Abstract
Two standardized enzyme immunoassays for the serological diagnosis of candidiasis were developed. The first one detects antimannan antibodies, while the second one detects mannan with a sensitivity of 0.1 ng/ml. These tests were applied to 162 serum samples retrospectively selected from 43 patients with mycologically and clinically proven candidiasis caused by Candida albicans. Forty-three serum samples were positive for mannan, and 63 had significant antibody levels. Strikingly, only five serum samples were simultaneously positive by both tests. When the results were analyzed per patient, 36 (84%) presented at least one serum positive by one test. For 30 of them, positivity by one test was always associated with negative results by the other test for any of the tested sera. For six patients whose sera were positive for either an antigen or an antibody response, a balance between positivity by each test was evidenced by kinetic analysis of sera drawn during the time course of the infection. Controls consisted of 98 serum samples from healthy individuals, 93 serum samples from patients hospitalized in intensive care units, and 39 serum samples from patients with deep mycoses. The sensitivities and specificities were 40 and 98% and 53 and 94% for mannanemia or antibody detection, respectively. These values reached 80 and 93%, respectively, when the results of both tests were combined. These observations, which clearly demonstrate a disparity between circulation of a given mannan catabolite and antimannan antibody response, suggest that use of both enzyme immunoassays may be useful for the routine diagnosis of candidiasis.
Yeasts of the genus Candida have been recognized as important agents of hospital-acquired infections. They have become the fourth most common isolate recovered from blood cultures in the United States (23). Similarly, the rates of candidemia have increased substantially in Europe as well (69, 70). Candidal infections occur on both medical and surgical services, but approximately half of them occur in surgical intensive care units. Depending on the hospital ward, the mortality rate attributable to candidemia ranges from 40 to 60% (46, 73). Difficulties in establishing an early and specific diagnosis of candidal infection are among the recognized reasons for such high mortality rates. The difficulties for clinical diagnosis lie in the absence of specific clinical signs (1, 4). Difficulties for biological diagnosis lie in the opportunistic character of yeasts. Their presence in normally colonized body sites of immunocompromised patients does not prove infection, and they are rarely isolated from infected deep organs or tissues including blood (43, 52, 55). Efforts have been made to find either antibodies against Candida albicans molecules or Candida-derived molecules whose presence in patient sera could indicate deep-tissue invasion. Tests have been developed to detect C. albicans proteins (35, 39, 71), metabolites (62), DNA (5, 15, 63), and polysaccharides. In this regard, a sensitive biochemical test for the detection of glucan, a major structural polysaccharide of the cell wall, has been made commercially available, and promising data from a large number of centers have been documented with a large number of serum samples from patients (29, 39, 40, 42). Like glucans, mannans are major components of the C. albicans cell wall, making up to 7% of the cell dry weight (26a). By contrast to glucans, mannans are noncovalently bound at the cell wall surface and are highly immunogenic (17). They correspond to a large and complex repertoire of mannopyranose units linked by either α-1,6, α-1,3, α-1,2, or β-1,2 linkages (61). Among these units, oligomannose sequences corresponding to epitopes specific for human and animal antibodies, either polyclonal or monoclonal, have been identified; antibody recognition depends on both the type of linkage connecting the mannose units and the length of the mannose chain (17, 19, 22, 32, 47, 61, 65). These epitopes may also be shared by the glycosidic moiety of a large number of different mannoproteins or glycolipids, reinforcing the quantitatively major character of mannose residues in C. albicans cells (64, 65). The use of mannan antigenemia (mannanemia) detection for the immunodiagnosis of systemic candidiasis was suggested by Weiner and Coats-Stephen (72) about two decades ago. Attempts to improve the immunological detection of mannan involved the use of immune complex dissociation by heating sera before performance of the test and the use of monoclonal antibodies that react with defined epitopes (21, 22, 53). These efforts resulted in standardization and a high level of specificity. These tests, however, like the commercially available Pastorex Candida, still lack sensitivity due to the rapid clearance of the antigen from patients’ sera and the test format (latex agglutination) (21, 37, 39, 50).
In contrast to mannanemia detection, tests based on antimannan antibody detection have been used less and less in the clinical diagnostic mycology laboratory because they have been described both as poorly specific and as poorly sensitive. The reasons for the poor specificity and sensitivity could be attributed to the elevated antibody titers in heavily colonized but uninfected hospitalized patients (44) and the possible lack of antibody response in infected immunocompromised patients (24). Although antimannan antibody and mannan antigenemia were used singly, to our knowledge, simultaneous assays for both components in the same sera have never been performed. Thus, in this study, sera from patients with documented candidiasis were tested for the presence of mannanemia and antimannan antibodies. To facilitate the combined detection of both mannan and its antibodies, we (i) developed a double-sandwich enzyme immunoassay (EIA) using the monoclonal antibody used in the Pastorex Candida, with increased sensitivity, and (ii) developed an EIA for the simultaneous detection of antibodies. A total of 162 serum samples from 43 hospitalized patients were retrospectively selected because of the presentation of clinical and mycological evidence of deep-seated candidiasis caused by C. albicans and were assessed by the methods that we developed for the presence of mannanemia and antimannan antibodies. Our data demonstrate that the developed EIA format increases the detection limit of mannan with increased sensitivity without adversely affecting the test specificity. A striking finding in this study is the observation that serum samples with a high mannanemia response had a low (undetectable) levels of antimannan antibodies and vice versa. This finding was consistent among patients in general and for a given patient during the time course of the disease.
MATERIALS AND METHODS
Patients.
Between January and December 1995, 162 serum samples were retrospectively collected in two different university hospitals from 43 patients (16 females and 27 males [mean age, 56 ± 17 years]) with proven candidiasis. The average number of serum samples per patient in this group was 3.7 ± 2 (Table 1). The following criteria were applied as retrospective selection rules when the laboratory and clinical files were examined: (i) positive culture of specimens from normally sterile sites (blood, bile, pericardial fluid, liver biopsy, drain, and wound specimens) for C. albicans; (ii) availability of serum samples obtained within a range of 1 week before and 2 weeks after positive cultures; (iii) the presence of risk factors (cancer and chemotherapy, abdominal surgery, AIDS, major health problems requiring hospitalization in intensive care units [ICUs], and use of broad-spectrum antibiotics, indwelling intravascular catheters, and hyperalimentation; and (iv) the presence of an infectious syndrome (namely, fever) that did not respond to antibacterial therapy but that did respond to antifungal therapy.
TABLE 1.
Underlying diseases, culture data, and results of antigen and antibody testing for patients with candidiasis
| Patient no. | Sexa | Age (yr) | Hospital ward | Underlying condition | No. of serum specimens | Site of C. albicans isolation | Peak antigen concn (ng/ml) by EIA | Maximum latex agglutination assay titer | Peak antibody response (AU) by EIA |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | ICU | Silicosis | 3 | Drain | 0.38 | 0 | 6 |
| 2 | M | 40 | Burns unit | Burns | 5 | Blood | 0.28 | 0 | 5 |
| 3 | F | 71 | Surgery | Colic cancer | 5 | Blood | 0.12 | 0 | 9 |
| 4 | M | 42 | ICU | Acute pancreatitis | 6 | Drain | 0 | 0 | 4 |
| 5 | M | 56 | Surgery | Colic perforation | 1 | Peritoneum | 0 | 0 | 2 |
| 6 | F | 65 | ICU | Cardiomyopathy | 1 | Blood | 0 | 0 | 1 |
| 7 | M | 74 | Surgery | Duodenopancreatectomy | 1 | Blood | 0 | 0 | 1 |
| 8 | M | 28 | Surgery | Splenic sarcoma | 2 | Blood | >10b | 8 | 8 |
| 9 | F | 33 | Infectious disease | AIDS | 11 | Blood | >10 | 64 | 6 |
| 10 | M | 71 | Surgery | Pancreatectomy | 1 | Blood | >10 | 2 | 6 |
| 11 | M | 43 | Digestive disease | AIDS | 10 | Blood | >10 | 512 | 0 |
| 12 | M | 59 | Digestive disease | AIDS | 2 | Blood | >10 | 1 | 0 |
| 13 | F | 58 | Surgery | Autolysis | 6 | Blood | >10 | 4 | 0 |
| 14 | F | 96 | ICU | Pneumopathy | 2 | Drain | 4.20 | 2 | 4 |
| 15 | M | 3 | Surgery | Neuroblastoma | 4 | Drain | 4.15 | 1 | 0 |
| 16 | M | 70 | ICU | Peritonitis | 4 | Peritoneum | 2.59 | 0 | 8 |
| 17 | M | 65 | Surgery | Peritonitis | 5 | Peritoneum | 1.00 | 0 | 8 |
| 18 | F | 65 | ICU | Adenocarcinoma | 5 | Blood | 0.86 | 2 | 5 |
| 19 | M | 30 | Infectious disease | AIDS | 3 | Blood | 0.69 | 0 | 0 |
| 20 | F | 47 | Surgery | Inguinal hernia | 1 | Kidney | 0 | 0 | 10 |
| 21 | F | 67 | ICU | Peritonitis | 3 | Drain | 0.23 | 0 | 10 |
| 22 | M | 54 | ICU | Pelvic abscess | 4 | Drain | 0 | 0 | 13 |
| 23 | M | 36 | Surgery | Silicosis | 5 | Drain | 0 | 0 | 17 |
| 24 | F | 71 | Surgery | Colectomy | 2 | Blood | 0 | 0 | 17 |
| 25 | M | 35 | Surgery | Hepatocellular carcinoma peritonitis | 5 | Bladder | 0 | 0 | 20 |
| Sound | |||||||||
| 26 | M | 58 | Surgery | 4 | Drain | 0 | 0 | 20 | |
| 27 | M | 58 | Digestive disease | Hysterectomy | 1 | Biopsy | 0 | 0 | 21 |
| 28 | M | 56 | Hematology | CML | 6 | Blood | 0 | 0 | 38 |
| 29 | M | 46 | Burns unit | Burns | 1 | Blood | 0 | 0 | 44 |
| 30 | M | 46 | Surgery | Cirrhosis | 7 | Biopsy specimen, blood | 0 | 0 | 53 |
| 31 | F | 35 | Infectious disease | AIDS, cholecystectomy | 1 | Blood | 0 | 0 | 53 |
| 32 | M | 64 | Infectious disease | Asthma | 1 | Blood | 0 | 0 | 55 |
| 33 | M | 57 | Surgery | Pulmonary lobectomy | 2 | Drain | 0.10 | 0 | 83 |
| 34 | M | 46 | Digestive disease | Cirrhosis | 2 | Drain | 0.32 | 0 | 100 |
| 35 | M | 74 | Surgery | Appendectomy | 5 | Blood | 0 | 0 | 100 |
| 36 | M | 70 | Surgery | Small intestine carcinoma | 4 | Drain, PLd | 0 | 0 | 100 |
| 37 | M | 56 | Surgery | Mesenteric fibroblastoma | 2 | Blood | 0 | 0 | 100 |
| 38 | F | 83 | Burns unit | Burns | 2 | Blood | 1.02 | 0 | 11 |
| 39 | M | 56 | Surgery | Liver transplantation | 6 | Blood | 3.50 | 0 | 12 (7)e |
| 40 | F | 72 | Surgery | Small intestine fistula | 3 | Blood | 2.00 | 1 | 53 (9) |
| 41 | M | 65 | Urology | Vesical carcinoma | 5 | Drain | 1.74 | 0 | 57 (21) |
| 42 | F | 62 | ICU | Peritonitis | 7 | Drain | 3.50 | 1 | 80 (13) |
| 43 | M | 67 | ICU | Septic shock | 6 | Drain | 8.53 | 2 | 83 (6) |
M, male; F, female.
Boldface indicates positive results.
CML, chronic myelocytic leukemia.
PL, pleural liquid.
Values in parentheses are antimannan antibody titer detected in the same sample which presented a peak of antigenemia.
Control sera.
Three groups of control sera were included in this study. (i) Group 1 comprised 93 serum specimens from 23 hospitalized patients (7 females and 16 males [mean age, 45 ± 12 years]) without evidence of invasive candidiasis. This group of patients was enrolled in a prospective study conducted in an ICU of Lille University Hospital for 6 months, the study was designed for the assessment of risk factors for nosocomial candidiasis. These patients were under clinical and mycological survey for periods ranging from 1 to 74 days (mean, 12 days). Samples of blood, oral swabs, urine, and stools were collected biweekly. We selected 23 patients. For 19 patients Candida colonization was documented in at least one body site, but there was no proven, probable, or even suspected Candida tissue invasion. In four patients, candidal colonization was not detected.
(ii) Group 2 consisted of 39 serum samples from patients with deep mycoses not caused by Candida. Twenty-two serum samples were retrospectively selected from 12 patients with invasive pulmonary aspergillosis (severe neutropenic patients with persistent fever, despite treatment with broad-spectrum antibacterial agents, and pulmonary infiltrates that developed on the chest roentgenogram). Invasive aspergillosis was also confirmed by the detection of Aspergillus galactomannan in sera. Three of 12 patients included in this group were infected with human immunodeficiency virus (HIV). Thirteen patients (one serum sample from each patient) were diagnosed with cryptococcal meningitis. Cryptococcal infection was confirmed by isolation of Cryptococcus neoformans from cerebrospinal fluid as well as detection of circulating antigen by the Pastorex Crypto latex agglutination test (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). Among these patients, six were known to have been infected with HIV for at least 3 months and two had undergone kidney transplantation. Four serum samples were obtained from four patients diagnosed with Pneumocystis carinii pneumonia. These sera were retrospectively obtained from two patients who had undergone bone marrow transplantation, one HIV-infected patient, and one patient who had undergone kidney transplant surgery. All patients were investigated for pulmonary disease, characterized by dyspnea, cough, and fever and accompanied by abnormal chest radiographs. In each case, the diagnosis of pneumonia was confirmed by the presence of P. carinii cysts in bronchoalveolar lavages.
(iii) Group 3 consisted of 98 serum samples from 98 healthy blood donors.
EIA detection of anti-C. albicans mannan antibodies in human sera.
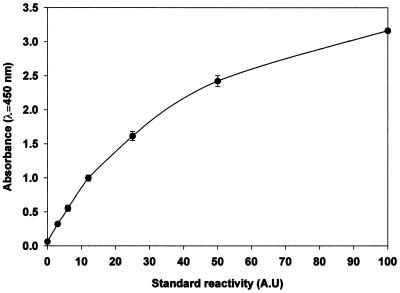
Microtiter plates were sensitized in an industrial setting with C. albicans cell wall mannan. The mannan was prepared from C. albicans VW32 grown in bioreactors under standard conditions used for the chemical and immunochemical analysis of this molecule (12). EIA was performed with BEP III automate (Behring Laboratories, Paris, France). For the serological diagnostic procedures already in use for Candida serology or other antimannan detection tests (51), each set of tests included a standard dilution which consisted of a serial twofold dilution of a pool of patients’ sera that strongly reacted with yeast mannan. These standard dilutions were aliquoted and stored at −30°C. For individual sera, 100 μl of serum diluted 1/8,000 was applied to each well, and the plate was incubated for 1 h at 37°C. After washing, 100 μl of horseradish peroxidase-conjugated anti-human immunoglobulins was then added, and the plates were incubated for 1 h at 37°C. After intensive washing, the reaction was revealed by 30 min of incubation in darkness with 200 μl of tetramethylbenzidine solution. The absorbance at a λ of 450/620 nm was measured. The results were reported in arbitrary units (AU) in relation to the results on the standard curve (Fig. 1).
FIG. 1.
EIA detection of anti-C. albicans mannan antibodies. The standard curve was obtained with serial dilutions of a pool of sera strongly reacting with mannan extracted from C. albicans VW32. Standard reactivity of 100 AU was determined as the dilution that gave the maximal absorbance at a λ of 450 nm.
By this test, preliminary experiments with 700 human serum specimens showed that a satisfactory correlation was obtained between immunofluorescence titers and EIA values (Spearman coefficient = 0.83; P = 0.0001) (data not shown). Ten AU corresponded to an immunofluorescence assay score of ≥400, which was considered indicative of candidiasis (49).
Detection of mannanemia.
Two procedures were used to detect mannanemia.
The first method used the commercially available latex agglutination test Pastorex Candida (Sanofi Diagnostics Pasteur) and was performed according to the manufacturer’s instructions. Three hundred microliters of patient sera was denatured with 100 μl of EDTA treatment solution, and the mixture was boiled for 3 min and centrifuged at 10,000 × g for 10 min. Forty microliters of supernatant was mixed in a plate well with 10 μl of latex particles.
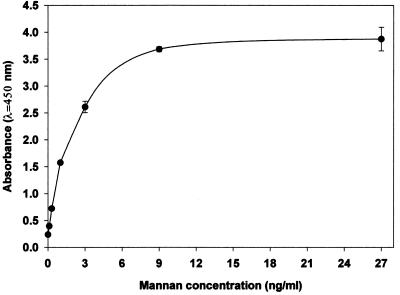
The second method used to detect mannanemia was an EIA that we developed by using the same monoclonal antibody (monoclonal antibody EBCA1) used to the sensitize latex particles in the Pastorex Candida test. The minimal epitope of this monoclonal antibody has been shown to correspond to α-linked mannopentaose of the C. albicans VW32 mannan acid-stable domain; this epitope is also present on numerous C. albicans mannoproteins (22). Microtiter plates were sensitized with monoclonal antibody EBCA1 in an industrial setting. Fifty microliters of supernatant, obtained from patient serum and treated as described above, was mixed in a plate well with 50 μl of horseradish peroxidase-conjugated EBCA1. After incubation for 90 min at 37°C, the plates were washed intensively and the reaction was revealed by 30 min of incubation in darkness with 200 μl of tetramethylbenzidine solution. The optical density was read at a λ of 450/620 nm on a PR2100 reader (Sanofi Diagnostics Pasteur). Reactions were performed in duplicate. Each experiment included a calibration curve for a pool of normal human sera supplemented with concentrations of mannan of 0.1 to 27 ng/ml (Fig. 2).
FIG. 2.
EIA detection of mannan extracted from C. albicans VW32 and spiked into a pool of negative sera. Standard deviations calculated from duplicates in three different experiments are noted by error bars.
Statistical analysis.
Data were analyzed with the SAS program. Sensitivity, specificity, and predictive values were calculated as described previously (54). The true-negative population included blood donors, noninfected hospitalized patients, and patients with deep mycoses not caused by a Candida sp.
RESULTS
Standardization of tests.
For each experiment for antimannan antibody detection, individual sera are tested in duplicate. The repeatability of the optical density (OD) values on a single microtiter plate corresponded to a coefficient of variation (CV) of <10%. As controls, each new set of experiments comprised sera from four patients exhibiting graded antimannan antibody levels. The interseries reproducibility obtained with these sera was examined after transformation of the OD through the standard curve as described above. This corresponded to a CV of <5% (n = 5). Concerning circulating mannan antigen detection, preliminary experiments showed that the sensitivity of detection was 0.1 ng/ml (the study was performed with sequential dilutions of a negative serum supplemented with 27 ng of mannan). In this study we considered 0.5 ng/ml to be the cutoff level since 99% of the controls had mannanemia values that were less than this value. The mannan concentrations in the tested sera were determined from the mean of the OD by using the sigmoid model curve provided with the reader. The intra-assay reproducibility expressed as the mean CV for the two control serum samples provided with the kit (negative and positive) was 3.32% (n = 5). Interseries reproducibility for control sera and test samples mannan concentrations showed a CV of <4%.
Mannan concentrations and antimannan antibody titers in patient and control sera.
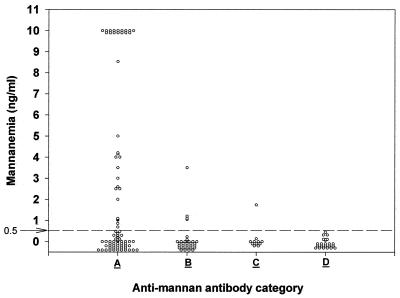
Figure 3 shows the results obtained with the 162 serum specimens drawn from the 43 patients with candidiasis tested by the EIA for the detection of both antibodies and circulating mannanemia. Individual antigenemia values (expressed in nanograms per milliliter) were plotted as a function of the antimannan antibody response roughly distributed according to the following four categories: A, the antibody response is weak or absent (<10 AU); B, the antibody response is moderately above the cutoff level (between 10 and 20 AU); C, the antibody response is medium (between 20 and 40 AU); and D, the antibody response is strong (>40 AU). Sixty-three (37%) of the serum specimens had antibody titers that exceeded 10 AU and only 43 (25%) displayed antigenemia values greater than 0.5 ng/ml (25%). Strikingly, only 5 of 162 serum specimens concomitantly had circulating mannan and significant antimannan antibodies.
FIG. 3.
Mannanemia in relation to antimannan antibody titers in 162 serum samples from 43 patients with systemic candidiasis. Antimannan antibody titers (reported in AU) were divided into the following four categories: A, 0 to 10 AU; B, 10 to 20 AU; C, 20 to 40 AU; D, >40 AU.
The results obtained for each of the 43 individual patients are summarized in Table 1. Table 1 indicates the maximal antigenemia and/or antibody response obtained for at least one of the retrospectively available serum samples. Patients have been classified into four groups according to the results observed by both tests. The first group (patients 1 to 7; 16%) corresponds to sera in which it was not possible to detect either antigens or antibodies; it is worthy of note that for three of the patients, only one sample was available. In the second group (patients 8 to 19; 28%), antigenemia was detected without evidence of an antibody response during the survey. In the third group (patients 20 to 37; 37%), no antigenemia was detectable but a significant antibody response was evidenced; for groups 2 and 3 which represented 66% of the patients, the striking disparity, i.e., lack of detectable antigen in the presence of significant amount of antibodies and vice versa, is evident. This was also observed for all sera drawn from a single patient during the survey. In the fourth group (patients 38 to 43; 13%), both antigenemia and antibody detection tests were positive. However, with the exception of five serum specimens (two drawn from patient 38 and one each drawn from patients 40, 41, and 42), we never observed positivity by both tests for the same serum sample.
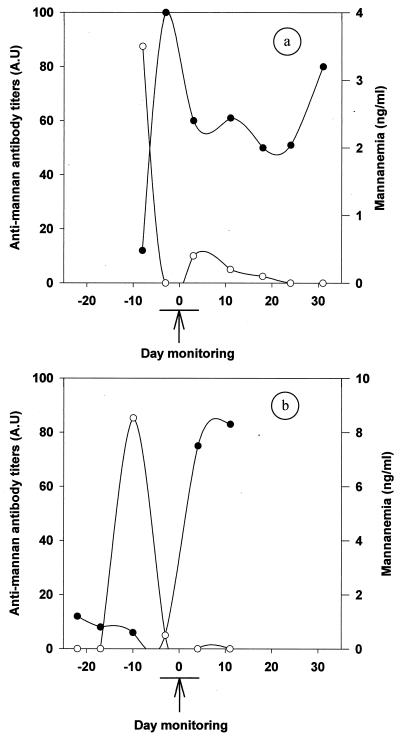
The complementation of the antigen and antibody detection tests was indeed evidenced when considering the kinetics of both parameters with sera drawn from individuals during the time course of the disease. Figure 4 shows the results corresponding to the mannanemia and antimannan antibody levels observed for patients 39 and 43 (see also Table 1). For patient 39 (Fig. 4a), a high mannan concentration was early detected in the serum available 1 week before the isolation of Candida from the patient’s blood (see arrow). However, it became negative 5 days later and remained under the threshold limit thereafter (<0.5 ng/ml). At the time of positive antigenemia, the antibody response was negative and sharply increased to reach a maximum within 5 days, with a concomitant decrease in mannanemia. For patient 43 (Fig. 4b), 3 weeks prior to the isolation of C. albicans, the antibody response was around the cutoff point (11%). Then, the antibody response gradually decreased during the following 3 weeks. During the same period, a strong antigenemia peak was observed with a single serum sample 10 days before the mycological isolation. The decrease in mannanemia preceded the onset of a strong antibody response concomitantly with mycological detection.
FIG. 4.
Examples of kinetic evolution of antigenemia (○) and antimannan antibody response (●) detected by EIA. Patients 39 (a) and 43 (b) had systemic candidiasis. The arrow marks the date of mycological isolation of C. albicans from blood and a drain. The curves are drawn by using the interpolate regression.
When a titer of 10 AU or more is considered to be a positive result by the antimannan antibody detection test, 2% (2 of 98) of healthy blood donors, 8.6% (2 of 23) of patients from an ICU, and 3.4% (1 of 29) of patients with deep mycoses not caused by Candida sp. had positive results (Table 2). When a threshold concentration of 0.5 ng of mannan per ml of serum was used as the limit for antigenemia, no positive results were detected for sera drawn from healthy blood donors, two of 29 (6.9%) patients with deep mycoses had positive results, and 1 of the 23 (4.3%) ICU control patients had a positive result.
TABLE 2.
Results of antibody and antigen testing for control populations
| Group or clinical diagnosis | No. of patients | No. of serum samples | No. of serum samples positive for the following:
|
|
|---|---|---|---|---|
| Mannanemia of >0.5 ng/ml | Antimannan antibody titer of ≥10 AU | |||
| Healthy blood donors | 98 | 98 | 0 | 2 |
| ICU-hospitalized patients without invasive candidiasis | 23 | 93 | 1 (0.68)a | 6 |
| Invasive pulmonary aspergillosis | 12 | 22 | 1 (1.45) | 0 |
| Cryptococcosis | 13 | 13 | 1 (0.57) | 1 (10) |
| P. carinii pneumonia | 4 | 4 | 0 | 0 |
Values in parentheses are the titers observed for the same sample which was antigen positive.
The sensitivity, specificity, and positive and negative predictive values calculated per patient for the Pastorex test and the mannanemia and antimannan antibody detection tests are shown in Table 3. The sensitivity of the mannanemia detection test increased from 28 to 40% by using an EIA format instead of a latex agglutination assay format. This increase in sensitivity did not reduce the specificity. As can be seen from the analysis of individual data, the combined use of EIA detection of antimannan antibodies and EIA detection of mannanemia allowed the detection of 80% of retrospectively mycologically proven deep Candida infections with a specificity of 93%.
TABLE 3.
Sensitivity, specificity, and predictive values for the detection of antigen and antimannan antibodiesa
| Statistic | Mannanemia Pastorex Candida | Mannanemia EIA | Antimannan antibody EIA | Combination of EIAs |
|---|---|---|---|---|
| Sensitivity (%) | 28 | 40 | 53 | 80 |
| Specificity (%) | 100 | 98 | 94 | 93 |
| Positive predictive value (%) | 100 | 85 | 72 | 78 |
| Negative predictive value (%) | 82 | 84 | 87 | 93 |
Results are calculated per patient according to the results of an analysis of 162 serum samples from 43 patients with systemic candidiasis and 230 serum samples from 150 controls.
DISCUSSION
Numerous approaches for the serological diagnosis of candidiasis have concentrated on the detection of C. albicans-derived molecules. These molecules were detected either on the basis of their antigenicity or through biochemical-enzymatic procedures. More recent progress has been made on the latter methods, and kits are commercially available for the detection of arabinitol (62) and glucans (29, 39, 40, 42), whereas PCR-based tests for Candida DNA detection are routinely performed in some laboratories (5, 15, 63). Immunological detection of C. albicans protein antigens of 47 and 48 kDa have represented promising advances (35, 39, 71), but the use of the commercially available assay (Directigen; Becton Dickinson) for the detection of the 48-kDa vacuolar enolase has been limited by its cost. In contrast, the Cand Tec latex agglutination test has been widely used as the first commercially available antigen detection test (3, 19, 31, 67); the still unknown nature and function of the target antigen have nonetheless impeded its further development. An interesting feature of the serological detection of C. albicans-derived antigens in patient sera, in contrast to the detection of nonimmunogenic molecules, is that the detected molecules may elicit an antibody response in infected patients. Depending on the pathophysiological importance of the antigen, joint consideration of patient antigenemia and antibody response can provide insight into the evolution of the infection. Such diagnostic strategies are commonly used in virology for the serological survey of either HIV or hepatitis B virus infections. These methods involved the kinetics of the serum antibody response to the p24, gp41, and gp120 of the hepatitis B surface, core, and e antigens, respectively, for the detection of antigenemia (14). Surprisingly, with the exception of the use of the approach for the detection of the 47-kDa antigen, which has been shown both to circulate and to elicit protective antibodies (36), such an approach has never been applied to the serological diagnosis of candidiasis. This is particularly true for the mannan, the major immunogen of the C. albicans cell wall, which for two decades has been shown to induce antibodies in humans and to circulate in patients’ sera (34). These diagnostic approaches have considered mannan to be a single molecule, but they failed to take into consideration its chemical and immunological complexity. Within the mannan, a large number of chemically defined sequences of mannose residues have been identified (61). Depending on the type of linkage between the mannose residues and the mannosyl chain length, mannan-derived oligomannosides have been shown to be involved in such basic processes as inhibition of lymphoproliferation (41), binding to epithelial cells and macrophages (6, 7, 16, 33, 59), induction of cytokines and arachidonic acid derivatives (2, 9), and induction of protective or nonprotective antibodies in animal models (19).
Polyclonal and then monoclonal antibodies have been used to detect circulating mannan in patients’ sera by either EIA (53), radioimmunoassay (72), latex agglutination (3, 18, 37), or coagglutination (30). Some of these epitopes have been preliminarily characterized, as for monoclonal antibodies AF1 and 5B2, which have been shown to correspond to β-1,2-linked mannopyraosyl units of the mannan acid-labile domain (11, 50). In the present study, we have used monoclonal antibody EBCA1, which is used to sensitize the latex particles involved in the commercially available test Pastorex Candida. Recent studies have shown that the monoclonal antibody EBCA1 minimal epitope was among a mixture of mannopentaoses present in the mannan acid-stable domain: an α-1,2-linked isomer and an isomer in which the fifth mannose was α-1,6 linked to the reducing unit of manno-α-1,2-tetraose (22). Therefore, monoclonal antibody EBCA1 epitopes fit with the more general structure Manα1-(2Manα1)n-2Man, (where n is ≥0), a type of mannopyranose chain that has been proposed to correspond to antigenic factor 1 (27, 61), which is ubiquitous in yeast species (66). The monoclonal antibody EBCA1 epitope has also been shown to be expressed on the glycan moiety of a large number of C. albicans mannoproteins which, if released, can be detected in patient sera. Previous studies on antigen detection with monoclonal antibody EBCA1 by the Pastorex latex agglutination test have shown a good specificity but a poor sensitivity (21, 39, 50). Therefore, we decided to increase the sensitivity of the test by developing an EIA format instead of a latex agglutination assay format. By using this method, the detection limit has been improved up to 0.1 ng of mannan per ml. As expected, this resulted in an increase in sensitivity which allowed us to detect antigenemia in 40% of the patients, whereas with the Pastorex system antigenemia could be detected in only 28% of the patients. This increase in sensitivity was not detrimental to specificity since only 3 of the 150 control serum specimens were positive; none of them was from healthy blood donors, 1 was drawn from a hospitalized colonized patient, and 2 were from patients infected with Aspergillus fumigatus and C. neoformans, respectively. Although no mycological evidence of candidiasis was found for these patients, the possibility that they could be infected or coinfected with C. albicans could not be completely ruled out. When the sensitivity of EIA is compared to that of Pastorex for each serum sample from patients with candidiasis, in general, values lower than 1.5 ng/ml failed to give a positive Pastorex test result. This resulted, however, in a limited gain in overall sensitivity since 43 serum samples were positive by EIA, whereas 35 were positive by Pastorex. Consideration of these results for each serum sample is more disappointing than those for each patient, but this illustrates one of the major limitations of mannanemia detection tests, which lies in the transient character of antigen circulation (25, 52). As a consequence, sensitivity is a function of the number of serum samples available from each patient. In this study, sensitivity of mannanemia detection dropped from 40 to 11% if data for patients for whom only one serum sample was available are considered. Several mechanisms have been proposed to explain this observation, among which we can find the quick degradation of mannose oligomers by serum mannosidases (10) or the binding of the mannose oligomers to soluble serum proteins (mannose binding protein C3) (20, 26, 60) or membranous receptors of phagocytes (16, 33, 59). However, the present study demonstrates that the detection of a given mannan catabolite in sera from candidiasis patients is inversely correlated to the presence of antimannan antibodies. Whether this phenomenon is restricted to the epitope that was detected (22) (the epitope has structural similarities to C. albicans mannan-derived O-linked oligomannosides that inhibit lymphocyte proliferation [41]) or fits with the more general phenomenon of antigen clearance by immune complexes remains to be established. These results also led us, like others recently (68), to reconsider the diagnostic value of antimannan antibody detection. We have found a specificity of 94% and a sensitivity of 53% for the EIA for antimannan antibody detection. It must be stressed that this sensitivity was calculated by including data for patients for whom the diagnosis was established by mannanemia detection. The combined tests had a sensitivity and specificity of 80 and 93%, respectively. With regard to the ability of both tests to diagnose C. albicans infection early in the course of infection, conclusions can be drawn only from data for the available sera due to the retrospective character of this study. For 18 of the 43 patients included in the study, at least one serum sample was available before mycological evidence of infection was gained. When considering the results for these sera, six patients presented with mannanemia (in the absence of significant antimannan antibody levels), nine patients presented with significant antimannan antibody levels (in the absence of mannanemia), and sera from the remaining three patients were negative by both tests. Evidence of antigenemia and an antibody response was gained an average of 6.2 and 7.3 days, respectively, before mycological isolation of C. albicans. However, this study is limited to patients infected with C. albicans only and patients infected with non-C. albicans Candida species were not included. Preliminary results obtained with sera from patients infected with Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei which, together with C. albicans, account for more than 95% of hospital Candida infections, are encouraging. This observation is not surprising since these different species of the Candida genus share both the EBCA1 epitope distribution on their mannan and mannoproteins (22) (for antigen detection tests) and a high level of cross-antigenic mannan reactivity (for antibody detection tests) (61). This study has shown that the combined performances of antigen and antibody detection by these tests were similar, irrespective of the immunosuppression status of the patient and the service. Evaluation of the utility of both tests in prospective studies enrolling large numbers of patients at risk for candidiasis (73) is necessary.
ACKNOWLEDGMENTS
We are grateful to Mahmoud A. Ghannoum and Donald Mackenzie for helpful suggestions on the manuscript. We thank also Gabriel Reboux (Besançon) for providing the sera from patients with cryptococcal meningitis and Laurence Richard and Nadine François for expert technical assistance.
This work was supported by a grant from the “Programme Hospitalier de Recherche Clinique du Ministère des Affaires Sociales, de la Santé et de la Ville.”
REFERENCES
- 1.Armstrong D. Problems in management of opportunistic fungal diseases. Rev Infect Dis. 1989;11(Suppl. 7):1591–1599. doi: 10.1093/clinids/11.supplement_7.s1591. [DOI] [PubMed] [Google Scholar]
- 2.Ashman R B, Papadimitriou J M. Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol Rev. 1995;59:646–672. doi: 10.1128/mr.59.4.646-672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey J W, Sada E, Brass C, Bennett J E. Diagnosis of systemic candidiasis by latex agglutination for serum antigen. J Clin Microbiol. 1985;21:749–752. doi: 10.1128/jcm.21.5.749-752.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey G P, editor. Candidiasis. Pathogenesis, diagnosis and treatment. 2nd ed. New York, N.Y: Raven Press; 1993. [Google Scholar]
- 5.Burnie J P, Golbang N, Matthews R C. Semiquantitative polymerase chain reaction enzyme immunoassay for diagnosis of disseminated candidiasis. Eur J Clin Microbiol Infect Dis. 1997;16:346–350. doi: 10.1007/BF01726361. [DOI] [PubMed] [Google Scholar]
- 6.Calderone R, Enache E, Eskandari T, Wadsworth E, Sturtevant J. Adherence of Candida albicans to mammalian cells in vitro: nutritional influences. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanisms. Tokyo, Japan: Salken Publishing Co.; 1997. pp. 75–83. [Google Scholar]
- 7.Calderone R A, Braun P C. Adherence and receptor relationships of Candida albicans. Microbiol Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone A. Cell wall of Candida albicans: its functions and its impact on the host. Curr Top Med Mycol. 1989;3:248–314. doi: 10.1007/978-1-4612-3624-5_10. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Ralston N V, Morgenthaler T I, Rohrbach M S, Limper A H. Candida albicans stimulates arachidonic acid liberation from alveolar macrophages through alpha-mannan and beta-glucan cell wall components. Infect Immun. 1994;62:3138–3145. doi: 10.1128/iai.62.8.3138-3145.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel P F, Winchester B, Warren C D. Mammalian alpha-mannosidases—multiple forms but a common purpose? Glycobiology. 1994;4:551–566. doi: 10.1093/glycob/4.5.551. [DOI] [PubMed] [Google Scholar]
- 11.De Bernardis F, Girmenia C, Boccanera M, Adriani D, Martino P, Cassone A. Use of a monoclonal antibody in a dot immunobinding assay for detection of a circulating mannoprotein of Candida spp. in neutropenic patients with invasive candidiasis. J Clin Microbiol. 1993;31:3142–3146. doi: 10.1128/jcm.31.12.3142-3146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faille C, Mackenzie D W, Michalski J C, Poulain D. Evaluation of an enzyme immunoassay using neoglycolipids constructed from Candida albicans oligomannosides to define the specificity of anti-mannan antibodies. Eur J Clin Microbiol Infect Dis. 1992;11:438–446. doi: 10.1007/BF01961859. [DOI] [PubMed] [Google Scholar]
- 13.Faille C, Wieruszeski J, Lepage G, Michalski J, Poulain D, Strecker G. 1H-NMR spectroscopy of manno-oligosaccharides of the β-1,2-linked series released from the phosphopeptidomannan of Candida albicans VW32 (serotype A) Biochem Biophys Res Commun. 1991;181:1251–1258. doi: 10.1016/0006-291x(91)92073-s. [DOI] [PubMed] [Google Scholar]
- 14.Field B, Knipe D, Holley P. Diagnostic virology. In: Press L-R, editor. Field virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Press; 1996. pp. 401–430. [Google Scholar]
- 15.Flahaut M, Sanglard D, Monod M, Bille J, Rossier M. Rapid detection of Candida albicans in clinical samples by DNA amplification of common regions from C. albicans-secreted aspartic proteinase genes. J Clin Microbiol. 1998;36:395–401. doi: 10.1128/jcm.36.2.395-401.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fradin C, Jouault T, Mallet A, Mallet J M, Camus D, Sinay P, Poulain D. Beta-1,2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J Leukoc Biol. 1996;60:81–87. doi: 10.1002/jlb.60.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Fukazawa Y. Antigenic structure of Candida albicans. Immunochemical basis of the serologic specificity of the mannans in yeasts. Immunol Ser. 1989;47:37–62. [PubMed] [Google Scholar]
- 18.Gentry L O, Wilkinson I D, Lea A S, Price M F. Latex agglutination test for detection of Candida antigen in patients with disseminated disease. Eur J Clin Microbiol. 1983;2:122–128. doi: 10.1007/BF02001577. [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidenreich F, Dierich M P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985;50:598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herent P, Stynen D, Hernando F, Fruit J, Poulain D. Retrospective evaluation of two latex agglutination tests for detection of circulating antigens during invasive candidiasis. J Clin Microbiol. 1992;30:2158–2164. doi: 10.1128/jcm.30.8.2158-2164.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquinot P M, Plancke Y, Sendid B, Strecker G, Poulain D. Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patients sera and distribution over Candida species. FEMS Microbiol Lett. 1999;166:131–138. doi: 10.1111/j.1574-6968.1998.tb13309.x. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis W R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 24.Jones J M. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3:32–45. doi: 10.1128/cmr.3.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappe R, Müller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29:1665–1669. doi: 10.1128/jcm.29.8.1665-1669.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitz D, Sthal P, Little J. The effect of a mannose binding protein on macrophage interaction with Candida albicans. Cell Mol Biol. 1992;38:407–412. [PubMed] [Google Scholar]
- 26a.Klis F. Cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Komido M, Watanabe M, Matsuda K, Suzuki M, Ikeda T, Oyamada H, Shibata N, Suzuki S. Structure of cell wall mannan of Candida kefyr IFO 0586. Infect Immun. 1994;62:4425–4431. doi: 10.1128/iai.62.10.4425-4431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H, Shibata N, Suzuki S. Evidence for oligomannosyl residues containing both β-1,2 and α-1,2 linkage as serotype A-specific epitope(s) in mannans of Candida albicans species. Infect Immun. 1992;60:2106–2109. doi: 10.1128/iai.60.5.2106-2109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohno S, Mitsutake K, Maesaki S, Yasuoka A, Miyazaki T, Kaku M, Koga H, Hara K. An evaluation of serodiagnostic tests in patients with candidemia: beta- glucan, mannan, candida antigen by Cand-Tec and d-arabinitol. Microbiol Immunol. 1993;37:207–212. doi: 10.1111/j.1348-0421.1993.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 30.Koshi G, Viswanathan A, Chandy M, Jairaj P S. Development of a coagglutination (COA) technic to detect Candida antigenemia. I. COA versus Candida isolation. Am J Clin Pathol. 1987;88:429–435. doi: 10.1093/ajcp/88.4.429. [DOI] [PubMed] [Google Scholar]
- 31.Lemieux C, St-Germain G, Vincelette J, Kaufman L, de Repentigny L. Collaborative evaluation of antigen detection by a commercial latex agglutination test and enzyme immunoassay in the diagnosis of invasive candidiasis. J Clin Microbiol. 1990;28:249–253. doi: 10.1128/jcm.28.2.249-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R K, Cutler J E. Chemical definition of an epitope/adhesin molecule on Candida albicans. J Biol Chem. 1993;268:18293–18299. [PubMed] [Google Scholar]
- 33.Marodi L, Schreiber S, Anderson D C, MacDermott R P, Korchak H M, Johnston R B., Jr Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Investig. 1993;91:2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez J P, Gil M L, Lopez-Ribot J L, Chaffin W L. Serologic response to cell wall mannoproteins and proteins of Candida albicans. Clin Microbiol Rev. 1998;11:121–141. doi: 10.1128/cmr.11.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews R, Burnie J P, Lee W. The application of epitope mapping in the development of a new serological test for systemic candidiasis. J Immunol Methods. 1991;143:73–79. doi: 10.1016/0022-1759(91)90274-j. [DOI] [PubMed] [Google Scholar]
- 36.Matthews R, Hodgetts S, Burnie J. Preliminary assessment of a human recombinant antibody fragment to hsp90 in murine invasive candidiasis. J Infect Dis. 1995;171:1668–1671. doi: 10.1093/infdis/171.6.1668. [DOI] [PubMed] [Google Scholar]
- 37.Meulemans L, Andremont A, Meunier F, Ceuppens A M, Garrigues M L, Stynen D. Pastorex Candida, a new latex agglutination test for mannan detection in serum of patients with invasive candidiasis. In: Tümbay E, Seeliger H P R, Ang Ö, editors. Candida and candidamycosis. New York, N.Y: Plenum Press; 1991. pp. 233–236. [Google Scholar]
- 38.Mitsutake K, Kohno S, Miyazaki T, Miyazaki H, Maesaki S, Koga H. Detection of Candida enolase antibody in patients with candidiasis. J Clin Lab Anal. 1994;8:207–210. doi: 10.1002/jcla.1860080405. [DOI] [PubMed] [Google Scholar]
- 39.Mitsutake K, Miyazaki T, Tashiro T, Yamamoto Y, Kakeya H, Otsubo T, Kawamura S, Hossain M A, Noda T, Hirakata Y, Kohno S. Enolase antigen, mannan antigen, Cand-Tec antigen, and beta-glucan in patients with candidemia. J Clin Microbiol. 1996;34:1918–1921. doi: 10.1128/jcm.34.8.1918-1921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki T, Kohno S, Mitsutake K, Maesaki S, Tanaka K, Ishikawa N, Hara K. Plasma (1→3)-beta-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol. 1995;33:3115–3118. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson R D, Shibata N, Podzorski R P, Herron M J. Candida mannan: chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin Microbiol Rev. 1991;4:1–19. doi: 10.1128/cmr.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, et al. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/s0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 43.Odds F C, editor. Candida and candidiasis. A review and bibliography. London, United Kingdom: Baillière Tindall; 1988. [Google Scholar]
- 44.Odds F C, Evans G. Distribution of pathogenic yeasts and humoral antibodies to Candida among hospital inpatients. J Clin Pathol. 1980;33:750–756. doi: 10.1136/jcp.33.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 1998;29:207–233. doi: 10.1016/s0968-4328(97)00072-3. [DOI] [PubMed] [Google Scholar]
- 46.Pittet D. Nosocomial bloodstream infections. In: Wenzel R P, editor. Prevention and control of nosocomial infections. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1993. pp. 512–555. [Google Scholar]
- 47.Poulain D, Faille C, Delaunoy C, Jacquinot P, Trinel P, Camus D. Probable presence of β(1-2)-linked oligomannosides that act as human immunoglobulin G3 epitopes and are distributed over a Candida albicans 14- to 18-kilodalton antigen. Infect Immun. 1993;61:1164–1166. doi: 10.1128/iai.61.3.1164-1166.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poulain D, Jouault T, Trinel P. Immunoreactivity of Candida albicans β-1,2-linked oligomannosides and phospholipomannan. In: Suzuki S, Suzuki M, editors. Fungal cells in biodefense mechanisms. Tokyo, Japan: Salken Publishing Co.; 1997. pp. 175–181. [Google Scholar]
- 49.Poulain D, Pinon J M. Diagnosis of systemic candidiasis: development of co-counterimmunoelectrophoresis. Eur J Clin Microbiol. 1986;5:420–426. doi: 10.1007/BF02075698. [DOI] [PubMed] [Google Scholar]
- 50.Poulain D, Robert R, Mesnard F, Sendid B, Lepage G, Camus D. Clearances of Candida albicans-derived alpha- and beta-linked mannose residues in sera from patients with candidiasis. Eur J Clin Microbiol Infect Dis. 1997;16:16–20. doi: 10.1007/BF01575114. [DOI] [PubMed] [Google Scholar]
- 51.Quinton J F, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan S, Colombel J F, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiss E, Morrison C J. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993;6:311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiss E, Stockman L, Kuykendall R J, Smith S J. Dissociation of mannan-serum complexes and detection of Candida albicans mannan by enzyme immunoassay variations. Clin Chem. 1982;28:306–310. [PubMed] [Google Scholar]
- 54.Sackett D L, Haynes R B, Tugwell P. Clinical epidemiology. The interpretation of diagnostic data. Boston, Mass: Little, Brown & Co.; 1985. pp. 59–138. [Google Scholar]
- 55.Saral R. Candida and Aspergillus infections in immunocompromised patients: an overview. Rev Infect Dis. 1991;13:487–492. doi: 10.1093/clinids/13.3.487. [DOI] [PubMed] [Google Scholar]
- 56.Shepherd M G. Cell envelope of Candida albicans. Crit Rev Microbiol. 1987;15:7–25. doi: 10.3109/10408418709104445. [DOI] [PubMed] [Google Scholar]
- 57.Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as β-1,2-linked oligomannosyl residues. Infect Immun. 1992;60:4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shibata N, Hisamichi K, Kobayashi H, Suzuki S. Complete assignment of 1H and 13C nuclear magnetic resonance chemical shifts of beta-1,2-linked mannooligosaccharides isolated from the phosphomannan of the pathogenic yeast Candida albicans NIH B-792 strain. Arch Biochem Biophys. 1993;302:113–117. doi: 10.1006/abbi.1993.1188. [DOI] [PubMed] [Google Scholar]
- 59.Stahl P D. The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol. 1990;2:317–318. doi: 10.1165/ajrcmb/2.4.317. [DOI] [PubMed] [Google Scholar]
- 60.Summerfield J A, Taylor M E. Mannose-binding proteins in human serum: identification of mannose-specific immunoglobulins and a calcium-dependent lectin, of broader carbohydrate specificity, secreted by hepatocytes. Biochim Biophys Acta. 1986;883:197–206. doi: 10.1016/0304-4165(86)90309-0. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki S. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr Top Med Mycol. 1997;8:57–70. [PubMed] [Google Scholar]
- 62.Switchenko A C, Miyada C G, Goodman T C, Walsh T J, Wong B, Becker M J, Ullman E F. An automated enzymatic method for measurement of d-arabinitol, a metabolite of pathogenic Candida species. J Clin Microbiol. 1994;32:92–97. doi: 10.1128/jcm.32.1.92-97.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talluri G, Mangone C, Freyle J, Shirazian D, Lehman H, Wise G J. Polymerase chain reaction used to detect candidemia in patients with candiduria. Urology. 1998;51:501–505. doi: 10.1016/s0090-4295(97)00641-9. [DOI] [PubMed] [Google Scholar]
- 64.Trinel P A, Borg-von-Zepelin M, Lepage G, Jouault T, Mackenzie D, Poulain D. Isolation and preliminary characterization of the 14- to 18-kilodalton Candida albicans antigen as a phospholipomannan containing beta-1,2-linked oligomannosides. Infect Immun. 1993;61:4398–4405. doi: 10.1128/iai.61.10.4398-4405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trinel P A, Faille C, Jacquinot P M, Cailliez J C, Poulain D. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect Immun. 1992;60:3845–3851. doi: 10.1128/iai.60.9.3845-3851.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuchiya T, Fukazawa Y, Taguchi M, Kase T, Shinoda T. Serologic aspects on yeast classification. Mycopathol Mycol Appl. 1974;53:77–91. doi: 10.1007/BF02127199. [DOI] [PubMed] [Google Scholar]
- 67.Van Deventer A J, Van Vliet H, Hop W, Goessens W. Diagnostic value of anti-Candida enolase antibodies. J Clin Microbiol. 1994;32:17–23. doi: 10.1128/jcm.32.1.17-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Deventer A J, Goessens W H, van Zeijl J H, Mouton J W, Michel M F, Verbrugh H A. Kinetics of anti-mannan antibodies useful in confirming invasive candidiasis in immunocompromised patients. Microbiol Immunol. 1996;40:125–131. doi: 10.1111/j.1348-0421.1996.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 69.Vincent J L, Bihari D J, Suter P M, Bruining H A, White J, Nicolas-Chanoin M H, Wolff M, Spencer R C, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 70.Voss A, Kluytmans J A, Koeleman J G, Spanjaard L, Vandenbroucke-Grauls C M, Verbrugh H A, Vos M C, Weersink A Y, Hoogkamp-Korstanje J A, Meis J F. Occurrence of yeast bloodstream infections between 1987 and 1995 in five Dutch university hospitals. Eur J Clin Microbiol Infect Dis. 1996;15:909–912. doi: 10.1007/BF01690507. [DOI] [PubMed] [Google Scholar]
- 71.Walsh T J, Hathorn J W, Sobel J D, Merz W G, Sanchez V, Maret S M, Buckley H R, Pfaller M A, Schaufele R, Sliva C, et al. Detection of circulating candida enolase by immunoassay in patients with cancer and invasive candidiasis. N Engl J Med. 1991;324:1026–1031. doi: 10.1056/NEJM199104113241504. [DOI] [PubMed] [Google Scholar]
- 72.Weiner M H, Coats-Stephen M. Immunodiagnosis of systemic candidiasis: mannan antigenemia detected by radioimmunoassay in experimental and human infections. J Infect Dis. 1979;140:989–993. doi: 10.1093/infdis/140.6.989. [DOI] [PubMed] [Google Scholar]
- 73.Wenzel R P. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995;20:1531–1534. doi: 10.1093/clinids/20.6.1531. [DOI] [PubMed] [Google Scholar]