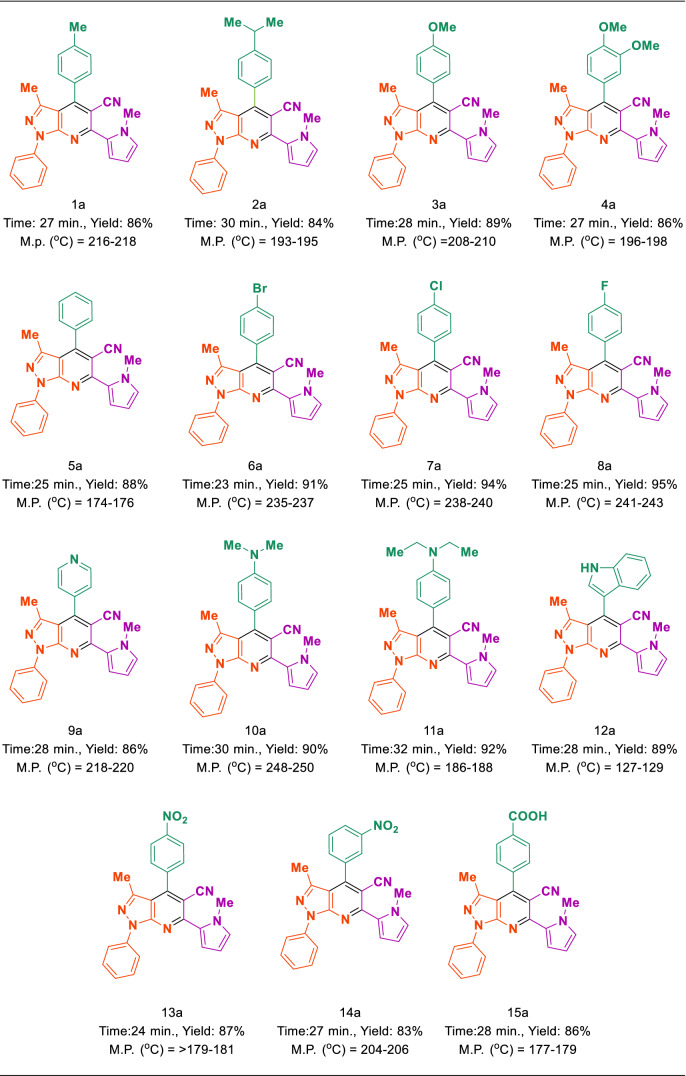
Abstract
In this paper, the MIL-53(Al)-NH2 metal–organic frameworks (MOFs) was prepared based on the anodic electrosynthesis under green conditions. The anodic electrosynthesis as an environmentally friendly procedure was performed in the aqueous solution, room temperature, atmospheric pressure, and in the short reaction time (30 min). Also, the employed procedure was accomplished without the need for the ex-situ salt and base/probase additives as cation source and ligand activating agent at the constant current mode (10.0 mA cm−2). The electrosynthesized MOFs was functionalized with phosphorus acid tags as a novel mesoporous catalyst. This mesoporous catalyst was successfully employed for synthesis of new series (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines by one-pot condensation reaction of 3-methyl-1-phenyl-1H-pyrazol-5-amine, 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile and various aromatic aldehydes (mono, bis and tripodal). This catalyst proceeded the organic synthetic reaction via a cooperative vinylogous anomeric based oxidation mechanism with a marginal decreasing its catalytic activity after recycling and reusability.
Subject terms: Chemistry, Materials science, Nanoscience and technology
Introduction
Metal–organic frameworks (MOFs) have been employed in various fields such as catalysts, electrocatalysts, supercapacitors, drug delivery, sensors, batteries, membranes, and absorbents due to the eye-catching features such as various synthesis methods, structural diversity, high surface area, and attractive architectures1–14. Besides, the potential functionalization and post-modification of MOFs have exposed the art of chemists in different fields15,16. The various documents have been reported for synthesis and electrosynthesis of MOFs as powder and thin films17–29. Behind the beneficial chemical synthesis procedures, the anodic and cathodic electrosynthesis methods are easy to observe and have been received a more acceptable niche and platform because of the environmentally friendly conditions30–34. This prominent glance comes from the one-step, green, and mild strategy that use of renewable electricity source instead of high temperature and high vacuum/pressure as a driving agent30–34. One of the MOFs having been synthesized via chemical and electrochemical procedures is MIL-53-(Al)-NH2, which has been used in different applications35–41. The main drawbacks of conventional chemical methods are the critical synthesis conditions such as high-pressure or -vacuum, prolonged time, and high temperature38–41. While, the anodic electrosynthesis method provides a mild and greener condition for this purpose35–37. In this method the anodic electrode plays the role of cation source for avoiding using of related cation salt which leads to the prevention of pore-blocking by the salt ions. Also, the electroreduction of water molecules on the counter electrode generates the hydroxide ions that lead to the in-situ deprotonation of ligands without the need for any ex-situ base/probase additive. Finally, the coordination and crystallization of MOF can be progressed by a green electricity source instead of high vacuum/pressure as a driving agent30–34. At the next step, the electrosynthesized MOFs was functionalized by phosphorous acid tags. Organphosphoric acid as a catalyst has been used for the oligomerization of light olefins, destructive alkylation of hydrocarbons and organic synthesis42,43. Furthermore, phosphoric acid and its derivatives have been also used in multinuclear NMR, adsorption and extraction44. Materials containing N(CH2PO3H2)2 moieties have environmental impacts worldwide in agricultural, chemical, and pharmaceutical applications45,46. Recently efficient methodologies have been reported for the preparation of metal–organic frameworks47,48, melamine and glycoluril49,50 and mesoporous materials (SBA-15) with phosphorous acid functional groups51.
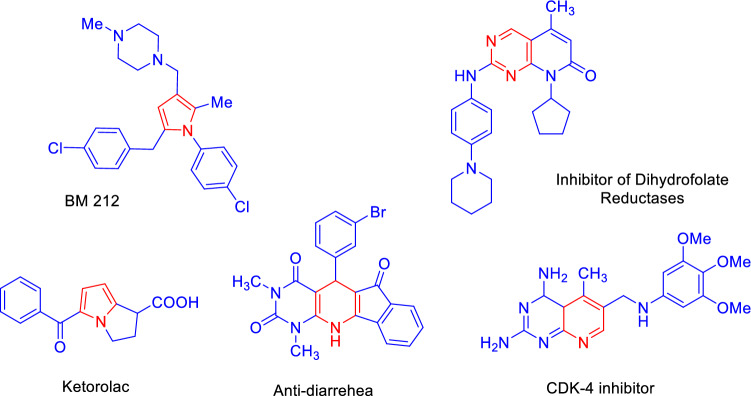
Lots of researches have been done to achieve a novel pyridine and other derivatives as drug candidates52,53. Aryl and heterocyclic compounds substituted with β-ketonitriles have shown a unique class of useful intermediates. Pyran, pyridine and other ring derivatives plays important role in nature and drug development. Molecules with pyran and pyridine moieties are also associated with several important biological and pharmacological activities such as antitumor, antipyretic, analgesic, antiallergic, anti-hypertension, bronchitis dilator, strengthen the heart, blood vessel dilator, antimalarial, anti-fungal, an adenosine kinase inhibitor, diuretic, tyrosine kinas' inhibitors and antibacterial activity54–59. Furthermore, these materials were also applied in the synthesis of BM 212, Ketorolac and other compounds as anti‐fungal and anti‐bacterial, and anti‐inflammatory inhibitors of dihydrofolate reductases, anti-diarrhoea and CDK-4 inhibitor respectively (Fig. 1)60.
Figure 1.
Structure of N-heterocycle rings as drug candidates.
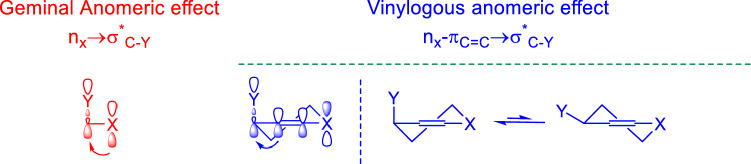
The anomeric interactions extended through double bonds has been named as a vinylogous anomeric effect. The geminal anomeric effect is compared with vinylogous anomeric (Fig. 2). As it can be seen in Fig. 2, in the vinylogous anomeric effect electron density is shared through a double bond but in the geminal anomeric effect donor and acceptor are germinal61. Recently anomeric based oxidation has been introduced for the latter step at the synthesis of susceptible molecules62,63. Since that reduction of substrates by NADH and NADPH2 have proceeded via a hydride transfer64–66. The key feature of the reduction mechanism is hydride transfer from carbon via an anomeric based oxidation mechanism. Herein, two cooperative vinylogous anomeric based oxidation mechanisms were suggested for the synthesis of new molecules with pyridine structure in the presence and absence of oxygen67,68.
Figure 2.
Geminal anomeric effect versus vinylogous anomeric effect61.
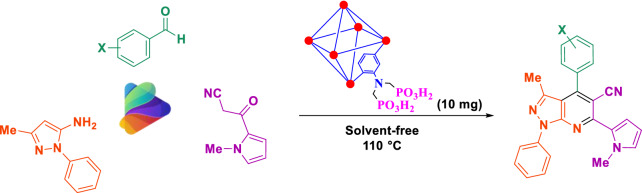
Since molecules with indole moieties are biological interest candidates69 and our background on the comprehensive reviewing of bis and tris indolyl methanes70, we decided to synthesis pyridines with both indole and pyrazole moieties. Therefore, in continuation of our investigations on the development of electrosynthesis of metal–organic frameworks (MOFs) and its chemical post-functionalization with phosphorous acid tags47–51, herein we wish to report a green methodology for preparing of new MIL-53(Al)-N(CH2PO3H2)2 as an efficient mesoporous catalyst. For this purpose, the MIL-53-(Al)-NH2 was successfully fabricated via anodic electrosynthesis technique in the aqueous solution, short reaction time (30 min), room temperature, and atmospheric pressure at the constant current mode (10.0 mA cm−2). It should be noted that the employed procedure was accomplished without the need for the related cation salt as a cation source and ex-situ base/probase additive for deprotonation of the ligand. The final mesoporous catalyst was prepared through functionalizing of MIL-53-(Al)-NH2 with the phosphorous acid tags and utilized for the synthesis of new (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines by the condensation reaction of 3-methyl-1-phenyl-1H-pyrazol-5-amine, 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile and various aromatic aldehydes (mono, bis and tripodal) under green conditions (Fig. 3).
Figure 3.
Synthesis of new (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines using MIL-53(Al)-N(CH2PO3H2)2.
Experimental
Materials
2-Amino terephthalic acid (NH2-H2BDC) (Merck, 95%), potassium nitrate (KNO3) (Sigma-Aldrich, 99%), DMF (Merck, 99%), phosphorous acid (Merck, 99%), 1-methyl-1H-pyrrole (Merck, 98%), 2-cyanoacetic acid (Merck, 98%), EtOH (Merck, 99%) were reagent-grade materials and employed as received without further purification.
Instrumental measurements
From the model of the BRUKER Ultrashield FT-NMR spectrometer (δ in ppm) were recorded 1H NMR (600 or 400 MHz), 13C NMR (151 or 101 MHz). Recorded on a Büchi B-545 apparatus in open capillary tubes were melting points. The PerkinElmer PE-1600-FTIR device was recorded for infrared spectra of compounds. SEM was performed using a scanning electron microscope for field publishing made by TE-SCAN. Thermal gravimetry (TG) and differential thermal gravimetric (DTG) were analyzed by a Perkin Elmer (Model: Pyris 1). BET and BJH were analyzed by BELSORP-mini ii high precision Surface area and pore size. Xrd was analyzed by ITAL STRUCTURE APD2000.
General procedure for the anodic electrosynthesis of MIL-53(Al)-NH2
In a typical anodic electrosynthesis procedure, (0.1 mmol, 0.127 g) potassium nitrate (KNO3) as a supporting electrolyte was dissolved in 45.0 mL distilled water (Solution A). Also, (8.2 mmol, 1.5 g) of 2-amino terephthalic acid (NH2-H2BDC) as a ligand was dissolved in the 5.0 mL EtOH (solution B) and added to solution A (10 Vol% EtOH). The precursor was stirred at room temperature for 15 min before the electrosynthesis. In the following, the prepared precursor transferred to the homemade undivided two-electrode cell consists of a cap glass bottle and two Aluminum plates (100.0 mm × 30.0 mm × 2.0 mm) as working (cation source) and the auxiliary electrodes. Electrosynthesis of MOF was performed by applying 10.0 mA cm−2 current densities for 30 min. The MIL-53-(Al)-NH3 powders were removed from the solution by centrifuge at 5000 rpm for 5 min and rinsed twice with distilled water and DMF. The final MIL-53-(Al)-NH3 was aged overnight at 100 °C.
General procedure for the preparation of MIL-53(Al)-N(CH2PO3H2)2
In a 50 mL round-bottomed flask, MIL-53(Al)-NH2 (0.5 g), paraformaldehyde (5.0 mmol, 0.27 g), phosphorous acid (4.0 mmol, 0.76 g), p-TSA (0.5 mmol, 0.08 g) and ethanol (25 mL) were added and refluxed for 18 h. After this time, a yellow precipitated was filtered off by centrifugation (1000 rpm, 10 min). The obtained solid residue was dried under vacuum to give MIL-53(Al)-N(CH2PO3H2)2 (Fig. 4).
Figure 4.
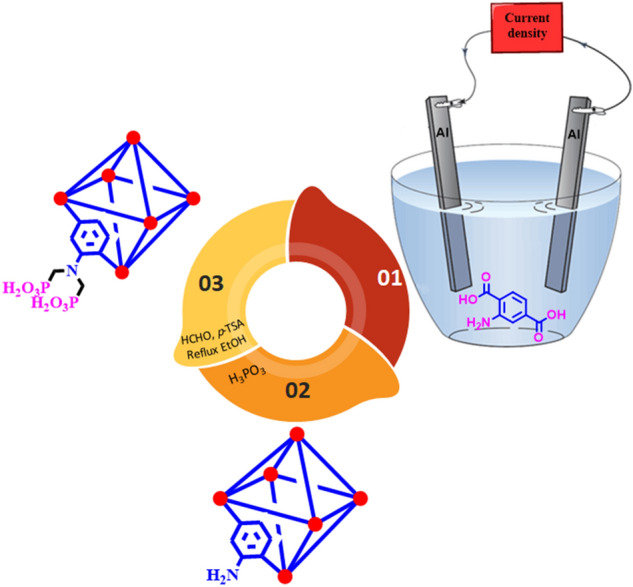
Preparation of MIL-53(Al)-N(CH2PO3H2)2.
General procedure for the synthesis of 3-methyl-6-(1-methyl-1H-pyrrol-3-yl)-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile derivatives using MIL-53(Al)-N(CH2PO3H2)2 as the catalyst
At first 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile was synthesized according to the previously reported literature (Fig. 5)71. Then, a mixture of 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.0 mmol, 0.174 g), aryl aldehyde (1.0 mmol), 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (1.0 mmol, 0.148 g) and MIL-53(Al)-N(CH2PO3H2)2 (10.0 mg) as catalyst were stirred under solvent-free conditions at 110 °C in a 25.0 mL round-bottomed. After completion of the reaction (monitor by TLC n-hexane: ethyl acetate; 7:3). The catalyst was separated by centrifugation (2000 rpm) after adding PEG (5.0 mL) for 5 min. Finally, the mixture was filtered off and washed with ethanol (3 × 10 mL) and the final pure product was obtained (Fig. 3).
Figure 5.
Preparation of 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile.
Spectral data
3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b] pyridine-5-carbonitrile (1a)
White solid; M.p: 216–218 °C; FT-IR (KBr): υ (cm−1) = 3282, 2955, 2923, 2219, 1575, 1556, 1506, 1488, 1415. 1H NMR (600 MHz, DMSO-d6) δ 8.13 (d, J = 7.8 Hz, 2H), 7.60 (t, J = 7.9 Hz, 2H), 7.54 (d, J = 7.9 Hz, 2H), 7.45 (d, J = 7.8 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.13 (s, 1H), 6.99 (dd, J = 3.8, 1.4 Hz, 1H), 6.21 (dd, J = 3.6, 2.7 Hz, 1H), 3.94 (s, 3H), 2.46 (s, 3H), 2.05 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 153.4, 152.4, 149.7, 144.40, 140.0, 138.5, 131.3, 129.7, 129.5, 129.3, 129.0, 128.6, 127.0, 121.8, 118.4, 115.9, 112.5, 108.1, 101.0, 39.5, 36.9, 21.4, 14.9, (See SI, Figs. S1–S3).
4-(4-isopropylphenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b] pyridine-5-carbonitrile (2a)
White solid; M.p: 193–195 °C; FT-IR (KBr): υ (cm−1) = 3067, 2959, 2929, 2866, 2221, 1572, 1558, 1541, 1507, 1415. 1H NMR (600 MHz, DMSO-d6) δ 8.13 (d, J = 7.7 Hz, 2H), 7.60 (t, J = 7.9 Hz, 2H), 7.57 (d, J = 8.1 Hz, 2H), 7.51 (d, J = 8.1 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.13 (s, 1H), 6.99 (dd, J = 3.8, 1.5 Hz, 1H), 6.21 (dd, J = 3.7, 2.7 Hz, 1H), 3.94 (s, 3H), 3.05 (d, J = 6.9 Hz, 1H), 2.04 (s, 3H), 1.31 (d, J = 6.9 Hz, 6H). 13C NMR (151 MHz, DMSO-d6) δ 153.3, 152.5, 150.7, 149.7, 144.4, 138.5, 131.5, 129.7, 129.5, 129.0, 128.6, 127.0, 126.8, 121.8, 118.4, 115.9, 112.6, 108.1, 100.9, 36.9, 33.7, 24.2, 14.8, (See SI, Figs. S4–S6).
4-(4-methoxyphenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b] pyridine-5-carbonitrile (3a)
White solid; M.p: 208–210 °C; FT-IR (KBr): υ (cm−1) = 3018, 2974, 2933, 2838, 2119, 1609, 1577, 1558, 1513, 1437, 1415. 1H NMR (600 MHz, DMSO-d6) δ 8.12 (d, J = 7.9 Hz, 2H), 7.59 (d, J = 8.1 Hz, 3H), 7.39 (t, J = 7.4 Hz, 1H), 7.18 (d, J = 8.6 Hz, 2H), 7.12 (s, 1H), 6.99 (dd, J = 3.7, 1.3 Hz, 1H), 6.22–6.20 (m, 1H), 3.94 (s, 3H), 3.89 (s, 3H), 2.08 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 160.9, 153.2, 152.5, 149.7, 144.4, 138.6, 131.1, 129.7, 128.9, 128.6, 127.0, 126.1, 121.7, 118.5, 115.9, 114.3, 112.7, 108.1, 101.1, 55.7, 36.9, 15.0, (See SI, Figs. S7–S9).
4-(3,4-dimethoxyphenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b] pyridine-5-carbonitrile (4a)
White solid; M.p: 196–198 °C; FT-IR (KBr): υ (cm−1) = 3128, 2992, 2960, 2834, 2224, 1600, 1568, 1516, 1490, 1439. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 7.7 Hz, 2H), 7.61 (t, J = 7.9 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.28 (s, 1H), 7.20 (s, 2H), 7.13 (s, 1H), 7.00 (dd, J = 3.8, 1.4 Hz, 1H), 6.22 (dd, J = 3.7, 2.7 Hz, 1H), 3.94 (s, 3H), 3.89 (s, 3H), 3.83 (s, 3H), 2.13 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 152.7, 152.0, 149.8, 149.2, 148.2, 144.0, 138.1, 129.2, 128.4, 128.1, 126.5, 125.7, 121.8, 121.3, 118.0, 115.4, 112.7, 112.2, 111.2, 107.6, 100.7, 55.7, 55.5, 36.4, 14.5, (See SI, Figs. S10–S12).
3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine-5 carbonitrile (5a)
Brown solid; M.p: 174–178 °C; FT-IR (KBr): υ (cm−1) = 2961, 2923, 2216, 1656, 1599, 1567, 1506, 1487, 1415. 1H NMR (400 MHz, DMSO-d6) δ 8.15–8.10 (m, 2H), 7.65–7.64 (m, 4H), 7.61–7.58 (m, 2H), 7.44–7.36 (m, 1H), 7.14 (dd, J = 2.6, 1.7 Hz, 1H), 7.00 (dd, J = 3.9, 1.7 Hz, 1H), 6.22 (dd, J = 3.9, 2.6 Hz, 1H), 3.95 (s, 3H), 2.02 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 152.7, 151.9, 149.2, 143.8, 138.0, 133.7, 129.8, 129.2, 128.8, 128.5, 128.4, 128.1, 126.6, 121.3, 117.8, 115.5, 112.0, 107.6, 100.4, 36.4, 14.2, (See SI, Figs. S13–S15).
4-(4-bromophenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (6a)
White solid; M.p: 235–237 °C; FT-IR (KBr): υ (cm−1) = 3063, 2983, 2953, 2214, 1995, 1574, 1557, 1506, 1485, 1412. 1H NMR (600 MHz, DMSO-d6) δ 8.12 (d, J = 7.9 Hz, 2H), 7.86 (d, J = 8.3 Hz, 2H), 7.64–7.59 (m, 4H), 7.40 (t, J = 7.4 Hz, 1H), 7.14 (s, 1H), 7.00 (dd, J = 3.8, 1.4 Hz, 1H), 6.22–6.21 (m, 1H), 3.94 (s, 3H), 2.06 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 152.4, 151.9, 149.7, 144.2, 138.5, 135.3, 133.0, 131.4, 129.7, 129.1, 129.1, 128.5, 127.1, 121.8, 118.2, 116.0, 112.4, 108.2, 100.9, 39.5, 36.9, 14.8, (See SI, Figs. S16–S18).
4-(4-chlorophenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (7a)
White solid; M.p: 238–240 °C; FT-IR (KBr): υ (cm−1) = 3083, 2980, 2964, 2216, 1595, 1573, 1555, 1506, 1486, 1413. 1H NMR (600 MHz, DMSO-d6) δ 8.12 (d, J = 7.8 Hz, 2H), 7.71 (q, J = 8.6 Hz, 4H), 7.61 (t, J = 7.9 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.14 (s, 1H), 7.00 (dd, J = 3.8, 1.5 Hz, 1H), 6.22 (dd, J = 3.7, 2.7 Hz, 1H), 3.94 (s, 3H), 2.06 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 152.4, 151.9, 149.7, 144.2, 138.5, 135.3, 133.0, 131.4, 129.7, 129.1, 129.1, 128.5, 127.1, 121.8, 118.2, 116.0, 112.43, 108.2, 100.9, 36.9, 14.8, (See SI, Figs. S19–S21).
4-(4-Fluorophenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (8a)
Light yellow solid; M.p.: 241–243 °C; FT-IR (KBr): υ (cm−1) = 3070, 2960, 2226, 1602, 1563, 1506, 1486, 1412. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 7.8 Hz, 2H), 7.60 (t, J = 7.9 Hz, 2H), 7.54 (d, J = 7.9 Hz, 2H), 7.45 (d, J = 7.8 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.13 (s, 1H), 6.99 (dd, J = 3.8, 1.4 Hz, 1H), 6.21 (dd, J = 3.6, 2.7 Hz, 1H), 3.94 (s, 3H), 2.46 (s, 3H), 2.05 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 164.1, 161.7, 151.9, 151.75, 149.2, 143.8, 138.0, 131.4, 131.4, 130.0, 129.3, 128.6, 128.0, 126.6, 121.3, 117.83, 115.7, 115.5, 115.4, 112.1, 107.7, 100.6, 36.4, 14.3, (See SI, Figs. S22–S24).
3-Methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-4-(pyridin-4-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (9a)
Pale orange solid; M.p: 218–220 °C; FT-IR (KBr): υ (cm−1) = 3041, 2958, 2233, 1596, 1568, 1554, 1540, 1505, 1413. 1H NMR (400 MHz, DMSO-d6) δ 8.87 (dd, J = 4.4, 1.6 Hz, 2H), 8.14–8.09 (m, 2H), 7.72 (dd, J = 4.4, 1.6 Hz, 2H), 7.61 (t, J = 8.0 Hz, 2H), 7.41 (t, J = 7.4 Hz, 1H), 7.17–7.14 (m, 1H), 7.02 (dd, J = 3.9, 1.7 Hz, 1H), 6.23 (dd, J = 3.9, 2.6 Hz, 1H), 3.95 (s, 3H), 2.04 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 151.8, 149.8, 149.6, 149.1, 143.5, 141.7, 137.9, 129.3, 129.2, 128.8, 127.9, 126.7, 123.5, 121.3, 119.9, 117.4, 115.6, 111.2, 107.8, 99.81, 36.5, 14.1, (See SI, Figs. S25–S27).
4-(4-(Dimethylamino)phenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (10a)s
Yellow solid; M.p: 248–250 °C; FT-IR (KBr): υ (cm−1) = 3107, 2906, 2814, 2220, 1605, 1530, 1504, 1486. 1H NMR (400 MHz, DMSO-d6) δ 8.16–8.07 (m, 2H), 7.59 (t, J = 8.0 Hz, 2H), 7.51–7.44 (m, 2H), 7.43–7.34 (m, 1H), 7.10 (t, J = 2.1 Hz, 1H), 6.97 (dd, J = 3.9, 1.7 Hz, 1H), 6.93–6.86 (m, 2H), 6.20 (dd, J = 3.9, 2.6 Hz, 1H), 3.92 (s, 3H), 3.04 (s, 6H), 2.16 (s, 3H), (See SI, Figs. S28–S29).
4-(4-(Diethylamino)phenyl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (11a)
Orange solid; M.p: 186–188 °C; FT-IR (KBr): υ (cm−1) = 2969, 2894, 2870, 2213, 1604, 1568, 1521, 1506, 1484. 1H NMR (400 MHz, DMSO-d6) δ 8.12 (d, J = 7.7 Hz, 2H), 7.59 (t, J = 7.9 Hz, 2H), 7.45 (d, J = 8.8 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 7.11–7.09 (m, 1H), 6.97 (dd, J = 3.9, 1.6 Hz, 1H), 6.84 (d, J = 8.9 Hz, 2H), 6.20 (dd, J = 3.8, 2.6 Hz, 1H), 3.92 (s, 3H), 3.45 (q, J = 6.9 Hz, 4H), 2.19 (s, 3H), 1.17 (t, J = 7.0 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 153.5, 152.3, 149.4, 148.5, 144.0, 138.1, 130.9, 129.2, 128.3, 128.1, 126.4, 121.2, 118.9, 118.6, 115.3, 112.1, 110.3, 107.5, 100.1, 43.6, 36.3, 15.0, 12.3, (See SI, Figs. S30–S32).
4-(1H-Indol-3-yl)-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (12a)
Light brown solid; M.p: 127–129 °C; FT-IR (KBr): υ (cm−1) = 3414, 3114, 3062, 2971, 2930, 2218, 1618, 1596, 1558, 1507, 1486, 1415. 1H NMR (400 MHz, DMSO-d6) δ 11.94 (s, 1H), 8.19–8.11 (m, 2H), 7.96 (s, 1H), 7.60 (t, J = 8.0 Hz, 3H), 7.42 (dd, J = 16.1, 7.7 Hz, 2H), 7.24 (t, J = 7.2 Hz, 1H), 7.17–7.10 (m, 2H), 6.99 (dd, J = 3.9, 1.7 Hz, 1H), 6.21 (dd, J = 3.9, 2.6 Hz, 1H), 3.96 (s, 3H), 2.07 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 152.4, 149.5, 147.2, 144.2, 138.1, 135.9, 129.2, 128.2, 127.6, 126.5, 126.2, 122.1, 121.3, 120.2, 119.2, 118.6, 115.3, 113.0, 112.2, 107.9, 107.5, 101.3, 36.35, 14.3, (See SI, Figs. S33–S35).
3-Methyl-6-(1-methyl-1H-pyrrol-2-yl)-4-(4-nitrophenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (13a)
Brown solid; M.p: 179–181 °C; FT-IR (KBr): υ (cm−1) = 3071, 2952, 2220, 1598, 1567, 1557, 1507, 1439, 1415. 1H NMR (400 MHz, DMSO-d6) δ 8.49 (d, J = 8.3 Hz, 1H), 8.13 (t, J = 7.7 Hz, 3H), 7.98 (d, J = 8.1 Hz, 1H), 7.61 (s, 2H), 7.43–7.37 (m, 2H), 7.15 (s, 1H), 7.05–7.01 (m, 1H), 6.23 (s, 1H), 3.95 (s, 3H), 2.02 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 151.8, 150.3, 149.1, 148.3, 143.6, 140.1, 138.0, 130.6, 130.3, 129.3, 129.2, 128.8, 126.7, 124.9, 123.6, 122.5, 121.34, 119.8, 117.5, 115.6, 107.8, 36.5, 14.3, (See SI, Figs. S36–S38).
3-Methyl-6-(1-methyl-1H-pyrrol-2-yl)-4-(3-nitrophenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14a)
Yellow solid; M.p: 204–206 °C; FT-IR (KBr): υ (cm−1) = 3078, 2955, 2925, 2857, 2214, 1669, 1567, 1535, 1489, 1419. 1H NMR (600 MHz, DMSO-d6) δ 8.60 (s, 1H), 8.52–8.49 (m, 1H), 8.16 (d, J = 7.6 Hz, 1H), 8.13 (d, J = 7.7 Hz, 2H), 7.96 (t, J = 8.0 Hz, 1H), 7.62 (t, J = 7.9 Hz, 2H), 7.41 (t, J = 7.4 Hz, 1H), 7.15 (s, 1H), 7.03 (dd, J = 3.8, 1.5 Hz, 1H), 6.23 (dd, J = 3.7, 2.7 Hz, 1H), 3.95 (s, 3H), 2.03 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 152.4, 151.9, 149.7, 144.2, 138.5, 135.3, 133.0, 131.4, 129.7, 129.1, 129.1, 128.5, 127.1, 121.8, 118.2, 116.0, 112.4, 108.2, 100.9, 36.9, 14.8, (See SI, Figs. S39–S41).
4-(5-Cyano-3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridin-4-yl)benzoic acid (15a)
Beige solid; M.p: 177–179 °C; FT-IR (KBr): υ (cm−1) = 3420, 3107, 2971, 2931, 2217, 1709, 1666, 1599, 1556, 1508, 1439, 1419. 1H NMR (400 MHz, DMSO-d6) δ 8.14–8.11 (m, 4H), 7.66 (d, J = 8.1 Hz, 2H), 7.61 (t, J = 7.9 Hz, 2H), 7.40 (t, J = 7.4 Hz, 1H), 7.15–7.11 (m, 1H), 7.01 (dd, J = 3.9, 1.6 Hz, 1H), 6.22 (dd, J = 3.8, 2.6 Hz, 1H), 3.95 (s, 3H), 2.03 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 167.8, 152.9, 152.4, 149.7, 144.3, 138.5, 136.4, 129.7, 129.6, 129.1, 128.6, 127.1, 121.8, 118.2, 116.0, 112.3, 108.2, 100.7, 36.9, 14.7, (See SI, Figs. S42–S44).
4,4'-(1,4-Phenylene)bis(3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile) (16a)
Beige solid; dec.: 320 °C; FT-IR (KBr): υ (cm−1) = 3064, 2949, 2220, 1646, 1566, 1507, 1436, 1417. 1H NMR (400 MHz, DMSO-d6) δ 1H NMR (400 MHz, DMSO) δ 8.16 (d, J = 8.4 Hz, 4H), 7.93 (d, J = 8.7 Hz, 3H), 7.66–7.58 (m, 5H), 7.41 (d, J = 7.0 Hz, 2H), 7.16 (s, 2H), 7.07 (dd, J = 3.7, 1.4 Hz, 2H), 6.27–6.23 (m, 2H), 3.98 (s, 6H), 2.15 (s, 3H), 2.12 (s, 3H), (See SI, Figs. S45–S46).
4,4'-(1,3-Phenylene)bis(3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile) (17a)ss
Brown solid; M.p: 248–250 °C; FT-IR (KBr): υ (cm−1) = 3463, 2950, 2220, 1660, 1599, 1570, 1558, 1506, 1490, 1416. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 7.8 Hz, 4H), 7.91 (d, J = 7.9 Hz, 2H), 7.63–7.59 (m, 5H), 7.40 (t, J = 7.4 Hz, 3H), 7.14 (d, J = 1.9 Hz, 2H), 7.01 (dd, J = 3.9, 1.6 Hz, 2H), 6.22 (dd, J = 3.7, 2.7 Hz, 2H), 3.96 (s, 6H), 2.13 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 151.8, 151.8, 149.1, 144.0, 138.0, 134.4, 129.9, 129.3, 129.0, 128.7, 128.5, 128.0, 126.6, 121.3, 117.7, 115.5, 112.1, 107.7, 100.6, 36.5, 14.0, (See SI, Figs. S47–S49).
4,4',4''-(((1,3,5-Triazine-2,4,6-triyl)tris(oxy))tris(benzene-4,1-diyl))tris(3-methyl-6-(1-methyl-1H-pyrrol-2-yl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile) (18a)s
Brown solid; M.p: 131–133 °C; FT-IR (KBr): υ (cm−1) = 3118, 2928, 2877, 2806, 2217, 1662, 1612, 1593, 1561, 1516, 1487, 1439. 1H NMR (400 MHz, DMSO-d6) δ 8.15–8.09 (m, 6H), 7.60 (dd, J = 10.9, 5.1 Hz, 6H), 7.46 (d, J = 8.6 Hz, 6H), 7.39 (t, J = 7.4 Hz, 3H), 7.13–7.10 (m, 3H), 7.00 (d, J = 1.8 Hz, 3H), 6.98 (dd, J = 3.3, 2.1 Hz, 6H), 6.21 (dd, J = 3.9, 2.6 Hz, 3H), 3.93 (s, 9H), 2.11 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ 162.2, 159.1, 153.2, 152.0, 149.3, 144.0, 138.1, 130.7, 129.2, 129.0, 128.3, 128.2, 126.4, 123.8, 123.2, 121.2, 118.1, 115.4, 115.2, 112.2, 107.6, 100.5, 30.5, 14.4, (See SI, Figs. S50–S52).
Results and discussion
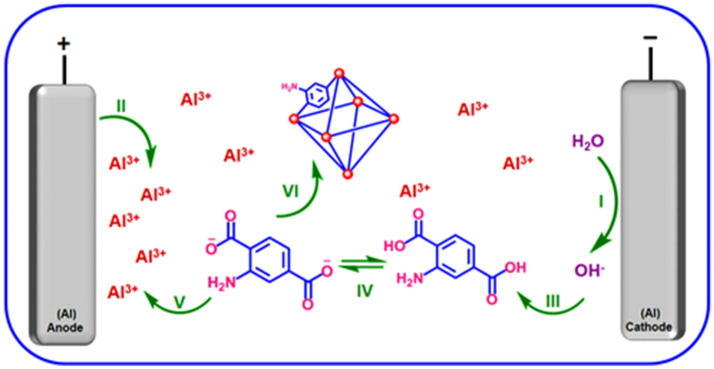
In order to develop the catalytic application of metal–organic frameworks (MOFs), MIL-53(Al)-NH2 was prepared by anodic electrosynthesis technique (Fig. 6). Post-functionalization of MIL-53(Al)-NH2 for preparing of MIL-53(Al)-N(CH2PO3H2)2 was occurred by using paraformaldehyde under refluxing ethanol and presence p-TSA. To shed light of this issue, at the first step, we employed an anodic electrosynthesis procedure for the preparation of MIL-53(Al)-NH2. This mode has accomplished by inserting two aluminium metallic plates in the presence of H2BDC-NH2 and KNO3 as ligand and supporting electrolyte, respectively at room temperature in an EtOH/H2O (10/90, v/v) mixture under stirring. Aluminium metal plays the role of cation source and also the anodic and cathodic electrodes. Upon the establishment of the current density (10.0 mA cm−2 at 30 min), the increased pH at the cathode surface thanks to the in-situ electrogeneration of hydroxide ions makes deprotonation of ligands by electroreduction of water molecules. On the other hand, the anode surface starts to oxidize in order to the preparation of aluminum cations to coordinate with the available ligands. The activated ligands can be coordinated to the available cations to starting the crystallinity of MIL-53(Al)-NH2 (Fig. 6). This process has some advantages as in-situ deprotonation of ligand, in-situ cation electrogeneration, cation-controlled release, and avoiding of the pore-blocking due to the absence of the related cation salt. The MIL-53(Al)-NH2 has prepared without the need of any ex-situ base/probase and cation salt additive, at room temperature, atmospheric pressure, and short reaction time. At the second step, the post-modification of the prepared MIL-53(Al)-NH2 with the phosphorous acid tags was performed in the percent of p-TSA and paraformaldehyde under refluxing ethanol. To shed light of the more details, the full characterization of the MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 as a porous material catalyst was evaluated by FT-IR, X-ray diffraction analysis (XRD), energy-dispersive X-ray spectroscopy (EDX), FE-SEM, elemental mapping, thermal gravimetric (TG) and derivative thermal gravimetric (DTG) techniques.
Figure 6.
Preparation of MIL-53(Al)-NH2 using anodic electrosynthesis method.
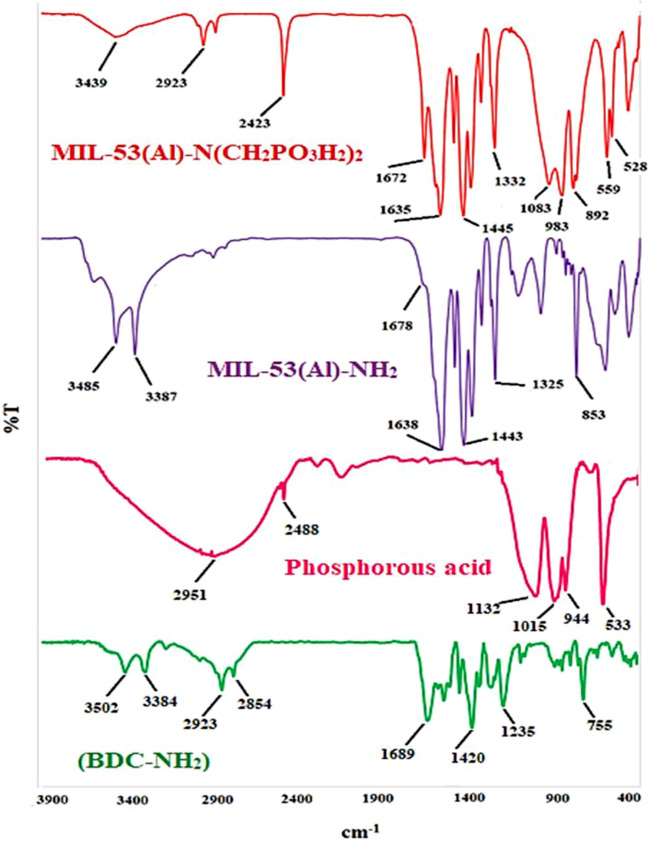
The FT-IR analysis was surveyed for verification of the functional groups and bonding properties in the synthesized MOF as a catalyst. The FT-IR spectra of 2-amino terephthalic acid as susceptible ligand, phosphorus acid, MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 MOFs were shown in Fig. 7. The broad peak 2600–3500 cm−1 is related to the OH of PO3H2 functional groups. Also, the absence of the OH group’s (carboxylic acid) peak 2488–3500 cm−1 in the ligand spectrum. Then, the absence of free C=O functional groups shifting of the characteristic couple bands from 1689 cm−1 in the ligand spectrum to the 1672 cm−1 in the MOFs spectra can be assigned to the coordination of the COO– groups with the aluminium metallic cations. Besides, the presence of the two sharp bands at 3485 and 3387 cm−1 in the MIL-53(Al)-NH2 spectrum indicates the symmetric and asymmetric vibrations of the NH2 groups35–37,72–78. The absorption bands at 944 and 1015 cm−1 are related to P-O bond stretching and the band at 1132 cm−1 is related to P=O. The FT-IR spectrum difference between MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 verified the structure of the desired post-synthesized catalyst.
Figure 7.
FT-IR analysis of 2-amino terephthalic acid, phosphorous acid, MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2.
To study the purity and crystallinity of the synthesized MOF structures, the XRD patterns were recorded. According to the XRD profile (Fig. 8), the MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 are isoreticualr materials with suitable crystallinity and without any impurity or contaminants. Comparing the three characteristic peaks located at the 2θ = 9.27°, 12.40°, and 17.50°, 25.10° with previously reported data is approved their structures35–37,72–78.
Figure 8.
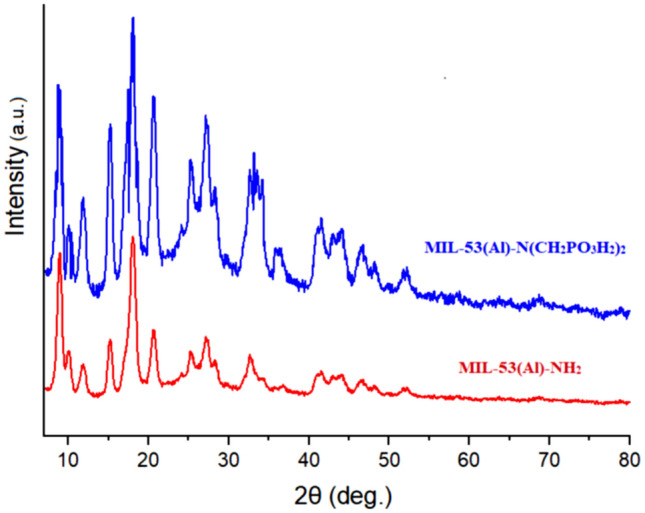
XRD pattern of MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2.
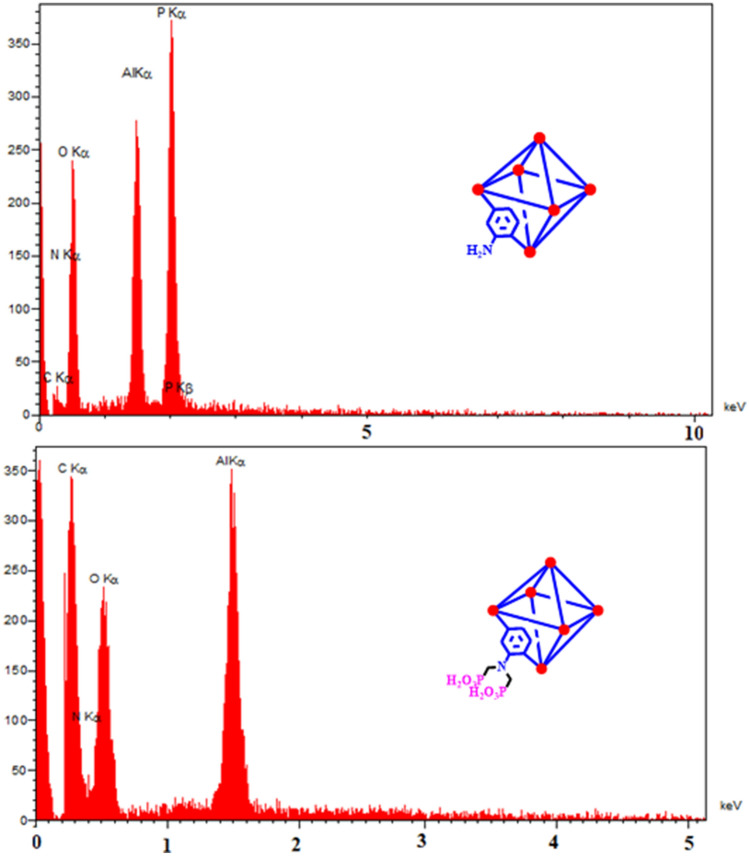
For more details, the EDX analysis was also accomplished for elemental analysis and elemental dispersity of prepared MOFs (Fig. 9). The emerged elemental peaks approved the existence of C, O, N, and Al elements in the MIL-53(Al)-NH2 structure and P element in the MIL-53(Al)-N(CH2PO3H2)2 structure. Besides, the EDX mapping images indicates the uniform and homogeneous distribution of elements (Fig. 10).
Figure 9.
EDX analysis and elemental mapping of electro-synthesized MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2.
Figure 10.
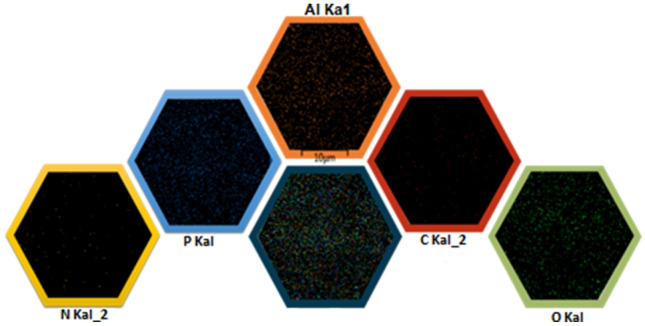
Elemental maps (EDX) of C (red); N (yellow); Al (orange); P (blue) and O (green) atoms for MIL-53(Al)-N(CH2PO3H2.
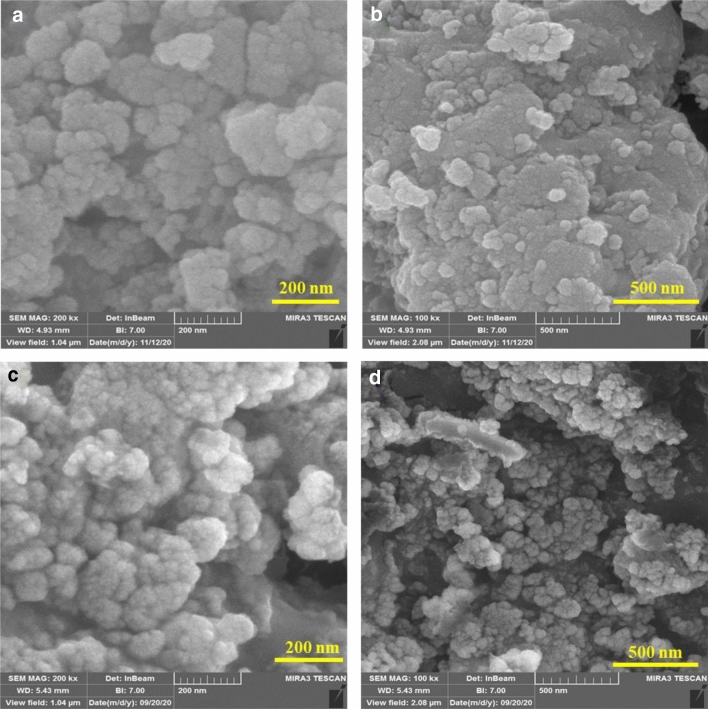
Also, FE-SEM images of electro-synthesized MIL-53(Al)-NH2 and its post-functionalized one were recorded for investigation of their morphologies (Fig. 11). The obtained images exhibit the uniform cauliflower-shaped nanoparticles with an average diameter size of around 33.0 nm. The increasing of the EtOH/H2O ratio than pure H2O solvent in the electrosynthesis procedure can lead to the lower crystallinity of structure and smaller particles32–34.
Figure 11.
FE-SEM images of MIL-53(Al)-NH2 (a,b) and MIL-53(Al)-N(CH2PO3H2)2(c,d).
The specific surface area and pore size distribution are considered as significant parameters of the prepared porous materials and can be affected on the catalysis performance. In this way, the N2 adsorption/desorption technique was employed for the investigation of the MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 structure porosity (Fig. 12). As can be seen, the N2 adsorption/desorption plots exhibit the IV-type isotherms with the hysteresis loops as a feature of the mesoporous materials. It is noteworthy, the created mesophase is consistent with the fact that increased EtOH/H2O ratio in the synthesis process can lead to mesoporosity structures32–34. According to the obtained BET results, the specific surface areas of MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 structure are 204.75 and 132.19 m2 g−1, respectively. Also, according to the obtained BJH information, the pore size of MIL-53(Al)-NH2 and MIL-53(Al)-N(CH2PO3H2)2 structure was determined to be 4.2 nm and 3.2 nm, respectively. It should be noted that the surface area and pore size of functionalized MOF was decreased. Thus, it can be assigned to the presence of the larger phosphorus acid tags than amine groups during the post-modification process.
Figure 12.
N2 adsorption/desorption isotherm and BJH of MIL-53(Al)-N(CH2PO3H2)2 and MIL-53(Al)-N(CH2PO3H2)2.
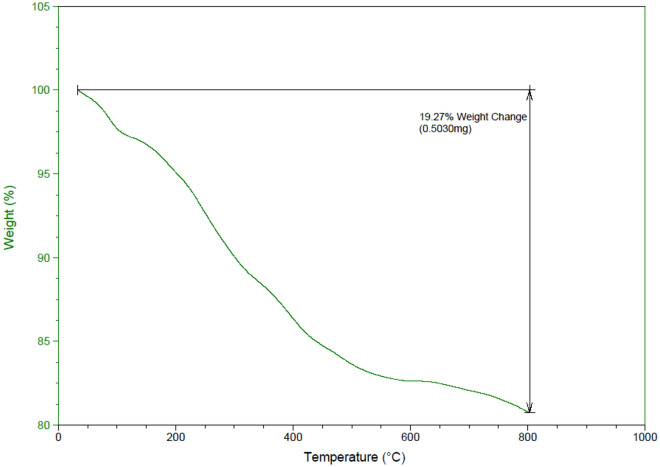
The thermogravimetric (TGA) analysis for MIL-53(Al)-N(CH2PO3H2)2 was performed to study at range of 50–800 °C, with a temperature increase rate of 10 °C min−1 in CO2 atmosphere (Fig. 13). The first distinguished weight loss step in the temperature zone of 50–100 °C can be assigned to the evaporation and removal of solvents. The second distinguished weight loss step in the temperature zone of 150–450 °C can be assigned to the decomposition of the organic section of material includes the ligand and phosphorus acid which leads to the degradation and collapse of the framework.
Figure 13.
Thermogravimetric (TGA) analysis of MIL-53(Al)-N(CH2PO3H2)2.
After identification of MIL-53(Al)-N(CH2PO3H2)2 as mesoporous hybrid-catalyst, we tested its catalytic activity in the preparation of novel organic and biological interest candidates (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines derivatives. Optimization of reaction for synthesis of target molecules were done by one-pot reaction of benzaldehyde (1.0 mmol, 0.106 g), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.0 mmol, 0.174 g) and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (1.0 mmol, 0.148 g) as a model reaction. The optimized data is listed in Table 1. As shown, the most optimal one-pot reaction for the preparation of (N-methyl-pyrrol)-pyrazolo[3,4- b]pyridines is reported in the presence of 10.0 mg MIL-53(Al)-N(CH2PO3H2)2 at 110 °C under solvent-free condition (Table 1 entry 4). The obtained data for the model reaction using other amounts of catalyst and temperature shows that the yield and time were not improved (Table 1 entries 1–8 except 4). The model reaction was also studied by using several solvents such as EtOH, CH2Cl2, CHCl3, EtOAc, CH3CN, PEG, n-Hexane, H2O and DMF (5.0 mL) and solvent-free condition in the presence of 10.0 mg of catalyst. The results of the reaction did not improve (Table 1, entries 9–17).
Table 1.
Effect of different amounts of catalysts, temperature and solvent (5 mL) in the synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines.
 | |||||
|---|---|---|---|---|---|
| Entry | Amount of cat. (mg) | Temp. (°C) | Time (min) | Solvent | Yielda (%) |
| 1 | – | 110 | 60 | – | Trace |
| 2 | 5 | 110 | 60 | – | 35 |
| 3 | 7.5 | 110 | 60 | – | 55 |
| 4 | 10 | 110 | 25 | – | 88 |
| 5 | 15 | 110 | 25 | – | 88 |
| 6 | 10 | 75 | 60 | – | 65 |
| 7 | 10 | 50 | 60 | – | 40 |
| 8 | 10 | r.t | 60 | – | Trace |
| 9 | 10 | Reflux | 60 | EtOH | 52 |
| 10 | 10 | Reflux | 60 | CH2Cl2 | Trace |
| 11 | 10 | Reflux | 60 | CHCl3 | 45 |
| 12 | 10 | Reflux | 60 | EtOAc | 48 |
| 13 | 10 | Reflux | 60 | CH3CN | 55 |
| 14 | 10 | 110 | 60 | PEG | 65 |
| 15 | 10 | Reflux | 60 | n-Hexane | Trace |
| 16 | 10 | Reflux | 60 | H2O | 45 |
| 17 | 10 | 110 | 60 | DMF | 55 |
aReaction conditions: benzaldehyde (1.0 mmol, 0.106 g), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.0 mmol, 0.174 g) and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (1.0 mmol, 0.148 g).
After optimizing the reaction conditions, MIL-53(Al)-N(CH2PO3H2)2 (10.0 mg) is applied to synthesis a good range of new biological and pharmacological interest candidates using various aromatic aldehyde derivatives (trephetaldehyde, iso-trephetaldehyde, tripodal-aldehyde, heterocycle, bearing electron-donating and electron-withdrawing groups), 3-methyl-1-phenyl-1H-pyrazol-5-amine and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile. As shown in Tables 2 and 3, the obtained results indicated that MIL-53(Al)-N(CH2PO3H2)2 is appropriate for the preparation of target molecules in high to excellent yields (65–92%) within relatively short reaction times (20–40 min). Interestingly, when we synthesized (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines in the percent indole aldehyde as a biological and pharmacological compound (Table 2). Also, the preparation of bis and tris-(N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines by the condensation of 3-methyl-1-phenyl-1H-pyrazol-5-amine and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile with terephthaldehyde, iso-terephthaldehyde and tripodal aldehyde using MIL-53(Al)-N(CH2PO3H2)2 (10.0 mg) at 110 °C under solvent-free condition (Table 3).
Table 2.
Synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines using MIL-53(Al)-N(CH2PO3H2)2 (10 mg) at 110 °C under solvent-free condition.
Table 3.
Synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines using MIL-53(Al)-N(CH2PO3H2)2 (10 mg) at 110 °C under solvent-free condition.
In suggested mechanism, aldehyde is activated with PO3H2 tags of MIL-53(Al)-N(CH2PO3H2)2 and intermediate (I) is prepared by reaction of 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile with the loss of H2O. In the second step, phenyl pyrazol-5-amine react with intermediate (I) to gives intermediate (II) after tautomerization. Then, intermediate (II) gives intermediates (III) after intramolecular cyclization and loss of one molecule of H2O. Then, the corresponding product (IV) is produced via a cooperative vinylogous anomeric based oxidation mechanism both in the presence and absence of oxygen (pathways A and B in Fig. 14)62,63,67,68,79. It should be mentioned that the reaction in the absence of oxygen and under both nitrogen and argon atmospheres have proceeded as same as in the presence of oxygen. Recently, we have comprehensively showed that the hetero atoms have stereoelectronic effects of common origin and that anomeric effect is one of them80.
Figure 14.
Plausible mechanisms for the synthesis (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines using MIL-53(Al)-N(CH2PO3H2)2.
To evaluate the performance of MIL-53(Al)-N(CH2PO3H2)2 as a catalyst for the synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines, we have tested various acid catalysts (organic and inorganic) in the reaction of benzaldehyde (1.0 mmol, 0.106 g), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.0 mmol, 0.174 g) and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (1.0 mmol, 0.148 g) in Table 4. The obtained results which are collected in Table 4 shows that, MIL-53(Al)-N(CH2PO3H2)2 is the best catalyst for the synthesis of a novel (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines.
Table 4.
Evaluation of various catalyst for the synthesis of novel (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines in comparison with MIL-53(Al)-N(CH2PO3H2)2.
 | ||||
|---|---|---|---|---|
| Entry | Catalyst | (mg) | Time (min) | Yield (%) |
| 1 | [PVI-SO3H]FeCl481 | 10 | 60 | 55 |
| 2 | NaHSO4 | 10 mol% | 60 | 28 |
| 3 | Fe3O4 | 10 | 60 | 35 |
| 4 | SSA82 | 10 | 60 | 45 |
| 5 | [Fe3O4@SiO2@(CH2)3-DABCO-SO3H]Cl283 | 10 | 60 | 35 |
| 6 | FeCl3 | 10 mol% | 60 | 45 |
| 7 | TrBr84 | 10 mol% | 60 | Trace |
| 8 | GTBSA85 | 10 mol% | 60 | 65 |
| 9 | Mg(NO3)2·6H2O | 10 mol% | 60 | Trace |
| 10 | [Py-SO3H]Cl86 | 10 mol% | 60 | 65 |
| 11 | NH4NO3 | 10 mol% | 60 | – |
| 12 | MIL-100(Cr)/NHEtN(CH2PO3H2)247 | 10 | 60 | 75 |
| 13 | APVPB87 | 10 | 60 | 45 |
| 14 | MHMHPA50 | 10 mol% | 60 | 55 |
| 15 | MIL-53(Al)-N(CH2PO3H2)2This work | 10 | 25 | 88 |
The obtained results of catalytic activity and reusability of MIL-53(Al)-N(CH2PO3H2)2 were added in (Fig. 15). As above said, MIL-53(Al)-N(CH2PO3H2)2 was separated by centrifugation and reused without significantly reducing its catalytic activity. For this purpose, recyclability of the catalyst was also studied on the one-pot reaction of benzaldehyde (1.0 mmol, 0.106 g), 3-methyl-1-phenyl-1H-pyrazol-5-amine (1.0 mmol, 0.174 g) and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (1.0 mmol, 0.148 g) as a model reaction under the above-mentioned optimized reaction conditions. Therefore, MIL-53(Al)-N(CH2PO3H2)2 can be reused up to five times without noticeable changes in its catalytic activity.
Figure 15.
Reusability of MIL-53(Al)-N(CH2PO3H2)2.
The large scale synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines by the reaction of aldehyde (20 mmol), 3-methyl-1-phenyl-1H-pyrazol-5-amine (20 mmol, 3.48 g) and 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile (20 mmol, 2.96 g) using MIL-53(Al)-N(CH2PO3H2)2 (1 g) at 110 °C (Table 5).
Table 5.
Large scale synthesis of (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines.
| Entry | Product | Time (min) | Yield (%) | M.p. °C |
|---|---|---|---|---|
| 1 |  |
55 | 75 | 216–218 |
| 2 |  |
60 | 65 | 209–211 |
Conclusion
In this study, electrosynthesis of MIL-53(Al)-NH2 as a metal–organic framework was presented. The anodic electrosynthesis as an environmentally friendly technique procedure was performed in the aqueous solution, room temperature, and atmospheric pressure in the shortest possible time. Also, the employed electrochemical technique provided a promising procedure for the preparation of mesoporous catalyst with a shorter time and high yield. Then its functionalization with the phosphorus acid tags through the post-modification process has occurred. It is noteworthy, this procedure was accomplished without the need for the ex-situ salt and base/pre-base additives as cation source and ligand activating agent, respectively. MIL-53(Al)-N(CH2PO3H2)2 as an efficient catalyst was used for the synthesis of the novel (N-methyl-pyrrol)-pyrazolo[3,4-b]pyridines as biological interest molecules via a cooperative vinylogous anomeric based oxidation mechanism. Short reaction time, clean profile of reaction, recycling and reusing of catalyst are the major advantages of the presented work. We think that the present work can open up a promising insight for developing of anomeric effect in the course of organic synthesis.
Supplementary Information
Acknowledgements
We thank the Bu-Ali Sina University and Iran National Science Foundation (INSF) (Grant Number: 98001912) for financial support.
Author contributions
S.K., H.S. S.A and M.Z.; methodology, validation, investigation, writing the original draft. M.A.Z.; supervision, resources, project administration, funding acquisition, conceptualization, writing-review. D.N., H.S and J.A. supervision, conceptualization of electrosynthesis parts and writing-review and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahmoud Zarei, Email: mahmoud8103@yahoo.com.
Mohammad Ali Zolfigol, Email: zolfi@basu.ac.ir.
Davood Nematollahi, Email: nemat@basu.ac.ir.
Saber Alizadeh, Email: s.alizadeh93@basu.ac.ir.
Hu Shi, Email: hshi@sxu.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-97801-7.
References
- 1.Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341:1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- 2.Long JR, Yaghi OM. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009;38:1213–1214. doi: 10.1039/b903811f. [DOI] [PubMed] [Google Scholar]
- 3.Sumida K, Liang K, Reboul J, Ibarra IA, Furukawa S, Falcaro P. Sol–gel processing of metal–organic frameworks. Chem. Mater. 2017;29:2626–2645. doi: 10.1021/acs.chemmater.6b03934. [DOI] [Google Scholar]
- 4.Czaja AU, Trukhan N, Müller U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009;38:1284–1293. doi: 10.1039/b804680h. [DOI] [PubMed] [Google Scholar]
- 5.Mueller U, Schubert M, Teich F, Puetter H, Schierle-Arndt K, Pastre J. Metal–organic frameworks-prospective industrial applications. J. Mater. Chem. 2006;16:626–636. doi: 10.1039/B511962F. [DOI] [Google Scholar]
- 6.Silva P, Vilela SM, Tomé JP, Paz FAA. Multifunctional metal–organic frameworks: from academia to industrial applications. Chem. Soc. Rev. 2015;44:6774–6803. doi: 10.1039/C5CS00307E. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Zhou Y, Liu S, Xu M. The applications of metal− organic frameworks in electrochemical sensors. ChemElectroChem. 2018;5:6–19. doi: 10.1002/celc.201700931. [DOI] [Google Scholar]
- 8.Sheberla D, Bachman JC, Elias JS, Sun CJ, Shao-Horn Y, Dincă M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017;16:220–224. doi: 10.1038/nmat4766. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Wöll C. Surface-supported metal–organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017;46:5730–5770. doi: 10.1039/C7CS00315C. [DOI] [PubMed] [Google Scholar]
- 10.Huang K, Wang B, Guo S, Li K. Micropatterned ultrathin MOF membranes with enhanced molecular sieving property. Angew. Chem. Int. Ed. 2018;57:13892–13896. doi: 10.1002/anie.201809872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu ZG, Chen SC, Fu WQ, Zheng Q, Zhang J. Epitaxial growth of MOF thin film for modifying the dielectric layer in organic field-effect transistors. ACS Appl. Mater. Interfaces. 2017;9:7259–7264. doi: 10.1021/acsami.6b14541. [DOI] [PubMed] [Google Scholar]
- 12.Ghamari F, Raoufi D, Alizadeh S, Arjomandi J, Nematollahi D. Construction of highly efficient new binder-free bimetallic metal–organic framework symmetric supercapacitors: CONSIDERING surface statistical and morphological analyses. J. Mater. Chem. A. 2021;9:15381–15393. doi: 10.1039/D1TA01724A. [DOI] [Google Scholar]
- 13.Zhang X, Kitao T, Piga D, Hongu R, Bracco S, Comotti A, Sozzani P, Uemura T. Carbonization of single polyacrylonitrile chains in coordination nanospaces. Chem. Sci. 2020;11:10844–10849. doi: 10.1039/D0SC02048F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochizuki S, Kitao T, Uemura T. Controlled polymerizations using metal–organic frameworks. Chem. Commun. 2018;54:11843–11856. doi: 10.1039/C8CC06415F. [DOI] [PubMed] [Google Scholar]
- 15.Kong X, Deng H, Yan F, Kim J, Swisher JA, Smit B, Yaghi OM, Reimer JA. Mapping of functional groups in metal-organic frameworks. Science. 2013;341:882–885. doi: 10.1126/science.1238339. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Cohen SM. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009;38:1315–1329. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]
- 17.Stassen I, Styles M, Van Assche T, Campagnol N, Fransaer J, Denayer J, Tan JC, Falcaro P, De Vos D, Ameloot R. Electrochemical film deposition of the zirconium metal-organic framework UiO-66 and application in a miniaturized sorbent trap. Chem. Mater. 2015;27:1801–1807. doi: 10.1021/cm504806p. [DOI] [Google Scholar]
- 18.Li M, Dinca M. Reductive electrosynthesis of crystalline metal–organic frameworks. J. Am. Chem. Soc. 2011;133:12926–12929. doi: 10.1021/ja2041546. [DOI] [PubMed] [Google Scholar]
- 19.Falcaro P, Buso D, Hill AJ, Doherty CM. Patterning techniques for metal organic frameworks. Adv. Mater. 2012;24:3153–3168. doi: 10.1002/adma.201200485. [DOI] [PubMed] [Google Scholar]
- 20.Zacher D, Shekhah O, Wöll C, Fischer RA. Thin films of metal–organic frameworks. Chem. Soc. Rev. 2009;38:1418–1429. doi: 10.1039/b805038b. [DOI] [PubMed] [Google Scholar]
- 21.Betard A, Fischer RA. Metal–organic framework thin films: from fundamentals to applications. Chem. Rev. 2012;112:1055–1083. doi: 10.1021/cr200167v. [DOI] [PubMed] [Google Scholar]
- 22.Soury S, Bahrami A, Alizadeh S, Shahna FG, Nematollahi D. Development of a needle trap device packed with zinc based metal-organic framework sorbent for the sampling and analysis of polycyclic aromatic hydrocarbons in the air. Microchem. J. 2019;148:346–354. doi: 10.1016/j.microc.2019.05.019. [DOI] [Google Scholar]
- 23.Firoozichahak A, Bahrami A, Ghorbani Shahna F, Alizadeh S, Nematollahi D, Farhadian M. UIO-66-NH2 packed needle trap for accurate and reliable sampling and analysis of the halogenated volatile organic compounds in air. Int. J. Environ. Anal. Chem. 2021;101:263–280. doi: 10.1080/03067319.2019.1664497. [DOI] [Google Scholar]
- 24.Firoozichahak A, Bahrami A, Ghorbani Shahna F, Alizadeh S, Nematollahi D, Farhadian M. Development of a needle trap device packed with titanium-based metal-organic framework sorbent for extraction of phenolic derivatives in air. J. Sep. Sci. 2020;43:1011–1018. doi: 10.1002/jssc.201900938. [DOI] [PubMed] [Google Scholar]
- 25.Pirmohammadi Z, Bahrami A, Nematollahi D, Alizadeh S, Ghorbani Shahna F, Rahimpoor R. Determination of urinary methylhippuric acids using MIL-53-NH2(Al) metal–organic framework in microextraction by packed sorbent followed by HPLC–UV analysis. Biomed. Chromatogr. 2020;34:e4725. doi: 10.1002/bmc.4725. [DOI] [PubMed] [Google Scholar]
- 26.Saedi N, Bahrami A, Ghorbani Shahna F, Habibi Mohraz M, Farhadian M, Alizadeh S. A needle trap device packed with MIL-100(Fe) metal organic frameworks for efficient headspace sampling and analysis of urinary BTEXs. Biomed. Chromatogr. 2020;34:e4800. doi: 10.1002/bmc.4800. [DOI] [PubMed] [Google Scholar]
- 27.Langari AAA, Firoozichahak A, Alizadeh S, Nematollahi D, Farhadian M. Efficient extraction of aromatic amines in the air by the needle trap device packed with the zirconium based metal-organic framework sorbent. RSC Adv. 2020;10:13562–13572. doi: 10.1039/D0RA00687D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimpoor R, Firoozichahak A, Nematollahi D, Alizadeh S, Alizadeh PM, Langari AAA. Bio-monitoring of non-metabolized BTEX compounds in urine by dynamic headspace-needle trap device packed with 3D Ni/Co-BTC bimetallic metal-organic framework as an efficient absorbent. Microchem. J. 2021;166:106229. doi: 10.1016/j.microc.2021.106229. [DOI] [Google Scholar]
- 29.Rahimpoor R, Firoozichahak A, Nematollahi D, Alizadeh S, Alizadeh PM, Langari AAA. Determination of halogenated hydrocarbons in urine samples using a needle trap device packed with Ni/Zn–BTC bi-MMOF via the dynamic headspace method. RSC Adv. 2021;11:21537–21547. doi: 10.1039/D1RA03227E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Kutubi H, Gascon J, Sudhölter EJ, Rassaei L. Electrosynthesis of metal–organic frameworks: challenges and opportunities. ChemElectroChem. 2015;2:462–474. doi: 10.1002/celc.201402429. [DOI] [Google Scholar]
- 31.Ji L, Wang J, Wu K, Yang N. Tunable electrochemistry of electrosynthesized copper metal–organic frameworks. Adv. Funct. Mater. 2018;28:1706961. doi: 10.1002/adfm.201706961. [DOI] [Google Scholar]
- 32.Li WJ, Tu M, Cao R, Fischer RA. Metal–organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives. J. Mater. Chem. A. 2016;4:12356–12369. doi: 10.1039/C6TA02118B. [DOI] [Google Scholar]
- 33.Alizadeh S, Nematollahi D. Electrochemically assisted self-assembly technique for the fabrication of mesoporous metal–organic framework thin films: composition of 3D hexagonally packed crystals with 2D honeycomb-like mesopores. J. Am. Chem. Soc. 2017;139:4753–4761. doi: 10.1021/jacs.6b12564. [DOI] [PubMed] [Google Scholar]
- 34.Alizadeh S, Nematollahi D. Convergent and divergent paired electrodeposition of metal-organic framework thin films. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-50390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez Joaristi A, Juan-Alcañiz J, Serra-Crespo P, Kapteijn F, Gascon J. Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ metal organic frameworks. Crystal Growth Des. 2012;12:3489–3498. doi: 10.1021/cg300552w. [DOI] [Google Scholar]
- 36.Cheng X, Zhang A, Hou K, Liu M, Wang Y, Song C, Zhang G, Guo X. Erratum: Size-and morphology-controlled NH2-MIL-53(Al) prepared in DMF water mixed solvents. Dalton Trans. 2014;43:44944505. doi: 10.1039/c3dt51322j. [DOI] [PubMed] [Google Scholar]
- 37.Hod I, Bury W, Karlin DM, Deria P, Kung CW, Katz MJ, So M, Klahr B, Jin D, Chung YW, Odom TW. Directed growth of electroactive metal-organic framework thin films using electrophoretic deposition. Adv. Mater. 2014;26:6295–6300. doi: 10.1002/adma.201401940. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Wang J, Du T, Zhang W, Zhu W, Yang C, Yue T, Sun J, Li T, Wang J. NH2-MIL-53 (Al) metal–organic framework as the smart platform for simultaneous high-performance detection and removal of Hg2+ Inorg. Chem. 2019;58:12573–12581. doi: 10.1021/acs.inorgchem.9b01242. [DOI] [PubMed] [Google Scholar]
- 39.Bolinois L, Kundu T, Wang X, Wang Y, Hu Z, Koh K, Zhao D. Breathing-induced new phase transition in an MIL-53 (Al)–NH2 metal–organic framework under high methane pressures. Chem. Commun. 2017;53:8118–8121. doi: 10.1039/C7CC02743E. [DOI] [PubMed] [Google Scholar]
- 40.Emam HE, Ahmed HB, El-Deib HR, El-Dars FM, Abdelhameed RM. Non-invasive route for desulfurization of fuel using infrared-assisted MIL-53(Al)-NH2 containing fabric. J. Colloid Interface Sci. 2019;556:193–205. doi: 10.1016/j.jcis.2019.08.051. [DOI] [PubMed] [Google Scholar]
- 41.Guan Y, Xia M, Wang X, Cao W, Marchetti A. Water-based preparation of nano-sized NH2-MIL-53(Al) frameworks for enhanced dye removal. Inorg. Chim. Acta. 2019;484:180–184. doi: 10.1016/j.ica.2018.09.036. [DOI] [Google Scholar]
- 42.Meng T, Hao Y-N, Zheng L, Cao M. Organophosphoric acid-derived CoP quantum dots@S, N-codoped graphite carbon as a trifunctional electrocatalyst for overall water splitting and Zn–air batteries. Nanoscale. 2018;10:14613–14626. doi: 10.1039/C8NR03299H. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Liu BY, Yang G, Chai Z. Synthesis of 2-aminophosphates via SN2-type ring openings of aziridines with organophosphoric acids. Org. Lett. 2019;21:4475–4479. doi: 10.1021/acs.orglett.9b01302. [DOI] [PubMed] [Google Scholar]
- 44.Lopes TR, Cipriano DF, Goncalves GR, Honorato HA, Schettino MA, Jr, Cunha AG, Emmerich FG, Freitas JC. Multinuclear magnetic resonance study on the occurrence of phosphorus in activated carbons prepared by chemical activation of lignocellulosic residues from the babassu production. J. Environ. Chem. Eng. 2017;5:6016–6029. doi: 10.1016/j.jece.2017.11.028. [DOI] [Google Scholar]
- 45.Das S, Behera SS, Murmu BM, Mohapatra RK, Mandal D, Samantray R, Parhi PK, Senanayake G. Extraction of scandium (III) from acidic solutions using organo-phosphoric acid reagents: A comparative study. Sep. Purif. Technol. 2018;202:248–258. doi: 10.1016/j.seppur.2018.03.023. [DOI] [Google Scholar]
- 46.Sato T, Watanabe K, Nagase H, Kito H, Niikawa M, Yoshioka Y. Investigation of the hemolytic effects of various organophosphoric acid triesters (OPEs) and their structure-activity relationship. Toxicol. Environ. Chem. 1997;59:305–313. doi: 10.1080/02772249709358444. [DOI] [Google Scholar]
- 47.Sepehrmansouri H, Zarei M, Zolfigol MA, Moosavi-Zare AR, Rostamnia S, Moradi S. Multilinker phosphorous acid anchored En/MIL-100(Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido [4, 5-b] quinolines. Mol. Catal. 2020;481:110303. doi: 10.1016/j.mcat.2019.01.023. [DOI] [Google Scholar]
- 48.Babaee S, Zarei M, Sepehrmansourie H, Zolfigol MA, Rostamnia S. Synthesis of metal–organic frameworks MIL-101(Cr)-NH2 containing phosphorous acid functional groups: Application for the synthesis of N-Amino-2-pyridone and pyrano [2, 3-c] pyrazole derivatives via a cooperative vinylogous anomeric-based oxidation. ACS Omega. 2020;5:6240–6249. doi: 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moradi S, Zolfigol MA, Zarei M, Alonso DA, Khoshnood A. Synthesis of a biological-based glycoluril with phosphorous acid tags as a new nanostructured catalyst: Application for the synthesis of novel natural henna-based compounds. ChemistrySelect. 2018;3:3042–3047. doi: 10.1002/slct.201702544. [DOI] [Google Scholar]
- 50.Afsar J, Zolfigol MA, Khazaei A, Zarei M, Gu Y, Alonso DA, Khoshnood A. Synthesis and application of melamine-based nano catalyst with phosphonic acid tags in the synthesis of (3´-indolyl) pyrazolo [3, 4-b] pyridines via vinylogous anomeric based oxidation. Mol. Catal. 2020;482:110666. doi: 10.1016/j.mcat.2019.110666. [DOI] [Google Scholar]
- 51.Jalili F, Zarei M, Zolfigol MA, Rostamnia S, Moosavi-Zare AR. SBA-15/PrN (CH2PO3H2)2 as a novel and efficient mesoporous solid acid catalyst with phosphorous acid tags and its application on the synthesis of new pyrimido [4,5-b] quinolones and pyrido [2,3-d] pyrimidines via anomeric based oxidation. Microporous Mesoporous Mater. 2020;294:109865. doi: 10.1016/j.micromeso.2019.109865. [DOI] [Google Scholar]
- 52.Hargrove TY, Wawrzak Z, Alexander PW, Chaplin JH, Keenan M, Charman SA, Perez CJ, Waterman MR, Chatelain E, Lepesheva GI. Complexes of Trypanosoma cruzi sterol 14α-demethylase (CYP51) with two pyridine-based drug candidates for Chagas disease: structural basis for pathogen selectivity. J. Biol. Chem. 2013;288:31602–31615. doi: 10.1074/jbc.M113.497990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaban T, Matiychuk V, Ogurtsov V, Chaban I, Nektegayev I. Development of effective anti-inflammatory drug candidates among novel thiazolopyridines. Ukrain. Biochem. J. 2020;92:132–139. doi: 10.15407/ubj92.02.132. [DOI] [Google Scholar]
- 54.Adibian F, Pourali AR, Maleki B, Baghayeri M, Amiri A. One-pot synthesis of dihydro-1H-indeno [1,2-b] pyridines and tetrahydrobenzo [b] pyran derivatives using a new and efficient nanocomposite catalyst based on N-butylsulfonate-functionalized MMWCNTs-D-NH2. Polyhedron. 2020;175:114179. doi: 10.1016/j.poly.2019.114179. [DOI] [Google Scholar]
- 55.Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA. Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido [2,3-d] pyrimidine. J. Med. Chem. 1980;23:327–329. doi: 10.1021/jm00177a025. [DOI] [PubMed] [Google Scholar]
- 56.Deb ML, Bhuyan PJ. Synthesis of novel classes of pyrido [2,3-d]-pyrimidines, Pyrano [2,3-d] pyrimidines, and pteridines. Synth. Commun. 2006;36:3085–3090. doi: 10.1080/00397910600775622. [DOI] [Google Scholar]
- 57.Devi I, Borah HN, Bhuyan PJ. Studies on uracils: a facile one-pot synthesis of oxazino [4,5-d]-, pyrano [2,3-d]-, pyrido [2,3-d]-and pyrimido [4,5-d] pyrimidines using microwave irradiation in the solid state. Tetrahedron Lett. 2004;45:2405–2408. doi: 10.1016/j.tetlet.2004.01.094. [DOI] [Google Scholar]
- 58.Ibrahim BA, Mohareb RM. Uses of ethyl benzoyl acetate for the synthesis of thiophene, pyran, and pyridine derivatives with antitumor activities. J. Heterocycl. Chem. 2020;57:4023–4035. doi: 10.1002/jhet.4112. [DOI] [Google Scholar]
- 59.Mohareb RM, Mikhail IR. Synthesis of Thiazole, Thiophene, pyran and pyridine derivatives derived from 3-phenyl-1H-pyrazol-5(4H)-one with anti-proliferative, tyrosine kinase and PIM-1 kinase inhibitions. Lett. Drug Des. Discov. 2020;17:485–501. doi: 10.2174/1570180816666190618105907. [DOI] [Google Scholar]
- 60.Allais C, Grassot JM, Rodriguez J, Constantieux T. Metal-free multicomponent syntheses of pyridines. Chem. Rev. 2014;114:10829–10868. doi: 10.1021/cr500099b. [DOI] [PubMed] [Google Scholar]
- 61.Alabugin IV. Stereoelectronic Effects: A Bridge Between Structure and Reactivity. Wiley; 2016. [Google Scholar]
- 62.Yarie M. Catalytic anomeric based oxidation. Iranian Journal of Catalysis. 2017;7:85–88. [Google Scholar]
- 63.Yarie M. Spotlight: Catalytic vinylogous anomeric based oxidation (Part I) Iran. J. Catal. 2020;10:79–83. [Google Scholar]
- 64.Bai CB, Wang NX, Xing Y, Lan X-W. Progress on chiral NAD(P)H model compounds. Synlett. 2017;13:402–414. [Google Scholar]
- 65.He T, Shi R, Gong Y, Jiang G, Liu M, Qian S, Wang Z. Base-promoted cascade approach for the preparation of reduced knoevenagel adducts using hantzsch esters as reducing agent in water. Synlett. 2016;27:1864–1869. doi: 10.1055/s-0035-1562099. [DOI] [Google Scholar]
- 66.Hamasaka G, Tsuji H, Uozumi Y. Organoborane-catalyzed hydrogenation of unactivated aldehydes with a Hantzsch ester as a synthetic NAD(P)H analogue. Synlett. 2015;26:2037–2041. doi: 10.1055/s-0034-1378846. [DOI] [Google Scholar]
- 67.Bodaghifard MA, Shafi S. Ionic liquid-immobilized hybrid nanomaterial: An efficient catalyst in the synthesis of benzimidazoles and benzothiazoles via anomeric-based oxidation. J. Iran. Chem. Soc. 2021;18:677–687. doi: 10.1007/s13738-020-02055-1. [DOI] [Google Scholar]
- 68.Baghery S, Zolfigol MA, Maleki F. [TEATNM] and [TEATCM] as novel catalysts for the synthesis of pyridine-3, 5-dicarbonitriles via anomeric-based oxidation. New J. Chem. 2017;41:9276–9290. doi: 10.1039/C7NJ01934C. [DOI] [Google Scholar]
- 69.Shiri M. Indoles in multicomponent processes (MCPs) Chem. Rev. 2012;112:3508–3549. doi: 10.1021/cr2003954. [DOI] [PubMed] [Google Scholar]
- 70.Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z. Bis-and trisindolylmethanes (BIMs and TIMs) Chem. Rev. 2010;110:2250–2293. doi: 10.1021/cr900195a. [DOI] [PubMed] [Google Scholar]
- 71.Al-Matar HM, Adam AY, Khalil KD, Elnagdi MH. Studies with 3-oxoalkanenitriles: Novel rearrangements observed while exploring the utility of 2-cyanoacetyl-1-methylpyrrole as a precursor to pyrrole substituted heterocyclic compounds. ARKIVOC. 2012;6:1–15. doi: 10.3998/ark.5550190.0013.601. [DOI] [Google Scholar]
- 72.Zhang S, Zhou L, Chen M. Amine-functionalized MIL-53(Al) with embedded ruthenium nanoparticles as a highly efficient catalyst for the hydrolytic dehydrogenation of ammonia borane. RSC Adv. 2018;8:12282–12291. doi: 10.1039/C8RA01507D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Xu Y, Li S, Xu F, Zhang Q. Ratio fluorescence detection of tetracycline by a Eu3+/NH2-MIL-53(Al) composite. RSC Adv. 2021;11:2397–2404. doi: 10.1039/D0RA09185E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu S, Ni Y. NH2-MIL-53(Al) nanocrystals: A fluorescent probe for the fast detection of aromatic nitro-compounds and ions in aqueous systems. Analyst. 2019;144:1687–1695. doi: 10.1039/C8AN01976B. [DOI] [PubMed] [Google Scholar]
- 75.Li C, Xiong Z, Zhang J, Wu C. The strengthening role of the amino group in metal–organic framework MIL-53(Al) for methylene blue and malachite green dye adsorption. J. Chem. Eng. Data. 2015;60:3414–3422. doi: 10.1021/acs.jced.5b00692. [DOI] [Google Scholar]
- 76.Seoane B, Téllez C, Coronas J, Staudt C. NH2-MIL-53(Al) and NH2-MIL-101(Al) in sulfur-containing copolyimide mixed matrix membranes for gas separation. Sep. Purif. Technol. 2013;111:72–81. doi: 10.1016/j.seppur.2013.03.034. [DOI] [Google Scholar]
- 77.Serra-Crespo P, Van Der Veen MA, Gobechiya E, Houthoofd K, Filinchuk Y, Kirschhock CE, Martens JA, Sels BF, De Vos DE, Kapteijn F, Gascon J. NH2-MIL-53(Al): A high-contrast reversible solid-state nonlinear optical switch. J. Am. Chem. Soc. 2012;134:8314–8317. doi: 10.1021/ja300655f. [DOI] [PubMed] [Google Scholar]
- 78.Stavitski E, Pidko EA, Couck S, Remy T, Hensen EJ, Weckhuysen BM, Denayer J, Gascon J, Kapteijn F. Complexity behind CO2 capture on NH2-MIL-53(Al) Langmuir. 2011;27:3970–3976. doi: 10.1021/la1045207. [DOI] [PubMed] [Google Scholar]
- 79.Babaee S, Zolfigol MA, Zarei M, Zamanian J. 1,10-Phenanthroline-based molten salt as a bifunctional sulfonic acid catalyst: Application to the synthesis of N-heterocycle compounds via anomeric based oxidation. ChemistrySelect. 2018;3:8947–8954. doi: 10.1002/slct.201801476. [DOI] [Google Scholar]
- 80.Alabugin IV, Kuhn L, Medvedev MG, Krivoshchapov NV, Vil VA, Yaremenko IA, Mehaffy P, Yarie M, Terentev AO, Zolfigol MA. Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone. Chem. Soc. Rev. 2021 doi: 10.1039/D1CS00386K. [DOI] [PubMed] [Google Scholar]
- 81.Sepehrmansourie H, Zarei M, Zolfigol MA, Babaee S, Rostamnia S. Application of novel nanomagnetic metal–organic frameworks as a catalyst for the synthesis of new pyridines and 1,4-dihydropyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-84005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zolfigol MA. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron. 2001;57:9509–9511. doi: 10.1016/S0040-4020(01)00960-7. [DOI] [Google Scholar]
- 83.Rajabi-Salek M, Zolfigol MA, Zarei M. Synthesis of a novel DABCO-based nanomagnetic catalyst with sulfonic acid tags: Application to the synthesis of diverse spiropyrans. Res. Chem. Intermed. 2018;44:5255–5269. doi: 10.1007/s11164-018-3421-1. [DOI] [Google Scholar]
- 84.Zarei M, Zolfigol MA, Moosavi-Zare AR, Noroozizadeh E. Trityl bromide versus nano-magnetic catalyst in the synthesis of henna-based xanthenes and bis-coumarins. J. Iran. Chem. Soc. 2017;14:2187–2198. doi: 10.1007/s13738-017-1155-4. [DOI] [Google Scholar]
- 85.Zarei M, Sepehrmansourie H, Zolfigol MA, Karamian R, Farida SHM. Novel nano-size and crab-like biological-based glycoluril with sulfonic acid tags as a reusable catalyst: Its application to the synthesis of new mono-and bis-spiropyrans and their in vitro biological studies. New J. Chem. 2018;42:14308–14317. doi: 10.1039/C8NJ02470G. [DOI] [Google Scholar]
- 86.Moosavi-Zare AR, Zolfigol MA, Zarei M, Zare A, Khakyzadeh V, Hasaninejad A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel-Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes. Appl. Catal. A. 2013;467:61–68. doi: 10.1016/j.apcata.2013.07.004. [DOI] [Google Scholar]
- 87.Noroozizadeh E, Moosavi-Zare AR, Zolfigol MA, Zarei M, Karamian R, Asadbegy M, Yari S, Farida SHM. Synthesis of bis-coumarins over acetic acid functionalized poly (4-vinylpyridinum) bromide (APVPB) as a green and efficient catalyst under solvent-free conditions and their biological activity. J. Iran. Chem. Soc. 2018;15:471–481. doi: 10.1007/s13738-017-1247-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.