Abstract
Purpose:
Our primary purpose was to calculate the minimal clinically important difference (MCID) for the PROMIS Upper Extremity (UE) Computer Adaptive Test (CAT) version 2.0 (v2.0) for a non-shoulder hand and upper extremity population. Secondarily, we calculated the PROMIS Physical Function (PF) CAT v2.0 and the QuickDASH MCID.
Methods:
Adult patients treated by one of five fellowship-trained hand surgeons between March 2015 and September 2019 at an academic tertiary institution were identified. The PROMIS UE CAT v2.0, PROMIS PF CAT v2.0, and QuickDASH were collected via tablet computer. Inclusion required response to at least one of the instruments both at baseline and follow-up (6 ± 4 weeks), and a response to the anchor question “Compared to your first evaluation at the University Orthopaedic Center, how would you describe your physical function level now?”. An additional anchor question assessing treatment-related improvement was also asked. MCID was calculated using an anchor-based approach using the mean change groups reporting no change and slight change for both anchor questions, and with the 1/2 standard deviation (SD) method.
Results:
Of 2106 participants, mean age was 48 ± 17 years, 53% were female, and 53% were recovering from surgery. Of these patients, 381 completed the PROMISE UE CAT v2.0, 497 completed the PROMISE PF CAT v2.0, and 2018 completed the QuickDASH. The score change between baseline and follow-up was significantly different between anchor groups for both anchor-based MCID calculations. Anchor-based MCID values were 3.0 to 4.0 for the UE CAT, 2.1 to 3.6 for the PF CAT, and 10.3 for the QuickDASH. MCID values per the 1/2 SD method were 4.1, 4.1, and 10.2, respectively.
Conclusions:
We propose MCID ranges of 3.0 to 4.1 for the PROMIS UE CAT v2.0, and 2.1 to 4.1 for the PROMIS PF CAT v2.0. The observed QuickDASH MCID values (10.2 to 10.3) are within the range of previously published values.
Clinical Relevance Statement:
These MCID estimates will aid in interpreting clinical outcomes and in powering clinical studies.
Keywords: Minimal clinically important difference (MCID), PROMIS, Upper extremity (UE) CAT, Physical Function (PF) CAT, QuickDASH/qDASH, Version 2.0
Introduction:
Collection of patient-reported outcome measures (PROMs) has recently become more common, including within the subspecialty of hand and upper extremity surgery. This trend is related to efforts to better understand the quality of health care delivered in the United States.1–5 With the goal of standardizing and simplifying the administration, interpretation, and utility of PROMs, the National Institutes of Health (NIH) developed the Patient-Reported Outcomes Measurement Information System (PROMIS) platform. In the realm of hand and upper extremity surgery, the PROMIS Upper Extremity (UE) CAT6–11 and the PROMIS Physical Function (PF)6,8,9,12,13 CAT have recently been used with increasing frequency to measure upper extremity function. The origin of the abbreviated version of the Disabilities of the Arm, Hand, and Shoulder (QuickDASH) predates the PROMIS instruments, but remains a commonly used instrument to measure upper extremity disability and function among hand and upper extremity populations as well.9,14–19
The QuickDASH is static in that it was designed using a fixed number of questions without the capacity to accommodate updates,14 whereas PROMIS instruments may be updated to potentially improve performance through refinement of their question banks and CAT algorithms. As an example, the performance characteristics of the PROMIS UE CAT were slightly improved for a general hand and upper extremity population through a recent update from version 1.2 to the current version (v2.0).11,20 Specifically, the ceiling and floor effects of 10.8% and 1.6%, respectively, for v1.26 were improved to 6.9% and 1%, respectively, with the v2.0 update.11 However, unlike for the PROMIS PF CAT in which scores on different versions are interchangeable, one downside of this upgrade to the PROMIS UE CAT is that scores on version 1.2 and version 2.0 are not interchangeable.21
The minimal clinically important difference (MCID) reflects the smallest change in a PROM score that a patient may perceive as beneficial. Considering the MCID is important when interpreting whether changes in PROM scores are clinically relevant at a population level, or when determining whether a statistically significant difference in scores between two treatment groups is clinically meaningful. With a recent increased emphasis upon patient-centered care,22–24 determination of the MCID for the current version of the PROMIS UE CAT (version 2.0) will be useful to inform sample size calculations in the design clinical studies and will be important for clinicians when interpreting the results of outcomes studies.
The primary purpose of this study was to determine the MCID of the PROMIS UE CAT version 2.0 for a non-shoulder hand and upper extremity patient population. Our secondary purpose was to provide MCID estimates for the PROMIS PF CAT version 2.0, as well as the QuickDASH.
Methods:
This retrospective cohort study was approved by the Institutional Review Board at our institution. We included adult patients (≥ 18 years of age) evaluated by one of five fellowship-trained orthopaedic hand surgeons at a tertiary academic medical center between March 2015 and September 2019. The QuickDASH was administered for all clinic patients at each visit for the whole study period, whereas we initiated collection of the PROMIS UE CAT v2.0 in April 2017. Patients evaluated for non-shoulder hand and upper extremity pathology were initially identified by an electronic data query for visits performed by each of the five surgeons. Manual chart review of clinical notes was performed to extract baseline patient characteristics, and to record which patients were recovering from a surgery performed within one year prior to the baseline visit or between the baseline and follow-up visits. The baseline visit was defined as the first visit with a recorded questionnaire within the study period. Similarly, we recorded which patients had received an injection within three months of the baseline visit, or between the baseline and follow-up visits. Patients seen for pathology of the lower extremity (e.g. neuroma management or lower extremity nerve decompression) or the shoulder were excluded.
Patients were asked to complete outcomes instruments using a tablet computer. Instruments were completed by patients in clinic, and/or in the preoperative holding area, which included the PROMIS UE CAT v2.0, the PROMIS PF CAT v2.0, and the abbreviated version of the Disabilities of the Arm, Shoulder, and Hand (QuickDASH). In addition, responses to two anchor questions are routinely collected at all follow-up appointments for all patients in our practice. Anchor Question 1 asks ‘Compared to your FIRST EVALUATION at the University Orthopaedic Center, how would you describe your physical function now?’. Possible responses on a 7-item Likert scale include “Much worse”, “Worse”, “Slightly worse”, “No change”, “Slightly improved”, “Improved”, or “Much improved”. Anchor Question 2 asks “How much relief and/or improvement do you feel you have experienced as a result of your treatment?”. Responses are limited to one of the following items on a Likert scale: “Unsure”, “My symptoms are worse”, “No relief/improvement”, “Little relief/improvement”, “Some relief/improvement”, or “Great relief/improvement”. These outcomes and anchor question data were integrated into our institution’s medical record via a secure wireless interface in an automated fashion. Patients with a response at baseline and at follow-up of 6 ± 4 weeks for the QuickDASH, plus a response to Anchor Question 1, were included. This relatively short follow-up period was chosen for multiple reasons: 1) clinically, this would typically capture the majority of patients’ first and second postoperative follow-up visits, 2) smaller levels of incremental improvement may occur between a shorter interval as compared to a longer interval (e.g. at 1 year follow-up, most patients would likely have improved more than a minimal amount), and 3) this time frame has been used previously for anchor-based MCID calculations.25 Patients with baseline and follow-up responses for the PROMIS UE CAT v2.0 or PROMIS PF CAT v2.0, plus a response to either anchor question, were recorded. For patients with multiple follow-up visits, the scores from the visit closest to 6 weeks from the baseline visit was used. Each individual patient was allowed to contribute only one data point to the anchor-based MCID calculations (e.g. if a patient was seen at a new patient visit then six weeks later, then seen four months thereafter and again 6 weeks after that, only the first pair of visits was considered).
Statistical analysis included calculation of descriptive statistics for patient baseline characteristics. Categorical variables were compared between anchor question groups reporting no change versus minimal change using Chi-square or Fisher Exact tests, and continuous variables were compared with t-tests. Outcomes scores for each instrument were summarized both at baseline and at follow-up, then compared using the Wilcoxon rank-sum test. Similarly, score changes for pertinent anchor groups were also compared with the Wilcoxon rank-sum test. Categorical data were compared using the Fisher exact or Chi-square tests, as indicted. All pertinent tests were 2-sided, and a significance level of 0.05 was used throughout.
Anchor-based MCID estimates were calculated for each instrument (UE CAT, PF CAT, and QuickDASH) using Anchor Question 1 as the mean difference between the “No change” group and the combined group that was comprised of patients reporting “Slightly improved” and “Improved”. Anchor-based MCID estimates were also calculated for each instrument using Anchor Question 2 as the mean difference between the “No relief/improvement” group and the combined group of patients reporting “Little relief/improvement” and “Some relief/improvement”. MCID estimates using the 1/2 standard deviation (SD) method were also calculated using the score change, as described previously.17,26 Although many MCID calculation methods exist including distribution-, anchor-, or opinion-based methods,27,28 we opted to use the anchor-based approach because this calculation method directly takes into account patient input on rating meaningful change. We also reported MCID estimates using the 1/2 SD method given its widespread use across many different fields of medicine.26,29–31 The specific formulae used for anchor-based and 1/2 SD MCID calculations are provided in Appendix I.
Results:
The initial electronic search identified 3294 patients that had completed baseline and follow-up PROMs and had responded to Anchor Question 1 during the study period. The following exclusions were made following manual chart review: 632 duplicate patients; 386 patients that lacked follow-up in the appropriate time frame; 160 minors; and 9 patients treated for pathology of the shoulder, cervical spine, or lower extremity.
The final cohort was comprised of 2,106 patients of which 381 completed the PROMISE UE CAT v2.0, 497 completed the PROMISE PF CAT v2.0, and 2018 completed the QuickDASH. Notably, 339 (16%) of the included patients responded to Anchor Question 1 but not Anchor Question 2. Mean age was 48 ± 17 years, and 1113 (53%) were female. Additional demographic data, including comparisons between groups reporting “No Change” and “Slightly Improved”/“Improved” on Anchor Question 1, are provided in Table 1.
Table 1.
Baseline Patient Characteristics
| Variable | Sub Category | # Missing | Summary | P-valuea | Statistical Test Useda | ||
|---|---|---|---|---|---|---|---|
| Entire Cohort N = 2106 | ‘Slightly Improved’ + ‘Improved’ Group for Anchor Question 1 N=1093 | ‘No Change’ Group for Anchor Question 1 N=533 | |||||
| Age (years) | Mean (SD) | 0 | 47.5 (16.9) | 46.7 (17.3) | 48.7 (16.4) | <0.05 | t-test |
| - Median (IQR) | - | 48 (33, 61) | 47 (32, 60) | 48 (36, 62) | - | - | |
| - Range | - | (18, 91) | (18, 91) | (18, 90) | - | - | |
| Provider | Provider A | 0 | 460 (22%) | 204 (18.7%) | 147 (26.6%) | <0.05 | Chi-square |
| Provider B | - | 554 (26%) | 318 (29.1%) | 136 (24.6%) | - | - | |
| Provider C | - | 285 (14%) | 130 (11.9%) | 87 (15.7%) | - | - | |
| Provider D | - | 217 (10%) | 117 (10.7%) | 45 (8.1%) | - | - | |
| Provider E | - | 543 (26%) | 291 (26.6%) | 130 (23.5%) | - | - | |
| Provider F | - | 47 (2%) | 33 (3%) | 8 (1.4%) | - | - | |
| Sex | Female | 0 | 1113 (53%) | 551 (50.4%) | 292 (52.8%) | 0.36 | Chi-square |
| Male | - | 993 (47%) | 542 (49.6%) | 261 (47.2%) | - | - | |
| Race | American Indian or Alaska Native | 2 | 22 (1%) | 12 (1.1%) | 6 (1.1%) | 0.63 | Fisher Exact |
| Asian | - | 37 (2%) | 20 (1.8%) | 12 (2.2%) | - | - | |
| Black or African American | - | 32 (2%) | 14 (1.3%) | 11 (2%) | - | - | |
| Choose not to disclose | - | 20 (1%) | 6 (0.5%) | 6 (1.1%) | - | - | |
| Native Hawaiian / Other Pacific Islander | - | 18 (1%) | 11 (1%) | 3 (0.5%) | - | - | |
| Other | - | 182 (9%) | 106 (9.7%) | 45 (8.1%) | - | - | |
| Unknown | - | 1 (0%) | 1 (0.1%) | 0 (0%) | - | - | |
| White or Caucasian | - | 1792 (85%) | 921 (84.4%) | 470 (85%) | - | - | |
| Marital Status | Divorced | 0 | 138 (7%) | 76 (7%) | 33 (6%) | 0.58 | Chi-square |
| Legally Separated | - | 20 (1%) | 9 (0.8%) | 6 (1.1%) | - | - | |
| Life Partner/Domestic Partner | - | 59 (3%) | 35 (3.2%) | 12 (2.2%) | - | - | |
| Married | - | 1183 (56%) | 592 (54.2%) | 328 (59.3%) | - | - | |
| Other | - | 28 (1%) | 17 (1.6%) | 7 (1.3%) | - | - | |
| Single | - | 573 (27%) | 311 (28.5%) | 139 (25.1%) | - | - | |
| Unknown | - | 32 (2%) | 17 (1.6%) | 9 (1.6%) | - | - | |
| Widowed | - | 73 (3%) | 36 (3.3%) | 19 (3.4%) | - | - | |
| Alcohol Use | Never | 190 | 4 (0%) | 1 (0.1%) | 2 (0.4%) | 0.20 | Fisher Exact |
| No | - | 893 (47%) | 464 (46.9%) | 231 (45.4%) | - | - | |
| Not Asked | - | 247 (13%) | 115 (11.6%) | 72 (14.1%) | - | - | |
| Not Currently | - | 1 (0%) | 0 (0%) | 1 (0.2%) | - | - | |
| Yes | - | 771 (40%) | 410 (41.4%) | 203 (39.9%) | - | - | |
| Tobacco Use | Never | 80 | 1430 (71%) | 738 (70%) | 367 (69.1%) | 0.16 | Fisher Exact |
| Not Asked | - | 3 (0%) | 3 (0.3%) | 0 (0%) | - | - | |
| Passive | - | 5 (0%) | 4 (0.4%) | 0 (0%) | - | - | |
| Quit | - | 338 (17%) | 162 (15.4%) | 100 (18.8%) | - | - | |
| Yes | - | 250 (12%) | 147 (13.9%) | 64 (12.1%) | - | - | |
Comparisons were made between the ‘No Change’ group on Anchor Question 1 and the combined ‘Slightly Improved’ and ‘Improved’ groups on Anchor Question 1.
Out of the entire cohort, 53% of patients (1118/2106) were recovering from surgery. Of those recovering from surgery, 78% (872/1118) underwent surgery between the baseline and follow-up visits, 27.8% (311/1118) underwent surgery within the 1 year prior to the baseline visit, and 5.8% (65/1118) underwent surgery both before the baseline visit and between their baseline and follow-up visits. The most common surgical procedures were carpal tunnel release (21% of the cohort), mass excision (11%), and distal radius open reduction internal fixation (6%). A summary of surgical procedures performed on patients within 1 year of the baseline visit, or between baseline and follow-up visits, is provided in Table 2. Of the 47% (988/2106) of patients treated nonoperatively, 24% (239/988) received a corticosteroid injection within three months of the baseline visit, or between the baseline and follow-up visits.
Table 2.
Summary of Surgical Procedures
| Procedure | n | Percent |
|---|---|---|
| Carpal tunnel release (open or endoscopic) | 274 | 20.8 |
| Mass or foreign body excision | 143 | 10.8 |
| Distal radius fracture operative fixation | 84 | 6.4 |
| Trigger digit release | 78 | 5.9 |
| Ligament reconstruction and tendon interposition | 65 | 4.9 |
| Phalanx or interphalangeal joint fracture operative fixation | 56 | 4.2 |
| Tendon transfer, repair, or lengthening | 51 | 3.9 |
| Cubital tunnel release with or without transposition | 46 | 3.5 |
| Amputation or replantation of digit(s) | 31 | 2.4 |
| Metacarpal or metacarpal phalangeal joint fracture operative fixation | 30 | 2.3 |
| Metacarpophalangeal or interphalangeal joint arthroplasty or fusion | 27 | 2.0 |
| Removal of hardware | 27 | 2.0 |
| Fasciectomy, partial palmar or palm only | 26 | 2.0 |
| Extensive reconstruction of upper extremity after crush or gunshot injury | 22 | 1.7 |
| Scaphoid fracture or nonunion operative fixation | 21 | 1.6 |
| Digital ulnar collateral ligament repair | 21 | 1.6 |
| Operative treatment for ulnar impingement and/or TFCC tear | 21 | 1.6 |
| Neuroplasty (major peripheral or digital nerve other than carpal/cubital tunnel) | 20 | 1.5 |
| Irrigation and debridement | 20 | 1.5 |
| Forearm fracture operative fixation | 18 | 1.4 |
| Repair of tendon and nerve and/or artery repair | 16 | 1.2 |
| Carpometacarpal fracture-dislocation operative fixation | 16 | 1.2 |
| Scapholunate ligament repair | 14 | 1.1 |
| Elbow fracture or LCL operative fixation, other than distal humerus | 14 | 1.1 |
| First extensor compartment release | 12 | 0.9 |
| Biceps or triceps repair | 11 | 0.8 |
| Nerve transfer or repair | 11 | 0.8 |
| Digital joint soft tissue repair | 11 | 0.8 |
| Radial head arthroplasty, ORIF, or excision | 10 | 0.8 |
| Carpal excision (partial or complete) | 10 | 0.8 |
| Elbow or wrist total arthroplasty or fusion | 10 | 0.8 |
| ECU centralization, debridement | 9 | 0.7 |
| Humerus operative fixation, distal or midshaft, | 9 | 0.7 |
| Laceration exploration and repair | 7 | 0.5 |
| Epicondylectomy or epicondyle debridement | 7 | 0.5 |
| Bursa excision | 6 | 0.5 |
| Intercarpal fusion | 6 | 0.5 |
| Flap coverage or skin graft | 6 | 0.5 |
| Perilunate operative fixation | 6 | 0.5 |
| Elbow or Wrist Arthroscopy | 5 | 0.4 |
| Fasciotomies | 3 | 0.2 |
| Other* | 18 | 1.4 |
Abbreviations: LCL - Lateral collateral ligament; TFCC - Triangular fibrocartilage complex.
Includes 4+5 extracompartmental artery bone graft to the lunate, biopsy, thrombosis excision, capitate shortening osteotomy, isolated radial styloidectomy, distal radioulnar joint stabilization, external fixator removal, nail ablation, mallet pinning, electrode implant placement, and tenosynovectomy
PROMIS UE CAT, PROMIS PF CAT, and QuickDASH scores all improved significantly between baseline and follow-up visits (p < 0.05 for each comparison; Table 3). Score change between baseline and follow-up is reported for both anchor questions by anchor group in Table 4, along with associated sample sizes. The score change for each instrument was significantly different between “No change” and the combined “Slightly improved” plus “Improved” groups for Anchor Question 1 (p < 0.05). Similarly, the score change for each instrument was significantly different between “No relief/improvement” and the combined “Slight relief/improvement” and “Some relief/improvement” groups for Anchor Question 2 (p < 0.05).
Table 3.
Summary of Baseline and Follow-Up Scores
| Statistic | Baseline Score | Follow-Up Score | P-value | |
|---|---|---|---|---|
|
PROMIS UE CAT v2.0 (N = 381) |
Mean (SD) | 35.3 (9.9) | 36.8 (9.9) | < 0.05a |
| Median (IQR) | 33.7 (28.0, 40.5) | 35.0 (30.2, 43.0) | - | |
| Range | (14.7, 61) | (14.7, 61) | - | |
|
PROMIS PF CAT v2.0 (N = 497) |
Mean (SD) | 41.9 (9.8) | 43.7 (9.6) | <0.05a |
| Median (IQR) | 41.3 (34.6, 48.2) | 42.9 (36.6, 50.1) | - | |
| Range | (19.1, 75.6) | (14.7, 75.6) | - | |
|
QuickDASH (N = 2018) |
Mean (SD) | 45.7 (22.6) | 39.2 (22.0) | <0.05a |
| Median (IQR) | 45.5 (27.3, 63.6) | 36.4 (22.7, 56.8) | - | |
| Range | (0, 100) | (0, 97.7) | - |
Statistical significance was based upon the student’s t-test.
Table 4.
Summary of Score Change by Anchor Group for the Entire Cohort*
| Instrument | Score Change by Anchor Question 1 Group Mean ± SD (N) |
p-value | Score Change by Anchor Question 2 Group Mean ± SD (N) |
p-value | ||
|---|---|---|---|---|---|---|
| No change | ‘Slightly Improved’ & ‘Improved’ | No relief/Improvement | Slight & Some relief/Improvement | |||
| PROMIS UE CAT v2.0 | −0.93 ± 6.53 (86) | 3.09 ± 7.89 (215) | < 0.05 | −1.52 ± 7.82 (70) | 1.47 ± 7.62 (251) | < 0.05 |
| PROMIS PF CAT v2.0 | 0.28 ± 6.97 (132) | 3.92 ± 8.34 (247) | < 0.05 | −0.46 ± 6.83 (117) | 1.59 ± 8.22 (304) | < 0.05 |
| QuickDASH | −2.19 ± 17.42 (533) | −12.45 ± 18.74 (1052) | < 0.05 | 3.52 ± 18.43 (422) | −6.81 ± 19.73 (1270) | < 0.05 |
| Total Number of Patients (N) | 751 | 1514 | - | 609 | 1825 | - |
Outcomes data represent Mean ± SD (N).
Comparisons are made between anchor groups using the Wilcoxon rank-sum test.
MCID estimates are illustrated in Table 5. Specifically, anchor-based MCID estimates using Anchor Question 1 were 4.0 for the UE CAT, 3.6 for the PF CAT, and 10.3 for the QuickDASH. MCID estimates using Anchor Question 2 were 3.0, 2.1, and 10.6, respectively. MCID estimates using the 1/2 SD method were 4.1, 4.1, and 10.2, respectively. These MCID values are summarized in Table 5.
Table 5.
MCID Values
| Anchor Question 1 | Anchor Question 2 | 1/2 SD Method | |
|---|---|---|---|
| PROMIS UE CAT v2.0 |
4.0 (N = 301) |
3.0 (N = 321) |
4.1 (N = 381) |
| PROMIS PF CAT v2.0 |
3.6 (N = 379) |
2.1 (N = 421) |
4.1 (N = 497) |
| QuickDASH |
10.3 (N = 1585) |
10.3 (N = 1692) |
10.2 (N = 2018) |
Discussion:
The main finding is that an estimate range for the MCID of the Patient-Reported Outcomes Measurement Information System (PROMIS) UE CAT version 2.0 in a non-shoulder hand and upper extremity patient population was 3.0 to 4.1. Secondary findings include MCID estimate ranges for the PROMIS PF CAT version 2.0 of 2.1 to 4.1 and QuickDASH, 10.2 to 10.3.
Although we were unable to identify prior studies elucidating the MCID of the PROMIS UE CAT v2.0, several studies have reported MCID estimates for the prior version (v1.2). With the caveat that scores are not interchangeable between v1.2 and v2.0 for the UE CAT,21 estimates in the current hand surgery literature include a value of 3.4 for a carpal tunnel release postoperative cohort using the 1/2 SD method,32 and an anchor-based estimate of 2.1 for a general hand surgery population.25 These values are provided for reference purposes rather than for direct comparison to the findings of the current study. Compared to a rough estimate of 5.0 for the MCID of PROMIS instruments, which can be calculated using the 1/2 standard deviation method and knowledge that their standard deviation is designed to be 10 in a normative population,33 we note that our estimate range is slightly smaller than this crude estimate. Since anchor-based calculation methods directly incorporate patient input regarding a global rating of change, in contrast to the 1/2 SD method, which does not consider patient input on change, it is possible that treatments that afford improvements as low as 3.0 to 4.1 points on the PROMIS UE CAT v2.0 may be clinically relevant at a population level for hand surgery patients. Notably, it is not commonplace to utilize MCID values for individual patients, but rather for populations of patients. Therefore, we would caution against applying these MCID values to individuals, for example in the clinic setting when reviewing outcomes scores over time.
Regarding our secondary findings, MCID estimate ranges observed in the current study for the PROMIS PF CAT v2.0 (2.1 to 4.1) and QuickDASH (10.2 to 10.3) are comparable to estimates in the published literature. Lee and Calfee elucidated an MCID range of 3.5 to 3.9 points for the PROMIS PF CAT among patients with thumb carpometacarpal arthritis using a combination of anchor-based and distribution-based estimates.13 The authors grouped patients with responses to both v1.2 and v2.0 on the PF CAT, as appropriate given the interchangeability of scores between versions of this instrument.21 In a patient sample recovering from nonoperatively managed distal radius fractures, Sandvall et al derived an MCID range of 3.6 to 4.6 on the PROMIS PF CAT (combined versions 1.2 and 2.0).34 Our estimates for the PROMIS PF CAT MCID are also similar to those described among populations beyond hand and upper extremity surgery. Specifically, estimates in the current study are similar to the range of estimates described in a cancer population (range 4 to 6, depending on the method of calculation)35 and are subjectively and slightly lower than the estimate of 4.6 calculated by Kazmers et al for a carpal tunnel release population using the 1/2 SD method.32 Also using the 1/2 SD method, Ho et al calculated an MCID value of 4.2 in an orthopaedic foot and ankle population, which is also subjectively similar to our estimate range.29 Regarding QuickDASH MCID estimates, the range in the current study is consistent with that of previously-reported values for upper extremity patients (8 to 19), albeit on the lower end of the range.17–19,36 Specifically, two studies conducted on an upper extremity physical therapy sample calculated QuickDASH MCID estimates of 8 points19 and 11 points,17 and an anchor-based estimate of 14 was determined by Sorensen et al. in a general hand surgery sample.18 The main purpose of providing PF CAT and QuickDASH MCID estimates in the current study was to allow for comparison to other literature, which demonstrates that these values fall within published ranges and may facilitate interpretation of our primary outcome.
Our study has limitations that warrant discussion. Retrospective identification of patients by procedure code may introduce potential for selection bias. Although no gold standard MCID calculation method exists, MCID may be calculated several ways. This includes distribution-, anchor-, or opinion-based methods, each with their own specific assumptions and various statistical thresholds that can be utilized, which further expands the number of potential ways the MCID may be estimated.27,28 Use of different anchor questions or anchor types may affect MCID values,27 although our results represent estimates from two separate anchor questions. Further, MCID estimates obtained using anchor-based methods may fall within random noise of the instrument, with additional potential effects of recall bias when patients answer anchor questions.37 To this end, we provided 1/2 SD estimates which may reflect the variability in measurement of outcomes with the studied instruments, however, distribution-based methods are limited by the absence of patient input regarding a global rating of improvement. Therefore, the distribution-based estimates may reflect a threshold of random noise in measurement that establishes the floor for the magnitude of potential meaningful change. Taking these factors into consideration, we report a range of MCID estimates for clinical use rather than one distinct value. Also worth mention is the balance between performing a study on a homogenous study population versus performing a study in which the results can be generalized to a wider population. By including a wide spectrum of non-shoulder hand and upper extremity patients, including those managed operatively and nonoperatively, our results may be more generalizable to a similarly wide patient population. However, the generalizability of our results to a shoulder population remains unclear.
In conclusion, we have calculated values for the minimal clinically important difference (MCID) for the PROMIS Upper Extremity CAT version 2.0 for a general non-shoulder hand and upper extremity patient population that received care at a single tertiary academic institution. We have also provided MCID estimates for the PROMIS PF CAT version 2.0 and QuickDASH and have observed that these values are subjectively similar to estimates in the published literature. These MCID estimate ranges may useful when interpreting clinical outcomes for operative and nonoperative hand surgery patients, and for powering prospective outcomes trials.
Acknowledgements:
This investigation was supported by the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
Appendix 1 –
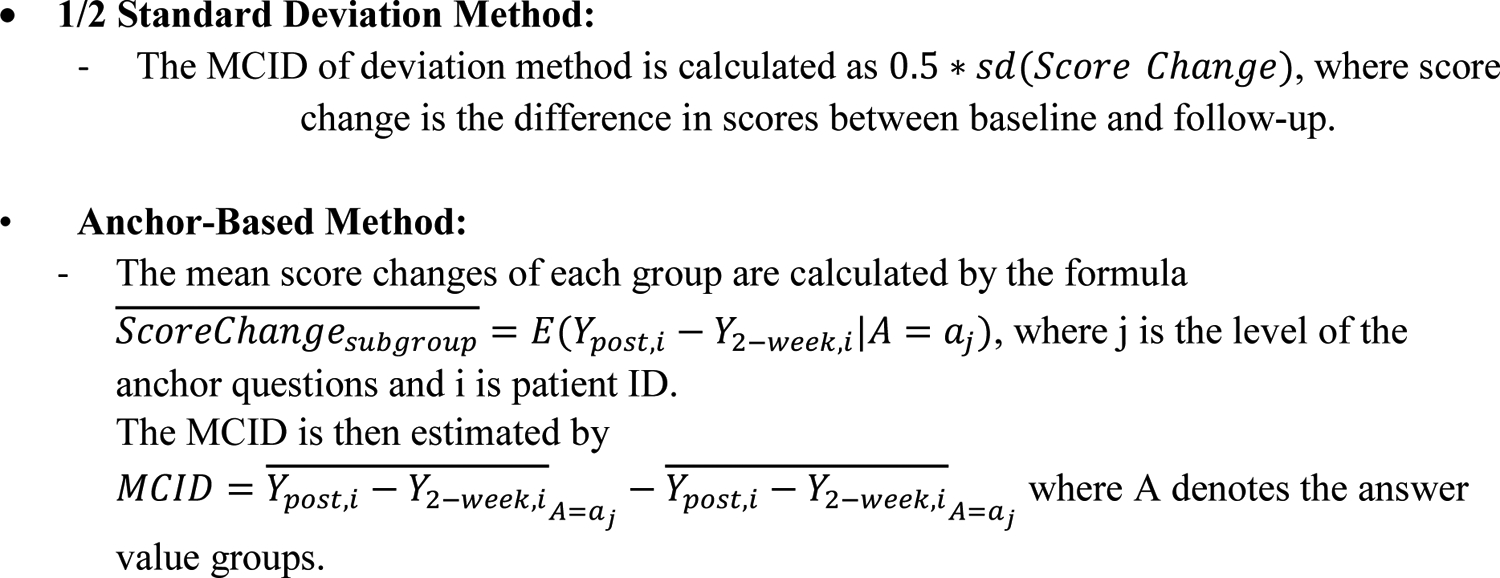
Equations used for MCID calculations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Smith MV, Calfee RP, Baumgarten KM, Brophy RH, Wright RW. Upper extremity-specific measures of disability and outcomes in orthopaedic surgery. J Bone Joint Surg Am. 2012;94(3):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoang-Kim A, Pegreffi F, Moroni A, Ladd A. Measuring wrist and hand function: Common scales and checklists. Injury. 2011;42(3):253–258. [DOI] [PubMed] [Google Scholar]
- 3.Alderman AK, Chung KC. Measuring outcomes in hand surgery. Clin Plast Surg. 2008;35(2):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KC, Burns PB, Sears ED. Outcomes research in hand surgery: Where have we been and where should we go? J Hand Surg Am. 2006;31(8):1373–1379. [DOI] [PubMed] [Google Scholar]
- 5.Hand Surgery Quality C Candidate quality measures for hand surgery. J Hand Surg Am. 2017;42(11):859–866 e853. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann JT, Hung M, Voss MW, Crum AB, Bounsanga J, Tyser AR. Evaluation of the patient-reported outcomes measurement information system upper extremity computer adaptive test. J Hand Surg Am. 2016;41(7):739–744 e734. [DOI] [PubMed] [Google Scholar]
- 7.Beleckas CM, Padovano A, Guattery J, Chamberlain AM, Keener JD, Calfee RP. Performance of patient-reported outcomes measurement information system (promis) upper extremity (ue) versus physical function (pf) computer adaptive tests (cats) in upper extremity clinics. J Hand Surg Am. 2017;42(11):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazmers NH, Hung M, Rane AA, Bounsanga J, Weng C, Tyser AR. Association of physical function, anxiety, and pain interference in nonshoulder upper extremity patients using the promis platform. J Hand Surg Am. 2017;42(10):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the patient-reported outcomes measurement information system. J Hand Surg Am. 2014;39(6):1160–1165. [DOI] [PubMed] [Google Scholar]
- 10.Patient-reported outcomes measurement information system - a brief guide to the promis physical function instruments. 2015. [June 6, 2018]; Available from: https://www.assessmentcenter.net/documents/PROMIS%20Physical%20Function%20Scoring%20Manual.pdf.
- 11.Tyser AR, Hung M, Bounsanga J, Voss MW, Kazmers NH. Evaluation of version 2.0 of the promis upper extremity computer adaptive test in nonshoulder upper extremity patients. J Hand Surg Am. 2019;44(4):267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandvall B, Okoroafor UC, Gerull W, Guattery J, Calfee RP. Minimal clinically important difference for promis physical function in patients with distal radius fractures. J Hand Surg Am. 2019;44(6):454–459 e451. [DOI] [PubMed] [Google Scholar]
- 13.Lee DJ, Calfee RP. The minimal clinically important difference for promis physical function in patients with thumb carpometacarpal arthritis. Hand (N Y). 2019;1558944719880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaton DE, Wright JG, Katz JN, Upper Extremity Collaborative G. Development of the quickdash: Comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046. [DOI] [PubMed] [Google Scholar]
- 15.Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the arm, shoulder, and hand questionnaire (dash) and its short version (quickdash), shoulder pain and disability index (spadi), american shoulder and elbow surgeons (ases) society standardized shoulder assessment form, constant (murley) score (cs), simple shoulder test (sst), oxford shoulder score (oss), shoulder disability questionnaire (sdq), and western ontario shoulder instability index (wosi). Arthritis Care Res (Hoboken). 2011;63 Suppl 11(S174–188. [DOI] [PubMed] [Google Scholar]
- 16.Hung M, Saltzman CL, Greene T, et al. The responsiveness of the promis instruments and the qdash in an upper extremity population. J Patient Rep Outcomes. 2017;1(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polson K, Reid D, McNair PJ, Larmer P. Responsiveness, minimal importance difference and minimal detectable change scores of the shortened disability arm shoulder hand (quickdash) questionnaire. Man Ther. 2010;15(4):404–407. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am. 2013;38(4):641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the arm, shoulder, and hand questionnaire (quickdash) and numeric pain rating scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18(6):920–926. [DOI] [PubMed] [Google Scholar]
- 20.Kaat AJ, Buckenmaier CT 3rd, Cook KF, et al. The expansion and validation of a new upper extremity item bank for the patient-reported outcomes measurement information system(r) (promis). J Patient Rep Outcomes. 2019;3(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northwestern university. Healthmeasures - interpret scores: Promis. 2019. [3/23/2020]; Available from: http://www.healthmeasures.net/score-and-interpret/interpret-scores/promis.
- 22.Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan SS, Kendall E, Sav A, et al. Patient-centered approaches to health care: A systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567–596. [DOI] [PubMed] [Google Scholar]
- 24.Dwamena F, Holmes-Rovner M, Gaulden CM, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12(CD003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazmers NH, Qiu Y, Yoo M, Stephens AR, Tyser AR, Zhang Y. The minimal clinically important difference of the promis and quickdash instruments in a nonshoulder hand and upper extremity patient population. J Hand Surg Am. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 27.Copay AG, Subach BR, Glassman SD, Polly DW Jr., Schuler TC. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J. 2007;7(5):541–546. [DOI] [PubMed] [Google Scholar]
- 28.Cook CE. Clinimetrics corner: The minimal clinically important change score (mcid): A necessary pretense. J Man Manip Ther. 2008;16(4):E82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho B, Houck JR, Flemister AS, et al. Preoperative promis scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37(9):911–918. [DOI] [PubMed] [Google Scholar]
- 30.Katz P, Morris A, Trupin L, Yazdany J, Yelin E. Disability in valued life activities among individuals with systemic lupus erythematosus. Arthritis Rheum. 2008;59(4):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asher AL, Kerezoudis P, Mummaneni PV, et al. Defining the minimum clinically important difference for grade i degenerative lumbar spondylolisthesis: Insights from the quality outcomes database. Neurosurg Focus. 2018;44(1):E2. [DOI] [PubMed] [Google Scholar]
- 32.Kazmers NH, Hung M, Bounsanga J, Voss MW, Howenstein A, Tyser AR. Minimal clinically important difference after carpal tunnel release using the promis platform. J Hand Surg Am. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Healthmeasures - interpret scores: Promis. Northwestern University; 2019. [2/22/2019]; Available from: http://www.healthmeasures.net/score-and-interpret/interpret-scores/promis. [Google Scholar]
- 34.Sandvall B, Okoroafor UC, Gerull W, Guattery J, Calfee RP. Minimal clinically important difference for promis physical function in patients with distal radius fractures. J Hand Surg Am. 2019; [DOI] [PubMed] [Google Scholar]
- 35.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (dash) and its shortened version (quickdash). J Orthop Sports Phys Ther. 2014;44(1):30–39. [DOI] [PubMed] [Google Scholar]
- 37.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. [DOI] [PubMed] [Google Scholar]


