Abstract
The endocannabinoids (eCBs) 2-arachidonoylglycerol and anandamide are among the best studied lipid messengers in the brain. By activating cannabinoid receptors in the central nervous system (CNS), eCBs tune synaptic function thereby influencing a variety of physiological and behavioral processes. Extensive research conducted over the last few decades has considerably enhanced our understanding of the molecular mechanisms and physiological functions of the eCB system. It is now well-established that eCBs are synthesized by postsynaptic neurons and serve as retrograde messengers that suppress neurotransmitter release at central synapses. While the detailed mechanisms by which eCBs gate synaptic function and behavioral processes are relatively well characterized, the mechanisms governing eCB transport at central synapses remain ill defined. Recently, several studies have begun to unravel the mechanisms governing intracellular and intercellular eCB transport. In this Review, we will focus on new advances in the mechanisms of intracellular and synaptic eCB transport in the CNS.
Introduction
Since the discovery of cannabinoid receptors (CBRs) and their endogenous lipid agonists, the endocannabinoid (eCB) system has been the focus of extensive research. It is now well-established that eCBs are ubiquitous retrograde messengers that depress neurotransmitters release at both excitatory and inhibitory synapses in the central nervous system (CNS) (Alger, 2012; Kano, Ohno-Shosaku, Hashimotodani, Uchigashima, & Watanabe, 2009; Katona & Freund, 2012). By modulating synaptic strength and plasticity throughout the CNS, eCBs are implicated in the regulation of an array of cognitive and physiological processes including learning and memory (Kruk-Slomka, Dzik, Budzynska, & Biala, 2017; Mechoulam & Parker, 2013), pain (Paulsen & Burrell, 2019; Schlosburg, Kinsey, & Lichtman, 2009; Zogopoulos, Vasileiou, Patsouris, & Theocharis, 2013), stress (Lutz, Marsicano, Maldonado, & Hillard, 2015; Morena, Patel, Bains, & Hill, 2016), reward (Parsons & Hurd, 2015; Sagheddu, Muntoni, Pistis, & Melis, 2015), and feeding behaviors (Bermudez-Silva, Viveros, McPartland, & Rodriguez de Fonseca, 2010; Tarragon & Moreno, 2019). In addition to controlling synaptic function in the mature brain, eCBs also regulate synaptic maturation and neurogenesis during development (Berghuis et al., 2007; Harkany et al., 2007). Consequently, dysregulation of eCB signaling is implicated in psychiatric (e.g., anxiety and depression) (Hillard, Weinlander, & Stuhr, 2012; Mechoulam & Parker, 2013), neurological (e.g., Epilepsy, Alzheimer disease and Huntington’s disease) (Chen et al., 2012; Cristino, Bisogno, & Di Marzo, 2020; Dvorzhak, Semtner, Faber, & Grantyn, 2013), and neurodevelopment disorders such as autism (Foldy, Malenka, & Sudhof, 2013). Consequently, pharmacological modulation of the eCB system constitutes a potential therapeutic strategy to treat psychiatric and neurological disorders (Cristino et al., 2020).
The eCB system is composed of two G-protein coupled receptors (GPCRs), commonly named cannabinoid type 1 and 2 receptors (CB1Rs and CB2Rs, respectively), their endogenous lipid agonists, 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide or AEA) and the enzymes involved in their synthesis and degradation. CB1Rs are highly expressed throughout the CNS (Egertova & Elphick, 2000; Herkenham et al., 1991; Matsuda, Bonner, & Lolait, 1993) and consequently the behavioral and physiological effects of eCBs are largely mediated by CB1Rs. Activation of these receptors, which are predominantly localized on synaptic terminals, plays a major role in controlling neurotransmitter release and synaptic plasticity (Castillo, Younts, Chavez, & Hashimotodani, 2012). Although CB2Rs are mainly expressed in peripheral tissues and immune cells including brain microglia (Munro, Thomas, & Abu-Shaar, 1993; Van Sickle et al., 2005), some evidence suggests neuronal CB2R expression in discrete brain areas (Jordan & Xi, 2019; Liu et al., 2017; Van Sickle et al., 2005; Zhang et al., 2014). However, it should be noted that the expression of CB2R in the healthy brain remains controversial, although its expression in neurons can be induced following neuronal insults (Haider et al., 2020; Viscomi et al., 2009).
The predominant mechanism by which eCBs control synaptic function is via retrograde signaling (Fig. 1). eCBs are synthesized and released by postsynaptic neurons in response to neuronal activation. Once released, eCBs traverse the synaptic cleft, activate pre-synaptic CB1Rs and suppress neurotransmitter release (Fig. 1) (Alger, 2012; Chevaleyre, Takahashi, & Castillo, 2006; Freund, Katona, & Piomelli, 2003; Kano et al., 2009; Katona & Freund, 2012). In addition to retrograde signaling, eCBs also serve as autocrine messengers that control intrinsic neuronal excitability by activating postsynaptic CB1Rs (Bacci, Huguenard, & Prince, 2004; Marinelli, Pacioni, Cannich, Marsicano, & Bacci, 2009), thereby gating several membrane currents (Gantz & Bean, 2017). The magnitude and spatiotemporal profile of eCB signaling is tightly regulated by the synthesis (Hashimotodani et al., 2013; Tanimura et al., 2010), degradation (Hashimotodani, Ohno-Shosaku, & Kano, 2007), and transport of eCBs (Haj-Dahmane et al., 2018). The detailed mechanisms regulating eCBs synthesis and degradation are well established and were the subject of previous reviews (Ahn, McKinney, & Cravatt, 2008; Mechoulam & Parker, 2013). This review will discuss our current understanding of the mechanisms underlying eCB transport and its impact upon synaptic eCB signaling.
Figure 1. Model of retrograde 2-AG signaling at central synapses.
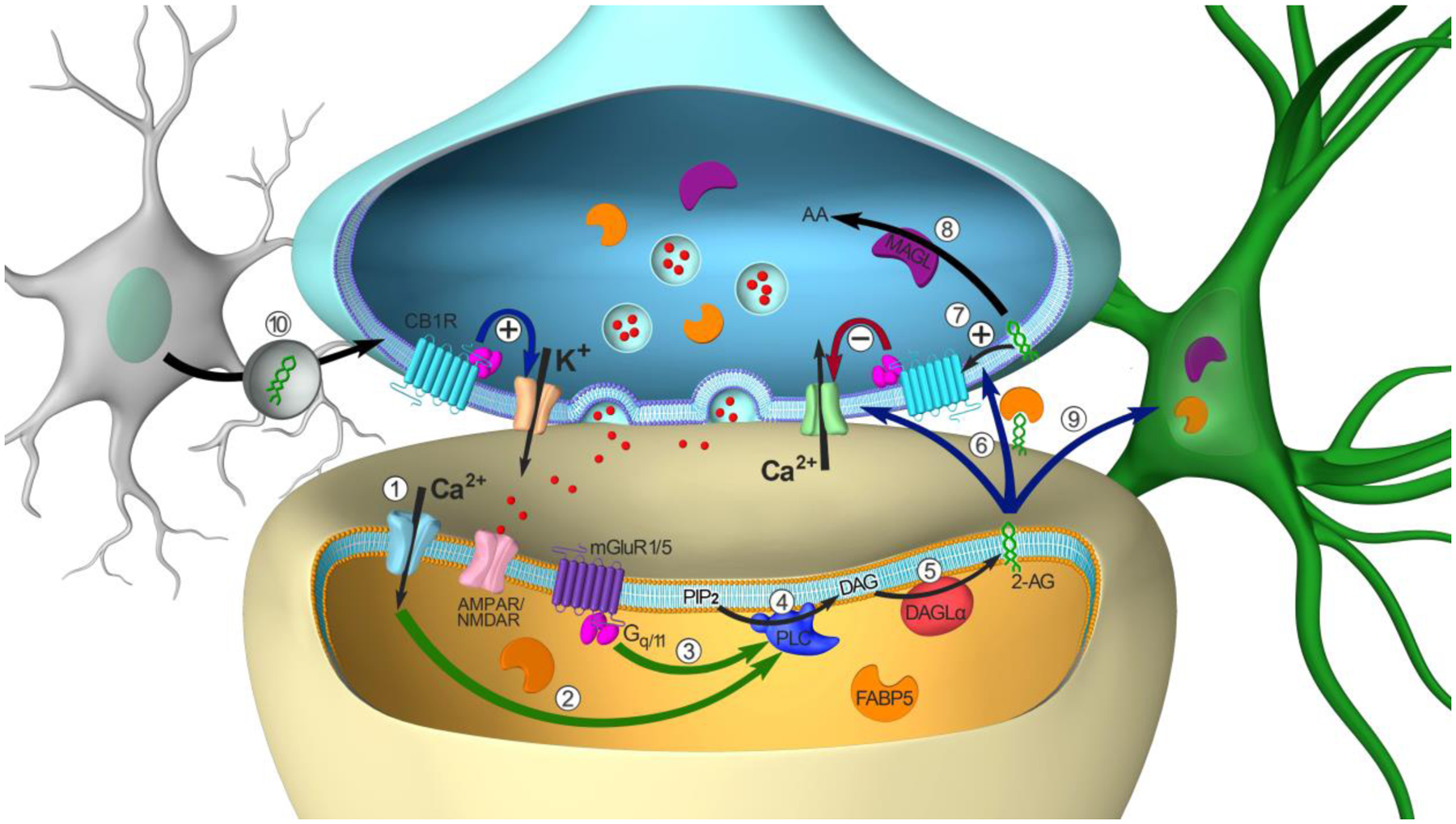
(Step 1) Depolarization of postsynaptic neurons triggers Ca2+ influx through voltage-dependent Ca2+ channels. (Step 2) The increase in intracellular Ca2+ activates various enzymes involved in eCB synthesis, including PLC. (Step 3) Activation of GPCRs coupled to the Gq/11 family, including metabotropic glutamate receptor type 1 and 5 (mGluR1/5), triggers PLC activation. (Step 4) PLC hydrolyzes PIP2 into DAG. (Step 5) DAGLα converts DAG into 2-AG. (Step 6) 2-AG is released in the synaptic cleft. Once released, 2-AG translocates across the synaptic cleft via distinct potential mechanisms discussed in this review. (Step 7) 2-AG activates presynaptic CB1Rs resulting in inhibition of voltage-dependent Ca2+ channels and the suppression of neurotransmitter release. (Step 8) 2-AG is then inactivated via hydrolysis into arachidonic acid (AA) and glycerol by MAGL localized in presynaptic terminals. (Step 9) MAGL in astrocytes also contributes to the termination of synaptic 2-AG signaling. (Step 10) AEA released from microglia via macrovesicles may also contribute to eCB signaling at central synapses.
Synaptic eCB signaling
The first evidence that eCBs function as retrograde messengers originated from electrophysiological studies of depolarization-induced suppression of inhibition (DSI), a form of short-term synaptic plasticity discovered by Alger and Marty at GABA synapses in the hippocampus and cerebellum, respectively (Llano, Leresche, & Marty, 1991; Pitler & Alger, 1994). These studies established that activation of postsynaptic neurons triggers calcium (Ca2+)-dependent eCB synthesis and release, which in turn activate presynaptic CB1Rs and inhibit GABA release, thereby inducing DSI (Ohno-Shosaku, Maejima, & Kano, 2001; Wilson & Nicoll, 2001). Concurrent to the discovery of DSI, Kreitzer and Regehr reported that eCBs also mediate depolarization-induced suppression of excitation (DSE), a form of short-term plasticity at glutamate synapses in the cerebellum (Kreitzer & Regehr, 2001). Following these seminal publications, eCB-mediated DSI and DSE have been reported throughout the brain (for review, see (Ohno-Shosaku & Kano, 2014)).
At most synapses, Ca2+ influx during neuronal activation stimulates Ca2+-dependent phospholipase Cβ (PLCβ), leading to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and the generation 1,2-diacylglycerol (DAG) (Fig. 1). DAG is then converted into 2-AG by diacylglycerol lipase α (DAGLα) localized on the inner leaflet of the postsynaptic plasma membrane (Yoshida et al., 2006). 2-AG is subsequently released and acts as a retrograde messenger by activating presynaptic CB1Rs to mediate DSI (Hashimotodani et al., 2007; Hashimotodani, Ohno-Shosaku, Maejima, Fukami, & Kano, 2008; Kim & Alger, 2004) and DSE (Hashimotodani et al., 2013; Pan et al., 2009; Uchigashima et al., 2007). Pharmacological inhibition or genetic deletion of DAGLα profoundly reduces the magnitude of DSI (Hashimotodani et al., 2007; Hashimotodani et al., 2008) and DSE (Hashimotodani et al., 2013), thus establishing its role as the key biosynthetic enzyme for 2-AG in the brain. 2-AG signaling is terminated by hydrolysis into arachidonic acid and glycerol by the enzyme monoacylglycerol lipase (MAGL), located in presynaptic terminals and surrounding astrocytes (Fig. 1) (Blankman, Simon, & Cravatt, 2007; Dinh, Freund, & Piomelli, 2002; Viader et al., 2015). Inhibition of 2-AG degradation facilitates and enhances the duration of DSI and DSE (Pan et al., 2009; Straiker et al., 2009; Zhong et al., 2011). In the case of both DSI and DSE, the transient activation of CB1Rs induces direct G-protein (likely through the βγ subunits) mediated inhibition of voltage-dependent Ca2+ channels (VDCC) (Brown, Brenowitz, & Regehr, 2003; Wilson & Nicoll, 2001) and subsequent depression of neurotransmitter release (Fig. 1).
In addition to mediating short-term synaptic plasticity (i.e. DSI and DSE), eCBs also induce presynaptic forms of long-term depression (eCB-LTD) at excitatory (Gerdeman, Ronesi, & Lovinger, 2002; Haj-Dahmane & Shen, 2010; Robbe, Kopf, Remaury, Bockaert, & Manzoni, 2002) and inhibitory synapses (Chevaleyre & Castillo, 2003; Marsicano et al., 2002). Examination of the mechanisms underpinning eCB-LTD reveals that activation of CB1Rs is required during the induction but not the expression of the LTD. The expression of eCB-LTD is predominantly mediated by Gi/o-dependent inhibition of the adenylate cyclase/cAMP/PKA signaling pathway (Chevaleyre et al., 2006; Haj-Dahmane & Shen, 2010; Heifets & Castillo, 2009) and may involve functional alteration of the presynaptic proteins Rab3B/RIM1a (Chevaleyre, Heifets, Kaeser, Sudhof, & Castillo, 2007; Tsetsenis et al., 2011) or inhibition of P/Q-type VDCCs (Mato, Lafourcade, Robbe, Bakiri, & Manzoni, 2008). Since these early studies, eCB-mediated short and long-term synaptic plasticity has been reported throughout the brain (for review, see (Araque, Castillo, Manzoni, & Tonini, 2017)). As such, eCBs are ubiquitous retrograde messengers critically involved in finetuning synaptic plasticity.
The synthesis of eCBs can also be induced by activation of numerous neurotransmitter receptors, in particular Gq/11-coupled GPCRs. Stimulation of these receptors, including group I metabotropic glutamate receptors (mGluR1/5s) (Maejima et al., 2005), M1 muscarinic receptors (Kim, Isokawa, Ledent, & Alger, 2002), α1-adrenergic receptors (Haj-Dahmane & Shen, 2014), and orexin receptors (Haj-Dahmane & Shen, 2005) mobilizes 2-AG via activation of PLCβ. The PLCβ-driven 2-AG serves both as a retrograde signal that induces transient or long-lasting depression of neurotransmitter release and as an autocrine messenger that regulates the intrinsic excitability of postsynaptic neurons by gating membrane channels (Gantz & Bean, 2017).
Besides “on demand” eCB synthesis, there is growing consensus that 2-AG and AEA are tonically synthesized in the brain. In the absence of neuronal activation, pharmacological inhibition or genetic deletion of MAGL or the AEA hydrolyzing enzyme fatty acid amide hydrolase (FAAH) increases brain 2-AG and AEA levels, respectively (Booker et al., 2012; Cravatt et al., 2001; Hashimotodani et al., 2007; Tanimura et al., 2010). Inhibition of these enzymes potentiates synaptic transmission via CB1R-dependent mechanisms, indicating that tonic eCB signaling controls basal synaptic transmission (Haj-Dahmane et al., 2018; Hentges, Low, & Williams, 2005; Robbe et al., 2002). However, the precise physiological roles and the mechanisms controlling tonic eCB synthesis remain poorly understood.
Unlike classical neurotransmitters, which are released by vesicle exocytosis and diffuse across the synaptic cleft to activate their receptors (Sudhof, 2013), eCBs are not stored in synaptic vesicles for future release. Instead, the current model of eCB signaling posits that eCBs are released into the synaptic cleft immediately upon their synthesis (Alger, 2012). In the following sections, we will discuss the potential mechanisms that mediate the release, intracellular and synaptic transport of eCBs at central synapses.
Membrane transport of eCBs
The rapid nature of retrograde eCB signaling (Wilson, Kunos, & Nicoll, 2001) necessitates efficient eCB transport across the lipid bilayer away from their biosynthetic enzymes, followed by translocation across the synaptic cleft to activate presynaptic CB1Rs. The mechanisms underlying the membrane transport of eCBs have been extensively investigated, leading to two prevailing models in which eCBs are transported either by simple diffusion (Fasia, Karava, & Siafaka-Kapadai, 2003; Glaser et al., 2003) or facilitated diffusion by a putative transmembrane transporter (Beltramo et al., 1997; Di Marzo et al., 1994). The simple diffusion model is supported by the observation that eCBs are uncharged lipids that readily partition into and diffuse across protein-free bilayers (Kaczocha, Lin, et al., 2012; Lynch & Reggio, 2006; Tian, Guo, Yao, Yang, & Makriyannis, 2005). Furthermore, eCB transport across cell membranes is observed universally in all cell-types regardless of tissue origin, and the kinetics of eCB uptake appear to be non-saturable (Fowler, 2013; Glaser et al., 2003; Kaczocha, Hermann, Glaser, Bojesen, & Deutsch, 2006) (for a critical discussion of eCB transport studies, see (Glaser, Kaczocha, & Deutsch, 2005)). Evidence supporting the existence of a putative membrane transporter is based largely upon the inhibition of eCB transport by structural analogs of eCBs as well as its temperature-dependence (Beltramo et al., 1997; Chicca, Marazzi, Nicolussi, & Gertsch, 2012; Di Marzo et al., 1994; Fegley et al., 2004; Moore et al., 2005; Piomelli et al., 1999). Further support for the existence of a putative membrane transporter originates from studies demonstrating that the site of action of eCB transport inhibitors is predominantly at the plasma membrane (Ligresti et al., 2010; Oddi et al., 2005). However, it is worth noting that these inhibitors target additional proteins involved in eCB transport and metabolism (e.g., FAAH and FABP5), which has made it challenging to distinguish between the contributions of the putative membrane transporter versus other proteins that modulate eCB uptake (Alexander & Cravatt, 2006; Glaser et al., 2003; Kaczocha et al., 2006; Kaczocha, Vivieca, Sun, Glaser, & Deutsch, 2012). More recently, Gertsch and colleagues identified small molecule inhibitors that are selective for the putative eCB membrane transporter (Chicca et al., 2012; Nicolussi, Chicca, et al., 2014; Nicolussi, Viveros-Paredes, et al., 2014). Future studies that leverage such pharmacological tools will be necessary to characterize the putative eCB membrane transporter and determine the precise contributions of this putative protein and simple diffusion toward eCB membrane transport.
Intracellular eCB Transport
The cytosol presents an energetic barrier that limits intracellular lipid (e.g., fatty acid and eCB) diffusion to various cellular organelles (Weisiger, 1996, 2002) including the endoplasmic reticulum and lipid droplets/adiposomes, the sites of AEA hydrolysis and accumulation (Cravatt et al., 1996; Deutsch & Chin, 1993; Giang & Cravatt, 1997; Glaser et al., 2003; Kaczocha, Glaser, Chae, Brown, & Deutsch, 2010; Oddi et al., 2008). The spatial separation between its site of signaling at the plasma membrane and intracellular inactivation suggests the existence of mechanisms that rapidly translocate AEA across the cytosol to FAAH for hydrolysis. Indeed, over the last decade, several classes of proteins have been identified as intracellular carriers for AEA and 2-AG (for a comprehensive review, see (Maccarrone, Dainese, & Oddi, 2010)). Fatty acid binding proteins (FABPs) were first to be identified as intracellular carriers for AEA (Kaczocha, Glaser, & Deutsch, 2009). The mammalian brain expresses three members of the FABP family: FABP3, FABP5, and FABP7 (Furuhashi & Hotamisligil, 2008). AEA displays highest affinity for FABP7, followed by FABP5 and FABP3, and a similar pattern is observed for 2-AG (Elmes et al., 2015; Kaczocha, Vivieca, et al., 2012). Accordingly, overexpression of FABP5 and FABP7, but not FABP3, enhances the cellular uptake of AEA (Kaczocha et al., 2009). The adult brain abundantly expresses FABP5 while FABP7 undergoes postnatal downregulation (Owada, Yoshimoto, & Kondo, 1996), suggesting that FABP5 may serve as the major FABP that regulates brain AEA metabolism. Indeed, pharmacological or genetic inhibition of FABP5 reduces the cellular uptake and metabolism of AEA, and elevates its levels in the brain (Bjorklund, Blomqvist, Hedlin, Persson, & Fowler, 2014; Kaczocha et al., 2015; Kaczocha et al., 2014; Kaczocha, Vivieca, et al., 2012; Yu, Levi, Casadesus, Kunos, & Noy, 2014). FABP5 deletion also produces CB1R-mediated antinociceptive effects (Berger et al., 2012; Kaczocha et al., 2015; Kaczocha et al., 2014; Peng et al., 2017). Compared to AEA, relatively little is known about the regulation of 2-AG transport by FABPs although a recent study reported that FABP5 inhibition elevates 2-AG levels and modulates 2-AG signaling in the dorsal raphe nucleus (DRn) (Haj-Dahmane et al., 2018).
FAAH-like anandamide transporter (FLAT), a truncated variant of FAAH that lacks its membrane anchoring N-terminus, was identified as another intracellular carrier for AEA (Fu et al., 2011). FLAT expression was reported throughout the brain and in numerous peripheral tissues including the liver, intestine, and pancreas. In contrast to membrane-bound FAAH, FLAT is a peripheral membrane protein that is largely devoid of catalytic activity but retains the ability to drive AEA accumulation in cells, presumably by facilitating its transport from the plasma membrane to intracellular FAAH (Fu et al., 2011). The FLAT-selective inhibitor ARN272 elevates tissue AEA levels and produces CB1R-mediated antinociceptive effects in a variety of pain models, suggesting a role for FLAT in AEA inactivation in vivo (Fu et al., 2011). Although the contribution of FLAT to AEA signaling and its potential as a therapeutic target warrants further investigation, it is noteworthy that follow up studies failed to detect FLAT expression in mammalian cells or tissues (Bjorklund et al., 2014; Leung, Elmes, Glaser, Deutsch, & Kaczocha, 2013). Consequently, the functional relevance of FLAT as an eCB carrier remains to be clarified (Fowler, 2014).
Maccarrone and colleagues identified heat shock protein 70.2 (Hsp70) and serum albumin as additional intracellular AEA transport proteins (Oddi et al., 2009). Compared to Hsp70, AEA bound to albumin with ~5-fold higher affinity, which is consistent with its known high affinity for AEA (Bojesen & Hansen, 2003). Overexpression of Hsp70 in SH-SY5Y neuroblastoma cells led to a 5-fold increase in AEA uptake, consistent with its function as an intracellular AEA carrier. To date, the relative contributions of Hsp70 and albumin toward eCB transport and inactivation in vivo have not been investigated.
Sterol carrier protein-2 (SCP2) is a lipid chaperone that also binds AEA (Liedhegner, Vogt, Sem, Cunningham, & Hillard, 2014). In silico docking approaches demonstrated that SCP2 exhibits greater affinity for AEA compared to 2-AG and expectedly, AEA was able to compete with cholesterol for binding to SCP2. Overexpression of SCP2 increased the cellular uptake of AEA, demonstrating a functional role for SCP2 in AEA accumulation (Liedhegner et al., 2014). The influence of SCP2 upon AEA metabolism in vivo is not known, however deletion of SCP2 in concert with the liver-specific FABP1 isoform did not alter brain AEA levels (Martin et al., 2019).
Employing a photoactivatable chemical probe based upon the structure of AEA, Cravatt and colleagues performed a proteome-wide interaction analysis and identified nucleobindin-1 (NUCB1) as a novel AEA binding protein (Niphakis et al., 2015). In addition to AEA, 2-AG and arachidonic acid were also identified as NUCB1 ligands. Pharmacological or genetic inhibition of NUCB1 markedly elevated AEA levels in cells (Niphakis et al., 2015). Interestingly, previous work has demonstrated that NUCB1 is largely a Golgi apparatus resident protein (Lavoie, Meerloo, Lin, & Farquhar, 2002) and while expressed in brain neurons, NUCB1 appears to exclusively localize to the Golgi apparatus (Tulke et al., 2016). Consequently, the precise mechanism(s) through which NUCB1 regulates cellular AEA metabolism and the contribution of this protein towards eCB signaling and inactivation in vivo awaits elucidation.
Outside of the brain, retinol-binding protein 2 was recently identified as an intestinal binding protein for 2-AG but not AEA (Lee et al., 2020). Moreover, the liver-specific FABP1 binds to AEA and 2-AG and its deletion results in elevated brain AEA and 2-AG levels (Huang et al., 2016; Martin et al., 2016). Mechanistically, FABP1 deletion elevates circulating levels of arachidonic acid, which is postulated to diffuse into the brain and increase the levels of AEA and 2-AG precursors leading to augmented AEA and 2-AG biosynthesis (Martin et al., 2016). FABP2, an FABP isoform expressed in the intestine, was likewise demonstrated to interact with AEA and 2-AG albeit with low affinity (Lai, Katz, Bernard, Storch, & Stark, 2020). Although not a binding protein, caveolae-mediated endocytosis has been proposed as another mechanism that contributes to AEA internalization (McFarland, Bardell, Yates, Placzek, & Barker, 2008; McFarland et al., 2004). Accordingly, pharmacological or genetic inhibition of components of the endocytic machinery reduced AEA cellular accumulation. Collectively, multiple proteins have been identified as intracellular AEA and 2-AG carriers and in a subset of cases their inhibition was shown to augment eCB signaling in vivo.
Extracellular and synaptic transport of eCBs
Once released, eCBs must traverse the synaptic cleft to rapidly activate CB1Rs and control synaptic transmission and plasticity. Similar to the cytosol, the hydrophilic property of the synaptic cleft presents an energetic barrier that impedes efficient eCB diffusion, suggesting the existence of mechanisms that facilitate extracellular/synaptic eCB transport. The discovery that FABPs function as intracellular eCB carriers (Kaczocha et al., 2009) raises the possibility that this family of proteins may also contribute to synaptic eCB transport. In order to serve as extracellular carriers, FABPs must be secreted into the extracellular milieu. Interestingly, results from previous studies have reported the secretion of FABPs from various cell-types through nonconventional mechanisms (Ertunc et al., 2015; Josephrajan et al., 2019; Villeneuve et al., 2018). Furthermore, proteomic analysis identified FABP5 as a component of human cerebrospinal fluid (Chiasserini et al., 2014), supporting a potential extracellular role for this protein. Consistent with this notion, we recently demonstrated that FABP5 is released by primary astrocytes in vitro, localizes to synapses, and is indispensable for retrograde 2-AG signaling at glutamate synapses of the DRn (Haj-Dahmane et al., 2018). Pharmacological inhibition or genetic deletion of FABP5 blocked 2-AG mediated DSE as well as Gq/11-driven retrograde 2-AG signaling. Inhibition of FABP5 also resulted in a blockade of tonic 2-AG-mediated control of excitatory synaptic transmission in the DRn (Haj-Dahmane et al., 2018). These effects were not mediated by impaired presynaptic CB1R function nor a deficit in 2-AG synthesis, thereby supporting the model that FABP5 may serve as a synaptic carrier for 2-AG at glutamate synapses.
Extracellular vesicles (EVs) have been proposed as an additional mechanism that mediates the extracellular and synaptic transport of eCBs. Thus, in an early study conducted in microglia, Gabrialli et al. demonstrated that microvesicles released from activated microglia were enriched in AEA. Importantly, addition of EVs isolated from activated microglia to a primary culture of GABA neurons inhibited GABAergic synaptic transmission through CB1R-dependent mechanisms (Gabrielli et al., 2015). Such findings have led to the suggestion that EVs can mediate the extracellular transport of eCBs released from microglia and enable microglia to modulate synaptic function. Further support for the concept that EVs contribute to eCB signaling stems from the recent demonstration that cocaine-driven retrograde eCB transport in midbrain dopamine neurons requires the release of non-synaptic EVs via a sigma 1 receptor (Sig1R)-dependent mechanism. Mechanistically, the secretion of EVs involves the dissociation of intracellular Sig1R from ADP-ribosylation factor 6 (ARF6), a G-protein that regulates EV trafficking (Nakamura et al., 2019). Inhibition of Sig1R or ARF6 suppressed cocaine-driven retrograde 2-AG mediated control of GABAergic synaptic transmission. Interestingly, while inhibition of EV release impaired cocaine-induced 2-AG signaling, it was dispensable for tonic eCB release, suggesting that distinct mechanisms may govern these temporally-distinct modes of eCB transport.
Future directions
Although some recent progress has been made in elucidating potential mechanisms underlying synaptic eCB transport in the brain, significant gaps still remain. For the FABP5-mediated extracellular/synaptic eCB transport model, key questions that require further investigation include the cellular origin of FABP5 and its mechanism(s) of secretion. For instance, although astrocytes maintained in primary culture secrete FABP5 while neurons do not (Haj-Dahmane et al., 2018), it is currently not known whether astrocytes constitute the major source of synaptic FABP5 in vivo. Furthermore, because neuronal activity drives eCB mobilization and release, it remains to be elucidated whether FABP5 secretion likewise requires neuronal and/or astrocytic activation. Previous studies have identified several nonconventional mechanisms underlying the secretion of peripherally-expressed FABPs (Ertunc et al., 2015; Josephrajan et al., 2019; Villeneuve et al., 2018); whether similar mechanisms govern FABP5 secretion in the brain will require further investigation. To date, FABP5 has been implicated in controlling retrograde 2-AG signaling (Haj-Dahmane et al., 2018), however it remains to be determined whether this function extends to the synaptic transport of AEA, which mediates retrograde signaling in the striatum and amygdala (Adermark & Lovinger, 2007; Gerdeman et al., 2002; Ramikie et al., 2014). In addition to FABP5, FABP3 and FABP7 are expressed in the brain and could in theory contribute to synaptic eCB transport. Furthermore, as noted above, several additional intracellular carriers have been identified and it is conceivable that a subset of these proteins (or as yet to be identified carriers) could contribute to synaptic eCB transport.
Additional studies are also required to address several key questions regarding the microvesicle and non-synaptic EV-mediated extracellular transport of eCBs. For instance, while activated microglia release AEA-containing microvesicles that stimulate CB1Rs when applied to neuronal cultures in vitro, the precise role of this mode of transport in gating synaptic eCB signaling in vivo remains to be determined. In addition, it is still not known whether this mechanism underlies the transport of microglial 2-AG. Similarly, although non-synaptic EVs transport 2-AG released from midbrain neurons following cocaine and GPCR activation, it is currently not known whether this mechanism contributes to retrograde 2-AG and AEA transport induced by neuronal activation. Importantly, given the slow kinetics of EV release (Cashikar & Hanson, 2019; Sung et al., 2020; Verweij et al., 2018) and the rapid time scale of eCB-mediated short-term synaptic plasticity (i.e. DSI and DSE) (Kreitzer & Regehr, 2001; Wilson et al., 2001), it is conceivable that EVs may be best suited to gate eCB-mediated synaptic function that necessitates eCB release over longer time scales. Additionally, it remains to be determined whether EVs represent a common mechanism for 2-AG and AEA transport that extends to other brain areas. The finding that cocaine application triggers EV release in the midbrain raises the question as to whether other drugs of abuse that enhance eCB signaling employ this mode of 2-AG transport.
There is growing consensus that eCBs mediate crosstalk between neurons and astrocytes by activating astrocytic CB1Rs (Araque et al., 2017). Furthermore, astrocytic MAGL contributes to the metabolism of 2-AG following its release by postsynaptic neurons (Viader et al., 2015). This raises the intriguing and yet unexplored possibility that overlapping or distinct mechanisms may govern 2-AG transport to these distinct cell-types. Clearly, future studies are needed to further define the precise mechanisms underlying synaptic eCB transport in the brain.
Acknowledgments
Research in the authors’ laboratories is supported in part by the National Institutes of Health grants MH122461, DA045863, DA035949, and DA048002 (to MK) & AA026601, DA045863, and MH122461 (to SHD).
Abbreviations
- eCB
Endocannabinoid
- CNS
Central nervous system
- AEA
Anandamide
- 2-AG
2-arachidonoylglycerol
- DSE
Depolarization-induced suppression of excitation
- DSI
Depolarization-induced suppression of inhibition
- LTD
Long-term depression
- CB1R
Cannabinoid receptor 1
- CB2R
Cannabinoid receptor 2
- DAGLα
Diacylglycerol lipase α
- DAG
Diacylglycerol
- AA
Arachidonic acid
- FAAH
Fatty acid amide hydrolase
- MAGL
Monoacylglycerol lipase
- FABP
Fatty acid binding protein
- EV
Extracellular vesicle
- VDCC
Voltage-dependent Ca2+ channels
- GPCR
G-protein coupled receptor
- PLC
Phospholipase C
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- FLAT
FAAH-like anandamide transporter
- Hsp70
Heat shock protein 70.2
- SCP-2
Sterol carrier protein-2
- NUCB1
Nucleobindin-1
- Sig1R
Sigma 1 receptor
- ARF6
ADP-ribosylation factor 6
Footnotes
Data Availability Statement
Data sharing is not applicable to this article because no new data were created or analysed in this study.
Competing Interests Statement: none
References
- Adermark L, & Lovinger DM (2007). Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc Natl Acad Sci U S A, 104(51), 20564–20569. doi:0706873104 [pii] 10.1073/pnas.0706873104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, & Cravatt BF (2008). Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev, 108(5), 1687–1707. doi: 10.1021/cr0782067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, & Cravatt BF (2006). The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc, 128(30), 9699–9704. doi: 10.1021/ja062999h [DOI] [PubMed] [Google Scholar]
- Alger BE (2012). Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J Physiol, 590(10), 2203–2212. doi: 10.1113/jphysiol.2011.220855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Castillo PE, Manzoni OJ, & Tonini R (2017). Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology, 124, 13–24. doi: 10.1016/j.neuropharm.2017.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, & Prince DA (2004). Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature, 431(7006), 312–316. doi: 10.1038/nature02913 [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, & Piomelli D (1997). Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science, 277(5329), 1094–1097. doi: 10.1126/science.277.5329.1094 [DOI] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, … Deutsch DG (2012). Targeting fatty acid binding protein (FABP) anandamide transporters - a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One, 7(12), e50968. doi: 10.1371/journal.pone.0050968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, … Harkany T (2007). Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science, 316(5828), 1212–1216. doi: 10.1126/science.1137406 [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Viveros MP, McPartland JM, & Rodriguez de Fonseca F (2010). The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav, 95(4), 375–382. doi: 10.1016/j.pbb.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Bjorklund E, Blomqvist A, Hedlin J, Persson E, & Fowler CJ (2014). Involvement of fatty acid amide hydrolase and fatty acid binding protein 5 in the uptake of anandamide by cell lines with different levels of fatty acid amide hydrolase expression: a pharmacological study. PLoS One, 9(7), e103479. doi: 10.1371/journal.pone.0103479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, & Cravatt BF (2007). A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol, 14(12), 1347–1356. doi:S1074–5521(07)00399–7 [pii] 10.1016/j.chembiol.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen IN, & Hansen HS (2003). Binding of anandamide to bovine serum albumin. J Lipid Res, 44(9), 1790–1794. doi: 10.1194/jlr.M300170-JLR200 [DOI] [PubMed] [Google Scholar]
- Booker L, Kinsey SG, Abdullah RA, Blankman JL, Long JZ, Ezzili C, … Lichtman AH (2012). The fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the nervous system to reverse LPS-induced tactile allodynia in mice. Br J Pharmacol, 165(8), 2485–2496. doi: 10.1111/j.1476-5381.2011.01445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, & Regehr WG (2003). Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci, 6(10), 1048–1057. doi: 10.1038/nn1126 [DOI] [PubMed] [Google Scholar]
- Cashikar AG, & Hanson PI (2019). A cell-based assay for CD63-containing extracellular vesicles. PLoS One, 14(7), e0220007. doi: 10.1371/journal.pone.0220007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, & Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron, 76(1), 70–81. doi: 10.1016/j.neuron.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, … Chen C (2012). Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep, 2(5), 1329–1339. doi: 10.1016/j.celrep.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, & Castillo PE (2003). Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron, 38(3), 461–472. doi: 10.1016/s0896-6273(03)00235-6 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, & Castillo PE (2007). Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron, 54(5), 801–812. doi: 10.1016/j.neuron.2007.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, & Castillo PE (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci, 29, 37–76. doi: 10.1146/annurev.neuro.29.051605.112834 [DOI] [PubMed] [Google Scholar]
- Chiasserini D, van Weering JR, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, … Jimenez CR (2014). Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics, 106, 191–204. doi: 10.1016/j.jprot.2014.04.028 [DOI] [PubMed] [Google Scholar]
- Chicca A, Marazzi J, Nicolussi S, & Gertsch J (2012). Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem, 287(41), 34660–34682. doi: 10.1074/jbc.M112.373241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, & Lichtman AH (2001). Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A, 98(16), 9371–9376. doi: 10.1073/pnas.161191698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, & Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature, 384(6604), 83–87. doi: 10.1038/384083a0 [DOI] [PubMed] [Google Scholar]
- Cristino L, Bisogno T, & Di Marzo V (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol, 16(1), 9–29. doi: 10.1038/s41582-019-0284-z [DOI] [PubMed] [Google Scholar]
- Deutsch DG, & Chin SA (1993). Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol, 46(5), 791–796. doi: 10.1016/0006-2952(93)90486-g [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, & Piomelli D (1994). Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature, 372(6507), 686–691. doi: 10.1038/372686a0 [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, & Piomelli D (2002). A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids, 121(1–2), 149–158. [DOI] [PubMed] [Google Scholar]
- Dvorzhak A, Semtner M, Faber DS, & Grantyn R (2013). Tonic mGluR5/CB1-dependent suppression of inhibition as a pathophysiological hallmark in the striatum of mice carrying a mutant form of huntingtin. J Physiol, 591(4), 1145–1166. doi: 10.1113/jphysiol.2012.241018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, & Elphick MR (2000). Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol, 422(2), 159–171. doi: [DOI] [PubMed] [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, … Deutsch DG (2015). Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem, 290(14), 8711–8721. doi: 10.1074/jbc.M114.618447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, … Hotamisligil GS (2015). Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res, 56(2), 423–434. doi: 10.1194/jlr.M055798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasia L, Karava V, & Siafaka-Kapadai A (2003). Uptake and metabolism of [3H]anandamide by rabbit platelets. Lack of transporter? Eur J Biochem, 270(17), 3498–3506. doi: 10.1046/j.1432-1033.2003.03724.x [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, & Piomelli D (2004). Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A, 101(23), 8756–8761. doi: 10.1073/pnas.0400997101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Malenka RC, & Sudhof TC (2013). Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron, 78(3), 498–509. doi: 10.1016/j.neuron.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ (2013). Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J, 280(9), 1895–1904. doi: 10.1111/febs.12212 [DOI] [PubMed] [Google Scholar]
- Fowler CJ (2014). Has FLAT fallen flat? Trends Pharmacol Sci, 35(2), 51–52. doi: 10.1016/j.tips.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, & Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev, 83(3), 1017–1066. doi: 10.1152/physrev.00004.2003 [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, … Piomelli D (2011). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci, 15(1), 64–69. doi: 10.1038/nn.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, & Hotamisligil GS (2008). Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov, 7(6), 489–503. doi: 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, … Verderio C (2015). Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep, 16(2), 213–220. doi: 10.15252/embr.201439668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, & Bean BP (2017). Cell-Autonomous Excitation of Midbrain Dopamine Neurons by Endocannabinoid-Dependent Lipid Signaling. Neuron, 93(6), 1375–1387 e1372. doi: 10.1016/j.neuron.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, & Lovinger DM (2002). Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci, 5(5), 446–451. doi: 10.1038/nn832 [DOI] [PubMed] [Google Scholar]
- Giang DK, & Cravatt BF (1997). Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci U S A, 94(6), 2238–2242. doi: 10.1073/pnas.94.6.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, & Deutsch DG (2003). Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A, 100(7), 4269–4274. doi: 10.1073/pnas.0730816100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M, & Deutsch DG (2005). Anandamide transport: a critical review. Life Sci, 77(14), 1584–1604. doi: 10.1016/j.lfs.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Haider A, Gobbi L, Kretz J, Ullmer C, Brink A, Honer M, … Ametamey SM (2020). Identification and Preclinical Development of a 2,5,6-Trisubstituted Fluorinated Pyridine Derivative as a Radioligand for the Positron Emission Tomography Imaging of Cannabinoid Type 2 Receptors. J Med Chem, 63(18), 10287–10306. doi: 10.1021/acs.jmedchem.0c00778 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, & Shen RY (2005). The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci, 25(4), 896–905. doi: 10.1523/JNEUROSCI.3258-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, & Shen RY (2010). Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol, 588(Pt 14), 2589–2604. doi: 10.1113/jphysiol.2010.190066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, & Shen RY (2014). Chronic stress impairs alpha1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. J Neurosci, 34(44), 14560–14570. doi: 10.1523/JNEUROSCI.1310-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY, Elmes MW, Studholme K, Kanjiya MP, Bogdan D, … Kaczocha M (2018). Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc Natl Acad Sci U S A, 115(13), 3482–3487. doi: 10.1073/pnas.1721339115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, & Mackie K (2007). The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci, 28(2), 83–92. doi: 10.1016/j.tips.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, & Kano M (2007). Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci, 27(5), 1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, & Kano M (2008). Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology, 54(1), 58–67. doi: 10.1016/j.neuropharm.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tanimura A, Kita Y, Sano Y, Shimizu T, … Kano M (2013). Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde signalling: evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J Physiol, 591(19), 4765–4776. doi: 10.1113/jphysiol.2013.254474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, & Castillo PE (2009). Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol, 71, 283–306. doi: 10.1146/annurev.physiol.010908.163149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, & Williams JT (2005). Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci, 25(42), 9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, & Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci, 11(2), 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Weinlander KM, & Stuhr KL (2012). Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience, 204, 207–229. doi: 10.1016/j.neuroscience.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, McIntosh AL, Martin GG, Landrock D, Chung S, Landrock KK, … Schroeder F (2016). FABP1: A Novel Hepatic Endocannabinoid and Cannabinoid Binding Protein. Biochemistry, 55(37), 5243–5255. doi: 10.1021/acs.biochem.6b00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, & Xi ZX (2019). Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci Biobehav Rev, 98, 208–220. doi: 10.1016/j.neubiorev.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephrajan A, Hertzel AV, Bohm EK, McBurney MW, Imai SI, Mashek DG, … Bernlohr DA (2019). Unconventional Secretion of Adipocyte Fatty Acid Binding Protein 4 Is Mediated By Autophagic Proteins in a Sirtuin-1-Dependent Manner. Diabetes, 68(9), 1767–1777. doi: 10.2337/db18-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Chae J, Brown DA, & Deutsch DG (2010). Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem, 285(4), 2796–2806. doi: 10.1074/jbc.M109.058461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, & Deutsch DG (2009). Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A, 106(15), 6375–6380. doi: 10.1073/pnas.0901515106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O’Rourke J, … Thanos PK (2015). Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Mol Pain, 11, 52. doi: 10.1186/s12990-015-0056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Hermann A, Glaser ST, Bojesen IN, & Deutsch DG (2006). Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem, 281(14), 9066–9075. doi: 10.1074/jbc.M509721200 [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Lin Q, Nelson LD, McKinney MK, Cravatt BF, London E, & Deutsch DG (2012). Anandamide externally added to lipid vesicles containing trapped fatty acid amide hydrolase (FAAH) is readily hydrolyzed in a sterol-modulated fashion. ACS Chem Neurosci, 3(5), 364–368. doi: 10.1021/cn300001w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Rebecchi MJ, Ralph BP, Teng YH, Berger WT, Galbavy W, … Ojima I (2014). Inhibition of fatty acid binding proteins elevates brain anandamide levels and produces analgesia. PLoS One, 9(4), e94200. doi: 10.1371/journal.pone.0094200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, & Deutsch DG (2012). Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem, 287(5), 3415–3424. doi: 10.1074/jbc.M111.304907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, & Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol Rev, 89(1), 309–380. doi: 10.1152/physrev.00019.2008 [DOI] [PubMed] [Google Scholar]
- Katona I, & Freund TF (2012). Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci, 35, 529–558. doi: 10.1146/annurev-neuro-062111-150420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Alger BE (2004). Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci, 7(7), 697–698. doi: 10.1038/nn1262 [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, & Alger BE (2002). Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci, 22(23), 10182–10191. doi:22/23/10182 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, & Regehr WG (2001). Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron, 29(3), 717–727. doi: 10.1016/s0896-6273(01)00246-x [DOI] [PubMed] [Google Scholar]
- Kruk-Slomka M, Dzik A, Budzynska B, & Biala G (2017). Endocannabinoid System: the Direct and Indirect Involvement in the Memory and Learning Processes-a Short Review. Mol Neurobiol, 54(10), 8332–8347. doi: 10.1007/s12035-016-0313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MP, Katz FS, Bernard C, Storch J, & Stark RE (2020). Two fatty acid-binding proteins expressed in the intestine interact differently with endocannabinoids. Protein Sci, 29(7), 1606–1617. doi: 10.1002/pro.3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie C, Meerloo T, Lin P, & Farquhar MG (2002). Calnuc, an EF-hand Ca(2+)-binding protein, is stored and processed in the Golgi and secreted by the constitutive-like pathway in AtT20 cells. Mol Endocrinol, 16(11), 2462–2474. doi: 10.1210/me.2002-0079 [DOI] [PubMed] [Google Scholar]
- Lee SA, Yang KJZ, Brun PJ, Silvaroli JA, Yuen JJ, Shmarakov I, … Blaner WS (2020). Retinol-binding protein 2 (RBP2) binds monoacylglycerols and modulates gut endocrine signaling and body weight. Sci Adv, 6(11), eaay8937. doi: 10.1126/sciadv.aay8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K, Elmes MW, Glaser ST, Deutsch DG, & Kaczocha M (2013). Role of FAAH-like anandamide transporter in anandamide inactivation. PLoS One, 8(11), e79355. doi: 10.1371/journal.pone.0079355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedhegner ES, Vogt CD, Sem DS, Cunningham CW, & Hillard CJ (2014). Sterol carrier protein-2: binding protein for endocannabinoids. Mol Neurobiol, 50(1), 149–158. doi: 10.1007/s12035-014-8651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A, De Petrocellis L, Hernan Perez de la Ossa D, Aberturas R, Cristino L, Moriello AS, … Di Marzo V. (2010). Exploiting nanotechnologies and TRPV1 channels to investigate the putative anandamide membrane transporter. PLoS One, 5(4), e10239. doi: 10.1371/journal.pone.0010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Zhang HY, Tagliaferro P, Chung M, Dennis E, … Onaivi ES (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep, 7(1), 17410. doi: 10.1038/s41598-017-17796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, & Marty A (1991). Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron, 6(4), 565–574. doi: 10.1016/0896-6273(91)90059-9 [DOI] [PubMed] [Google Scholar]
- Lutz B, Marsicano G, Maldonado R, & Hillard CJ (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci, 16(12), 705–718. doi: 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DL, & Reggio PH (2006). Cannabinoid CB1 receptor recognition of endocannabinoids via the lipid bilayer: molecular dynamics simulations of CB1 transmembrane helix 6 and anandamide in a phospholipid bilayer. J Comput Aided Mol Des, 20(7–8), 495–509. doi: 10.1007/s10822-006-9068-9 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Dainese E, & Oddi S (2010). Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci, 35(11), 601–608. doi: 10.1016/j.tibs.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, … Kano M (2005). Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci, 25(29), 6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Cannich A, Marsicano G, & Bacci A (2009). Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci, 12(12), 1488–1490. doi: 10.1038/nn.2430 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, … Lutz B (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418(6897), 530–534. doi: 10.1038/nature00839 [DOI] [PubMed] [Google Scholar]
- Martin GG, Chung S, Landrock D, Landrock KK, Huang H, Dangott LJ, … Schroeder F (2016). FABP-1 gene ablation impacts brain endocannabinoid system in male mice. J Neurochem, 138(3), 407–422. doi: 10.1111/jnc.13664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Seeger DR, McIntosh AL, Milligan S, Chung S, Landrock D, … Schroeder F (2019). Sterol Carrier Protein-2/Sterol Carrier Protein-x/Fatty Acid Binding Protein-1 Ablation Impacts Response of Brain Endocannabinoid to High-Fat Diet. Lipids, 54(10), 583–601. doi: 10.1002/lipd.12192 [DOI] [PubMed] [Google Scholar]
- Mato S, Lafourcade M, Robbe D, Bakiri Y, & Manzoni OJ (2008). Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca++ channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology, 54(1), 87–94. doi: 10.1016/j.neuropharm.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, & Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol, 327(4), 535–550. doi: 10.1002/cne.903270406 [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Bardell TK, Yates ML, Placzek EA, & Barker EL (2008). RNA interference-mediated knockdown of dynamin 2 reduces endocannabinoid uptake into neuronal dCAD cells. Mol Pharmacol, 74(1), 101–108. doi: 10.1124/mol.108.044834 [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, & Barker EL (2004). A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem, 279(40), 41991–41997. doi: 10.1074/jbc.M407250200 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, & Parker LA (2013). The endocannabinoid system and the brain. Annu Rev Psychol, 64, 21–47. doi: 10.1146/annurev-psych-113011-143739 [DOI] [PubMed] [Google Scholar]
- Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, … Felder CC (2005). Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc Natl Acad Sci U S A, 102(49), 17852–17857. doi: 10.1073/pnas.0507470102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, & Hill MN (2016). Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology, 41(1), 80–102. doi: 10.1038/npp.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, & Abu-Shaar M (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365(6441), 61–65. doi: 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, … Lupica CR (2019). Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife, 8doi: 10.7554/eLife.47209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolussi S, Chicca A, Rau M, Rihs S, Soeberdt M, Abels C, & Gertsch J (2014). Correlating FAAH and anandamide cellular uptake inhibition using N-alkylcarbamate inhibitors: from ultrapotent to hyperpotent. Biochem Pharmacol, 92(4), 669–689. doi: 10.1016/j.bcp.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Nicolussi S, Viveros-Paredes JM, Gachet MS, Rau M, Flores-Soto ME, Blunder M, & Gertsch J (2014). Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol Res, 80, 52–65. doi: 10.1016/j.phrs.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Lum KM, Cognetta AB 3rd, Correia BE, Ichu TA, Olucha J, … Cravatt BF (2015). A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell, 161(7), 1668–1680. doi: 10.1016/j.cell.2015.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Bari M, Battista N, Barsacchi D, Cozzani I, & Maccarrone M (2005). Confocal microscopy and biochemical analysis reveal spatial and functional separation between anandamide uptake and hydrolysis in human keratinocytes. Cell Mol Life Sci, 62(3), 386–395. doi: 10.1007/s00018-004-4446-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, … Maccarrone M (2009). Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol, 16(6), 624–632. doi: 10.1016/j.chembiol.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquariello N, De Simone C, Rapino C, Dainese E, … Maccarrone M (2008). Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci, 65(5), 840–850. doi: 10.1007/s00018-008-7494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, & Kano M (2014). Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol, 29, 1–8. doi: 10.1016/j.conb.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, & Kano M (2001). Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron, 29(3), 729–738. doi: 10.1016/s0896-6273(01)00247-1 [DOI] [PubMed] [Google Scholar]
- Owada Y, Yoshimoto T, & Kondo H (1996). Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J Chem Neuroanat, 12(2), 113–122. doi: 10.1016/s0891-0618(96)00192-5 [DOI] [PubMed] [Google Scholar]
- Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, & Liu QS (2009). Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther, 331(2), 591–597. doi: 10.1124/jpet.109.158162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, & Hurd YL (2015). Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci, 16(10), 579–594. doi: 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RT, & Burrell BD (2019). Comparative studies of endocannabinoid modulation of pain. Philos Trans R Soc Lond B Biol Sci, 374(1785), 20190279. doi: 10.1098/rstb.2019.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Studholme K, Kanjiya MP, Luk J, Bogdan D, Elmes MW, … Kaczocha M (2017). Fatty-acid-binding protein inhibition produces analgesic effects through peripheral and central mechanisms. Mol Pain, 13, 1744806917697007. doi: 10.1177/1744806917697007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, & Makriyannis A (1999). Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci U S A, 96(10), 5802–5807. doi: 10.1073/pnas.96.10.5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, & Alger BE (1994). Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron, 13(6), 1447–1455. doi: 10.1016/0896-6273(94)90430-8 [DOI] [PubMed] [Google Scholar]
- Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, … Patel S (2014). Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron, 81(5), 1111–1125. doi: 10.1016/j.neuron.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, & Manzoni OJ (2002). Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A, 99(12), 8384–8388. doi: 10.1073/pnas.122149199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu C, Muntoni AL, Pistis M, & Melis M (2015). Endocannabinoid Signaling in Motivation, Reward, and Addiction: Influences on Mesocorticolimbic Dopamine Function. Int Rev Neurobiol, 125, 257–302. doi: 10.1016/bs.irn.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, & Lichtman AH (2009). Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J, 11(1), 39–44. doi: 10.1208/s12248-008-9075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, & Mackie K (2009). Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol, 76(6), 1220–1227. doi: 10.1124/mol.109.059030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron, 80(3), 675–690. doi: 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, von Lersner A, Guerrero J, Krystofiak ES, Inman D, Pelletier R, … Weaver AM (2020). A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun, 11(1), 2092. doi: 10.1038/s41467-020-15747-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, … Kano M (2010). The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron, 65(3), 320–327. doi: 10.1016/j.neuron.2010.01.021 [DOI] [PubMed] [Google Scholar]
- Tarragon E, & Moreno JJ (2019). Cannabinoids, Chemical Senses, and Regulation of Feeding Behavior. Chem Senses, 44(2), 73–89. doi: 10.1093/chemse/bjy068 [DOI] [PubMed] [Google Scholar]
- Tian X, Guo J, Yao F, Yang DP, & Makriyannis A (2005). The conformation, location, and dynamic properties of the endocannabinoid ligand anandamide in a membrane bilayer. J Biol Chem, 280(33), 29788–29795. doi: 10.1074/jbc.M502925200 [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, & Sudhof TC (2011). Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci U S A, 108(34), 14300–14305. doi: 10.1073/pnas.1112237108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulke S, Williams P, Hellysaz A, Ilegems E, Wendel M, & Broberger C (2016). Nucleobindin 1 (NUCB1) is a Golgi-resident marker of neurons. Neuroscience, 314, 179–188. doi: 10.1016/j.neuroscience.2015.11.062 [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, & Watanabe M (2007). Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci, 27(14), 3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, … Sharkey KA (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310(5746), 329–332. doi: 10.1126/science.1115740 [DOI] [PubMed] [Google Scholar]
- Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, … Pegtel DM (2018). Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol, 217(3), 1129–1142. doi: 10.1083/jcb.201703206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Blankman JL, Zhong P, Liu X, Schlosburg JE, Joslyn CM, … Cravatt BF (2015). Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep, 12(5), 798–808. doi: 10.1016/j.celrep.2015.06.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, … Schekman R (2018). Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J Cell Biol, 217(2), 649–665. doi: 10.1083/jcb.201705047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, … Maccarrone M (2009). Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci, 29(14), 4564–4570. doi:29/14/4564 [pii] 10.1523/JNEUROSCI.0786-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger RA (1996). When is a carrier not a membrane carrier? The cytoplasmic transport of amphipathic molecules. Hepatology, 24(5), 1288–1295. doi: 10.1002/hep.510240550 [DOI] [PubMed] [Google Scholar]
- Weisiger RA (2002). Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Mol Cell Biochem, 239(1–2), 35–43. [PubMed] [Google Scholar]
- Wilson RI, Kunos G, & Nicoll RA (2001). Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron, 31(3), 453–462. doi: 10.1016/s0896-6273(01)00372-5 [DOI] [PubMed] [Google Scholar]
- Wilson RI, & Nicoll RA (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature, 410(6828), 588–592. doi: 10.1038/35069076 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, & Watanabe M (2006). Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci, 26(18), 4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Levi L, Casadesus G, Kunos G, & Noy N (2014). Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) in the brain. J Biol Chem, 289(18), 12748–12758. doi: 10.1074/jbc.M114.559062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, … Xi ZX (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A, 111(46), E5007–5015. doi: 10.1073/pnas.1413210111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Pan B, Gao XP, Blankman JL, Cravatt BF, & Liu QS (2011). Genetic deletion of monoacylglycerol lipase alters endocannabinoid-mediated retrograde synaptic depression in the cerebellum. J Physiol, 589(Pt 20), 4847–4855. doi: 10.1113/jphysiol.2011.215509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogopoulos P, Vasileiou I, Patsouris E, & Theocharis SE (2013). The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol, 27(1), 64–80. doi: 10.1111/fcp.12008 [DOI] [PubMed] [Google Scholar]


