Abstract
Introduction:
SLC2A1 polymorphism may play a role in the smooth muscle cell proliferation and extracellular matrix synthesis in vessels. However, the role of SLC2A1 polymorphism on diabetic cardiovascular disease (CVD) have not yet been identified. In this study, we aimed to investigate the association between SLC2A1 HaeIII polymorphism and CVD in Korean patients with type 2 diabetes mellitus (T2DM) according to disease duration.
Methods:
A total of 846 patients with T2DM who visited the Chungbuk National University Hospital were investigated. The HaeIII polymorphism of SLC2A1 gene was determined by real time polymerase chain reaction method. Genotyping results were presented GG, AG, or AA. Subgroup analysis was performed according to duration of T2DM (⩽10, 11–20, >20 years).
Results:
The AA genotype was significantly associated with higher prevalence of CVD in patients with DM duration less than 10 years (26.3% vs 9.2%, p = 0.014). There was no significant association between SLC2A1 HaeIII polymorphism and other diabetic complications including, retinopathy, nephropathy, neuropathy, cerebrovascular disease, and peripheral artery disease.
Conclusions:
The SLC2A1 HaeIII polymorphism was associated with CVD in Korean patients with T2DM with short disease duration.
Keywords: SLC2A1 polymorphism, type 2 diabetes mellitus, cardiovascular disease, diabetic complications
Introduction
Cardiovascular disease (CVD) is the main cause of morbidity and mortality, and affect the quality of life in patients with diabetes mellitus (DM). There is some evidence of a relationship between diabetic CVD and genetic factors. Some patients with a long duration of DM do not experience complications, indicating the existence of specific, genetically defined predisposing factors.1
The solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1) polymorphism has been related with diabetic microvascular complications in several studies.2,3 The association with SLC2A1 polymorphism and CVD has been investigated in several studies.4–6 However, the role of SLC2A1 polymorphism in the development of CVD in type 2 diabetes mellitus (T2DM) remains controversy. Moreover, few studies have evaluated the association with SLC2A1 HaeIII polymorphism and CVD in patients with T2DM. Therefore, in this study, we investigated the association between the HaeIII polymorphism in the SLC2A1 gene and CVD in Korean patients with T2DM according to disease duration.
Methods
Study populations
This study was conducted simultaneously with our previous study.7 A total of 846 patients with T2DM who visited the Chungbuk National University Hospital were included in the analysis. Diagnosis of DM was defined according to the American Diabetes Association guidelines.8 Demographic data and laboratory data were collected for each patient. All participants provided written informed consent. This study was approved by the Institutional Review Board of the Chungbuk National University Hospital (IRB No. 2018-03-034-001). The current study was conducted according to the guidelines administered by the Declaration of Helsinki.
Diabetic complications
Diabetic complications, both microvascular (such as retinopathy, nephropathy, and neuropathy) and macrovascular (including CVD, cerebrovascular disease, and peripheral artery disease) complications, were evaluated. Retinopathy and neuropathy were assessed based on medical records. Nephropathy was defined according to eGFR, with a cutoff value of 60 mL/min/1.73 m2. CVD (history of stable angina, unstable angina, myocardial infarction, and intervention of coronary artery disease) were evaluated based on medical records and the results of coronary angiographic data. Cerebrovascular disease was also assessed based on medical records and, if possible, magnetic resonance imaging results. Peripheral artery disease was defined as an ankle-brachial index value below 1.20.
Genotyping
The polymorphism of SLC2A1 (rs1385129, A/G) were detected using TaqMan probe-based real-time polymerase chain reaction (PCR). The mixture of PCR primers and fluorescent probes for rs1385129 (ThermoFisher TaqMan™ SNP Genotyping Assay, Cat# 4351379, Waltham, MA, USA) was used for PCR amplification with a genotyping master mix (ThermoFisher, Cat# 4371353, Waltham, MA, USA) in a 25 µL reaction. Reactions were cycled using the following parameters: preheating at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Fluorescence was detected at the end of every cycle and genotype data were automatically generated by a BioRad CFX96 Real-Time PCR system (BioRad, Hercules, CA, USA).
Statistical analysis
The probability of Hardy–Weinberg equilibrium was tested using the chi-squared test. Data were expressed as the mean ± standard deviation (SD) or as percentages for categorical variables. The baseline characteristics were compared using Student’s t-test for continuous variables and the chi-squared test for categorical parameters. The chi-squared test was used to evaluate differences in prevalence according to genotype. Multiple logistic regression analyses were performed to evaluate the risk factors associated with CVD. All statistical analyses were performed using the SPSS for Windows software (22.0; IBM Corp., Armonk, NY, USA). Significance was set at p < 0.05.
Results
Characteristics of the study populations
The mean age and BMI of the total study subjects (n = 846) were 61.3 ± 12.3 years and 25.7 ± 3.8 kg/m2, respectively. The duration of diabetes was 10.3 ± 7.9 years and about half of the patients had a family history of diabetes and hypertension. The genotypic distribution of SLC2A1 met the Hardy–Weinberg equilibrium. The frequency of the SLC2A1 HaeIII genotypes in the study subjects was as follows: GG, 62.1% (n = 525); AG, 33.9% (n = 287); and AA, 4.0% (n = 34). Among the study subjects, 812 patients carried the GG or AG genotypes and 34 patients carried the AA genotype. No statistically significant differences were noted in baseline characteristics except hypertension (50.6% in the GG + AG group vs 70.6% in the AA group, p = 0.022).
SLC2A1 HaeIII polymorphism and CVD according to duration of diabetes
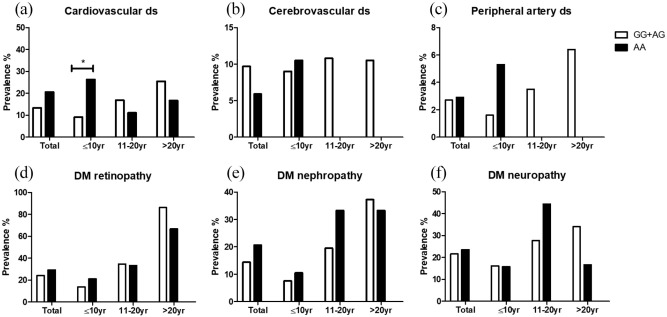
There was a tendency to a higher prevalence of CVD in the AA group than in the GG + AG group however, this result did not reach statistical significance (20.6% vs 13.4%, p = 0.234) (Figure 1(a)). A subgroup analysis according to the duration of diabetes showed a significantly higher prevalence of cardiovascular disease in the AA group compared with the GG + AG group (26.3% vs 9.2%, p = 0.014) in patients with a duration of diabetes that was ⩽10 years. In subgroups with a disease duration of 11–20 and >20 years, the prevalence of cardiovascular disease was insignificantly different between the two groups. These results are presented in Figure 1(a). Multiple logistic regression analysis was performed in patients with DM duration ⩽10 years. Non-G allele was significantly associated with the higher risk of CVD (OR 3.954, 95% CI 1.238–12.627, p = 0.020) after adjusting for other clinical parameters including HTN, age, sex, and HbA1c (data not shown).
Figure 1.
SLC2A1 genotypes and prevalence of diabetic complications among groups according to duration of diabetes.
SLC2A1 HaeIII polymorphism and other diabetic complications
The associations between SLC2A1 HaeIII polymorphism and other diabetic complications are presented in Figure 1(b) to (f). In the whole cohort, the prevalence of macrovascular complications, including cerebrovascular disease and peripheral artery disease, in the GG + AG group and AA group was 9.7% versus 5.9% and 2.7% versus 2.9%, respectively. Regarding microvascular complications, the prevalence of retinopathy was 24.1% in the GG + AG group and 29.4% in the AA group. The prevalence of nephropathy and neuropathy was also similar between the two groups (14.4% vs 20.6% and 21.6% vs 23.5%, respectively). A subgroup analysis performed according to disease duration revealed the absence of significant differences between the two groups regarding the five complications mentioned above.
Discussion
In this study, we investigated the association between the SLC2A1 HaeIII polymorphism and CVD in Korean patients with T2DM according to disease duration. The AA genotype was significantly associated with a higher prevalence of CVD, especially in patients with DM duration equal or less than 10 years. The incidence of CVD in T2DM is relatively low during the first 10 years of DM duration, after which it increase rapidly about over 10 years of DM duration.9 For this reason, we analyzed the association between SLC2A1 HaeIII polymorphism and CVD according to the DM duration in order to exclude the influence of DM duration on CVD. Other diabetic complications did not show significant differences in prevalence according to genotype in this study. To the best of our knowledge, this is the first study to evaluate association between association between the SLC2A1 HaeIII polymorphism and CVD in Asian population.
There were few studies regarding the role of genetic variant of SLC2A1 in susceptibility to CVD in T2DM. Rufino et al.10 reported that the SLC2A1 XbaI polymorphism was associated with vascular calcification in nondiabetic uremic patients. Osadnik et al.11 showed that the functional (rs710218) SLC2A1 polymorphism was a risk factor for in-stent restenosis. Another study performed in West Indian patients with T2DM showed the associations between the HaeIII SLC2A1 polymorphism and development of DM, diabetic nephropathy and CVD.5 In contrary, SLC2A1 polymorphism was not a determinant for cardiovascular risk factors in Mediterranean T2DM.6 This inconsistent finding might be explained by differences in population, number of subject, and genetic background.
In our study, the prevalence of CVD was significantly different between different genotype groups. There are several possible reasons affect to results in present study. First, number of patients especially with AA genotype was small and after dividing the patients according to disease duration, number of patient were more decreased. Second, in this study, we evaluated only in Korean patients therefore, there might be differences by ethnicity. Third, risk factors affect CVD such as glycemic control, dyslipidemia, and hypertension changed as increasing the duration of DM.
The present study had several strengths. First, the number of subjects included was relatively large compared with previous studies. Second, we performed a comprehensive evaluation of the diabetic complications, including both micro- and macrovascular complications, according to disease duration. However, there were several limitations to our study. We collected data retrospectively by reviewing medical records; therefore, data may be missing and recall biases may have occurred. Furthermore, the minor allele frequency was low (4%) despite the relatively large number of participants included in the study. Finally, the fact that the subjects were exclusively Korean patients with T2DM was also a limitation of this study.
Conclusions
The present study demonstrated that the HaeIII polymorphism in SLC2A1 was associated with the prevalence of early cardiovascular complications in Korean patients with T2DM. The HaeIII polymorphism could be used as a predictive marker of diabetic cardiovascular complications. Further studies using other larger multiethnic cohorts and experimental models are required to validate our results.
Footnotes
Author contributions: H.J.J. selected samples and sent them for testing. D-.H.L. performed the statistical analysis and drafted the manuscript. G.W.W and Y.H.L. contributed to sample measurement. J.S.S., E.J.K., and T.K.O. contributed to the discussion and reviewed the manuscript. H.J.J. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Study Ethics approval was obtained from the Institutional Review Board of the Chungbuk National University Hospital.
ORCID iD: Dong-Hwa Lee  https://orcid.org/0000-0002-1552-3205
https://orcid.org/0000-0002-1552-3205
Availability of data and materials: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Zintzaras E, Uhlig K, Koukoulis GN, et al. Methylenetetrahydrofolate reductase gene polymorphism as a risk factor for diabetic nephropathy: a meta-analysis. J Hum Genet 2007; 52: 881–890. [DOI] [PubMed] [Google Scholar]
- 2.Amini S, Javanmardi M, Mokarizadeh A, et al. Association of HaeIII single nucleotide polymorphisms in the SLC2A1 gene with risk of diabetic nephropathy; evidence from Kurdish patients with type 2 diabetes mellitus. QJM 2016; 109: 399–404. [DOI] [PubMed] [Google Scholar]
- 3.Ng ZX, Kuppusamy UR, Tajunisah I, et al. Investigation of SLC2A1 26177A/G gene polymorphism via high resolution melting curve analysis in Malaysian patients with diabetic retinopathy. J Diabetes Complications 2012; 26: 388–392. [DOI] [PubMed] [Google Scholar]
- 4.Liou T-H, Pi-Sunyer FX, Laferrère B.Physical disability and obesity. Nutr Rev 2005; 63: 321–331. [DOI] [PubMed] [Google Scholar]
- 5.Lal S, Mistry K.Glucose transporter 1(GLUT1) gene frequency distribution of XbaIG>T and HaeIIIT>C polymorphisms among different West Indian patients with type 2 diabetes mellitus. Gene Rep 2017; 6: 36–40. [Google Scholar]
- 6.Gutierrez C, Vendrell J, Pastor R, et al. GLUT1 gene polymorphism in non-insulin-dependent diabetes mellitus: genetic susceptibility relationship with cardiovascular risk factors and microangiopathic complications in a Mediterranean population. Diabetes Res Clin Pract 1998; 41: 113–120. [DOI] [PubMed] [Google Scholar]
- 7.Lee DH, Won GW, Lee YH, et al. Associations between the HaeIII single nucleotide polymorphism in the SLC2A1 gene and diabetic nephropathy in Korean patients with type 2 diabetes mellitus. J Korean Med Sci 2019; 34: e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 2020; 43: S14–S31. [DOI] [PubMed] [Google Scholar]
- 9.Deshpande AD, Harris-Hayes M, Schootman M.Epidemiology of diabetes and diabetes-related complications. Phys Ther 2008; 88: 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rufino M, Hernandez D, Barrios Y, et al. The GLUT-1 XbaI gene polymorphism is associated with vascular calcifications in nondiabetic uremic patients. Nephron Clin Pract 2008; 108: c182–c187. [DOI] [PubMed] [Google Scholar]
- 11.Osadnik T, Strzelczyk J, Bujak K, et al. Functional polymorphism rs710218 in the gene coding GLUT1 protein is associated with in-stent restenosis. Biomark Med 2015; 9: 743–750. [DOI] [PubMed] [Google Scholar]