Abstract
Dysphagia is a common and frequently undetected complication of many neurological disorders and of sarcopoenia in ageing persons. Spontaneous swallowing frequency (SSF) has been mooted as a possible tool to classify dysphagia risk. We conducted a review of the literature to describe SSF in both the healthy population and in disease-specific populations, in order to consider its utility as a screening tool to identify dysphagia. We searched Medline, Embase, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials databases. Metadata were extracted, collated and analysed to give quantitative insight. Three hundred and twelve articles were retrieved, with 19 meeting inclusion and quality criteria. Heterogeneity between studies was high (I2 = 99%). Mean SSF in healthy younger sub-groups was 0.98/min [CI: 0.67; 1.42]. In the Parkinson’s sub-group, mean SSF was 0.59/min [0.40; 0.87]. Mean SSF in healthy older, higher risk and dysphagic populations were similar (0.21/min [0.09; 0.52], 0.26/min [0.10; 0.72] and 0.30/min [0.16; 0.54], respectively). SSF is a novel, non-invasive clinical variable which warrants further exploration as to its potential to identify persons at risk of dysphagia. Larger, well-conducted studies are needed to develop objective, standardised methods for detecting SSF, and develop normative values in healthy populations.
Keywords: swallow frequency, deglutition, deglutition disorders, dysphagia, screening
Introduction
Spontaneous swallowing is an aerodigestive reflex supporting airway protection (Shaker, 1995). Swallowing impairment, or dysphagia, is a common and frequently undetected complication of many neurological and structural disorders in children and adults. Entry of food/liquid below the true vocal cords is known as aspiration and is a common consequence of dysphagia. Dysphagia and aspiration are the pre-eminent risk factors for pneumonia, each with odds ratios >10 (Harkness et al., 1990; Vergis et al., 2001).
With 479,564 finished consultant episodes and >3 million bed days, pneumonia is the most common cause for UK hospital admissions (HES data: 2015-2016), and the commonest healthcare-associated infection (HCAI) in Europe, accounting for 26% of all HCAI (ECDPC, 2013). But while mass screening is often provided by nurses and/or speech and language therapists (SALTs) in the stroke unit, widespread screening of at-risk clinical populations such as in older people’s medicine is logistically impossible.
Swallow screening tools are well-used in dysphagia screening; these include questionnaires, observations and clinical history. However outside of the stroke population, there is no consensus on best practice; each professional chooses their preferred technique (Etges et al., 2014). Questionnaires are an effective way to screen community and secondary care patients (Park et al., 2015) but remain subjective with varying degrees of diagnostic accuracy due largely to the unconscious nature of the process. There is currently no validated bedside screening tool for automated screening of dysphagia and aspiration risk.
We and others are assessing the utility of spontaneous swallowing frequency (SSF) (Crary et al., 2014) in this role. SSF is the rate of swallowing over a prolonged period without purposeful intervention; reduced SSF is mooted as an indicator of dysphagia in clinical populations that include: post-stroke (Crary et al., 2014); head and neck cancer (Kamarunas et al., 2019); Parkinson’s disease (Pehlivan et al., 1996); and older persons (Crary et al., 2013; Murray et al., 1996; Tanaka et al., 2013). Recent advances in technology have enabled an assortment of cheap, non-invasive swallow detectors. Surface EMG, sound analysis and other techniques may offer low-cost and simple screening that might be deployed with only limited training.
Yet despite swallowing being one of the most common and readily observed of all physiological processes, there is no consensus as to a normal SSF, even in a healthy adult population. To illustrate the uncertainty, early research conducted by Lear et al. (1965) suggesting that humans swallow 203-1008 times per day (0.14–0.7/min), with a mean frequency of 585 per day. Whereas recently, Rudney et al. (1995) reported that a healthy human will swallow spontaneously 18-400 times an hour (0.3–6.7/min). These two normal ranges for SSF cover two orders of magnitude and are barely compatible with one another. A cursory review of the literature suggests further variability with age, aetiologies and comorbidities. In the context of developing a dysphagia screening test, there is no scope to move forward without better understanding this aspect of basic swallow physiology.
In this review we assess the literature reporting spontaneous swallow rates in healthy populations and in populations with, or at risk of developing, dysphagia. We wish to provide original insight into use of SSF as a screening tool, by:
- Reporting spontaneous swallowing frequency of persons in normal and clinically relevant sub-groups.
- Discussing the implications; specifically, the clinical utility of SSF as a screening tool to identify dysphagia.
Methods
Search strategy and selection criteria
We conducted electronic searches to identify relevant primary research articles using the following databases: MEDLINE, Embase, Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials. See Table 1 for the full search strategy.
Table 1.
Electronic Search Strategies to Identify Primary Research Articles on Spontaneous Swallow Frequency.
| Database | Search Strategy |
|---|---|
| Ovid MEDLINE (R) 1946–2019 | 1. (Swallow* adj2 frequency).mp |
| 2. (Swallow* adj2 rate).mp | |
| 3. Spontaneous swallow*.mp | |
| 4. Swallow* per.mp | |
| 5. 1 or 2 or 3 or 4 | |
| 6. Deglutition disorders | |
| 7. Pneumonia, aspiration/or respiratory aspiration/ | |
| 8. Dysphagia.mp | |
| 9. 6 or 7 or 8 | |
| 10. 5 and 9 | |
| EMBASE 1980–2019 | 1. (Swallow* adj2 frequency).mp |
| 2. (Swallow* adj2 rate).mp | |
| 3. Spontaneous swallow*.mp | |
| 4. Swallow* per.mp | |
| 5. 1 or 2 or 3 or 4 | |
| 6. Deglutition disorders/ | |
| 7. Pneumonia, aspiration/or respiratory aspiration/ | |
| 8. Dysphagia.mp | |
| 9. 6 or 7 or 8 | |
| 10. 5 and 9 |
Citations were excluded by a single rater (JB) if they: had no abstract; included no human data; or made no reference to spontaneous swallow rate. Abstracts and where appropriate full articles were then reviewed in more detail. Papers were excluded if they presented only an abstract and not a full paper; included data only from sleeping subjects (i.e. no awake control); did not measure SSF directly (i.e. SSF was predicted using other variables) and if they only reported data from populations under the age of 18 years.
Data extraction
Where articles met the inclusion criteria, a single rater (JB) extracted the following data: sample size; study population/aetiology; study controls; population ages; swallow frequency; SD of swallow frequency between subjects; swallow identification method; length of swallowing recordings; statistical significance of SSF between observational and/or intervention cohorts; and additional information where appropriate–for example, correlational statistics and study information.
Quality assessment and data extraction
Two independent raters (JB and CE) rated each paper as poor, fair or good quality according to the NHLBI Study Quality Assessment tools (NIH, 2014) (see Appendix A). One rater has expertise in scientific methodology (JB) and the second is an expert in dysphagia screening (CE). Where ratings were different, the raters deliberated and agreed on a final rating. Eligible data were extracted from each paper. Ineligible data included nocturnal measurements, obstructed measurements (e.g. tracheotomy tube present), invasive measurements (e.g. endoscopy) and participants <18 years of age.
Data analysis
The data samples were then categorised into sub-groups, each with a minimum of four studies to allow for effective meta-analysis (Fu et al., 2011). As such, the following sub-groups were formed as shown in Tables 2 and 3:
Table 2.
Observational Studies Measuring Spontaneous Swallow Frequency in Patients with, or at Risk of Developing, Dysphagia.
| Author, year | Aetiology | N | Sub-group | Swallow rate (/min) | p | Additional details | Swallow identification method |
|---|---|---|---|---|---|---|---|
| Crary et al. (2014) | Stroke, known dysphagia | 26 | Dysphagia | 0.23 (±0.15) | <0.0001 | Acute stroke patients screened using SSF (via acoustic analysis) vs. standard clinical screening protocols for dysphagia identification | Microphone taped lateral inferior to the cricoid and connected to a digital recorder (Crary et al., 2013) |
|
Stroke, n o dysphagia |
36 |
High-risk |
0.55 (±0.3) |
||||
| Niimi et al. (2018) | Stroke, high SP | 17 | High-risk | 0.51 ± 0.34 | 0.016 | To determine the relationship between SSF and salivary substance p (SP) levels. SP is known to act as a neurotransmitter in the swallowing reflex. Low levels of SP in saliva attenuate the swallowing reflex. SSF data collected for 1-hr per condition | Microphone placed onto the anterolateral side of the neck |
| Stroke, low SP |
23 |
High-risk |
0.27 ± 0.19 |
||||
| Murray et al. (1996) | Older, hospitalised, full cohort | 47 | N/A N/A N/A |
0.89 (±0.85) | Investigation of SSF in the predication of aspiration of food and liquid, following dysphagia categorisation using fiberoptic endoscopic evaluation of swallowing (FEES) | Fiberoptic endoscopic evaluation of swallowing (FEES) | |
| Older, hospitalised, aspiration | 29 | 0.72 (±0.78) | |||||
| Older, hospitalised, no aspiration | 18 | 1.16 (±0.91) | |||||
| Older, normal |
17 |
N/A 2.82 (±1.71) |
|||||
|
Young, normal |
5 |
N/A 2.96 (±0.88) |
|||||
| Crary et al. (2013) | Older, normal | 11 | Healthy old | 0.47 | <0.0001 | To evaluate an acoustic recording technique as a measure to estimate SSF. No significant differences in spontaneous swallow frequency were observed between the multichannel physiologic recordings and the acoustic recordings (0.85 vs. 0.81 sw/min) | Multichannel recordings including surface EMG, swallow apnoea and cervical auscultation. Microphone for acoustic recordings attached just below the lateral cricoid cartilage |
|
Young, normal |
18 |
Healthy yng |
1.02 |
||||
| Tanaka et al. (2013) | Older, normal | 20 | Healthy old | 0.16 (±0.08) | 0.023 | SSF in older people during daily life: a comparison of (1) older persons versus young, and (2) older bedridden versus older semi-bedridden. Recorded for 1-hr each time | Laryngeal microphone and digital voice recorder |
| Bedridden | 10 | High-risk | 0.11 (±0.06) | ||||
|
Semi-bedridden |
10 |
Healthy old |
0.2 (±0.09) |
||||
| Young, normal | 15 | Healthy yng | 0.68 (±0.33) | <0.0001 | |||
| Trocello et al. 2015 | Wilson's disease, dysphagic | 2 | N/A | 0.97 | >0.05 | Examination of hypersialorrhea in Wilson’s disease and association with dysphagia severity. SSF recorded for 10 mins | Stethoscope attached to the neck and a microphone connected to a rhinolaryngeal stroboscope |
| Wilson's disease, non-dysphagic | 6 | N/A | 1.35 | ||||
|
Young, normal |
10 |
Healthy yng |
1.70 |
||||
| Kamarunas et al. 2019 | Oropharyngeal dysphagia, post-CVA | 9 | Dysphagia | 0.73 (±0.75) | 0.48 | Group comparisons on SSF without vibration intervention. Data used as baseline information for study aiming to evaluate whether sensory stimulation could excite an impaired swallowing system (via use of SSF) | 1) Hyolaryngeal elevation (piezoelectric accelerometer peaks) 2) respiratory apnoea (inductive plethysmography - absence of ribcage/abdomen movement) 3) note from trained observer |
| Oropharyngeal dysphagia, post-radiation for H&N cancer | 4 | Dysphagia | 0.7 (±1.15) | ||||
|
Healthy control |
10 |
N/A |
Taken from Mulheren and Ludlow (2017) |
||||
| Pehlivan et al. (1996) | Parkinson’s | 21 | Parkinson’s | 0.8 | <0.05 | Use of “Digital Phagometer” (piezoelectric sensor and digital event counter) to measure SSF in patients with Parkinson’s | Piezoelectric sensor placed at the coniotomy region between the thyroid and cricoid cartilages |
|
Healthy control |
21 |
Healthy yng |
1.18 |
||||
| Marks and Weinreich (2001) | Parkinson’s | 28 | Parkinson’s | 0.55 (±0.32) | Use of an electret microphone to measure SSF to give an indication of drooling in patients with Parkinson’s | Microphone positioned over the centre of the cricoid cartilage | |
|
Healthy, age-matched control |
8 |
Healthy old |
0.13 (±0.03) |
||||
| Kalf et al. (2011) | Parkinson’s, droolers | 15 | Parkinson’s | 0.51 (±0.39) | 0.346 | Factors potentially contributing to drooling, including SSF, examined in Parkinson’s patients with and without diurnal saliva loss | EMG, motion sensor (at larynx) and video |
|
Parkinson’s, non-droolers |
15 |
Parkinson’s |
0.4 (±0.26) |
Table 3.
Interventional studies measuring spontaneous swallow frequency in patients with, or at risk of developing, dysphagia.
| Author, year | Aetiology | n | Sub-group | Swallow rate (/min) | P | Additional details | Swallow identification method |
|---|---|---|---|---|---|---|---|
| Brady et al. (2016) | Known or suspected dysphagia, OSL: Normal/mild, baseline | 19 |
Dysphagia | 0.53 | Investigation of the relationship between SSF, accumulated oropharyngeal secretion levels (OSLs) and gustatory stimulation (GS). Moderate relationship between SSF at rest and OSL (Pearson correlation 0.47; p = 0.01) | Visually by two raters (inspection of larynx) with assistance from surface EMG. | |
| Known or suspected dysphagia, OSL: Normal/mild, post-GS | N/A | 2.63 | |||||
| Known or suspected dysphagia, OSL: severe/profound, baseline | 8 |
Dysphagia | 0.06 | ||||
|
Known or suspected dysphagia, OSL: Severe/profound, post-GS |
N/A |
1.75 |
|||||
| Konradi et al. 2015 | Acute neurogenic dysphagia, baseline | 19 | Dysphagia | 0.28 (±0.26) | To investigate the direct effect of facio-oral tract therapy on SSF of non- tracheotomised patients with acute neurogenic dysphagia | Visually and by palpation during laryngeal elevation | |
| Acute neurogenic dysphagia, during intervention | N/A | 0.47 (±0.32) | 0.037 | ||||
| Acute neurogenic dysphagia, follow-up (5 mins later) | N/A |
0.33 (±0.28) |
0.44 |
||||
| Kothari et al. 2017 | Brain injury, with dysphagia, baseline | 10 | Dysphagia | 0.12 (±0.05) | <0.001 | To determine if external subglottic air flow (ESAF) influences swallowing frequency in severely dysphagic tracheotomised patients with brain injury | Visually by occupational therapists |
| Brain injury, with dysphagia, during E-SAF | N/A |
0.42 (±0.14) |
|||||
| Seidl et al. (2005) | Neurogenic dysphagia, with TT | 10 | N/A | 0.08 (±0.16) | 0.001 | Comparing SSF in patients with neurogenic dysphagia with or without tracheotomy tubes (TTs) to assess the underlying mechanisms of dysphagia to improve rehabilitation strategies. SSF was assessed by counting elevation of the larynx over 5-min | Observations were videotaped and evaluated by 2 independent investigators |
| Neurogenic dysphagia, without TT |
Dysphagia |
0.33 (±0.3) |
|||||
| Seidl (2007) | Neurological disorders, baseline (day 1) | 10 | High-risk | 0.09 (±0.2) | 0.043 | To investigate the success of facio-oral therapy in patients with neurological disorders. SSF was assessed by counting elevation of the larynx | Observations were videotaped and evaluated by 2 independent investigators |
| Neurological disorders, final day of therapy (day 15) | N/A |
0.47 (±0.35) |
|||||
| Kamarunas et al. (2019)a | Oropharyngeal dysphagia, 30 Hz stimuation | 13 | N/A | Swallow rate not reported Sham vs.: 30 Hz 70 Hz 110 Hz 70+100 Hz 150 Hz |
<0.001 | To examine if laryngeal stimulation increases SSF and if so, discover the optimal rate of vibration using a device. The device showed the potential to increase SSF at 70 Hz and 110 Hz frequencies | 1) Hyolaryngeal elevation (piezoelectric accelerometer peaks) 2) respiratory apnoea (inductive plethysmography - absence of ribcage/abdomen movement) 3) note from trained observer |
| Oropharyngeal dysphagia, 70 Hz stimuation | >0.025 | ||||||
| Oropharyngeal dysphagia, 110 Hz stimuation | <0.001 | ||||||
| Oropharyngeal dysphagia, 70+110 Hz stimuation | >0.025 | ||||||
| Oropharyngeal dysphagia, 150 Hz stimuation |
>0.025 |
||||||
| Mulheren and Ludlow (2017) | Healthy adults, baseline | 10 | Healthy yng | 1.27 (±1.22) | To determine if vibration in comparison to a sham device increases SSF and enhances cortical hemodynamic responses to swallows. SSF analysed over 20-min periods in each condition | 1) Hyolaryngeal elevation (rapid accelerometer change) 2) respiratory apnoea (inductive plethysmography - absence of ribcage/abdomen movement) 3) note from trained observer |
|
| Healthy adults, 30 Hz stimulation | Healthy yng | Swallow rate not reported for comparisons. Sham vs.: 30 Hz 70 Hz 110 Hz 70+100 Hz 150 Hz |
>0.05 | ||||
| Healthy adults, 70 Hz stimulation | 0.05 | ||||||
| Healthy adults, 110 Hz stimulation | >0.05 | ||||||
| Healthy adults, 70 + 110 Hz stimulation | >0.05 | ||||||
| Healthy adults, 150 Hz stimulation |
0.043 |
||||||
| Theurer et al. (2005) | Healthy adults, baseline | 4 | Healthy yng | 0.86 (±0.18) | To determine whether air-pulse trains delivered to the peritonsillar area facilitate swallowing in adults – measured as SSF over a 5-min period | Laryngeal and respiratory movements using pressure transducers from expanding bellows | |
| Healthy adults, unilateral (R) | N/A | 1.95 (±0.89) | |||||
| Healthy adults, unilateral (L) | N/A | ∼1.6(±0.18) | |||||
| Healthy adults, bilateral | N/A | 2.35 (±1.32) | |||||
| Healthy adults, hand | N/A |
0.55 (±0.38) |
|||||
| South et al. (2010) | Parkinson’s, baseline | 23 | Parkinson’s | 0.62 (±0.57) | Assessing the swallow frequency of patients with Parkinson’s at baseline, during gum chewing (GC) and post-CG (5-min each) | Laryngeal bellows and respiratory bellows | |
| Parkinson’s, GC | N/A | 2.99 (±0.6) | <0.0001 | ||||
| Parkinson’s, post-GC | N/A |
1.4 (±0.51) |
<0.0011 |
||||
| D’Angelo et al. (2014) | Healthy adults, awake | 11 11 |
Healthy yng | 0.47 (±0.37) | Examining the effects of anaesthesia and hypercapnia on swallowing-breathing coordination | 1) Pressure catheter (inserted nasally into the hypopharyngeal area) 2) EMG (using wire electrodes inserted into the genioglossus muscle) 3) pneumotachograph (to measure respiratory flow) |
|
| Healthy adults, anaesthesia | N/A | 0.03 (±0.06) | <0.001 | ||||
| Healthy adults, hypercapnia |
N/A |
0.37 (±0.4) |
>0.05 |
aRepeated in both tables as paper included data from both observational and interventional sub-studies.
Healthy younger below 60 years of age with no documented comorbidities; Healthy older of 60 years or more with no documented comorbidities; dysphagic, with documented dysphagia, with or without documented comorbidities; Parkinson’s, with a diagnosis of Parkinson’s disease, without documented dysphagia; Higher risk, with aetiologies associated in the literature with dysphagia that do not fit into the other categories, e.g. post stroke patients without documented dysphagia. None of these aetiologies individually had the four studies required to form a separate sub-group. We acknowledge that this sub-group is the most inhomogeneous in composition.
We report each analysis within each sub-group in turn and for the entire dataset, all sub-groups as a whole.
Estimate of population spontaneous swallow frequency
To give quantitative insight, a meta-analysis was conducted using the Meta and Metafor packages from the R programming language (R Core Team, 2017); the outcome measure was the log-transform of the mean spontaneous swallow frequency (SSF) expressed in units of swallows per minute. We estimated the central value and confidence intervals using a random effects model and inverse variance weighting, with an ad hoc variance correction combining the approaches of Hartung and Knapp (2001) and Jackson et al. (2017).
Assessment of study heterogeneity
Heterogeneity is a measure of inconsistency between the studies making up a meta-analysis. High heterogeneity means the studies are so different that they cannot be considered as samples from the same population. A random-effects meta-analysis is indicated and the results must be treated with caution; a pooled estimate of the outcome measure may not be meaningful.
Results
Literature retrieval
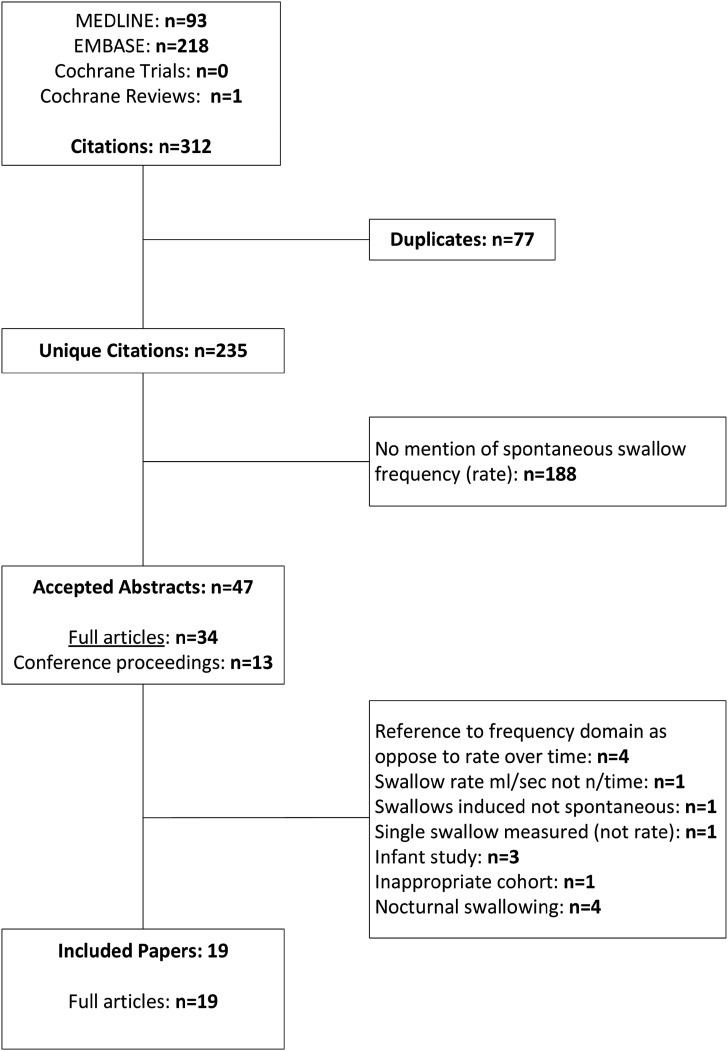
From 312 abstracts found by our search strategy, 19 full papers were included in this review (Figure 1). They are summarised in Tables 2 and 3.
Figure 1.
Flow chart illustrating the abstracts identified and reviewed.
19 papers reported a mixture of observational (n = 9 case–control or cross-sectional) and interventional (n = 10, non-randomised pre-post intervention) studies. Five were of good quality, six of fair/good quality, seven of fair quality, with one poor quality paper (Appendix A). The raters agreed that the poor paper (Marks & Weinreich, 2001) failed in key areas: an inadequate definition of the study population; no sample size justification; risk of bias from lack of blinding; and insufficient detail RE controls and inclusion/exclusion criteria.
Small samples of highly disparate aetiologies were reported, often n = 10 or fewer with the largest single cohort being n = 47. This increases the likelihood that significant comorbidities be present in some samples and not in others. For example, hypersialorrhea is prevalent in patients with Parkinson’s disease (PD) and Wilson’s disease, which may increase swallowing frequency even in those with swallowing difficulties.
Tables 2 and 3 present detail of the studies. There was no standardised way in which to measure spontaneous swallowing and little consistency in identification of dysphagia, timing of observations or methods for detecting swallows. There was a single report (Murray et al., 1996) of Fibre-endoscopic Evaluation of Swallow (FEES) which we excluded from analysis since the FEES investigation is invasive; this likely cannot be considered comparable with the other non-invasive methods.
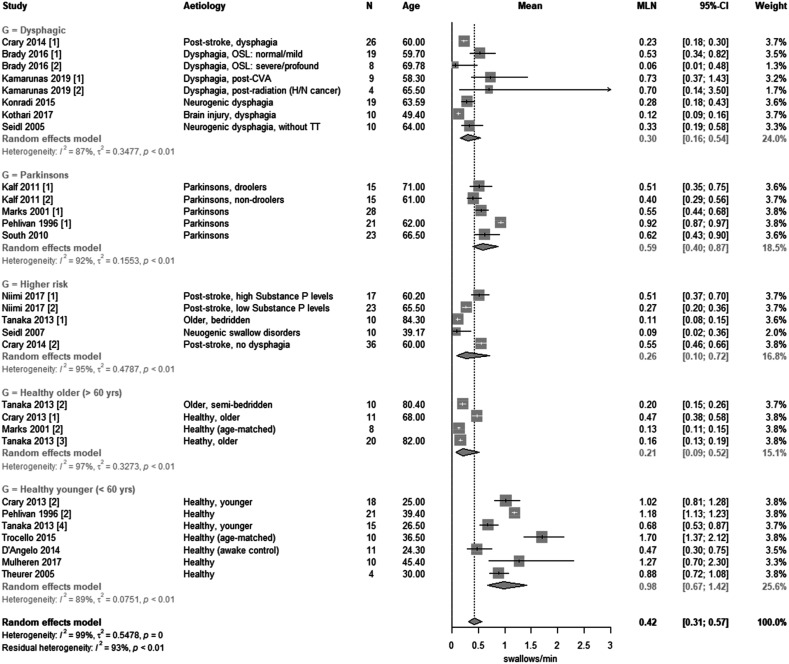
For 29 samples in 19 papers, there was an estimate of spontaneous swallow frequency in either a healthy or clinically relevant population (11 samples were excluded – Tables 2 and 3). The meta-analysis of log transformed SSF is presented in Figure 2.
Figure 2.
Forest plot of the means of study sub-groups from papers categorised as having been diagnosed with dysphagia, Parkinson’s disease, of being of higher risk of dysphagia, being healthy older (>60 years) and healthy younger (<60 years).
The estimates of SSF are visibly variable between and within all sub-groups (Figure 2). The high overall heterogeneity (I2 = 99%, τ2 = 0.55) is anticipated since the studies represent a range of ages, normal volunteers and clinical conditions. Importantly, heterogeneity remains high within each sub-group (dysphagic, I2 = 87%; Parkinson’s, I2 = 92%; higher risk, I2 = 95%; healthy older, I2 = 97%; healthy younger, I2 = 89%). This implies that the large differences in SSF between studies cannot be explained by random variability alone. Nevertheless we believe the data gives a representative picture of previous research into the use of SSF as a screening tool for dysphagia.
The best estimate of overall SSF is 0.42 [0.31; 0.57]/min; this is of limited importance since there are anticipated differences between the sub-groups. To consider the sub-groups, the range of SSF in healthy younger was 0.47–1.7/min, healthy older 0.13–0.47, Parkinson’s 0.4–0.92, higher risk (i.e. post-stroke, bedridden, neurogenic swallow disorders) 0.09–0.55 and dysphagic 0.06–0.73. Notably Murray et al. (1996) with his instrumental FEES assessment reported a dramatically higher swallowing rate in both healthy young (SSF: 2.82 [±1.71]) and healthy older (SSF: 2.96 [±0.88]) volunteers than any other sub-group.
Turning to the sub-group analysis, SSF was similar in dysphagic (0.30 [0.16; 0.54]), higher risk (0.26 [0.10; 0.72]) and healthy older (0.21 [0.09; 0.527]) sub-groups. Whereas, Parkinson’s sub-groups had an SSF of 0.59 [0.40; 0.87] and healthy younger sub-groups average SSF was higher still at 0.98 [0.67; 1.42].
Discussion
In this manuscript, we wished to report the spontaneous swallowing frequency of persons in normal and clinically relevant sub-groups and discuss the implications of the previous work.
Summary of Evidence
Swallowing is a well-described physiological process. As with any physiological measurement, separate measurements of the underlying process ought to show some degree of agreement. Yet this is entirely absent from the literature; heterogeneity among comparable sub-groups of subjects ranges from I2 = 87%–97%. In broad terms, the great majority of variability is between rather than within studies and the studies are not comparable.
We concede that age and comorbidities are potential confounding factors, and indeed, we cannot be certain that the patient sub-groups (dysphagia; PD; high-risk) are comparable. In particular, the high-risk sub-group includes a range of aetiologies. However, even among the healthy young and healthy old sub-groups, there are reports of such vastly different swallow frequency that the studies are inconsistent with each other.
Spontaneous Swallow Frequency
Given this summary, we suggest that data on swallow frequency from different centres, using different technologies or different methodologies, should not be compared. Which (if any) measure of SSF agrees with a physiologically accurate reference standard remains unknown, and so we cannot report normative values for SSF in either healthy or clinically relevant populations.
However, from the intervention studies in Table 3, we note that there is often a repeatable difference between clinical populations within the same study and within individual patients when their health status has changed. It appears that differences in a swallow index measured in the same centre using the same methods and study design are sensitive to clinical condition or to intervention. This seems reasonable since in this situation, the major methodological differences are controlled.
Effect of age and Gender on Spontaneous Swallow Frequency
Despite high heterogeneity, there is a negative association of SSF with age. Healthy young (<60 years) have a mean SSF of 0.98 from seven studies while healthy old have a mean SSF of 0.21 from four studies. There is no overlap between the two sub-groups; simply, older people swallow less. This is in agreement with Crary et al. (2013) and Tanaka et al. (2013), both of whom report significantly higher SSF in young healthy subjects when compared to older healthy subjects using the same technology (see Table 2). However, even in the healthy young sub-group, mean SSFs between studies are highly variable and we cannot say what a true ‘normal’ SSF is.
The reducing SSF with age may be implicated in dysphagia. If so, it is surprising that SSF appears similar across the healthy and unhealthy older population, irrespective of dysphagia risk. As with all correlation studies, cause and effect is difficult to assess; it is possible that a completely different age-related process causes dysphagia and that swallow frequency is irrelevant.
Finally, one conference abstract reported a higher rate of spontaneous swallow in women (Bradley et al., 2012). No full papers have yet been published assessing the effect of gender on SSF, which remains an open question.
The Clinical Utility of Spontaneous Swallow Frequency
In the role of SSF as a screening tool for dysphagia, a ‘red flag’ threshold for concern would be a helpful addition to the literature. Unfortunately, the data do not support such a definition; the healthy younger sub-group had the highest swallow frequency, and the healthy older sub-group had the lowest. Dysphagia has previously been associated with a lower SSF, but SSF in Parkinson’s disease was consistently higher than the global average. Therefore, it would be easy to conclude that the SSF measurement has no clinical utility. However, until the community can agree on how to measure spontaneous swallow frequency accurately, we cannot say for sure that measurements of the underlying physiological process have no clinical value.
If we consider the diagnosis of dysphagia as our criterion, then a validated clinical assessment by SALT may be a more appropriate reference standard. Crary et al. (2014) compared SSF to a clinical assessment, reporting that a threshold of 0.4 swallows/min showed high sensitivity (0.96) but low specificity (0.68) for dysphagia. The workers suggest SSF may be a useful screening tool but lacks the specificity for a definitive diagnosis. Our data would agree; those at heightened risk of dysphagia have a much lower SSF than younger healthy participants. With the exception of PD, the mean SSF was in every other case lower than Crary’s threshold. Note however that this is the mean SSF; sensitivity and specificity are defined at a patient-by-patient level.
Repeated Measurements Within Patients to Monitor Interventions
While it remains unclear what is a normal swallow rate or a clinically significant change in rate, multiple studies suggest that pre- and post-intervention measurements using consistent methods give useful insight. Interventions to increase swallowing frequency have been used to aid in secretion management and have increased understanding in conditions where hypersalivation and resultant drooling are common: Parkinson’s (Kalf et al., 2011; South et al., 2010) and Wilson’s Disease (Trocello et al., 2015). Parkinson’s disease is also associated with increased risk of dysphagia. More than 80% of patients develop dysphagia following disease onset (Suttrup & Warnecke, 2016). Figure 2 suggests that PD patients have a lower SSF than healthy younger subjects but they swallow more regularly than other at-risk sub-groups. This may be explained by known and unknown cases of hypersalivation in this patient population (Kalf et al., 2011). In addition, confounding conditions such as dysphagia may not have been diagnosed, and are not well-reported. This makes it difficult to determine the value of SSF in this population.
Interventions to stimulate swallows appear successful. Four studies were effective at increasing SSF in cohorts with dysphagia; two found facio-oral tract therapy to significantly increase swallow rate (Konradi et al., 2015; Seidl, 2007), one used gustatory stimulation (Brady et al., 2016) and one successfully applied vibrations to stimulation the larynx in a dysphagic cohort (Kamarunas et al., 2019). Studies reported that these interventions have the potential to improve swallow function in those with dysphagia via the measurement of SSF, but no studies report long term outcomes. We observe that these studies were often not blinded or randomised so were open to bias, and it is difficult to know to what improvement should be considered clinically significant.
SSF Measurement Methodologies
Accurate measurement of SSF may be an appropriate reference for basic research in swallow physiology, particularly the effects of healthy ageing. A variety of techniques were used to measure spontaneous swallows, most frequently with the single or combined use of electromyography (EMG), acoustic/sound recording, respiratory bellows/transducers (around the ribcage), bellows/transducers located around the larynx or scoring by a trained observer. Other techniques included auscultation, fiberoptic endoscopic evaluation (FEES) and magnetic resonance imaging (MRI).
A clinically useful measure of SSF should have good validity, that is, should give comparable results to a reference standard. A reference for the measurement of SSF (i.e. the detection of swallows over prolonged periods of time) does not currently exist. Nevertheless any clinical physiological measurement – sphygmomanometry, for example – is predicated on the idea that the measurement indicates the underlying blood pressure irrespective of the technology. There is consensus as to what constitutes a swallow, and so there is objectively a ‘correct’ SSF, at least in principle. If measurements disagree to the extent reported here, we cannot avoid the conclusion that some (or possibly all) technologies are generating incorrect data.
To resolve these issues, comparative studies between several measures of SSF are needed. Only one study compared two systems for measuring SSF (Crary et al., 2013), the first an acoustic recording technique, the comparator a comprehensive multichannel physiologic recording with surface EMG, swallow apnoea, and cervical auscultation. There was no significant difference in SSF between the two methods (0.85/min vs. 0.81/min). Superficially, this suggests that a microphone alone might be used to record swallows. Perhaps more importantly, the consistency between two different technologies might suggest that the technology works well and that other methodological factors (for example, the use of long-term ambulatory use versus lab-based investigations with prescribed activities) are responsible for the poor agreement between centres.
FEES is a well-used and reliable method for assessing a single swallow and may be an appropriate reference standard for an event-by-event comparison, a form of criterion validity. Large-scale validation studies of SSF instrumentation are required before systematic trials in clinical populations, with data involving known swallows and potential artefacts. In order to ensure high specificity with regards to swallow detection, designs must rule out noise artefacts in addition to reliably identifying all swallows.
Conclusion
SSF is a novel, non-invasive clinical variable which warrants further explorations as to its potential to identify persons at risk of dysphagia. Larger, well-conducted studies are needed to develop objective, standardised methods for detecting SSF, and develop normative values in healthy populations in order to answer this question.
Appendix A. NHLBI NIH Study Quality Assessment Tool Outcome.
| Paper | Year | Design | Criteria Questions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Rater #1 | Rater #2 | ||
| Crary | 2013 | Case control | Y | Y | N | Y | Y | Y | NA | Y | Y | CD | Y | Y | Fair | Good | ||
| Crary | 2014 | Cross-sectional | Y | Y | Y | Y | N | Y | Y | NA | Y | N | Y | Y | Y | NA | Good | Good |
| Kalf | 2011 | Case control | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good | Good | ||
| Marks | 2001 | Case control | Y | Y | N | Y | N | Y | NA | Y | Y | N | N | NA | Poor | Poor | ||
| Murray | 1996 | Case control | Y | Y | N | Y | Y | Y | NA | Y | Y | Y | NR | N | Fair | Fair | ||
| Niimi | 2018 | Cross-sectional | Y | Y | NR | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good | Good |
| Pehlivan | 1996 | Case control | Y | Y | N | N | Y | Y | NA | Y | Y | Y | N | NA | Fair | Fair | ||
| Tanaka | 2013 | Case control | Y | Y | N | NA | Y | Y | NA | Y | Y | Y | Y | Y | Fair | Fair | ||
| Trocello | 2015 | Case control | Y | Y | N | Y | N | Y | NR | Y | Y | Y | N | Y | Fair | Fair | ||
| Interventional | ||||||||||||||||||
| Brady | 2016 | Pre-post | Y | Y | Y | NR | CD | Y | Y | N | Y | Y | N | NA | Fair | Good | ||
| D’Angelo | 2014 | Controlled intervention | Y | Y | N | N | N | Y | Y | Y | Y | N | Y | N | Y | Y | Fair | Fair |
| Kamarunas | 2018 | Controlled intervention | N | NA | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | NA | Fair | Fair |
| Konradi | 2015 | Pre-post | Y | Y | Y | Y | CD | N | Y | Y | Y | Y | Y | NA | Fair | Good | ||
| Kothari | 2017 | Pre-post | Y | Y | Y | Y | CD | Y | Y | N | Y | Y | Y | NA | Good | Good | ||
| Mulheren | 2017 | Controlled intervention | N | NA | N | N | N | Y | Y | Y | Y | Y | Y | N | Y | NA | Fair | Good |
| Seidl | 2005 | Pre-post | Y | Y | Y | CD | CD | Y | Y | N | Y | Y | N | NA | Fair | Fair | ||
| Seidl | 2007 | Pre-post | Y | Y | Y | Y | CD | Y | Y | Y | Y | Y | Y | Y | Good | Good | ||
| South | 2010 | Controlled intervention | N | NA | N | N | Y | Y | Y | Y | Y | Y | Y | NA | Y | NA | Fair | Good |
| Theurer | 2005 | Controlled intervention | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y | N | NA | CD | Fair | Good |
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Joseph M. Bulmer https://doi.org/ 0000-0001-7778-0840
Michael J. Drinnan https://doi.org/ 0000-0002-2181-8202
References
- Bradley J. A., Paulson E. S., Kharofa J., Li X., Schultz C., Wang D. (2012). Patient and tumor characteristics and pretreatment swallowing frequency and duration in head-and-neck cancer patients. International Journal of Radiation Oncology Biology Physics, 84. 10.1016/j.ijrobp.2012.07.1366. [DOI] [Google Scholar]
- Brady S. L., Wesling M. W., Donzelli J. J., Kaszuba S. (2016). Swallowing frequency: impact of accumulated oropharyngeal secretion levels and gustatory stimulation. Ear, Nose, and Throat Journal, 95(2), E7-E9. 10.1177/014556131609500203. [DOI] [PubMed] [Google Scholar]
- Crary M., Carnaby G., Sia I. (2014). Spontaneous swallow frequency compared with clinical screening in the identification of dysphagia in acute stroke. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 23(8), 2047-2053. https://www.sciencedirect.com/science/article/pii/S1052305714001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary M. A., Sura L., Carnaby G. (2013). Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency. Dysphagia, 28(1), 86-94. 10.1007/s00455-012-9416-y. [DOI] [PubMed] [Google Scholar]
- D'Angelo O. M., Diaz-Gil D., Nunn D., Simons J. C., Gianatasio C., Mueller N., Meyer M. J., Pierce E., Rosow C., Eikermann M. (2014). Anesthesia and increased hypercarbic drive impair the coordination between breathing and swallowing. Anesthesiology, 121(6), 1175-1183. 10.1097/ALN.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges C., Scheeren B., Gomes E., Codas L. B. (2014). Screening tools for dysphagia: A systematic review. Codas, 26(5), 343-349. https://www.scielo.br/scielo.php?pid=S2317-17822014000500343&script=sci_arttext&tlng=es. [DOI] [PubMed] [Google Scholar]
- Fu R., Gartlehner G., Grant M., Shamliyan T., Sedrakyan A., Wilt T. J., Griffith L., Oremus M., Raina P., Ismaila A., Santaguida P., Lau J., Trikalinos T. A. (2011). Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. Journal of Clinical Epidemiology, 64(11), 1187-1197. 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Harkness G., Bentley D., Roghmann K. J. (1990). Risk factors for nosocomial pneumonia in the elderly. The American Journal of Medicine, 89(4), 457-463. https://www.sciencedirect.com/science/article/pii/000293439090376O. [DOI] [PubMed] [Google Scholar]
- Hartung J., Knapp G. (2001). On tests of the overall treatment effect in meta-analysis with normally distributed responses. Statistics in Medicine, 20(12), 1771-1782. 10.1002/sim.791. [DOI] [PubMed] [Google Scholar]
- Jackson D., Law M., Rücker G., Schwarzer G. (2017). The Hartung‐Knapp modification for random‐effects meta‐analysis: A useful refinement but are there any residual concerns? Statistics in Medicine, 36(25), 3923-3934. 10.1002/sim.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalf J. G., Munneke M., van den Engel-Hoek L., de Swart B. J., Borm G. F., Bloem B. R., Zwarts M. J. (2011). Pathophysiology of diurnal drooling in Parkinson’s disease. Movement Disorders, 26(9), 1670-1676. 10.1002/mds.23720. [DOI] [PubMed] [Google Scholar]
- Kamarunas E., Wong S. M., Ludlow C. L. (2019). Laryngeal vibration increases spontaneous swallowing rates in chronic oropharyngeal dysphagia: a proof-of-principle pilot study. Dysphagia, 34(5), 640-653. 10.1007/s00455-018-9962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi J., LerchCataldo A.M., Kerz T. (2015). Direct effects of facio-oral tract therapy on swallowing frequency of non-tracheotomised patients with acute neurogenic dysphagia. SAGE Open Medicine, 3, 205031211557895. 10.1177/2050312115578958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari M., Bjerrum K., Nielsen L. H., Jensen J., Nielsen J. F. (2017). Influence of external subglottic air flow on dysphagic tracheotomized patients with severe brain injury. Annals of Otology, Rhinology & Laryngology, 126(3), 199-204. 10.1177/0003489416683192. [DOI] [PubMed] [Google Scholar]
- Lear C.S., Flanagan J. B., Jr., Mooreess C. F. (1965). The frequency of deglutition in man. Archives of Oral Biology, 10, 83-100. https://www.sciencedirect.com/science/article/pii/0003996965900609. [DOI] [PubMed] [Google Scholar]
- Marks L., Weinreich J. (2001). Drooling in Parkinson’s disease: a novel tool for assessment of swallow frequency. International Journal of Language & Communication disorders, (36), 288-291. 10.3109/13682820109177899. [DOI] [PubMed] [Google Scholar]
- Mulheren R. W., Ludlow C. L. (2017). Vibration over the larynx increases swallowing and cortical activation for swallowing. Journal of Neurophysiology, 118(3), 1698-1708. 10.1152/jn.00244.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Langmore S. E., Ginsberg S., Dostie A. (1996). The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia, 11, 99-103. https://link.springer.com/content/pdf/10.1007/BF00417898.pdf. [DOI] [PubMed] [Google Scholar]
- Niimi M., Hashimoto G., Hara T., Yamada N., Abo M., Fujigasaki H., Ide T. (2018). Relationship between frequency of spontaneous swallowing and salivary substance P level in patients with acute stroke. Dysphagia, 33(4), 414-418. 10.1007/s00455-017-9867-2. [DOI] [PubMed] [Google Scholar]
- Park Y., Bang H. L., Han H. R., Chang H. K. (2015). Dysphagia screening measures for use in nursing homes: a systematic review. Synapse.Koreamed.Org, 45(1), 1-13. 10.4040/jkan.2015.45.1.1. [DOI] [PubMed] [Google Scholar]
- Pehlivan M., Yüceyar N., Ertekin C., Celebi G., Ertaş M., Kalayci T., Aydoĝdu I. (1996). An electronic device measuring the frequency of spontaneous swallowing: digital phagometer. Dysphagia, 11, 259-264. https://link.springer.com/content/pdf/10.1007/BF00265212.pdf. [DOI] [PubMed] [Google Scholar]
- Rudney J., Ji Z., Larson C. J. (1995). The prediction of saliva swallowing frequency in humans from estimates of salivary flow rate and the volume of saliva swallowed. Archives of Oral Biology, 40(6), 507-512 https://www.sciencedirect.com/science/article/pii/0003996995000049. [DOI] [PubMed] [Google Scholar]
- Seidl R. O., Nusser-Müller-Busch R., Ernst A. (2005). The influence of tracheotomy tubes on the swallowing frequency in neurogenic dysphagia. Otolaryngology-Head and Neck Surgery, 132(3), 484-486. 10.1016/j.otohns.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Seidl R. O., Nusser-Müller-Busch R., Hollweg W., Westhofen M., Ernst A. (2007). Pilot study of a neurophysiological dysphagia therapy for neurological patients. Clinical Rehabilitation, 21(8), 686-697. 10.1177/0269215507076393. [DOI] [PubMed] [Google Scholar]
- Shaker R. (1995). Airway protective mechanisms: current concepts. Dysphagia, 10(4), 216-227. https://link.springer.com/content/pdf/10.1007/BF00431413.pdf. [DOI] [PubMed] [Google Scholar]
- South A., Somers S., Neurology M. J. (2010). Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology, 74(15), 1198-1202. https://n.neurology.org/content/74/15/1198.short. [DOI] [PubMed] [Google Scholar]
- Suttrup I, Warnecke T. (2016). Dysphagia in Parkinson’s disease. Dysphagia, 31, 24-32. 10.1007/s00455-015-9671-9. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Nohara K., Kotani Y., Matsumura M., Sakai T. (2013). Swallowing frequency in elderly people during daily life. Journal of Oral Rehabilitation, 40(10), 744-750. 10.1111/joor.12085. [DOI] [PubMed] [Google Scholar]
- Theurer J. A., Bihari F., Barr A. M., Martin R. E. (2005). Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia, 20(4), 254-260. 10.1007/s00455-005-0021-1. [DOI] [PubMed] [Google Scholar]
- Trocello J.-M., Osmani K., Pernon M., Chevaillier G., de Brugière C., Remy P., Wenisch E., Cousin C., Girardot-Tinant N., Woimant F. (2015). Hypersialorrhea in Wilson’s Disease. Dysphagia, 30(5), 489-495. 10.1007/s00455-015-9627-0. [DOI] [PubMed] [Google Scholar]
- Vergis E., Brennen C., Wagener M., Muder R. R. (2001). Pneumonia in long-term care: a prospective case-control study of risk factors and impact on survival. Arch Intern Med, 161(19), 2378-2381. https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/649245. [DOI] [PubMed] [Google Scholar]