Abstract
Background:
The real-world effect of intermittently scanned continuous glucose monitoring on glucose control in type 2 diabetes treated with basal insulin is uncertain. This retrospective real-world study aimed to evaluate change in glycated hemoglobin (HbA1c) amongst adults with type 2 diabetes managed with basal insulin starting flash glucose monitoring.
Methods:
Medical records were reviewed for adults with type 2 diabetes treated with basal insulin for ⩾1 year and using FreeStyle LibreTM Flash Glucose Monitoring for ⩾3 months. Prior to device use an HbA1c 8.0%–12.0% was recorded and a further HbA1c result was recorded 3–6 months (90–194 days) after starting device use.
Results:
Medical records (n = 91) analyzed from six Canadian diabetes centers showed HbA1c significantly decreased by 0.8% ± 1.1 (mean ± SD, [p < 0.0001]) from mean baseline HbA1c 8.9% ± 0.9 to 8.1% ± 1.0 at 3–6 months after initiating flash glucose monitoring. HbA1c improvement was not independently associated with age, BMI, insulin use duration, or sex.
Conclusion:
This Canadian real-world retrospective study showed significantly reduced HbA1c following initiation of flash glucose monitoring technology to further support management of type 2 diabetes treated with basal insulin.
Keywords: Flash sensor glucose monitoring, HbA1c, insulin, type 2 diabetes
Introduction
Globally, an HbA1c measurement remains the accepted gold standard parameter for evaluating overall glycemic control. There is a growing body of evidence for improvements in glycemic control using continuous glucose monitoring technology in patients with type 2 diabetes managed with intensive insulin therapy.1–4 However, any effect in type 2 diabetes treated with basal insulin alone is less certain.5 This retrospective real-world study aimed to evaluate the impact of flash glucose monitoring on HbA1c in this specific population.
Methods
This retrospective non-interventional single-arm chart review study was conducted by six diabetes centers in Canada. Each center searched their databases for eligible patient medical records for individuals aged 18 years or older with type 2 diabetes, on a basal only insulin regimen for 1 year or more, using FreeStyle Libre® Flash Glucose Monitoring System (Abbott, Diabetes Care, Witney, UK) for 3 months or more when data were collected, had an HbA1c measurement between 8.0% and 12.0% (64–108 mmol/mol) 3 months or less prior to device use and an HbA1c result recorded 3–6 months after. Final eligibility for inclusion was determined by statistical analysis of the extracted information.
All HbA1c measurements used in the analysis were from the medical records. A baseline HbA1c result was defined as measured 90 days or less before the patient commenced use of the device (index date). The HbA1c result after commencing device use was defined as being measured between 90 and 194 days after the index date. Sites also extracted information for: age, sex, BMI, concomitant disease/diabetes complications, insulin use duration, and medications.
This chart review study collected retrospective, anony-mized data from medical records, the protocol was submitted to and approved by an accredited centralized institutional review board, informed consent was not required.
Outcomes
Primary outcome was evaluation of HbA1c change from baseline to an HbA1c measurement taken between 3 and 6 months (90 and 194 days) after commencing the device. The primary endpoint was analyzed for the following prespecified subgroups: age (<65 and ⩾65 years), baseline HbA1c (<9.0 and ⩾9.0%), BMI (<30 and ⩾30 kg/m2), sex, frequency of blood glucose testing, and insulin use duration (<4 and ⩾4 years).
Statistical analysis
Differences between HbA1c at baseline and 90–194 days after initiation of device use were assessed by a paired t test. To detect a change in HbA1c of 0.35% (3.8 mmol/mol) with a power of 80% (at p < 0.05), a total of 78 medical records were needed, based on an SD of change in HbA1c of 1.1%.6 Data analysis was performed by qualified statisticians at Abbott Diabetes Care (UK) using version 9.4 of SAS.
Results
Medical records (n = 103) were identified by six diabetes centers in Canada and data were extracted for the period November 2017–May 2020. Three medical records did not meet the inclusion criteria, four medical records omitted a baseline HbA1c within 90 days of device initiation and two did not have an HbA1c result 90–194 days after starting device use. Three medical records were retrospectively excluded as prandial insulin was used after device use initiation. From the 91 medical records included in the primary endpoint analysis, mean age was 64.3 years, mean baseline HbA1c was 8.9%, mean BMI 30.5 kg/m2, and 63 (69.2%) records were for male patients. Glucose-lowering medication was prescribed in 87 (95.6%) records and GLP1-agonists in 44 (48.4%), in addition to basal insulin.
Primary end point
HbA1c significantly decreased by 0.8% ± 1.1 mean ± SD (95% confidence interval for change –1.1to –0.6 [−9.1 mmol/mol ± 12.1, −11.6 to −6.6], p < 0.0001) from baseline HbA1c 8.9% ± 0.9 (74.1 mmol/mol ± 9.7) to 8.1% ± 1.0 (65.0 mmol/mol ± 10.5) 3–6 months after initiation of device use.
The mean number of days between device initiation and final HbA1c values was 123.0 days (median 118.0). A sensitivity analysis for change in HbA1c for different time windows of the final HbA1c value (121–149, 107–163, and 90–180 days) showed change in HbA1c remained the same ([−0.8%, p = 0.0002], [−0.7%, p < 0.0001], and [−0.8%, p < 0.0001], respectively).
Comparing baseline HbA1c to HbA1c levels for each month of the 3–6 month period after the index date (months 3–4, 4–5, 5–6, and 5½–6½), HbA1c change remained similar ([−0.8%, p < 0.0001], [−0.8%, p = 0.0002], [−0.7%, p = 0.0299], and [−1.1%, p = 0.0475], respectively).
Subgroup analysis
HbA1c improvement was observed by age (<65 years, p < 0.0001 and ⩾65 years, p = 0.0001), baseline HbA1c (<9% and ⩾9.0%, p < 0.0001), BMI (<30 and ⩾30 kg/m2, p < 0.0001), insulin use duration (<4 and ⩾4 year, p < 0.0001), and sex (female [n = 28, p = 0.0147] and male [n = 63, p < 0.0001]), shown in Figure 1. Change in HbA1c was more pronounced in baseline levels ⩾9.0% (−1.6% ± 1.3 mean ± SD, p < 0.0001). Blood glucose testing frequency data was available for 57% (n = 52/91) of medical records. The majority (n = 47/52) of testing was ⩽2 tests/day and HbA1c reduced (−1.1 ± 1.2, mean ± SD, p < 0.0001). For >2 tests/day (n = 5) final HbA1c fell by 1.3% ± 1.4 without reaching significance (p = 0.1161).
Figure 1.
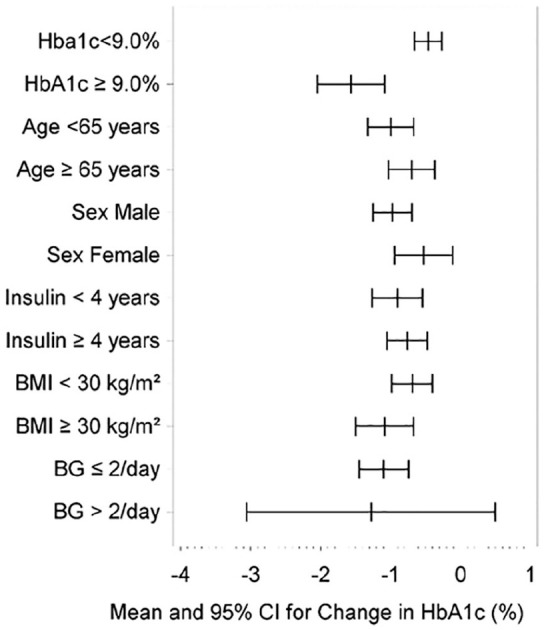
Change in HbA1c by baseline HbA1c, age group, sex, duration of insulin therapy, BMI, and BG test frequency at baseline (mean and 95% confidence interval).
Discussion
The last decade has seen a rapid advance in safe and accurate glucose monitoring technology. Evidence is growing for glycemic benefit associated with glucose monitoring technology use in type 2 diabetes, however, more recent studies excluded basal only insulin use.1,2,4 The current retrospective chart review study evaluated the impact on HbA1c of initiating flash glucose monitoring in type 2 diabetes managed with a basal insulin only regimen in Canada. The observed significant decrease in HbA1c of 0.8% supports the 0.6% reduction reported for flash glucose monitoring use in type 2 diabetes treated with basal insulin by a recent American study and findings from three European studies and one study from Israel in type 2 diabetes and basal-bolus insulin therapy.3,4,7 Vigersky et al.5 reported a 1.2% HbA1c reduction (within the intervention group) at 6 months for a prospective randomized controlled trial analyzing intermittent use of real-time CGM in a type 2 diabetes cohort using either basal insulin or glucose lowering medication.
A recent meta-analysis suggests that a mean HbA1c reduction of 0.7% could be expected for a mean baseline HbA1c of 8.9%.8 Furthermore, flash glucose monitoring use in type 1 and type 2 diabetes is associated with a 0.4% decrease in HbA1c for each 1% increase in baseline levels over 7.2% (4 mmol/mol for each 11 mmol/mol over 55 mmol/mol), which supports the HbA1c change in the current study.9 The observed change in HbA1c was more marked at baseline HbA1c levels above 9%, a pattern which has been noted by others.4,9
Similarly, to other recent studies assessing flash glucose monitoring use in type 2 diabetes, HbA1c reduction in the current study was not associated with age.4 HbA1c reduction was also observed with low baseline frequency of blood glucose testing, supporting data from France, and the view that minimal blood glucose testing frequency may not influence uptake or benefit from flash glucose monitoring use.4
Baseline demographics and characteristic data for age, BMI, duration of insulin use are comparable to those reported for CGM studies in a similar population.5,10,11 The observed baseline HbA1c is comparable to an RCT in a similar population10 and up to 1% (11 mmol/mol) higher than both a large multinational prospective study11 in a similar cohort and a randomized controlled trial in type 2 diabetes with basal insulin or glucose lowering medication.5 This elevated baseline HbA1c was not unexpected as insulin initiation in type 2 diabetes is often delayed in Canada and less than half of the type 2 diabetes population achieve glycemic targets.12 Extracted medical history data were comparable to other studies in type 2 diabetes and use of oral medications in addition to basal insulin reflects current practice in Canada.
The current study methodology enabled an evaluation of the impact of the device in real-life routine clinical settings in multiple geographical areas of Canada. The number of medical records available for review was limited by the exclusion of prandial insulin use, a common next therapy step, after initiation of the device. Additionally, the study design limited the attributable benefits associated with flash glucose monitoring and standard diabetes care as these were not evaluated against a control group or through propensity score matching. The retrospective study approach also prevented more individualized data capture, such as changes in medication, meaning an evaluation of therapy adjustments and the primary endpoint was not possible. However, the influence on patient behavior/inclusion was minimized allowing the observed results to be generalized and applied in other routine practice settings. Lastly, although the demonstrated decrease in HbA1c in the current study is notable, mean HbA1c at study end remains above the recommended target and further studies are warranted in this population.
Conclusions
In conclusion, this retrospective study showed a significantly reduced HbA1c following the initiation of flash glucose monitoring technology to support management of type 2 diabetes treated with basal insulin in Canada.
Acknowledgments
The authors would like to thank Marcus Green and Zoe Welsh for statistical support (Statistics, Abbott, Diabetes Care, UK), Amanda Cartmale (Scientific Affairs Manager, Abbott, Diabetes Care, UK) for editorial support with manuscript preparation, and all those who contributed to the collection of data at the study sites; Dr Tom Elliott (Chief Investigator), BC Diabetes, Vancouver, British Columbia; Dr Alexander Abitbol, LMC Diabetes & Endocrinology Ltd, Toronto, Ontario; Dr Sorin Beca, My Endo Diabetes and Endocrinology Centers, Markham, Ontario; Dr Rajendra Beharry, Beharry Medical Center, Toronto, Ontario; Dr Michael Tsoukas, Clinique Medicale Hygea, Montreal, Quebec; and Dr Alexandro Zarruk, West Island Metabolic Unit, Pierrefonds, Quebec.
Footnotes
Data availability: The datasets generated during and/or analyzed in the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A. Abitbol reports grants from Abbott Canada, during the conduct of the study; grants and personal fees from Sanofi, Novo Nordisk, Janssen, AstraZeneca, Eli Lilly, Senseonics, and Merck; personal fees from Boehringer Ingelheim; grants from Amgen, Gilead, Pfizer, Zealand, and Dexcom; and grants from Zucara; outside the submitted work; S. Beca has nothing to disclose; R Beharry has nothing to disclose; T. Elliot has nothing to disclose; M. Tsoukas reports personal fees from Abbott during the conduct of the study; A. Zarruk has nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Abbott, Diabetes Care, Witney UK in collaboration with the chief investigator designed the study protocol. Abbott, Diabetes Care provided all study materials, was involved in data analysis and reporting but was not part of the authors’ interpretation of the study results. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017; 8(1): 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily injections. A randomized trial. Ann Intern Med 2017; 167(6): 365–374. [DOI] [PubMed] [Google Scholar]
- 3.Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 2019; 42(7): 1178–1184. [DOI] [PubMed] [Google Scholar]
- 4.Kröger J, Fasching P, Hanaire H.Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther 2020; 11(1): 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigersky RA, Fonda SJ, Chellappa M, et al. Sort and long term effect of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbott, Diabetes Care. Data on file 2015. [Google Scholar]
- 7.Miller E, Brandner L, Wright E.84-LB: HbA1c reduction after initiation of the FreeStyle Libre System in type 2 diabetes patients on long-acting insulin or noninsulin therapy. Diabetes 2020; 69(Suppl. 1). 10.2337/db20-84-LB (accessed 26 May 2021). [DOI] [Google Scholar]
- 8.Evans M, Welsh Z, Ells S, et al. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2020; 11(1): 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castellana M, Parisi C, Di Molfetta S, et al. Efficacy and safety of flash glucose monitoring in patients with type 1 and type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care 2020; 8(1): e001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilany J, Bhandari H, Nabriski D, et al. Effect of prandial treatment timing adjustment, based on continuous glucose monitoring, in patients with type 2 diabetes uncontrolled with once-daily basal insulin: a randomized, phase IV study. Diabetes Obes Metab 2018; 20(5): 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray KK, Kendall DM, Zhao Z, et al. A multinational observational study assessing insulin use: understanding the determinants associated with progression of therapy. Diabetes Obes Metab 2019; 21(5): 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berard L, Antonishyn N, Arcudi K, et al. Insulin matters: a practical approach to basal insulin management in type 2 diabetes. Diabetes Ther 2018; 9(2): 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]


