Abstract
Background
Atrial fibrillation (AF) is a risk factor for cognitive impairment and dementia in patients with stroke history. However, the association between AF and cognitive impairment in broader populations is less clear.
Objective
To systematically review and quantitatively synthesize the existing evidence regarding the association of AF with cognitive impairment of any severity and etiology and dementia.
Methods
Medline, Scopus, and Cochrane Central were searched in order to identify studies investigating the association between AF and cognitive impairment (or dementia) cross-sectionally and longitudinally. Studies encompassing and analyzing exclusively patients with stroke history were excluded. A random-effects model meta-analysis was conducted. Potential sources of between-study heterogeneity were investigated via subgroup and meta-regression analyses. Sensitivity analyses including only studies reporting data on stroke-free patients, vascular dementia, and Alzheimer’s disease were performed.
Results
In total, 43 studies were included. In the pooled analysis, AF was significantly associated with dementia (adjusted OR, 1.6; 95% CI, 1.3 to 2.1; I2, 31%) and the combined endpoint of cognitive impairment or dementia (pooled adjusted OR, 1.5; 95% CI, 1.4 to 1.8; I2, 34%). The results were significant, even when studies including only stroke-free patients were pooled together (unadjusted OR, 2.2; 95% CI, 1.4 to 3.5; I2, 96%), but the heterogeneity rates were high. AF was significantly associated with increased risk of both vascular (adjusted OR, 1.7; 95% CI, 1.2 to 2.3; I2, 43%) and Alzheimer’s dementia (adjusted HR, 1.4; 95% CI, 1.2 to 1.6; I2, 42%).
Conclusion
AF increases the risk of cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer’s disease. Future studies should employ interventions that may delay or even prevent cognitive decline in AF patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-021-06954-8.
KEY WORDS: atrial fibrillation, dementia, systematic review, meta-analysis
Introduction
Atrial fibrillation (AF) and dementia frequently coexist, affecting predominantly the elderly.1 As global population aging is accelerated, the burden of both disorders is projected to increase dramatically. Specifically, the number of patients affected by dementia is expected to increase to 131 million by 2050, while the corresponding number for AF is more than 100 million individuals.2–5 Prior studies have shown that AF is a risk factor for cognitive impairment and dementia in patients with stroke history, since AF-related brain infarcts may lead to a step-wise decline in cognitive function,6, 7 as part of the vascular contributions to cognitive impairment and dementia.8 Specifically, a previous meta-analysis by Kwok et al. showed that AF confers an increased risk of dementia not only in patients with stroke history, but also in broader populations.9 At the same time, a number of recent observational studies examined the relationship between AF and cognitive impairment in stroke-free patients reporting equivocal results.10–12 Interestingly, Saglietto et al., in their meta-analysis, pooled together adjusted data from separate studies, showing that AF increased the risk of dementia independently from prior history of cerebrovascular accident.13 However, a more rigorous analysis including only studies with stroke-free patients would provide more solid conclusions. In this context, Liu et al. attempted to explore the association of AF with dementia in patients without stroke at baseline, but the analysis may have led to overestimated results due to use of a fixed-effects instead of a random-effects model.14 Additionally, no previous meta-analysis has systematically explored the association of AF with specific subtypes of dementia (i.e., vascular dementia and Alzheimer’s disease). Filling this knowledge gap and examining whether an independent association exists between AF and cognitive impairment may help reduce the burden of both disorders on patients and healthcare systems.
With this study, we aim to systematically review and quantitatively synthesize the existing evidence regarding the association of AF with cognitive impairment of any severity and etiology, including dementia of any severity and etiology. Since dementia represents the more severely affected subgroup of individuals with cognitive impairment, a subgroup that may demonstrate unique associations with AF, we perform separate syntheses for dementia.
Materials and Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines15 and registered in Prospero (117069):
Data Sources—Search Strategy
The electronic databases Medline, Scopus, and Cochrane Central were searched for relevant studies by two independent reviewers (CAP, NZ). The complete search algorithm for each database is presented in Supplementary File 1. The search terms we used result in retrieving studies that include participants with various neurodegenerative dementias, vascular dementia, and mild cognitive impairment (retrievable since it contains “cognitive impairment,” and VCID or VCI). Therefore, our use of the term cognitive impairment (CI) should not be confused with mild cognitive impairment (MCI). The reference lists of the included studies and relevant reviews were also examined for further eligible studies.
Study Selection
A study was deemed to be eligible for this systematic review if the following inclusion criteria were met: (i) investigating the association between AF and cognitive impairment (and/or dementia)16; reporting event rates or relative risk (RR)/odds ratio (OR) or hazard ratios (HR) for the comparison groups; (iii) all observational study designs were accepted (cohort, cross-sectional, prospective, retrospective); and (iv) published in any language up to December 2019. Studies encompassing and analyzing solely patients with stroke history were excluded. A summary of eligibility criteria can be found in Supplementary Table 1.
Two independent reviewers assessed the eligibility of the potentially included studies, according to the inclusion criteria (AAM, DGK). Disagreements were resolved by consensus.
Data Extraction and Quality Assessment
Pre-specified forms were used to extract the epidemiological and clinical data of the included studies. Data extraction was performed by two independent reviewers (CAP, CAT). Any discrepancies were resolved by the involvement of a third reviewer (LP). When duplicated populations were identified and the studies reporting on them used the same outcome measure, only the larger study was included in the analysis.
The assessment of risk of bias of the individual studies was performed independently by two investigators using the QUIPS tool (CAP, CAT).17 Each of the following bias domains was critically appraised as low, moderate, or high risk: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis, and reporting. To rate the quality of evidence of individual studies, the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach was used.
Data Synthesis and Analysis
In the primary analysis, the combined endpoint of cognitive impairment or dementia was assessed. Given a lack of uniformity for criteria of cognitive impairment, a separate analysis for the better-defined outcome of dementia was also performed. Different effect measures (unadjusted OR/HR and adjusted OR/HR) were pooled separately. The adjusted effect measures were used as reported in the original studies, irrespective of the number and kind of confounders used in their multivariate analyses. When associations with cognitive impairment and dementia were both reported, the outcome for the larger population was included in the primary analysis (individuals with dementia comprise a subgroup of those with cognitive impairment). Potential sources of between-study heterogeneity were investigated via subgroup analysis (cross-sectional vs. cohort studies) and meta-regression analysis (explanatory variables: age, hypertension (HTN), coronary artery disease (CAD), diabetes mellitus (DM), and hyperlipidemia). Several sensitivity analyses were also performed: (i) including only studies with stroke-free patients, in order to assess whether AF is associated with CI/dementia for reasons other than inducing clinical strokes; (ii) including only studies with prospective design; (iii) including only studies that implemented sensitive neuropsychological testing for detection of cognitive impairment milder than dementia and did not rely solely on MMSE, since the latter is insensitive for detection of mild cognitive impairment (MCI) and even more so for subtle forms of cognitive impairment due to ceiling effects.18–20 Specifically, in this sensitivity analysis, we included studies that used a global score derived from scores of three or more sensitive neuropsychological tests for detection of cognitive impairment21 [examples of tests that were combined for a global score were Delayed Word Recall Test (DWRT), Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale–Revised, Word Fluency Test (WFT), logical memory immediate and delayed recall, incidental learning from the Wechsler Memory Scale-III, trail making test parts A and B, WAIS-R digits span backward, Boston naming test, and animal naming].19, 20 We also included studies22–24 that used singular sensitive diagnostic tests for detection of MCI, specifically, Montreal Cognitive Assessment (MoCA) or Modified Mini-Mental State Examination (3MS).19, 20 Finally, studies that adopted the modified Petersen criteria for diagnosis of MCI were included in this analysis, since their implementation requires careful clinical review of cognitive symptoms and cognitive testing25; (iv) including only studies that implemented established criteria for diagnosis of dementia (such as the National Institute on Aging-Alzheimer’s Association workgroups criteria; or the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences criteria; or the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; or the Diagnostic and Statistical Manual of Mental Disorders-IV criteria); or a single diagnostic tool with adequate sensitivity, such as the informant questionnaire on cognitive decline in the elderly (IQCODE). From this sensitivity analysis, we excluded studies which determined dementia diagnosis based on patient record (ICD-9/10) diagnoses, without direct assessment of patients; (v) including only studies reporting data for Alzheimer’s dementia (AD); (vi) including only studies reporting data for vascular dementia (VaD); (vii) including only studies with a number of patients > 500. The random-effects model was used to account for heterogeneity within and between studies. Heterogeneity was assessed with the Higgins I-squared statistic (I2). Funnel plots were used to illustrate the publication bias risk, and the risk was quantified with the Egger’s regression test. A forest plot was used to graphically display the effect size in each study as well as in the pooled estimate. To test the effect of study design (cross-sectional vs. cohort) on the outcome, a subgroup analysis was performed. A p value < 0.05 was considered significant. All statistical analyses were performed using Revman version 5.3 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) and Comprehensive Meta-analysis version 3 (Englewood, NJ).
Results
Study Selection and Study Characteristics
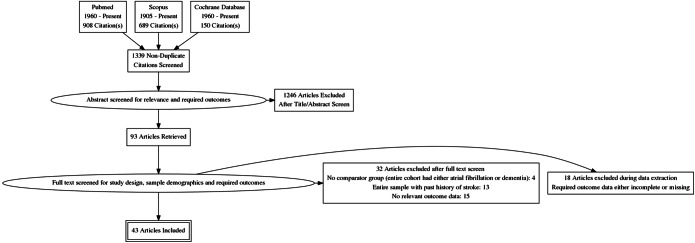
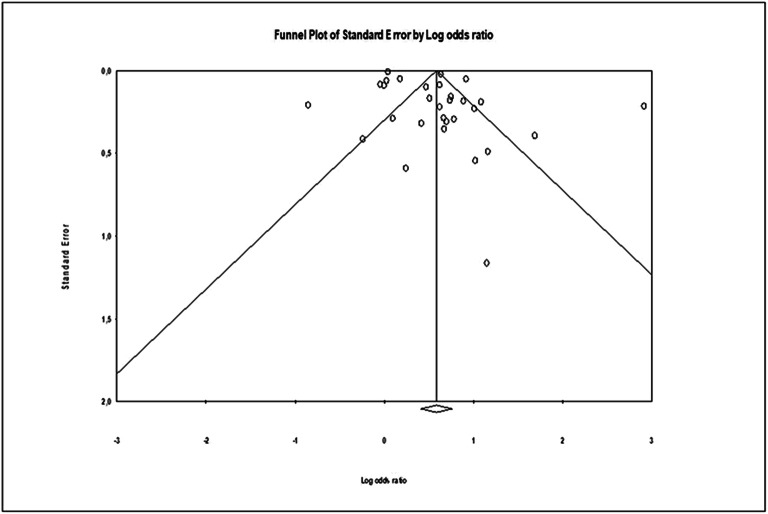
In total, 43 studies were included in this systematic review.1, 10–12, 16, 21–58 A detailed flow diagram is presented in Figure 1. Nineteen studies reported cross-sectional analyses,1, 22–28, 32, 33, 35, 36, 38, 40, 41, 43, 44, 49, 58 20 studies had prospective design,10–12, 16, 21, 34, 37, 39, , 45–48, 50–53, 55–57 and four were retrospective.29–31, 54 The number of enrolled patients ranged from 57 to 1,627,631 among the included studies. Hypertension (HTN), coronary artery disease (CAD), and diabetes rates among different studies ranged from 1 to 99%, 3.3 to 74.6%, and 4.4 to 37.2%, respectively. The percentage of patients with stroke history was reported in 23 studies (231.564 patients) and ranged from 1 to 24.7%,10, 12, 16, 21, 25–27, 29–31, 33, 34, 36, 40, 45, 47–50, 52–54, 56 while 14 studies reported data on stroke-free patients10–12, 23, 24, 28, 32, 34, 37, 41, , 51, 55, 57 (325.494 patients). Although different diagnostic methods were used to identify AF and cognitive impairment, electrocardiogram and Mini-Mental State Examination (MMSE) were the most commonly used tests. The baseline characteristics of the included studies are presented in Table 1 and Supplementary Table 2. The overall risk of bias was found to be low or moderate in most studies. The most common cause of bias was related to the measurement of outcome (i.e., cognitive impairment and dementia). Details for risk of bias assessment of individual studies are shown in Supplementary Table 3. Details for the GRADE rating of individual studies can be found in Supplementary Table 3. Egger’s test (p = 0.01) and visual inspection of the funnel plot (Fig. 2) indicated that publication bias may exist due to potentially unpublished small studies.
Figure 1.
PRISMA flow chart. The selection process is reported according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Table 1.
Baseline Demographics and Baseline Characteristics of Included Studies (AF/no AF)
| Author/Year | Country | No. of patients | Study design | Follow-up, years (Mean)a | Age, years (Mean) |
Stroke History (%) | AF ascertainment | Cognitive impairment ascertainment | Dementia ascertainment | Comprehensiveness of assessment for cognitive impairment/dementia |
|---|---|---|---|---|---|---|---|---|---|---|
| Ott 1997c | Netherlands |
6584 (157/6151) |
Cross-sectional | NA | ΝΑ | ΝΑ | EKG | MMSE |
MMSE; Geriatric Mental Schedule; CAMDEN; DSM III; brain MRI; examination by neurologist; medical records; criteria with a subdiagnosis of Alzheimer's disease based on NINCDS-ADRDA; criteria with subdiagnosis of vascular dementia in accordance with NINDS-AIREN |
Cognitive impairment: No Dementia: Yes |
| Cacciatore 1998 | Italy |
1075 (88 vs 987) |
Cross-sectional | NA |
73.9 (NA) |
0 | NA | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Tilvis 2004 | Finland |
629 (61/568) |
Prospective | 5 | NA |
8.6 (NA) |
History, medical records | MMSE | CDR |
Cognitive impairment: No Dementia: No |
| Debette 2006 | France |
83 (32 /51) |
Cross-sectional | NA |
62 (NA) |
NA | NA | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Joswiak 2006 | Poland |
2314 (547/1467) |
Cross-sectional | NA | 76 | NA | EKG | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Forti 2007 | Italy |
431 (13/418) |
Prospective | 4 |
75 (NA) |
NA | History | MMSE | NINCDS-ADRDA criteria, CDR, Petersen criteria for MCI |
Cognitive impairment: No Dementia: Yes |
| Rastas 2007 | Finland |
339 (72/267) |
Prospective | 3.5 |
88 (NA) |
20 (NA) |
Medical records | MMSE | DSM-III Revised criteria, neurologist’s clinical examination, MMSE, and Short Portable Mental Status Questionnaire tests; CDR, Actives of Daily Living, and Instrumental Activities of Daily Living scales; delirium excluded; history/interviews; consensus by two neurologists needed |
Cognitive impairment: No Dementia: Yes |
| Bilato 2009 | Italy |
1576 (135/1441) |
Retrospective | 4 |
76 (79/74) |
4.5 (8.8/4.2) |
EKG | MMSE, clock drawing tests | NA |
Cognitive impairment: No Dementia: NA |
| Peters 2009 | UK |
3369 (190/3146) |
Prospective | 1.8 |
84 (NA) |
6.5 (NA) |
NA | NA | MMSE, clock drawing tests; DSM-IV criteria; brain CT assessed by two neuroradiologists and modified ischaemic score/Hachinski ischaemic score |
Cognitive impairment: NA Dementia: Yes |
| Bunch 2010b | USA |
37025 (10161/26864) |
Retrospective | 5 |
60.7 (68/58) |
3.6 (4.7/3.2) |
Medical Records, ICD-9 codes | NA | Medical Records, ICD-9 codes |
Cognitive impairment: NA Dementia: No |
| Dublin 2011 | USA |
3045 (132/2913) |
Prospective | 6.8 |
74.1 (77/74) |
0 | ICD Codes | NA | Cognitive Abilities Screening Instrument, DSM-IV, NINCDS-ADRDA |
Cognitive impairment: NA Dementia: Yes |
| Marengoni 2011 | Sweden |
685 (68/617) |
Prospective | 4 |
78 (NA) |
NA | ICD codes, history, medical records | MMSE | DSM-III |
Cognitive impairment: No Dementia: Yes |
| Marzona 2012 | USA |
31506 (27864/3068) |
Prospective | 4.7 (median) |
66.5 (66.3/69.4) |
21d (27.6/20.5) |
EKG | MMSE | MMSE, reported dementia |
Cognitive Impairment: No Dementia: No |
| Yoshihara 2012 | Brazil |
1524 (37/1487) |
Cross-sectional | NA |
72.2 (77.7/72.1) |
NA | EKG | NA | DSM-IV, Diagnostic tool developed by the 10/66 Dementia Research Group and validated for low income countries, community screening instrument for dementia (CSI-D), modified version of the CERAD ten word list, community directed version of the geriatric mental state |
Cognitive Impairment: NA Dementia: Yes |
| Polidoro 2013 | Italy |
140 (70/70) |
Cross-sectional | NA |
79.2 (79.3/79,1) |
21.4 (22.9/20) |
EKG | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Bellomo 2012 | Italy | 57 (26/31) | Cross-sectional | NA |
71.8 (71.4/72.1) |
0 | EKG | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Habeych 2015 | USA | 17062 (898/16164) | Case-control | NA | NA | NA | NA | NA | ICD-9 codes |
Cognitive impairment: NA Dementia: No |
| Haring 2013 | USA | 6433 (255/6178) | Prospective | 8.6 | NA | 0 | NA | 3MSE | Modified Consortium to Establish a Registry for Alzheimer's Disease (CERAD) battery of neuropsychological tests and standardized interviews, DSM-IV |
Cognitive impairment: Yes Dementia: Yes |
| Author/Year | Country | No. of patients | Study design | Follow-up, years (Mean)a |
Age, years (Mean) |
Stroke History (%) | AF ascertainment | Cognitive impairment ascertainment | Dementia ascertainment | Comprehensiveness of assessment for cognitive disorder/dementia |
| Salehi 2013 | Iran |
189 (93/96) |
Prospective | 1 (overall) |
71.2 (71.1/71.3) |
0 | EKG | NA | DSM-IV |
Cognitive impairment: NA Dementia: yes |
| Horstmann 2014 | Germany |
718 (165/ 623) |
Cross-sectional | NA |
66.8 (75.5/64.5) |
0 | History, EKG, holter monitoring | IQCODE >3.44 | IQCODE ≥4 |
Cognitive impairment: Yes Dementia: Yes |
| Alosco 2015 | USA |
187 (60/127) |
Cross-sectional | NA | 68.5 (70.9 /67.3) | 0 | History | Modified MMSE, Trail making test A and B, Digit symbol coding, boston naming test, Animal fluency test, CVLT-II long delay free recall and total recognition hits | NA |
Cognitive Impairment: yes Dementia: NA |
| Annweiler 2015 | France |
267 (58/209) |
Cross-sectional | NA |
83.3 (NA) |
19.9 (NA) | EKG, History | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Rusanen 2014 | Finland |
1510 (NA) |
Prospective | 25.5 |
50.3 (NA) |
7.2 (NA) |
ICD codes | MMSE | MMSE, Finnish version of CERAD |
Cognitive impairment: NA Dementia: Yes |
| Ding 2018 | Sweden |
2658 (243/2442) |
Prospective | 9 |
73.1 (80.9/72.3) |
4.3d (13.2/3.4) |
ICD codes | MMSE | DSM-IV, NINCDS-ADRDA , NINDS-AIREN |
Cognitive function: No Dementia: Yes |
| Elias 2006 | USA |
1011 (59/952) |
Prospective | 30 | 61.4 (68/61) | 0 | EKG, holter monitoring, history |
Multiple tests from the Wechsler adult intelligence scale and Halstead-Reitan Battery for the assessment of multiple cognitive domains |
NA |
Cognitive impairment: Yes Dementia: NA |
| De Bruijn 2015c | Netherlands |
6514 (318/6196) |
Prospective | 20 (overall) |
68.6 (75.7/68.3) |
3d (NA) |
EKG | NA | Cambridge Examination for Mental Disorders of the Elderly |
Cognitive impairment: NA Dementia: Yes |
| Di Nisio 2015 | Italy |
309 (103 /206) |
Cross-sectional | NA |
77.5 (77.7/77.4) |
4.5 (24.3/12.2) |
EKG |
MMSE, Rey Auditory Verbal Learning Test, Rey-Osterrieth Complex Figure, Corsi block-tapping task, digit span test, verbal span test, Babcock’s story, Raven’s pro- gressive matrices and attentive matrices |
DSM-IV, NINDS-ADRDA, NINDS-AIREN criteria |
Cognitive impairment: Yes Dementia: Yes |
| Jefferson 2015 | USA |
9720 (583/9137) |
Cross-sectional | NA | NA | 0 | EKG | Petersen criteria, CDR, MMSE, Digit Span Forward, Digit Symbol, Trail Making Test (A, B), Digit Span Backward, Boston Naming Test, Animal Naming, Vegetable Naming, Logical Memory (Immediate/Delayed Recall) | NA |
Cognitive impairment: Yes Dementia: NA |
| Liao 2015 | Taiwan | 665330 (332665/332665) | Prospective | 12 |
70.3 (70.3/70.3) |
24.7 (32.3/17.2) |
ICD-9 codes | NA | ICD-9 codes |
Cognitive impairment: NA Dementia: No |
| Bunch 2016b | USA |
6030 (3000/3030) |
Retrospective | 7.1 (median) | 69.3 (69.3/69.3) |
5.1 (5.2/4.9) |
EKG | NA | MMSE |
Cognitive impairment: NA Dementia: No |
| Coma 2016 | Spain |
881 (187/794) |
Cross-sectional | NA |
72.6 (NA) |
12 (14 11.5) |
NA | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Dugger 2016 | USA |
17008 (1003/16005) |
Cross-sectional | NA | NA | NA | NA | NA | Uniform Data Set (UDS), composed of standardized clinical evaluations at Alzheimer’s Disease Centers (ADCs) funded by the National Institute of Aging |
Cognitive impairment: NA Dementia: Yes |
| Ma 2016 | China |
5067 (174/ 4893) |
Cross-sectional | NA |
72.1 (NA) |
24.1 (NA) |
History | Modified Petersen criteria | NA |
Cognitive impairment: Yes Dementia: NA |
| Marzona 2016 | Italy |
1627631 (27431/1600200) |
Prospective | 12 |
75.3 (78.4/75.2) |
1 (7.3/0.9) |
EKG | NA | ICD-9 codes |
Cognitive impairment: NA Dementia: No |
| Pulignano 2016 | Italy |
331 (98/233) |
Prospective | 1 |
78 (78/77) |
10 (11.2/9.4) |
EKG | MMSE | NA |
Cognitive impairment: No Dementia: NA |
| Graff -Radford 2016 | USA |
1044 (141/903) |
Cross-sectional | NA |
77.8 (NA) |
5.4 (9.9/4.7) |
EKG | NA | Petersen criteria for MCI |
Cognitive impairment:NA Dementia:No |
| Walters 2016 | UK |
800013 (24763/775250) |
Retrospective | 5 (median) | 65.6 ±6.1(NA) | 4.9(NA) | Medical records | NA | ICD-10 |
Cognitive impairment: NA Dementia: No |
| Alonso 2017c | USA |
6432 (611/5821) |
Cross-sectional | NA |
76.2 (79/76) |
4.5 (10/4) |
EKG during study visit or any prior visit, or ICD-9 code at any point | NA |
NIA-AA and NINDS-AIREN criteria |
Cognitive impairment: NA Dementia: Yes |
| Yang 2017 | China |
188 (72 /116) |
Cross-sectional | NA |
66.3 (68.9/64.6) |
0 | History | The Beijing version of MoCA | NA |
Cognitive impairment: Yes Dementia: NA |
| Singh-Manoux 2017 | UK |
7428 (414/7014) |
Prospective | 14.7 |
55.7 (58.8/55.5) |
15 (NA) |
EKG, ICD codes | Cognitive test battery, comprising of the following cognitive tests: 20-word free recall for memory, Alice Heim 4-1 for reasoning, measures of phonemic and semantic fluency. A global cognitive score was calculated based on the above | ICD-10 codes |
Cognitive impairment: Yes Dementia: No |
| Pavel 2018 | Russia |
100 (48/50) |
Cross-sectional | NA |
77 (78/76) |
NA | EKG | MoCA, FCSRT | MoCA, FCSRT |
Cognitive impairment: Yes Dementia: No |
| Chen 2018c | USA | 12515 (2106/10409) | Prospective | 20.2 |
56.9 (59.3/56,4) |
2 (1/2) |
EKG, ICD-9 codes | Cognitive battery comprising of delayed word recall (DWR), digit symbol substitution (DSS), subtest of the Wechsler Memory Scale-Revised, first letter word fluency |
DSM-V, NIA-AA, ICD-9 codes |
Cognitive impairment: Yes Dementia: Yes |
| Dongmin Kim 2019 | Korea |
262611 (10435/252176) |
Prospective | 8 (overall) |
70.7 (71.7/70.7) |
0 | NA | NA | KDSQ, ICD-10 codes |
Cognitive impairment: NA Dementia: No |
aUnless otherwise specified
bStudies with overlapping population
cStudies with overlapping population
dData for stroke-free patients were also reported
CAD Coronary Artery Disease; CDR Clinical Dementia Rating; CVLT-II California Verbal Learning Test-II; EKG Electrocardiogram; FCSRT Free and Cued Selective Reminding Test; GMS Geriatric Mental Schedule; HLD Hyperlipidemia; HTN Hypertension; ICD International Classification of Diseases; IQCODE Informant Questionnaire on Cognitive Decline in the Elderly; KDSQ Korean Dementia Screening Questionnaire; MMSE Multi-Mental State Exam; MoCA Montreal Cognitive Assessment; NA Not Applicable
Figure 2.
Publication bias assessment. Funnel plot with pseudo 95% confidence limits. Log, logarithm.
Synthesis of Individual Results
Composite Endpoint of Dementia or Cognitive Impairment
In total, 31 studies reported unadjusted OR for the combined endpoint of dementia or cognitive impairment.1, 10–12, 21–26, 28, 29, 31–33, 35–38, 40–46, 49–52 In the pooled analysis, AF was associated with increased risk for the combined endpoint (pooled unadjusted OR, 1.6; 95% CI, 1.4 to 1.9; df = 30) with significant between-study heterogeneity (I2, 97%) (Supplementary Figure 1). When adjusted effect measures were pooled together, AF was found to be a strong predictor of the combined endpoint with low between-study heterogeneity (15 studies: pooled adjusted OR, 1.5; 95% CI, 1.4 to 1.8; df = 14; I2, 34%; Supplementary Figure 2 and 18 studies: pooled adjusted HR, 1.4; 95% CI, 1.2 to 1.5; df = 17; I2, 92%; Supplementary Figure 3). The test for subgroup differences showed that there was no statistically significant subgroup effect (p = 0.24), suggesting that study design did not affect the outcome (Supplementary Figure).
The results of meta-regression analysis can be found in Supplementary file 2.
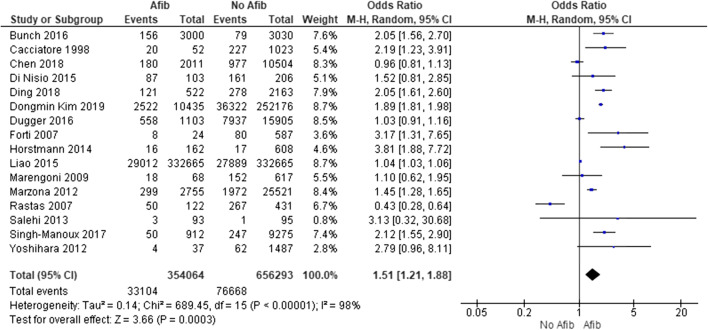
Dementia
Sixteen studies reported unadjusted data on the association of AF with dementia.10–12, 16, 21, 30, 32, 36, 38, 39, 41, 44–46, 51, 52 In the pooled analysis, AF was strongly associated with dementia (pooled unadjusted OR, 1.5; 95% CI, 1.2 to 1.9; df = 15) with significant between-study heterogeneity (I2, 98%) (Fig. 3). The association remained significant, after pooling together adjusted effect measures (8 studies: adjusted OR, 1.6; 95% CI, 1.3 to 2.1; df = 7; I2, 31%; Supplementary Figure 5; and 17 studies: adjusted HR, 1.4; 95% CI, 1.2 to 1.5; df=16; I2, 92% Supplementary Figure 6).
Figure 3.
Meta-analysis results. Forest plot demonstrating pooled unadjusted odds ratio for dementia. Afib, atrial fibrillation; CI, confidence interval.
Sensitivity Analysis
Studies with Stroke-Free Patients
In total, 325.494 patients without stroke at baseline were included in the analysis. Among the 14 studies that reported data on stroke-free patients,10–12, 23, 24, 28, 32, 34, 37, 41, , 51, 55, 57 AF was associated with a higher risk of cognitive impairment or dementia (11 studies: unadjusted OR, 2.2; 95% CI, 1.4 to 3.5; df = 10; I2, 96%; Supplementary Figure 7, 7 studies: adjusted HR, 1.4; 95% CI, 1.1 to 1.7; df = 6; I2, 87%; Supplementary Figure 8).
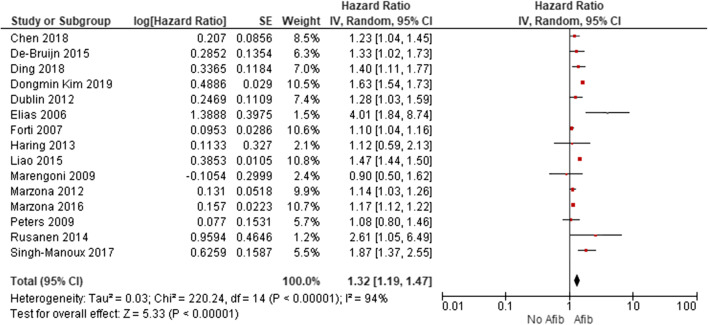
Studies with Prospective Design
Significant results were yielded for the combined endpoint of cognitive impairment and/or dementia, even when the analysis was limited to prospective studies (15 studies: adjusted HR, 1.3; 95% CI, 1.2 to 1.5; df = 14; I2, 94%) (Fig. 4).
Figure 4.
Sensitivity analysis. Forest plot demonstrating pooled adjusted hazard ratio including only prospective studies. Afib, atrial fibrillation; CI, confidence interval.
Studies with Comprehensive Cognitive Testing
Among the 9 studies with comprehensive cognitive testing,21–25, 36, 41, 52, 57 AF was associated with higher risk of cognitive dysfunction (unadjusted OR, 1.8; 95% CI, 1.2 to 2.6; I2, 88%) (Supplementary Figure 9).
Studies with Comprehensive Dementia Diagnosis
AF was found to be an independent predictor of dementia, when the analysis was restricted to studies that used high-quality criteria for the diagnosis of dementia and reported adjusted data (9 studies: adjusted HR, 1.3; 95% CI, 1.2 to 1.4; I2, 0%) (Supplementary Figure 10).
Alzheimer’s Dementia and Vascular Dementia
Thirteen studies provided data for the association of AF with AD1, 10, 11, 25, 26, 30, 31, 34, 36, 38, 46, 56 and 5 studies for the association with VaD.1, 26, 31, 36, 38 AF was associated with an increased risk for both types of dementia (7 studies: adjusted HR for AD, 1.4; 95% CI, 1.1 to 1.6; df = 6; I2, 42%; Supplementary Figure 11, adjusted OR for VaD, 1.7; 95% CI, 1.2 to 2.3; df = 4; I2, 43%; Supplementary Figure 12)
Studies with Number of Patients > 500
Among the 11 studies that included in their analysis more than 500 patients and reported adjusted data, AF was significantly associated with the combined endpoint of cognitive impairment or dementia (adjusted pooled OR, 1.5; 95% CI, 1.4 to 1.7; df = 10; I2 = 25%) (Supplementary Figure 13).
Discussion
This was a systematic review and meta-analysis of 43 observational studies that examined the association of AF with CI and dementia. To the best of our knowledge, this is the most updated meta-analysis on the topic with the largest patient population (more than 3.5 million patients), providing for the first time separate analyses and pooled data for the association of AF with VaD and AD, as well. The main finding of our study is that the presence of AF is associated with an increased risk of cognitive impairment and dementia. Interestingly, this association remained significant even when data from prospective studies were pooled together. Moreover, the sensitivity analyses for the association of AF with vascular dementia and Alzheimer’s disease yielded significant results for both subtypes of dementia. Finally, given the great heterogeneity in the methods used to ascertain CI and dementia across the included studies, we performed separate analyses including only studies that implemented sensitive neuropsychological testing for detection of cognitive impairment and established criteria for diagnosis of dementia. These sub-analyses allow drawing more solid conclusions on the association of AF with CI and dementia. In light of the high incidence of AF, our findings have important implications for patients and healthcare systems given the morbidity and quality-of-life implications, as well as the economic and social burden associated with poor cognitive outcomes during aging.
However, some of the analyses of our study were limited by a high degree of heterogeneity. Even if we tried to adjust for them, by using a random-effects model, performing multiple subgroup, sensitivity, and meta-regression analyses, there was still significant heterogeneity in the analyses. We think the reason for that was mainly the different status and management of patients with AF among the different studies. Prior studies have shown that anticoagulation has a positive impact in decreasing the risk for dementia and cognitive impairment in patients with AF. We were not able to adjust for the anticoagulation status (and type) in our analysis. Similarly, prior studies have shown that higher CHADS2 and CHA2DS2-VASc scores are associated with dementia risk in AF patients.37, 45 The amount of time that patients spent in AF vs sinus rhythm and the overall left ventricular function are likely associated with the risk for cognitive decline, given the increased risk for thromboembolic events and the decreased cardiac output.59
Our findings are in agreement with most of the previous studies. A recent study demonstrated an independent association between AF and cognitive impairment but included only patients with previous history of stroke, which is per se a known risk factor for cognitive decline.60 Whether AF is independently associated with cognitive impairment and dementia in the absence of known cerebrovascular accident history had remained an unanswered question for many years, with several studies reporting conflicting results.38, This important question was not addressed by a recently published meta-analysis by Islam et al., since a subgroup analysis including only studies with stroke-free patients was not performed.61 Seven years ago, a large meta-analysis published in 2013 demonstrated that patients with AF had approximately 40% higher risk to develop cognitive impairment regardless of the presence of history of clinical stroke or not.62 Contrary to our meta-analysis, the impact of clinically important moderator variables (e.g., age, hypertension, diabetes) on study effect measure was not explored. Since then, several population-based studies have been published. A recent study by Kim et al., which was included in our meta-analysis, was performed on a population-based cohort with more than 10,000 participants and showed that incident AF increased the risk of dementia (HR, 1.52; 95% CI, 1.43 to 1.63). The results remained significant after censoring for stroke (HR, 1.27; 95% CI, 1.18 to 1.37).11 Our work expands previous knowledge by exploring the independent association between AF and cognitive decline in a much larger patient population (more than 3.5 million patients) of whom the vast majority had no prior history of clinical stroke. Another intriguing finding of our meta-analysis was that AF was independently associated with an increased risk of AD, supporting the hypothesis that there may be a causality link between vascular disease risk factors and AD.63
The underlying mechanisms that could explain an association between AF and cognitive impairment independent of clinical stroke have not been elucidated, but the leading hypothesis is that chronic AF may lead to silent strokes that gradually deteriorate brain function.64 Apart from cardioembolic events, AF may lead to cerebral hypoperfusion due to the irregular rhythm and subsequent decrease in cardiac output predisposing to silent strokes and cognitive decline., 64 Despite the fact that the data on the association of AF with cognitive dysfunction are observational, and thus causality cannot be established, it is tempting to hypothesize that the prevention and aggressive treatment of these pathogenic mechanisms may be beneficial for AF patients. Interestingly, in a randomized trial, patients with restored sinus rhythm after AF ablation had significantly lower rates of dementia, compared to those who remained in AF.65 Additionally, a previous study by Cacciatore et al. demonstrated that both low (< 50 beats per minute) and fast (> 90 beats per minute) ventricular rate responses were significant predictors of dementia in patients with AF.32 Taking these lines of evidence together, a reasonable strategy for patients with AF would be to aim at maintaining heart rate within normal range in order to reduce the risk for cognitive impairment. Prospective randomized controlled clinical trials are needed to clarify whether aiming for lower heart rates than those currently recommended by guidelines (< 110 beats per minute), or even converting to sinus rhythm, may provide incremental benefit to patients at higher risk for cognitive impairment (i.e., stroke patients).66
The effect of anticoagulation on cognition in patients with AF is equivocal. A meta-analysis of three observational studies showed that the incidence rates of dementia were not different in the group treated with anticoagulants (pooled OR, 0.89; 95% CI, 0.47 to 1.69).67 In contrast, a recent large study by Kim et al. showed that AF patients taking oral anticoagulants had lower risk for dementia, compared to the no-anticoagulation group (HR, 0.61; 95% CI, 0.54 to 0.68).11 Another important finding of this study was that the risk for dementia gradually increased with rising CHA2DS2-VASc score (HR, 1.11; 95% CI, 1.07 to 1.14; for each 1-point increment of the score). This finding suggests that patients with higher scores should be closely monitored for signs of cognitive decline. Whether high CHA2DS2-VASc scores can guide initiation of anticoagulation without a diagnosis of AF is a hypothesis that should be further investigated in the future.
Strengths and Limitations
This is the largest meta-analysis to date on this topic, and the only study with detailed subgroup analyses across the whole spectrum of cognitive impairment and dementia subtypes. By following the most rigorous guidelines for data extraction and analysis, the results of this analysis may be considered as statistically robust. However, our study has also a number of limitations. First, this was a meta-analysis of population-based cross-sectional and cohort-based/case control studies and thus should be interpreted within the context of observational research and its inherent limitations (e.g., some studies included a small percentage of patients with stroke history). Second, some of the studies were not designed to study the impact of AF on dementia and cognitive impairment and this might have led to less than rigorous ascertainment of dementia diagnosis and cognitive impairment or in their reporting in the original investigations. Third, ascertainment for AF, cognitive impairment, and dementia relied on widely different methods and definitions across studies. Fourth, the adjusted effect measures used in our meta-analysis were adjusted for different covariates across the original studies. Fifth, many of the analyses were limited by high degrees of heterogeneity which we tried to address by using a random-effects model, analyzing separately unadjusted and adjusted OR/HR, and performing multiple subgroup, sensitivity, and meta-regression analyses. In the stroke-free sub-analysis, where a high degree of heterogeneity was reported, a meta-regression analysis was limited by the fact that most of the included studies did not provide sufficient data for this patient population. Finally, despite the meticulous search of the existing literature by two independent reviewers, a reference librarian was not involved in the study selection process.
Conclusion
Our findings suggest that AF may be associated with an increased risk of both cognitive impairment and dementia in a broad population including mainly stroke-free patients and studies with very low rates of stroke. Physicians should be particularly alert to identify early clinical manifestations of cognitive decline in this patient population. If such symptoms are present, an interdisciplinary approach (heart-stroke team with cardiologists, neurologists, and geriatricians) may be required to effectively manage and treat these patients. Future studies should explore the effect of interventions that may delay or even prevent cognitive decline in AF patients.
Supplementary Information
(DOCX 31 kb)
(DOCX 26 kb)
(DOCX 27 kb)
(DOCX 44 kb)
(DOCX 22 kb)
(DOCX 26.9 kb)
(DOCX 25 kb)
(DOCX 22 kb)
(DOCX 24.1 kb)
(DOCX 22.7 kb)
(DOCX 62 kb)
(DOCX 57 kb)
(DOCX 24 kb)
(DOCX 8 kb)
(DOCX 14 kb)
(DOCX 29 kb)
(DOCX 11 kb)
(DOCX 8 kb)
Acknowledgements
The present study was supported in part by the Intramural Program of the National Institute on Aging, National Institutes of Health.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28(2):316–321. doi: 10.1161/01.STR.28.2.316. [DOI] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114;(2):119-125. [DOI] [PubMed]
- 4.Ponjoan A, Garre-Olmo J, Blanch J, et al. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol. 2019;11:217–228. doi: 10.2147/CLEP.S186590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CX, Brown A, Tse H-F, et al. Epidemiology of atrial fibrillation: the Australian and Asia-Pacific perspective. Heart Lung Circ. 2017;26(9):870–879. doi: 10.1016/j.hlc.2017.05.120. [DOI] [PubMed] [Google Scholar]
- 6.Chander RJ, Lim L, Handa S, et al. Atrial fibrillation is independently associated with cognitive impairment after ischemic stroke. J Alzheimers Dis. 2017;60(3):867–875. doi: 10.3233/JAD-170313. [DOI] [PubMed] [Google Scholar]
- 7.Douiri A, McKevitt C, Emmett ES, Rudd AG, Wolfe CDA. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation. 2013;128(12):1341–1348. doi: 10.1161/CIRCULATIONAHA.113.002236. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76(10):914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 10.Ding M, Fratiglioni L, Johnell K, et al. Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population-based study. Neurology. 2018;91(19):e1732–e1740. doi: 10.1212/WNL.0000000000006456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Yang P-S, Yu HT, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J. 2019;40(28):2313–2323. doi: 10.1093/eurheartj/ehz386. [DOI] [PubMed] [Google Scholar]
- 12.Marzona I, O’Donnell M, Teo K, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. C Can Med Assoc J = J l’Association medicale Can. 2012;184(6):E329–36. doi: 10.1503/cmaj.111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saglietto A, Matta M, Gaita F, Jacobs V, Bunch TJ, Anselmino M. Stroke-independent contribution of atrial fibrillation to dementia: a meta-analysis. Open Hear. 2019;6(1):e000984. doi: 10.1136/openhrt-2018-000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D-S, Chen J, Jian W-M, Zhang G-R, Liu Z-R. The association of atrial fibrillation and dementia incidence: a meta-analysis of prospective cohort studies. J Geriatr Cardiol. 2019;16(3):298–306. doi: 10.11909/j.issn.1671-5411.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastas S, Verkkoniemi A, Polvikoski T, et al. Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38(5):1454–1460. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 18.Pendlebury ST, Cuthbertson FC, Welch SJV, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41(6):1290–1293. doi: 10.1161/STROKEAHA.110.579888. [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Patten R, Britton K, Tremont G. Comparing the Mini-Mental State Examination and the modified Mini-Mental State Examination in the detection of mild cognitive impairment in older adults. Int psychogeriatrics. 2019;31(5):693–701. doi: 10.1017/S1041610218001023. [DOI] [PubMed] [Google Scholar]
- 21.Chen LY, Norby FL, Gottesman RF, et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc 2018;7;(6). [DOI] [PMC free article] [PubMed]
- 22.Krupenin P, Gabitova M, Bordovsky S, et al. Impact of atrial fibrillation on the rate of mild cognitive impairment in the elderly. J Neurol Sci. 2018;394:75–77. doi: 10.1016/j.jns.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Alosco ML, Spitznagel MB, Sweet LH, Josephson R, Hughes J, Gunstad J. Atrial fibrillation exacerbates cognitive dysfunction and cerebral perfusion in heart failure. PACE - Pacing Clin Electrophysiol. 2015;38(2):178–186. doi: 10.1111/pace.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Niu W, Zang X, Lin M, Zhao Y. The association between atrial fibrillation and cognitive function in patients with heart failure. Eur J Cardiovasc Nurs. 2017;16(2):104–112. doi: 10.1177/1474515116641299. [DOI] [PubMed] [Google Scholar]
- 25.Ma F, Wu T, Zhao J, et al. Prevalence of mild cognitive impairment and its subtypes among Chinese older adults: Role of vascular risk factors. Dement Geriatr Cogn Disord. 2016;41(5-6):261–272. doi: 10.1159/000446507. [DOI] [PubMed] [Google Scholar]
- 26.Alonso A, Knopman DS, Gottesman RF, et al. Correlates of dementia and mild cognitive impairment in patients with atrial fibrillation: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J Am Heart Assoc 2017;6;(7). [DOI] [PMC free article] [PubMed]
- 27.Annweiler C, Ferland G, Barberger-Gateau P, Brangier A, Rolland Y, Beauchet O. Vitamin K antagonists and cognitive impairment: results from a cross-sectional pilot study among geriatric patients. J Gerontol A Biol Sci Med Sci. 2015;70(1):97–101. doi: 10.1093/gerona/glu133. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo A, De Benedetto G, Fossati C, et al. Atrial fibrillation (AF) and cognitive impairment in the elderly: a case-control study. Arch Gerontol Geriatr. 2012;55(2):247–250. doi: 10.1016/j.archger.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Bilato C, Corti M-C, Baggio G, et al. Prevalence, functional impact, and mortality of atrial fibrillation in an older Italian population (from the Pro.V.A. study) Am J Cardiol. 2009;104(8):1092–1097. doi: 10.1016/j.amjcard.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 30.Bunch TJ, May HT, Bair TL, et al. Atrial fibrillation patients treated with long-term warfarin anticoagulation have higher rates of all dementia types compared with patients receiving long-term warfarin for other indications. J Am Heart Assoc 2016;5;(7). [DOI] [PMC free article] [PubMed]
- 31.Bunch TJ, Weiss JP, Crandall BG, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer’s dementia. Hear Rhythm. 2010;7(4):433–437. doi: 10.1016/j.hrthm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Cacciatore F, Testa G, Langellotto A, et al. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10-year study. Dement Geriatr Cogn Disord. 2012;34(3-4):143–148. doi: 10.1159/000342195. [DOI] [PubMed] [Google Scholar]
- 33.Coma M, Gonzalez-Moneo MJ, Enjuanes C, et al. Effect of permanent atrial fibrillation on cognitive function in patients with chronic heart failure. Am J Cardiol. 2016;117(2):233–239. doi: 10.1016/j.amjcard.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 34.de Bruijn RFAG, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72(11):1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 35.Debette S, Bauters C, Leys D, Lamblin N, Pasquier F, de Groote P. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13(4):205–208. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 36.Di Nisio M, Prisciandaro M, Rutjes AWS, Russi I, Maiorini L, Porreca E. Dementia in patients with atrial fibrillation and the value of the Hachinski ischemic score. Geriatr Gerontol Int. 2015;15(6):770–777. doi: 10.1111/ggi.12349. [DOI] [PubMed] [Google Scholar]
- 37.Dublin S, Anderson ML, Heckbert SR, et al. Neuropathologic changes associated with atrial fibrillation in a population-based autopsy cohort. J Gerontol A Biol Sci Med Sci. 2014;69(5):609–615. doi: 10.1093/gerona/glt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dugger BN, Malek-Ahmadi M, Monsell SE, et al. A cross-sectional analysis of late-life cardiovascular factors and their relation to clinically defined neurodegenerative diseases. Alzheimer Dis Assoc Disord. 2016;30(3):223–229. doi: 10.1097/WAD.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forti P, Maioli F, Pisacane N, Rietti E, Montesi F, Ravaglia G. Atrial fibrillation and risk of dementia in non-demented elderly subjects with and without mild cognitive impairment (MCI) Arch Gerontol Geriatr. 2007;44(Suppl 1):155–165. doi: 10.1016/j.archger.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Graff-Radford J, Madhavan M, Vemuri P, et al. Atrial fibrillation, cognitive impairment, and neuroimaging. Alzheimers Dement. 2016;12(4):391–398. doi: 10.1016/j.jalz.2015.08.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horstmann S, Rizos T, Rauch G, Fuchs M, Arden C, Veltkamp R. Atrial fibrillation and prestroke cognitive impairment in stroke. J Neurol. 2014;261(3):546–553. doi: 10.1007/s00415-013-7233-3. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson AL, Hohman TJ, Liu D, et al. Adverse vascular risk is related to cognitive decline in older adults. J Alzheimers Dis 2015;44;(4):1361-1373. [DOI] [PMC free article] [PubMed]
- 43.Jozwiak A, Guzik P, Mathew A, Wykretowicz A, Wysocki H. Association of atrial fibrillation and focal neurologic deficits with impaired cognitive function in hospitalized patients > or = 65 years of age. Am J Cardiol. 2006;98(9):1238–1241. doi: 10.1016/j.amjcard.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 44.Kawabata-Yoshihara LA, Scazufca M, De Souza Santos I, et al. Atrial fibrillation and dementia: results from the São Paulo ageing & health study. Arq Bras Cardiol. 2012;99(6):1108–1114. doi: 10.1590/S0066-782X2012005000106. [DOI] [PubMed] [Google Scholar]
- 45.Liao J-N, Chao T-F, Liu C-J, et al. Risk and prediction of dementia in patients with atrial fibrillation - a nationwide population-based cohort study. Int J Cardiol. 2015;199:25–30. doi: 10.1016/j.ijcard.2015.06.170. [DOI] [PubMed] [Google Scholar]
- 46.Marengoni A, Qiu C, Winblad B, Fratiglioni L. Atrial fibrillation, stroke and dementia in the very old: a population-based study. Neurobiol Aging. 2011;32(7):1336–1337. doi: 10.1016/j.neurobiolaging.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Marzona I, Baviera M, Vannini T, et al. Risk of dementia and death in patients with atrial fibrillation: a competing risk analysis of a population-based cohort. Int J Cardiol. 2016;220:440–444. doi: 10.1016/j.ijcard.2016.06.235. [DOI] [PubMed] [Google Scholar]
- 48.Peters R, Poulter R, Beckett N, et al. Cardiovascular and biochemical risk factors for incident dementia in the Hypertension in the Very Elderly Trial. J Hypertens. 2009;27(10):2055–2062. doi: 10.1097/HJH.0b013e32832f4f02. [DOI] [PubMed] [Google Scholar]
- 49.Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr. 2013;57(3):325–327. doi: 10.1016/j.archger.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Pulignano G, Del Sindaco D, Tinti MD, et al. Atrial fibrillation, cognitive impairment, frailty and disability in older heart failure patients. J Cardiovasc Med (Hagerstown) 2016;17(8):616–623. doi: 10.2459/JCM.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 51.Salehi R, Enamzadeh E, Goldust M. Study of cognitive disorders in stroke-free patients with a history of atrial fibrillation. Pakistan J Biol Sci PJBS. 2013;16(1):44–47. doi: 10.3923/pjbs.2013.44.47. [DOI] [PubMed] [Google Scholar]
- 52.Singh-Manoux A, Fayosse A, Sabia S, et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J. 2017;38(34):2612–2618. doi: 10.1093/eurheartj/ehx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilvis RS, Kähönen-Väre MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59(3):268–274. doi: 10.1093/gerona/59.3.M268. [DOI] [PubMed] [Google Scholar]
- 54.Walters K., Hardoon S., Petersen I., et al. Predicting dementia risk in primary care: development and validation of the dementia risk score using routinely collected data. BMC Med 2016;14;(1). [DOI] [PMC free article] [PubMed]
- 55.Elias MF, Sullivan LM, Elias PK, et al. Atrial fibrillation is associated with lower cognitive performance in the Framingham offspring men. J stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2006;15(5):214–222. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Rusanen M, Kivipelto M, Levälahti E, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis. 2014;42(1):183–191. doi: 10.3233/JAD-132363. [DOI] [PubMed] [Google Scholar]
- 57.Haring B, Leng X, Robinson J, et al. Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women’s Health Initiative Memory Study. J Am Heart Assoc. 2013;2(6):e000369. doi: 10.1161/JAHA.113.000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Habeych ME, Castilla-Puentes R. Comorbid medical conditions in vascular dementia: a matched case-control study. J Nerv Ment Dis. 2015;203(8):604–608. doi: 10.1097/NMD.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 59.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 60.Kokkinidis DG, Zareifopoulos N, Theochari CA, et al. Association between atrial fibrillation and cognitive impairment in individuals with prior stroke: a meta-analysis and meta-regression analysis. Stroke. 2020;51(6):1662–1666. doi: 10.1161/STROKEAHA.119.027815. [DOI] [PubMed] [Google Scholar]
- 61.Islam MM, Poly TN, Walther BA, et al. Association between atrial fibrillation and dementia: a meta-analysis. Front Aging Neurosci. 2019;11:305. doi: 10.3389/fnagi.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5_Part_1):338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigurdsson S, Aspelund T, Kjartansson O, et al. Incidence of brain infarcts, cognitive change, and risk of dementia in the general population: the AGES-Reykjavik Study (Age Gene/Environment Susceptibility-Reykjavik Study) Stroke. 2017;48(9):2353–2360. doi: 10.1161/STROKEAHA.117.017357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bunch TJ, Crandall BG, Weiss JP, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(8):839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 66.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2016;50(5):e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 67.Moffitt P, Lane DA, Park H, O’Connell J, Quinn TJ. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: systematic review. Age Ageing. 2016;45(6):767–775. doi: 10.1093/ageing/afw104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 31 kb)
(DOCX 26 kb)
(DOCX 27 kb)
(DOCX 44 kb)
(DOCX 22 kb)
(DOCX 26.9 kb)
(DOCX 25 kb)
(DOCX 22 kb)
(DOCX 24.1 kb)
(DOCX 22.7 kb)
(DOCX 62 kb)
(DOCX 57 kb)
(DOCX 24 kb)
(DOCX 8 kb)
(DOCX 14 kb)
(DOCX 29 kb)
(DOCX 11 kb)
(DOCX 8 kb)