Abstract
There are no relevant meta-analyses that have assessed the safety of the sodium-glucose transporter 2 (SGLT2) inhibitors in different chronic diseases. We aimed at evaluating the safety of four SGLT2 inhibitors in three chronic diseases by meta-analysis of the large randomized trials of SGLT2 inhibitors. We performed random-effects meta-analysis and carried out subgroup analysis according to type of underlying diseases and type of SGLT2 inhibitors. SGLT2 inhibitors versus placebo significantly reduced the risk of acute kidney injury (RR 0.75, 95% CI 0.66–0.85), and showed the reduced trend in the risk of severe hypoglycemia (RR 0.86, 95% CI 0.71–1.03). SGLT2 inhibitors significantly increased the risks of diabetic ketoacidosis (RR 2.57), genital infection (RR 3.75), and volume depletion (RR 1.14); and showed the increased trends in the risks of fracture (RR 1.07), amputation (RR 1.21), and urinary tract infection (RR 1.07). These effects exhibited by SGLT2 inhibitors were consistent across three chronic diseases (i.e. type 2 diabetes, chronic heart failure, and chronic kidney disease) and four SGLT2 inhibitors (i.e. dapagliflozin, empagliflozin, ertugliflozin, and canagliflozin) (all Psubgroup > 0.05). These findings will guide that specific adverse events are monitored when SGLT2 inhibitors are used in clinical practice.
Keywords: SGLT2 inhibitors, acute kidney injury, diabetic ketoacidosis, genital infection, volume depletion
Introduction
Large randomized trials of sodium-glucose transporter 2 (SGLT2) inhibitors have been conducted on the purpose of assessing the cardiorenal or death endpoints. However, those individual trials are underpowered to evaluate the specific endpoints relevant with safety. Although there have been meta-analysis studies1–4 published which have assessed the safety of SGLT2 inhibitors in type 2 diabetes (T2D), these studies have produced the inconsistent findings. Moreover, there is a lack of relevant meta-analyses that have assessed the safety of the sodium-glucose transporter 2 (SGLT2) inhibitors in different chronic diseases. Thus, we included all the large randomized trials of SGLT2 inhibitors including three recently published trials (i.e. DAPA-CKD,5 EMPEROR-Reduced,6 and VERTIS CV7), and carried out this meta-analysis to evaluate the safety of four SGLT2 inhibitors in three chronic diseases.
Methods
In this study we included the large randomized placebo-controlled trials of SGLT2 inhibitors that aimed at assessing cardiovascular or renal outcomes in patients with T2D, or in patients with chronic heart failure (CHF), or in patients with chronic kidney disease (CKD). The eight safety outcomes of interest were fracture, diabetic ketoacidosis, amputation, urinary tract infection, genital infection, acute kidney injury, severe hypoglycemia, and volume depletion. We used a random-effects model to perform meta-analysis, to generate pooled risk ratios (RRs) and 95% confidence intervals (CIs). Heterogeneity was estimated by I2. We performed subgroup analysis respectively stratified by different chronic diseases and different SGLT2 inhibitors, and tested the subgroup effects using Cochran’s Q test. P value <0.05 means statistical significance.
Results
After literature search (search until September 25th, 2020; Supplemental Appendix 1, pp 1–2) and study selection (Supplemental Figure S1 in Appendix 1), we included eight large randomized trials5–12 of SGLT2 inhibitors: DAPA-CKD5 assessing dapagliflozin in CKD patients, EMPEROR-Reduced6 assessing empagliflozin in CHF patients, DAPA-HF8 assessing dapagliflozin in CHF patients, VERTIS CV7 assessing ertugliflozin in T2D patients, CREDENCE9 assessing canagliflozin in T2D patients, DECLARE–TIMI 5810 assessing dapagliflozin in T2D patients, CANVAS Program11 assessing canagliflozin in T2D patients, and EMPA-REG OUTCOME12 assessing empagliflozin in T2D patients. All the included studies had the low bias risk (Supplemental Figure S2 in Appendix 1), and involved a total of 33,124 participants in the SGLT2 inhibitor group and 26,568 participants in the placebo group. Supplemental Appendix 2 presents the original data used for meta-analysis.
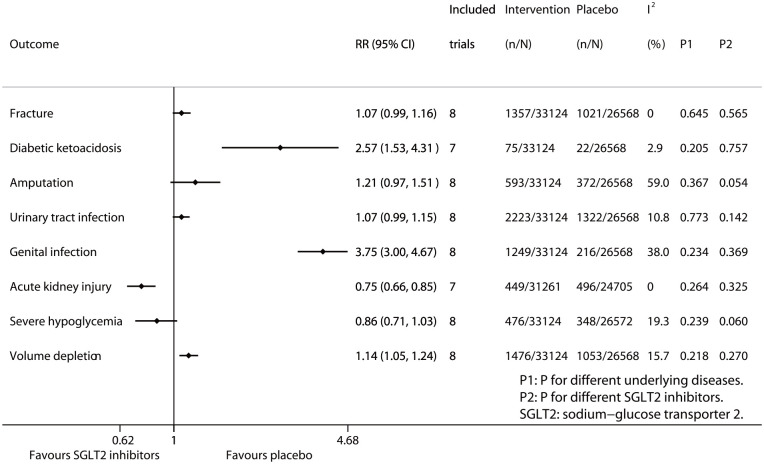
Figure 1 summarizes the results of meta-analysis and subgroup analysis. Compared with placebo SGLT2 inhibitors significantly reduced the risk of acute kidney injury (RR 0.75, 95% CI 0.66–0.85; p for drug effect <0.001), while SGLT2 inhibitors showed the reduced trend in the risk of severe hypoglycemia (RR 0.86, 95% CI 0.71–1.03; p for drug effect = 0.096). On the contrary, SGLT2 inhibitors significantly increased the risks of diabetic ketoacidosis (RR 2.57, 95% CI 1.53–4.31; P for drug effect <0.001), genital infection (RR 3.75, 95% CI 3.00–4.67; P for drug effect <0.001), and volume depletion (RR 1.14, 95% CI 1.05–1.24; p for drug effect = 0.002); while SGLT2 inhibitors showed the increased trends in the risks of fracture (RR 1.07, 95% CI 0.99–1.16; P for drug effect = 0.081), amputation (RR 1.21, 95% CI 0.97–1.51; P for drug effect = 0.085), and urinary tract infection (RR 1.07, 95% CI 0.99–1.15; p for drug effect = 0.074). The above results of meta-analysis are detailed in Supplemental Figures S3–S10 in Appendix 1. The results of subgroup analysis according to different diseases (Psubgroup ranged from 0.205 to 0.773; Supplemental Figures S11–S18 in Appendix 1) and different SGLT2 inhibitors (Psubgroup ranged from 0.054 to 0.757; Supplemental Figures S19–S26 in Appendix 1) suggest that the effects of SGLT2 inhibitors on the eight safety outcomes assessed in the study were consistent across three chronic diseases (i.e. T2D, CHF, and CKD) and four SGLT2 inhibitors (i.e. dapagliflozin, empagliflozin, ertugliflozin, and canagliflozin).
Figure 1.
Meta-analysis of SGLT2 inhibitors and eight safety endpoints.
Discussion
This study is the first one that evaluated the safety of four SGLT2 inhibitors (i.e. dapagliflozin, empagliflozin, ertugliflozin, and canagliflozin) in three chronic diseases (i.e. T2D, CHF, and CKD), and has two key findings. First, SGLT2 inhibitors versus placebo significantly reduced the risk of acute kidney injury (RR 0.75) and showed the reduced trend in the risk of severe hypoglycemia, regardless of type of chronic diseases and type of SGLT2 inhibitors. Second, SGLT2 inhibitors versus placebo significantly increased the risks of diabetic ketoacidosis (RR 2.57), genital infection (RR 3.75), and volume depletion (RR 1.14), and showed the increased trends in the risks of fracture, amputation, and urinary tract infection, regardless of type of chronic diseases and type of SGLT2 inhibitors. Compared with prior meta-analysis studies1–4 which assessed the safety of SGLT2 inhibitors only in T2D, our study included three recently published trials5–7 and therefore assessed the safety of different SGLT2 inhibitors in three chronic diseases.
One strength of this meta-analysis is that all the original studies included were with high quality. On the contrary, one weakness is that the studies included in the meta-analysis were conducted in patient populations with very considerable differences in their baseline characteristics, including the comorbidities. Thus, the findings identified by this meta-analysis need to be further verified in well-designed studies minimizing ascertainment bias.
In conclusion, SGLT2 inhibitors significantly reduce the risk of acute kidney injury, and show the reduced trend in the risk of severe hypoglycemia; whereas this drug class significantly increase the risks of diabetic ketoacidosis, genital infection, and volume depletion, and show the increased trends in the risks of fracture, amputation, and urinary tract infection, regardless of type of underlying diseases and type of SGLT2 inhibitors. These findings will guide that specific adverse events are monitored when SGLT2 inhibitors are used in clinical practice.
Supplemental Material
Supplemental material, sj-pdf-1-dvr-10.1177_14791641211011016 for Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors by Mei Qiu, Liang-Liang Ding, Miao Zhang and Hai-Rong Zhou in Diabetes & Vascular Disease Research
Supplemental material, sj-xlsx-2-dvr-10.1177_14791641211011016 for Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors by Mei Qiu, Liang-Liang Ding, Miao Zhang and Hai-Rong Zhou in Diabetes & Vascular Disease Research
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Shenzhen Key Medical Discipline Construction Fund (SZXK063).
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. All the original data for this meta-analysis study were collected from published articles. Thus, ethics approval was not required.
ORCID iD: Mei Qiu  https://orcid.org/0000-0001-5013-657X
https://orcid.org/0000-0001-5013-657X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 2020; 173: 278–286. [DOI] [PubMed] [Google Scholar]
- 2.Huang CY, Lee JK.Sodium-glucose co-transporter-2 inhibitors and major adverse limb events: a trial-level meta-analysis including 51 713 individuals. Diabetes Obes Metab 2020; 22(12): 2348–2355. [DOI] [PubMed] [Google Scholar]
- 3.Heyward J, Mansour O, Olson L, et al. Association between sodium-glucose cotransporter 2 (SGLT2) inhibitors and lower extremity amputation: a systematic review and meta-analysis. PLoS One 2020; 15: e234065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita S, Kuno T, Takagi H, et al. Risk of amputation associated with sodium-glucose co-transporter 2 inhibitors: a meta-analysis of five randomized controlled trials. Diabetes Res Clin Pract 2020; 163: 108136. [DOI] [PubMed] [Google Scholar]
- 5.Heerspink H, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383(15): 1436–1446. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383(15): 1413–1424. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383(15): 1425–1435. [DOI] [PubMed] [Google Scholar]
- 8.McMurray J, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381(21): 1995–2008. [DOI] [PubMed] [Google Scholar]
- 9.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dvr-10.1177_14791641211011016 for Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors by Mei Qiu, Liang-Liang Ding, Miao Zhang and Hai-Rong Zhou in Diabetes & Vascular Disease Research
Supplemental material, sj-xlsx-2-dvr-10.1177_14791641211011016 for Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors by Mei Qiu, Liang-Liang Ding, Miao Zhang and Hai-Rong Zhou in Diabetes & Vascular Disease Research