein R.. Cardiovascular mortality after type 1 and type 2 myocardial infarction in young adults. J Am Coll Cardiol 2020;75:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malmasi S, Sandor NL, Hosomura N, Goldberg M, Skentzos S, Turchin A.. Canary: an NLP platform for clinicians and researchers. Appl Clin Inform 2017;08:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger AK, Duval S, Jacobs DR, Barber C, Vazquez G, Lee S, Luepker RV.. Relation of length of hospital stay in acute myocardial infarction to post-discharge mortality. Am J Cardiol 2008;101:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, Shadick NS, Mehta NN.. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc 2015;4:e001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, Gabriel SE.. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011;70:482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofmann U, Frantz S.. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 2015;116:354–367. [DOI] [PubMed] [Google Scholar]
- 26. Abou-Raya A, Abou-Raya S.. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev 2006;5:331–337. [DOI] [PubMed] [Google Scholar]
- 27. Santos-Zas I, Lemarié J, Tedgui A, Ait-Oufella H.. Adaptive immune responses contribute to post-ischemic cardiac remodeling. Front Cardiovasc Med 2019;5:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ziegler L, Frumento P, Wallén H, de Faire U, Gigante B.. The predictive role of interleukin 6 trans-signalling in middle-aged men and women at low-intermediate risk of cardiovascular events. Eur J Prev Cardiol 2020;27:122–129. [DOI] [PubMed] [Google Scholar]
- 29. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 30. Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE.. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol 2012;110:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen J, Lam SH, Shang Q, Wong C-K, Li EK, Wong P, Kun EW, Cheng IT, Li M, Li TK, Zhu TY, Lee JJ-W, Chang M, Lee AP-W, Tam L-S.. Underestimation of risk of carotid subclinical atherosclerosis by cardiovascular risk scores in patients with psoriatic arthritis. J Rheumatol 2018;45:218–226. [DOI] [PubMed] [Google Scholar]
- 32. Crowson CS, Gabriel SE, Semb AG, van Riel PLCM, Karpouzas G, Dessein PH, Hitchon C, Pascual-Ramos V, Kitas GD, Douglas K, Sandoo A, Rollefstad S, Ikdahl E, Kvien TK, Arts E, Fransen J, Tsang L, El-Gabalawy H, Yáñez IC, Matteson EL, Rantapää-Dahlqvist S, Wållberg-Jonsson S, Innala L, Sfikakis PP, Zampeli E, Gonzalez-Gay MA, Corrales A, van de Laar M, Vonkeman H, Meek I, Husni E, Overman R, Colunga I, Galarza D; Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford) 2017;56:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drosos GC, Konstantonis G, Sfikakis PP, Tektonidou MG.. Underperformance of clinical risk scores in identifying vascular ultrasound-based high cardiovascular risk in systemic lupus erythematosus. Eur J Prev Cardiol 2020; [DOI] [PubMed] [Google Scholar]
- 34. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, Plutzky J, Cannon CP, Nasir K, Di Carli MF, Bhatt DL, Blankstein R.. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG-MI Registry. J Am Coll Cardiol 2018;71:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fyfe T, Baxter RH, Cochran KM, Booth EM.. Plasma-lipid changes after myocardial infarction. Lancet 1971;298:997–1001. [DOI] [PubMed] [Google Scholar]
- 36. Pitt B, Loscalzo J, Ycas J, Raichlen JS.. Lipid levels after acute coronary syndromes. J Am Coll Cardiol 2008;51:1440–1445. [DOI] [PubMed] [Google Scholar]
- 37. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ.. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be available upon request, subject to institutional policies