Abstract
Background and Aims
This retrospective study aimed to investigate the impact of positive hepatitis B core antibody (anti-HBc) and metabolic disorders on clinical characteristics of hepatocellular carcinoma (HCC) patients in an HBV-endemic area.
Methods
A total of 1950 consecutive patients newly diagnosed with HCC between 2002 and 2015 were included. Patient records were reviewed. We compared non-viral and non-alcoholic HCC patients with other etiological groups for HCC. In addition, we compared HCC patients with negative hepatitis B surface antigen (HBsAg) and positive anti-HBc to those with negative HBsAg and negative anti-HBc, and to those with HBV.
Results
The prevalence of non-viral and non-alcoholic HCC increased from 7% in 2002–2011 to 12% in 2012–2015. The proportion of non-viral and non-alcoholic HCC gradually increased with age. Patients with non-viral and non-alcoholic HCC exhibited higher rates of metabolic disorders and preserved liver function. The rate of anti-HBc positivity was similarly high in all HCC etiological groups. The clinical features of HCC patients with negative HBsAg and positive anti-HBc were similar to those with negative HBsAg and negative anti-HBc, but significantly different from those with HBV HCC. Regarding tumor characteristics, patients in the non-viral and non-alcoholic HCC group had more advanced stages of tumors (mUICC stage III–V and BCLC stage C/D). There was no significant difference in overall survival among the patient groups. The presence of anti-HBc did not affect patient survival.
Conclusion
Patients with non-viral and non-alcoholic HCC had a relatively high prevalence of metabolic disorders and preserved liver function. However, they had advanced tumor stage compared to patients from other etiological groups. Anti-HBc positivity did not affect the clinical characteristics or prognosis of non-HBV HCC patients in this study.
Keywords: hepatocellular carcinoma, cryptogenic hepatocellular carcinoma, metabolic disease, anti–hepatitis B core antibody, hepatitis B virus
Introduction
Hepatocellular carcinoma (HCC), the second major cause of cancer death worldwide, usually is caused by chronic liver disease of different etiologies, such as hepatitis B virus (HBV) or hepatitis C virus (HCV) infection or alcohol abuse.1,2 Although hepatitis B or C virus infection is the main cause of HCC in the world, its prevalence is expected to decrease due to potent antiviral therapies. Concurrently, interest has grown in cryptogenic HCC. The prevalence of cryptogenic HCC is reported to be approximately 9–10% in South Korea.3,4
Globally, non-alcoholic fatty liver disease (NAFLD) has become distinguished as one of the major causes of chronic liver disease and thus has also been considered as a contributing factor for development of HCC. Recent studies have indicated that metabolic disorders closely related to NAFLD including obesity and diabetes also have an independent role in increasing risk of HCC.5,6 HBV infection can be involved in the process of carcinogenesis, even in patients with negative hepatitis B surface antigen (HBsAg). First, after a sufficient period of chronic HBV infection, patients who achieve HBsAg seroclearance and whose viral DNA is no longer detectable can have advanced hepatic fibrosis, an independent risk factor for HCC.7 Second, persistent occult HBV infection (OBI), defined as detectable HBV DNA in sera or liver tissues, but no detectable HBsAg can cause continuous liver necroinflammation, even at a very low level of inflammatory activity, which potentially leads to HCC. Moreover, HBV also can induce carcinogenesis directly without liver cirrhosis (LC) through DNA integration into the host genome.8,9 In these situations, the anti–hepatitis B core antibody (anti-HBc) is positive. Therefore, positive anti-HBc can provide information to speculate the role of HBV infection in the development of HCC.
In Western countries, many cases of cryptogenic HCC have been considered to have evolved from NAFLD or non-alcoholic steatohepatitis (NASHs).10 Although NAFLD and metabolic factors have increased as the lifestyle in South Korea has become more Westernized, the characteristics of cryptogenic HCC can be different from that of Western countries because of different viral etiologies. Therefore, this study aimed to evaluate the impact of metabolic disorders and HBV as potential causes of cryptogenic HCC based on clinical characteristics and prognosis of HCC patients in an HBV-endemic area.
Methods
Patients
The study population was composed of 1960 consecutive patients newly diagnosed with HCC enrolled between January 2002 and December 2015 at a tertiary center in South Korea. Patients were excluded if they were diagnosed with HCC of rare etiology, such as autoimmune hepatitis, primary biliary cirrhosis, Wilson’s disease, and Budd–Chiari syndrome (N = 10). The etiology of HCC was determined by clinical and serological evaluation. HBsAg-positive patients were classified into the HBV HCC group and patients with anti–HCV-positive and/or detectable HCV RNA were classified into the HCV HCC group, regardless of alcohol intake. The amount of alcohol was estimated based on patient report. Patients with significant alcohol intake exceeding 30 g/day for men and 20 g/day for women were classified into the alcohol HCC group.11 The non-viral and non-alcoholic HCC group was composed of patients who were negative for HBsAg and anti-HCV Ab and had no significant alcohol consumption. Patients with both HBV and HCV infection were evenly distributed in the HBV and HCV groups (N = 16). The patients were followed until January 2018. This study was approved by the Institutional Review Board/Ethics Committee of Seoul St Mary’s Hospital of The Catholic University of Korea (KC17RESI0679). Informed consent was not required because this retrospective study used only patient records.
Clinical Assessment
Clinical data were collected at the time of HCC diagnosis. Metabolic risk factors were diabetes, hypertension, and obesity as assessed by body mass index (BMI). Total cholesterol and triglyceride levels were also checked. Diabetes was defined as fasting glucose ≥ 126 mg/dL or usage of current anti-diabetes medication. Hypertension was defined when systolic blood pressure was 140 mmHg or above, or diastolic blood pressure was 90 mmHg or above, or usage of current anti-hypertensive medication. Aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 (FIB-4) score, non-invasive serum fibrosis markers, were calculated with the following formulas: APRI = (AST/upper limit of normal AST/platelet count) × 100 and FIB-4 = (age × AST)/[platelet count × (ALT)1/2]. LC was diagnosed when any of the following were present: cirrhotic configuration in imaging studies (nodular liver surface or hypertrophy of caudate lobe), thrombocytopenia (< 150,000 /mL), or findings suggesting portal hypertension (varix, ascites, or splenomegaly).12 HCC was diagnosed when typical radiologic findings were identified in patients with risk factors for HCC or when it was histologically proven. The Modified Union for International Cancer Control (mUICC) and Barcelona Clinic Liver Cancer (BCLC) staging were complementarily applied for staging of HCC according to the guidelines of The Korean Liver Cancer Association and National Cancer Center.13
Statistical Analyses
Continuous variables were expressed as mean ± SD or median (range). Student’s t-test or Mann–Whitney U test was used for comparison of continuous variables, and the chi-square test or Fisher exact test was used for comparing categorical variables. The cumulative overall survival (OS) rate was evaluated using the Kaplan–Meier method, and the differences were analyzed using the log-rank test. Univariate and multivariate analyses with the Cox proportional hazard model were used to identify predictors for survival. Two-tailed P values ≤ .05 were considered significant. Data were analyzed using SPSS 20.0.
Results
Etiologies of Hepatocellular Carcinoma
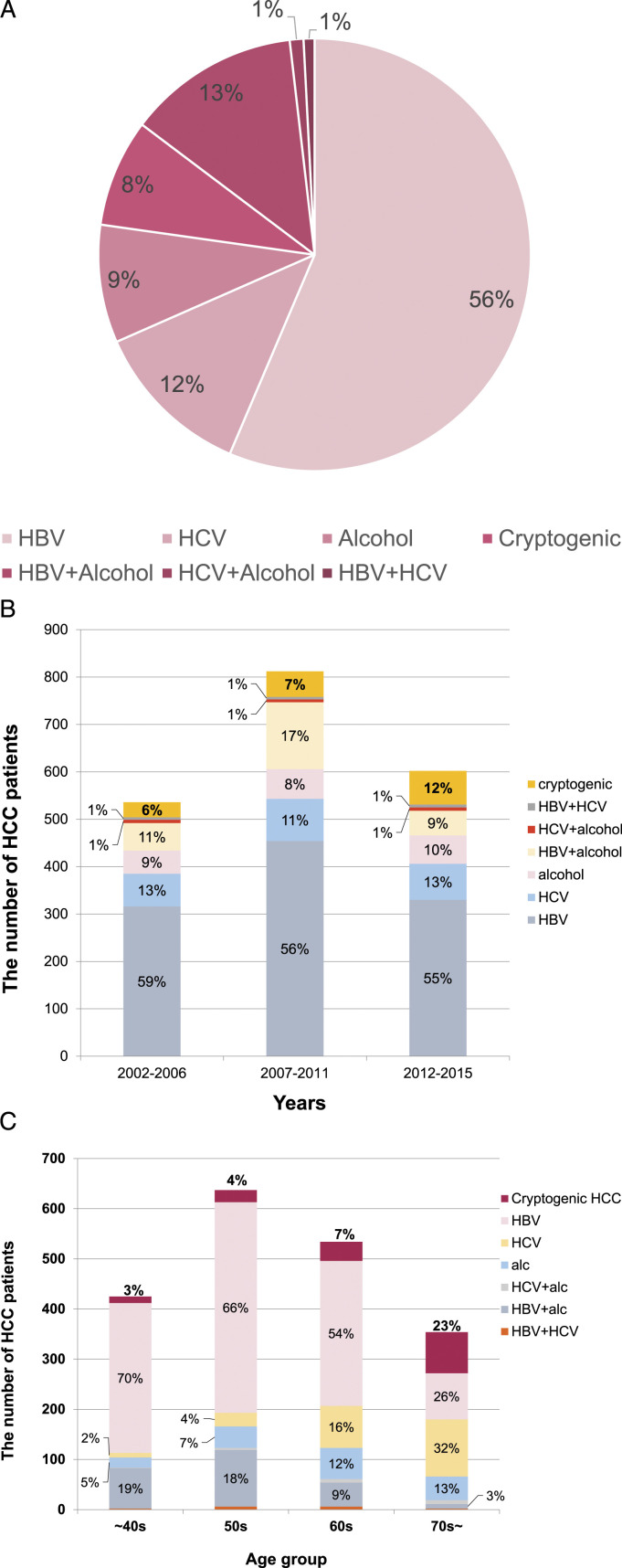
In the 1950 patients, HBV was a causative factor in 70.1%, HCV in 13.8%, alcohol in 22.7%, and non-viral and non-alcoholic cause in 8.1% (Figure 1(a)). For changes in causes of HCC over time, the proportion of HBV decreased from 73% in 2002–2011 to 64% in 2012–2015, while there was no meaningful change with time in the proportion of HCV (15% in 2002–2006, to 12% in 2007–2011, and 15% in 2012–2015) or alcohol (21%, 26%, and 19%, respectively). However, the proportion of non-viral and non-alcoholic HCC increased from 7% in 2002–2011 to 12% in 2012–2015 (Figure 1(b)). The proportion of non-viral and non-alcoholic HCC gradually increased with age, reaching 23% in over 70s (Figure 1(c)).
Figure 1.
The proportion of HCC etiologies. (a) Etiologies of total HCC patients. (b) Change in composition ratio of HCC etiologies according to time. (c) Change in proportion of non-viral and non-alcoholic HCC according to age group. The proportion of non-viral and non-alcoholic and HCV-related HCC increased with age, while the proportion of HBV- and alcohol-related HCC was highest before 50s and in the 50s, respectively.
Comparison of Hepatocellular Carcinoma Patients Among Etiological Groups
Clinical characteristics of the HCC patients according to etiology are shown in Supplementary Table 1. Patients in the non-viral and non-alcoholic HCC group had the highest prevalence of diabetes and hypercholesterolemia compared to the other groups. They had the lowest proportion of LC and Child–Turcotte–Pugh (CTP) class B/C. Regarding tumor characteristics (Supplementary Table 2), more patients in the non-viral and non-alcoholic HCC group had tumors in advanced stages (mUICC stage III–V and BCLC stage C/D). Curative treatment modalities as the first line of therapy including surgical resection, RFA/PEI, and liver transplantation were performed minimally in the patients with non-viral and non-alcoholic HCC.
Anti–Hepatitis B Core Antibody Status in Hepatocellular Carcinoma Patients
Among the HCC patients with negative HBsAg, the proportion of positive anti-HBc patients was 81.3%. According to the etiologies, 81.4% of patients with available results and 79.7% had positive anti-HBc in the HCV and alcoholic HCC groups, respectively. The status of anti-HBc was positive in 82.9% of non-viral and non-alcoholic HCC group. There was no significant difference in proportion of patients who were anti–HBc-positive between the non-viral and non-alcoholic HCC and HCV HCC groups (P = .773) or between the non-viral and non-alcoholic HCC and alcohol HCC groups (P = .547). Identification of HBV DNA level was possible in 211 (54.5%) patients with negative HBsAg and positive anti-HBc, of which 13 (6.2%) had a detectable level (median = 45 IU/mL, range = 10.0–2.1 × 103).
Clinical Characteristics According to Status of Hepatitis B Surface Antigen and Anti–Hepatitis B Core Antibody
In this study, anti-HBc positivity was similarly high in all etiological groups. Thus, to identify the clinical impact of past or occult HBV infection on clinical characteristics of HCC patients with negative HBsAg, we divided patients with non-HBV HCC by the results of anti-HBc and compared the patients with negative HBsAg and positive anti-HBc to those with negative HBsAg and anti-HBc, and to those with HBV (Table 1). There was no significant difference in clinical characteristics between HBsAg-negative patients with positive anti-HBc and those with negative anti-HBc, except that the proportion of females was higher in patients with negative HBsAg and anti-HBc. However, patients in the HBV HCC group were significantly younger and had the lowest prevalence of diabetes, hypertension, and hypertriglyceridemia compared to the other groups.
Table 1.
Clinical Characteristics According to Status of HBsAg and Anti-HBc.
| HBsAg (−) and | HBV (n = 1367) | HBsAg (−) and anti-HBc (+) vs | |||
|---|---|---|---|---|---|
| anti-HBc (+) (n = 387) | anti-HBc (−) (n = 89) | HBsAg (−) and anti-HBc (−) | HBV | ||
| P value | P value | ||||
| Age | 67 ± 11 | 65 ± 12 | 55 ± 10 | .230 | < .001 |
| Gender (male) | 296 (76.5) | 57 (64.0) | 1083 (79.2) | .022 | .261 |
| Diabetes | 138 (35.7) | 36 (40.4) | 258 (18.9) | .464 | < .001 |
| Hypertension | 167 (43.2) | 39 (43.8) | 350 (25.6) | 1.000 | < .001 |
| Total cholesterol > 200 mg/dLa | 33 (8.8) | 9 (10.3) | 149 (11.3) | .679 | .186 |
| Triglyceride > 150 mg/dLa | 42 (11.2) | 12 (13.8) | 75 (5.7) | .464 | < .001 |
| BMI ≥ 25a | 127 (33.6) | 29 (34.5) | 410 (30.6) | .899 | .285 |
| BMI ≥ 30a | 16 (4.2) | 5 (6.0) | 47 (3.5) | .560 | .535 |
| AFP, median (Q1–Q3), ng/mL | 17 (4.9–3.0 × 102) | 18.5 (5.6–1.7 × 102) | 76 (10.2–9.1 × 102) | .445 | .395 |
| APRI ≥ 1.5 | 125 (32.3) | 31 (34.8) | 496 (36.3) | .166 | .821 |
| FIB-4 ≥ 3.25 | 238 (61.5) | 64 (71.9) | 735 (53.8) | .008 | .001 |
| Prothrombin time (INR) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | .857 | .057 |
| CTP score | 6 ± 1 | 6 ± 1 | 6 ± 1 | .122 | .225 |
| CTP class (A/B/C) | 303/73/11 (78.3/18.9/2.8) | 61/24/4 (68.5/27.0/4.5) | 1088/244/35 (79.6/17.8/2.6) | .145 | .849 |
| Liver cirrhosis | 259 (66.9) | 64 (71.9) | 1009 (73.8) | .382 | .008 |
Values are expressed as means ± standard deviation or number (%).
aSome patients lacked clinical information at the time of HCC diagnosis. Abbreviations: anti-HBc, anti–hepatitis B core antibody; FIB-4, fibrosis-4; HBsAg, hepatitis B surface antigens; HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; AFP, alpha-fetoprotein; APRI, aspartate aminotransferase-to-platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CTP, Child–Turcotte–Pugh.
Tumor Characteristics According to Status of Hepatitis B Surface Antigen and Anti–Hepatitis B Core Antibody
Tumor characteristics were not different between the HBsAg-negative patients with positive anti-HBc and those with negative anti-HBc (Table 2). Patients with HBV had a significantly larger proportion of portal vein thrombosis of tumor and extrahepatic metastasis than did patients with negative HBsAg. Consequently, significantly more patients with HBV had tumors in advanced stages (mUICC-IV/V and BCLC-C/D).
Table 2.
Tumor Characteristics According to Status of HBsAg and Anti-HBc.
| HBsAg (−) and | HBV (n = 1367) | HBsAg (−) and anti-HBc (+) vs | |||
|---|---|---|---|---|---|
| anti-HBc (+) (n = 387) | anti-HBc (−) (n = 89) | HBsAg (−) and anti-HBc (−) | HBV | ||
| P value | P value | ||||
| Uni-nodular tumor, n (%) | 225 (58.1) | 58 (65.2) | 729 (53.3) | .234 | .094 |
| Largest tumor size, ≤ 5 cm, n (%) | 238 (61.5) | 63 (70.8) | 775 (56.7) | .114 | .103 |
| Portal vein thrombosis, n (%) | 89 (23.0) | 24 (27.0) | 440 (32.2) | .490 | < .001 |
| Extrahepatic metastasis, n (%)a | 46 (11.9) | 9 (10.1) | 219 (16.1) | .716 | .045 |
| mUICC staging, n (%)a | .965 | .009 | |||
| I | 70 (18.1) | 18 (20.2) | 217 (15.9) | ||
| II | 157 (40.6) | 36 (40.4) | 489 (35.8) | ||
| III | 80 (20.7) | 17 (19.1) | 259 (18.9) | ||
| IV/V | 80 (20.7) | 18 (20.2) | 402 (29.4) | ||
| BCLC staging, n (%) | .185 | < .001 | |||
| 0/A | 171 (44.2) | 44 (49.4) | 599 (43.8) | ||
| B | 106 (27.4) | 16 (18.0) | 253 (18.5) | ||
| C/D | 110 (28.4) | 29 (32.6) | 515 (37.7) | ||
| 1st treatment, n (%) | |||||
| Curative therapyb | 76 (19.6) | 19 (21.3) | 202 (14.8) | .769 | .022 |
| Surgical resection | 11 (2.8) | 2 (2.2) | 50 (3.7) | ||
| Liver transplantation | 4 (1.0) | 1 (1.1) | 16 (1.2) | ||
| RFA/PEI | 61 (15.8) | 16 (18.0) | 136 (9.9) | ||
Values are expressed as number (%).
Tumor characteristics were evaluated as a result of MRI or CT scans used at the time of HCC diagnosis.
aSome patients lacked clinical information at the time of HCC diagnosis.
bPatients not indicated for curative therapies were applied to palliative therapies including transarterial chemoembolization, transarterial radioembolization, radiotherapy, systemic treatments, or conservative treatment. Abbreviations: anti-HBc, anti–hepatitis B core antibody; BCLC, Barcelona Clinic Liver Cancer; HBsAg, hepatitis B surface antigens; HBV, hepatitis B virus, mUICC, Modified Union for International Cancer Control; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation.
Overall Survival
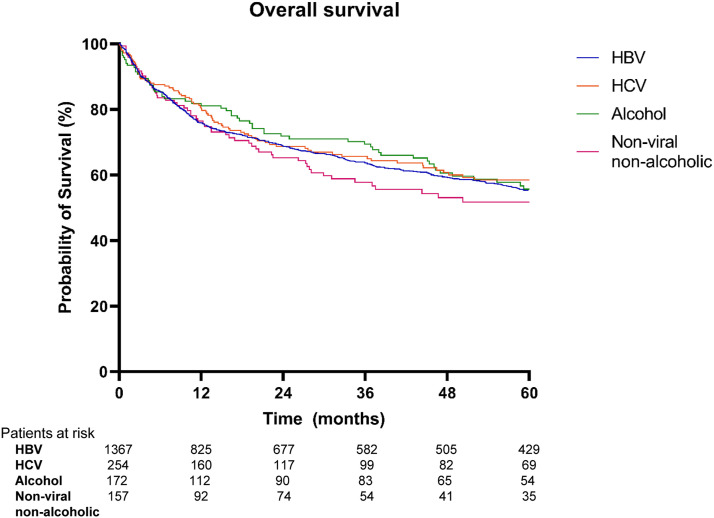
During the mean follow-up period of 40.4 ± 47.8 months, 768 patients (39.4%) died, 422 (21.6%) remained alive, and 760 (39.0%) were lost to follow-up. The cumulative survival rate at 5 years was 55.3% in the HBV group, 58.2% in the HCV group, 55.7% in the alcohol group, and 51.7% in the non-viral and non-alcoholic group. There was no significant difference in the OS rate among the groups (Figure 2). In patients with non-HBV HCC (Table 3), univariate analysis showed that low BMI (< 25), CTP class B/C, and presence of portal vein thrombosis in tumors were significantly associated with poor OS. The presence of anti-HBc did not affect patient survival. In multivariate analysis, CTP class A (P < .001, hazard ratio (HR) = .44, 95% CI: .37–.52) and presence of portal vein thrombosis of tumor (P < .001, HR = 5.18, 95% CI: 4.39–6.11) remained independently predictive of OS.
Figure 2.
Kaplan–Meier curves of overall survival according to etiological group of HCC.
Table 3.
Predictors for Overall Survival in Patients With Non-HBV HCC.
| Univariate | Multivariate | ||
|---|---|---|---|
| P value | HR (95% CI) | P value | |
| Age (years), ≥ 60 vs < 60 | .143 | ||
| Sex, male vs female | .188 | ||
| Diabetes, positive vs negative | .358 | ||
| Hypertension, positive vs negative | .443 | ||
| Total cholesterol > 200 vs < 200 mg/dL | .301 | ||
| Triglyceride > 150 vs < 150 mg/dL | .335 | ||
| BMI, ≥ 25 vs < 25 | .002 | .92 (.77–1.10) | .381 |
| CTP class, A vs B/C | < .001 | .44 (.37–.52) | < .001 |
| Portal vein thrombosis of tumor, yes vs no | < .001 | 5.18 (4.39–6.11) | < .001 |
| Anti-HBc, positive vs negative | .693 | ||
Discussion
Since the prevalence of cryptogenic HCC is increasing worldwide, attempts to clarify the cause in patients with cryptogenic HCC who are not candidates for screening are important. This large cohort study with a long-term follow-up evaluated the effects of metabolic disorders closely related to NAFLD or NASH and past or occult HBV infection, represented by positive anti-HBc, as potential etiologies of HCC on clinical characteristics or prognosis of HCC patients in an HBV-endemic area. Patients with non-viral and non-alcoholic HCC had higher prevalence of metabolic disorder and preserved liver function compared to those with other etiologies of HCC. When non-HBV HCC patients were divided by the results of anti-HBc, there was no significant difference in clinical or tumor characteristics between the two groups. In addition, the characteristics of HCC patients with negative HBsAg and positive anti-HBc differed from those of patients with HBV HCC.
Several clinical features of patients with non-viral and non-alcoholic HCC suggested that they had characteristics of NAFLD-related HCC in this study. First, the prevalence of metabolic disorders including diabetes, hypertension, hypercholesterolemia, and hypertriglyceridemia in the non-viral and non-alcoholic HCC group tended to be higher than the other HCC groups. Second, the proportion of non-viral and non-alcoholic HCC gradually increased according to age. The prevalence of diabetes and metabolic syndrome remarkably increased to 29.8 and 37.7%, respectively, in patients over 65 years of age according to a survey performed in South Korea.14,15 A previous study showed that the risk of HCC increased in association with diagnosis of diabetes, longer duration of diabetes, and increasing number of metabolic comorbidities.6 In addition, the proportion of patients with CTP class A was highest and that of patients with liver cirrhosis was lowest in the non-viral and non-alcoholic HCC group. There has been accumulating evidence showing that NAFLD could directly induce the carcinogenesis of HCC, with a reported 10–75% incidence of HCC from a non-cirrhotic liver.16
Previous studies demonstrated the role of anti-HBc positivity in the development of HCC. Past HBV infection as indicated by positive anti-HBc was a potential etiology in more than 40% of non-viral HCC cases in an HBV-endemic area and significantly associated with the development of HCC in patients with chronic liver disease, regardless of etiology in a meta-analysis.17,18 However, this study showed that anti-HBc positivity has no clinical significance in non-HBV HCC patients. There is still controversy about the role of anti-HBc positivity in HCC patients. A study from the United States demonstrated that neither past HBV infection nor OBI affects HCC development in patients with chronic hepatitis C.19 Furthermore, the specificity of serological anti-HBc testing for detecting OBI can be low due to the high prevalence of past HBV infection in HBV-endemic areas. The proportion of anti-HBc positivity did not agree well with that of OBI defined based on liver tissue with positive HBV DNA20 Further studies are needed to elucidate uncertainty about the effect of anti-HBc positivity in HCC patients.
Of note, patients with HBV HCC had a significantly lower prevalence of metabolic disorders compared to the other etiological groups in this study. The association between HBV infection and metabolic syndrome has been evaluated in previous studies, and although still controversial, many of them showed that HBV infection is associated with low risk of metabolic disorders.21 The proportion of patients who had metabolic syndrome was significantly lower in patients with HBV infection than patients with HCV infection or uninfected subjects.22 HBV infection was an independent predictor for lower risk of fatty liver after adjustment for factors including alcohol consumption and metabolic factors.23 Additionally, the proportion of patients with metabolic syndrome decreased in a linear trend as the HBV viral load increased.24 Despite the inverse relationship between metabolic factors and HBV infection, it has been reported that HCC is increased when metabolic factors accompanied CHB, especially in men.25,26
Patients with non-viral and non-alcoholic HCC had significantly higher proportion of advanced stage tumors compared to the other groups, suggesting that HCC screening was insufficient in these patients. However, the OS rate was not different among the HCC groups. Preserved liver function in patients with non-viral and non-alcoholic HCC might lead to a survival benefit because the presence of LC and high CTP score are significantly associated with poor OS in HCC patients. However, since we did not evaluate other variables related to the survival of HCC patients, further study is needed.
This study has some limitations largely due to its retrospective nature. There would be inaccuracies in identifying metabolic risk factors because those were evaluated based on interviewing patients or obtaining laboratory results at the time of diagnosis of HCC. This study was performed at a single tertiary center, thus possibly introducing bias in the results. We tried to overcome these limitations by including as many patients as possible for a long period of time. There were no conclusive results for the role of OBI in non-viral and non-alcoholic HCC because this study could not evaluate OBI enough via HBV DNA in sera or liver tissue. The test for HBV DNA was not routinely measured in clinical practice when patients were negative for HBsAg.
In conclusion, patients with non-viral and non-alcoholic HCC had a higher prevalence of metabolic disorder and preserved liver function compared to patients with other etiologies of HCC. The proportion of advanced stage tumors was higher in them. Anti-HBc positivity did not affect clinical characteristics or prognosis of non-HBV HCC patients. Further study is needed to elucidate possible etiologies of non-viral and non-alcoholic HCC in HBV endemic area.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211039758 for Effects of Positive Hepatitis B Core Antibody and Metabolic Disorders in Hepatocellular Carcinoma in an Endemic Area of Hepatitis B Virus by Hae Lim Lee, Si Hyun Bae, Jaejun Lee, Pil Soo Sung, Sung Won Lee, Jeong Won Jang, Jungmin Lee, Jong Young Choi, Nam Ik Han and Seung Kew Yoon in Cancer Control
Appendix
Abbreviations
- anti-HBc
anti–hepatitis B core antibody
- APRI
aspartate aminotransferase to platelet ratio index
- BCLC
Barcelona Clinic Liver Cancer
- BMI
body mass index
- CI
confidence interval
- CTP
Child–Turcotte–Pugh
- FIB-4
fibrosis-4
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- LC
liver cirrhosis
- mUICC
Modified Union for International Cancer Control
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OBI
occult HBV infection
- OS
overall survival
Author Contributions: Study concept and design: Jungmin Lee, Hae Lim Lee, and Si Hyun Bae; collecting and interpreting data: Jungmin Lee, Hae Lim Lee, and Si Hyun Bae; contributed reagents/materials/analysis tools: Hae Lim Lee, Si Hyun Bae, Jaejun Lee, Pil Soo Sung, Sung Won Lee, Jeong Won Jang, Jong Young Choi, Nam Ik Han, Seung Kew Yoon, and Jungmin Lee; Wrote the paper: Jungmin Lee, Hae Lim Lee, and Si Hyun Bae. All authors have approved this final version of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the Institutional Review Board/Ethics Committee of Seoul St. Mary’s Hospital of The Catholic University of Korea (KC17RESI0679).
Guarantor: JL
Informed Consent: Informed consent for patient information to be published in this article was not obtained. It was confirmed that consent forms might be waived for this study using only patient records by the Institutional Review Board/Ethics Committee.
Supplementary Material: Supplementary material for this article is available online.
ORCID iD
Jungmin Lee https://orcid.org/0000-0003-4153-3834
References
- 1.Liao SF, Yang HI, Lee MH, et al. Fifteen-year population attributable fractions and causal pies of risk factors for newly developed hepatocellular carcinomas in 11,801 men in Taiwan. PloS One. 2012;7:e34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Canc. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 3.Kwak HW, Park JW, Nam BH, et al. Clinical outcomes of a cohort series of patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2014;29:820-829. [DOI] [PubMed] [Google Scholar]
- 4.Kwak HW, Park JW, Koh YH, et al. Clinical characteristics of patients with cryptogenic hepatocellular carcinoma in a hepatitis B virus-endemic area. Liver Cancer. 2016;5:21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon TG, King LY, Chong DQ, et al. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology. 2018;67:1797-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531-1537. [DOI] [PubMed] [Google Scholar]
- 8.Cabrerizo M, Bartolome J, Caramelo C, et al. Molecular analysis of hepatitis B virus DNA in serum and peripheral blood mononuclear cells from hepatitis B surface antigen-negative cases. Hepatology. 2000;32:116-123. [DOI] [PubMed] [Google Scholar]
- 9.Wong DK, Huang FY, Lai CL, et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology. 2011;54:829-836. [DOI] [PubMed] [Google Scholar]
- 10.Giannini EG, Bodini G, Furnari M, et al. NASH-related and cryptogenic cirrhosis similarities extend beyond cirrhosis. J Hepatol. 2018;69:972-973. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328-357. [DOI] [PubMed] [Google Scholar]
- 12.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13(3):227-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korean Diabetes Association and Korean Diabetes Research Foundation . Diabetes Fact Sheet in Korea 2018. Korean Diabetes Association; 2019. http://www.diabetes.or.kr/pro/news/admin.php?category=A&code=admin&number=1666&mode=view. [Google Scholar]
- 15.Korean Society of Cardiometabolic Syndrome . Metabolic Syndrome Fact Sheet in Korea 2018; 2019. http://kscms.org/en/uploads/Metabolic_Syndrome.pdf. [Google Scholar]
- 16.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1-14. [DOI] [PubMed] [Google Scholar]
- 17.Lee SB, Kim KM, An J, et al. Clinical characteristics and potential aetiologies of non-B non-C hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int. 2016;36:1351-1361. [DOI] [PubMed] [Google Scholar]
- 18.Coppola N, Onorato L, Sagnelli C, et al. Association between anti-HBc positivity and hepatocellular carcinoma in HBsAg-negative subjects with chronic liver disease: a meta-analysis. Medicine (Baltim). 2016;95:e4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lok AS, Everhart JE, Di Bisceglie AM, et al. Occult and previous hepatitis B virus infection are not associated with hepatocellular carcinoma in United States patients with chronic hepatitis C. Hepatology. 2011;54:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim CW, Park JW, Kim SH, et al. Noncirrhotic hepatocellular carcinoma: etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol. 2017;10:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther. 2020;51:216-230. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YH, Kee KM, Wang JH, et al. Association between chronic viral hepatitis and metabolic syndrome in southern Taiwan: a large population-based study. Aliment Pharmacol Ther. 2018;48:993-1002. [DOI] [PubMed] [Google Scholar]
- 23.Wong VW-S, Wong GL-H, Chu WC-W, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [DOI] [PubMed] [Google Scholar]
- 24.Kuo YH, Tsai MC, Kee KM, et al. Associated factors for metabolic syndrome in the older adults with chronic virus hepatitis in the community. PloS One. 2016;11:e0155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu MW, Lin CL, Liu CJ, et al. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: a large cohort study. Gastroenterology. 2017;153(4):1006-1017. [DOI] [PubMed] [Google Scholar]
- 26.Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211039758 for Effects of Positive Hepatitis B Core Antibody and Metabolic Disorders in Hepatocellular Carcinoma in an Endemic Area of Hepatitis B Virus by Hae Lim Lee, Si Hyun Bae, Jaejun Lee, Pil Soo Sung, Sung Won Lee, Jeong Won Jang, Jungmin Lee, Jong Young Choi, Nam Ik Han and Seung Kew Yoon in Cancer Control