Prescribing therapeutically equivalent, lower-cost generic drugs is among the most cost-effective health care interventions available in the USA.1 Such cost savings are important for Medicaid, since increased spending on brand-name prescription drugs in recent years has led several programs to reduce their services or limit eligibility requirements.2 One class of drugs for which generic prescribing could be particularly impactful are pain medications, since opioid analgesics are commonly prescribed in Medicaid.3
We sought to determine the rate of generic utilization and potential savings from generic substitution across state Medicaid programs for two widely popular classes of opioid formulations with therapeutically equivalent generic versions: oxycodone-acetaminophen and hydrocodone-acetaminophen.4
METHODS
The Centers for Medicare & Medicaid Services (CMS) provides quarterly data on drug use by state Medicaid programs. We extracted the total number of units (tablets or capsules only) dispensed for all brand-name and generic oxycodone-acetaminophen (e.g., Percocet) and hydrocodone-acetaminophen (e.g., Vicodin) in 48 states and the District of Columbia (1991–2018). The primary measure was the generic dispensing rate: generic opioid units divided by the total units of brand-name and generic opioids.5
We then estimated potential savings from generic substitution using calculated state-specific prices of generic oxycodone available in Medicaid starting in 1998, the year generic oxycodone became available. The generic price per dosage unit was determined as follows: total generic reimbursement/total generic dosage units (plus $0.02 to account for the price of acetaminophen). We applied the calculated generic price to the number of units dispensed for brand-name prescriptions with equivalent dosage. All savings were expressed in 2018 US dollars adjusted for medical inflation (from the US Bureau of Labor Statistics).
Under federal statute, manufacturers must pay basic and inflationary rebates to state Medicaid programs.6 SSR Health publishes estimates of quarterly Medicaid rebates for select drug products, which allows for an approximation of the total federal rebate. Using the SSR Health database, we extrapolated average Medicaid-specific net WAC discounts for Percocet to estimate annual rebates from 1998 to 2018.
RESULTS
Ten brand-name formulations containing oxycodone-acetaminophen and 19 containing hydrocodone-acetaminophen were prescribed in Medicaid during the study period. Among branded drugs, Endocet (57%) and Roxicet (33%) were the most commonly prescribed oxycodone-acetaminophen combination; Lorcet (31%) and Vicodin (28%) were the most common hydrocodone-acetaminophen formulation.
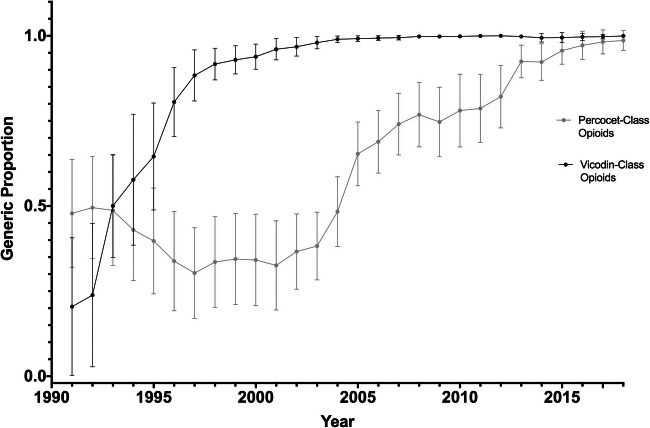
The time to reach a 90% average generic dispensing rate from initial approval of the generic product was 31.2 years (1981–2012) for oxycodone-acetaminophen and 13.0 years (1984–1997) for hydrocodone-acetaminophen (Fig. 1). There was substantial variation across states in generic utilization for both opioid classes, especially in the initial years after generic approval. New Jersey, New York, and Pennsylvania had particularly low average generic dispensing rates for oxycodone-acetaminophen from 2008 to 2018 (72%, 73%, and 76% respectively) compared to the overall average (88%) (Table 1).
Figure 1.
State-level variation in generic proportions of oxycodone-acetaminophen tablet and capsule opioid formulations and hydrocodone-acetaminophen tablet and capsule opioid formulations, 1991 to 2018. Generic dispensing rates were calculated with brand-name and generic oxycodone-acetaminophen and hydrocodone-acetaminophen units dispensed from 1991 to 2018. Error bars denote one standard deviation from the mean generic utilization ratio across 48 states, and the District of Columbia. Arizona, and Tennessee data were not available for these states for the full study period.
Table 1.
Variation in Generic Dispensing Rates for Brand-Name Oxycodone-Acetaminophen Tablet and Capsule Formulations, 2008–2018
| State | Average generic dispensing rate (%) |
|---|---|
| NJ | 72.3 |
| NY | 72.5 |
| PA | 75.7 |
| KY | 77.4 |
| HI | 79.9 |
| DE | 80.1 |
| AL | 81.6 |
| UT | 82.4 |
| GA | 82.7 |
| MS | 83.7 |
| MD | 85.0 |
| AK | 85.1 |
| MO | 85.4 |
| OK | 85.5 |
| DC | 85.5 |
| MT | 86.0 |
| WV | 86.0 |
| WY | 86.2 |
| LA | 86.5 |
| KS | 86.6 |
| SC | 86.6 |
| TX | 86.8 |
| AR | 87.1 |
| NV | 87.3 |
| ID | 87.9 |
| WA | 88.1 |
| NC | 88.2 |
| OR | 88.3 |
| NE | 88.7 |
| VA | 89.4 |
| CA | 90.1 |
| CT | 90.6 |
| FL | 91.3 |
| CO | 91.4 |
| IL | 91.4 |
| IA | 91.5 |
| MI | 91.9 |
| VT | 92.4 |
| OH | 92.7 |
| MA | 93.0 |
| NM | 93.9 |
| WI | 94.1 |
| MN | 94.3 |
| IN | 94.5 |
| ND | 95.2 |
| SD | 95.6 |
| NH | 95.9 |
| RI | 96.3 |
| ME | 97.9 |
For the 10 brand-name oxycodone-acetaminophen drugs, the total spending reported by Medicaid from 1998 to 2018 was $820 million and the estimated spending with generic substitution would have been $581 million, an estimated difference of $239 million, or $108 million accounting for average rebates from SSR Health. For the 19 brand-name hydrocodone-acetaminophen drugs, the total Medicaid spending was $117 million and the estimated spending with generic substitution would have been $78 million, an estimated difference of $39 million, or $18 million with the rebate.
DISCUSSION
Despite the availability of inexpensive generic oxycodone-acetaminophen and hydrocodone-acetaminophen since the 1980s, use of brand-name versions of these combination products remained common for at least a decade—for oxycodone-acetaminophen through 2012—and showed wide variation across Medicaid programs. The majority of brand-name prescribing came from other brand-name products with the same active ingredients but clinically irrelevant variations in formulation (e.g., capsule vs. tablet) or dose (e.g., 300 vs. 325 mg of acetaminophen), making automatic substitution of generics by pharmacists impossible.
Medicaid programs take steps to encourage prescribing of available generic alternatives to expensive brand-name drugs. Yet, we found that brand-name Percocet (9% of all brand-name oxycodone-acetaminophen prescriptions) and Vicodin (28% of all brand-name hydrocodone-acetaminophen prescriptions) continued to be widely dispensed despite the existence of interchangeable generic versions.
A limitation of our study is that we applied average rebate estimates for Percocet to other oxycodone-acetaminophen and hydrocodone-acetaminophen products. However, our estimates are likely conservative, as Percocet would have experienced higher total rebates given its extended time on market.
We found extensive variability in the use of generic combination opioids, notwithstanding mandatory state laws to substitute generic versions of brand-name drugs. As Medicaid and other payers experience financial strains from increasing prescription drug costs, excess spending could be curbed by limiting coverage of expensive brand-name combination drugs that do not provide additional benefits over their generic constituents and enacting laws that provide pharmacists more flexibility in facilitating generic substitution.
Funding
Research funded by Arnold Ventures; Dr. Kesselheim’s work also funded by the Harvard-MIT Center for Regulatory Science
Compliance with ethical standards
Conflict of Interest
Dr. Kesselheim reports having served in the past as an expert witness on behalf of a class of states in multidistrict litigation against opioid manufacturers (2018–2019).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Association for Accessible Medicines. 2017 Generic drug access & savings in the U.S. report. Available at: https://accessiblemeds.org/resources/blog/2017-generic-drug-access-and-savings-us-report Accessed December 1, 2019.
- 2.Barlas S. States try to control Medicaid pharmaceutical costs: numerous, diverse cost pressures force myriad reform efforts. P T. 2015;40(4):260–262. [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser Family Foundation. Utilization and spending trends in Medicaid outpatient prescription drugs. Available at: https://www.kff.org/medicaid/issue-brief/utilization-and-spending-trends-in-medicaid-outpatient-prescription-drugs/ Accessed December 2, 2019.
- 4.Office of Inspector General. Opioids in Ohio Medicaid: review of extreme use and prescribing. Available at: https://oig.hhs.gov/oei/reports/oei-05-18-00010.pdf Accessed December 2, 2019.
- 5.Liberman JN, Roebuck MC. Prescription drug costs and the generic dispensing ratio. J Manag Care Pharm. 2010;16(7):502–506. doi: 10.18553/jmcp.2010.16.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medicaid. Medicaid Drug Rebate Program. Available at: https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/index.html Accessed December 2, 2019.