Abstract
Purpose:
The aim of this study was to evaluate the demographic characteristics, clinical and pathological factors, and the outcome of cancer and COVID-19 patients in Mexico.
Patients and methods:
A prospective, multicentric study was performed through a digital platform to have a national registry of patients with cancer and positive SARS-CoV-2 test results through reverse transcription quantitative polymerase chain reaction (RT-qPCR). We performed the analysis through a multivariate logistic regression model and Cox proportional hazard model.
Results:
From May to December 2020, 599 patients were registered with an average age of 56 years with 59.3% female; 27.2% had hypertension. The most frequent diagnoses were breast cancer (30.4%), lymphoma (14.7%), and colorectal cancer (14.0%); 72.1% of patients had active cancer and 23.5% of patients (141/599) were deceased, the majority of which were men (51.7%). This study found that the prognostic factors that reduced the odds of death were gender (OR = 0.42, p = 0.031) and oxygen saturation (OR = 0.90, p = 0.0001); meanwhile, poor ECOG (OR = 5.4, p = 0.0001), active disease (OR = 3.9, p = 0.041), dyspnea (OR = 2.5, p = 0.027), and nausea (OR = 4.0, p = 0.028) increased the odds of death. In the meantime, the factors that reduce survival time were age (HR = 1.36, p = 0.035), COPD (HR = 8.30, p = 0.004), having palliative treatment (HR = 10.70, p = 0.002), and active cancer without treatment (HR = 8.68, p = 0.008).
Conclusion:
Mortality in cancer patients with COVID-19 is determined by prognostic factors whose identification is necessary. In our cancer population, we have observed that being female, younger, non-COPD, with non-active cancer, good performance status, and high oxygen levels reduce the probability of death.
Keywords: cancer patients, COVID-19, death, Latin America, prognostic factors, survival
Introduction
The severity of the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to a pandemic crisis, with dramatic loss of human life worldwide. According to a survey from the World Health Organization, 90% of countries report one or more disruptions to essential health services.1 Until 11 June 2021, there have been about 175 million cases with 3,775,180 deaths.2 During the same period, Mexico had 2,413,742 cases and 223,568 deaths.3
In general population, elderly males, presence of diabetes, and obesity have been identified as biological vulnerabilities for more severe COVID-19 outcomes.4 On the contrary, cancer is among the top causes of death.5 Higher mortality of COVID-19 seems to be related to cancer, but until today, there is no consistency on data.6 Reports about racial/ethnic disparities found that Latinos at the United States bear a disproportionate burden of COVID-19-related outcomes. One possible explanation is that they underline many comorbidities.7,8
Available data of prognostic factors in Latin American cancer patients with COVID-19 are currently limited. A recent report from the COVID-19 and Cancer Consortium (CCC-19), which included 4966 patients (where only 15% were Hispanic), showed that besides age, male gender, obesity, and comorbidities, being Hispanic, having a worse performance status, a hematological malignancy, and recent chemotherapy are associated with more severe COVID-19.9
Mexico is an upper-middle-income country where cancer has remained the third leading cause of death.10 On one hand, 10.4% of the population had diabetes and 25.5% had hypertension; on the other hand, 65% of the population has overweight and 30% has obesity.11,12 The aim of this study was to evaluate the specific demographic characteristics and clinical factors associated with survival and death of cancer patients with SARS-CoV-2 from the north, center, and south of Mexico.
Methods
Between March and April 2020, oncologists from public and private institutions serving cancer patients of all 32 states of Mexico and serving patients infected with COVID-19 were invited to the study. After their confirmation, two meetings were held to establish the relevant variables for the current study from the clinical and epidemiologic point of view and its definition. A digital platform was created (https://www.oncovid-19.org) with restricted access to enter the information of the participating centers across the country. Three pilot tests were performed and reviewed by experts to streamline the registry process. This was a prospectively planned study, but the data collection could be retrospective, after the infection by SARS-CoV-2.
Patients
The inclusion criteria were subjects ⩾18 years old, any sex, with confirmed cancer diagnosis, and positive SARS-CoV-2 test results through reverse transcription quantitative polymerase chain reaction (RT-qPCR). We excluded patients with cancer whose diagnoses were 10 or more years ago without any recurrence. The obtained information spans from May to December 2020. Once the information of all institutions was received, the database was validated up to the cutoff date. Given the sensible nature of the study, personal identification data of individual patients were not included. This protocol was approved by the Review Board of the Instituto Nacional de Cancerología (Rev/0016/20). Informed consent was waived.
Definitions and outcomes
The following definitions were considered for this analysis: the functionality was measured through Eastern Cooperative Oncology Group (ECOG), which was clustered in two categories: low functionality (ECOG 2, 3, and 4) and high functionality (ECOG 0 and 1). The type of malignancy was divided in two categories: solid tumors and hematologic. Then, the clinical stage was defined as non-metastatic and metastatic. For hematologic malignancies that are not anatomically staged, like leukemias, they were considered disseminated at diagnosis, with some exceptions. The cancer status was categorized as in remission or without evidence and active disease. The ‘treatment’ variable clustered all patients who receive systemic (neoadjuvant, adjuvant, palliative, or maintenance) or radical (surgery or radiotherapy) management. Also, the ‘non-treatment’ variable included cases that were on vigilance or recently diagnosed, therefore, without an allocated treatment. Regarding comorbidities, chronic kidney disease was defined as the presence of kidney damage or an estimated glomerular filtration rate less than 60 ml/min/per 1.73 m2, persisting for 3 months or more;13 meanwhile, chronic obstructive pulmonary disease (COPD) includes emphysema and chronic bronchitis. Finally, both COVID-19 comorbidities and related symptoms were defined as present or absent. The follow-up time was calculated as the difference between initial COVID-19 symptom date and the patient outcome date (death or last visit, accordingly).
Statistical analysis
The data were represented as absolute and relative frequency tables for the categorical variables, and as medians and interquartile range (IQR) for the quantitative variables. For the analysis, patients were divided according to their outcome (death or alive). The possible differences were initially established with chi-square test/Wilcoxon’s test and Fisher’s exact test, as appropriate. A p value ⩽0.05 was considered to determine the study-relevant variables exclusively. A logistic regression was done to obtain a final model with the factors that are related to the patient outcome. During the final multivariate analysis, the p < 0.05 value was considered as statistically significant. Kaplan–Meier curves and log-rank test were done for all the categorical predictors, and for the continuous variables, we used a univariate Cox proportional hazard regression, to explore whether to include them in the Cox final model (we consider them if the test has a p ⩽ 0.2). After the final model, we describe the predictors with a p value <0.05. Stata V16 was the program used for statistical analysis.14
Results
General population profile
Upon information delivery cutoff date, five institutions from Mexico City and seven states participated. The data included 599 cancer patients infected with SARS-CoV-2, mostly from Mexico City from the Instituto Nacional de Cancerología (n = 313, 52.2%); Hospital de Oncología Centro Médico Nacional Siglo XXI, IMSS (n = 87, 14.5%); Centro Médico ABC (n = 86, 14.3%); and Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (n = 33, 5.5%), followed by the north of Mexico (Baja California Norte, Sonora, and Nuevo León), center of the country (that includes data from Guanajuato y Querétaro), and the southeastern part of Mexico (includes Veracruz, Oaxaca, and Yucatán).
The median age of the included patients in this study was 56 years (IQR = 45–66 years), with 59.3% of patients being female and 68.4% had good functionality (ECOG 0 and 1). Regarding addictions, up to 25% of patients were smokers or consumed alcoholic beverages. On the contrary, 24.2% of patients did not present additional comorbidities apart from cancer and SARS-CoV-2 infection. It is relevant that the median body mass index (BMI) of the population can be categorized as overweight with 26.4 (IQR = 23.7–29.4), whereas 22.4% had obesity (BMI ⩾ 30). Meanwhile, high blood pressure (HBP) was reported on 27.2% of patients, followed by diabetes mellitus, dyslipidemia, chronic kidney failure, and cardiac disease with 19.5%, 5.7%, 4.0%, and 3.4%, respectively.
The most frequent neoplasias were breast cancer (n = 122, 30.4%), lymphoma (n = 59, 14.7%), colorectal (n = 56, 14.0%), prostate (n = 32, 8.0%), and cervical cancer (n = 30, 7.5%). From the whole group, only 24.4% of the cases were on remission. Among active cases, 34.2% showed metastatic disease. From hematologic tumors (n = 103), 34% have no disease activity.
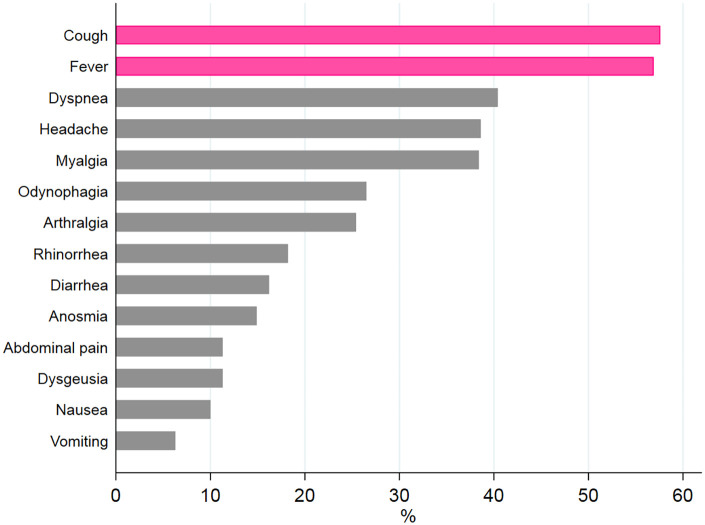
On the overall population, the most frequent COVID-19 symptoms were cough (58%) and fever (56.8%), whereas dyspnea was shown in 45.8% (see Figure 1). When considering deceased patients, the cough and fever reached 77.1% and 76.3%, respectively.
Figure 1.
COVID-19 distribution symptoms in the overall oncology group.
The pink color shows the main role of cough and fever, followed by dyspnea, headache, and myalgia.
Outcomes
The oncologic patient sample included in this study had a median follow-up of 22 days (10–47 days). We observed that 23.5% of patients died (141/599). Men died more frequently (30.9% versus 19.6%, p = 0.002) and the eldest (54 versus 62.5 years, p = 0.0001). Our analysis highlights a high frequency of diabetes and kidney failure on deceased patients (p = 0.01 and p = 0.001, respectively). Meanwhile, BMI was not relevant on the statistic evaluation (p = 0.095). A greater death frequency was shown on the group with low ECOG functionality (51.6%, p = 0.0001).
Death frequency was reduced on the patient group without oncologic treatment (13.4% versus 28.3%). A similar situation was observed with the survivor group (p = 0.0001). In addition, patients with hematologic tumors were deceased more frequently than those with solid tumors (33.3% versus 22.4%, p = 0.020). Lymphoma was the most frequent diagnosis among the hematologic group, and breast cancer was the most frequent solid tumor, with significant death frequency differences among the oncologic diagnosis (p = 0.0001, 33.9% versus 12.7%).
Patients receiving treatment were grouped as follows: chemotherapy (39.1%), hormone therapy (9.9%), and targeted therapy and immunotherapy (7.41% and 3.0%, respectively). Meanwhile, 7.2% had gone through surgery and 4.2% under radiotherapy. The remaining 29.2% had no treatment description.
Treatment of COVID-19 was as follows: antibiotics (57.2%, n = 343), oseltamivir (8.0%, n = 48), remdesivir (0.3%, n = 2), lopinavir/ritonavir (1.5%, n = 9), atazanavir/ritonavir (0.25%, n = 1), hydroxychloroquine (2.2%, n = 13), chloroquine (3.2%, n = 19), tocilizumab (3.0%, n = 18), ivermectin (2.3%, n = 14), ruxolitinib (0.3%, n = 2), convalescent plasma therapy (1.5, n = 9), anticoagulants (34.9%, n = 209), and steroids (30.9%, n = 185).
In total, 342 patients met the composite COVID-19 severe illness; 248 (72.5%) were admitted to the hospital whereas 57 (23.0%) required mechanical ventilation. COVID-19 treatments for patients under mechanical ventilation were antibiotics (90.8%), anticoagulants (70.8%), steroids (66.1%), antivirals (30.8%), and interleukin-6 (IL-6) receptor antagonist (9.2%)
Table 1 shows the comparison between surviving versus deceased patients; it includes only the characteristics with statistically significant results. Nonetheless, the comparative analysis also included BMI (p = 0.095), alcoholism (p = 0.109), HBP (p = 0.364), heart disease (p = 0.067), dyslipidemia (p = 0.151), chronic obstructive lung disease (p = 0.428), asthma (p = 0.147), human immunodeficiency virus infection (p = 0.601), and other COVID-19 symptoms such as diarrhea (p = 0.212), dysgeusia (p = 0.06), odynophagia (p = 0.134), anosmia (p = 0.858), and headache (p = 0.629), besides the oncological treatments such as chemotherapy, hormonotherapy, immunotherapy, and target therapies (p > 0.05)
Table 1.
Demographic, clinical, and treatment characteristics according to the outcome of COVID-19 patients.
| Characteristics | Total | Alive patients | Deceased patients | p |
|---|---|---|---|---|
| n = 599 | n = 441 (75.8%) | n = 141 (24.2%) | ||
| Age | 0.0001 | |||
| Median (IQR) | 56 (45–66) | 54 (44–64) | 62.5 (48–70) | |
| Sex | 0.002 | |||
| Female | 355 (59.3) | 278 (80.3) | 68 (19.6) | |
| Male | 244 (40.7) | 163 (69.1) | 73 (30.9) | |
| ECOG | 0.0001 | |||
| High functionality | 342 (68.4) | 292 (87.9) | 40 (12.0) | |
| Low functionality | 158 (31.6) | 73 (48.3) | 78 (51.6) | |
| Unknown | 99 (16.5) | 76 (76.8) | 23 (23.2) | |
| Tobacco use | 0.033 | |||
| No | 442 (73.8) | 335 (78.4) | 92 (21.5) | |
| Yes | 151 (25.2) | 104 (69.8) | 45 (30.2) | |
| Unknown | 6 (1.0) | 2 (33.3) | 4 (66.7) | |
| Diabetes mellitus | 0.01 | |||
| No | 451 (75.3) | 348 (79.3) | 91 (20.7) | |
| Yes | 117 (19.5) | 76 (67.9) | 36 (32.1) | |
| Unknown | 31 (5.2) | 17 (54.8) | 14 (45.2) | |
| Chronic kidney disease | 0.001 | |||
| No | 544 (90.8) | 413 (78.2) | 115 (21.8) | |
| Yes | 24 (4.0) | 11 (47.8) | 12 (52.2) | |
| Unknown | 31 (5.2) | 17 (54.8) | 14 (45.2) | |
| Cancer status | 0.013 | |||
| Active disease | 432 (72.1) | 304 (72.9) | 113 (27.1) | |
| Disease in remittance | 146 (24.4) | 123 (84.8) | 22 (15.2) | |
| Unknown | 21 (3.5) | 14 (70.0) | 6 (30.0) | |
| Clinical stage | 0.0001 | |||
| Non-advanced | 309 (60.1) | 253 (83.2) | 51 (16.8) | |
| Advanced | 205 (39.2) | 137 (69.2) | 61 (30.8) | |
| Not available | 85 (14.2) | 51 (63.7) | 29 (36.2) | |
| Oncologic diagnosis | 0.020 | |||
| Solid tumors | 496 (82.8) | 375 (77.6) | 108 (22.4) | |
| Hematologic | 103 (17.2) | 66 (66.7) | 33 (33.3) | |
| Type of treatment | 0.0001 | |||
| With treatment | 338 (64.4) | 233 (71.7) | 92 (28.3) | |
| Without treatment | 187 (35.6) | 161 (86.6) | 25 (13.4) | |
| COVID-19 symptoms | ||||
| Cough | 0.0001 | |||
| No | 231 (38.6) | 201 (87.0) | 30 (13.0) | |
| Yes | 345 (57.6) | 227 (69.2) | 101 (30.8) | |
| Unknown | 23 (3.8) | 13 (56.5) | 10 (43.5) | |
| Fever | 0.0001 | |||
| No | 239 (39.9) | 208 (87.0) | 31 (13.0) | |
| Yes | 341 (56.9) | 224 (69.2) | 100 (30.9) | |
| Unknown | 19 (3.2) | 9 (47.4) | 10 (52.6) | |
| Dyspnea | 0.0001 | |||
| No | 331 (55.3) | 302 (91.8) | 27 (8.1) | |
| Yes | 242 (40.4) | 123 (54.2) | 104 (45.8) | |
| Unknown | 26 (4.3) | 16 (61.5) | 10 (38.5) | |
| Nausea | 0.025 | |||
| No | 445 (74.3) | 340 (78.9) | 91 (21.1) | |
| Yes | 60 (10.0) | 34 (58.6) | 24 (41.4) | |
| Unknown | 94 (15.7) | 67 (72.0) | 26 (28.0) | |
| Vomiting | 0.002 | |||
| No | 496 (82.8) | 376 (78.2) | 104 (21.7) | |
| Yes | 38 (6.3) | 20 (54.0) | 17 (45.9) | |
| Unknown | 65 (10.8) | 45 (69.2) | 20 (30.8) | |
| Abdominal pain | 0.001 | |||
| No | 465 (77.6) | 356 (78.9) | 95 (21.1) | |
| Yes | 68 (11.3) | 39 (60.0) | 26 (40.0) | |
| Unknown | 66 (11.0) | 46 (69.7) | 20 (30.3) | |
| Rhinorrhea | 0.003 | |||
| No | 462 (77.1) | 355 (79.1) | 94 (20.9) | |
| Yes | 109 (18.2) | 68 (64.8) | 37 (35.2) | |
| Unknown | 28 (4.7) | 18 (64.3) | 10 (35.7) | |
| Myalgia | 0.026 | |||
| No | 345 (57.6) | 265 (79.8) | 67 (20.2) | |
| Yes | 230 (38.4) | 162 (71.7) | 64 (28.3) | |
| Unknown | 24 (4.0) | 14 (58.3) | 10 (41.7) | |
| Arthralgia | 0.005 | |||
| No | 381 (63.6) | 294 (80.3) | 72 (19.7) | |
| Yes | 152 (25.4) | 101 (67.3) | 49 (32.7) | |
| Unknown | 66 (11.0) | 46 (69.7) | 20 (30.3) | |
| Oxygen saturation (%) | 90 (84–95) | 93 (89–95) | 80 (70–87) | 0.00001 |
COVID-19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range.
Information was not complete for some categories; therefore, the total amount may not be reached in all cases.
Table 2 shows the characteristics that were included on the multivariate analysis to detect related factors associated to death in oncologic patients infected with SARS-CoV-2. It can be observed that six out of all evaluated variables were statistically significant on the final model, and two of them reduced the odds of death.
Table 2.
Multivariate analysis of oncologic patients with COVID-19.
| Death | Odds ratio | p > |z| | 95% Confidence interval | |
|---|---|---|---|---|
| Age | 1.003 | 0.769 | 0.98 | 1.03 |
| Sex | ||||
| Female | 0.42 | 0.031 | 0.19 | 0.92 |
| Male | Ref. | |||
| ECOG | ||||
| Low functionality | 5.42 | 0.0001 | 2.41 | 12.16 |
| High functionality | Ref. | |||
| Tobacco use | ||||
| Yes | 1.33 | 0.496 | 0.58 | 3.07 |
| No | Ref. | |||
| Diabetes mellitus | ||||
| Yes | 1.13 | 0.807 | 0.43 | 2.94 |
| No | Ref. | |||
| Chronic kidney disease | ||||
| Yes | 1.99 | 0.472 | 0.30 | 13.05 |
| No | Ref. | |||
| Clinical stage | ||||
| Advanced | 0.85 | 0.696 | 0.39 | 1.87 |
| Non-advanced | Ref. | |||
| Oncologic diagnosis | ||||
| Hematologic | 0.83 | 0.717 | 0.30 | 2.29 |
| Solid | Ref. | |||
| Treatment | ||||
| Without treatment | 1.88 | 0.234 | 0.67 | 5.30 |
| Ongoing treatment | Ref. | |||
| Cancer status | ||||
| Active disease | 3.94 | 0.041 | 1.05 | 14.73 |
| Disease in remittance | Ref. | |||
| COVID-19 symptoms | ||||
| Cough | ||||
| Yes | 1.82 | 0.186 | 0.75 | 4.42 |
| No | Ref. | |||
| Fever | ||||
| Yes | 1.47 | 0.376 | 0.63 | 3.46 |
| No | Ref. | |||
| Dyspnea | ||||
| Yes | 2.49 | 0.027 | 1.10 | 5.59 |
| No | Ref. | |||
| Nausea | ||||
| Yes | 4.03 | 0.028 | 1.17 | 13.97 |
| No | Ref. | |||
| Vomiting | ||||
| Yes | 0.74 | 0.682 | 0.17 | 3.12 |
| No | Ref. | |||
| Abdominal pain | ||||
| Yes | 0.65 | 0.444 | 0.22 | 1.94 |
| No | Ref. | |||
| Rhinorrhea | ||||
| Yes | 1.43 | 0.44 | 0.57 | 3.58 |
| No | Ref. | |||
| Myalgia | ||||
| Yes | 0.40 | 0.17 | 0.11 | 1.48 |
| No | Ref. | |||
| Arthralgia | ||||
| Yes | 2.54 | 0.16 | 0.68 | 9.51 |
| No | Ref. | |||
| Oxygen saturation (%) | 0.90 | 0.0001 | 0.87 | 0.94 |
COVID-19, coronavirus disease 2019; ECOG, Eastern Cooperative Oncology Group; Ref, reference category.
The bold values represent p < 0.05.
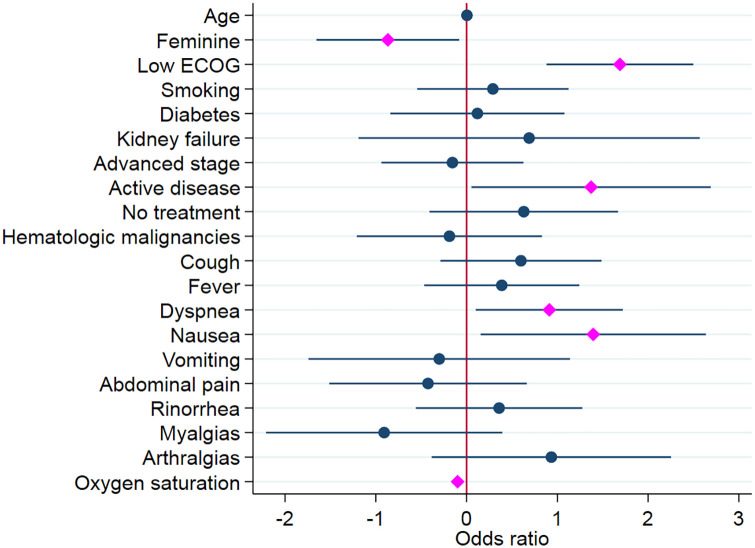
According to these results, the odds of death for a woman are half when compared with the odds of death for a man (p = 0.031) when adjusted for other variables. Also, we observed that for each additional unit of arterial oxygen saturation, the odds of death are reduced [odds ratio (OR) = 0.905645, p = 0.0001]. On the other hand, the odds for poor ECOG (2, 3, and 4) are 5.4 times greater than the odds of death for patients with good performance status (p = 0.0001). Regarding active disease, the odds of these patients are 3.9 times greater than the odds of patients in remission (p = 0.041). On the specific case of the dyspnea and nausea symptoms, the odds of death are 2.4 and 4.0, respectively, in contrast with those who lack these symptoms (p = 0.027 and 0.028, respectively). The regression coefficients are graphically represented in Figure 2.
Figure 2.
Logistic regression of oncologic patients infected with SARS-CoV-2.
ORs that correspond with p values <0.05 were marked with pink, whereas those with p ⩾ 0.05 were colored blue and the 95% confidence interval ranges were colored navy for both groups.
Survival
Cox proportional hazard model suggests shorter patient survival times in older patients [hazard ratio (HR) = 1.3692, p = 0.035], low ECOG (HR = 8.6593, p = 0.017), presence of COPD (HR = 8.3088, p = 0.004), dyspnea (HR = 2.9377, p = 0.008), being in a palliative treatment (HR = 10.7043, p = 0.002), and having active cancer without treatment (HR = 8.685, p = 0.008). In contrast, having higher oxygen saturation led to longer patient survival times (HR = 0.9529, p = 0.0001).
Discussion
Emerging diseases suppose a serious health problem on a global scale. According to the World Health Organization, the current pandemic caused by the SARS-CoV-2 virus, disease-causing zoonotic agent for COVID-19, has wreaked havoc across all levels of health systems.1
The information coming from the United States suggests that ethnic and racial minorities (e.g. Hispanics) have an increased trend of acquiring COVID-19 and presenting greater complications, including death.15 This is partly due to the difficulty of accessing health services, inadequate social distancing, and high comorbidity frequency among this population.7,15 Sosa-Rubí et al. reached similar conclusions after analyzing 373,963 COVID-19 non-cancer patients from Mexico. Apart from stressing the impact of social and economic vulnerability, they reported that the odds of requiring hospitalization facing COVID-19 and diabetes mellitus was 38.4%, reaching 42.9% if another comorbidity was added such as obesity, HBP, heart disease, or kidney failure.8
On April 2020, facing the lack of information regarding the management and prognosis of oncology patients infected with COVID-19, the National Cancer Institute (Instituto Nacional de Cancerología) proposed a collaborative agreement with all federative entities to collect information of cancer patients infected with SARS-CoV-2 confirmed by RT-qPCR. This work had no financing and is the effort of physicians worried by the vulnerability of their patients; regardless of the increased workload generated by this emergency, they voluntarily gathered the information that is analyzed in this work.
Previous reports describe that comparing patients with cancer to non-cancer patients, apart from being more susceptible to SARS-CoV-2 infections, cancer patients are at an increased risk of more severe sequelae.16–18 This study analyzes the prognostic factors related to Latin American patients, specifically Mexican patients, with cancer and with a SARS-CoV-2 infection during 2020.
The greatest number of registered cases of this study corresponds to the Metropolitan Area of Mexico City. This is due to the concentration of cancer attention sites along with its population density (around 20 million inhabitants) and, although this first report observes a low percentage of patients from other states (12.7%), it helps to reflect the situation of the infection country-wise.
The study population showed a female predominance (60%) with an age average of 56 years. This is contrary to other published series19–21 where the average age was 66 years without gender majority. This situation may probably be due to the fact that 30.4% of cases had been diagnosed with breast cancer, which is developed earlier in Latin American women when compared with other populations.22
Although it is a ‘younger’ group, it was found that one out of every three patients had HBP and one out of every five had diabetes mellitus. Overweight, which is present in 50% of patients, and obesity, observed in one out of every four, were discarded from the univariate analysis as a relevant factor for death. This coincides with the study of Al-Salameh et al.,23 where they do not relate it to death, although they do relate it to admission to the intensive care unit. The effect of diabetes mellitus and chronic kidney failure was diluted upon adjustment in the final model; meanwhile, COPD reduces the survival time (HR = 8.3088 p = 0.004).
Given the cohort’s general characteristics, it was observed that over half of patients, 60.1%, were on non-metastatic stages; almost 70% of them had an ECOG status between 0 and 1. Also, it was found that 72.1% of patients had active cancer, where a third had metastatic disease; 64% of patients within the cohort were receiving oncologic treatment. However, it is worth noting that 23.5% of patients were deceased (141/599), which represents almost double of the findings of Kuderer et al.,20 which included 928 patients from the United States, Canada, and Spain. However, our death frequency is very similar to the UK cohort (n = 800), which was reported as 28%.21
When adjusted for other variables, the logistic regression showed that being male (p = 0.031) increased the odds of death, which coincides with previously published studies, both in the general and in the oncologic populations.9,24 A CCC-19 study reported that having active cancer and ECOG 2, 3, or 4 was associated to a negative prognosis in cancer patients infected with SARS-CoV-2.20 In this study, we observed an increase of between 3 and 5 for the odds of death in patients with active cancer and a poor ECOG score. Although a third of the population had an ECOG ⩾ 2, it was observed that this parameter has great influence when determining a patient’s death. We also observed that the fatality rate was not associated to advanced stage as other reports; we believe that is because 30.4% of our cohort had breast cancer. Some reports have shown that women with breast cancer have fewer severe forms of COVID-19 and less likely to die.25,26 Regarding the oncologic treatment, when performing the step-by-step regression, it was detected that receiving treatment increased the odds of death. However, this effect was diluted upon inclusion on the multivariate analysis and variable adjustment. Our results were like some previous reports where the oncological treatments such as chemotherapy did not increase adverse outcomes.27 The same behavior was noted with hematologic versus solid tumors: univariate analysis detected that the frequency of death in hematologic tumors was higher (p = 0.020). However, after the multivariate analysis, the difference did not withhold its significance and the odds of death were not increased unlike other studies9,19,21,25 maybe as a consequence of the high rate of hematological patients on surveillance (34.3%). On the contrary, subjective symptoms such as vomiting, nausea, and abdominal pain could be attributed not only to the oncological treatment and the cancer itself but also to COVID-19, and despite we are unable to identify the cause, we found that nausea increased the odds of death.
By Cox regression, age was an important factor in reducing survival time with an HR of 1.36 per year (p = 0.035), as well as being in palliative treatment (HR = 10.7043, p = 0.002) or having active cancer without treatment (HR = 8.685, p = 0.008).
It is worth stressing that, out of the full cohort, 342 patients presented severe COVID-19 (according to the pre-established definition),28 where over two-thirds of the patients required hospitalization. We observed that the mortality for this specific subgroup was 31.9%, similar to the results reported by Desai et al.29 after the meta-analysis, including 2922 patients with a 30-day mortality rate of 30% [95% confidence interval (CI) = 25–35%].
The aim of this study was to have a broad picture of specific demographics and clinical factors associated with death by COVID-19 in a ‘vulnerable’ patient population, the one with cancer. It is important to remark, that this study has some limitations as selection bias, as no control with non-cancer patients but most important, there is no control with cancer patients without COVID-19. In the last months, we have learned that not all cancer patients have risk for fatality among COVID-19 infection.25 Cancer is a heterogeneous disease, where besides the treatment type, primary tumor subtype, stage, age, and gender also play a role.30
Another limitation is that the presented results on cancer patients with COVID-19 in Mexico cannot be generalized to the whole Mexican population due to most of the data coming from the National Cancer Institute, which is one of the largest oncology centers of the country. Furthermore, the collected information from hospitals and the patient outcomes has been partial given their organization: they remitted patients to COVID-19-exclusive hospitals, which limited the information analysis.
In this study, we observed that there is a high frequency of death on cancer patients infected with SARS-CoV-2. However, though we adjusted our models, we cannot ensure that this is only due to cancer-related or host-related factors. There is a need to analyze the role of the viral infection per se. Also, this report did not analyze the impact of cancer treatments (meaning, if the dose intensity was maintained or if the treatment was delayed given the fear of patient infection) regarding cancer and COVID-19.
To the best of our knowledge, this is the largest series of Latin American cancer patients with COVID-19 infection reported thus far. We would like to stress the relevance of the study being multicentric. This allows us to observe that 7 out of every 10 patients had active cancer, in which case one out of every four died.
Even in Mexico, which is labeled as a medium–high income country, it is not always possible to follow international guidelines31 to reduce the effects of COVID-19 in cancer patients. The health infrastructure does not allow telemedicine across all levels (with the goal of reducing hospital visits) or intravenous-to-oral therapy change. Another important aspect is that social distancing is not always feasible on cities with high population density. Thus, we must prioritize oncology care through better strategies with the goal of refining institutional protocols based on our own information.
Conclusion
This study shows the prognosis factors related to death in Mexican cancer patients with SARS-CoV-2 infection. In our population, we found characteristics reported previously as gender and age, performance status, active cancer, and oxygen saturation; nevertheless, we did not find that other comorbidities besides COPD increase the probability of death. Meanwhile, we understand how COVID-19 affects our cancer patients; we must be aware of the importance of improving health policies in patients with oncology diseases.
Acknowledgments
The authors thank all the institutions’ and hospitals’ staff. The authors also thank Universidad la Salle for the support in the creation of the digital platform (https://www.oncovid-19.org).
Footnotes
Author contributions: ER-G conceived the original idea, study conception, design, revision, and approval of the final version of the manuscript. AP-N contributed substantially to study conception and design, drafting, revision, analysis, data interpretation, and approval of the final version of the manuscript. JA-B contributed to study conception and design, critical revisions, relevant contributions, and revision and approval of the final version of the manuscript. PC-J contributed to critical revision of the draft and approval of the final version of the manuscript. AM-G contributed to study conception and design, revision of the draft, and approval of the final version of the manuscript. SRR conceived the original idea, revisions, relevant contributions to the content and format of the manuscript, and approval of the final version of the manuscript. JJS contributed to study conception and design, critical revisions, relevant contributions, and revision and approval of the final version of the manuscript. RG-C contributed to study conception and approval of the final version of the manuscript. DSG involved in design, critical revisions, relevant contributions, and approval of the final version of the manuscript. HMF involved in drafting and approval of the final version of the manuscript. GAB contributed to critical revisions and approval of the final version of the manuscript. AEA helped in investigation, critical revisions, and approval of the final version of the manuscript. NVM provided relevant contributions, and helped in the revision and approval of the final version of the manuscript. EAF-F contributed to critical revision of the draft and approval of the final version of the manuscript. RAV helped in investigation, contributions to the content and format of the manuscript, and approval of the final version of the manuscript. DV-C conceived the original idea, design, revision, and approval of the final version of the manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Adriana Peña-Nieves  https://orcid.org/0000-0003-0632-0585
https://orcid.org/0000-0003-0632-0585
Contributor Information
Erika Ruiz-Garcia, Department of Medical Oncology & Translational Medicine Laboratory, Instituto Nacional de Cancerología, Av. San Fernando N. 22, ZC 14080 Mexico City, Mexico.
Adriana Peña-Nieves, Translational Medicine Laboratory, Instituto Nacional de Cancerología, Mexico City, Mexico.
Jorge Alegria-Baños, Centro Oncológico Hospital Medica Sur, Mexico City, Mexico & Facultad de Ciencias Químicas, Universidad La Salle, Mexico City, Mexico.
Patricia Cornejo-Juarez, Department of Infectious Disease, Instituto Nacional de Cancerología, Mexico City, Mexico.
Abelardo Meneses-García, Translational Medicine Laboratory, Instituto Nacional de Cancerología, Mexico City, Mexico.
Samuel Rivera Rivera, Department of Medical Oncology, Hospital de Oncología CM SXXI, IMSS, Mexico City, Mexico.
Juan José Sánchez, Department of Medical Oncology, Hospital de Oncología CM SXXI, IMSS, Mexico City, Mexico.
Raquel Gerson-Cwilich, Department of Medical Oncology, Centro Médico ABC, Mexico City, Mexico.
Daniela Shveid Gerson, Department of Medical Oncology, Centro Médico ABC, Mexico City, Mexico.
Heriberto Medina Franco, Department of Surgery, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Gabriela Alejandra Buerba, Department of Surgery, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico.
Alicia Acosta Espinoza, Hospital ISSSTE/ISSSTECALI, Mexicali, Mexico.
Norma Valencia Mijares, Hospital Regional de Alta Especialidad de Oaxaca, UMAA #1, IMSS, Oaxaca, Mexico.
Edith A. Fernández-Figueroa, Computational and Integrative Genomics, Instituto Nacional de Medicina Genómica, Mexico City, Mexico
Roberto A. Vázquez, Facultad de Ingeniería, Universidad La Salle, Mexico City, Mexico
Diana Vilar-Compte, Department of Infectious Disease, Instituto Nacional de Cancerología, Av. San Fernando N. 22, ZC 14080 Mexico City, Mexico. Hospital Epidemiology, Instituto Nacional de Cancerología, Mexico City, Mexico.
References
- 1.World Health Organization. COVID-19 continues to disrupt essential health services in 90% of countries, https://www.who.int/news/item/23-04-2021-covid-19-continues-to-disrupt-essential-health-services-in-90-of-countries (2020, accessed 3 April 2021).
- 2.Johns Hopkins University (JHU). COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). The Center for Systems Science and Engineering (CSSE) at JHU, https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (2021, accessed 11 June 2021). [Google Scholar]
- 3.Secretaría de Salud, Gobierno de México. 22° Informe epidemiológico de la situación de COVID-19, 2021, https://www.gob.mx/cms/uploads/attachment/file/643068/Informe_COVID-19_2021.05.31.pdf
- 4.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 – COVID-NET, 14 States, March 1–30, 2020. Morb Mortal Wkly Rep 2020; 69: 458–464, http://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm?s_cid=mm6915e3_w (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424, http://doi.wiley.com/10.3322/caac.21492 (accessed 11 June 2021). [DOI] [PubMed] [Google Scholar]
- 6.Corti C, Curigliano G.Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol 2021; 32: 569–571, https://linkinghub.elsevier.com/retrieve/pii/S0923753421000107 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020; 323: 2466–2467, https://jamanetwork.com/journals/jama/fullarticle/2766098 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosa-Rubí SG, Seiglie JA, Chivardi C, et al. Incremental risk of developing severe COVID-19 among Mexican patients with diabetes attributed to social and health care access disadvantages. Diabetes Care 2021; 44: 373–380, http://care.diabetesjournals.org/lookup/doi/10.2337/dc20-2192 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol 2021; 32: 787–800, https://linkinghub.elsevier.com/retrieve/pii/S0923753421008747 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohar-Betancourt A, Reynoso-Noverón N, Armas-Texta D, et al. Cancer trends in Mexico: essential data for the creation and follow-up of public policies. J Glob Oncol 2017; 3: 740–748, https://ascopubs.org/doi/10.1200/JGO.2016.007476 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campos-Nonato I, Hernández-Barrera L, Pedroza-Tobias A.Hypertension in Mexican adults: prevalence, diagnosis and type of treatment. Ensanut MC 2016. Salud Publica Mex 2018; 60: 233–243. [DOI] [PubMed] [Google Scholar]
- 12.Levaillant M, Lièvre G, Baert G.Ending diabetes in Mexico. Lancet 2019; 394: 467–468, https://linkinghub.elsevier.com/retrieve/pii/S0140673619316629 (accessed 11 June 2021). [DOI] [PubMed] [Google Scholar]
- 13.Chapter 1: definition and classification of CKD. Kidney Int Suppl 2013; 3: 19–62, https://linkinghub.elsevier.com/retrieve/pii/S2157171615311011 (accessed 30 July 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC, 2019, https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ [Google Scholar]
- 15.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020; 6: 1108–1110, https://jamanetwork.com/journals/jamaoncology/fullarticle/2763673 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernabe-Ramirez C, Velazquez AI, Olazagasti C, et al. The HOLA COVID-19 study: an international effort to determine how COVID-19 has impacted oncology practices in Latin America. Cancer Cell 2020; 38: 605–608, https://linkinghub.elsevier.com/retrieve/pii/S1535610820305444 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21: 335–357, https://linkinghub.elsevier.com/retrieve/pii/S1470204520300966 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol 2020; 6: 557–559, https://ascopubs.org/doi/10.1200/GO.20.00097 (accessed 13 August 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov 2020; 10: 783–791, http://cancerdiscovery.aacrjournals.org/lookup/doi/10.1158/2159-8290.CD-20-0422 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020; 395: 1907–1918, https://linkinghub.elsevier.com/retrieve/pii/S0140673620311879 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020; 395: 1919–1926, https://linkinghub.elsevier.com/retrieve/pii/S0140673620311739 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justo N, Wilking N, Jönsson B, et al. A review of breast cancer care and outcomes in Latin America. Oncologist 2013; 18: 248–256, https://onlinelibrary.wiley.com/doi/abs/10.1634/theoncologist.2012-0373 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Salameh A, Lanoix J-P, Bennis Y, et al. The association between body mass index class and coronavirus disease 2019 outcomes. Int J Obes 2021; 45: 700–705, http://www.nature.com/articles/s41366-020-00721-1 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059, https://jamanetwork.com/journals/jama/fullarticle/2765184 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Baldwin E, Zhang X, et al. Comparison and impact of COVID-19 for patients with cancer: a survival analysis of fatality rate controlling for age, sex and cancer type. BMJ Health Care Inform 2021; 28: e100341, https://informatics.bmj.com/lookup/doi/10.1136/bmjhci-2021-100341 (accessed 13 August 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathelin C, Ame S, Anyanwu S, et al. Breast cancer management during the COVID-19 pandemic: The Senologic International Society Survey. Eur J Breast Health 2021; 17: 188–196, http://cms.galenos.com.tr/Uploads/Article_47125/ejbh-17-188-En.pdf (accessed 16 August 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol 2020; 38: 3538–3546, https://ascopubs.org/doi/10.1200/JCO.20.01307 (accessed 16 August 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi RT.The multidimensional challenge of treating coronavirus disease 2019 (COVID-19): remdesivir is a foot in the door. Clin Infect Dis 2020: ciaa1132, https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1132/5879440 (accessed 11 June 2021). [DOI] [PMC free article] [PubMed]
- 29.Desai A, Gupta R, Advani S, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta-analysis of cohort studies. Cancer 2021; 127: 1459–1468. [DOI] [PubMed] [Google Scholar]
- 30.Curigliano G.Cancer patients and risk of mortality for COVID-19. Cancer Cell 2020; 38: 161–163, https://linkinghub.elsevier.com/retrieve/pii/S1535610820303676 (accessed 13 August 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ESMO. Cancer patient management during the COVID-19 pandemic, 2020, https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic