Abstract
Background:
Extended interval dosing of natalizumab is associated with significantly lower progressive multifocal leukoencephalopathy risk compared with every-4-week (Q4W) dosing in patients with relapsing-remitting multiple sclerosis. Previous studies have suggested that natalizumab effectiveness is maintained in patients who switch from Q4W to extended interval dosing but have been limited by a lack of well-matched patient cohorts.
Methods:
Tysabri Observational Program (TOP) data as of November 2019 were used to identify patients with relapsing-remitting multiple sclerosis treated with natalizumab Q4W and those with a single physician-indicated dosing change from Q4W to every-6-week (Q6W) dosing after ⩾1 year of Q4W treatment. Patients were propensity score matched at the time of the switch from Q4W to Q6W dosing. Clinical outcomes (annualized relapse rate and probability of remaining relapse free or free of 24-week confirmed disability worsening) and safety outcomes were assessed for the two cohorts.
Results:
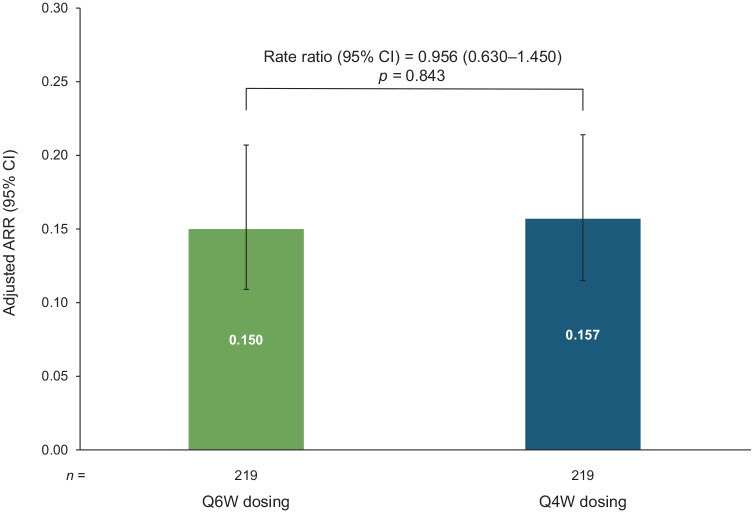
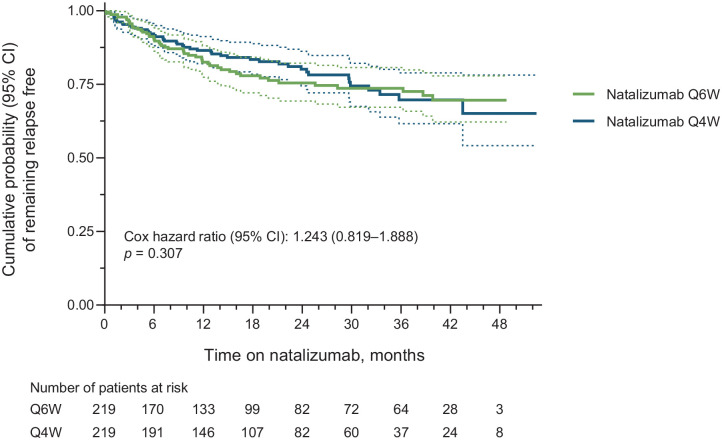
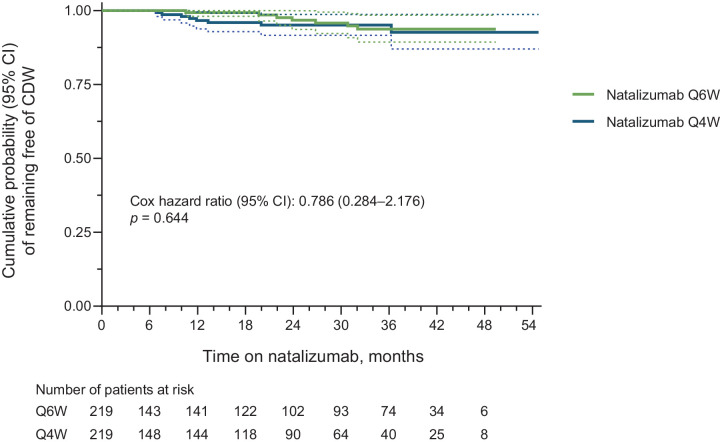
This study included 219 pairs of propensity score–matched Q6W and Q4W patients. Annualized relapse rates were similar for Q6W (0.150) and Q4W (0.157) patients. The probability of remaining relapse free [hazard ratio = 1.243 (95% confidence interval = 0.819–1.888); p = 0.307] and of remaining free of 24-week confirmed disability worsening [hazard ratio = 0.786 (95% confidence interval = 0.284–2.176); p = 0.644] did not differ significantly between Q6W and Q4W patients. Summarized safety results for the matched Q6W and Q4W patients are also presented.
Conclusion:
These real-world findings in well-matched patient cohorts from TOP demonstrate that natalizumab effectiveness is maintained in patients who switch to Q6W dosing after ⩾1 year of Q4W dosing.
ClinicalTrials.gov identifier:
Keywords: clinical outcomes, extended interval dosing, multiple sclerosis, natalizumab, real-world evidence
Introduction
Natalizumab administered intravenously every 4 weeks (Q4W) is an efficacious treatment for relapsing forms of multiple sclerosis (MS), as demonstrated by randomized clinical trials and real-world evidence.1–4 However, natalizumab treatment is also associated with increased risk of progressive multifocal leukoencephalopathy (PML) in anti–JC virus (JCV) antibody-positive patients.5,6
Some clinicians have explored using natalizumab extended interval dosing (EID) with the aim of reducing PML risk by balancing adequate immunosuppressive activity to control MS disease with allowing sufficient immune surveillance in the central nervous system to maintain JCV suppression.7,8
An analysis of the Tysabri Outreach: United Commitment to Health (TOUCH) Prescribing Program safety database demonstrated that natalizumab EID (average dosing interval of approximately 6 weeks) is associated with a significantly lower risk of PML than Q4W dosing.9 However, TOUCH does not include MS disease activity measures and therefore cannot provide evidence as to whether natalizumab EID decreases treatment effectiveness.
Independent real-world studies suggest that the effectiveness of natalizumab is maintained in patients who initiate treatment on standard interval dosing (SID) regimens and later switch to EID.7,8,10,11 However, the unmatched patient cohorts and variable definitions of EID employed limit the generalizability of these results, and there remains a vital need for comparative clinical effectiveness data for SID and EID dosing in well-matched real-world populations with well-defined dosing intervals.
The Tysabri Observational Program (TOP) is an ongoing, multinational prospective study of the safety and effectiveness of natalizumab in patients with relapsing-remitting multiple sclerosis (RRMS) in real-world clinical practice, with long-term safety and efficacy data collected from more than 6000 natalizumab-treated patients.3 Data collected in TOP include the exact dates of natalizumab infusions (since 2014), physician-intended dosing frequency, and the dates of deliberate dosing frequency changes, facilitating comparative assessments of patients on different dosing regimens.
In the present study, clinical outcomes were compared for patients in TOP who were treated with natalizumab Q4W for ⩾1 year and then switched to every-6-week (Q6W) dosing with propensity score–matched patients who remained on Q4W dosing.
Methods
Patients and study design
TOP (ClinicalTrials.gov identifier: NCT00493298) is an ongoing, open-label, multicenter, prospective observational study of the safety and effectiveness of natalizumab in patients with RRMS treated in real-world clinical practice settings.3,12 Details of the TOP study design have previously been published.12 Briefly, patients enrolled in TOP are required to have a diagnosis of RRMS and to have received ⩽3 doses of natalizumab in their lifetime at the time of enrollment. Patient demographic and clinical characteristics are recorded at enrollment, with ongoing patient assessments documented at regularly scheduled clinical practice visits (approximately every 6 months). Safety and relapse data may also be collected at unscheduled visits to evaluate new or worsening neurological symptoms. The TOP study protocol was approved by each center’s independent ethics committee, and all patients provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
TOP data available as of November 2019 were used to identify patients with Q4W and Q6W dosing histories based on physicians’ intended dosing on study case report forms. The Q6W group included patients who had a single physician-intended change in frequency of dosing from natalizumab Q4W to Q6W after ⩾1 year of Q4W treatment. Patients in the Q4W group had no intentional dosing other than Q4W. Patients with any natalizumab dosing interval ⩾12 weeks (a dosing gap) or < 3 weeks (overdosing) during the Q4W dosing period were excluded from the analysis. All eligible patients were also required to have sufficient demographic and disease characteristic information for propensity score matching.
Propensity score matching
All patients in the Q4W group who had sufficient follow-up to match a given Q6W patient, defined as on-treatment follow-up duration greater than or equal to the pre-switch Q4W treatment period for that Q6W patient, were considered potential matches. Propensity scores for Q6W patients and all potential matching Q4W patients were derived using logistic regression with age, sex, baseline Expanded Disability Status Scale (EDSS) score, baseline anti-JCV antibody status (positive/negative), time from MS onset, natalizumab exposure duration, and relapse activity (both prior to TOP enrollment and while on Q4W dosing in TOP) as covariates.
Q6W and Q4W patients were matched at the time of the dosing switch (for Q6W patients) or at the corresponding exposure-matched time point (for Q4W patients) using propensity score–based caliper matching.13 Patients were matched 1:1 with replacement of Q4W patients to achieve the best possible match for all Q6W patients.
Statistical analysis
Annualized relapse rates (ARRs) in the matched populations were calculated by negative binomial regression using generalized estimating equations for robust standard error estimation to derive 95% confidence intervals (CIs). Hazard ratios (HRs) of time to first relapse and time to 24-week confirmed disability worsening (CDW, defined as an increase of ⩾0.5 point from a baseline EDSS score of ⩾6.0, ⩾1.0 point from a baseline EDSS score of ⩾1.0 to < 6.0, or ⩾1.5 points from a baseline EDSS score of 0.0, confirmed after 24 weeks) were estimated using the Cox and Kaplan–Meier methods with no additional adjustments. Assuming a sample size of 236 patients per arm, power = 0.8, and α = 0.05, a significant difference in ARR for Q6W versus Q4W would be detected at a rate ratio of 1.93.
Safety
Safety outcomes were summarized for Q6W and Q4W patients using descriptive statistics. The unique (i.e. not duplicated) Q4W patient cohort was used for the safety assessment to avoid potential duplication of safety events in the matched group.
Results
Patients
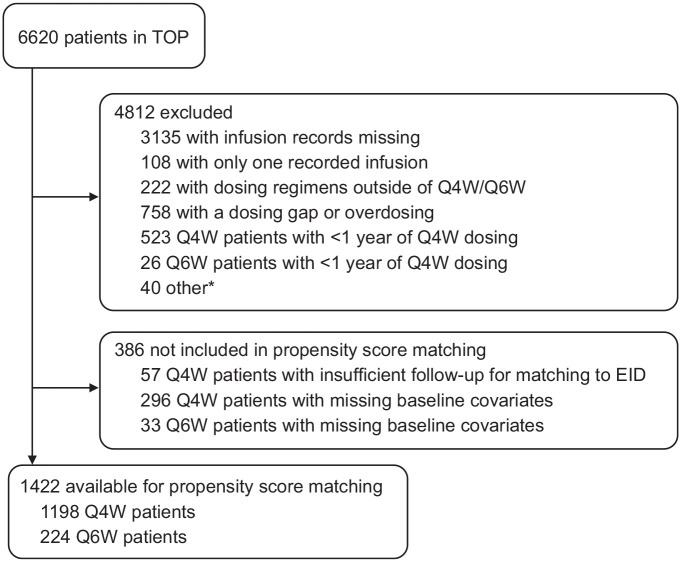
TOP data as of November 2019 included information on 6620 patients. A total of 3485 patients had nonmissing dosing records and were considered for inclusion, of whom 1422 met the study inclusion criteria and had sufficient follow-up and baseline information for propensity score matching (Figure 1). Of these, 224 patients had a single physician-intended change in dosing frequency from Q4W to Q6W (unique Q6W patients). The 1198 unique Q4W patients had all intentional dosing indicated as Q4W and had sufficient on-natalizumab follow-up to be considered potential matches for any Q6W patient at the time of the latter patient’s switch from Q4W to Q6W dosing.
Figure 1.
Tysabri Observational Program (TOP) patients included in propensity score matching.
EID, extended interval dosing; Q4W, every 4 weeks; Q6W, every 6 weeks.
*Other includes infusion records with missing dates (n = 13), no Q4W infusion history (switch occurred prior to 2014; n = 5), and multiple switches between Q4W and Q6W (n = 22).
Assessing all potential Q4W matches for each Q6W patient separately and allowing duplication of Q4W patients to achieve the best possible match yielded 33,295 potential Q4W matches for the 224 unique Q6W patients (Table 1). Propensity score–based 1:1 caliper matching produced 219 pairs of Q6W and Q4W patients for the effectiveness comparisons. Each of the 219 Q6W patients included were unique; 196 of the 219 Q4W patients included in the analyses were unique and 23 were matched with replacement to > 1 Q6W patient to minimize the difference between propensity scores and achieve the best match.
Table 1.
Demographic and disease characteristics at time of switch to Q6W dosing (Q6W patients) or matching exposure time point (Q4W patients) before and after propensity score matching.
| Covariatea | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| Q6W (n = 224) |
Q4W (n = 33,295) |
Standardized difference | Q6W (n = 219) |
Q4W (n = 219) |
Standardized difference | |
| Age, mean (SD) | 39.9 (9.50) | 43.1 (9.64) | −0.334 | 39.9 (9.55) | 40.6 (9.4) | −0.007 |
| Female, n (%) | 151 (67.4) | 23,844 (71.6) | −0.091 | 147 (67.1) | 147 (67.1) | 0.000 |
| Patients with relapses on Q4W prior to Q6W switch, n (%)b | 96 (41.5) | 13,237 (39.8) | 0.063 | 92 (42.0) | 95 (43.3) | −0.028 |
| EDSS score, mean (SD) | 3.29 (1.34) | 3.51 (1.53) | −0.149 | 3.31 (1.35) | 3.16 (1.44) | 0.110 |
| Number of relapses in the year prior to initiation of natalizumab, mean (SD) | 2.00 (0.89) | 2.06 (0.99) | −0.072 | 2.00 (0.89) | 1.95 (0.83) | 0.053 |
| Duration of MS symptoms, mean (SD), years | 14.02 (7.21) | 14.49 (6.86) | −0.068 | 13.87 (7.20) | 14.50 (7.70) | −0.084 |
| Natalizumab exposure, mean (SD), years | 4.32 (2.25) | 5.36 (2.30) | −0.458 | 4.36 (2.26) | 4.41 (2.28) | −0.020 |
| Anti-JCV antibody positive at baseline, n (%) | 76 (33.9) | 9637 (28.9) | 0.108 | 74 (33.8) | 68 (31.1) | 0.059 |
EDSS, Expanded Disability Status Scale; JCV, JC virus; MS, multiple sclerosis; Q4W, every 4 weeks; Q6W, every 6 weeks; SD, standard deviation.
Covariates shown for all potentially matched patients with replacement of Q4W patients to achieve best match.
For Q6W patients, number of patients experiencing relapse prior to the Q4W to Q6W switch time point; for Q4W patients, number of patients experiencing relapse during the matched exposure period prior to the Q4W to Q6W switch time point.
Demographic and disease covariates were well balanced between the Q6W and Q4W dosing groups after propensity score matching, with the absolute value of all standardized differences ⩽0.110 (Table 1). Mean (standard deviation) follow-up times for the matched Q6W and Q4W patients after the Q4W/Q6W switch time point were 2.00 (1.30) and 1.89 (1.15) years, respectively.
Clinical outcomes
In the matched treatment cohorts, over the follow-up period after the switch from Q4W to Q6W, most patients were free from relapse [Q6W, 171 of 219 (78.1%) Q4W, 178 of 219 (81.3%)] and ARRs did not differ significantly between groups [0.150 for Q6W versus 0.157 for Q4W; rate ratio 0.956 (95% CI = 0.630–1.450); p = 0.843; Figure 2].
Figure 2.
Annualized relapse rates (ARRs) in propensity score–matched Q6W and Q4W patients (all matched patients). ARRs in the matched population calculated with negative binomial regression. CIs derived using generalized estimating equation for robust standard errors.
ARR, annualized relapse rate; CI, confidence interval; Q4W, every 4 weeks; Q6W, every 6 weeks.
The Kaplan–Meier and Cox proportional-hazards estimated probability of remaining free of relapses did not differ significantly between the matched Q6W and Q4W cohorts [HR = 1.243 (95% CI = 0.819–1.888); p = 0.307; Figure 3]. Similarly, Q6W and Q4W patients did not differ in the probability of remaining free of 24-week CDW [HR = 0.786 (95% CI = 0.284–2.176); p = 0.644; Figure 4].
Figure 3.
Cumulative probability of remaining relapse free in propensity score–matched Q6W and Q4W patients (all matched patients). Cox p-value equivalent to log-rank test. Dashed lines indicate the 95% CIs.
CI, confidence interval; Q4W, every 4 weeks; Q6W, every 6 weeks.
Figure 4.
Cumulative probability of 24-week CDW in propensity score–matched Q6W and Q4W patients (all matched patients). CDW was defined as an increase of ⩾0.5 point from a baseline EDSS score of ⩾6.0, ⩾1.0 point from a baseline EDSS score of ⩾1.0 to < 6.0, or ⩾1.5 points from a baseline EDSS score of 0.0, confirmed after 24 weeks. Cox p-value equivalent to log-rank test. Dashed lines indicate the 95% CIs.
CDW, confirmed disability worsening; CI, confidence interval; EDSS, Expanded Disability Status Scale; Q4W, every 4 weeks; Q6W, every 6 weeks.
Safety
The safety population comprised the unique Q6W (n = 219) and Q4W (n = 196) patients. Serious adverse events (SAEs) were reported by 19 of 219 Q6W patients (8.7%) and 22 of 196 Q4W patients (11.2%; Table 2). In general, SAEs reported by Q6W and Q4W patients were similar. SAEs with a greater than twofold difference in incidence between dosing groups were infections and infestations [reported by 4 of 219 Q6W patients (1.8%) and 8 of 196 Q4W patients (4.1%)] and neoplasms [reported by 4 of 219 Q6W patients (1.8%) and 1 of 196 Q4W patients (0.5%)]. SAEs related to treatment were observed in 4 Q6W patients (1.8%) and 3 Q4W patients (1.5%). Supplemental Table e-1 lists all SAEs experienced by the Q6W and Q4W safety populations. There were two confirmed cases of PML, both in the Q6W treatment group.
Table 2.
Summary of safety outcomes in propensity score–matched Q6W and Q4W patients (unique patient sets).
| Outcome, n (%) | Treatment group |
|
|---|---|---|
| Q6W (n = 219) |
Q4W (n = 196) |
|
| Patients with ⩾1 SAE | 19 (8.7) | 22 (11.2) |
| Patients with ⩾1 treatment-related SAEa | 4 (1.8) | 3 (1.5) |
| SAEs occurring in > 1% of either group, n (%)b | ||
| Infections and infestations | 4 (1.8) | 8 (4.1) |
| Nervous system disorders | 4 (1.8) | 5 (2.6) |
| Gastrointestinal disorders | 1 (0.5) | 4 (2.0) |
| Neoplasmsc | 4 (1.8) | 1 (0.5) |
| Pregnancy, puerperium, and perinatal conditions | 4 (1.8) | 1 (0.5) |
| Injury, poisoning, and procedural complications | 2 (0.9) | 3 (1.5) |
| Musculoskeletal and connective tissue disorders | 3 (1.4) | 3 (1.5) |
| Hepatobiliary disorders | 1 (0.5) | 2 (1.0) |
| Infections requiring hospitalization | 2 (0.9) | 3 (1.5) |
| Malignancy | 2 (0.9) | 1 (0.5) |
| PML | 2 (0.9) | 0 |
| Deaths | 1 (0.5) | 0 |
PML, progressive multifocal leukoencephalopathy; Q4W, every 4 weeks; Q6W, every 6 weeks; SAE, serious adverse event.
Includes relation to study medication recorded as related, possibly related, or unknown.
Listed by System Organ Class.
Benign, malignant, and unspecified neoplasms, including cysts and polyps.
Case 1 was a 49-year-old male who had received 105 doses of natalizumab. The patient had prior exposure to the immunosuppressant cyclophosphamide (total exposure not specified), and 6 months prior to PML onset, his anti-JCV antibody index was 4.09. The physician-intended switch from Q4W to Q6W dosing occurred < 1 month prior to initial PML symptoms of worsening gait and dysarthria. The reporting physician indicated that the PML case occurred under Q4W dosing. The last magnetic resonance imaging (MRI) prior to PML symptom onset was conducted approximately 7 months before the switch to Q6W dosing (8 months before PML symptom onset). The patient had an EDSS score of 6.5 reported in TOP 6 months prior to PML symptom onset. Approximately 2 months after PML symptom onset, his EDSS score was 7.5 and ongoing symptoms of severe dysarthria, dysphagia, cognitive deficit, and unstable gait were reported. As of the last update (approximately 6 months after PML symptom onset), the patient was alive and in an intensive care unit with a Karnofsky score of 10. The outcome is considered PML without recovery.
Case 2 was a 48-year-old female who had received 48 doses of natalizumab. The patient had prior exposure to the immunosuppressant cyclophosphamide (total exposure not specified), and 8 months prior to PML onset, her anti-JCV antibody index was 1.17. The switch from Q4W to Q6W dosing occurred 15 months prior to the initial PML symptoms of decreased vision, anxiety, sensory problems, insomnia, worsening of stability, and cognitive function slowing. The reporting physician indicated that the PML case occurred under Q6W dosing. The patient had an EDSS score of 4.0 reported in TOP 3 months prior to PML symptom onset. Approximately 5 months after PML symptom onset, her EDSS score was 5.0 and her Karnofsky score was 60. Approximately 1 year after symptom onset, the PML-IRIS outcome was assessed by physician as recovered with sequelae; the patient’s EDSS score was 5.5 and her Karnofsky score was 70. As of the last update (approximately 2 years after PML symptom onset), the patient was alive, had a stable EDSS score, and was residing at home and receiving unspecified treatment for MS.
Discussion
In this study of well-matched natalizumab patients in TOP, there were no significant differences in ARR, risk of relapse, or risk of EDSS worsening between patients who switched to Q6W dosing after ⩾1 year of Q4W dosing and those who remained on Q4W dosing.
The main limitation of this analysis is that it is an observational study with the sample size limited to the available population in TOP. As such, the study was not powered to test the specific hypothesis of equivalence between EID and SID. The authors estimate that with the available sample size, this study had the power to detect a rate ratio of 1.93 in ARR as a significant difference with α = 0.05. Thus, it is important not to overinterpret the lack of statistically significant differences in clinical outcomes. The study conclusions are primarily inferred from and supported by the high similarity in point estimates observed between the Q6W and Q4W groups.
The ARRs observed here for the Q6W and Q4W cohorts (0.150 and 0.157, respectively) over approximately 2 years of follow-up compare favorably with other real-world observational studies of natalizumab effectiveness in patients over 1–2 years of treatment.2,14–16 This suggests that the similarities in ARR between Q6W dosing and Q4W dosing groups are not driven by atypical responses to natalizumab in this smaller, more selective patient population.
Real-world clinical and radiographic outcomes for patients on natalizumab EID versus SID regimens have been reported elsewhere.7,8,10,11,17 While all of these studies have found that natalizumab effectiveness is not diminished with EID, they are limited by variable definitions of EID, which include dosing intervals ranging from 5 to 8 weeks, and by a lack of well-matched EID and SID patient cohorts (Table 3). Since standardized MS disease activity measures, physician-intended dosing frequency, and dates of intentional dosing frequency changes are prospectively collected in TOP, the analyses described here could be focused on a specific EID practice of interest, be based on EID and SID patients exposure-matched at the time of the switch to EID, and account for covariates both prior to natalizumab initiation and during initial Q4W treatment.
Table 3.
Summary of previous studies of natalizumab EID versus SID.
| Publication | Sample sizes | Dosing definitions | Observed average dosing intervals | Prior SID exposure for EID group | Follow-up time | Efficacy outcomes | PML outcomes | Author conclusion |
|---|---|---|---|---|---|---|---|---|
| Zhovtis Ryerson et al.7 | SID: 1080 EID: 894 |
SID defined as Q4W EID defined as ⩾31 days |
SID: 30 days EID: 31–61 days |
⩾6 months Q4W dosing | Q4W: 27.39 doses (mean) EID: 40.07 doses (mean total); 24.96 (mean as EID) |
Proportion with no new MRI activity: 81% during Q4W 82% during EID Proportion with no new clinical activity: 74% during Q4W 75% during EID: Proportion with no new MRI activity and no new clinical activity: 62% during Q4W 78% during EID |
Q4W: 4 cases EID: no cases |
‘Dosing intervals up to 8 weeks and 5 days did not diminish effectiveness of natalizumab therapy’. |
| Yamout et al.8 | SID: 85 EID: 85 (all participants switched to EID) |
SID defined as Q4W EID defined as ⩾35 days |
SID: Not reported EID: 35–56 days |
Mean of 15.4 months Q4W dosing | Q4W: 15.4 months EID: 11.8 months |
Proportion relapse free: At end of Q4W 95.3% At end of EID 93.9% Proportion with no new MRI activity: 81.2% at end of Q4W 92.0% at end of EID Mean (SD) EDSS score: 2.56 (1.62) at end of Q4W 2.59 (1.61) at end of EID |
EID: no cases | ‘In patients treated with natalizumab, shifting from Q4W dosing to EID has no negative effect on efficacy as evidenced by relapse rate, disability progression, and MRI activity’. |
| Bomprezzi and Pawate10 | SID: 361 EID: 96 |
SID defined as Q4W EID defined as 42–56 days |
SID: not reported EID: not reported |
Mean of 22 months Q4W dosing (range, 12–24 months) | Q4W: 22 months (mean) EID: ⩾6 months (mean 20 months) |
Proportion relapsed: 13% during Q4W 13% during EID Proportion with new MRI activity: 11% during Q4W 9% during EID |
Not reported | ‘EID did not compromise the treatment effect as measured by relapse rate, and no significant breakthrough disease activity was observed’. |
| Clerico et al.11 | SID: 216 EID: 144 |
SID defined as < 35 days EID defined as ⩾35 days |
SID: 30.1 days during first 6 months of follow-up; 30.8 days overall EID: 43.4 days during first 6 months of follow-up; 42 days overall |
6 months SID | SID and EID: 2.2 years | ARR (95% CI): SID: 0.060 (0.033, 0.087) EID: 0.039 (0.017, 0.063) |
SID: no cases EID: no cases |
‘In conclusion, there is no evidence of a reduced efficacy of natalizumab in an EID setting’. |
| Chisari et al.17 | SID: 1254 EID: 838 |
SID defined as 28–32 days EID defined as 33–49 days |
SID: 30.8 days EID: 39.8 days |
⩾6 months | SID and EID: Follow-up assessments at 12 months and 24 months | Proportion relapsed at 12 months: SID: 4.3% EID: 3.5% Proportion relapsed at 24 months: SID: 3.7% EID: 3.1% Mean (SD) ARR at 12 months: SID: 0.10 (0.09) EID: 0.11 (0.08) Mean (SD) ARR at 24 months: SID: 0.09 (0.05) EID: 0.10 (0.05) Mean (SD) EDSS score at 12 months: SID: 3.1 (2.9) EID: 3.2 (2.5) Mean (SD) EDSS score at 24 months: SID: 3.3 (2.8) EID: 3.4 (2.1) |
Not reported | ‘The use of natalizumab with an extended interval schedule showed similar effectiveness compared with SID’. |
ARR, annualized relapse rate; CI, confidence interval; EDSS, Expanded Disability Status Scale; EID, extended interval dosing; MRI, magnetic resonance imaging; PML, progressive multifocal leukoencephalopathy; Q4W, every 4 weeks; SD, standard deviation; SID, standard interval dosing.
For this study, EID was defined specifically as Q6W dosing following ⩾1 year of Q4W dosing. This definition was selected for consistency with the EID regimen associated with lower PML risk versus SID in the TOUCH safety database analysis. In the TOUCH analysis, the average EID dosing interval was approximately 6 weeks (range, 35.0–43.0 days), and approximately 85% of the patients who switched to EID did so after ⩾1 year of Q4W dosing (median of 25 infusions prior to switch).9 The specification of an EID dosing interval of Q6W is supported by observations from two recent retrospective cohort studies of 838 and 144 patients in Italian clinical practice who received natalizumab EID over ⩽2 years, in which the median EID dosing intervals were 43 and 42 days, respectively.11,17
The focus on this particular EID regimen is also supported by efficacy modeling and simulations from natalizumab clinical trials. Pharmacokinetic/pharmacodynamic (PK/PD) model–based simulations of disease outcomes for patients initiating natalizumab at different dosing intervals concluded that initializing treatment on EID might not provide adequate disease control.18 However, more recent PK/PD model–based simulations predict that natalizumab efficacy may be maintained with dosing intervals ⩽6 weeks in patients who have taken natalizumab Q4W for ⩾1 year before switching to EID.19 An ongoing randomized prospective trial of natalizumab effectiveness in patients who switch to Q6W dosing after ⩾1 year of Q4W dosing compared with those who remain on Q4W dosing (NOVA, NCT03689972) is being conducted to assess the same EID regimen employed in this analysis of TOP.
While the definition of EID as Q6W dosing after ⩾1 year of Q4W dosing employed here was based on the safety and efficacy considerations specified above, TOP data indicate that this also reflects a common EID clinical practice. In this population, only 222 of 3485 TOP patients (6.4%) with available infusion records had any intentional dosing intervals other than Q4W and Q6W, suggesting that physician-intended changes from Q4W to dosing frequencies other than Q6W are uncommon. Data from TOP also indicate that most patients who switch to Q6W dosing do so after a stable period of Q4W dosing. Indeed, of the TOP patients with Q6W dosing records in these analyses, only 26 were excluded for having a Q4W dosing history of < 1 year. Thus, the results of this analysis are applicable to natalizumab EID in common clinical practice.
Propensity score matching at time of the switch to Q6W rather than at the initiation of natalizumab treatment permitted the inclusion of potentially important covariates not considered in other analyses of EID versus SID. Disease activity while on Q4W dosing prior to switch was included as a covariate, which should help to reduce potential bias caused by selection of patients for EID based on optimal early natalizumab outcomes. Anti-JCV antibody status was also included as a covariate, as PML risk is often the main consideration in switching patients from Q4W to Q6W dosing in clinical practice. The propensity score matching strategy utilized here yielded comparison populations that were extremely well matched (with all covariate standardized differences after matching ⩽ 0.110); thus, potential bias from any of the measured covariates is likely to be small. However, as TOP is a real-world data set, the study is still limited by potential remaining selection biases from unmeasured covariates. In particular, since MRI data are not recorded in TOP, such data were not available for use as a matching covariate.
TOP patients on Q6W and Q4W dosing appeared to have a similar incidence of all SAEs as well as SAEs related to treatment based on a qualitative assessment, though no hypothesis testing was planned or performed due to sample sizes. The SAEs observed in both groups were consistent with those observed in previous studies of patients in TOP3 and other real-world2 and clinical1 studies. Two cases of PML were observed in this study, both in the Q6W dosing group. It is worth noting that one of the PML cases occurred within 1 month after a switch to Q6W dosing and was recorded by the physician as a Q4W PML case. Thus, a major limitation of the current analysis with respect to safety summary is that patients could be included in the Q6W cohort who had very short Q6W exposure. Both PML cases were characterized by presence of known risk factors for PML,6 including positivity for anti-JCV antibodies, natalizumab exposure beyond 2 years, and prior use of immunosuppressants. These factors place both cases in the highest PML risk category. The cases highlight the fact that though EID has been shown to be associated with lower risk of PML,9 PML cases do still occur with EID.7,20–22 These findings reinforce the need for vigilant monitoring of natalizumab patients and consideration of all the known PML risk factors, including anti-JCV antibody index, prior immunosuppressant use, and exposure duration, even in the context of EID. It should be noted that the small sample sizes included here are not sufficient to compare safety outcomes, especially for rare events, in the propensity score–matched dosing cohorts. Further real-world studies (including TOP) with more patients and longer follow-up periods may provide additional insight into the real-world safety of Q6W versus Q4W dosing.
In conclusion, real-world clinical outcomes in TOP indicate that natalizumab effectiveness is maintained in propensity score–matched patients who switch to Q6W after ⩾1 year of Q4W dosing. These data may also be helpful in understanding the benefit-risk profile of Q6W dosing. The results of the ongoing NOVA randomized trial will provide a more complete picture of natalizumab effectiveness in patients with RRMS who switch to Q6W dosing after ⩾1 year of Q4W dosing.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864211042458 for No evidence for loss of natalizumab effectiveness with every-6-week dosing: a propensity score–matched comparison with every-4-week dosing in patients enrolled in the Tysabri Observational Program (TOP) by Helmut Butzkueven, Ludwig Kappos, Tim Spelman, Maria Trojano, Heinz Wiendl, Ray Su, Shirley Liao, Robert Hyde, Stephanie Licata, Pei-Ran Ho and Nolan Campbell in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors gratefully acknowledge the contributions of the TOP investigators. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript and take responsibility for the integrity of the work as a whole. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by John Watson, PhD, of Ashfield MedComms (Middletown, CT, USA), an Ashfield Health company, and was funded by Biogen. Joshua Safran of Ashfield MedComms copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the manuscript to the authors. The authors provided their final approval of all content.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Biogen.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HB: institution (Monash University) has received compensation for consulting, talks, advisory/steering board activities from Alfred Health, Biogen, Genzyme, Merck, and Novartis and research support from Biogen, Merck, MS Research Australia, National Health and Medical Research (Australia), Novartis, the Oxford Health Policy Forum, the Pennycook Foundation, and Roche. LK: institution (University Hospital Basel) has received and used exclusively for research support steering committee, advisory board, consultancy fees from Actelion, Addex, Bayer HealthCare, Biogen, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB, and Xenoport; speaker fees from Bayer HealthCare, Biogen, Merck, Novartis, Sanofi, and Teva; support of educational activities from Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; and grants from Bayer HealthCare, Biogen, F. Hoffmann-La Roche, Merck, Novartis, the European Union, Inno-Swiss, the Roche Research Foundations, the Swiss Multiple Sclerosis Society, and the Swiss National Research Foundation. TS: has received honoraria for consultancy and funding for travel from Biogen and Novartis. MT: has received compensation for consulting from Biogen, Merck Serono, Novartis, and Roche; speaker honoraria from Biogen, Merck Serono, Novartis, Roche, Sanofi Genzyme, and Teva; and research grants from Biogen, Merck Serono, Novartis, and Roche. HW: has received honoraria from AbbVie, Actelion, Alexion, Biogen, Cognomed, Evgen, F. Hoffmann-La Roche, MedDay, Merck Serono, Novartis, Roche Pharma AG, Sanofi Genzyme, and Teva and research support from Biogen, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi. RS, S Liao, S Licata, P-RH: former employees of and may hold stock and/or stock options in Biogen. RH, NC: employees of and may hold stock and/or stock options in Biogen.
ORCID iDs: Helmut Butzkueven  https://orcid.org/0000-0003-3940-8727
https://orcid.org/0000-0003-3940-8727
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Helmut Butzkueven, Department of Neuroscience, Central Clinical School, Monash University, Alfred Centre, Melbourne, VIC 3004, Australia. Department of Neurology, Box Hill Hospital, Monash University, Box Hill, VIC, Australia.
Ludwig Kappos, Research Center for Clinical Neuroimmunology and Neuroscience Basel, Departments of Medicine, Clinical Research, Biomedicine, and Biomedical Engineering, University Hospital and University of Basel, Basel, Switzerland.
Tim Spelman, Department of Medicine and Melbourne Brain Centre, Royal Melbourne Hospital, The University of Melbourne, Melbourne, VIC, Australia.
Maria Trojano, Department of Basic Medical Sciences, Neuroscience and Sense Organs, University of Bari, Bari, Italy.
Heinz Wiendl, Department of Neurology, University of Münster, Münster, Germany.
Ray Su, Biogen, Cambridge, MA, USA, at the time of this analysis.
Shirley Liao, Biogen, Cambridge, MA, USA, at the time of this analysis.
Robert Hyde, Biogen, Baar, Switzerland.
Stephanie Licata, Biogen, Cambridge, MA, USA, at the time of this analysis.
Pei-Ran Ho, Biogen, Cambridge, MA, USA, at the time of this analysis.
Nolan Campbell, Biogen, Cambridge, MA, USA.
References
- 1.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 2.Perumal J, Fox RJ, Balabanov R, et al. Outcomes of natalizumab treatment within 3 years of relapsing-remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol 2019; 19: 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 2020; 91: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigaut K, Fabacher T, Kremer L, et al. Long-term effect of natalizumab in patients with RRMS: TYSTEN cohort. Mult Scler 2020; 27: 729–741. [DOI] [PubMed] [Google Scholar]
- 5.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870–1880. [DOI] [PubMed] [Google Scholar]
- 6.Ho P-R, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16: 925–933. [DOI] [PubMed] [Google Scholar]
- 7.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 885–889. [DOI] [PubMed] [Google Scholar]
- 8.Yamout BI, Sahraian MA, Ayoubi NE, et al. Efficacy and safety of natalizumab extended interval dosing. Mult Scler Relat Disord 2018; 24: 113–116. [DOI] [PubMed] [Google Scholar]
- 9.Zhovtis Ryerson L, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019; 93: e1452–e1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: a two-center, 7-year experience. Ther Adv Neurol Disord 2014; 7: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerico M, De Mercanti SF, Signori A, et al. Extending the interval of natalizumab dosing: is efficacy preserved? Neurotherapeutics 2020; 17: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butzkueven H, Kappos L, Pellegrini F, et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J Neurol Neurosurg Psychiatry 2014; 85: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belachew S, Phan-Ba R, Bartholomé E, et al. Natalizumab induces a rapid improvement of disability status and ambulation after failure of previous therapy in relapsing-remitting multiple sclerosis. Eur J Neurol 2011; 18: 240–245. [DOI] [PubMed] [Google Scholar]
- 15.Melin A, Outteryck O, Collongues N, et al. Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 2012; 259: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 16.Totaro R, Lugaresi A, Bellantonio P, et al. Natalizumab treatment in multiple sclerosis patients: a multicenter experience in clinical practice in Italy. Int J Immunopathol Pharmacol 2014; 27: 147–154. [DOI] [PubMed] [Google Scholar]
- 17.Chisari CG, Grimaldi LM, Salemi G, et al. Clinical effectiveness of different natalizumab interval dosing schedules in a large Italian population of patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2020; 91: 1297–1303. [DOI] [PubMed] [Google Scholar]
- 18.Muralidharan KK, Steiner D, Amarante D, et al. Exposure–disease response analysis of natalizumab in subjects with multiple sclerosis. J Pharmacokinet Pharmacodyn 2017; 44: 263–275. [DOI] [PubMed] [Google Scholar]
- 19.Chang I, Muralidharan KK, Campbell N, et al. Modeling the efficacy of natalizumab in multiple sclerosis patients who switch from every-4-week dosing to extended-interval dosing. J Clin Pharmacol 2021; 61: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldassari LE, Jones SE, Clifford DB, et al. Progressive multifocal leukoencephalopathy with extended natalizumab dosing. Neurol Clin Pract 2018; 8: e12–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarpazza C, De Rossi N, Tabiadon G, et al. Four cases of natalizumab-related PML: a less severe course in extended interval dosing? Neurol Sci 2019; 40: 2119–2124. [DOI] [PubMed] [Google Scholar]
- 22.Hervás JV, Presas-Rodríguez S, Crespo-Cuevas AM, et al. Progressive multifocal leukoencephalopathy associated to natalizumab extended dosing regimen. Neurodegener Dis Manag 2015; 5: 399–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864211042458 for No evidence for loss of natalizumab effectiveness with every-6-week dosing: a propensity score–matched comparison with every-4-week dosing in patients enrolled in the Tysabri Observational Program (TOP) by Helmut Butzkueven, Ludwig Kappos, Tim Spelman, Maria Trojano, Heinz Wiendl, Ray Su, Shirley Liao, Robert Hyde, Stephanie Licata, Pei-Ran Ho and Nolan Campbell in Therapeutic Advances in Neurological Disorders