Abstract
Purpose:
To evaluate the long-term effectiveness of iTrack (Nova Eye Medical, Fremont, USA) ab-interno canaloplasty performed as a standalone procedure, or combined with cataract surgery, in reducing antiglaucoma medication dependence and maintaining intraocular pressure within target range in patients with controlled primary open-angle glaucoma.
Methods:
This is a retrospective, single-center, case series study of patients who underwent iTrack ab-interno canaloplasty as a standalone procedure (n = 34) or in combination with cataract surgery (n = 11). Eyes with controlled mild or moderate glaucoma (intraocular pressure ⩽17 mmHg) were included; those with prior glaucoma surgeries were excluded. The primary outcome was the mean reduction in glaucoma medication and the secondary outcome was the maintenance of controlled intraocular pressure postoperatively.
Results:
A total of 35 patients (45 eyes) with a mean age of 73 ± 9.7 years were included in the study. For all eyes, a significant decrease (61%) in the mean number of medications was seen at 36 months (1.89 ± 0.93 versus 0.60 ± 0.82; p < 0.001), with 56% (14/25) of eyes medication free. No significant difference was observed in the reduction of medication use whether iTrack was performed with or without cataract surgery at 12 and 36 months. Mean baseline intraocular pressure of 14.42 ± 2.2 mmHg for all eyes was maintained at the 12-, 24-, and 36-month follow-up visits (14.6 ± 3.65, 15.06 ± 2.26, and 14.19 ± 2.91, respectively), with no significant difference between the two groups (p = 0.08).
Conclusion:
The iTrack ab-interno canaloplasty significantly reduced medication dependency and maintained intraocular pressure within target range in patients with controlled mild or moderate primary open-angle glaucoma, while showing a good safety profile, both as a standalone procedure or in combination with cataract surgery.
Keywords: ab-interno canaloplasty, glaucoma, iTrack, MIGS, primary open-angle glaucoma, Schlemm’s canal, viscodilation
Introduction
Glaucoma is the leading cause of blindness worldwide and it is estimated that it will affect close to 112 million people worldwide by 2040.1 The main treatment goal for primary open-angle glaucoma (POAG), which is typically caused by the gradual blockage of aqueous outflow, is the reduction of intraocular pressure (IOP) to prevent progressive optic nerve damage and, ultimately, visual field deterioration. The traditional glaucoma treatment algorithm consists of topical antiglaucoma medications followed by laser treatment and penetrating filtration surgeries such as trabeculectomy and glaucoma tube drainage implants when IOP is not controlled with topical therapy.2 Over the last decade, a variety of minimally invasive glaucoma surgeries (MIGS) have been developed to fill the treatment gap between topical therapy and more invasive filtration surgery.
Although a wide variety of effective topical medications are available, patient nonadherence is a common problem that can lead to optic nerve damage and disease progression.3–6 Potential barriers which may inhibit adherence include difficulty administering eye drops, forgetfulness, cost of the medications, and side effects such as ocular surface disease that can negatively impact patient quality of life.7–11 Furthermore, ocular surface diseases may be initiated or aggravated by topical glaucoma medications which can divert fluid away from the natural outflow system and damage those structures within the eye responsible for maintaining aqueous outflow. For example, benzalkonium chloride, a preservative common to most topical medications, can cause apoptosis in the endothelial cells and along the trabecular columns.12 This can lead to fusion of the trabecular meshwork and subsequent collapse of Schlemm’s canal.13 For these reasons, patients with mild-moderate glaucoma who are well controlled with multiple antiglaucoma therapies may benefit from medication reduction.
The advent of MIGS has allowed a more nuanced approach to the surgical treatment of patients with mild-moderate glaucoma. However, the majority of MIGS options are focal in their approach and are thereby unable to address all aspects of the conventional outflow system. On the contrary, the pathophysiology of glaucoma is known to affect all aspects of the conventional outflow pathway, both proximal and distal. In human eyes, up to 75% of the resistance to aqueous humor outflow is localized within the trabecular meshwork.14 Greater contractility of Schlemm’s canal can also increase outflow resistance, with the canal in POAG eyes shown to be shorter, narrowed, and often collapsed.15–17 Another significant cause of increased outflow resistance in POAG eyes is herniations of the trabecular meshwork which can obstruct up to 90% of collector channels.18 The MIGS procedure iTrack ab-interno canaloplasty (Nova Eye Medical, Fremont, California) offers a comprehensive approach to lower IOP that effectively targets the three main sites of outflow resistance in the conventional outflow pathway: the trabecular meshwork, Schlemm’s canal, and the distal collector channels,19–23 therefore offering a theoretical advantage over MIGS procedures that are focal in their approach. Performed via an ab-interno approach to Schlemm’s canal, the iTrack canaloplasty microcatheter spares conjunctival manipulation and preserves the angle, allowing future procedures to be performed if necessary.
A fundamental component of the iTrack procedure is viscodilation, in which a high-molecular-weight HA-based (hyaluronic acid) ophthalmic viscosurgical device (OVD) is delivered into Schlemm’s canal in order to enlarge it and to open the distal outflow system. This process of viscodilation is further reinforced via 360-degree catheterization of Schlemm’s canal with the iTrack canaloplasty microcatheter. Featuring a 250-μm atraumatic bulbous tip, the microcatheter is designed to bypass collector channel ostia and to push trabecular meshwork herniations out of the ostia with minimal tissue trauma. It is also designed to deliver precise microquantities of high-molecular-weight HA-based OVD, at a constant pressure, with an average of more than 100 µl of OVD delivered over 360 degrees of the canal. The microcatheter features a fiber optic tip which enables the surgeon to visualize its position during circumnavigation of Schlemm’s canal.
In this case series study, we evaluate the effectiveness of iTrack ab-interno canaloplasty in reducing the use of IOP-lowering medications over 3 years while maintaining IOP within target range (⩽17 mmHg) in patients with mild-moderate POAG.
Methods
Study design and patients
Study design
Forty-five eyes of 35 consecutive patients with controlled mild or moderate POAG underwent ab-interno canaloplasty with the iTrack canaloplasty microcatheter, either as a standalone procedure (iTrack-alone) or in combination with cataract surgery (iTrack + phaco), and were included in this retrospective, single-center case series study. Outcome measures included the reduction in glaucoma medication use, maintenance of the controlled IOP, and adverse events at 12, 24, and 36 months postoperatively.
All patients underwent a complete baseline ophthalmic examination prior to surgery that included a history of glaucoma, medication use, IOP, best-corrected distance visual acuity (CDVA), gonioscopy, slit-lamp, and fundus examination. Postoperative follow-up examinations were at 12, 24, and 36 months. All relevant information was recorded at each follow-up visit, including IOP measurements, CDVA, slit-lamp examination, glaucoma medications, and adverse events. Gonioscopy was performed postoperatively, where required.
Patient selection
This study was performed in accordance with the principles stated in the Declaration of Helsinki and its amendments. An ethical board approval was obtained from the University of Oklahoma Health Sciences Center Institutional Review Board, with approval number #6173. This study was Health Insurance Portability and Accountability Act compliant and each patient provided written informed consent after the nature of the procedure and options had been fully discussed. Inclusion criteria were adult patients (18 years of age and older) with a diagnosis of mild or moderate POAG that was controlled at the preoperative visit (IOP ⩽ 17 mmHg). Disease severity was determined based on mean deviation (MD) from Humphrey visual field (HVF) testing: mild (>−6 dB), moderate (from −6 to −12 dB), and severe cases (<−12 dB).24 Confirmed evidence of characteristic changes to the optic nerve consistent with glaucomatous optic neuropathy and thinning of the retinal nerve fiber layer on ocular coherence tomography (OCT) was also evaluated to determine disease severity. Both phakic and pseudophakic patients were included. Exclusion criteria included patients who had undergone angle-based MIGS within the 6 months prior to the procedure, and patients who had prior MIGS-based procedure. Patients who had undergone laser trabeculoplasty (LTP) within the 6 months prior to the procedure were also excluded. Additional exclusion criteria included patients with any sign of angle disease, such as peripheral anterior synechiae, goniosynechiae, or angle recession, patients with neovascular disease, uveitis, and developmental or other forms of secondary glaucoma, such as steroid-induced glaucoma.
Surgical procedure
All surgeries were performed at the University of Oklahoma, Dean McGee Eye Institute by a single surgeon (M.A.K.) between October 2014 and July 2015. Prior to starting the surgery, the iTrack microcatheter was prepared and primed. In patients undergoing cataract surgery, the iTrack procedure was performed following cataract extraction and intraocular lens (IOL) implantation. A primary, clear, corneal incision was made temporally in both the standalone procedure and when combined with cataract surgery. The anterior chamber was filled with OVD to maintain anterior chamber stability. A paracentesis tract was made inferonasally. The tip of the iTrack microcatheter was placed into the anterior chamber and the microscope, as well as the patient’s head, was tilted into position for intraoperative gonioscopy. The gonioprism was placed on the cornea and the trabecular meshwork was visualized. A needle was used to make a 1- to 2-mm nasal goniotomy. The tip of the iTrack canaloplasty microcatheter was grasped in the anterior chamber with microsurgical forceps and directed through the goniotomy and into Schlemm’s canal. The iTrack canaloplasty microcatheter was then advanced around the entire circumference of the canal. The illuminated tip of the microcatheter was visualized through the conjunctiva as it passed temporally, and then gonioscopically, as it returned to the goniotomy site. The iTrack canaloplasty microcatheter was slowly withdrawn as microboluses of high-molecular-weight OVD were delivered in Schlemm’s canal via a process of pressurized viscodilation. The patient’s head and microscope were then returned to the primary position and any remaining OVD was removed from the anterior chamber, completing the procedure.
Statistical analysis
Statistical analysis was performed with latest version of SPSS for Windows (SPSS Inc., Chicago III, IL). Demographic and preoperative data were analyzed with independent t-test or Fisher exact test. The IOP, antiglaucomatous medication usage, and visual acuity data were compared with baseline values using repeated-measures analysis of variance (ANOVA) at months 12, 24, and 36 between the treatment groups (iTrack-alone and iTrack + phaco).
Results
Patient demographics
A total of 45 eyes of 35 patients with a mean age of 73 ± 9.7 years who met the study eligibility criteria and completed the preoperative visit were enrolled in the study. The majority of patients were female (n = 19, 54%). Of the total 45 eyes, 35 eyes (78%), 31 eyes (69%) eyes, and 25 eyes (56%) completed the 12-, 24-, and 36-month visit, respectively. Patients underwent iTrack ab-interno canaloplasty as either a standalone procedure (iTrack-alone, n = 11 eyes) or in combination with cataract surgery (iTrack + phaco, n = 34 eyes). Table 1 presents patient demographics and baseline characteristics. Patients were predominantly Caucasian (63%), with the majority (78%) diagnosed with mild POAG.
Table 1.
Demographic and baseline characteristics of patients with controlled primary open-angle glaucoma.
| n = 35 patients | |
|---|---|
| Age (years) | |
| Mean ± SD | 73.3 ± 9.7 |
| Median | 74 |
| Range | 45–90 |
| Gender, n (%) | |
| Female | 19 (54) |
| Male | 16 (46) |
| Race, n (%) | |
| Black | 12 (34) |
| Hispanic | 1 (3) |
| Asian | 0 |
| Caucasian | 22 (63) |
| Diagnosis | n = 45 eyes |
| POAG | 45 (100) |
| Mild | 35 (78) |
| Moderate | 10 (22) |
| Severe | 0 |
| Eye operated on, n (%) | |
| Left | 23 (51) |
| Right | 22 (49) |
| Pre-op (Mean ± SD) | |
| IOP | 14.4 ± 2.2 |
| Number of medications | 1.89 ± 0.9 |
IOP, intraocular pressure; POAG, primary open-angle glaucoma; SD, standard deviation.
Reduction in antiglaucoma medication use
Figure 1 and Table 2 summarize the data utilized to represent the reduction of antiglaucoma medication use. Baseline mean medication use prior to treatment was for all eyes (n = 45); 1.89 ± 0.93 which was significantly reduced by 82%, 76%, and 61%, respectively, at 12, 24, and 36 months postoperatively (p < 0.001).
Figure 1.
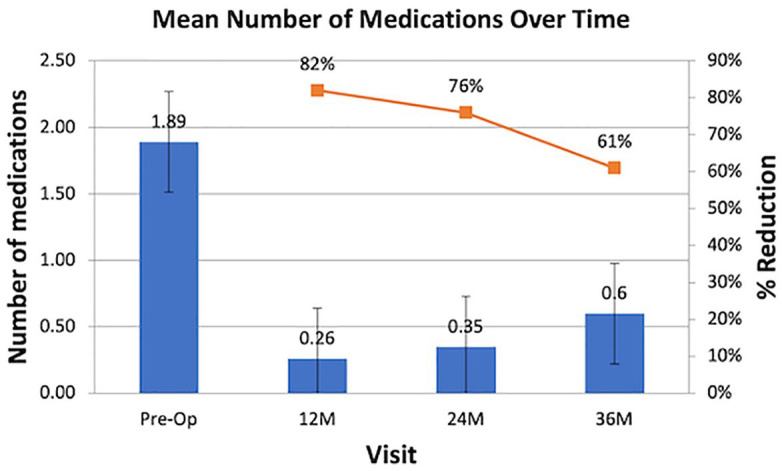
Reduction of antiglaucoma medications (all eyes).
Table 2.
Reduction of antiglaucoma medications (all eyes).
| N | 45 | 35 | 31 | 25 | p-value |
|---|---|---|---|---|---|
| Pre-op | 12M | 24M | 36M | ||
| Mean ± SD | 1.89 ± 0.93 | 0.26 ± 0.56 | 0.35 ± 0.55 | 0.60 ± 0.82 | <0.001 |
| Median | 2.0 | 0.0 | 0.0 | 0.0 | |
| Min | 0.0 | 0.0 | 0.0 | 0.0 | |
| Max | 4.0 | 2.0 | 2.0 | 3.0 | |
| % Reduction | 82.4% | 75.8% | 61% |
M, month; %, percentage; SD, standard deviation.
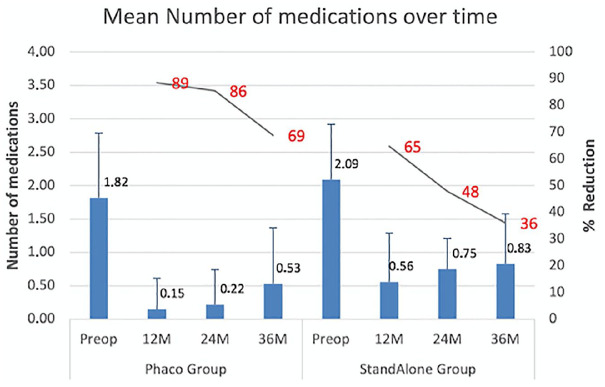
In both groups, there was a statistically significant reduction in the medication burden at each time point, with respect to baseline (Table 3 and Figure 2). For eyes in the iTrack-alone group (n = 11), medication use was reduced from 2.2 ± 0.14 at baseline to 0.53 ± 0.14 at 12 months, 0.62 ± 0.15 at 24 months, and 0.85 ± 0.16 at 36 months (p = 0.03). For eyes in the iTrack + phaco group (n = 34), medication use was reduced from 1.83 ± 0.1 at baseline to 0.16 ± 0.11 at 12 months, 0.24 ± 0.12 at 24 months, and 0.48 ± 0.13 at 36 months (p = 0.03). When comparing the reduction in medication use between the iTrack-alone and iTrack + phaco groups (Table 3 and Figure 2), there was no significant difference in the percentage reduction at 12 months (65% versus 88%; p = 0.0782), while it was significant at 24 months (48% versus 86%; p = 0.0125). At 36 months the difference was not significant (36% versus 69%; p = 0.1955) but the iTrack-alone group showed a high degree of variability and the confidence interval also included the iTrack + phaco group. The standard error of the iTrack-alone group is three times that of the iTrack + phaco group (31.1 versus 9.99).
Table 3.
Reduction of antiglaucoma medications, iTrack + phaco compared with iTrack-alone.
| Time point | iTrack + phaco | iTrack-alone | p-value on (% reduction) | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | % reduction | n | Mean ± SD | % reduction | ||
| Pre-op | 34 | 1.83 ± 0.1 | – | 11 | 2.2 ± 0.14 | – | – |
| 12M | 26 | 0.16 ± 0.11 | 88.50 | 9 | 0.53 ± 0.14 | 64.80 | 0.0782 |
| 24M | 23 | 0.24 ± 0.12 | 85.50 | 8 | 0.62 ± 0.15 | 47.9 | 0.0125 |
| 36M | 19 | 0.48 ± 0.13 | 68.80 | 6 | 0.85 ± 0.16 | 36.1 | 0.1955 |
M, month; %, percentage; Phaco, phacoemulsification; SD, standard deviation.
Figure 2.
Reduction of antiglaucoma medications, iTrack + phaco compared with iTrack-alone.
Free of antiglaucoma medications
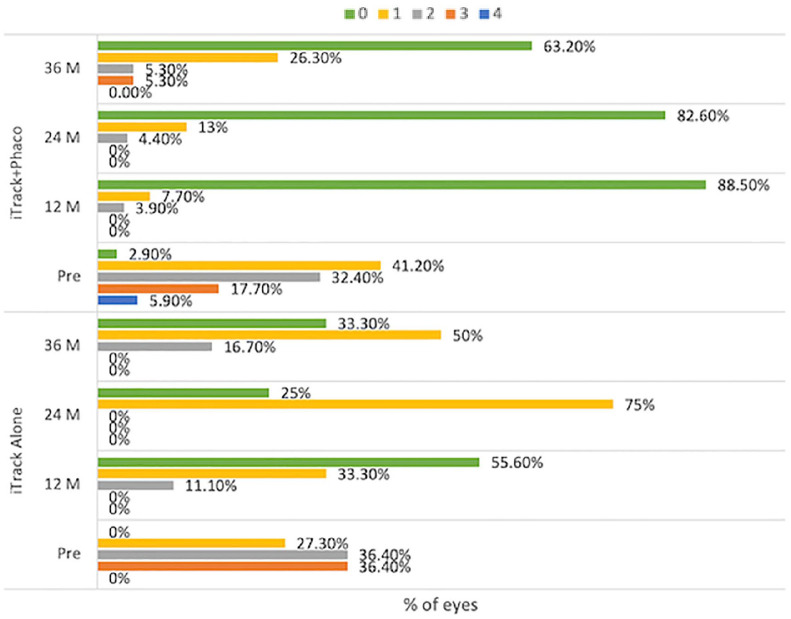
The percentage of all eyes that were medication-free was 80% (28 of 35 eyes) at 12 months, 68% (21 of 31 eyes) at 24 months, and 56% (14 of 25 eyes) at 36 months, as compared with 2% (1 of 45 eyes) at baseline (Table 4 and Figure 3).
Table 4.
Free of antiglaucoma medications, percentage of eyes.
| # of medications | iTrack + phaco | iTrack-alone | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre | 12M | 24M | 36M | Pre | 12M | 24M | 36M | |
| 0 | 1 (2.9%) | 23 (88.5%) | 19 (82.6%) | 12 (63.2%) | 0 (0%) | 5 (55.6%) | 2 (25%) | 2 (33.3%) |
| 1 | 14 (41.2%) | 2 (7.7%) | 3 (13%) | 5 (26.3%) | 3 (27.3%) | 3 (33.3%) | 6 (75%) | 3 (50%) |
| 2 | 11 (32.4%) | 1 (3.9%) | 1 (4.4%) | 1 (5.3%) | 4 (36.4%) | 1 (11.1%) | 0 (0%) | 1 (16.7%) |
| 3 | 6 (17.7%) | 0 (0%) | 0 (0%) | 1 (5.3%) | 4 (36.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 4 | 2 (5.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 34 (100%) | 26 (100%) | 23 (100%) | 19 (100%) | 11 (100%) | 9 (100%) | 8 (100%) | 6 (100%) |
M, month; %, percentage.
Figure 3.
Free of antiglaucoma medications, percentage of eyes.
Maintenance of IOP
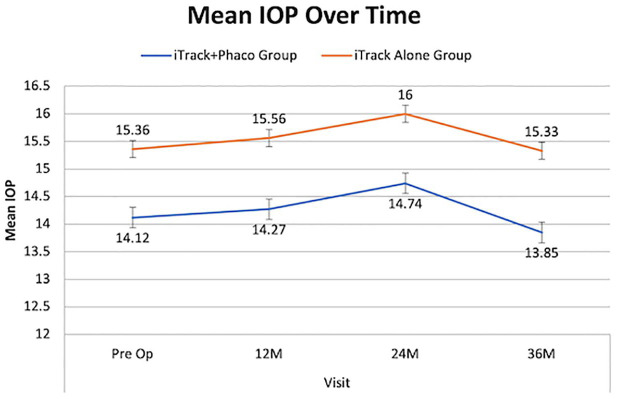
As shown in Table 5, the mean baseline IOP of all eyes (14.42 ± 2.2 mmHg) remained stable at the 12-, 24-, and 36-month follow-up visits: 14.60 ± 3.65 mmHg (n = 35), 15.06 ± 2.26 mmHg (n = 31), and 14.19 ± 2.91 mmHg (n = 26), respectively, with no significant difference between the two treatment groups (p = 0.08).
Table 5.
IOP compared for all eyes, iTrack + phaco and iTrack-alone.
| Time point | All eyes | iTrack + phaco | iTrack-alone | p-value | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD (mmHg) | n | Mean ± SD (mmHg) | n | Mean ± SD (mmHg) | ||
| Pre-op | 45 | 14.14 ± 2.2 | 34 | 14.1 ± 2.2 | 11 | 15.4 ± 2.0 | – |
| 12M | 35 | 14.6 ± 3.65 | 26 | 14.3 ± 3.8 | 9 | 15.6 ± 3.1 | 0.731 |
| 24M | 31 | 15.06 ± 2.26 | 23 | 14.7 ± 2.0 | 8 | 16.0 ± 2.8 | 0.935 |
| 36M | 26 | 14.19 ± 2.91 | 20 | 13.9 ± 2.5 | 6 | 15.3 ± 4.1 | 0.857 |
IOP, intraocular pressure; SD, standard deviation.
Mean IOP also remained stable in the iTrack-alone group (n = 11), ranging from 15.4 ± 2.0 mmHg at baseline to 15.6 ± 3.1 mmHg and 16.0 ± 2.8 mmHg at 12 and 24 months, respectively, before reducing to 15.3 ± 4.1 mmHg at 36 months. In the iTrack + phaco group (n = 34), mean IOP was 14.1 ± 2.2 mmHg, 14.3 ± 3.8 mmHg, and 14.7 ± 2.0 mmHg at baseline, 12 months, and 24 months, respectively, and fell significantly at 36 months to 13.9 ± 2.5 mmHg. Comparing IOP between the iTrack-alone and iTrack + phaco groups (Table 5 and Figure 4) highlighted that there was no statistically significant difference observed between the treatment groups.
Figure 4.
Maintenance of IOP: iTrack + phaco compared with iTrack-alone.
Safety outcomes
Overall, the frequency of surgical and post-surgical complications was low with no serious adverse events recorded. Adverse events were limited to intraoperative bleeding at the goniotomy site and postoperative microhyphema development, which were transient and resolved within 1 week without sequelae.
Discussion
In this retrospective case series study, iTrack ab-interno canaloplasty performed as a standalone procedure, or in conjunction with cataract surgery, was found to be a safe and effective option for reducing glaucoma medication dependence and maintaining IOP in patients with controlled mild-moderate POAG.
Although topical antiglaucoma medications are the first-line treatment for glaucoma, reducing the reliance on these medications in this patient population may provide significant benefits due to the numerous quality-of-life issues and complications associated with their long-term, chronic, administration. Moreover, patient compliance and adherence are known factors which complicate the use of antiglaucoma medications and thus negatively impact their overall effectiveness. Potential aspects which may limit adherence include difficulty administering eye drops, forgetfulness, cost of the medications, and side effects, such as ocular surface disease, that can negatively impact quality of life.7–11 Nonadherence, which has been reported in up to 80% of patients, can lead to optic nerve damage and disease progression.3–6 As such, the importance of reducing the medication burden in glaucoma patients, both in terms of maintaining the health of the eye and improving patient quality of life, should not be underestimated.
Clinical evidence indicates that ab-interno canaloplasty is safe and effective in lowering IOP and reducing medication burden in mild-moderate POAG.22,25,26 A consecutive case series by Gallardo et al.25 demonstrated the clinical efficacy and safety of iTrack ab-interno canaloplasty over a 12-month period. Overall, 84.9% of eyes experienced a reduction in IOP which was greater than 20%. The case series also assessed the efficacy of iTrack when performed as both a standalone procedure (n = 41) and in combination with cataract surgery (n = 34). At 12 months, when performed as a standalone procedure, mean IOP and baseline medication use were reduced by 32.8% (p < 0.001) and 51.1% (p < 0.001), respectively. When performed in conjunction with cataract surgery, mean IOP and baseline medication were reduced by 31.7% (p < 0.001) and 71.1% (p < 0.001), respectively. The percentage of eyes that were medication free at 12 months was 30.8% in the standalone group and 50% in the group where treatment was combined with cataract surgery.
Körber,27 performing ab-interno canaloplasty with iTrack, reported a reduction in mean IOP from 18.8 ± 5.63 at baseline (n = 23) to 14.73 ± 2.97 mmHg at 12 months (n = 11). Patient reliance on medications was also reduced, with the mean number of medications falling from 1.69 at baseline (n = 23) to 0.21 at the last follow-up visit (n = 11).27
Kazerounian et al.26 reported a reduction in mean IOP of 32.5% from 20.24 ± 5.92 mmHg (n = 25) at baseline to 13.67 ± 2.15 mmHg (n = 21, p < 0.001) at 24 months when ab-interno canaloplasty was performed as a standalone procedure, or in conjunction with cataract surgery. Medication reduction was also significantly reduced, falling from 1.92 ± 1.04 at baseline to 0.05 ± 0.23 at 24 months. Importantly, 80% of patients were medication-free at 24 months.26
In this case series study, the iTrack ab-interno canaloplasty procedure, performed with or without cataract surgery, was effective in reducing medication use in eyes with controlled POAG (baseline IOP ⩽17 mmHg) up to 36 months. Significant reductions were seen at all postoperative visits for all eyes (N = 45): 82% at 12 months, 76% at 24 months, and 61% at 36 months (p < 0.001). The percentage of all eyes that were medication free was 80% (28/35) at 12 months, 68% (21/31) at 24 months, and 56% (14/25) at 36 months versus 2% (1/45) at baseline.
We noted a nonsignificant trend toward a greater reduction in medication use in the iTrack + phaco group (69%) with respect to the iTrack-alone group (36%) at 36 months (p = 0.1955). While the mean values appear nonsignificant, statistical significance was not achieved due to a high standard deviation of the data. It is therefore possible that a statistically significant trend could have been identified with a larger sample size. In such a case, we hypothesize that a significant change could be attributable to the confounding effect of cataract surgery, which has been suggested to reduce outflow resistance by impacting the anterior chamber depth and the trabecular-iris angle.28,29
Maintenance of IOP was a secondary endpoint in the current study. As reported in the Advanced Glaucoma Intervention Study, eyes that consistently maintained IOP below 18 mmHg were the least likely to show progression of visual field loss.30 In this study, the mean baseline IOP of 14.42 ± 2.2 mmHg remained stable at the 12-, 24-, and 36-month follow-up visits and in both treatment groups. Therefore, for patients with controlled POAG, the ab-interno canaloplasty procedure, performed with or without cataract surgery, may be an effective option for reducing medication dependence while maintaining IOP within target range.
The findings of this study suggest that iTrack ab-interno canaloplasty offers a highly effective treatment option for patients with controlled, mild-moderate POAG and a necessity to reduce their medication burden. The efficacy and safety profile of ab-interno canaloplasty was comparable when performed as a standalone procedure or combined with cataract surgery.
Limitations of the present study include that it was a single-center, retrospective study with a relatively small patient cohort. Moreover, the high rate of attrition at months 24 and 36 can be attributed to the effective maintenance of IOP and few postoperative complications, which simplified the postoperative regimen and thus resulted in many patients returning to their referring eyecare provider for subsequent follow-up.
Conclusion
iTrack ab-interno canaloplasty is a comprehensive MIGS that is theorized to restore the physiological outflow pathways in patients with mild-moderate glaucoma via a tissue-sparing approach. Overall, ab-interno canaloplasty performed as a standalone procedure, and in combination with cataract surgery, significantly reduced dependency on antiglaucoma medications while maintaining preoperative IOP in patients with controlled, mild-moderate POAG. Thus, inclusion of the iTrack procedure in early glaucoma treatment algorithms may help to reduce the necessity for medications and delay the need for more aggressive surgeries. The promising results seen with this relatively small group of patients highlight the need for prospective, multicenter trials with a larger patient cohort in order to confirm these findings.
Footnotes
Conflict of interest statement: The author declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.K. is Chief Medical Consultant of Nova Eye Medical.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Nova Eye Medical, Fremont, California.
ORCID iD: Mahmoud A. Khaimi  https://orcid.org/0000-0002-8979-557X
https://orcid.org/0000-0002-8979-557X
References
- 1.Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma. Ophthalmology 2016; 123: P41–P111. [DOI] [PubMed] [Google Scholar]
- 3.Olthoff CMG, Schouten JSAG, van de Borne BW, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology 2005; 112: 953–961. [DOI] [PubMed] [Google Scholar]
- 4.Dreer LE, Girkin C, Mansberger SL.Determinants of medication adherence to topical glaucoma therapy. J Glaucoma 2012; 21: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology 2011; 118: 2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi GCM, Pasinetti GM, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol 2011; 21: 410–414. [DOI] [PubMed] [Google Scholar]
- 7.Davis SA, Sleath B, Carpenter DM, et al. Drop instillation and glaucoma. Curr Opin Ophthalmol 2018; 29: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GF, Hollander DA, Williams JM.Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin 2013; 29: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 9.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology 2015; 122: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mylla Boso AL, Gasperi E, Fernandes L, et al. Impact of ocular surface disease treatment in patients with glaucoma. Clin Ophthalmol 2020; 14: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur D, Gupta A, Singh G.Perspectives on quality of life in glaucoma. J Curr Glaucoma Pract 2012; 6: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammar DA, Kahook MY.Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol Vis 2011; 17: 1806–1813. [PMC free article] [PubMed] [Google Scholar]
- 13.Yan X, Li M, Chen Z, et al. Schlemm’s canal and trabecular meshwork in eyes with primary open angle glaucoma: a comparative study using high-frequency ultrasound biomicroscopy. PLoS ONE 2016; 11: e0145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel M, Picciani RG, Lee RK, et al. Aqueous humor dynamics: a review. Open Ophthalmol J 2010; 4: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J, Xu J, Wei A, et al. Spectral-domain optical coherence tomographic assessment of Schlemm’s canal in Chinese subjects with primary open-angle glaucoma. Ophthalmology 2013; 120: 709–715. [DOI] [PubMed] [Google Scholar]
- 16.Allingham RR, de Kater AW, Ethier CR.Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res 1996; 62: 101–109. [DOI] [PubMed] [Google Scholar]
- 17.Hann CR, Vercnocke AJ, Bentley MD, et al. Anatomic changes in Schlemm’s canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressures. Invest Ophthalmol Vis Sci 2014; 55: 5834–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha EDK, Xu J, Gong L, et al. Variations in active outflow along the trabecular outflow pathway. Exp Eye Res 2016; 146: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegmann R, Pienaar A, Grieshaber MC.Schlemm’s canal surgery: restoring physiological aqueous outflow. In: Grieshaber MC, Orgul S, Flammer J. (eds) Glaucoma therapy – State of the art. Basel: Association for Continuing Education in Ophthalmology, 2009, pp. 113–120. [Google Scholar]
- 20.Stegmann R, Pienaar A, Miller D.Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999; 25: 316–322. [DOI] [PubMed] [Google Scholar]
- 21.Grieshaber MC, Pienaar A, Olivier J, et al. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci 2010; 51: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 22.Khaimi MA.Canaloplasty: a minimally invasive and maximally effective glaucoma treatment. J Ophthalmol 2015; 2015: 485065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaimi MA, Dvorak JD, Ding K.An analysis of 3-year outcomes following canaloplasty for the treatment of open-angle glaucoma. J Ophthalmol 2017; 2017: 2904272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European glaucoma society terminology and guidelines for glaucoma, 4th edition– Chapter 2: classification and terminology Supported by the EGS Foundation: part 1: foreword; introduction; glossary; Chapter 2| classification and terminology. Br J Ophthalmol 2017; 101: 73–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallardo MJ, Supnet RA, Ahmed II, K. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol 2018; 12: 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazerounian S, Zimbelmann M, Lörtscher M, et al. Canaloplasty ab interno (AbiC) – 2-year-results of a novel minimally invasive glaucoma surgery (MIGS) technique. Klin Monatsbl Augenheilkd. Epub ahead of print 17November2020. DOI: 10.1055/a-1250-8431. [DOI] [PubMed] [Google Scholar]
- 27.Körber N.Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd 2018; 32: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berdahl JP.Cataract surgery to lower intraocular pressure. Middle East Afr J Ophthalmol 2009; 16: 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rękas M, Barchan-Kucia K, Konopińska J, et al. Analysis and modeling of anatomical changes of the anterior segment of the eye after cataract surgery with consideration of different phenotypes of eye structure. Curr Eye Res 2015; 40: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 30.Gaasterland DE, Ederer F, Beck A, et al. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]